Influence of MgO on Low Temperature Reduction and Mineralogical Changes of Sinter in Simulated COREX Shaft Furnace Reducing Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
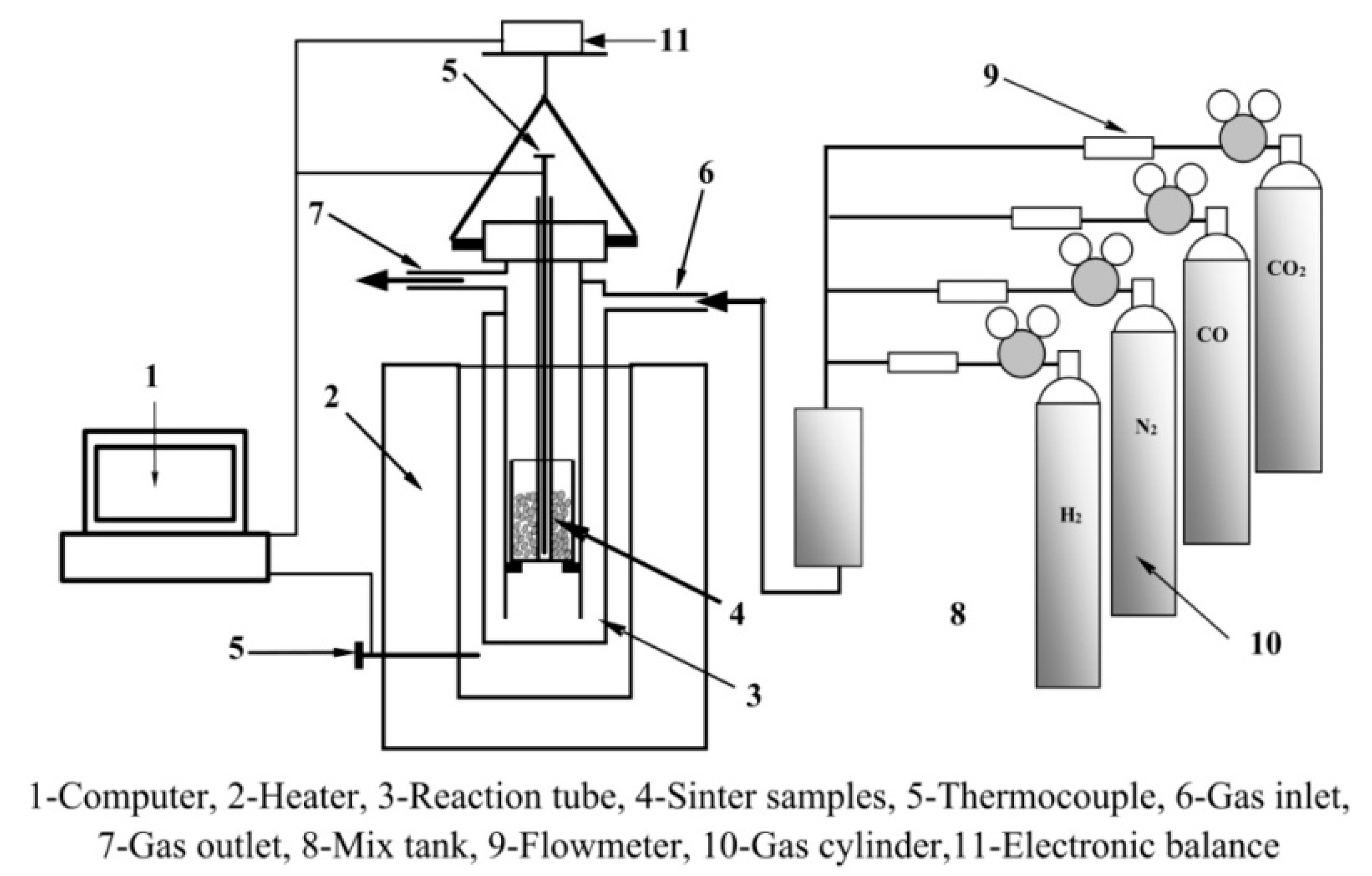
2.2. Methods
3. Results and Discussions
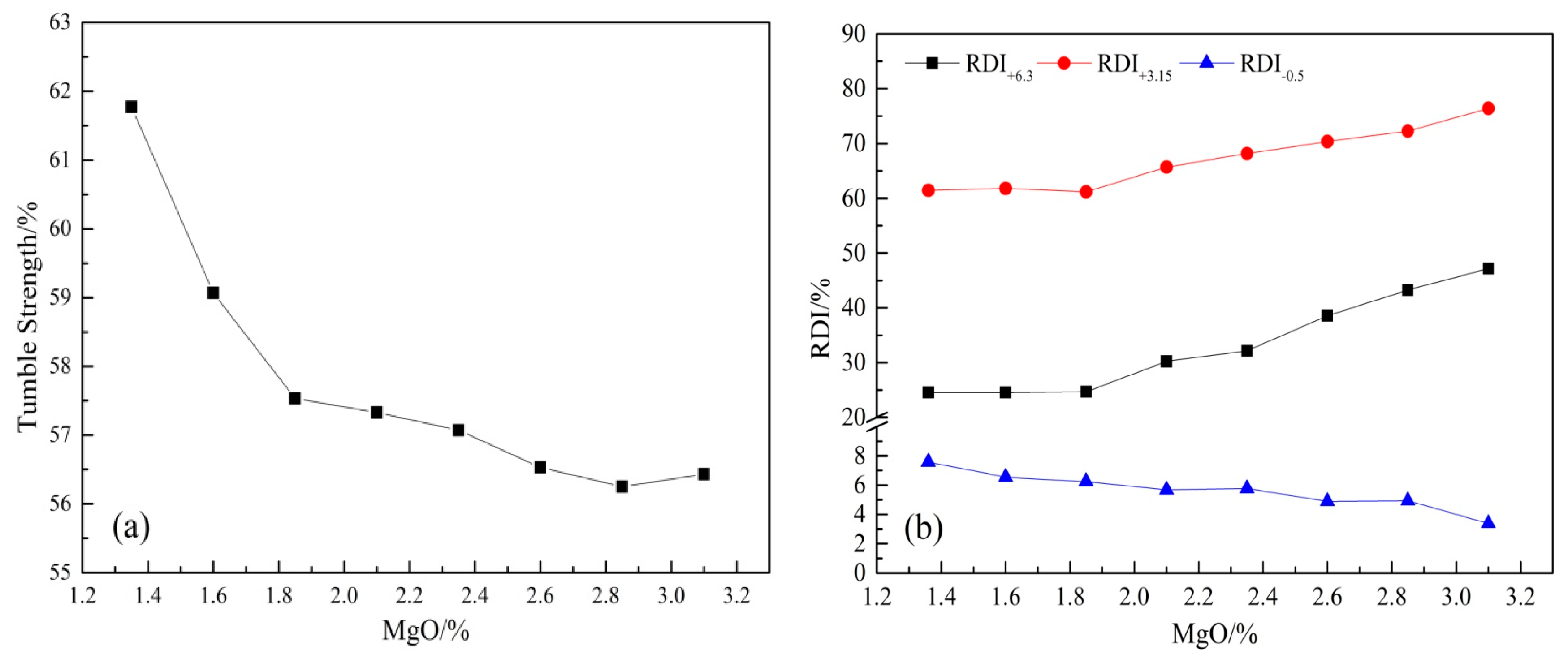
3.1. Effect of MgO on the Cold Strength and Low Temperature Reduction Degradation Performance of Sinter
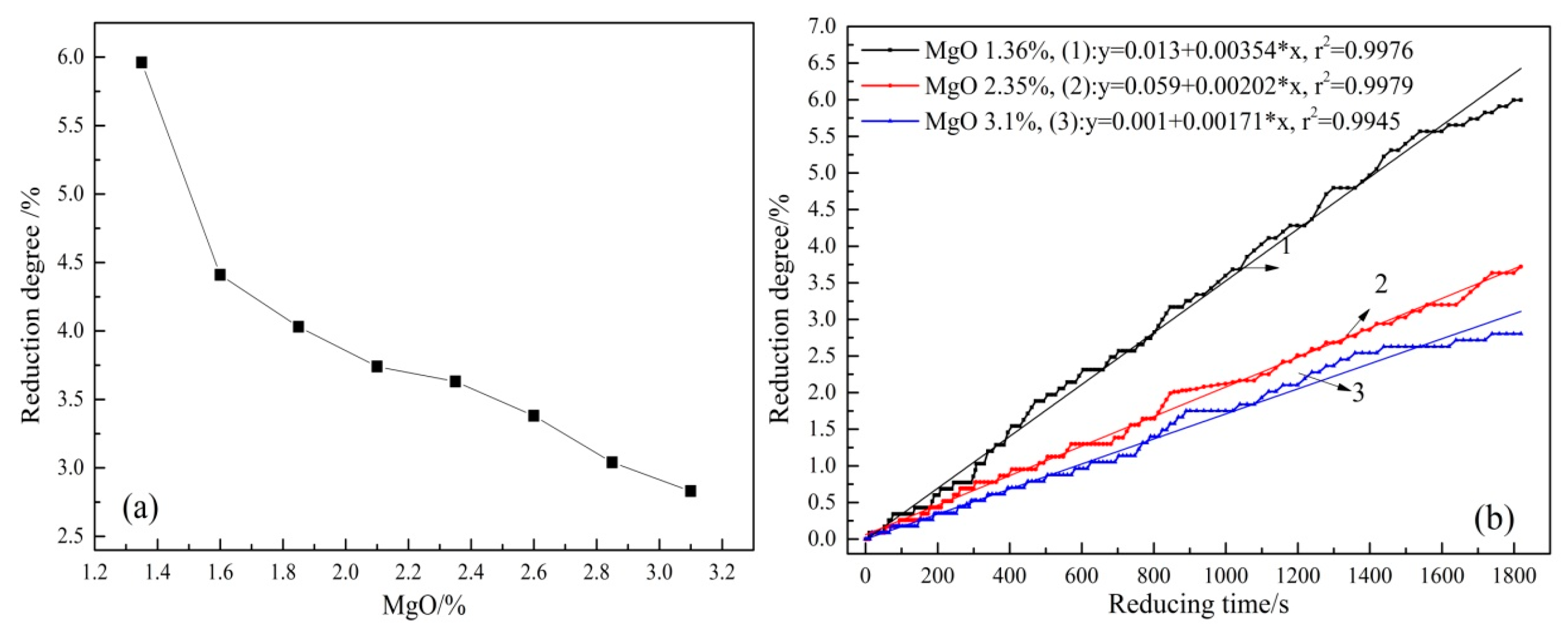
3.2. Effect of MgO on the Reduction Degree and Reduction Rate of Sinter at Low Temperature
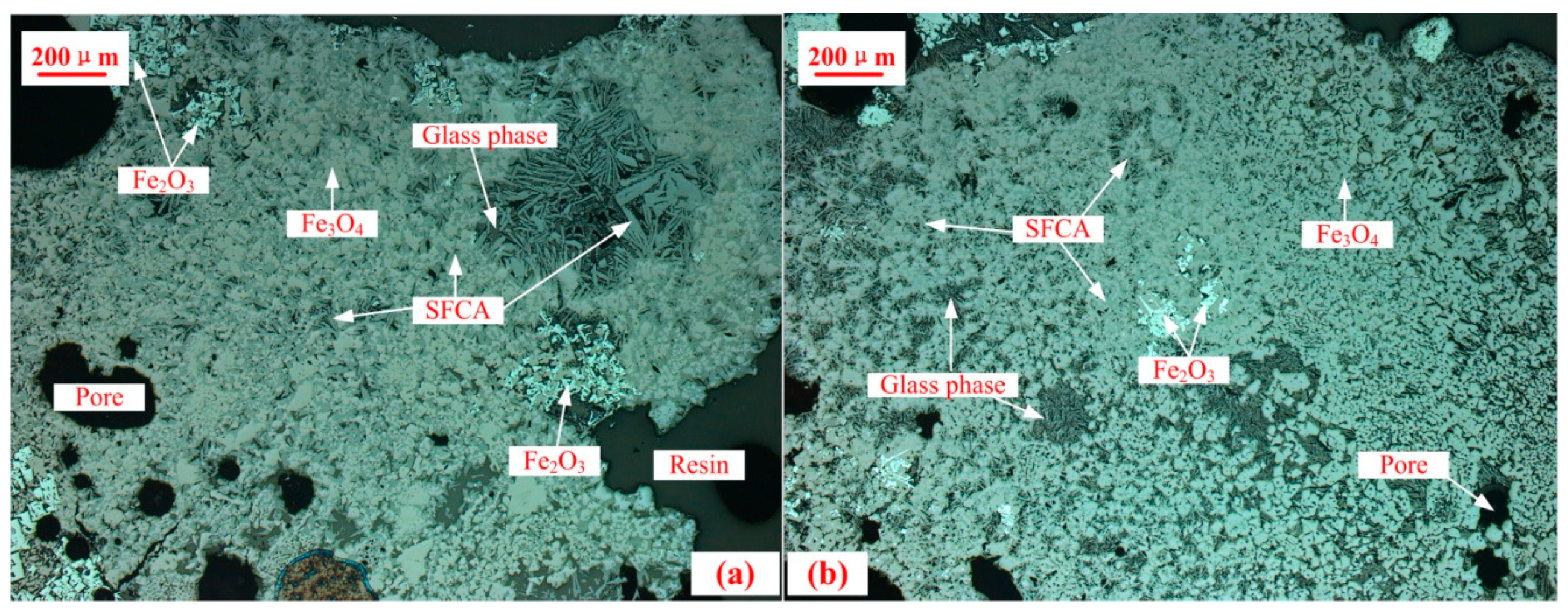
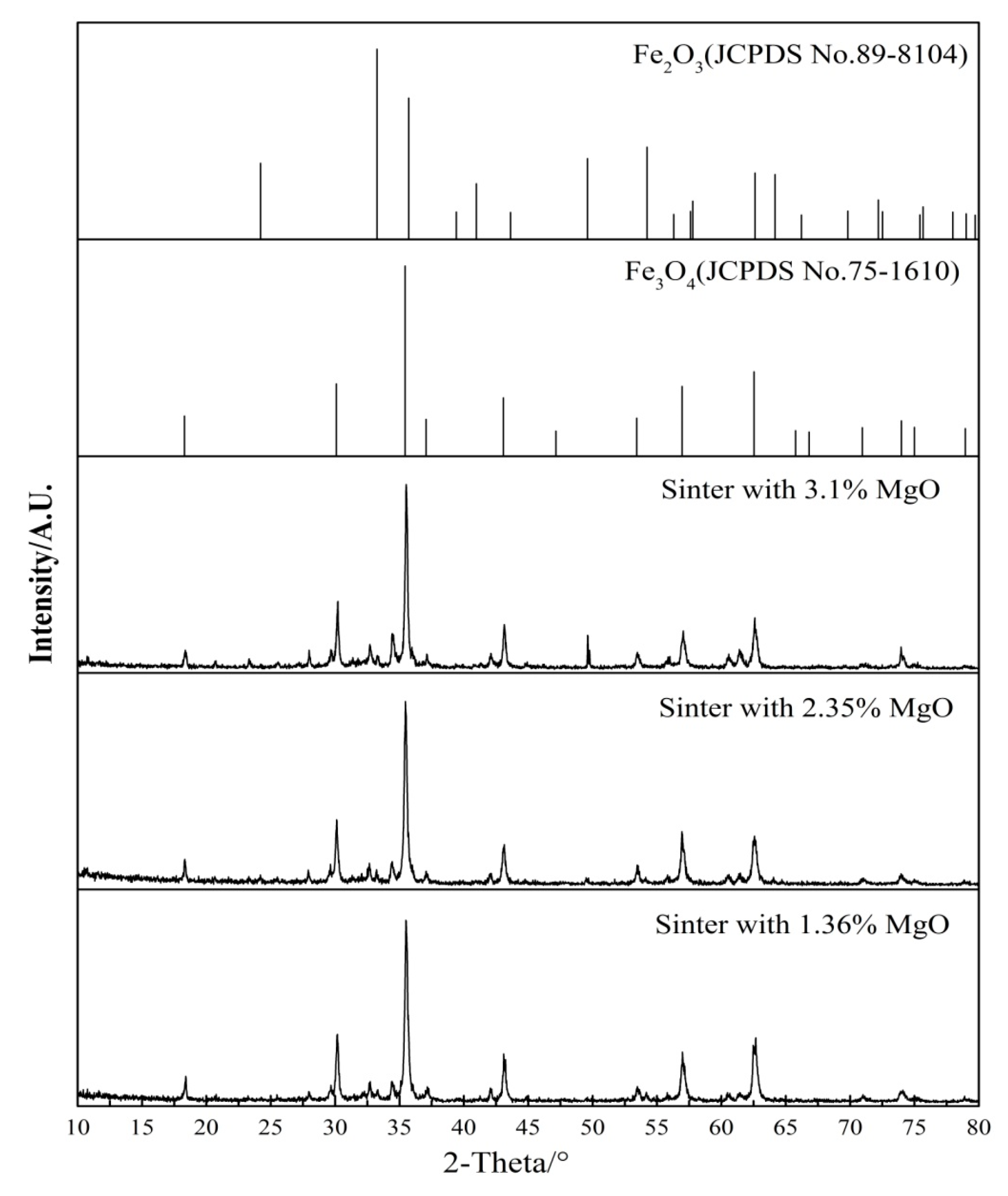
3.3. Microstructure and Mineral Compositions of Sinter with Different Content of MgO
3.4. Mineralogical Changes of Sinter with Different MgO Content after Reduction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shalimov, A. The COREX process for making high-quality steels at mini-mills. Metallurgist 2000, 44, 35–39. [Google Scholar]
- Kumar, P.P.; Barman, S.C.; Reddy, B.M.; Sekhar, V.R. Raw materials for COREX and their influence on furnace performance. Ironmak. Steelmak. 2009, 36, 87–90. [Google Scholar] [CrossRef]
- Kumar, P.P.; Gupta, D.; Naha, T.K.; Gupta, S.S. Factors affecting fuel rate in COREX process. Ironmak. Steelmak. 2013, 33, 293–298. [Google Scholar] [CrossRef]
- Guo, Y.L.; Xu, W.R.; Zhu, J.M.; Zhang, J.Y. The burden structure and its consumption in the melter gasifier of the COREX process. Metall. Mater. Trans. B 2013, 44, 1078–1085. [Google Scholar] [CrossRef]
- Shi, B.J.; Zhu, D.Q.; Pan, J.; Xue, Y.X. Reduction behaviors of sinter made from magnetite concentrates in reducing process simulated COREX shaft furnace. In Proceedings of the 8th International Symposium on High-Temperature Metallurgical Processing, San Diego, CA, USA, 26 February–2 March 2017. [Google Scholar]
- Tian, B.S.; Li, W.H. Production practice of blowing-in OY furnace of bayi steel. Xinjiang Iron Steel 2015, 4, 1–4. [Google Scholar]
- Asada, M.; Shima, M.; Omori, Y. Measurement of macro strain in the course of reduction of the skeletal hematite in sinter(sinter). Phys. Rev. C 1984, 30, 1776–1778. [Google Scholar]
- Loo, C.E.; Bristow, N.J. Mechanism of low-temperature reduction degradation of iron ore sinters. Trans. Inst. Min. Metall. Sect. C 1994, 103, 126–135. [Google Scholar]
- Nakajima, R.; Sumigama, T.; Wakimoto, K.; Nagano, S.; Kawata, H.; Sakurai, M. Reduction Degradation behavior of sinter in the blast furnace shaft (blast furnace phenomena). Tetsu-to-Hagane 1987, 73, 1964–1971. [Google Scholar] [CrossRef][Green Version]
- Panigrahy, S.C.; Verstraeten, P.; Dilewijns, J. Influence of MgO addition on mineralogy of iron ore sinter. Metall. Trans. B 1984, 15, 23–32. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, G.S.; Guo, W.; Li, X.G.; Shen, F.M. Effect of MgO on sintering process and metallurgical properties of sinter. Iron Steel 2006, 41, 1–5. [Google Scholar]
- Zhang, M.; Andrade, M.W. Effect of MgO and basicity on microstructure and metallurgical properties of iron ore sinter. In Characterization of Minerals Metals and Materials John; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Gan, Q.; He, Q.; Wen, Y.C. Study on influence of MgO on mineral composition and metallurgical properties of V-bearing titaniferous magnetite sinter. Iron Steel 2008, 43, 7–11. [Google Scholar]
- Guo, Y.F.; Guo, X.M. Effect of MgO on low temperature reduction process of hematite fines sinter. J. Iron Steel Res. 2017, 29, 697–703. [Google Scholar]
- Pan, J.; Shi, B.J.; Zhu, D.Q.; Mo, Y.P. Improving sintering performance of specularite concentrates by pre-briquetting process. ISIJ Int. 2016, 56, 777–785. [Google Scholar] [CrossRef]
- Shi, B.J.; Zhu, D.Q.; Pan, J.; Liu, X.Q. Combined effect of MgO and basicity varied by different dolomite and burnt lime addition on sintering performance of magnetite concentrates. Ironmak. Steelmak. 2019. [Google Scholar] [CrossRef]
- Yadav, U.S.; Pandey, B.D.; Das, B.K.; Jena, D.N. Influence of magnesia on sintering characteristics of iron ore. Ironmak. Steelmak. 2002, 29, 91–95. [Google Scholar] [CrossRef]
- Fan, X.H.; Li, W.Q.; Gan, M.; Chen, X.L.; Yuan, L.S.; Ji, Z.Y.; Yu, Z.Y.; Huang, X.X.; Su, D. Influence and mechanism of MgO on strength of high basicity sinter. J. Cent. South Univ. 2012, 43, 3325–3330. [Google Scholar]
- Li, Q.; Huang, Z.C.; Jiang, T.; Yang, Y.B.; Li, G.H. Effect of dolomite and serpentine on sinter quality and microstructure. Iron Steel 2006, 41, 10–14. [Google Scholar]
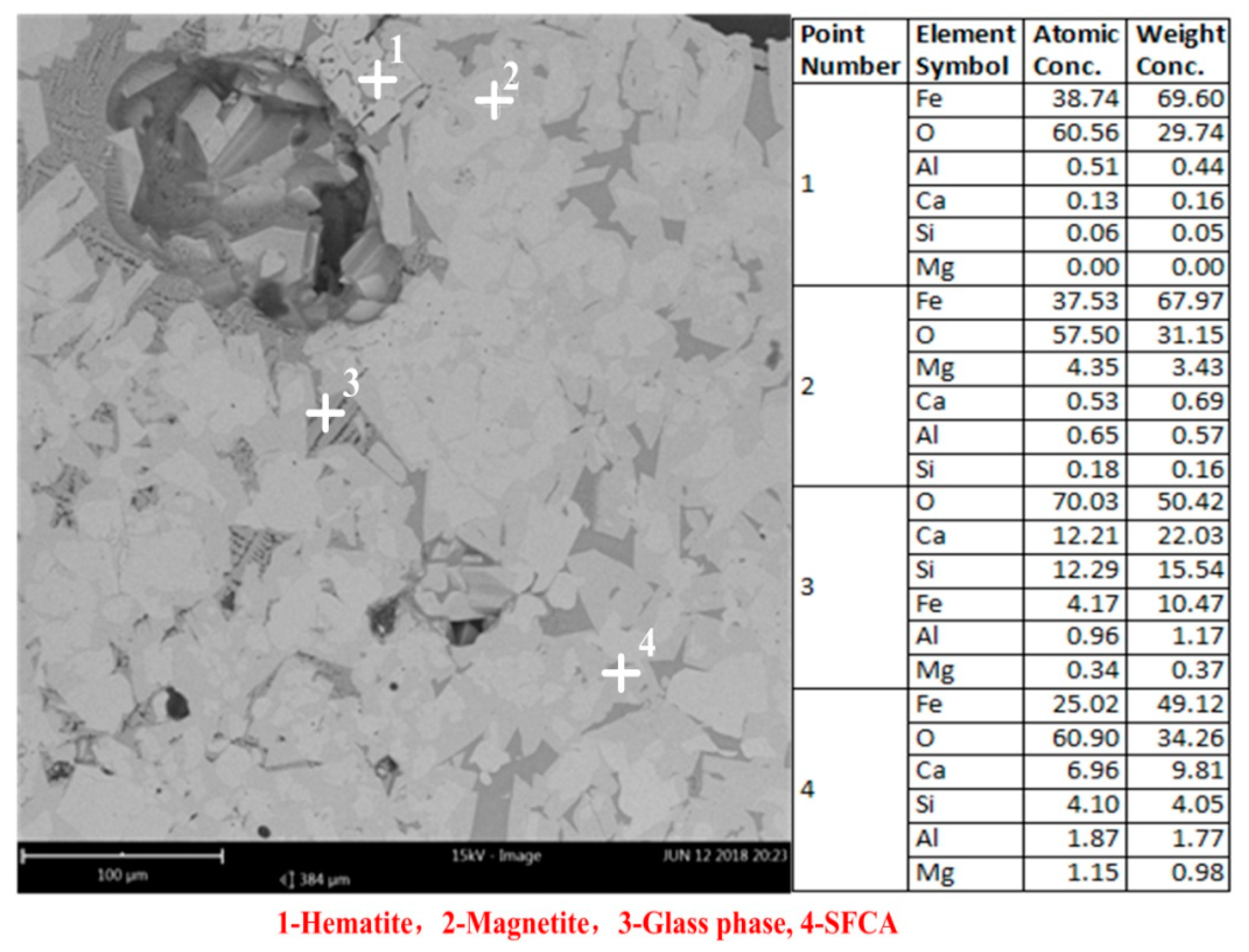



| MgO Content | Hematite | Magnetite | Glass Phase | SFCA | Porosity |
|---|---|---|---|---|---|
| 1.36% | 15.37 | 32.02 | 11.73 | 25.56 | 14.96 |
| 2.35% | 13.58 | 34.45 | 14.83 | 21.06 | 16.08 |
| 3.10% | 7.25 | 40.27 | 15.96 | 19.63 | 16.89 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, D.; Chou, J.; Shi, B.; Pan, J. Influence of MgO on Low Temperature Reduction and Mineralogical Changes of Sinter in Simulated COREX Shaft Furnace Reducing Conditions. Minerals 2019, 9, 272. https://doi.org/10.3390/min9050272
Zhu D, Chou J, Shi B, Pan J. Influence of MgO on Low Temperature Reduction and Mineralogical Changes of Sinter in Simulated COREX Shaft Furnace Reducing Conditions. Minerals. 2019; 9(5):272. https://doi.org/10.3390/min9050272
Chicago/Turabian StyleZhu, Deqing, Jianlei Chou, Benjing Shi, and Jian Pan. 2019. "Influence of MgO on Low Temperature Reduction and Mineralogical Changes of Sinter in Simulated COREX Shaft Furnace Reducing Conditions" Minerals 9, no. 5: 272. https://doi.org/10.3390/min9050272
APA StyleZhu, D., Chou, J., Shi, B., & Pan, J. (2019). Influence of MgO on Low Temperature Reduction and Mineralogical Changes of Sinter in Simulated COREX Shaft Furnace Reducing Conditions. Minerals, 9(5), 272. https://doi.org/10.3390/min9050272





