A New Slurry for Photocatalysis-Assisted Chemical Mechanical Polishing of Monocrystal Diamond
Abstract
1. Introduction
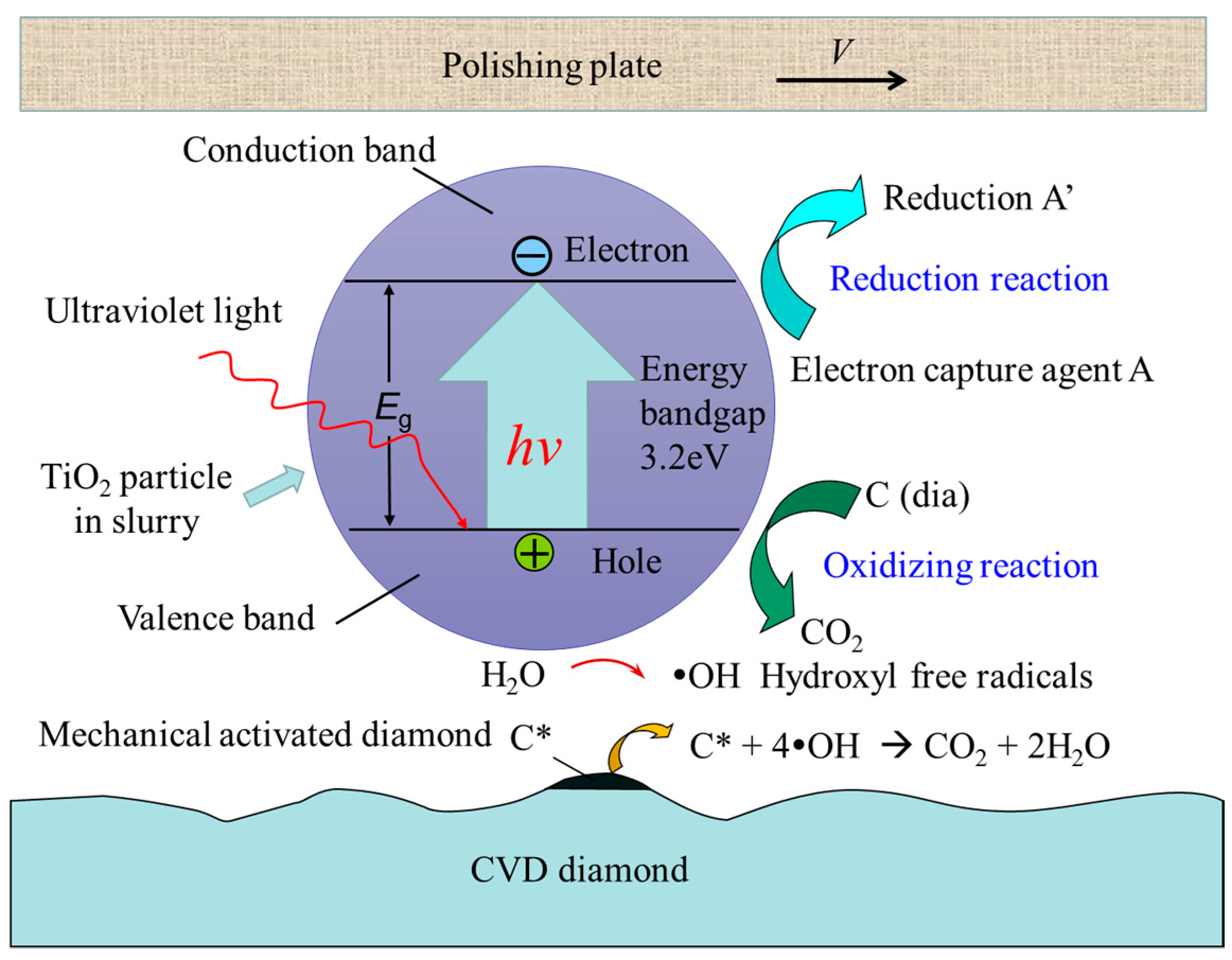
2. Principle, Methods and Experiments
2.1. Preparation of PCMP Slurry
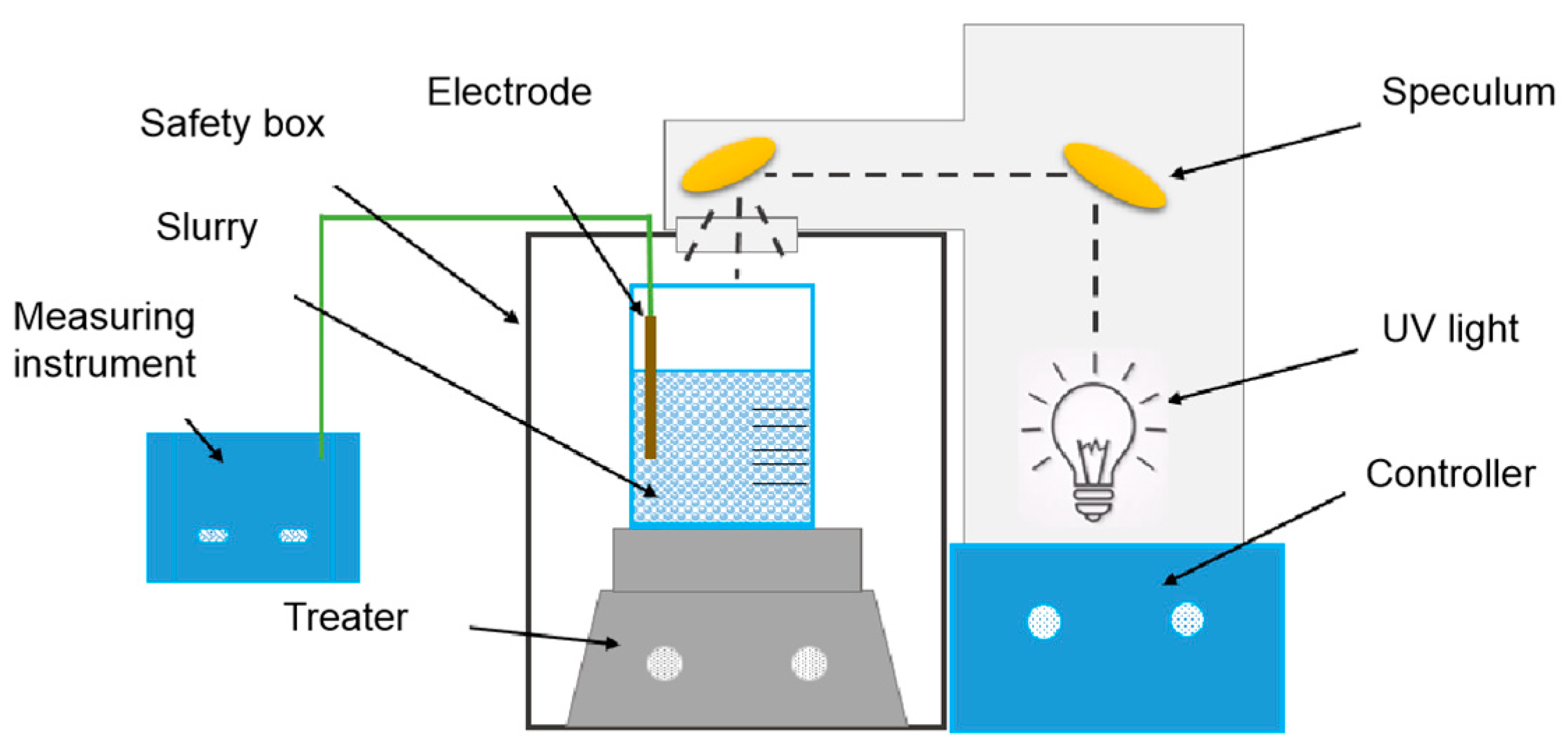
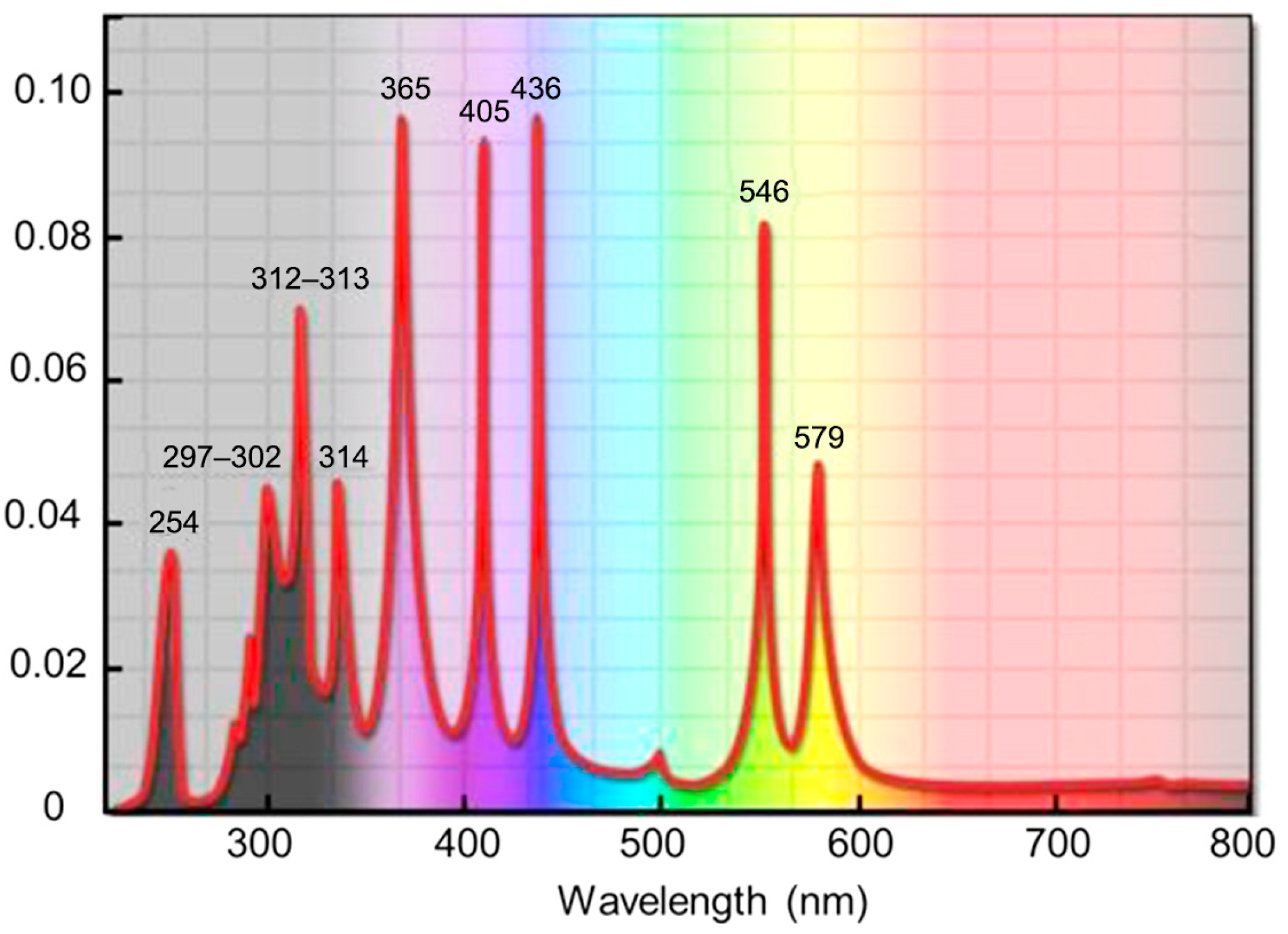
2.2. Characterization of PCMP Slurry
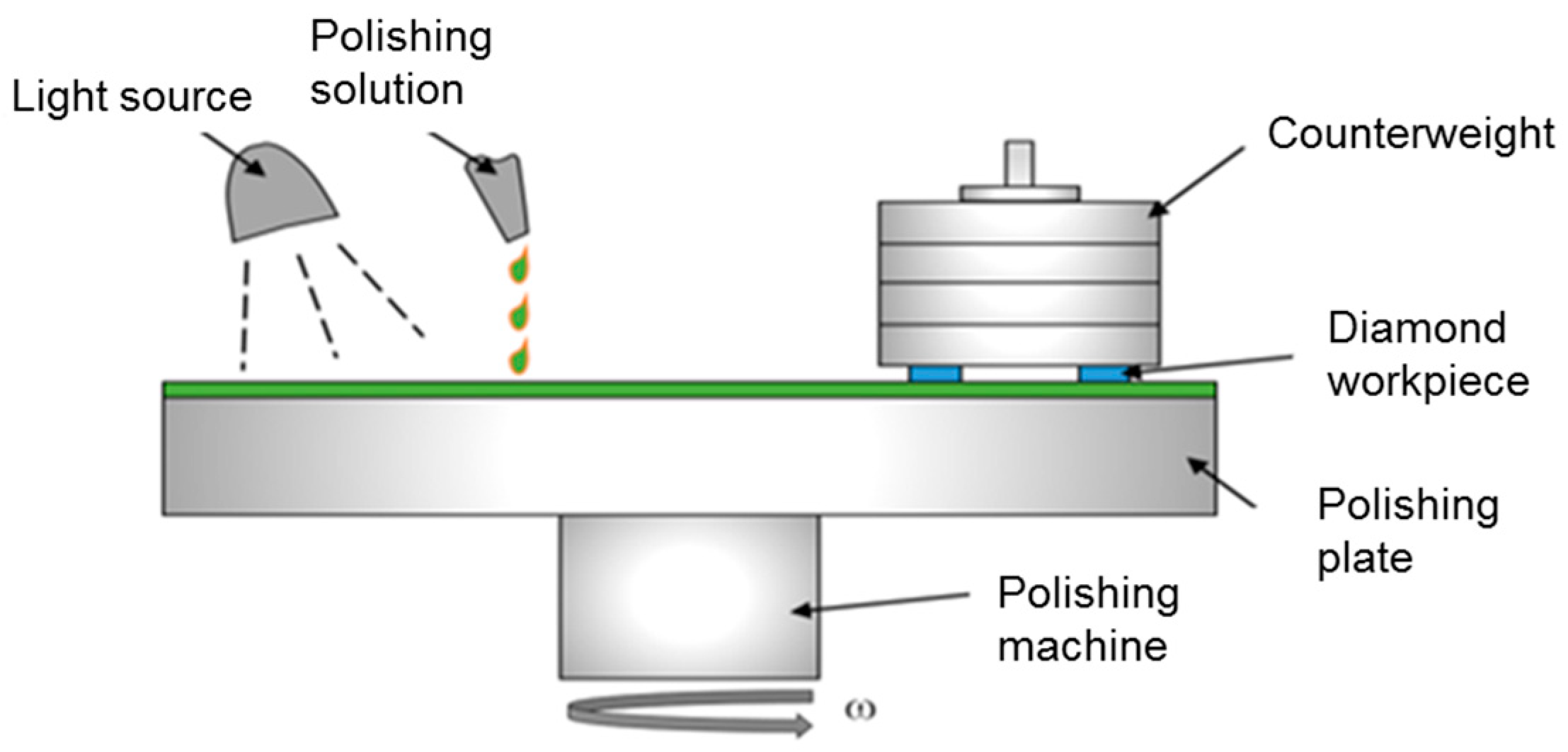
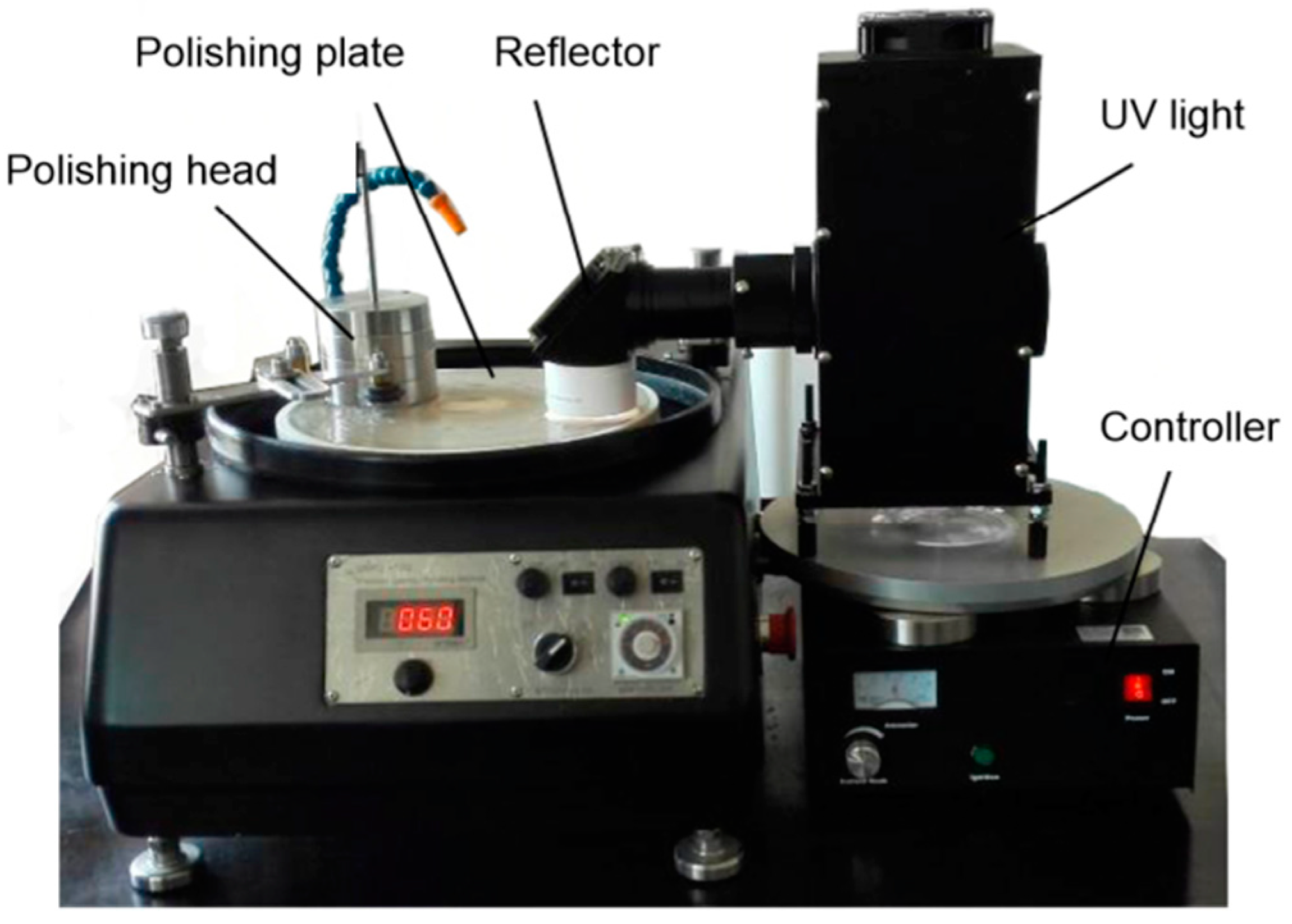
2.3. PCMP Experiments
3. Results and Discussion
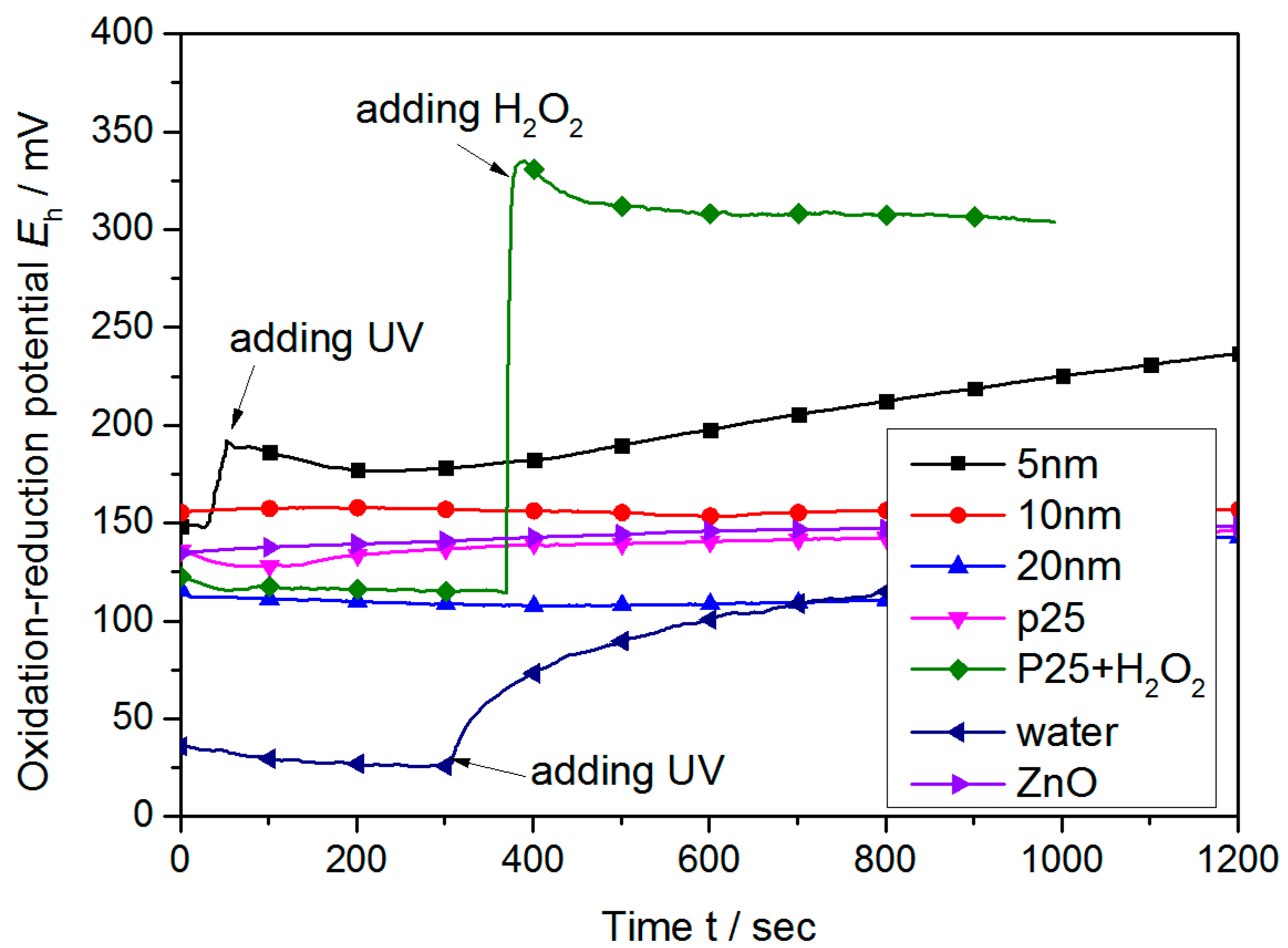
3.1. Oxidation–Reduction Potential of PCMP Slurry
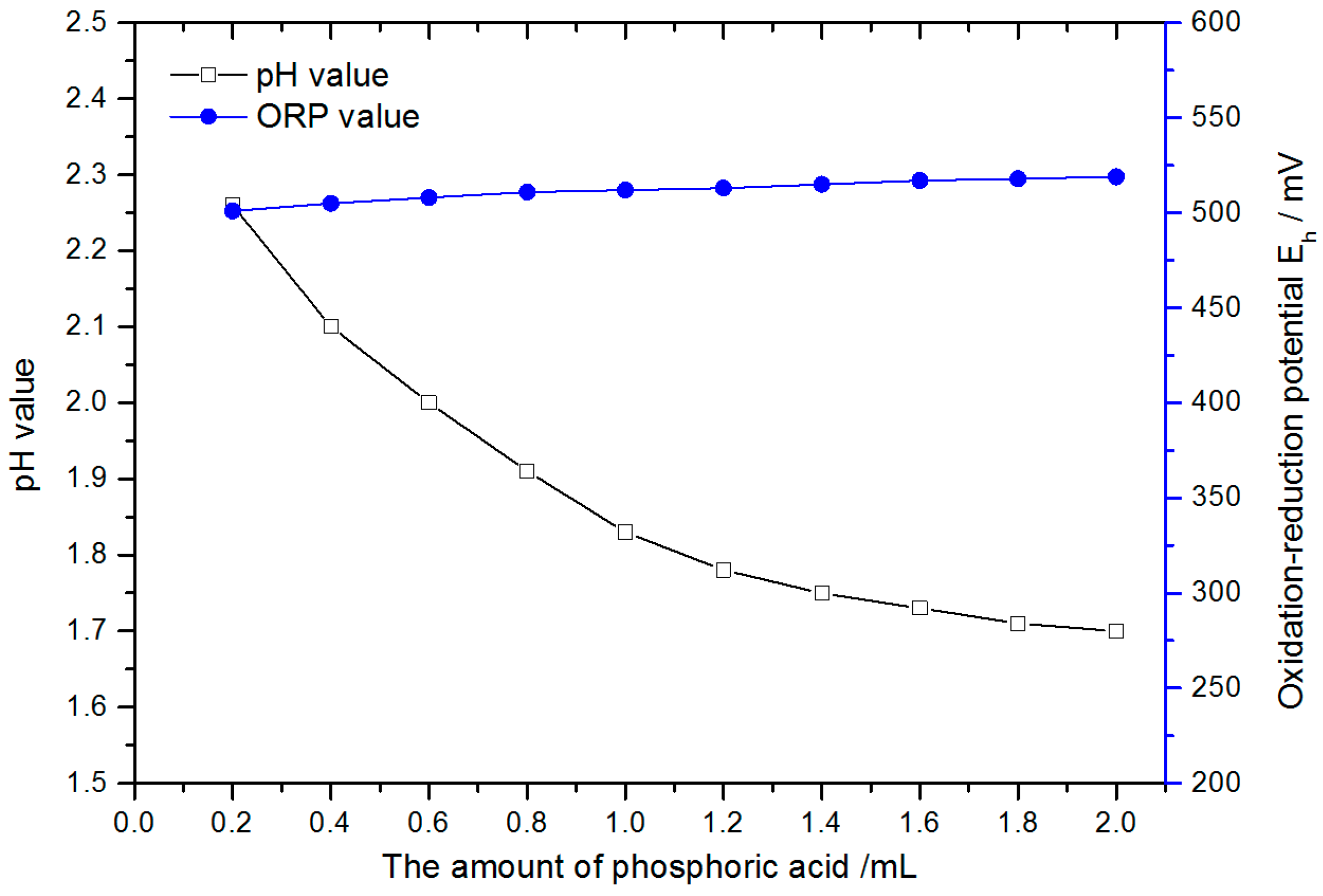
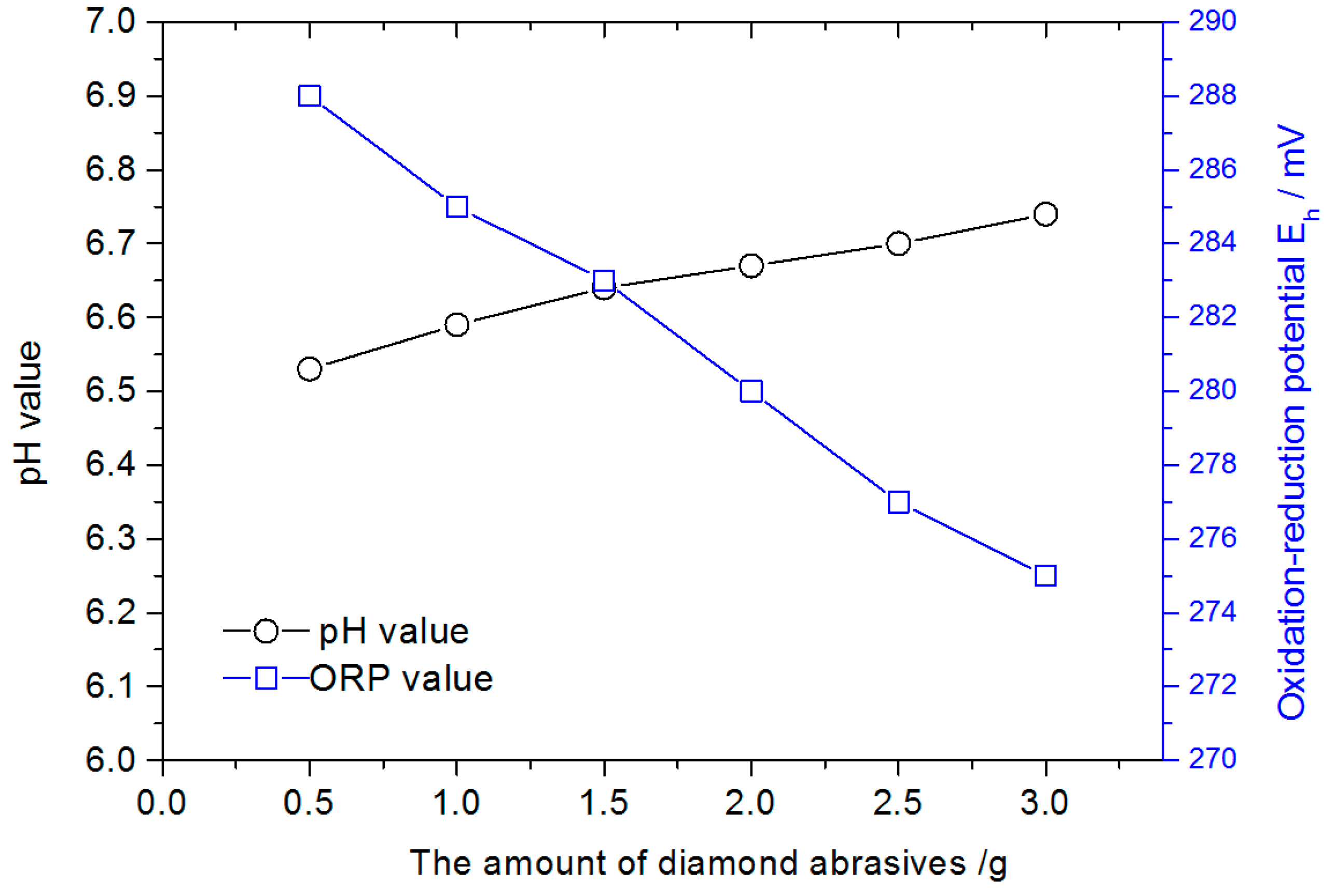
3.2. pH Value of PCMP Slurry
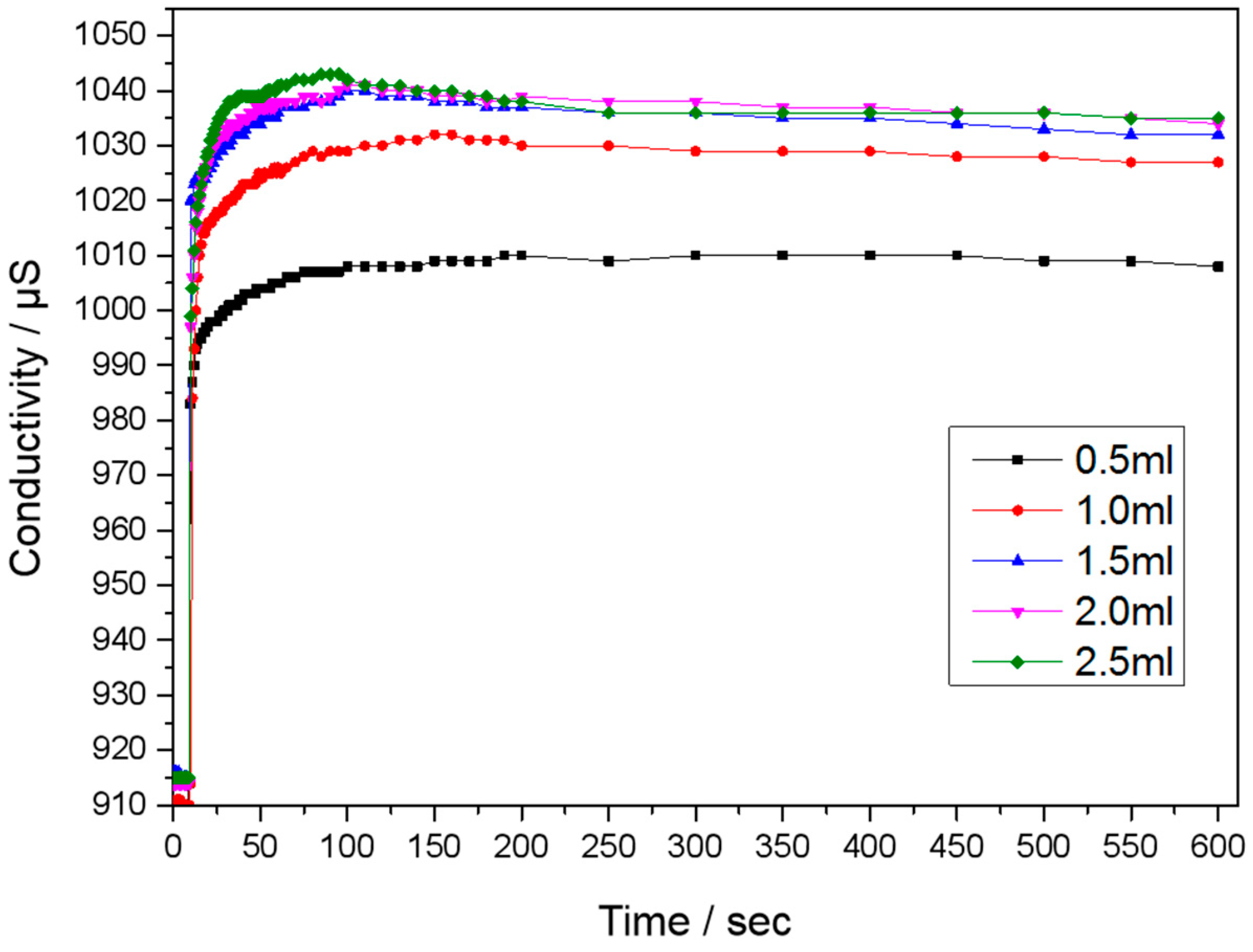
3.3. Conductivity of PCMP Slurry
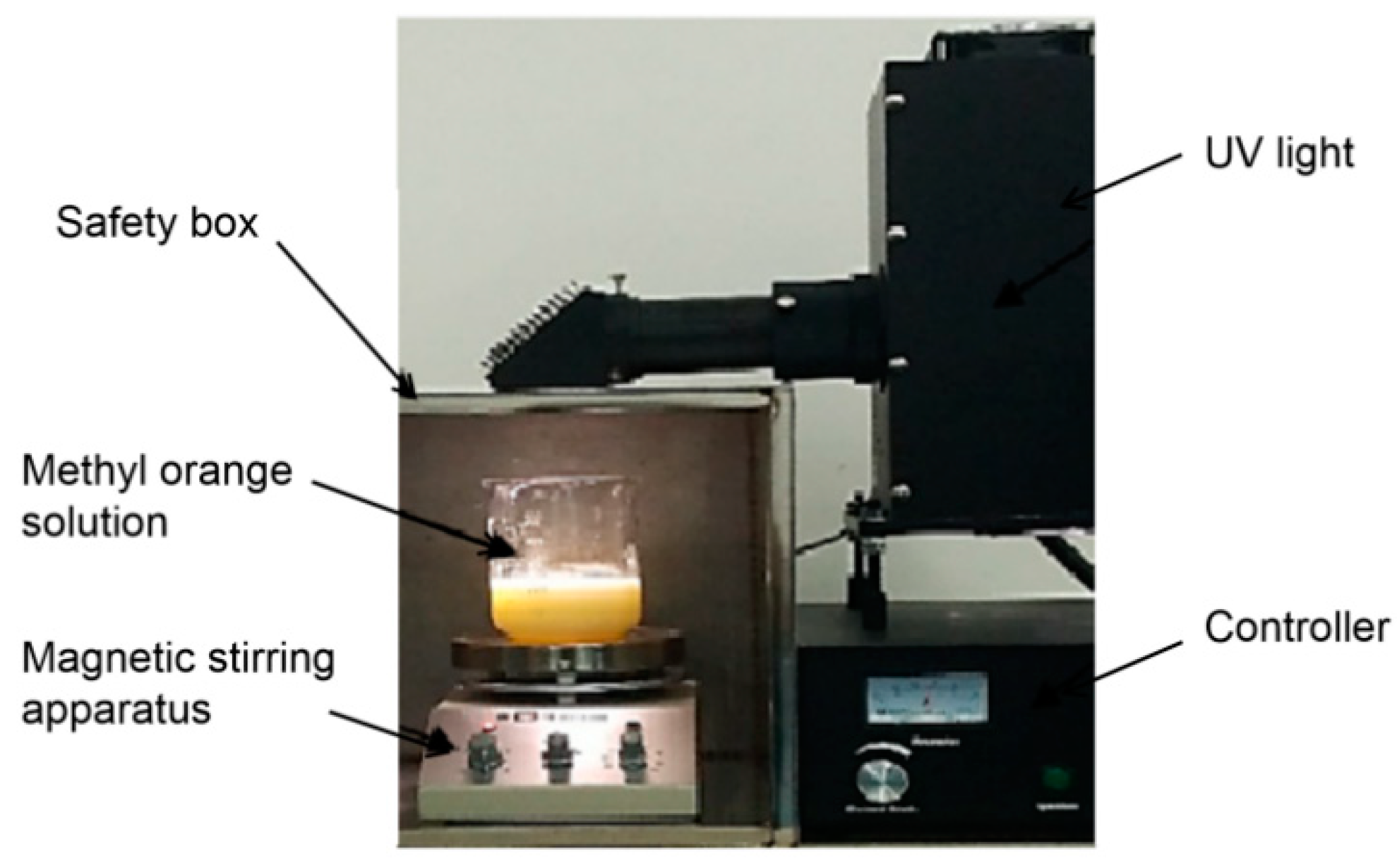
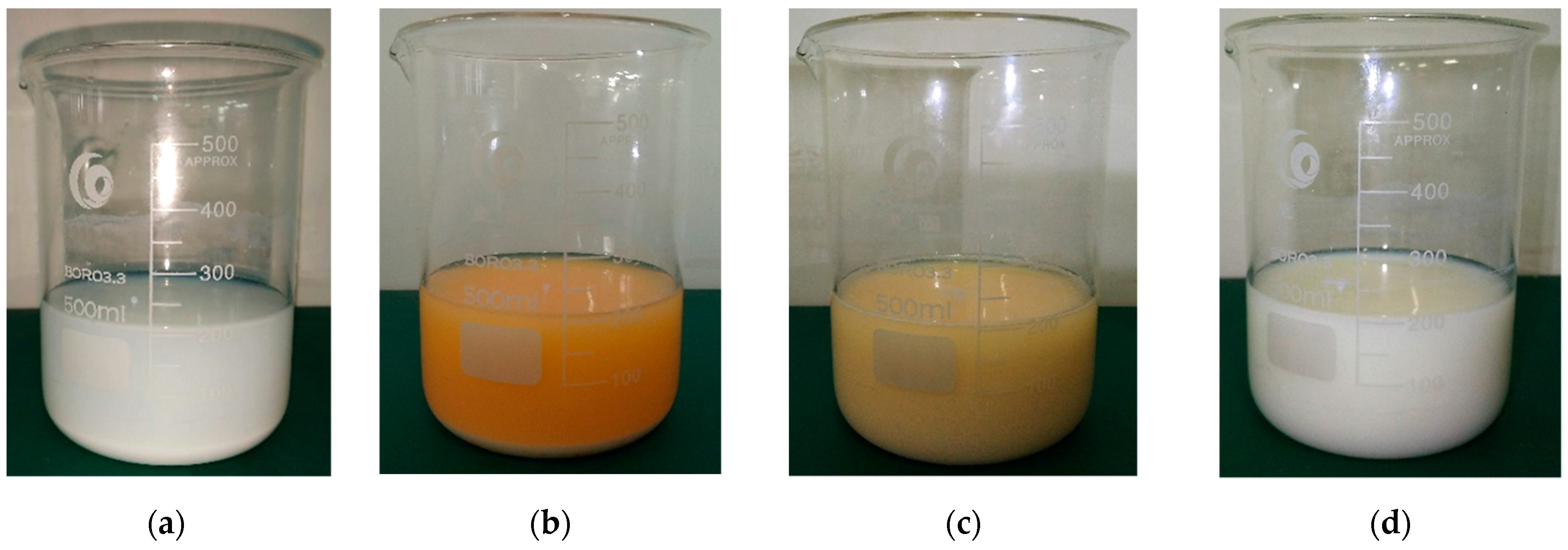
3.4. Oxidation Test of Methyl Orange
3.5. Polishing Diamond with PCMP Slurry
4. Conclusions
- (1)
- The removal of diamonds via photocatalysis-assisted chemical mechanical polishing is effective as it utilizes hydroxyl radical ·OH as an oxidant in the slurry. The ECMP slurry contains a photocatalyst, abrasive, electron capture agent, pH regulator and dispersant to achieve an optimal effect.
- (2)
- The maximum ORP is present in the 5 nm TiO2 and P25 TiO2 solutions. By incorporating H2O2 and H3PO4 into the slurry and exposing it to UV light, the oxidizability of the slurry increases. Both H2O2 and H3PO4 are neither detrimental to the environment nor to humans; however, K2FeO4 decomposes more easily than H2O2.
- (3)
- The ORP of slurry and the oxidation of the diamond can both be improved by acid condition. TiO2 powder and H2O2 can be used to boost slurry conductivity, but, as the TiO2 and H2O2 concentrations reach a particular threshold, the gain in conductivity stops.
- (4)
- Methyl orange is an appropriate reagent for determining whether a slurry is oxidizable because the UV light will cause the yellow color to disappear after 60 min.
- (5)
- Both P25 TiO2 and 5 nm TiO2 exhibit strong photocatalysis properties. Surface roughness can be decreased from Ra 33.6 nm to Ra 2.6 nm in 8 h using a slurry containing P25 TiO2. Moreover, PCMP can be used to remove mechanical scratches from diamond surfaces.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kubota, A.; Nagae, S.; Motoyama, S. High-precision mechanical polishing method for diamond substrate using micron-sized diamond abrasive grains. Diam. Relat. Mater. 2020, 101, 107644. [Google Scholar] [CrossRef]
- Hicks, M.L.; Pakpour-Tabrizi, A.C.; Jackman, R.B. Polishing, preparation and patterning of diamond for device applications. Diam. Relat. Mater. 2019, 97, 107424. [Google Scholar] [CrossRef]
- Mollart, T.P.; Wort, C.J.H.; Pickles, C.S.J. CVD diamond optical components, multi-s optical components pectral properties and performance at elevated temperatures. Proc. SPIE 2001, 4375, 180–198. [Google Scholar]
- Harris, D.C. Properties of diamond for window and dome applications. Proc. SPIE 1994, 2286, 218–228. [Google Scholar]
- Liao, L.; Zhang, Z.; Meng, F.; Liu, D.; Wu, B.; Li, Y.; Xie, W. A novel slurry for chemical mechanical polishing of single crystal diamond. Appl. Surf. Sci. 2021, 564, 150431. [Google Scholar] [CrossRef]
- Luo, H.; Ajmal, K.M.; Liu, W.; Yamamura, K.; Deng, H. Polishing and planarization of single crystal diamonds: State-of-the-art and perspectives. Int. J. Extrem. Manuf. 2021, 3, 022003. [Google Scholar] [CrossRef]
- Hird, J.R.; Field, J.E. Diamond polishing. R. Soc. Lond. Proc. Ser. A 2004, 460, 3547–3568. [Google Scholar] [CrossRef]
- Doronin, M.A.; Polyakov, S.N.; Kravchuk, K.S.; Molchanov, S.P.; Lomov, A.A.; Troschiev, S.Y.; Terentiev, S.A. Limits of single crystal diamond surface mechanical polishing. Diam. Relat. Mater. 2018, 87, 149–155. [Google Scholar] [CrossRef]
- Jarvis, M.R.; Pérez, R.; van Bouwelen, F.M.; Payne, M.C. Microscopic mechanism for mechanical polishing of diamond (110) surfaces. Phys. Rev. Lett. 1998, 80, 3428. [Google Scholar] [CrossRef]
- Suzuki, K.; Yasunaga, N.; Seki, Y. Dynamic friction polishing of diamond utilizing sliding wear by rotating metal disc. Proc. ASPE 1996, 14, 482–485. [Google Scholar]
- Lee, W.S.; Baik, Y.J.; Eun, K.Y.; Yoon, D.Y. Metallographic etching of polycrystalline diamond films by reaction with metal. Diam. Relat. Mater. 1995, 4, 989–995. [Google Scholar] [CrossRef]
- Choi, S.K.; Jung, D.Y.; Kweon, S.Y.; Jung, S.K. Surface characterization of diamond films polished by thermomechanical polishing method. Thin Solid Film. 1996, 279, 110–114. [Google Scholar] [CrossRef]
- Yuan, Z.; Jin, Z.; Ma, X.; Dong, B. Fabrication and characterization of FeNiCr matrix-TiC composite for polishing CVD diamond film. J. Mater. Sci. Technol. 2009, 25, 319–324. [Google Scholar]
- Konov, V.I. Laser in micro and nanoprocessing of diamond materials. Laser Photonics Rev. 2012, 6, 739–766. [Google Scholar] [CrossRef]
- Malshe, A.P.; Park, B.S.; Brown, W.D.; Naseem, H.A. A review of techniques for polishing and planarizing chemically vapor-deposited (CVD) diamond films and substrates. Diam. Relat. Mater. 1999, 8, 1198–1213. [Google Scholar] [CrossRef]
- Yamamura, K.; Emori, K.; Sun, R.; Ohkubo, Y.; Endo, K.; Yamada, H.; Chayahara, A.; Mokuno, Y. Damage-free highly efficient polishing of single-crystal diamond wafer by plasma-assisted polishing. CIRP Ann. 2018, 67, 353–356. [Google Scholar] [CrossRef]
- Mi, S.; Toros, A.; Graziosi, T.; Quack, N. Non-contact polishing of single crystal diamond by ion beam etching. Diam. Relat. Mater. 2019, 92, 248–252. [Google Scholar] [CrossRef]
- Yang, N.; Huang, W.; Lei, D. Control of nanoscale material removal in diamond polishing by using iron at low temperature. J. Mater. Process. Technol. 2020, 278, 116521. [Google Scholar] [CrossRef]
- El-Dasher, B.S.; Gray, J.J.; Tringe, J.W.; Biener, J.; Hamza, A.V.; Wild, C.; Wörner, E.; Koidl, P. Crystallographic anisotropy of wear on a polycrystalline diamond surface. Appl. Phys. Lett. 2006, 88, 241915. [Google Scholar] [CrossRef]
- Shi, Z.; Jin, Z.; Guo, X.; Yuan, S.; Guo, J. Insights into the atomistic behavior in diamond chemical mechanical polishing with OH environment using ReaxFF molecular dynamics simulation. Comput. Mater. Sci. 2019, 166, 136–142. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Wang, J. Modeling the effects of cohesive energy for single particle on the material removal in chemical mechanical polishing at atomic scale. Appl. Surf. Sci. 2007, 253, 9137–9141. [Google Scholar] [CrossRef]
- Charrier, G.; Lévy, S.; Vigneron, J.; Etcheberry, A.; Simon, N. Electroless oxidation of boron-doped diamond surfaces: Comparison between four oxidizing agents; Ce4+, MnO4−, H2O2 and S2O82−. Diam. Relat. Mater. 2011, 20, 944–950. [Google Scholar] [CrossRef]
- Zhou, J.; Niu, X.; Wang, Z.; Cui, Y.; Wang, J.; Yang, C.; Huo, Z.; Wang, R. Roles and mechanism analysis of chitosan as a green additive in low-tech node copper film chemical mechanical polishing. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124293. [Google Scholar] [CrossRef]
- Yuan, Z.; Jin, Z.; Zhang, Y.; Wen, Q. Chemical mechanical polishing slurries for chemically vapor-deposited diamond films. J. Manuf. Sci. Eng. 2013, 135, 041006. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Shi, C.; Liu, D.; Liu, L. Density functional theory analysis and novel green chemical mechanical polishing for potassium dihydrogen phosphate. Colloids Surf. A: Physicochem. Eng. Asp. 2023, 662, 131000. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, Z.; Liao, L.; Liu, J.; Su, H.; Wang, S.; Guo, D. Green chemical mechanical polishing of sapphire wafers using a novel slurry. Nanoscale 2020, 12, 22518–22526. [Google Scholar] [CrossRef]
- Fujishima, A. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Carey, J.H.; Lawrence, J.; Tosine, H.M. Photodechlorination of PCB’s in the presence of titanium dioxide in aqueous suspensions. Bull. Environ. Contam. Toxicol. 1976, 16, 697–701. [Google Scholar] [CrossRef]
- Magalhaes, P.; Andrade, L.; Nunes, O.C.; Mendes, A. Titanium dioxide photocatalysis: Fundamentals and application on photoinactivation. Rev. Adv. Mater. Sci. 2017, 51, 91–129. [Google Scholar]
- Bahnemann, D.W.; Hilgendorff, M.; Memming, R. Charge carrier dynamics at TiO2 particles: Reactivity of free and trapped holes. J. Phys. Chem. B 1997, 101, 4265–4275. [Google Scholar] [CrossRef]
- Chung, C.J.; Chiang, C.C.; Chen, C.H.; Hsiao, C.H.; Lin, H.I.; Hsieh, P.Y.; He, J.L. Photocatalytic TiO2 on copper alloy for antimicrobial purposes. Appl. Catal. B Environ. 2008, 85, 103–108. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdar, M.; He, J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef] [PubMed]
- Puma, G.L.; Bono, A.; Krishnaiah, D.; Collin, J.G. Preparation of titanium dioxide photocatalyst loaded onto activated carbon support using chemical vapor deposition: A review paper. J. Hazard. Mater. 2008, 157, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Al-Qaradawi, S.; Salman, S.R. Photocatalytic degradation of methyl orange as a model compound. J. Photochem. Photobiol. A Chem. 2002, 148, 161–168. [Google Scholar] [CrossRef]
- Liu, W.; Xiong, Q.; Lu, J.; Wang, X.; Yan, Q. Tribological behavior of single crystal diamond based on UV photocatalytic reaction. Tribol. Int. 2022, 175, 107806. [Google Scholar] [CrossRef]
- Zhang, L.; Hamers, R.J. Photocatalytic reduction of CO2 to CO by diamond nanoparticles. Diam. Relat. Mater. 2017, 78, 24–30. [Google Scholar] [CrossRef]







| Photocatalyst | Abrasive Materials | Electron Trapping Agent | pH Regulator |
|---|---|---|---|
| 5 nm TiO2 | Al2O3 | H2O2 | NaOH |
| 10 nm TiO2 | diamond | K2FeO4 | H3PO4 |
| 20 nm TiO2 | SiC | ||
| P25 TiO2 | |||
| ZnO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, J.; Zhao, Y.; Zhu, J.; Yuan, Z.; Du, H.; Wen, Q. A New Slurry for Photocatalysis-Assisted Chemical Mechanical Polishing of Monocrystal Diamond. Machines 2023, 11, 664. https://doi.org/10.3390/machines11060664
Shao J, Zhao Y, Zhu J, Yuan Z, Du H, Wen Q. A New Slurry for Photocatalysis-Assisted Chemical Mechanical Polishing of Monocrystal Diamond. Machines. 2023; 11(6):664. https://doi.org/10.3390/machines11060664
Chicago/Turabian StyleShao, Junyong, Yanjun Zhao, Jianhui Zhu, Zewei Yuan, Haiyang Du, and Quan Wen. 2023. "A New Slurry for Photocatalysis-Assisted Chemical Mechanical Polishing of Monocrystal Diamond" Machines 11, no. 6: 664. https://doi.org/10.3390/machines11060664
APA StyleShao, J., Zhao, Y., Zhu, J., Yuan, Z., Du, H., & Wen, Q. (2023). A New Slurry for Photocatalysis-Assisted Chemical Mechanical Polishing of Monocrystal Diamond. Machines, 11(6), 664. https://doi.org/10.3390/machines11060664








