The Kilmar Magnesite Deposits: Evaporitic Metasediments in the Grenville Supergroup, Morin Terrane, Quebec
Abstract
:1. Introduction
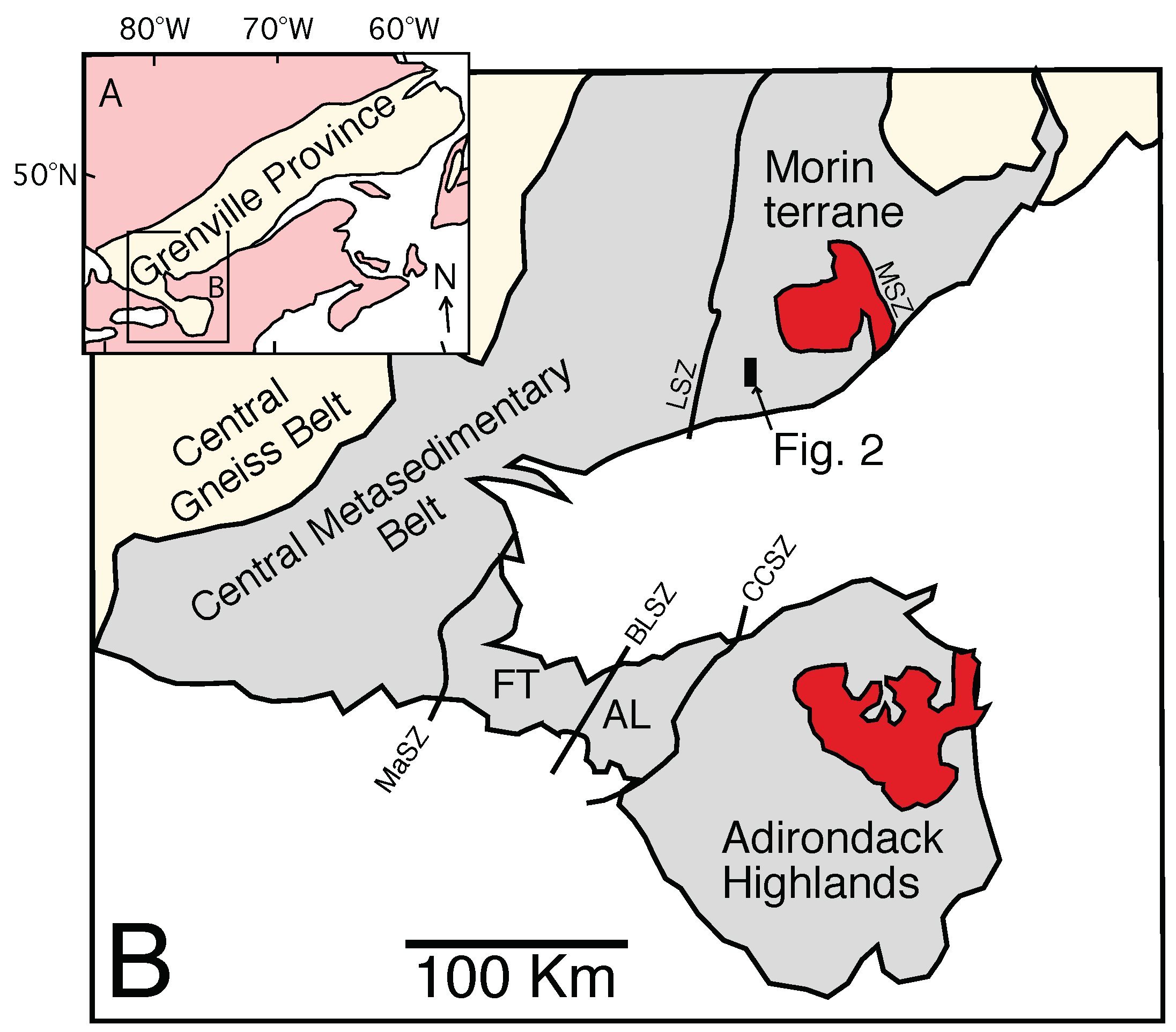
2. Geologic Setting
3. Materials and Methods
4. Results and Discussion
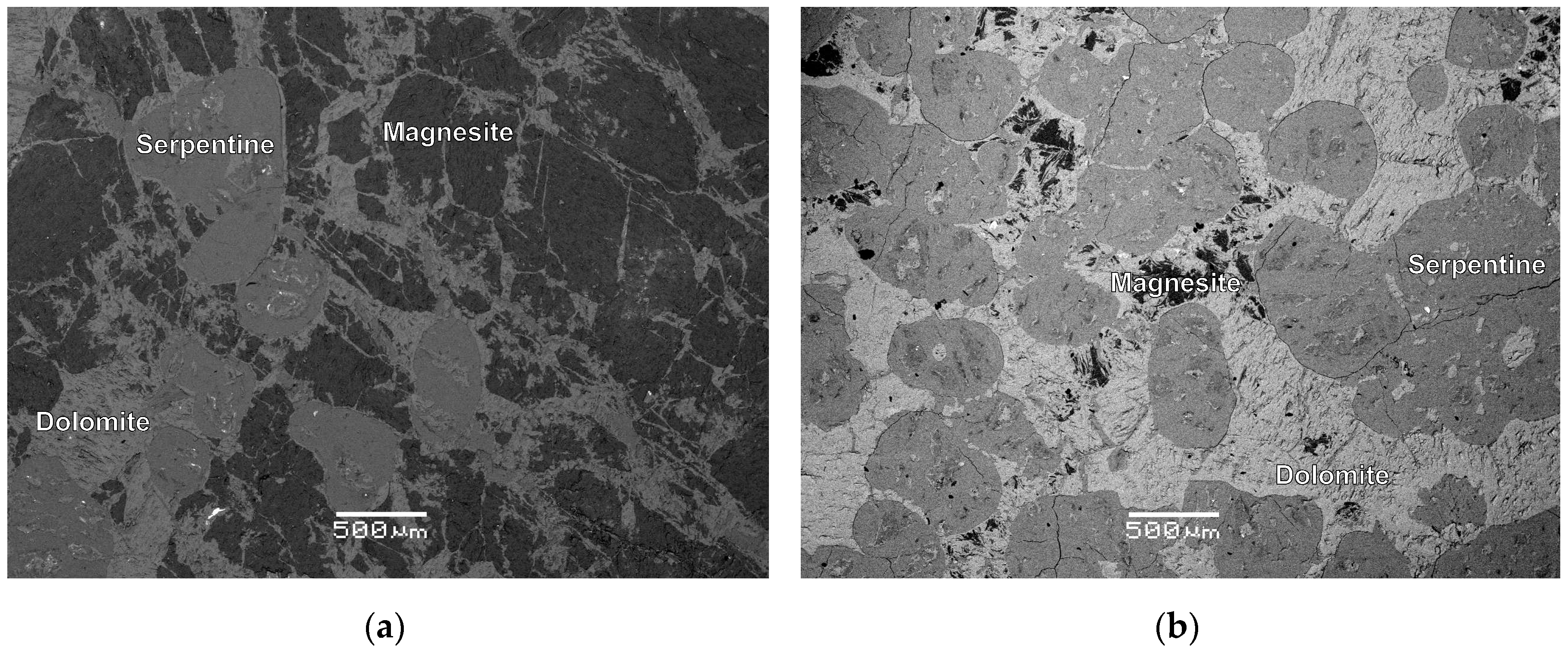
4.1. Outcrop Description and Mineralogy
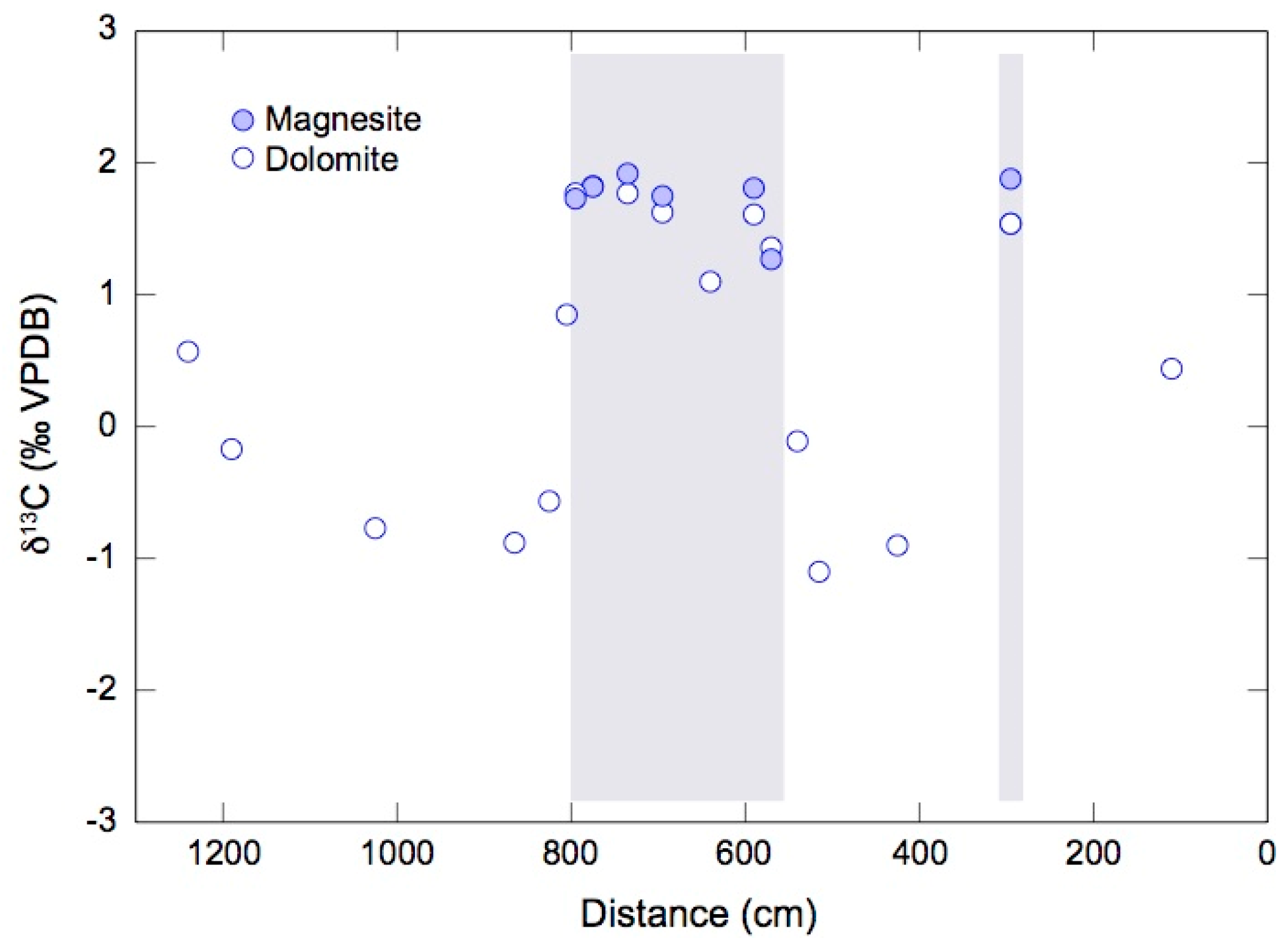
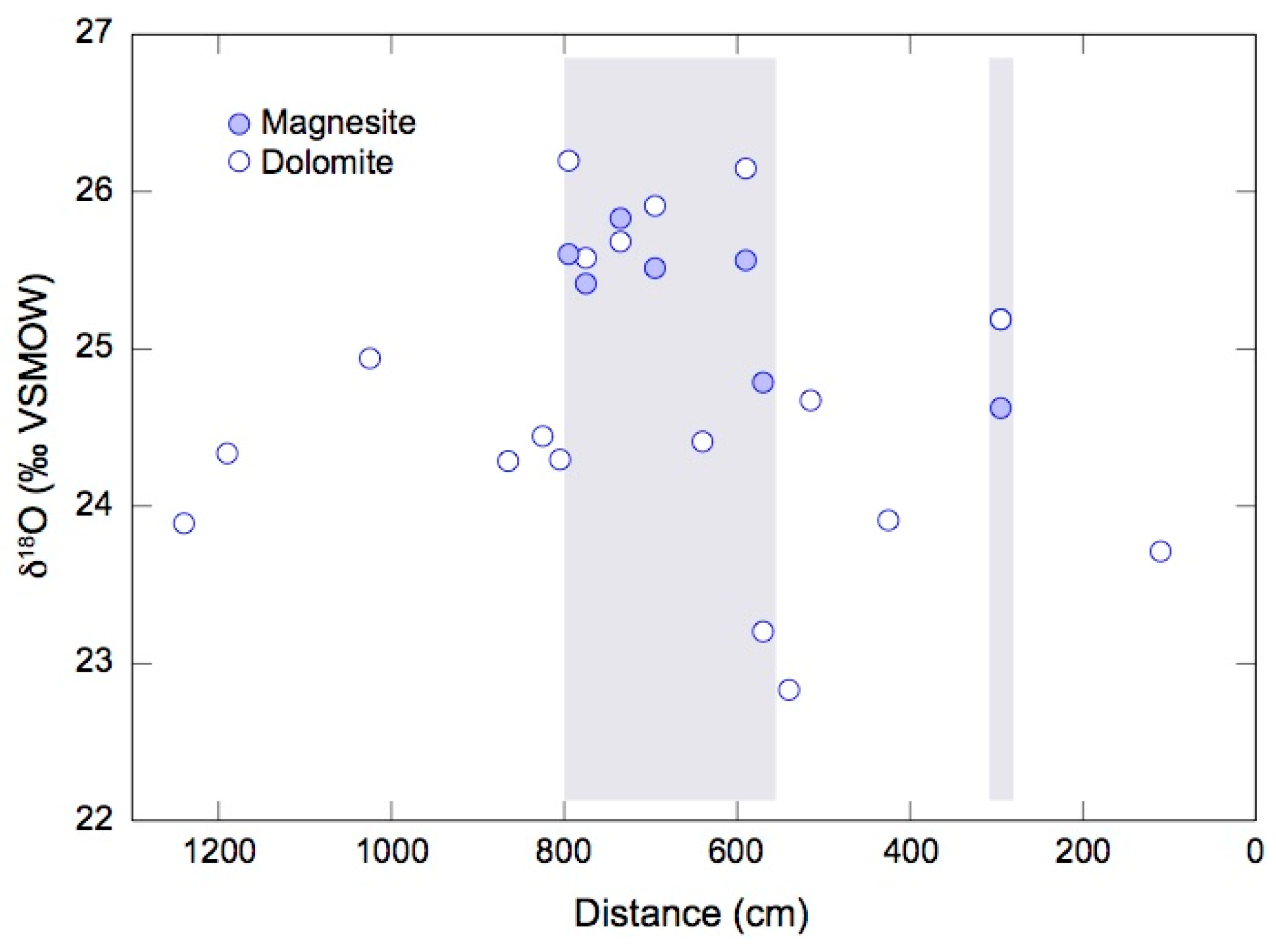
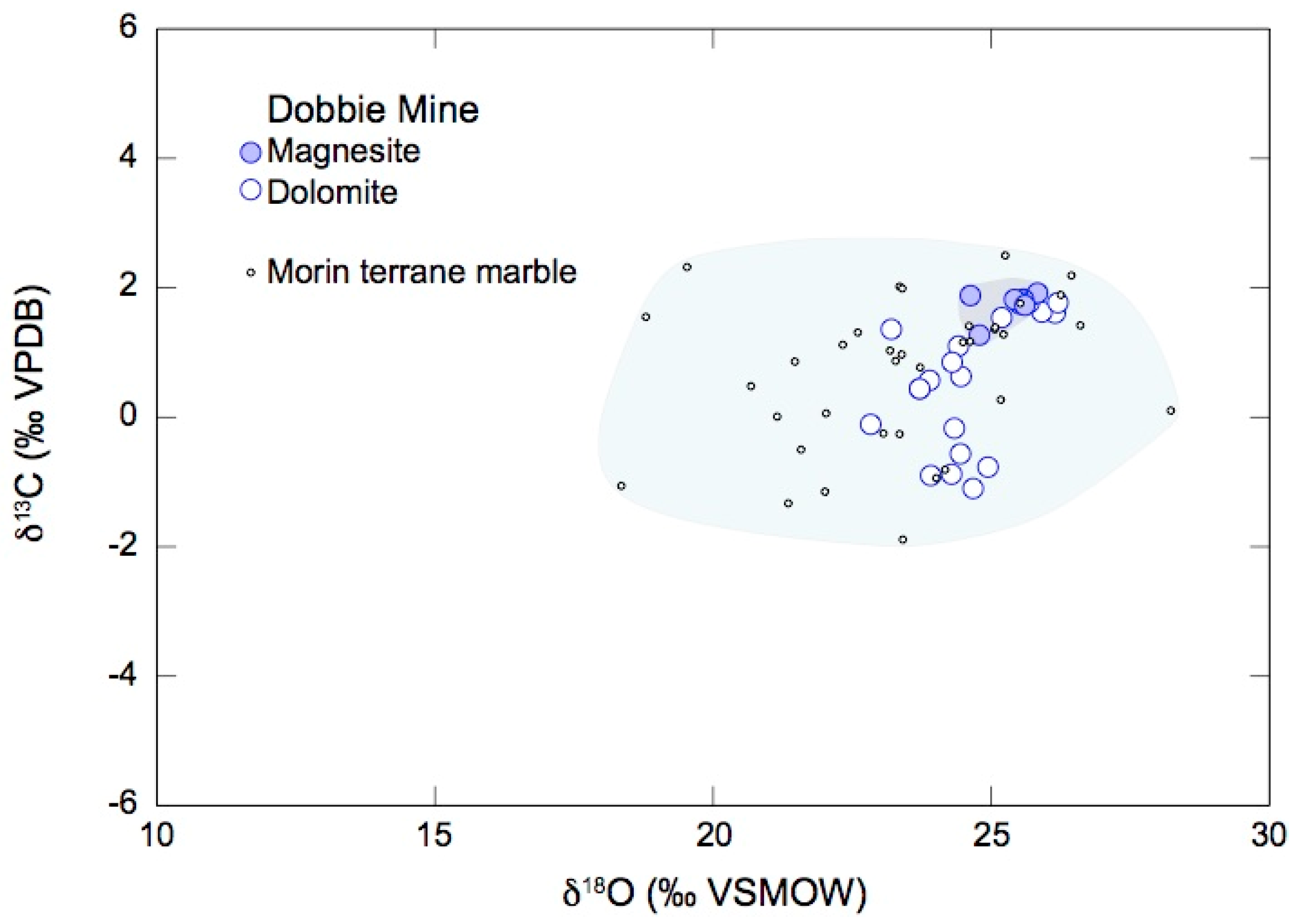
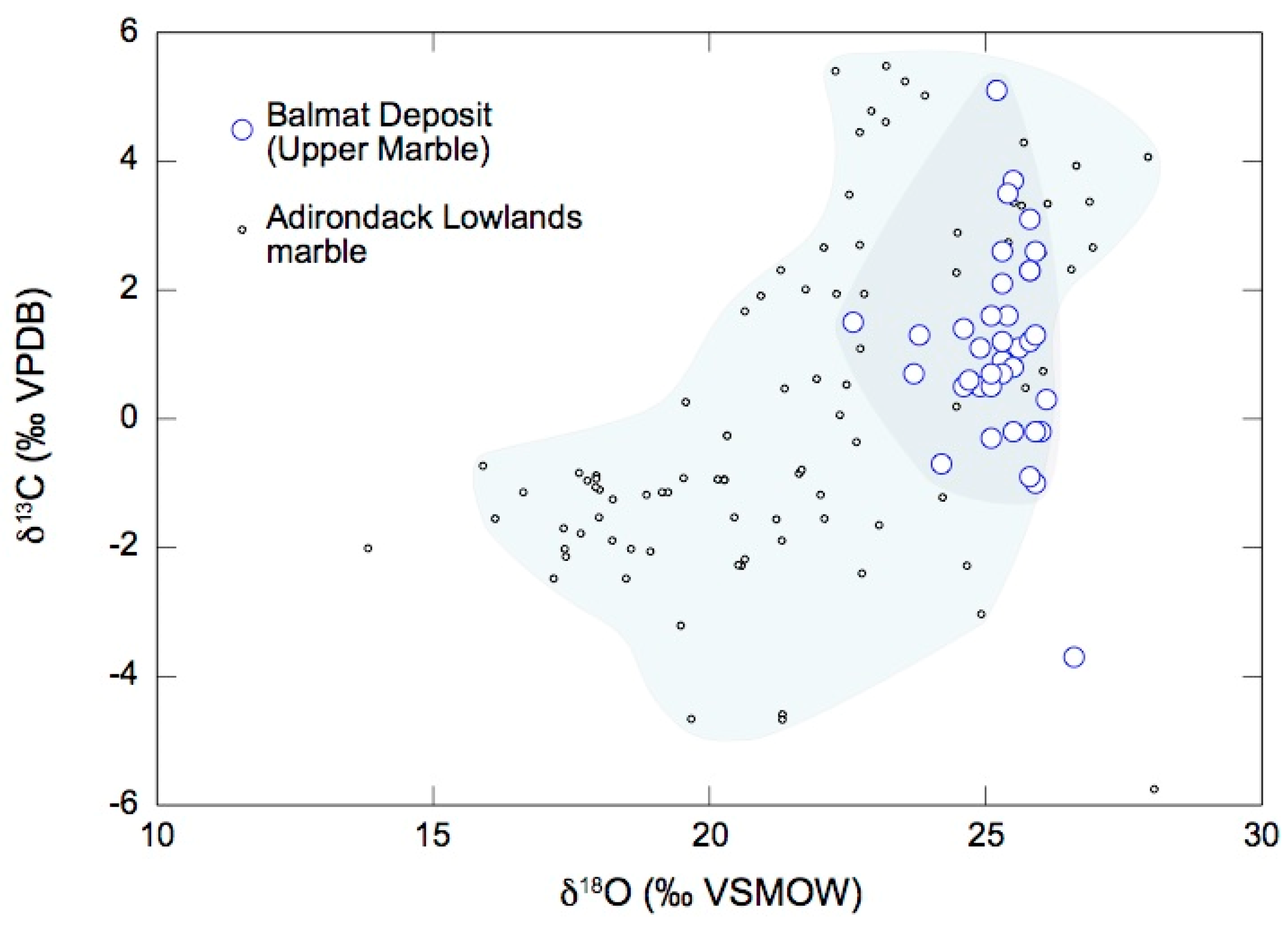
4.2. Oxygen and Carbon Isotope Ratios of Magnesite and Dolomite
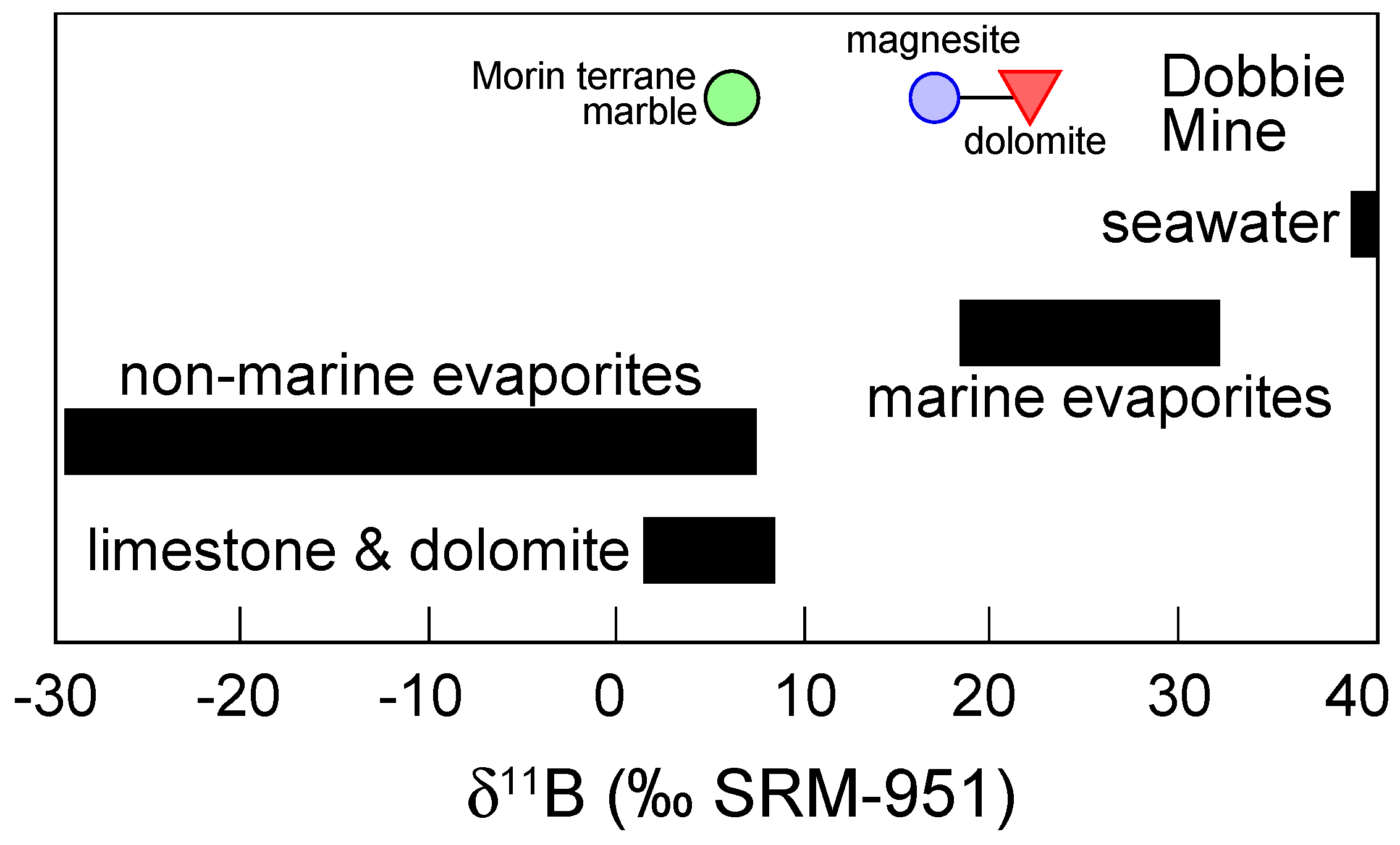
4.3. Boron Isotope Ratios
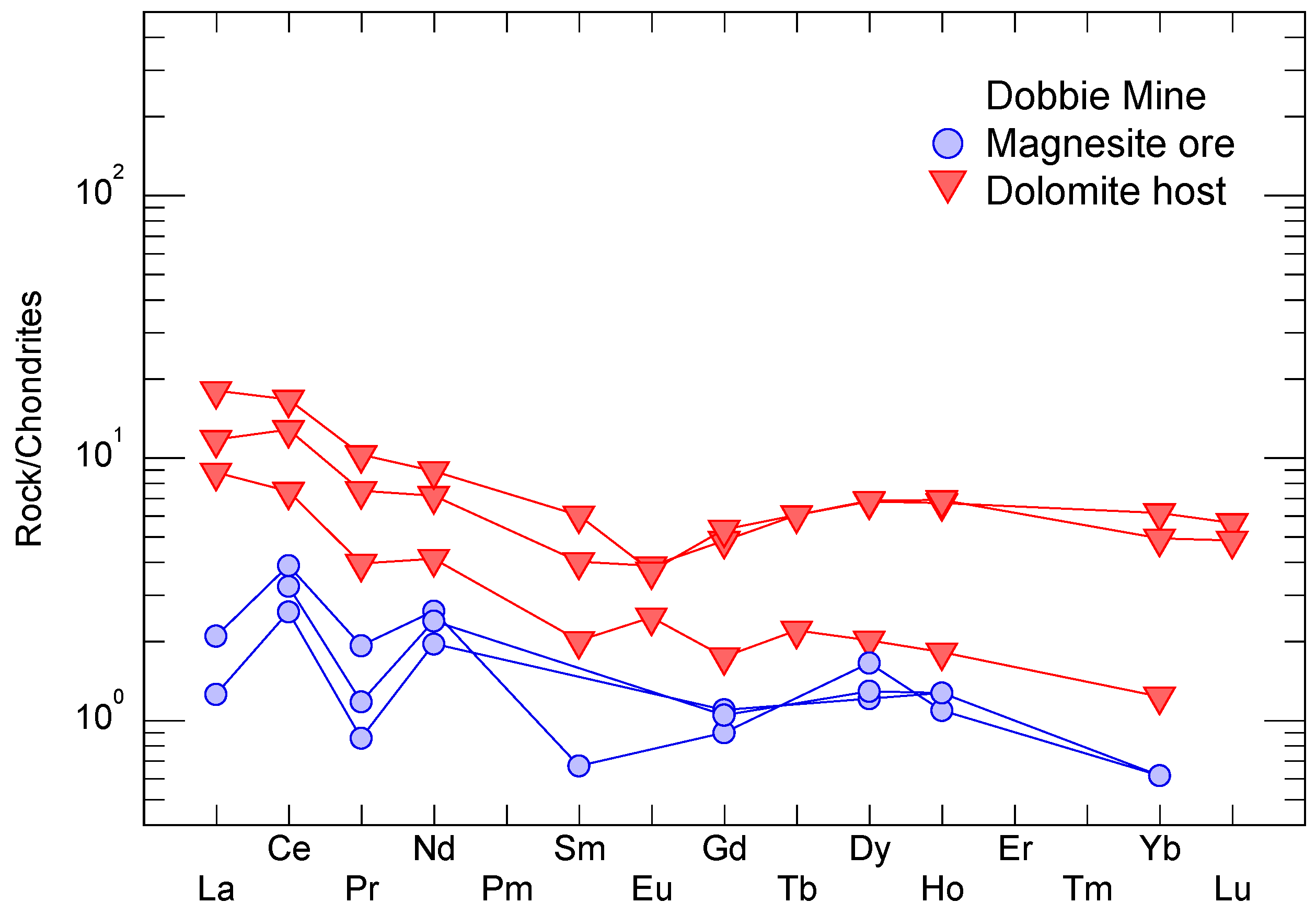
4.4. Major and Trace Elements
5. Discussion
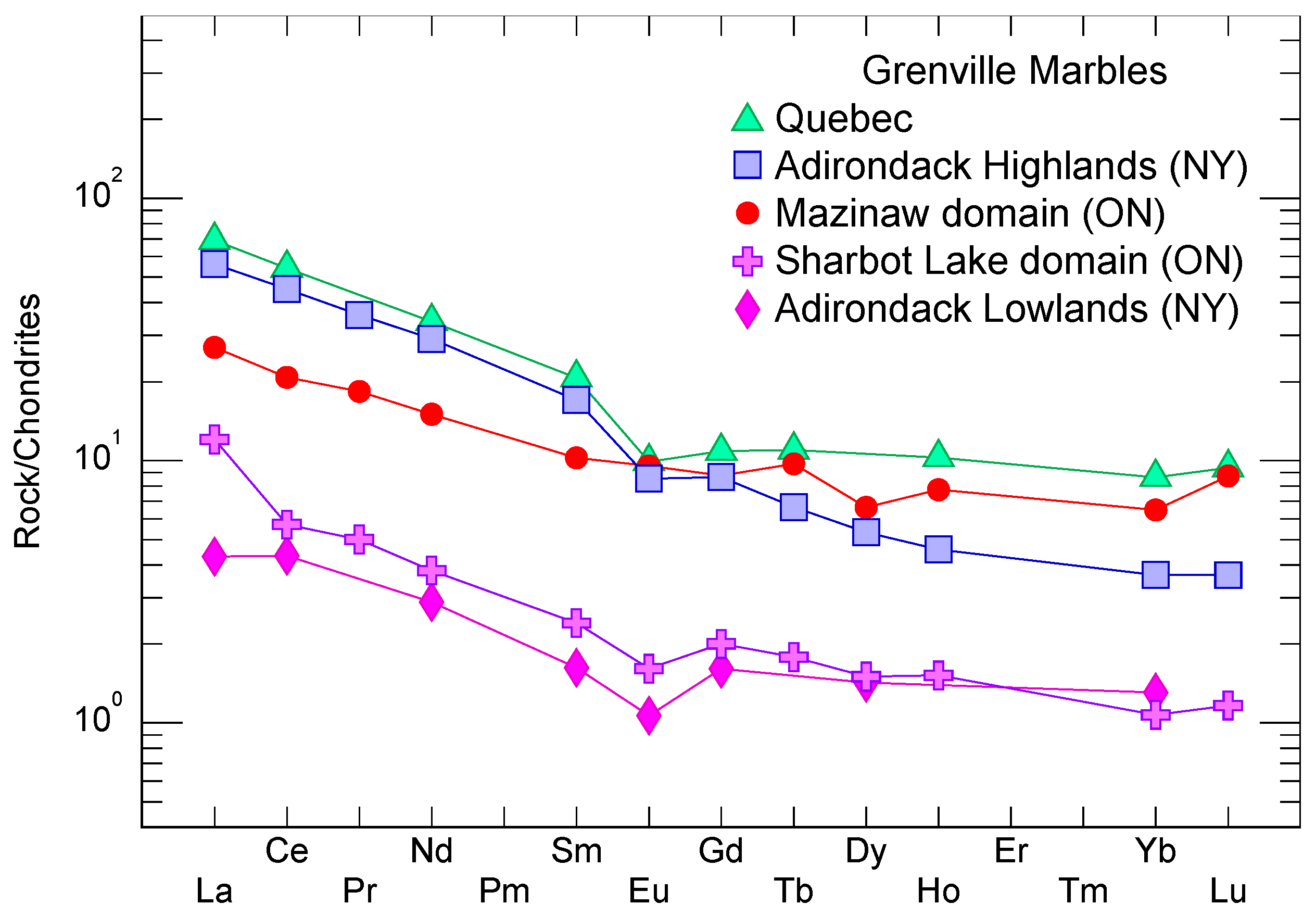
5.1. Comparison to Grenville Marbles in Nearby Terranes
5.2. Magnesite Ore Deposit Models
5.3. Regional Implications for Depositional Settings of Ores in Grenville Marbles
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perrault, S. The ups and downs of industrial mineral production in Quebec: A response to changing markets. Newsl. Mineral. Assoc. Can. 2003, 71, 1–23. [Google Scholar]
- Sabina, A.P. Rocks and Minerals for the Collector. Buckingham-Mont-Laurier-Grenville, Quebec; Hawkesbury-Ottawa, Ontario; Geological Survey of Canada: Ottawa, ON, Canada, 1986; p. 90.
- Wilson, M.E. Magnesite Deposits of Grenville District, Argenteuil County, Quebec; Geological Survey of Canada: Ottawa, ON, Canada, 1917; p. 88.
- Osborne, F.F. Magnesite-dolomite deposits, Grenville Township. In Quebec Bureau of Mines Annual Report, Part C Lachute Map-Area, Part III; Quebec Bureau of Mines: Québec, QC, Canada, 1938; pp. 65–87. [Google Scholar]
- Möller, P. Comparative geology of magnesite deposits and occurrences. In Magnesite: Geology, Mineralogy, Geochemistry, Formation of Mg-Carbonates; Möller, P., Ed.; Borntraeger: Berlin, Germany, 1989; pp. 1–13. [Google Scholar]
- Gauthier, M.; Chartrand, F. Metallogeny of the Grenville Province revisited. Can. J. Earth Sci. 2005, 42, 1719–1734. [Google Scholar] [CrossRef]
- Sasias, M.; Banville, R. Rapport de travaux statutaires, Projet Gatineau Zinc, propriete de Kilmar. In Mining Exploration File GM 64433; Ministère de l’Énergie et des Ressources naturelles du Québec: Québec, QC, Canada, 2009; p. 106. [Google Scholar]
- Martignole, J.; Schrijver, K. Tectonic setting and evolution of the Morin Anorthosite, Grenville Province, Quebec. Bull. Geol. Soc. Finl. 1970, 42, 165–209. [Google Scholar] [CrossRef]
- Doig, R. U-Pb zircon dates of Morin anorthosite suite rocks, Grenville Province, Quebec. J. Geol. 1991, 99, 729–738. [Google Scholar] [CrossRef]
- Peck, W.H. Reconnaissance geochronology and geochemistry of the Mont-Tremblant gneiss of the Morin terrane, Grenville Province, Québec. Geosphere 2012, 8, 1356–1365. [Google Scholar] [CrossRef]
- Wynne-Edwards, H.; Gregory, A.F.; Hay, P.W.; Giovanella, C.A.; Reinhardt, E.W. Mont Laurier and Kempt Lake Map-Areas, Quebec; A Preliminary Report on the Grenville Project; Geological Survey of Canada: Ottawa, ON, Canada, 1966; p. 32.
- Peck, W.H.; Quinan, M.P.; Selleck, B.W. Detrital zircon constraints on Grenville sedimentation at the margin of Laurentia. Precambrian Res. 2019, 331, 105342. [Google Scholar] [CrossRef]
- Davidson, A. An overview of Grenville Province geology, Canadian shield. In Geology of the Precambrian Superior and Grenville Provinces and Precambrian Fossils in North America; Lucas, S.B., St-Onge, M.R., Eds.; Geological Survey of Canada: Ottawa, ON, Canada, 1998; pp. 205–270. [Google Scholar]
- Peck, W.H.; DeAngelis, M.T.; Meredith, M.T.; Morin, E. Polymetamorphism of marbles in the Morin Terrane, Grenville Province, Quebec. Can. J. Earth Sci. 2005, 42, 1949–1965. [Google Scholar] [CrossRef]
- Peck, W.H.; Quinan, M.P. New age constraints on magmatism and metamorphism in the Morin terrane (Grenville Province, Quebec). Can. J. Earth Sci. under review.
- Katz, M.B. The nature and origin of the granulites of Mont Tremblant Park, Quebec. Geol. Soc. Am. Bull. 1969, 80, 2019–2037. [Google Scholar] [CrossRef]
- Anonymous. Magnesite deposits at Kilmar, Quebec. In The Geology of Industrial Minerals in Canada; Guillet, G.R., Martin, W., Eds.; Canadian Institute of Mining and Metallurgy: Westmount, QC, Canada, 1983; Volume 29, pp. 79–80. [Google Scholar]
- Al-Aasm, I.S.; Taylor, B.; South, B. Stable isotope analysis of multiple carbonate samples using selective acid extraction. Chem. Geol. Isot. Geosci. Sect. 1990, 80, 119–125. [Google Scholar] [CrossRef]
- McCrea, J.M. On the isotopic chemistry of carbonates and a paleotemperature scale. J. Chem. Phys. 1950, 18, 849–857. [Google Scholar] [CrossRef]
- Das Sharma, S.; Patil, D.; Gopalan, K. Temperature dependence of oxygen isotope fractionation of CO2 from magnesite-phosphoric acid reaction. Geochim. Cosmochim Acta 2002, 66, 589–593. [Google Scholar] [CrossRef]
- Baumgartner, L.P.; Valley, J.W. Stable isotope transport and contact metamorphic fluid flow. Rev. Mineral. Geochem. 2001, 43, 415–467. [Google Scholar] [CrossRef]
- Schauble, E.A.; Ghosh, P.; Eiler, J.M. Preferential formation of 13C–18O bonds in carbonate minerals, estimated using first-principles lattice dynamics. Geochim. Cosmochim. Acta 2006, 70, 2510–2529. [Google Scholar] [CrossRef]
- Marschall, H.R. Boron isotopes in the ocean floor realm and the mantle. In Boron Isotopes, Advances in Isotope Geochemistry; Marschall, H., Foster, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 189–215. [Google Scholar]
- Barth, S. Boron isotope variations in nature: A synthesis. Geol. Rundsch 1993, 82, 640–651. [Google Scholar] [CrossRef]
- Jiang, S.; Ni, P.; Ling, H.; Jiang, Y. Boron isotope geochemistry of borate and magnesite deposits in eastern Liaoning and Jilin Provinces of China. In Mineral Deposits at the Beginning of the 21st Century; Piestrzyñski, A., Ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 989–991. [Google Scholar]
- McDonough, W.F.; Sun, S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Lugli, S.; Torres-Ruiz, J.; Garuti, G.; Olmedo, F. Petrography and geochemistry of the Eugui magnesite deposit (Western Pyrenees, Spain): Evidence for the development of a peculiar zebra banding by dolomite replacement. Econ. Geol. 2000, 95, 1775–1791. [Google Scholar] [CrossRef]
- Easton, R. Regional Geochemical Variation in Grenvillian Carbonate Rocks: Implications for Mineral Exploration; Ontario Geological Survey: Sudbury, ON, Canada, 1995; pp. 6–18.
- Hauer, K. Protoliths, Diagenesis, and Depositional History of the Upper Marble. Adirondack Lowlands, New York. Ph.D. Thesis, Miami University, Oxford, OH, USA, 1995. [Google Scholar]
- Whitney, P.R.; Olmsted, J.F. Rare earth element metasomatism in hydrothermal systems: The Willsboro-Lewis wollastonite ores, New York, USA. Geochim. Cosmochim Acta 1998, 62, 2965–2977. [Google Scholar] [CrossRef]
- Easton, R.; Sykora, L.; van Haaften, S.; Meyn, H. Geochemical Data from Carbonate Rocks from the Central Metasedimentary Belt; Ontario Geological Survey: Sudbury, ON, Canada, 2007.
- Shaw, D.M.; Dostal, J.; Keays, R.R. Additional estimates of continental surface Precambrian shield composition in Canada. Geochim. Cosmochim Acta 1976, 40, 73–83. [Google Scholar] [CrossRef]
- Valley, J.; O’Neil, J.R. Fluid heterogeneity during granulite facies metamorphism in the Adirondacks: Stable isotope evidence. Contrib. Mineral. Petrol. 1984, 85, 158–173. [Google Scholar] [CrossRef]
- Peck, W.H.; Volkert, R.A.; Meredith, M.T.; Rader, E.L. Calcite-graphite thermometry of the Franklin marble, New Jersey Highlands. J. Geol. 2006, 114, 485–499. [Google Scholar] [CrossRef]
- Dunn, S.R.; Markley, M.J.; Kotikian, M.; Achenbach, K.; Montanye, B.; Peck, W.H. Geothermometry of the western half of the Central Metasedimentary Belt, Grenville Province, Ontario, and its implications. Am. Mineral. 2019, 104, 791–809. [Google Scholar] [CrossRef]
- Kitchen, N.E.; Valley, J. Carbon isotope thermometry in marbles of the Adirondack Mountains, New York. J. Metamorph. Geol. 1995, 13, 577–594. [Google Scholar] [CrossRef]
- Moecher, D.; Essene, E.; Valley, J. Stable isotopic and petrological constraints on scapolitization of the Whitestone meta-anorthosite, Grenville Province, Ontario. J. Metamorph. Geol. 1992, 10, 745–762. [Google Scholar] [CrossRef]
- Kretz, R. Oxygen and carbon isotopic composition of Grenville marble, and an appraisal of equilibrium in the distribution of isotopes between calcite and associated minerals, Otter Lake area, Quebec, Canada. Can. Mineral. 2001, 39, 1455–1472. [Google Scholar] [CrossRef]
- Tortorello, R.D.; Peck, W. Calcite-Graphite Thermometry Of Marbles in the Frontenac Terrane (Grenville Province, Ontario); Geological Society of America: Boulder, CO, USA, 2010; p. 160. [Google Scholar]
- Stiller, M.; Rounick, J.; Shasha, S. Extreme carbon-isotope enrichments in evaporating brines. Nature 1985, 316, 434. [Google Scholar] [CrossRef]
- Babel, M.; Schreiber, B. Geochemistry of evaporites and evolution of seawater. Treatise Geochem. 2014, 9, 483–560. [Google Scholar]
- Zachmann, D.; Johannes, W. Cryptocrystalline magnesite. In Magnesite: Geology, Mineralogy, Geochemistry, Formation of Mg-Carbonates; Möller, P., Ed.; Borntraeger: Berlin, Germany, 1989; pp. 15–28. [Google Scholar]
- Kralik, M.; Aharon, P.; Schroll, E.; Zachmann, D. Carbon and oxygen isotope systematics of magnesites: A review. In Magnesite: Geology, Mineralogy, Geochemistry, Formation of Mg-Carbonates; Möller, P., Ed.; Borntraeger: Berlin, Germany, 1989; pp. 197–223. [Google Scholar]
- Pohl, W. Genesis of magnesite deposits—Models and trends. Geol. Rundsch 1990, 79, 291–299. [Google Scholar] [CrossRef]
- Melezhik, V.A.; Fallick, A.E.; Medvedev, P.V.; Makarikhin, V.V. Palaeoproterozoic magnesite: Lithological and isotopic evidence for playa/sabkha environments. Sedimentology 2001, 48, 379–397. [Google Scholar] [CrossRef]
- Frank, T.D.; Fielding, C.R. Marine origin for Precambrian, carbonate-hosted magnesite? Geology 2003, 31, 1101–1104. [Google Scholar] [CrossRef]
- Ellmies, R.; Voigtländer, G.; Germann, K.; Krupenin, M.; Möller, P. Origin of giant stratabound deposits of magnesite and siderite in Riphean carbonate rocks of the Bashkir mega-anticline, western Urals. Geol. Rundsch 1999, 87, 589–602. [Google Scholar]
- Spötl, C.; Burns, S.J. Formation of 18O-depleted dolomite within a marine evaporitic sequence, Triassic Reichenhall Formation, Austria. Sedimentology 1991, 38, 1041–1057. [Google Scholar] [CrossRef]
- Cooke, D.R.; Bull, S.W.; Large, R.R.; McGoldrick, P.J. The importance of oxidized brines for the formation of Australian Proterozoic stratiform sediment-hosted Pb-Zn (Sedex) deposits. Econ. Geol. 2000, 95, 1–18. [Google Scholar] [CrossRef]
- Whelan, J.F.; Rye, R.O.; De Lorraine, W.; Ohmoto, H. Isotopic geochemistry of a mid-Proterozoic evaporite basin: Balmat, New York. Am. J. Sci. 1990, 290, 396–424. [Google Scholar] [CrossRef]
- Nantel, S. Les Tourmalinites et les Roches Riches en Tourmaline Dans la Partie sud de la Province de Grenville, Québec, et Leur Association Avec des Minéralisations en Zn et en Cu-Co Plus ou Moins Au; Ministère des Ressources Naturelles du Québec: Québec, QC, Canada, 1994; p. 26.
- Larivière, J. Hypogene Zinc Silicates, Oxides and Sulfides in Mesoproterozoic Grenville Supergroup Marbles of the Bryson-Renfrew Region (Quebec and Ontario): Distribution and Genetic Significance. Ph.D. Thesis, Université Du Québec À Montréal, Montreal, QC, Canada, 2012. [Google Scholar]
- Sangster, A.; Gauthier, M.; Gower, C. Metallogeny of structural zones, Grenville Province, northeastern North America. Precambrian Res 1992, 58, 401–426. [Google Scholar] [CrossRef]
- Whelan, J.F.; Rye, R.O.; deLorraine, W.F. The Balmat-Edwards zinc-lead deposits; synsedimentary ore from Mississippi valley-type fluids. Econ. Geol. 1984, 79, 239–265. [Google Scholar] [CrossRef]
- Rathkopf, C.A.; Peck, W.H. Stable Isotope Geochemistry of Marble-Hosted Zinc Deposits, Central Metasedimentary Belt, Grenville Province, Ontario; Geological Society of America: Boulder, CO, USA, 2010; p. 110. [Google Scholar]
- Peck, W.H.; Volkert, R.A.; Mansur, A.T.; Doverspike, B.A. Stable isotope and petrologic evidence for the origin of regional marble-hosted magnetite deposits and the zinc deposits at Franklin and Sterling Hill, New Jersey Highlands, United States. Econ. Geol. 2009, 104, 1037–1054. [Google Scholar] [CrossRef]


| Sample | L | δ13C (Dol)‰ | δ18O (Dol)‰ | δ13C (Mag)‰ | δ18O (Mag)‰ | δ11B‰ | %Mag | %Dol | %Serp | Distance (m) |
|---|---|---|---|---|---|---|---|---|---|---|
| GE-1 | D | 0.63 | 24.45 | 33 | 49 | 13 | 0 | |||
| GE-2 | D | 0.44 | 23.71 | 9 | 63 | 28 | 1.1 | |||
| GE-3 | M | 1.54 | 25.19 | 1.88 | 24.62 | 25 | 41 | 34 | 3.0 | |
| GE-5 | D | −0.90 | 23.91 | 10 | 55 | 34 | 4.3 | |||
| GE-6 | D | −1.10 | 24.67 | 10 | 43 | 47 | 5.2 | |||
| GE-7 | D | −0.11 | 22.83 | 8 | 91 | 1 | 5.4 | |||
| GE-8 | M | 1.36 | 23.20 | 1.27 | 24.79 | 12 | 49 | 39 | 5.7 | |
| GE-9 | M | 1.61 | 26.15 | 1.81 | 25.56 | 15.5 | 40 | 25 | 35 | 5.9 |
| GE-10 | D/M | 1.10 | 24.41 | 9 | 48 | 43 | 6.4 | |||
| GE-11 | M | 1.63 | 25.91 | 1.75 | 25.51 | 15.5 | 41 | 32 | 27 | 7.0 |
| GE-12 | M | 1.77 | 25.68 | 1.92 | 25.83 | 18.4 | 37 | 34 | 29 | 7.4 |
| GE-13 | M | 1.83 | 25.58 | 1.82 | 25.42 | 34 | 35 | 30 | 7.8 | |
| GE-14 | M | 1.77 | 26.20 | 1.73 | 25.60 | 56 | 18 | 26 | 8.0 | |
| GE-15 | D | 0.85 | 24.30 | 6 | 53 | 41 | 8.1 | |||
| GE-16 | D | −0.57 | 24.44 | 7 | 70 | 23 | 8.3 | |||
| GE-17 | D | −0.88 | 24.29 | Tr | 33 | 67 | 8.7 | |||
| GE-18 | D | −0.77 | 24.94 | 22.6 | 4 | 48 | 48 | 10.3 | ||
| GE-20 | D | −0.17 | 24.34 | 31 | 27 | 42 | 11.9 | |||
| GE-21 | D | 0.57 | 23.89 | 35 | 23 | 42 | 12.4 |
| Sample | L | SiO2 wt% | Al2O3 wt% | Fe2O3 wt% | MnO wt% | CaO wt% | V ppm | Ni ppm | Zn ppm | Rb ppm | Sr ppm | Y ppm | Zr ppm |
| GE-2 | D | 6.12 | 0.03 | 0.16 | 0.05 | 25.00 | 10 | <5 | 71 | <0.2 | 2920 | 12.5 | 2.8 |
| GE-7 | D | 16.20 | 0.05 | 0.47 | 0.05 | 18.50 | 10 | 7 | 253 | 0.4 | 2040 | 6.8 | 0.7 |
| GE-9 | M | 2.13 | 0.05 | 0.54 | 0.08 | 15.00 | 10 | 5 | 68 | <0.2 | 810 | 4.5 | <0.5 |
| GE-11 | M | 2.64 | 0.05 | 0.52 | 0.08 | 9.38 | 23 | 6 | 77 | 0.3 | 510 | 4.2 | <0.5 |
| GE-12 | M | 4.55 | 0.10 | 0.36 | 0.06 | 10.10 | 13 | <5 | 90 | 0.2 | 640 | 4.1 | <0.5 |
| GE-18 | D | 20.40 | 0.04 | 0.37 | 0.09 | 15.20 | 19 | <5 | 278 | 0.2 | 1100 | 14.2 | 3.1 |
| Sample | L | La ppm | Ce ppm | Pr ppm | Nd ppm | Sm ppm | Eu ppm | Gd ppm | Dy ppm | Ho ppm | Er ppm | Yb ppm | Lu ppm |
| GE-2 | D | 2.8 | 7.9 | 0.7 | 3.3 | 0.6 | 0.22 | 0.97 | 1.69 | 0.37 | 1.24 | 1 | 0.14 |
| GE-7 | D | 2.1 | 4.6 | 0.37 | 1.9 | 0.3 | 0.14 | 0.35 | 0.50 | 0.1 | 0.34 | 0.2 | <0.05 |
| GE-9 | M | 0.5 | 2.4 | 0.18 | 1.2 | 0.1 | <0.05 | 0.18 | 0.41 | 0.06 | 0.27 | 0.1 | <0.05 |
| GE-11 | M | 0.3 | 1.6 | 0.08 | 0.9 | <0.1 | <0.05 | 0.22 | 0.30 | 0.07 | 0.17 | 0.1 | <0.05 |
| GE-12 | M | <0.1 | 2.0 | 0.11 | 1.1 | <0.1 | <0.05 | 0.21 | 0.32 | 0.07 | 0.20 | 0.1 | <0.05 |
| GE-18 | D | 4.3 | 10.3 | 0.96 | 4.1 | 0.9 | 0.21 | 1.07 | 1.70 | 0.38 | 1.11 | 0.8 | 0.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peck, W.H.; Eppich, G.R. The Kilmar Magnesite Deposits: Evaporitic Metasediments in the Grenville Supergroup, Morin Terrane, Quebec. Minerals 2019, 9, 554. https://doi.org/10.3390/min9090554
Peck WH, Eppich GR. The Kilmar Magnesite Deposits: Evaporitic Metasediments in the Grenville Supergroup, Morin Terrane, Quebec. Minerals. 2019; 9(9):554. https://doi.org/10.3390/min9090554
Chicago/Turabian StylePeck, William H., and Gary R. Eppich. 2019. "The Kilmar Magnesite Deposits: Evaporitic Metasediments in the Grenville Supergroup, Morin Terrane, Quebec" Minerals 9, no. 9: 554. https://doi.org/10.3390/min9090554
APA StylePeck, W. H., & Eppich, G. R. (2019). The Kilmar Magnesite Deposits: Evaporitic Metasediments in the Grenville Supergroup, Morin Terrane, Quebec. Minerals, 9(9), 554. https://doi.org/10.3390/min9090554





