Facile Hydrothermal Synthesis of Nanocubic Pyrite Crystals Using Greigite Fe3S4 and Thiourea as Precursors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of the Greigite Precursor
2.3. Synthesis of Pyrite Nanocubic Crystals
3. Results and Discussion
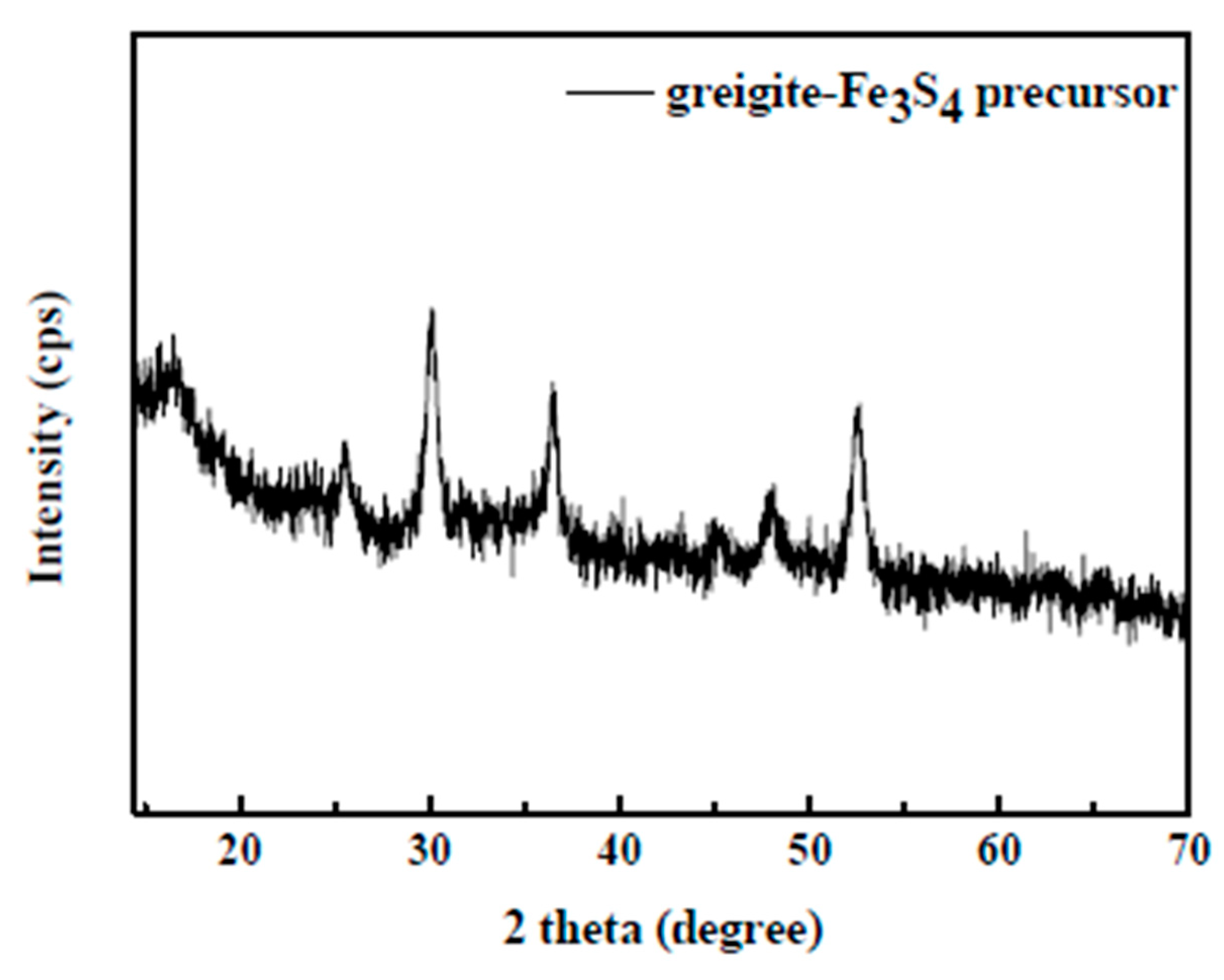
3.1. Characterization of the Greigite Fe3S4 Precursor
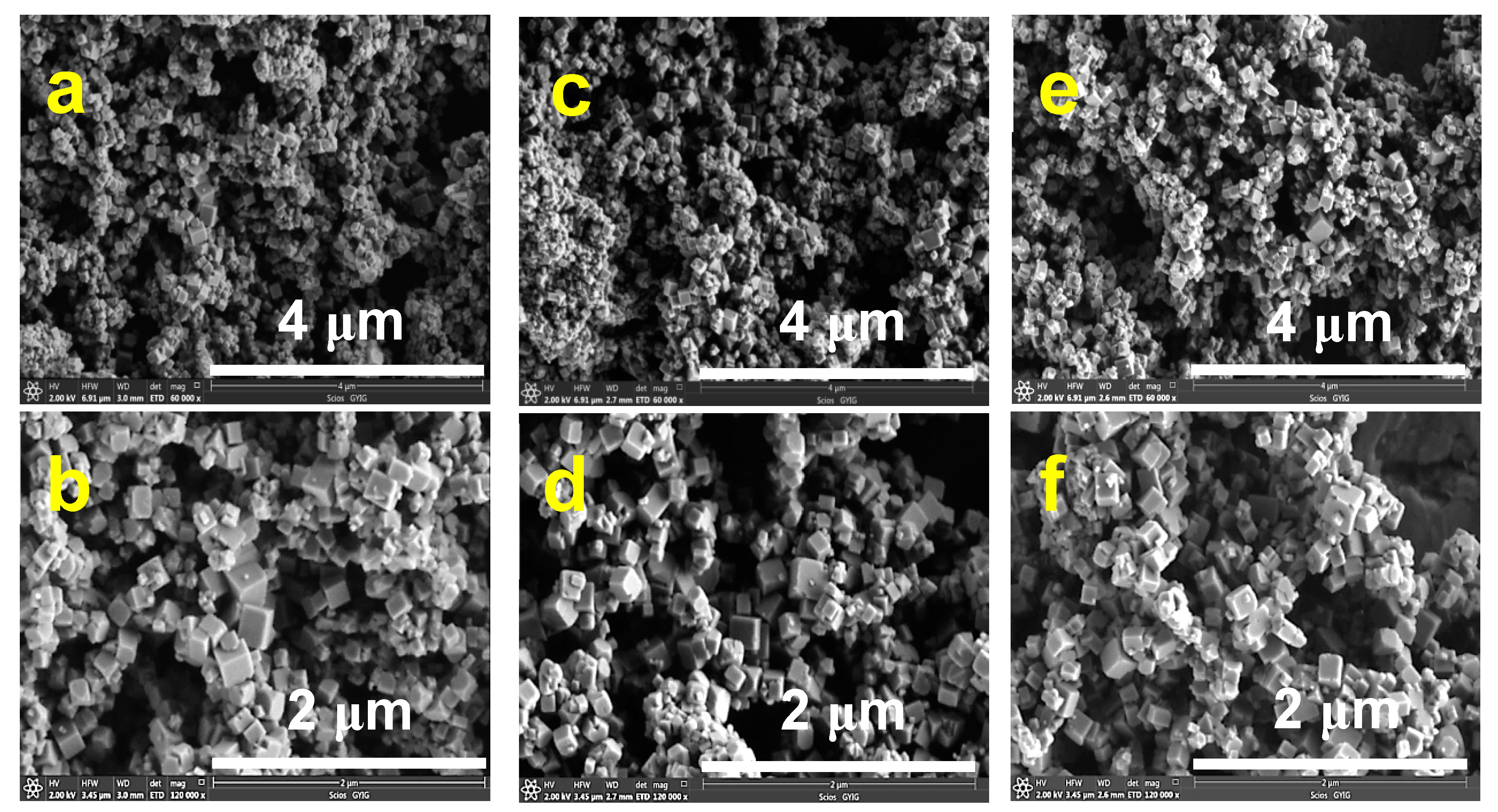
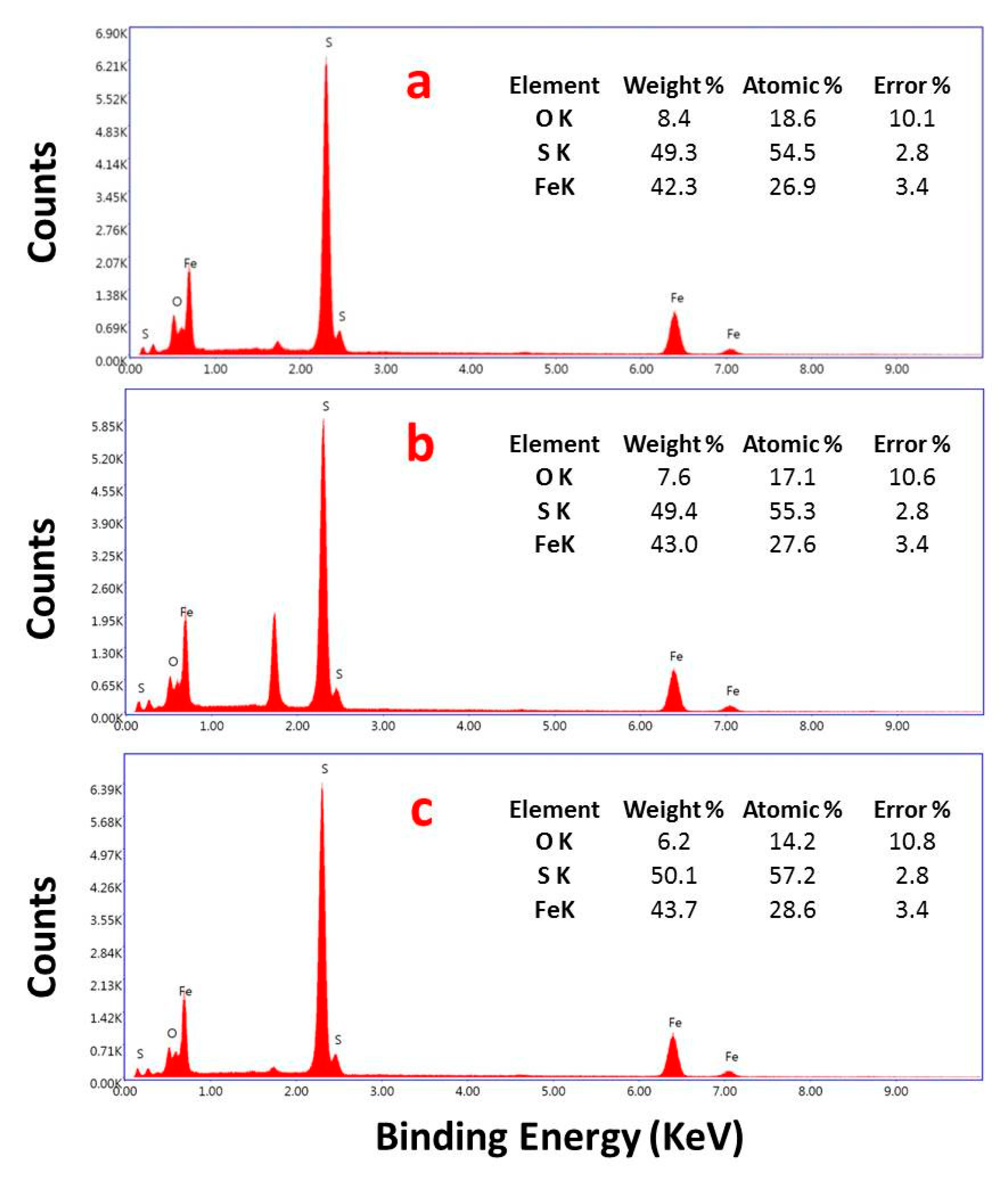
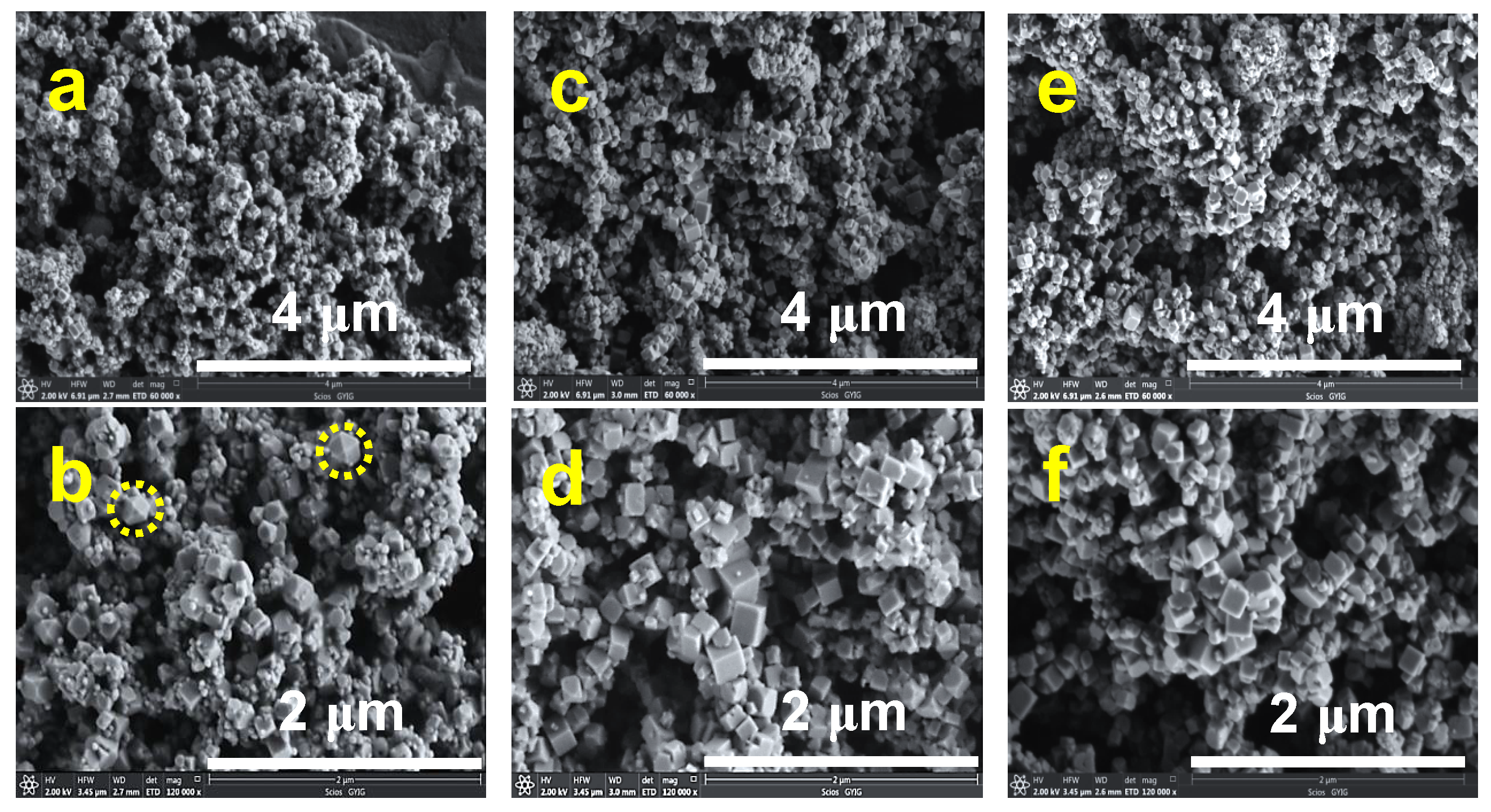
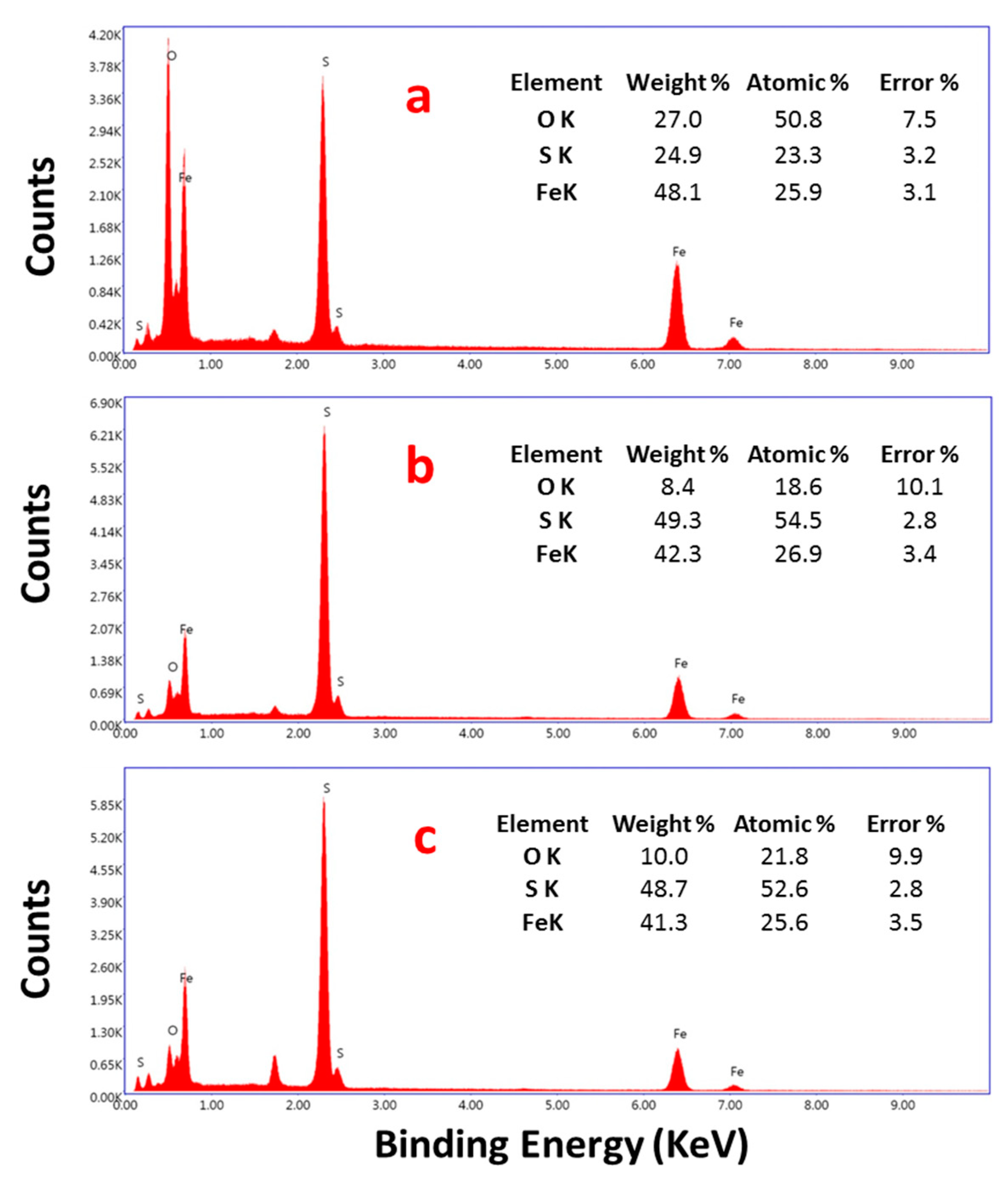
3.2. Characterization of Pyrite Nanocubes Prepared with Thiourea as the Sulfur Source
3.2.1. Effect of Hydrothermal Time
3.2.2. Effect of the Thiourea Concentration
3.3. Characterization of Pyrite Prepared with a Mixture of Na2S and S Powder as the Sulfur Source
3.4. Mechanism of Pyrite Formation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rickard, D.; Luther, G.W. Chemistry of iron sulfides. Chem. Rev. 2007, 107, 514–562. [Google Scholar] [CrossRef]
- Vaughan, D.J.; Corkhill, C.L. Mineralogy of Sulfides. Elements 2017, 13, 81–87. [Google Scholar] [CrossRef]
- Huang, F.; Gao, W.; Gao, S.; Meng, L.; Zhang, Z.; Yan, Y.; Ren, Y.; Li, Y.; Liu, K.; Xing, M.; et al. Morphology Evolution of Nano-Micron Pyrite: A Review. J. Nanosci. Nanotechnol. 2017, 17, 5980–5995. [Google Scholar] [CrossRef]
- Large, R.R.; Danyushevsky, L.; Hollit, C.; Maslennikov, V.; Meffre, S.; Gilbert, S.; Bull, S.; Scott, R.; Emsbo, P.; Thomas, H.; et al. Foster, Gold and Trace Element Zonation in Pyrite Using a Laser Imaging Technique: Implications for the Timing of Gold in Orogenic and Carlin-Style Sediment-Hosted Deposits. Econ. Geol. 2009, 104, 635–668. [Google Scholar] [CrossRef]
- Reich, M.; Kesler, S.E.; Utsunomiya, S.; Palenik, C.S.; Chryssoulis, S.L.; Ewing, R.C. Solubility of gold in arsenian pyrite. Geochim. Cosmochim. Acta 2005, 69, 2781–2796. [Google Scholar] [CrossRef]
- Deditius, A.P.; Reich, M.; Kesler, S.E.; Utsunomiya, S.; Chryssoulis, S.L.; Walshe, J.; Ewing, R.C. The coupled geochemistry of Au and As in pyrite from hydrothermal ore deposits. Geochim. Cosmochim. Acta 2014, 140, 644–670. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Ai, Z.; Zhang, L. Hydrothermal Synthesis of FeS2 as a High-Efficiency Fenton Reagent to Degrade Alachlor via Superoxide-Mediated Fe(II)/Fe(III) Cycle. ACS Appl. Mater. Interface 2015, 7, 28534–28544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yuan, S.H. Production of hydroxyl radicals from abiotic oxidation of pyrite by oxygen under circumneutral conditions in the presence of low-molecular-weight organic acids. Geochim. Cosmochim. Acta 2017, 218, 153–166. [Google Scholar] [CrossRef]
- Lee, W.; Batchelor, B. Abiotic, reductive dechlorination of chlorinated ethylenes by iron-bearing soil minerals. 2. Green rust. Environ. Sci. Technol. 2002, 36, 5348–5354. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Batchelor, B. Abiotic reductive dechlorination of chlorinated ethylenes by iron-bearing soil minerals. 1. Pyrite and magnetite. Environ. Sci. Technol. 2002, 36, 5147–5154. [Google Scholar] [CrossRef]
- Pham, H.T.; Kitsuneduka, M.; Hara, J.; Suto, K.; Inoue, C. Trichloroethylene transformation by natural mineral pyrite: The deciding role of oxygen. Environ. Sci. Technol. 2008, 42, 7470–7475. [Google Scholar] [CrossRef]
- Shukla, S.; Xing, G.; Ge, H.; Prabhakar, R.R.; Mathew, S.; Su, Z.; Nalla, V.; Venkatesan, T.; Mathews, N.; Sritharan, T.; et al. Origin of Photocarrier Losses in Iron Pyrite (FeS2) Nanocubes. ACS Nano 2016, 10, 4431–4440. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Ahmed, E.; Khan, Y.; Riaz, K.N.; Malik, M.A. Nanocrystalline Pyrite for Photovoltaic Applications. Chemistryselect 2018, 3, 6488–6524. [Google Scholar] [CrossRef]
- Cui, M.M.; Johannesson, K.H. Comparison of tungstate and tetrathiotungstate adsorption onto pyrite. Chem. Geol. 2017, 464, 57–68. [Google Scholar] [CrossRef]
- Kantar, C.; Ari, C.; Keskin, S. Comparison of different chelating agents to enhance reductive Cr(VI) removal by pyrite treatment procedure. Water Res. 2015, 76, 66–75. [Google Scholar] [CrossRef]
- Kirkeminde, A.; Ren, S. Thermodynamic control of iron pyrite nanocrystal synthesis with high photoactivity and stability. J. Mater. Chem. A 2013, 1, 49–54. [Google Scholar] [CrossRef]
- Kuo, T.R.; Chen, W.T.; Liao, H.J.; Yang, Y.H.; Yen, H.C.; Liao, T.W.; Wen, C.Y.; Lee, Y.C.; Chen, C.C.; Wang, D.Y. Improving Hydrogen Evolution Activity of Earth-Abundant Cobalt-Doped Iron Pyrite Catalysts by Surface Modification with Phosphide. Small 2017, 13, 1603356. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, X.; Suo, L.; Luo, C.; Gao, T.; Wang, C. Electrospun FeS2@Carbon Fiber Electrode as a High Energy Density Cathode for Rechargeable Lithium Batteries. ACS Nano 2016, 10, 1529–1538. [Google Scholar] [CrossRef]
- Igarashi, K.; Yamamura, Y.; Kuwabara, T. Natural synthesis of bioactive greigite by solid-gas reactions. Geochim. Cosmochim. Acta 2016, 191, 47–57. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Feng, X.; Cui, Y.; Yang, X. Hydrothermal synthesized micro/nano-sized pyrite used as cathode material to improve the electrochemical performance of thermal battery. J. Appl. Electrochem. 2014, 44, 1075–1080. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Wang, T. Shape controlled growth of pyrite FeS2 crystallites via a polymer-assisted hydrothermal route. Crystengcomm 2010, 12, 3797–3805. [Google Scholar] [CrossRef]
- Golsheikh, A.M.; Huang, N.M.; Lim, H.N.; Chia, C.H.; Harrison, I.; Muhamad, M.R. One-pot hydrothermal synthesis and characterization of FeS2 (pyrite)/graphene nanocomposite. Chem. Eng. J. 2013, 218, 276–284. [Google Scholar] [CrossRef]
- Wu, R.; Zheng, Y.F.; Zhang, X.G.; Sun, Y.F.; Xu, J.B.; Jian, J.K. Hydrothermal synthesis and crystal structure of pyrite. J. Cryst. Growth 2004, 266, 523–527. [Google Scholar] [CrossRef]
- Zou, J.; Gao, J. Preparation of Nanosize Iron Pyrite FeS2 and Its Properties. In Materials Research, Pts 1 and 2; Gu, Z.W., Han, Y.F., Pan, F.H., Wang, X.T., Weng, D., Zhou, S.X., Eds.; Trans Tech Publications Ltd.: Zurich, Switzerland, 2009; pp. 459–462. [Google Scholar]
- Luo, S.; Nie, X.; Yang, M.; Fu, Y.; Zeng, P.; Wan, Q. Sorption of Differently Charged Gold Nanoparticles on Synthetic Pyrite. Minerals 2018, 8, 428. [Google Scholar] [CrossRef]
- Liu, W.L.; Rui, X.H.; Tan, H.T.; Xu, C.; Yan, Q.Y.; Hng, H.H. Solvothermal synthesis of pyrite FeS2 nanocubes and their superior high rate lithium storage properties. RSC Adv. 2014, 4, 48770–48776. [Google Scholar] [CrossRef]
- Kar, S.; Chaudhuri, S. Solvothermal synthesis of nanocrystalline FeS2 with different morphologies. Chem. Phys. Lett. 2004, 398, 22–26. [Google Scholar] [CrossRef]
- Zhu, L.; Richardson, B.J.; Yu, Q. Controlled colloidal synthesis of iron pyrite FeS2 nanorods and quasi-cubic nanocrystal agglomerates. Nanoscale 2014, 6, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Peckler, L.T.; Muscat, A.J. Phase Pure Pyrite FeS2 Nanocubes Synthesized Using Oleylamine as Ligand, Solvent, and Reductant. Cryst. Growth Des. 2015, 15, 3565–3572. [Google Scholar] [CrossRef]
- Yu, B.B.; Zhang, X.; Jiang, Y.; Liu, J.; Gu, L.; Hu, J.S.; Wan, L.J. Solvent-Induced Oriented Attachment Growth of Air-Stable PhasePure Pyrite FeS2 Nanocrystals. J. Am. Chem. Soc. 2015, 137, 2211–2214. [Google Scholar] [CrossRef]
- Macpherson, H.A.; Stoldt, C.R. Iron Pyrite Nanocubes: Size and Shape Considerations for Photovoltaic Application. ACS Nano 2012, 6, 8940–8949. [Google Scholar] [CrossRef]
- Bai, Y.X.; Yeom, J.; Yang, M.; Cha, S.H.; Sun, K.; Kotov, N.A. Universal Synthesis of Single-Phase Pyrite FeS2 Nanoparticles, Nanowires, and Nanosheets. J. Phys. Chem. C 2013, 117, 2567–2573. [Google Scholar] [CrossRef]
- Morin, G.; Noël, V.; Menguy, N.; Brest, J.; Baptiste, B.; Tharaud, M.; Ona-Nguema, G.; Ikogou, M.; Viollier, E.; Juillot, F. Nickel accelerates pyrite nucleation at ambient temperature. Geochem. Perspect. Lett. 2017, 5, 6–11. [Google Scholar] [CrossRef]
- Caban-Acevedo, M.; Liang, D.; Chew, K.S.; DeGrave, J.P.; Kaiser, N.S.; Jin, S. Synthesis, Characterization, and Variable Range Hopping Transport of Pyrite (FeS2) Nanorods, Nanobelts, and Nanoplates. ACS Nano 2013, 7, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.D.; Xing, C.C.; Cao, K.; Zhang, L.; Liu, J.B.; Meng, L. Template-directed synthesis of pyrite (FeS2) nanorod arrays with an enhanced photoresponse. J. Mater. Chem. A 2014, 2, 9496–9505. [Google Scholar] [CrossRef]
- Berry, N.; Cheng, M.; Perkins, C.L.; Limpinsel, M.; Hemminger, J.C.; Law, M. Atmospheric-Pressure Chemical Vapor Deposition of Iron Pyrite Thin Films. Adv. Energy Mater. 2012, 2, 1124–1135. [Google Scholar] [CrossRef]
- Hsiao, S.-C.; Hsu, C.-M.; Chen, S.-Y.; Perng, Y.-H.; Chueh, Y.-L.; Chen, L.-J.; Chou, L.-H. Facile synthesis and characterization of high temperature phase FeS2 pyrite nanocrystals. Mater. Lett. 2012, 75, 152–154. [Google Scholar] [CrossRef]
- Yuan, B.; Luan, W.; Tu, S.-T. One-step synthesis of cubic FeS2 and flower-like FeSe2 particles by a solvothermal reduction process. Dalton Trans. 2012, 41, 772–776. [Google Scholar] [CrossRef]
- Xu, J.; Xue, H.; Yang, X.; Wei, H.; Li, W.; Li, Z.; Zhang, W.; Lee, C.-S. Synthesis of Honeycomb-like Mesoporous Pyrite FeS2 Microspheres as Efficient Counter Electrode in Quantum Dots Sensitized Solar Cells. Small 2014, 10, 4754–4759. [Google Scholar] [CrossRef]
- Chakraborty, B.; Show, B.; Jana, S.; Mitra, B.C.; Maji, S.K.; Adhikary, B.; Mukherjee, N.; Mondal, A. Cathodic and anodic deposition of FeS2 thin films and their application in electrochemical reduction and amperometric sensing of H2O2. Electrochim. Acta 2013, 94, 7–15. [Google Scholar] [CrossRef]
- Khabbaz, M.; Entezari, M.H. Degradation of Diclofenac by sonosynthesis of pyrite nanoparticles. J. Environ. Manag. 2017, 187, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Xian, H.Y.; Zhu, J.X.; Liang, X.L.; He, H.P. Morphology controllable syntheses of micro- and nano-iron pyrite mono- and poly-crystals: A review. RSC Adv. 2016, 6, 31988–31999. [Google Scholar] [CrossRef]
- Wang, Q.; Morse, J.W. Laboratory simulation of pyrite formation in anoxic sediments. ACS Symp. Ser. 1995, 612, 206–223. [Google Scholar] [CrossRef]
- Li, Q.; Wei, Q.; Zuo, W.; Huang, L.; Luo, W.; An, Q.; Pelenovich, V.O.; Mai, L.; Zhang, Q. Greigite Fe3S4 as a new anode material for high-performance sodium-ion batteries. Chem. Sci. 2017, 8, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, M.; Alfredsson, M.; Brodholt, J.; Wright, K.; Catlow, C.R.A. Arsenic incorporation into FeS2 pyrite and its influence on dissolution: A DFT study. Geochim. Cosmochim. Acta 2007, 71, 624–630. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Renock, D.; Ewing, R.C.; Ramana, C.V.; Becker, U.; Kesler, S.E. A proposed new type of arsenian pyrite: Composition, nanostructure and geological significance. Geochim. Cosmochim. Acta 2008, 72, 2919–2933. [Google Scholar] [CrossRef]
- Abraitis, P.K.; Pattrick, R.A.D.; Vaughan, D.J. Variations in the compositional, textural and electrical properties of natural pyrite: A review. Int. J. Miner. Process. 2004, 74, 41–59. [Google Scholar] [CrossRef]
- Xian, H.Y.; Zhu, J.X.; Tang, H.M.; Liang, X.L.; He, H.P.; Xi, Y.F. Aggregative growth of quasi-octahedral iron pyrite mesocrystals in a polyol solution through oriented attachment. Crystengcomm 2016, 18, 8823–8828. [Google Scholar] [CrossRef]
- Liang, Y.X.; Bai, P.P.; Zhou, J.; Wang, T.Q.; Luo, B.W.; Zheng, S.Q. An efficient precursor to synthesize various FeS2 nanostructures via a simple hydrothermal synthesis method. Crystengcomm 2016, 18, 6262–6271. [Google Scholar] [CrossRef]
 : pyrite,
: pyrite,  : hematite; and (c) XRD patterns of the as-synthesized pyrite at 24 h with different concentrations of Na2S and S powder as the sulfur source.
: hematite; and (c) XRD patterns of the as-synthesized pyrite at 24 h with different concentrations of Na2S and S powder as the sulfur source.
 : pyrite,
: pyrite,  : hematite; and (c) XRD patterns of the as-synthesized pyrite at 24 h with different concentrations of Na2S and S powder as the sulfur source.
: hematite; and (c) XRD patterns of the as-synthesized pyrite at 24 h with different concentrations of Na2S and S powder as the sulfur source.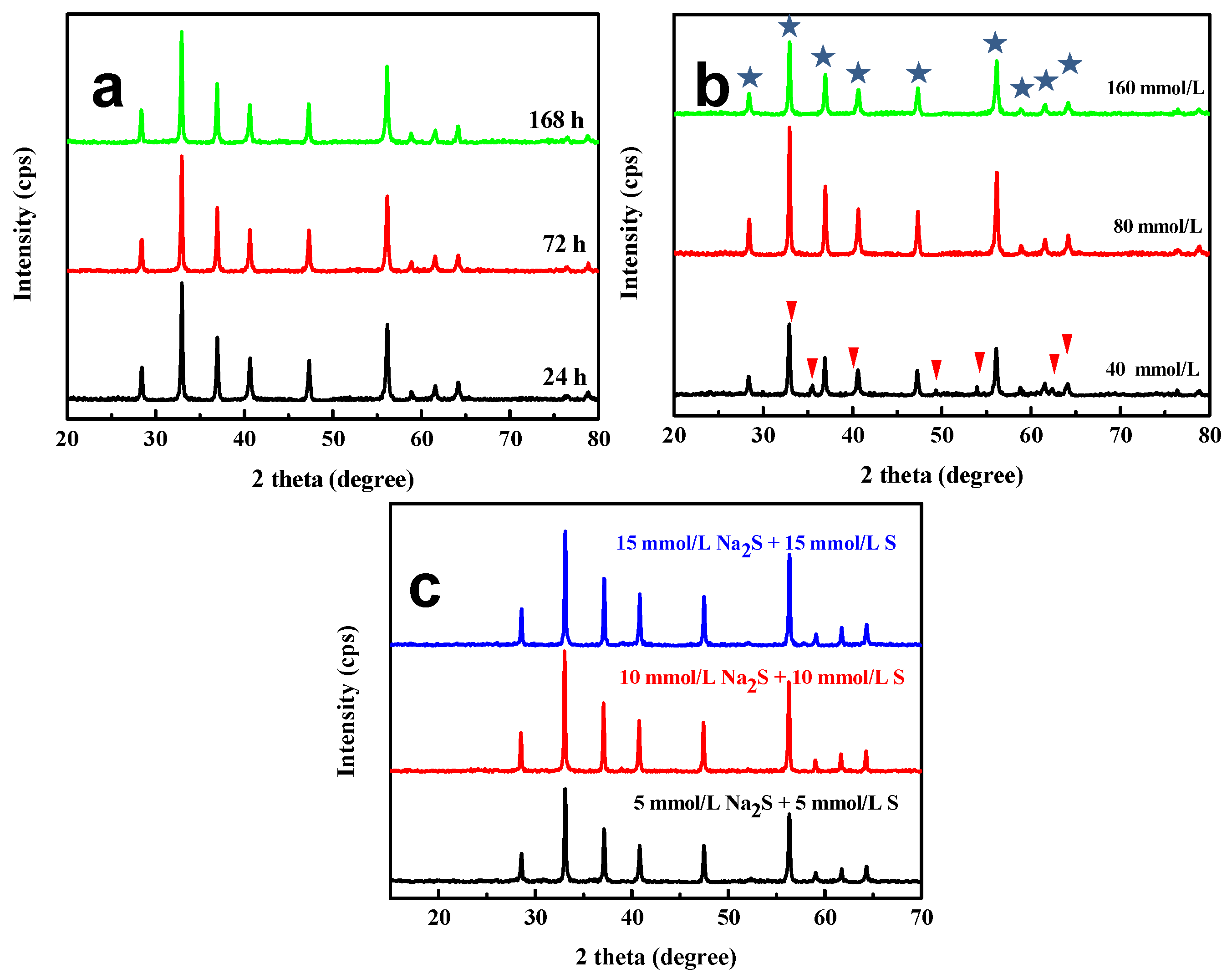

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, X.; Luo, S.; Yang, M.; Zeng, P.; Qin, Z.; Yu, W.; Wan, Q. Facile Hydrothermal Synthesis of Nanocubic Pyrite Crystals Using Greigite Fe3S4 and Thiourea as Precursors. Minerals 2019, 9, 273. https://doi.org/10.3390/min9050273
Nie X, Luo S, Yang M, Zeng P, Qin Z, Yu W, Wan Q. Facile Hydrothermal Synthesis of Nanocubic Pyrite Crystals Using Greigite Fe3S4 and Thiourea as Precursors. Minerals. 2019; 9(5):273. https://doi.org/10.3390/min9050273
Chicago/Turabian StyleNie, Xin, Suxing Luo, Meizhi Yang, Ping Zeng, Zonghua Qin, Wenbin Yu, and Quan Wan. 2019. "Facile Hydrothermal Synthesis of Nanocubic Pyrite Crystals Using Greigite Fe3S4 and Thiourea as Precursors" Minerals 9, no. 5: 273. https://doi.org/10.3390/min9050273
APA StyleNie, X., Luo, S., Yang, M., Zeng, P., Qin, Z., Yu, W., & Wan, Q. (2019). Facile Hydrothermal Synthesis of Nanocubic Pyrite Crystals Using Greigite Fe3S4 and Thiourea as Precursors. Minerals, 9(5), 273. https://doi.org/10.3390/min9050273






