Recovery of Metals from Waste Lithium Ion Battery Leachates Using Biogenic Hydrogen Sulfide
Abstract
1. Introduction
2. Materials and Methods
2.1. LIB Waste Processing and Composition
2.2. Leaching of LIBs
2.3. Sulfate Reducing Bioreactor for the Generation of Biogenic Hydrogen Sulfide
2.3.1. Preparation of SRB Inoculum
2.3.2. Bioreactor Operation
2.4. DNA Extraction and Analysis
2.5. Biogenic Sulfide Precipitation of Metals
2.6. Biogenic Sulfide Precipitation Efficiency
3. Results and Discussion
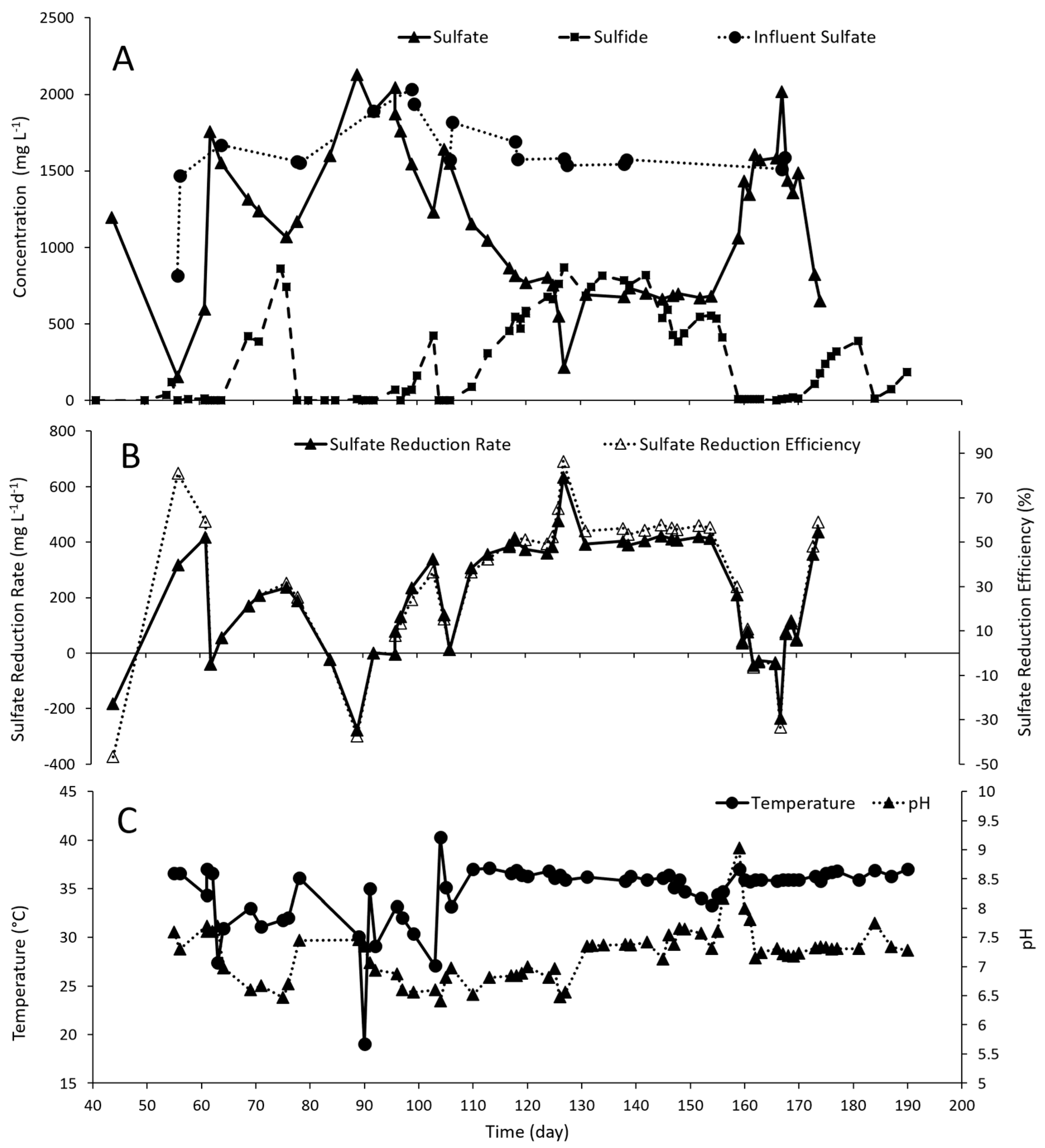
3.1. Bioreactor Performance and Troubleshooting
3.1.1. Dissolved Sulfide, pH and Temperature as Indicators of Microbial Activity
3.1.2. Installation of a Separate Sparging Reactor Facilitated Biogenic H2S Production in the FBR
3.1.3. Ratio of Sulfate to Lactate was Important for Optimising Sulfide Generation
3.1.4. Sulfate Reduction Rate and Efficiency
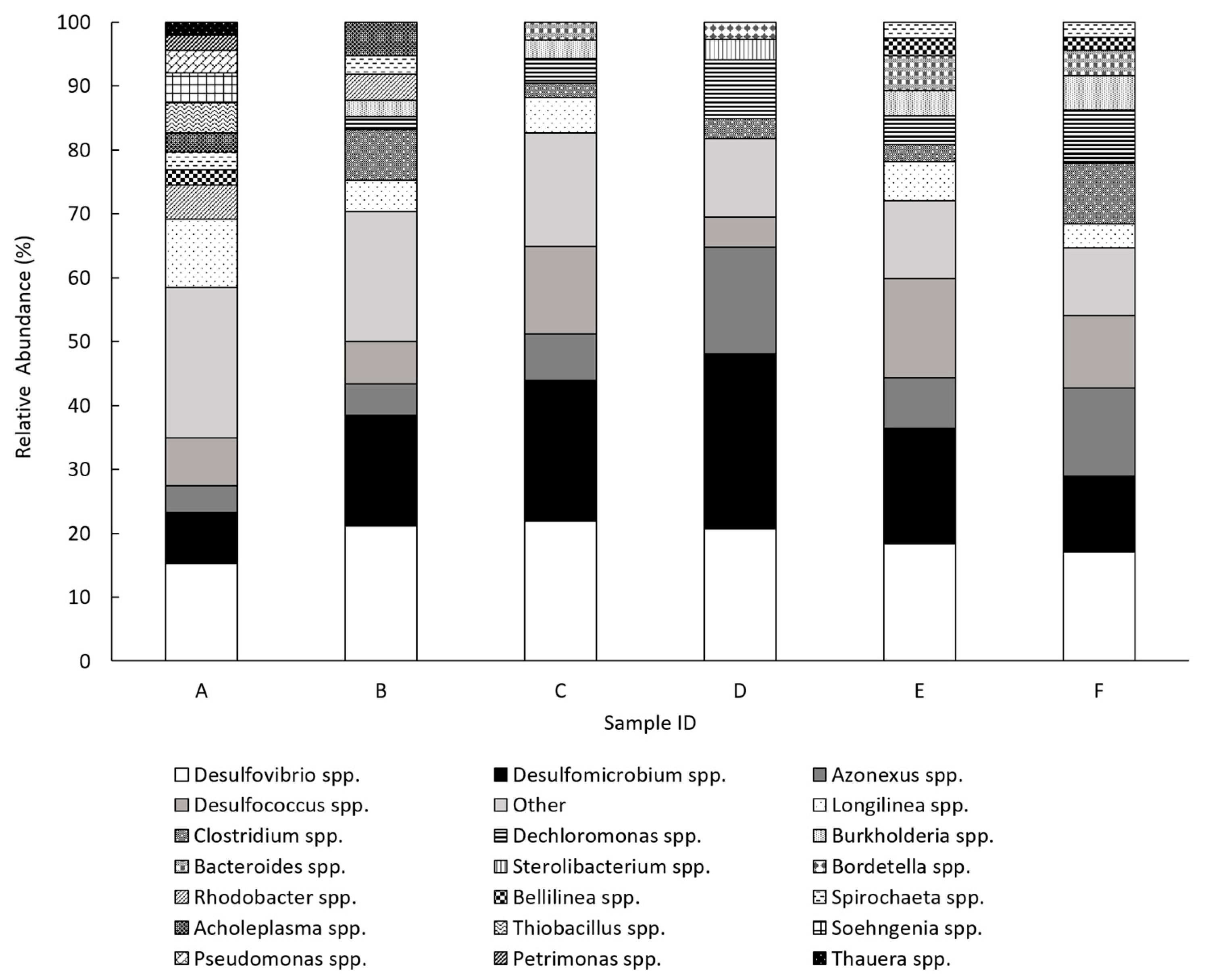
3.2. Microbial Community Structure
3.3. Metal Sulfide Precipitation
3.3.1. Sparging by the SR Immediately Removed All H2S, but the FBR Remained Active
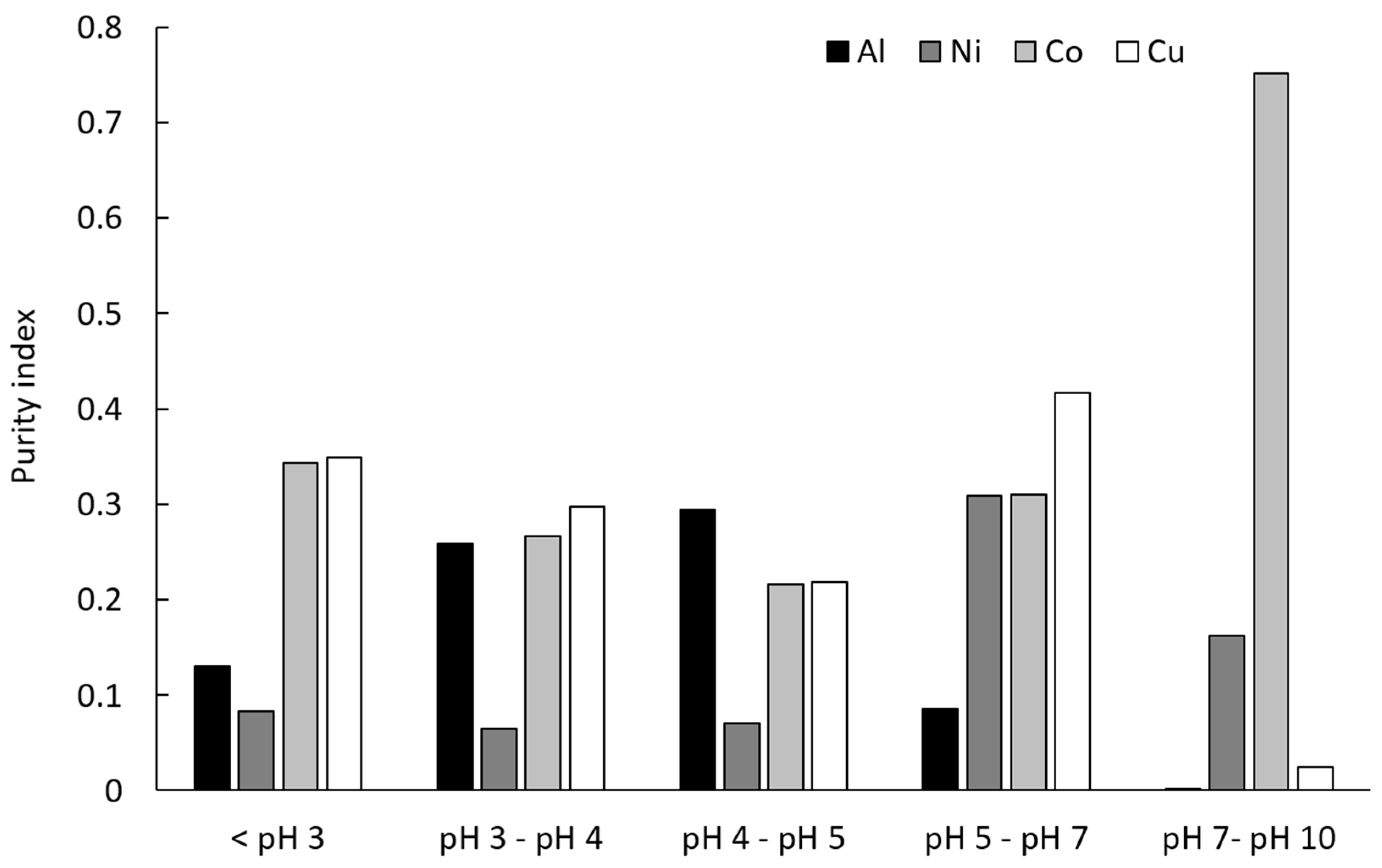
3.3.2. Recovery of Metals was pH Dependent
3.3.3. Purity Indices of Metal Precipitates
3.4. Implications of Findings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Desjardins, J. China Leading the Charge for Lithium-Ion Megafactories. Available online: https://www.mining.com/web/china-leading-the-charge-for-lithium-ion-megafactories/ (accessed on 13 August 2019).
- Randell, P. Waste Lithium-Ion Battery Projections; Final Report; Department of the Environment and Energy: Canberra, Australia, 2016.
- Hong, Y.; Valix, M. Bioleaching of electronic waste using acidophilic sulfur oxidising bacteria. J. Clean. Prod. 2014, 65, 465–472. [Google Scholar] [CrossRef]
- Ibietela, D.; Eucharia Uche, N. Effect of Spent Laptop Battery Waste on Soil Microorganisms. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 867–876. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, B.; Huang, K.; Wang, X.; Wang, D. Environmental impact assessment and end-of-life treatment policy analysis for Li-ion batteries and Ni-MH batteries. Int. J. Environ. Res. Public Health 2014, 11, 3185–3198. [Google Scholar] [CrossRef] [PubMed]
- Heelan, J.; Gratz, E.; Zheng, Z.; Wang, Q.; Chen, M.; Apelian, D.; Wang, Y. Current and Prospective Li-Ion Battery Recycling and Recovery Processes. JOM 2016, 68, 2632–2638. [Google Scholar] [CrossRef]
- Boxall, N.J.; Adamek, N.; Cheng, K.; Haque, N.; Bruckard, W.; Kaksonen, A. Multistage leaching of metals from spent lithium ion battery waste using electrochemically generated acidic lixiviant. Waste Manag. 2018, 74, 435–445. [Google Scholar] [CrossRef]
- King, S.; Boxall, N. Lithium battery recycling in Australia: Defining the status and identifying opportunities for the development of a new industry. J. Clean. Prod. 2019, 215, 1279–1287. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Moscardini, E.; Altimari, P.; Abo Atia, T.; Toro, L. Cobalt products from real waste fractions of end of life lithium ion batteries. Waste Manag. 2016, 51, 214–221. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, K. Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag. 2012, 32, 1575–1582. [Google Scholar] [CrossRef]
- Dolker, T.; Pant, D. Bioremediation of metals from lithium-ion battery (LIB) waste. In Waste Bioremediation; Springer: Berlin/Heidelberg, Germany, 2018; pp. 265–278. [Google Scholar]
- Lv, W.; Wang, Z.; Cao, H.; Zheng, X.; Jin, W.; Zhang, Y.; Sun, Z. A sustainable process for metal recycling from spent lithium-ion batteries using ammonium chloride. Waste Manag. 2018, 79, 545–553. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Jha, A.K.; Kumar, V.; Hait, J.; Pandey, B.D. Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone. Waste Manag. 2013, 33, 1890–1897. [Google Scholar] [CrossRef]
- Li, J.; Shi, P.; Wang, Z.; Chen, Y.; Chang, C.C. A combined recovery process of metals in spent lithium-ion batteries. Chemosphere 2009, 77, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhai, L.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Recovery of valuable metals from spent lithium-ion batteries by ultrasonic-assisted leaching process. J. Power Sources 2014, 262, 380–385. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Shen, B. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid. J. Hazard. Mater. 2015, 295, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qu, W.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Succinic acid-based leaching system: A sustainable process for recovery of valuable metals from spent Li-ion batteries. J. Power Sources 2015, 282, 544–551. [Google Scholar] [CrossRef]
- Lupi, C.; Pasquali, M. Electrolytic nickel recovery from lithium-ion batteries. Miner. Eng. 2003, 16, 537–542. [Google Scholar] [CrossRef]
- Chagnes, A.; Pospiech, B. A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries. J. Chem. Technol. Biotechnol. 2013, 88, 1191–1199. [Google Scholar] [CrossRef]
- Granata, G.; Moscardini, E.; Pagnanelli, F.; Trabucco, F.; Toro, L. Product recovery from Li-ion battery wastes coming from an industrial pre-treatment plant: Lab scale tests and process simulations. J. Power Sources 2012, 206, 393–401. [Google Scholar] [CrossRef]
- Baba, Y.; Kubota, F.; Goto, M.; Cattrall, R.; Kolev, S.P. Separation of cobalt(II) from manganese(II) using a polymer inclusion membrane with N-[N,N-di(2-ethylhexyl)aminocarbonylmethyl]glycine (D2EHAG) as the extractant/carrier. J. Chem. Technol. Biotechnol. 2016, 91, 1320–1326. [Google Scholar] [CrossRef]
- Garcia, E.M.; Taroc, H.A.; Matencio, T.; Domingues, R.Z.; dos Santos, J.A.F.; de Freitas, M. Electrochemical recycling of cobalt from spent cathodes of lithium-ion batteries: Its application as coating on SOFC interconnects. J. Appl. Electrochem. 2011, 41, 1373–1379. [Google Scholar] [CrossRef]
- Freitas, M.; Celante, V.G.; Pietre, M.K. Electrochemical recovery of cobalt and copper from spent Li-ion batteries as multilayer deposits. J. Power Sources 2010, 195, 3309–3315. [Google Scholar] [CrossRef]
- Freitas, M.; Garcia, E.M. Electrochemical recycling of cobalt from cathodes of spent lithium-ion batteries. J. Power Sources 2007, 171, 953–959. [Google Scholar] [CrossRef]
- Niu, Z.; Zou, Y.; Xin, B.; Chen, S.; Liu, C.; Li, Y. Process controls for improving bioleaching performance of both Li and Co from spent lithium ion batteries at high pulp density and its thermodynamics and kinetics exploration. Chemosphere 2014, 109, 92–98. [Google Scholar] [CrossRef]
- Zeng, G.; Deng, X.; Luo, S.; Luo, X.; Zou, J. A copper-catalyzed bioleaching process for enhancement of cobalt dissolution from spent lithium-ion batteries. J. Hazard. Mater. 2012, 199, 164–169. [Google Scholar] [CrossRef]
- Mishra, D.; Kim, D.J.; Ralph, D.E.; Ahn, J.G.; Rhee, Y.H. Bioleaching of metals from spent lithium ion secondary batteries using Acidithiobacillus ferrooxidans. Waste Manag. 2008, 28, 333–338. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, G.; Mao, Z.; Fang, Z.; Yang, C. Precipitation of valuable metals from bioleaching solution by biogenic sulfides. Miner. Eng. 2008, 22, 289–295. [Google Scholar] [CrossRef]
- Cibati, A.; Cheng, K.; Morris, C.; Ginige, M.; Sahinkaya, E.; Pagnanelli, F.; Kaksonen, A. Selective precipitation of metals from synthetic spent refinery catalyst leach liquor with biogenic H2S produced in a lactate-fed anaerobic baffled reactor. Hydrometallurgy 2013, 139, 154–161. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Lavonen, L.; Kuusenaho, M.; Kolli, A.; Närhi, H.; Vestola, E.; Puhakka, J.; Tuovinen, O.H. Bioleaching and recovery of metals from final slag waste of the copper smelting industry. Miner. Eng. 2011, 24, 1113–1121. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Puhakka, J. Sulfate reduction based bioprocesses for the treatment of acid mine drainage and the recovery of metals. Eng. Life Sci. 2007, 7, 541–564. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Boxall, N.J.; Gumulya, Y.; Khaleque, H.N.; Morris, C.; Bohu, T.; Cheng, K.Y.; Usher, K.; Lakaniemi, A.M. Recent progress in biohydrometallurgy and microbial characterisation. Hydrometallurgy 2018, 180, 7–25. [Google Scholar] [CrossRef]
- Campbell, L.; Postgate, J. Revision of the holotype strain of Desulfotomaculum ruminis (Coleman) Campbell and Postgate. Int. J. Syst. Evol. Microbiol. 1969, 19, 139–140. [Google Scholar] [CrossRef][Green Version]
- Dar, S.; Kleerebezem, R.; Stams, A.; Kuenen, J.; Muyzer, G. Competition and coexistence of sulfate-reducing bacteria, acetogens and methanogens in a lab-scale anaerobic bioreactor as affected by changing substrate to sulfate ratio. Appl. Microbiol. Biotechnol. 2008, 78, 1045–1055. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Riekkola-Vanhanen, M.; Puhakka, J. Optimization of metal sulphide precipitation in fluidized-bed treatment of acidic wastewater. Water Res. 2003, 37, 255–266. [Google Scholar] [CrossRef]
- McMahon, M. Development of a Sulfate Reducing Packed Bed Bioreactor for Use in a Sustainable Hydrogen Production Process. Master’s Thesis, Queen’s University, Kingston, ON, Canada, 2007. [Google Scholar]
- Ucar, D.; Bekmezci, O.; Kaksonen, A.; Sahinkaya, E. Sequential precipitation of Cu and Fe using a three-stage sulfidogenic fluidized-bed reactor system. Miner. Eng. 2011, 24, 1100–1105. [Google Scholar] [CrossRef]
- Cord-Ruwisch, R. A quick method for the determination of dissolved and precipitated sulfide in cultures of sulfate-reducing bacteria. J. Microbiol. Meth. 1985, 4, 33–36. [Google Scholar] [CrossRef]
- Sahinkaya, E.; Gungor, M. Comparison of sulfidogenic up-flow and down-flow fluidized-bed reactors for the biotreatment of acidic metal-containing wastewater. Bioresour. Technol. 2010, 101, 9508–9514. [Google Scholar] [CrossRef]
- Reis, M.; Almeida, J.; Lemos, P.; Carrondo, M. Effect of hydrogen sulfide on growth of sulfate reducing bacteria. Biotechnol. Bioeng. 1992, 40, 593–600. [Google Scholar] [CrossRef]
- Kaksonen, A.; Franzmann, P.; Puhakka, J. Effects of hydraulic retention time and sulfide toxicity on ethanol and acetate oxidation in sulfate-reducing metal-precipitating fluidized-bed reactor. Biotechnol. Bioeng. 2004, 86, 332–343. [Google Scholar] [CrossRef]
- Harada, H.; Uemura, S.; Momonoi, K. Interaction between sulfate-reducing bacteria and methane-producing bacteria in UASB reactors fed with low strength wastes containing different levels of sulfate. Water Res. 1994, 28, 355–367. [Google Scholar] [CrossRef]
- Özkaya, B.; Kaksonen, A.H.; Sahinkaya, E.; Puhakka, J.A. Fluidized bed reactor biotechnology for multiple environmental engineering solutions. Water Res. 2019, 150, 452–465. [Google Scholar] [CrossRef]
- Van der Meer, T.; Kinnunen, P.H.M.; Kaksonen, A.H.; Puhakka, J.A. Effect of fluidized-bed carrier material on biological ferric sulphate generation. Miner. Eng. 2007, 20, 782–792. [Google Scholar] [CrossRef]
- McCartney, D.; Oleszkiewicz, J. Competition between methanogens and sulfate reducers: Effect of COD:sulfate ratio and acclimation. Water Environ. Res. 1993, 65, 655–664. [Google Scholar] [CrossRef]
- Mendez, R.; ten Brummeler, E.; Hulshoffpol, L. Start up of UASB reactors treating sucrose containing substrates with low COD/sulfate ratio. Environ. Technol. Lett. 1989, 10, 83–90. [Google Scholar] [CrossRef]
- Bertolino, S.; Silva, L.; Aquino, S.; Leão, V. Comparison of UASB and fluidized-bed reactors for sulfate reduction. Braz. J. Chem. Eng. 2015, 32, 59–71. [Google Scholar] [CrossRef]
- Kiran, M.; Pakshirajan, K.; Das, G. An overview of sulfidogenic biological reactors for the simultaneous treatment of sulfate and heavy metal rich wastewater. Chem. Eng. Sci. 2017, 158, 606–620. [Google Scholar] [CrossRef]
- Marin, P.; Alkalay, D.; Guerrero, L.; Chamy, R.; Schiappacasse, M. Design and startup of an anaerobic fluidized bed reactor. Water Sci. Technol. 1999, 40, 63–70. [Google Scholar] [CrossRef]
- Achenbach, L.; Michaelidou, U.; Bruce, R.; Fryman, J.; Coates, J. Dechloromonas agitata gen. nov., sp. nov. and Dechlorosoma suillum gen. nov., sp. nov., two novel environmentally dominant (per) chlorate-reducing bacteria and their phylogenetic position. Int. J. Syst. Evol. Microbiol. 2001, 51, 527–533. [Google Scholar] [CrossRef]
- Wolterink, A.; Kim, S.; Muusse, M.; Kim, I.; Roholl, P.; van Ginkel, C.; Kengen, S. Dechloromonas hortensis sp. nov. and strain ASK-1, two novel (per) chlorate-reducing bacteria, and taxonomic description of strain GR-1. Int. J. Syst. Evol. Microbiol. 2005, 55, 2063–2068. [Google Scholar] [CrossRef]
- Collins, M.; Lawson, P.; Willems, A.; Cordoba, J.; Fernandez-Garayzabal, J.; Garcia, P.; Farrow, J. The phylogeny of the genus Clostridium: Proposal of five new genera and eleven new species combinations. Int. J. Syst. Evol. Microbiol. 1994, 44, 812–826. [Google Scholar] [CrossRef]
- Nunoura, T.; Hirai, M.; Miyazaki, M.; Kazama, H.; Makita, H.; Hirayama, H.; Takai, K. Isolation and Characterization of a Thermophilic, Obligately Anaerobic and Heterotrophic Marine Chloroflexi Bacterium from a Chloroflexi-dominated Microbial Community Associated with a Japanese Shallow Hydrothermal System, and Proposal for Thermomarinilinea lacunofontalis gen. nov., sp. nov. Microbes Environ. 2013, 28, 228–235. [Google Scholar]
- Yamada, T.; Imachi, H.; Ohashi, A.; Harada, H.; Hanada, S.; Kamagata, Y.; Sekiguchi, Y. Bellilinea caldifistulae gen. nov., sp. nov. and Longilinea arvoryzae gen. nov., sp. nov., strictly anaerobic, filamentous bacteria of the phylum Chloroflexi isolated from methanogenic propionate-degrading consortia. Int. J. Syst. Evol. Microbiol. 2007, 57, 2299–2306. [Google Scholar] [CrossRef]
- Devereux, R.; He, S.; Doyle, C.; Orkland, S.; Stahl, D.; LeGall, J.; Whitman, W. Diversity and origin of Desulfovibrio species: Phylogenetic definition of a family. J. Bacteriol. 1990, 172, 3609–3619. [Google Scholar] [CrossRef]
- Postgate, J.; Campbell, L. Classification of Desulfovibrio species, the nonsporulating sulfate-reducing bacteria. Bacteriol. Rev. 1966, 30, 732–738. [Google Scholar]
- Vita, N.; Valette, O.; Brasseur, G.; Lignon, S.; Denis, Y.; Ansaldi, M.; Pieulle, L. The primary pathway for lactate oxidation in Desulfovibrio vulgaris. Front. Microbiol. 2015, 6, 606. [Google Scholar] [CrossRef]
- Hippe, H.; Vainshtein, M.; Gogotova, G.; Stackebrandt, E. Reclassification of Desulfobacterium macestii as Desulfomicrobium macestii comb. nov. Int. J. Syst. Evol. Microbiol. 2003, 53, 1127–1130. [Google Scholar] [CrossRef]
- Rozanova, E.; Nazina, T.; Galushko, A. Isolation of a new genus of sulfate-reducing bacteria and description of a new species of this genus, Desulfomicrobium apsheronum gen. nov., sp. nov. Microbiology 1988, 57, 514–520. [Google Scholar]
- Reinhold-Hurek, B.; Hurek, T. Reassessment of the taxonomic structure of the diazotrophic genus Azoarcus sensu lato and description of three new genera and new species, Azovibrio restrictus gen. nov., sp. nov., Azospira oryzae gen. nov., sp. nov. and Azonexus fungiphilus gen. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 2000, 50, 649–660. [Google Scholar]
- Chou, J.; Jiang, S.; Cho, J.; Song, J.; Lin, M.; Chen, W. Azonexus hydrophilus sp. nov., a nifH gene-harbouring bacterium isolated from freshwater. Int. J. Syst. Evol. Microbiol. 2008, 58, 946–951. [Google Scholar] [CrossRef]
- Imhoff-Stuckle, D.; Pfennig, N. Isolation and characterization of a nicotinic acid-degrading sulfate-reducing bacterium, Desulfococcus niacini sp. nov. Arch. Microbiol. 1983, 136, 194–198. [Google Scholar] [CrossRef]
- Kuever, J.; Rainey, F.; Widdel, F. Bergey’s Manual of Systematics of Archaea and Bacteria. In Desulfococcus; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 1–5. [Google Scholar]
- Wolicka, D. Sulphate-Reducing Bacteria in Biological Treatment Wastewaters; Nova Science Publishers: New York, NY, USA, 2010. [Google Scholar]
- Imhoff, J.; Truper, H.; Pfennig, N. Rearrangement of the species and genera of the phototrophic purple nonsulfur bacteria. Int. J. Syst. Evol. Microbiol. 1984, 34, 340–343. [Google Scholar] [CrossRef]
- Tichi, M.; Tabita, F. Interactive control of Rhodobacter capsulatus redox-balancing systems during phototrophic metabolism. J. Bacteriol. 2001, 183, 6344–6354. [Google Scholar] [CrossRef]
- Dröge, S.; Fröhlich, J.; Radek, R.; König, H. Spirochaeta coccoides sp. nov., a novel coccoid spirochete from the hindgut of the termite Neotermes castaneus. Appl. Environ. Microbiol. 2006, 72, 392–397. [Google Scholar] [CrossRef]
- Leschine, S.; Paster, B.; Canale-Parola, E. Free-living Saccharolytic Spirochetes: The genus Spirochaeta. Prokaryotes 2006, 7, 195–210. [Google Scholar]
- Windsor, H.; Windsor, G.; Noordergraaf, J. The growth and long term survival of Acholeplasma laidlawii in media products used in biopharmaceutical manufacturing. Biologicals 2010, 38, 204–210. [Google Scholar] [CrossRef]
- Wexler, H. Bacteroides: The Good, the Bad, and the Nitty-Gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef]
- Martens, E.; Chiang, H.; Gordon, J. Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe 2008, 4, 447–457. [Google Scholar] [CrossRef]
- Eberl, L.; Vandamme, P. Members of the genus Burkholderia: Good and bad guys. F1000Research 2016, 2016, 5. [Google Scholar] [CrossRef]
- Hamad, M.; Austin, C.; Stewart, A.; Higgins, M.; Vázquez-Torres, A.; Voskuil, M. Adaptation and Antibiotic Tolerance of Anaerobic Burkholderia pseudomallei. Antimicrob. Agents Chemother. 2011, 55, 3313–3323. [Google Scholar] [CrossRef]
- van Houten, B. Microbial Aspects of Synthesis Gas Fed Bioreactors Treating Sulfate and Metal Rich Wastewaters. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2006. [Google Scholar]
- Elferink, S. Sulfate-Reducing Bacteria in Anaerobic Bioreactors. Ph.D. Thesis, Wageningen Agricultural University, Wageningen, The Netherlands, 1998. [Google Scholar]
- Elberson, M.; Sowers, K. Isolation of an aceticlastic strain of Methanosarcina siciliae from marine canyon sediments and emendation of the species description for Methanosarcina siciliae. Int. J. Syst. Evol. Microbiol. 1997, 47, 1258–1261. [Google Scholar] [CrossRef]
- Itoh, T.; Yoshikawa, N.; Takashina, T. Thermogymnomonas acidicola gen. nov., sp. nov., a novel thermoacidophilic, cell wall-less archaeon in the order Thermoplasmatales, isolated from a solfataric soil in Hakone, Japan. Int. J. Syst. Evol. Microbiol. 2007, 57, 2557–2561. [Google Scholar] [CrossRef]
- National Institute of Advanced Industrial Science and Technology. Atlas of Eh-pH Diagrams: Intercomparison of Thermodynamic Databases. 2005. Available online: https://www.nrc.gov/docs/ML1808/ML18089A638.pdf (accessed on 31 August 2019).
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Hydrometallurgical processing of spent LIBs in the presence of a reducing agent with emphasis on kinetics of leaching. Chem. Eng. J. 2015, 281, 418–427. [Google Scholar] [CrossRef]
- Falagán, C.; Yusta, I.; Sánchez-España, J.; Johnson, D.B. Biologically-induced precipitation of aluminium in synthetic acid mine water. Miner. Eng. 2017, 106, 79–85. [Google Scholar] [CrossRef]
- Sahinkaya, E.; Gunes, F.; Ucar, D.; Kaksonen, A. Sulfidogenic fluidized bed treatment of real acid mine drainage water. Bioresour. Technol. 2011, 102, 683–689. [Google Scholar] [CrossRef]
- Lewis, A. Review of metal sulphide precipitation. Hydrometallurgy 2010, 104, 222–234. [Google Scholar] [CrossRef]



| Metal | Metal Content in LIB Waste (%) | Commodity Price ($US/ton) | Metal Value Contained in a ton of LIBs ($US) | Fraction of the Metal Value Contained in a ton of LIBs (%) |
|---|---|---|---|---|
| Cobalt | 20.4 | 33,000 | 6732 | 84.9 |
| Lithium * | 2.67 | 16,500 | 441 | 3.3 |
| Nickel | 5.42 | 13,546 | 734 | 5.0 |
| Manganese * | 1.22 | 2060 | 25 | 0.2 |
| Copper | 11.7 | 5941 | 695 | 5.5 |
| Aluminium | 6.72 | 1797 | 121 | 1.1 |
| Total | 8748 | 100 | ||
| Sample ID (Day of Operation) | Sample Source | DNA Concentration (ng μL−1) | Dissolved Sulfide Concentration in Reactor at the Time of Sampling (mg L−1) |
|---|---|---|---|
| A (105) | GAC | 2.16 | n.d. |
| B (106) | Concentrated suspension | 5.58 | n.d. |
| C (126) | GAC | 4.50 | 762 |
| D (126) | Concentrated suspension | 6.92 | 762 |
| E (146) | GAC | 7.68 | 590 |
| F (147) | Concentrated suspension | 6.54 | 590 |
| Genus | Sample Detection | Description | References |
|---|---|---|---|
| Bacteria | |||
| Dechloromonas | B, C, D, E, F | Anaerobic, Gram-negative bacteria capable of complete acetate oxidation by dissimilatory (per)chlorate reduction to chloride. | [50,51] |
| Clostridium | B, C, D, E, F | A diverse genus of anaerobic, spore forming, Gram-positive bacteria found in soils, animals and humans (both pathogenically and commensally). | [52] |
| Longilinea | A, B, C, E, F | Anaerobic Gram-negative bacteria which utilise proteins and some sugars for growth. Growth is enhanced in co-culture with hydrogen scavenging methanogens. | [53,54] |
| Bellilinea | A, E, F | Anaerobic Gram-negative bacteria which utilise carbohydrates for growth. Growth is enhanced in co-culture with hydrogen scavenging methanogens. | [54] |
| Desulfovibrio | All | Anaerobic Gram-negative SRB. They incompletely oxidise organic substrates to acetate. | [55,56,57] |
| Desulfomicrobium | All | Anaerobic Gram-negative SRB. They incompletely oxidise organic substrates to acetate. | [58,59] |
| Azonexus | All | Facultative aerobic Gram-negative bacteria. Capable of nitrogen fixation. | [60,61] |
| Desulfococcus | All | Strictly anaerobic Gram-negative SRB. They completely oxidise organic substrates. | [62,63,64] |
| Rhodobacter | A, B | Gram-negative bacteria with a range of metabolic capabilities. Main species of this genus are photosynthetic purple bacteria. | [65,66] |
| Spirochaeta | A, B, E, F | Anaerobic, saccharolytic and helical shaped bacteria. | [67,68] |
| Acholeplasma | A, B | Wall-less bacteria with uncertain classifications as either saprotrophic or pathogenic. | [69] |
| Bacteroides | C, E, F | Anaerobic Gram-negative bacteria largely found in the mammalian gastrointestinal microbiota. They break down complex molecules (such as plant and host-derived glycans) to simpler ones. | [70,71] |
| Burkholderia | B, C, E, F | Gram-negative bacteria which are aerobic but can often tolerate anaerobic conditions. They include animal, human and plant pathogens, as well as environmentally important species involved in nitrogen fixation, promoting plant growth and killing pest organisms. | [72,73] |
| Archaea | |||
| Methanobacterium | All | Methanogenic archaea known to grow on hydrogen and carbon dioxide. They grow without oxygen and are often present in anaerobic bioreactors. They exhibit similar growth kinetics to Desulfovibrio. | [74,75] |
| Methanosarcina | A, C, E | Archaeal genus containing species of acetoclastic methanogens that play a pivitol role in anaerobic consortia. | [76] |
| Thermogymnomonas | C, E | Aerobic, acidophilic and heterotrophic archaea which lack a cell wall. | [77] |
| Metal | Initial Leachate Metals (mg L−1) | Precipitation Efficiency (%) |
|---|---|---|
| Al | 5420 | 99.9 |
| Ni | 4160 | 99.9 |
| Co | 17,400 | 99.9 |
| Li | 2470 | 0.00 |
| Fe | 1140 | 99.5 |
| Mg | 34.6 | 49.1 |
| Mn | 987 | 98.9 |
| Cd | 91.3 | 98.6 |
| Zn | 78.7 | 98.4 |
| Cu | 9600 | 99.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvert, G.; Kaksonen, A.H.; Cheng, K.Y.; Van Yken, J.; Chang, B.; Boxall, N.J. Recovery of Metals from Waste Lithium Ion Battery Leachates Using Biogenic Hydrogen Sulfide. Minerals 2019, 9, 563. https://doi.org/10.3390/min9090563
Calvert G, Kaksonen AH, Cheng KY, Van Yken J, Chang B, Boxall NJ. Recovery of Metals from Waste Lithium Ion Battery Leachates Using Biogenic Hydrogen Sulfide. Minerals. 2019; 9(9):563. https://doi.org/10.3390/min9090563
Chicago/Turabian StyleCalvert, Giles, Anna H. Kaksonen, Ka Yu Cheng, Jonovan Van Yken, Barbara Chang, and Naomi J. Boxall. 2019. "Recovery of Metals from Waste Lithium Ion Battery Leachates Using Biogenic Hydrogen Sulfide" Minerals 9, no. 9: 563. https://doi.org/10.3390/min9090563
APA StyleCalvert, G., Kaksonen, A. H., Cheng, K. Y., Van Yken, J., Chang, B., & Boxall, N. J. (2019). Recovery of Metals from Waste Lithium Ion Battery Leachates Using Biogenic Hydrogen Sulfide. Minerals, 9(9), 563. https://doi.org/10.3390/min9090563







