Limits to the Validity of Thermal-Pressure Equations of State
Abstract
:1. Introduction
2. Methods
3. Thermal-Pressure EoS
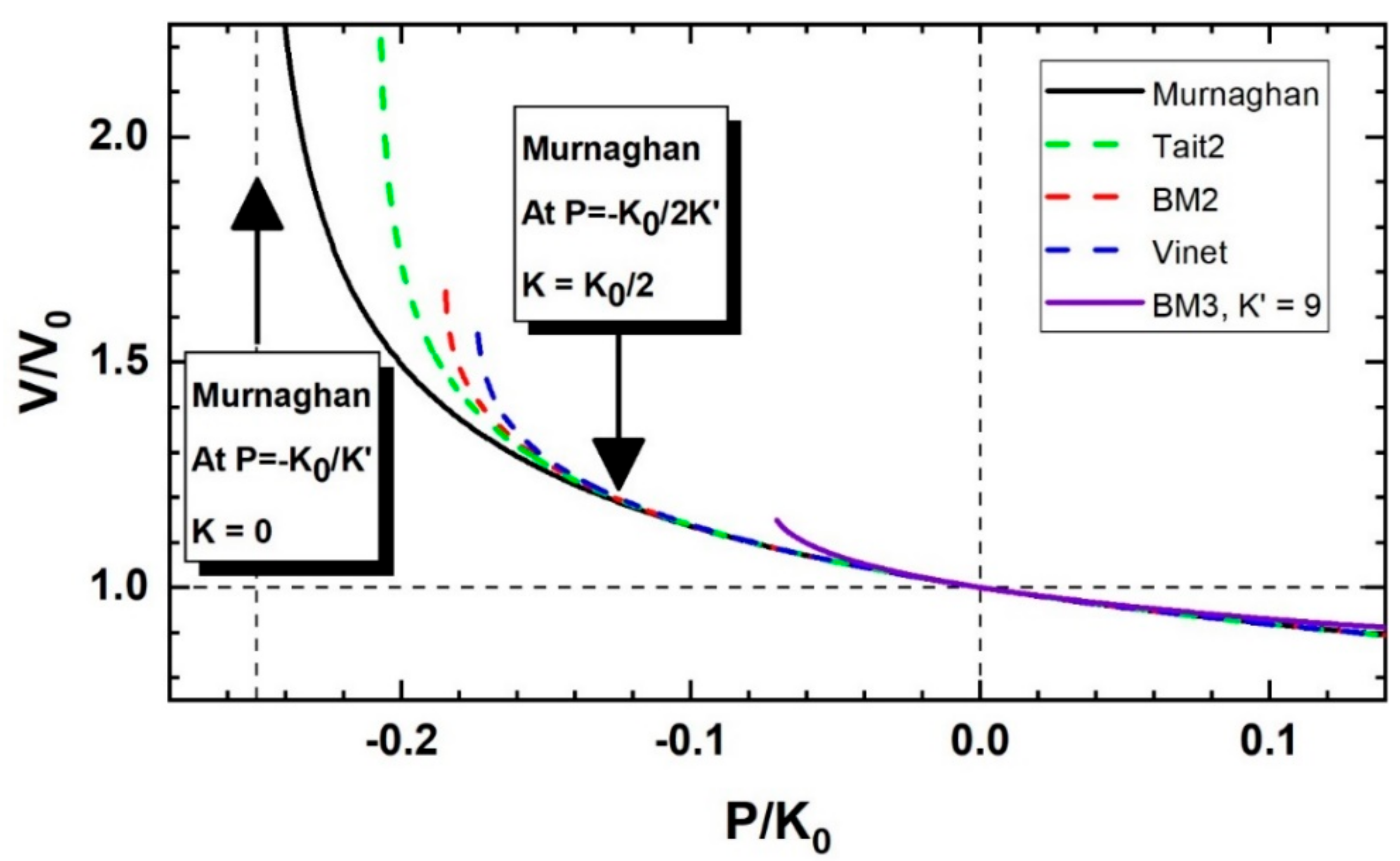
3.1. Limitations to Isothermal EoS in Expansion
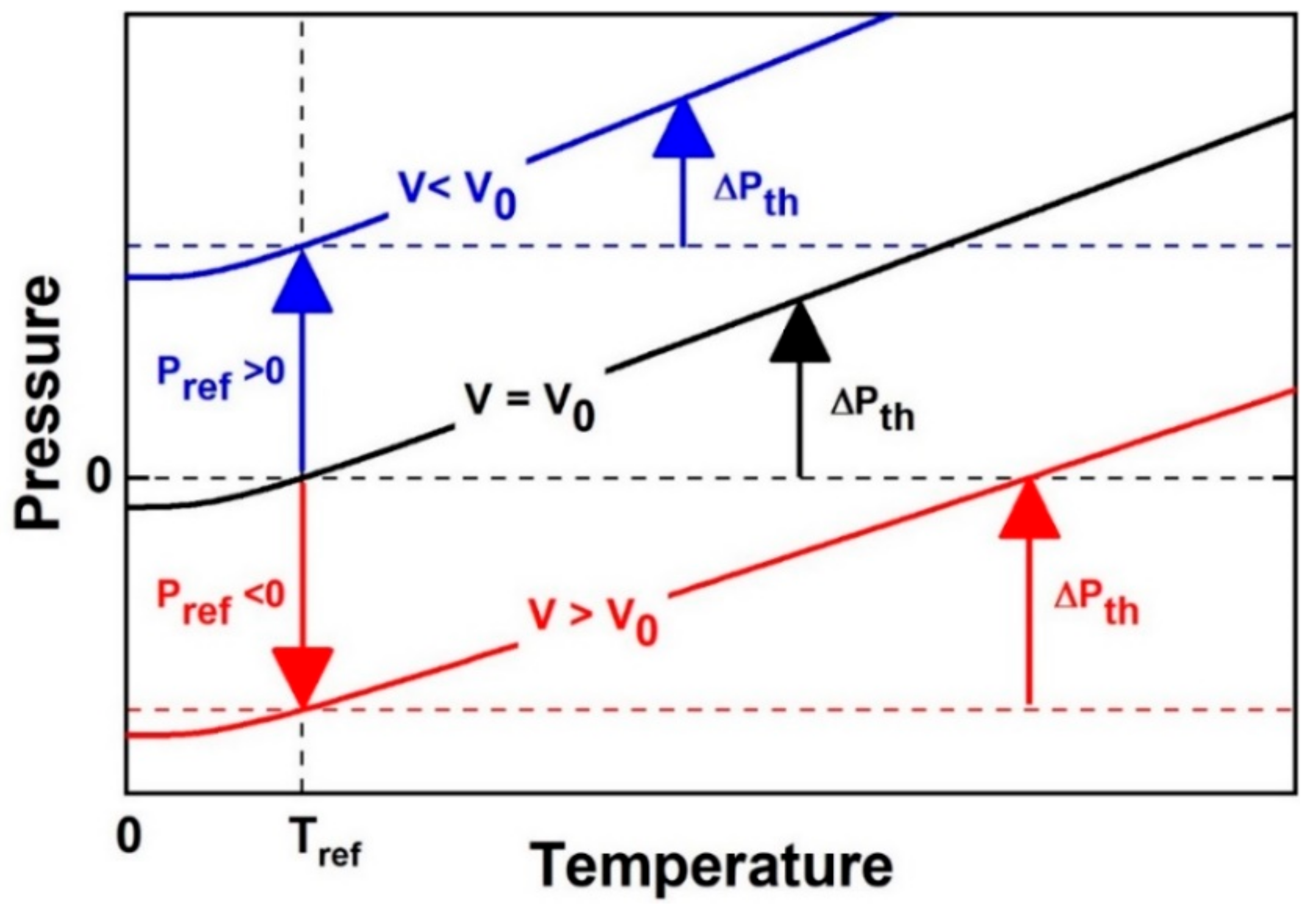
3.2. Limitations to Thermal-Pressure EoS
4. Mie-Grüneisen-Debye EoS
4.1. Limitations to MGD EoS from Expansion
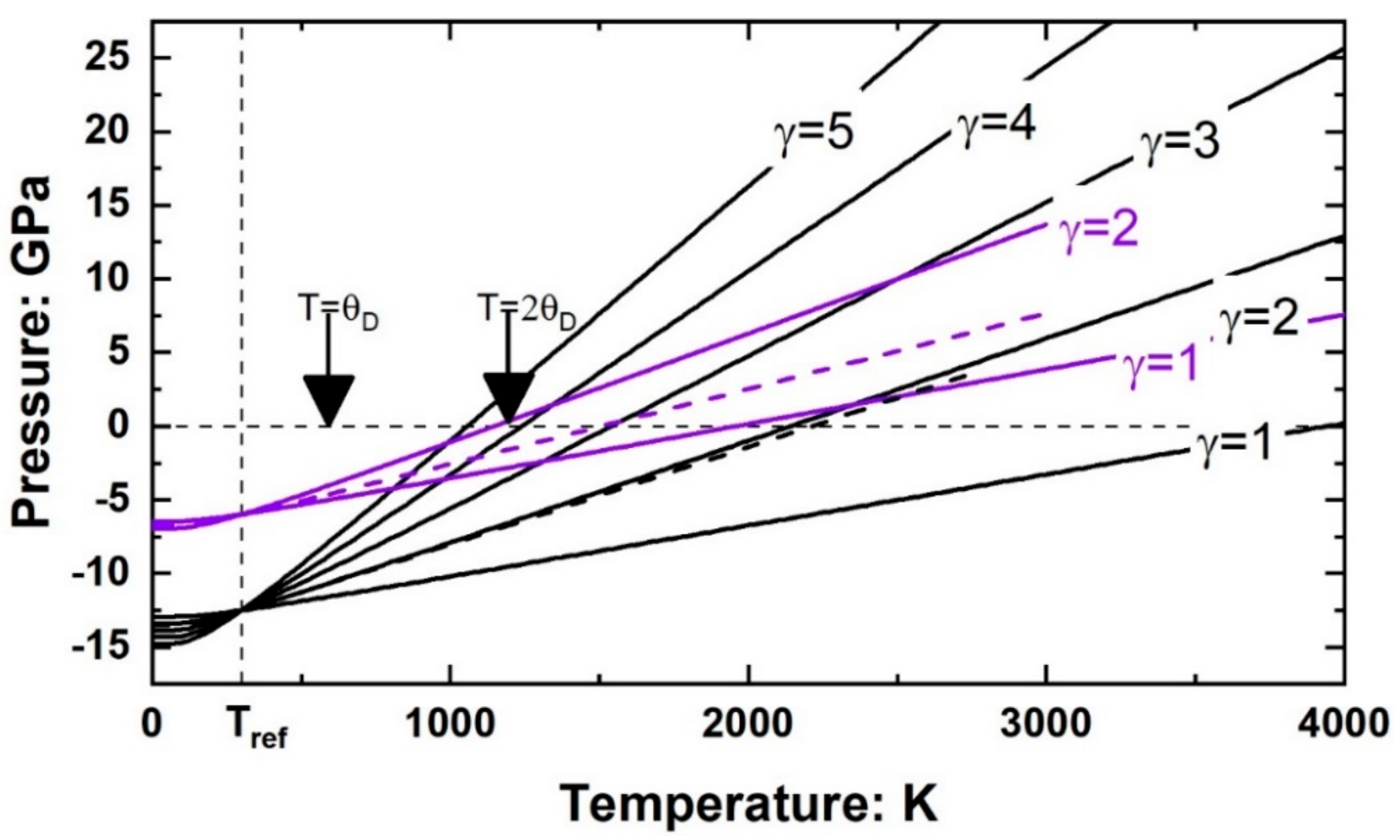
4.2. The Consequences of q
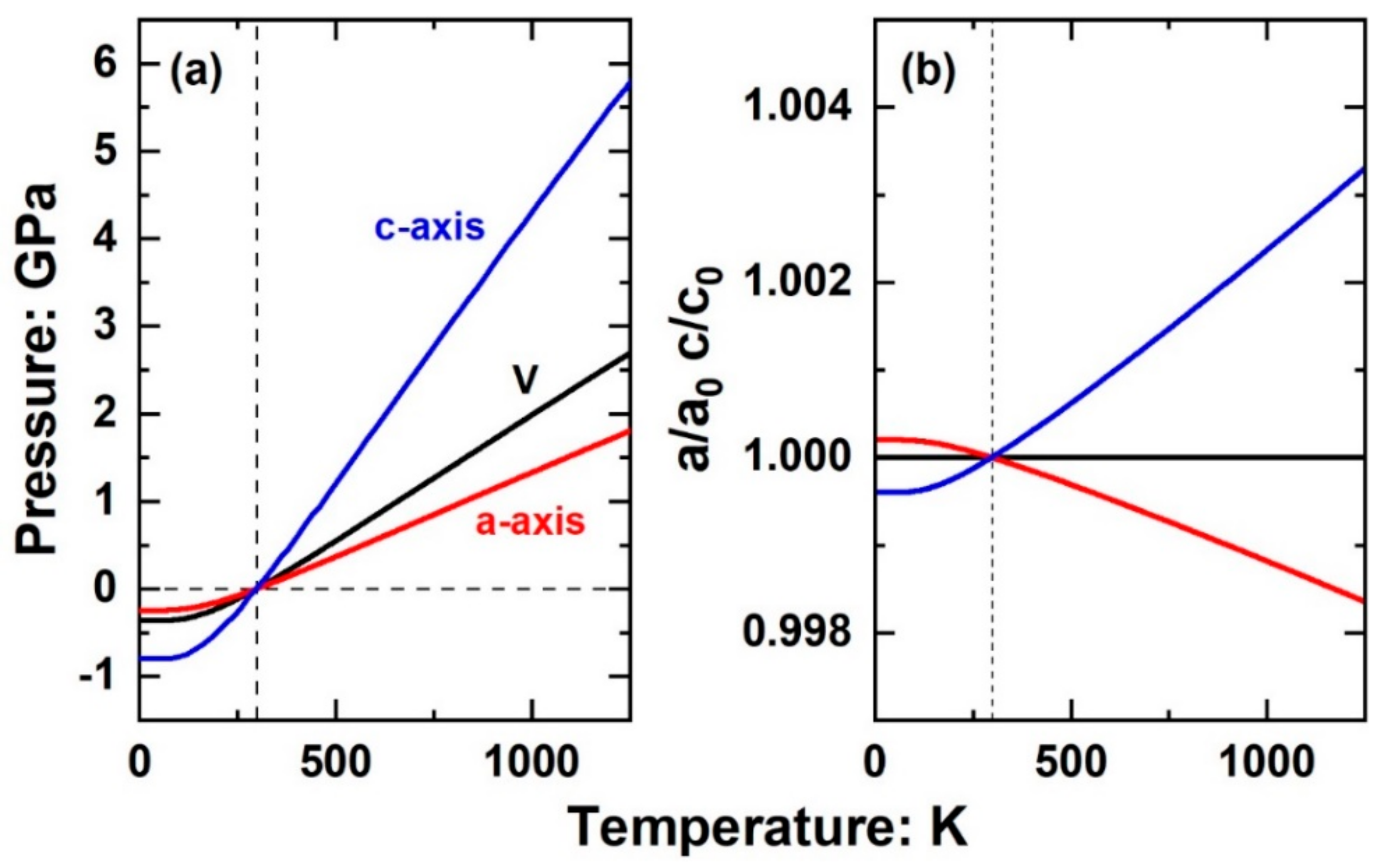
4.3. The Consequences of Anisotropic Pth
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anderson, O.L. Equations of State of Solids for Geophysics and Ceramic Science; Oxford University Press: Oxford, UK, 1995; p. 432. [Google Scholar]
- Navrotsky, A. Presented at the MSA Centennial Symposium, Washington, DC, USA, 20–21 June 2019.
- Wagner, F.; Sohl, F.; Hussmann, H.; Grott, M.; Rauer, H. Interior structure models of solid exoplanets using material laws in the infinite pressure limit. Icarus 2011, 214, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Elkins-Tanton, L.; Seager, S. Coreless Terrestrial Exoplanets. Astrophys. J. 2008, 688, 628–635. [Google Scholar] [CrossRef] [Green Version]
- Duffy, T.; Madhusudham, N.; Lee, K.K.M. Mineralogy of super-Earth planets. In Treatise on Geophysics, 2nd ed.; Elsevier: Oxford, UK, 2015; Volume 2, pp. 149–178. [Google Scholar] [CrossRef]
- Angel, R.J.; Gonzalez-Platas, J.; Alvaro, M. EosFit7c and a Fortran module (library) for equation of state calculations. Z. für Krist. 2014, 229, 405–419. [Google Scholar] [CrossRef]
- Angel, R.J.; Alvaro, M.; Nestola, F. 40 years of mineral elasticity: a critical review and a new parameterisation of Equations of State for mantle olivines and diamond inclusions. Phys. Chem. Miner. 2018, 45, 95–113. [Google Scholar] [CrossRef]
- Angel, R.J.; Jackson, J.M. Elasticity and equation of state of orthoenstatite, MgSiO3. Am. Mineral. 2002, 87, 558–561. [Google Scholar] [CrossRef]
- Webb, S. The elasticity of the upper mantle orthosilicates olivine and garnet to 3 GPa. Phys. Chem. Miner. 1989, 16, 684–692. [Google Scholar] [CrossRef]
- Murnaghan, F. Finite deformations of an elastic solid. Am. J. Math. 1937, 49, 235–260. [Google Scholar] [CrossRef]
- Angel, R.J. Equations of state. In High-Pressure and High-Temperature Crystal Chemistry; Hazen, R.M., Downs, R.T., Eds.; MSA: Chantilly, VA, USA, 2000; Volume 41, pp. 35–60. [Google Scholar]
- Birch, F. Finite elastic strain of cubic crystals. Phys. Rev. 1947, 71, 809–824. [Google Scholar] [CrossRef]
- Kroll, H.; Kirfel, A.; Heinemann, R.; Barbier, B. Volume thermal expansion and related thermophysical parameters in the Mg,Fe olivine solid-solution series. Eur. J. Mineral. 2012, 24, 935–956. [Google Scholar] [CrossRef]
- Gonzalez-Platas, J.; Alvaro, M.; Nestola, F.; Angel, R.J. EosFit7-GUI: A new GUI tool for equation of state calculations, analyses, and teaching. J. Appl. Crystallogr. 2016, 49, 1377–1382. [Google Scholar] [CrossRef]
- Hardy, R.J. Temperature and pressure dependence of intrinsic anharmonic and quantum corrections to the equation of state. J. Geophys. Res. B 1980, 85, 7011–7015. [Google Scholar] [CrossRef]
- Holland, T.J.B.; Powell, R. An improved and extended internally consistent thermodynamic dataset for phases of petrological interest, involving a new equation of state for solids. J. Metamorph. Geol. 2011, 29, 333–383. [Google Scholar] [CrossRef]
- Nye, J.F. Physical Properties of Crystals; Oxford University Press: Oxford, UK, 1957; p. 329. [Google Scholar]
- Hazen, R.M.; Finger, L.W. Comparative Crystal Chemistry; John Wiley and Sons: New York, NY, USA, 1982; p. 231. [Google Scholar]
- Stangarone, C.; Alvaro, M.; Angel, R.; Prencipe, M.; Mihailova, B.D. Determination of the phonon-mode Grüneisen tensors of zircon by DFT simulations. Eur. J. Mineral. 2019, 2019. [Google Scholar] [CrossRef]
- Zaffiro, G. Elastic Geobarometry: In-Situ Single-Crystal X-ray Diffraction Measurements of Inclusions Trapped in Host Minerals to Determine the Entrapment Conditions. Ph.D. Thesis, University of Pavia, Pavia, Italy, 2019. [Google Scholar]
- Grüneisen, E. Zustand des festen Körpers. Handb. der Phys. 1926, 1, 1–52. [Google Scholar]
- Key, S.W. Grüneisen tensor for anisotropic materials. J. Appl. Phys. 1967, 38, 2923–2928. [Google Scholar] [CrossRef]
- Cantrell, J.H. Generalized Grüneisen tensor from solid nonlinearity parameters. Phys. Rev. B 1980, 21, 4191–4195. [Google Scholar] [CrossRef]
- Angel, R.J.; Murri, M.; Mihailova, B.; Alvaro, M. Stress, strain and Raman shifts. Z. für Krist. 2019, 234, 129–140. [Google Scholar] [CrossRef]
- Barron, T.H.K.; Collins, J.F.; Smith, T.W.; White, G.K. Thermal expansion, Grüneisen functions and static lattice properties of quartz. J. Phys. C Solid State Phys. 1982, 15, 4311–4326. [Google Scholar] [CrossRef]
- Angel, R.J.; Sochalski-Kolbus, L.M.; Tribaudino, M. Tilts and tetrahedra: The origin of anisotropy of feldspars. Am. Mineral. 2012, 97, 765–778. [Google Scholar] [CrossRef]
- Zaffiro, G.; Angel, R.J.; Alvaro, M. Constraints on the Equations of State of stiff anisotropic minerals: Rutile, and the implications for rutile elastic barometry. Mineral. Mag. 2019, 83, 339–347. [Google Scholar] [CrossRef]
- Blackman, M. The theory of the specific heat of solids. Rep. Prog. Phys. 1941, 8, 11–30. [Google Scholar] [CrossRef]
- Murshed, M.M.; Zhao, P.; Fischer, M.; Huq, A.; Alekseev, E.; Gesing, T.M. Thermal expansion modeling of framework-type Na[AsW2O9] and K[AsW2O9]. Mater. Res. Bull. 2016, 84, 273–282. [Google Scholar] [CrossRef]
- Murshed, M.M.; Mendive, C.B.; Curti, M.; Šehović, M.; Friedrich, A.; Fischer, M.; Gesing, T.M. Thermal expansion of mullite-type Bi2Al4O9: A study by X-ray diffraction,vibrational spectroscopy and density functional theory. J. Solid State Chem. 2015, 229, 87–96. [Google Scholar] [CrossRef]
- Fei, Y.; Murphy, C.; Shibazaki, Y.; Shahar, A.; Huang, H. Thermal equation of state of hcp-iron: Constraint on the density deficit of Earth’s solid inner core. Geophys. Res. Lett. 2016, 43, 6837–6843. [Google Scholar] [CrossRef]
- Munn, R.W. Role of the elastic constants in negative thermal expansion of axial solids. J. Phys. C Solid State Phys. 1972, 5, 535–542. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angel, R.J.; Miozzi, F.; Alvaro, M. Limits to the Validity of Thermal-Pressure Equations of State. Minerals 2019, 9, 562. https://doi.org/10.3390/min9090562
Angel RJ, Miozzi F, Alvaro M. Limits to the Validity of Thermal-Pressure Equations of State. Minerals. 2019; 9(9):562. https://doi.org/10.3390/min9090562
Chicago/Turabian StyleAngel, Ross J., Francesca Miozzi, and Matteo Alvaro. 2019. "Limits to the Validity of Thermal-Pressure Equations of State" Minerals 9, no. 9: 562. https://doi.org/10.3390/min9090562
APA StyleAngel, R. J., Miozzi, F., & Alvaro, M. (2019). Limits to the Validity of Thermal-Pressure Equations of State. Minerals, 9(9), 562. https://doi.org/10.3390/min9090562





