He-Ar Isotopes and Trace Gas Compositions of Fluid Inclusions in Massive Sulphides from the Yushui Copper-Polymetallic Deposit, South China: Metallogenic Implications
Abstract
1. Introduction
2. Geological Setting
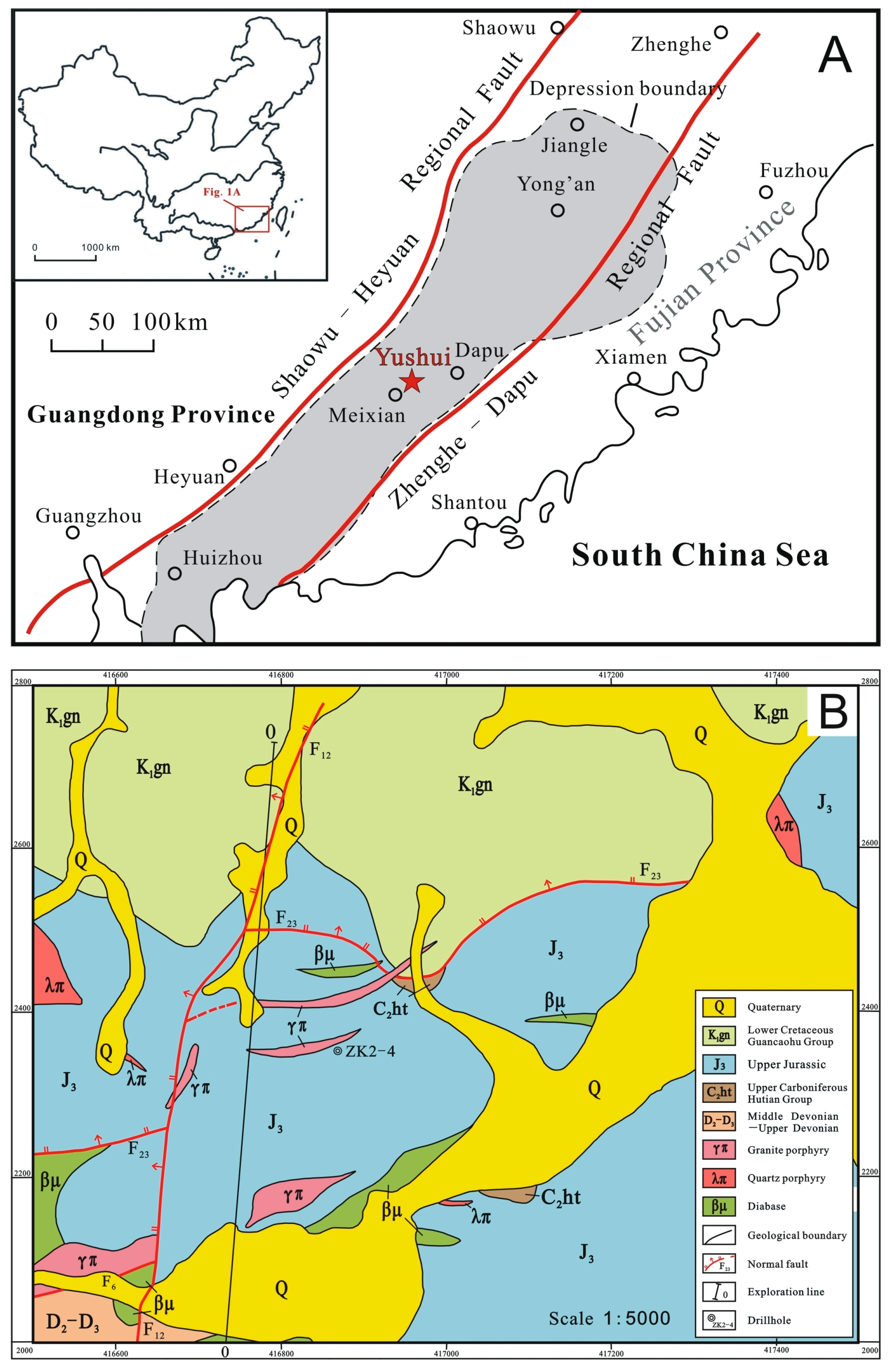
2.1. Regional Background of the Yushui Deposit
- (1)
- Middle Devonian–Lower Carboniferous (D2-C1): primarily medium- to fine-grained quartz sandstone, pebbly quartz sandstone, conglomerate with thin-bedded mudstone and siltstone, representing clastic rock formation characterized by neritic-littoral facies. This set of strata tilt to the northeast, with a dip angle of 25 to 30 degrees and a thickness greater than 300 m [73].
- (2)
- Upper Carboniferous Hutian Group (C2ht): dominated by dolomite with dolomitic limestone and limestone, or accompanied by thin layers of irregular light-colored chert, quartz sandstone, and mudstone, suggesting a neritic to littoral facies of carbonate rocks, overlying the Middle Devonian–Lower Carboniferous clastic rocks with a low-angle unconformity and a thickness of 200 to 350 m [73].
- (3)
- Upper Jurassic Gaojiping Group (J3gj): continental volcanic rocks, mainly consisted of mafic, intermediate to felsic lavas and clastic rocks overlying the Upper Carboniferous Hutian Group (with a nearly parallel, eruptive unconformity); approximately 30–80 m in thickness.
- (4)
- Lower Cretaceous Guancaohu Group (K1gn): represented by lacustrine volcano-sedimentary clastic rocks (with purple-red color), overlying the Upper Jurassic Gaojiping Group with an angular unconformity. The strata tilt to the north with a thickness of 150–200 m.
- (5)
- Quaternary (Q): eluvium, alluvium, and diluvium.
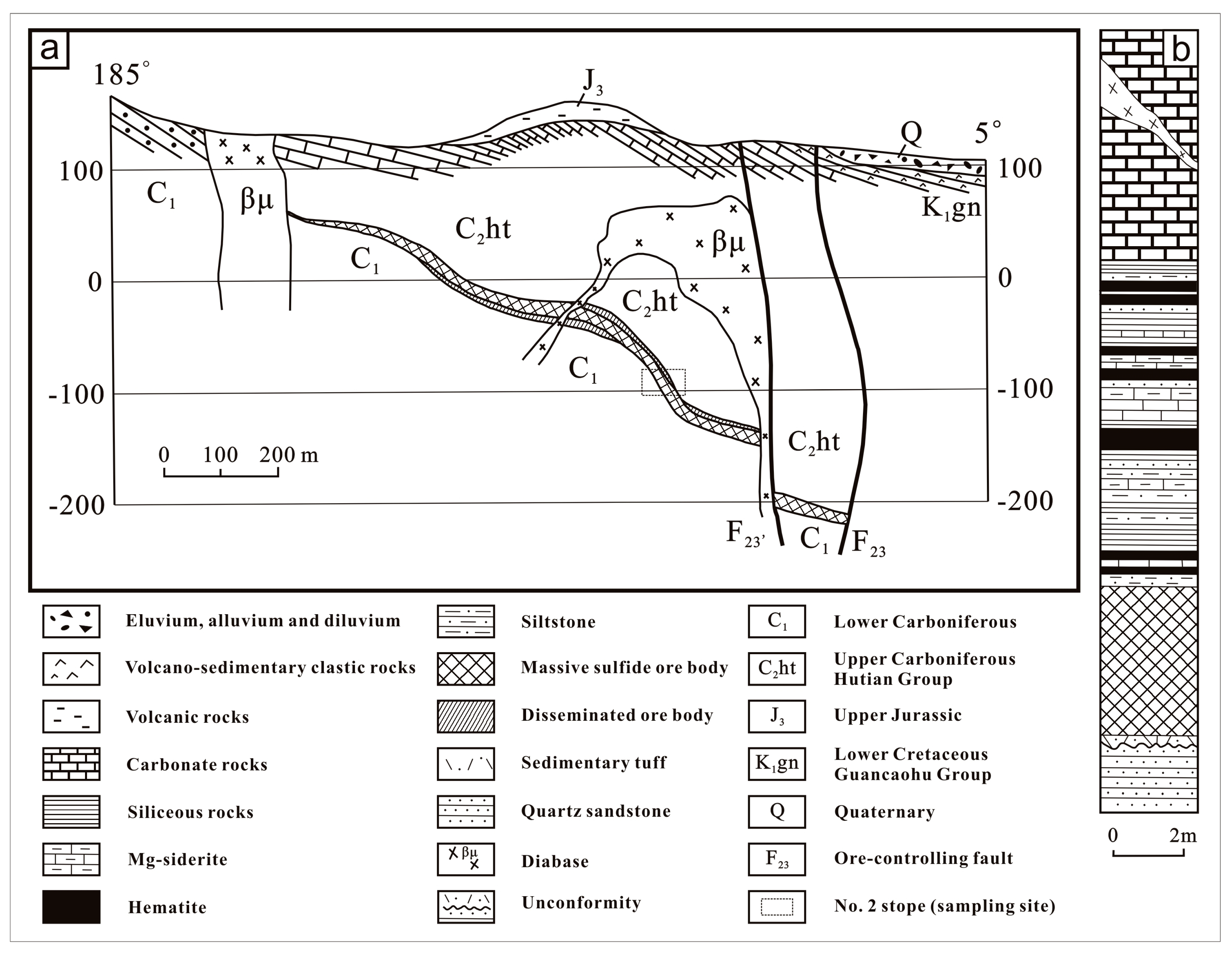
2.2. Occurrences and Distribution of Ore Bodies
- (1)
- Ore-bearing tuffaceous sedimentary layer, with a thickness of 5–30 cm. The tuffaceous sedimentary rocks occur interbedded with thin layers of Cu-rich sulphide ores, showing microlaminations. Feldspar and quartz occur as phenoclasts in the tuffaceous layers. This layer unconformably overlies the Carboniferous clastic rocks.
- (2)
- Bedded/stratiform massive sulphides, with a thickness of 0.1–9.4 m. This layer has the highest Cu grade locally reaching more than 50 wt. %. Silver occurs predominantly in the copper-rich ores and exhibits a positive correlation with Cu grades (but not with Pb or Zn contents) [13]. The grain sizes of sulphide minerals are very fine (mostly less than 0.1 mm), displaying laminated structures. Moreover, flow structures and plastic deformation can also be observed in such laminated layers [2,3,73].
- (3)
- Hematite–chert layer, closely associated with the massive sulphide ores, 0.1–3.7 m in thickness. It is a mixed sedimentary layer with very fine intercalations of exhalative materials and sediments. The hematite-bearing chert/siliceous mudstone locally occurs with siderite, siltstone, (sandy) dolomite, and more rarely thin-bedded sulphide ore layer. All these geological characteristics mentioned above indicate that the Yushui copper-polymetallic deposit has the typical features of a seafloor sedimentary-exhalative deposit [1,15,74,75].
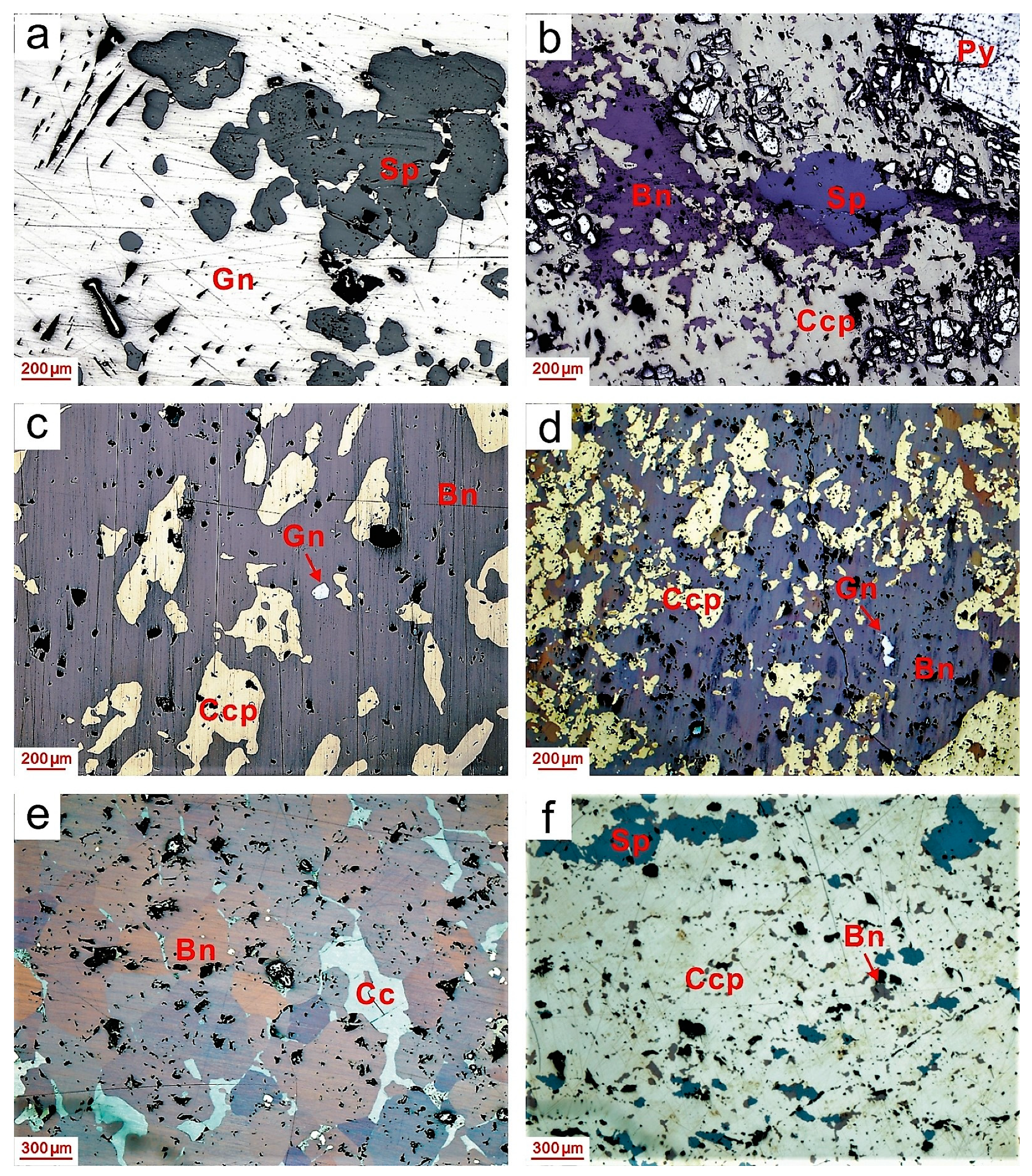
3. Sampling and Analytical Methods
4. Results
4.1. He-Ar Concentrations and Isotopic Ratios
4.2. Trace Gas Analysis of Fluid Inclusions
5. Discussion
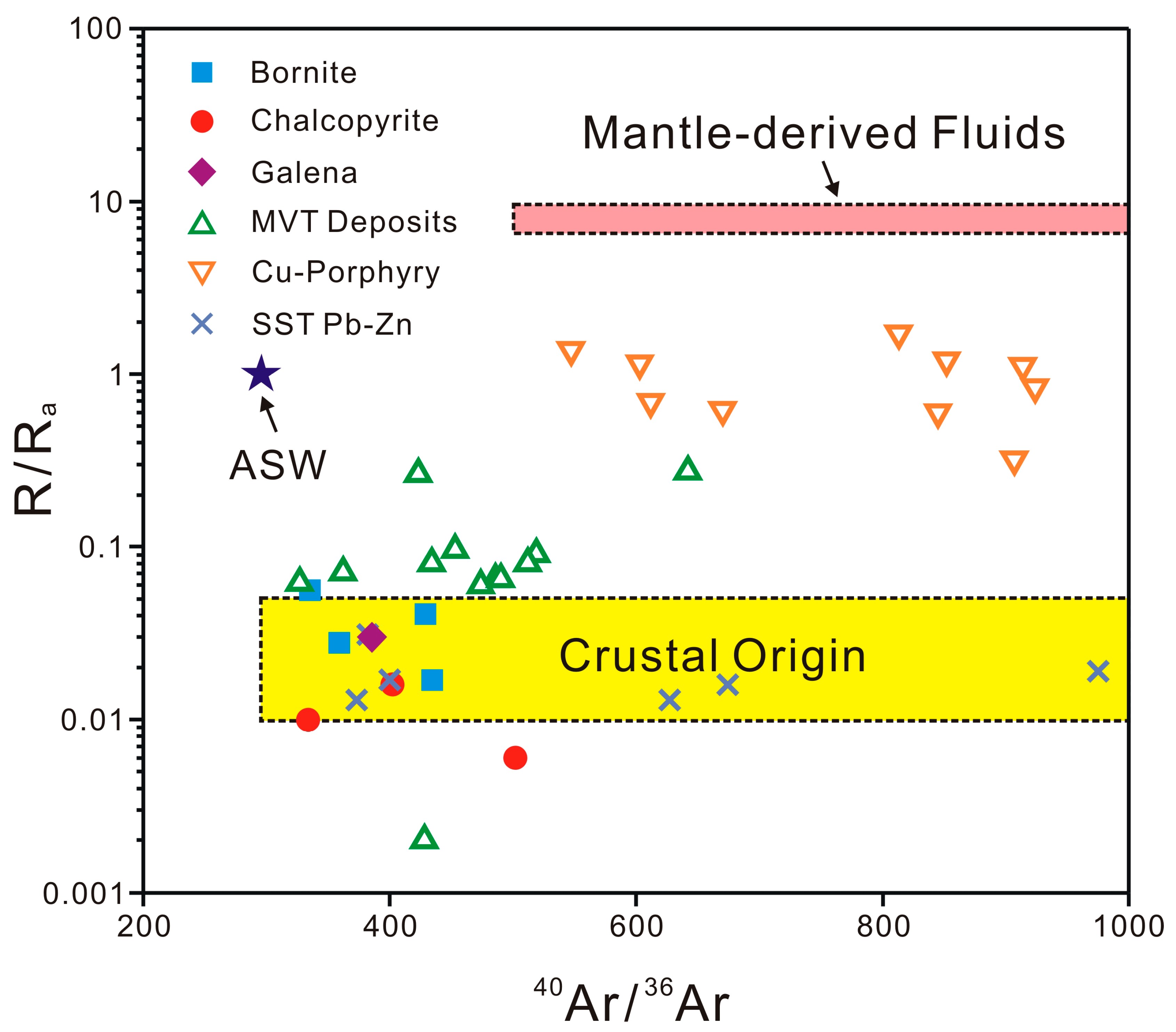
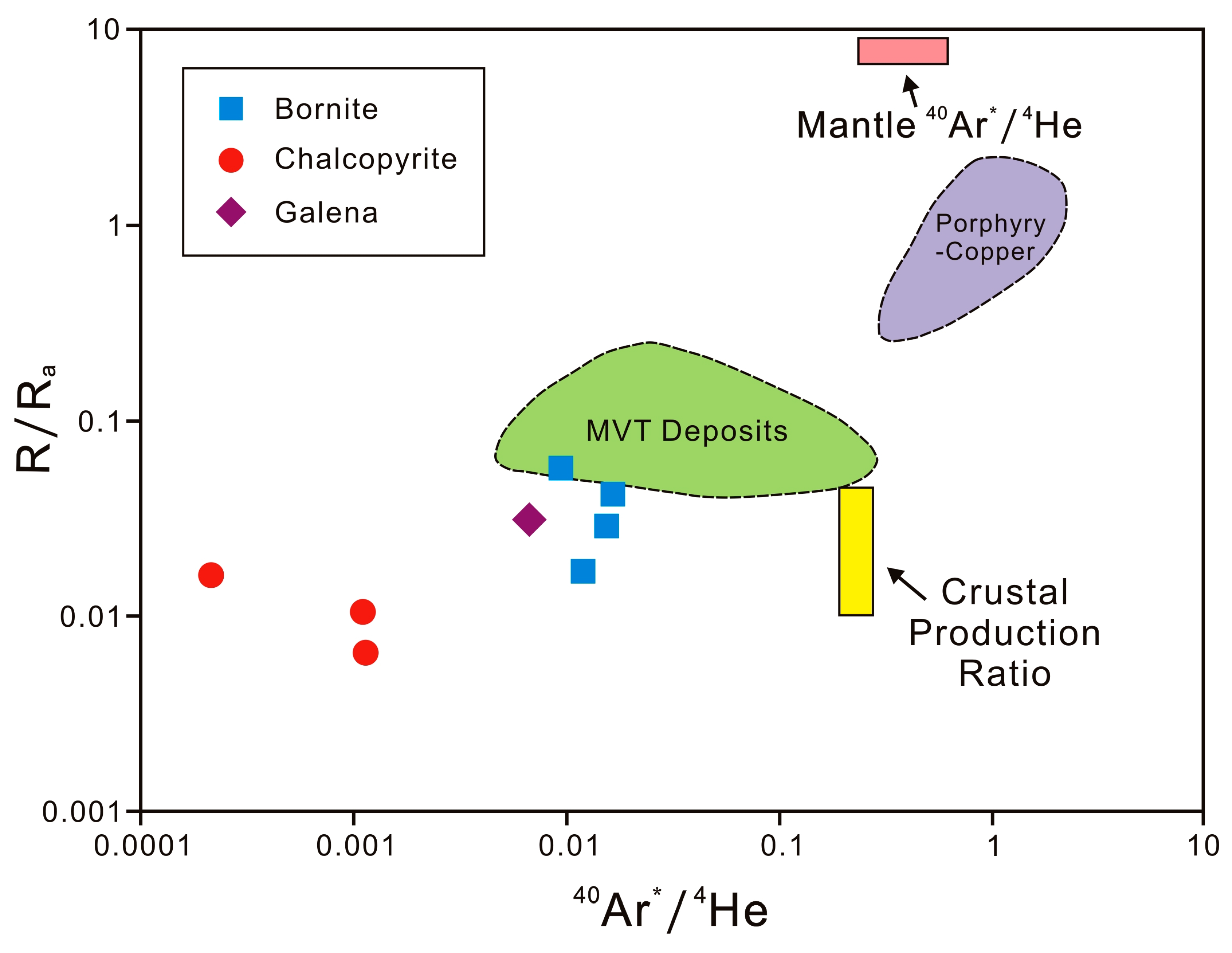
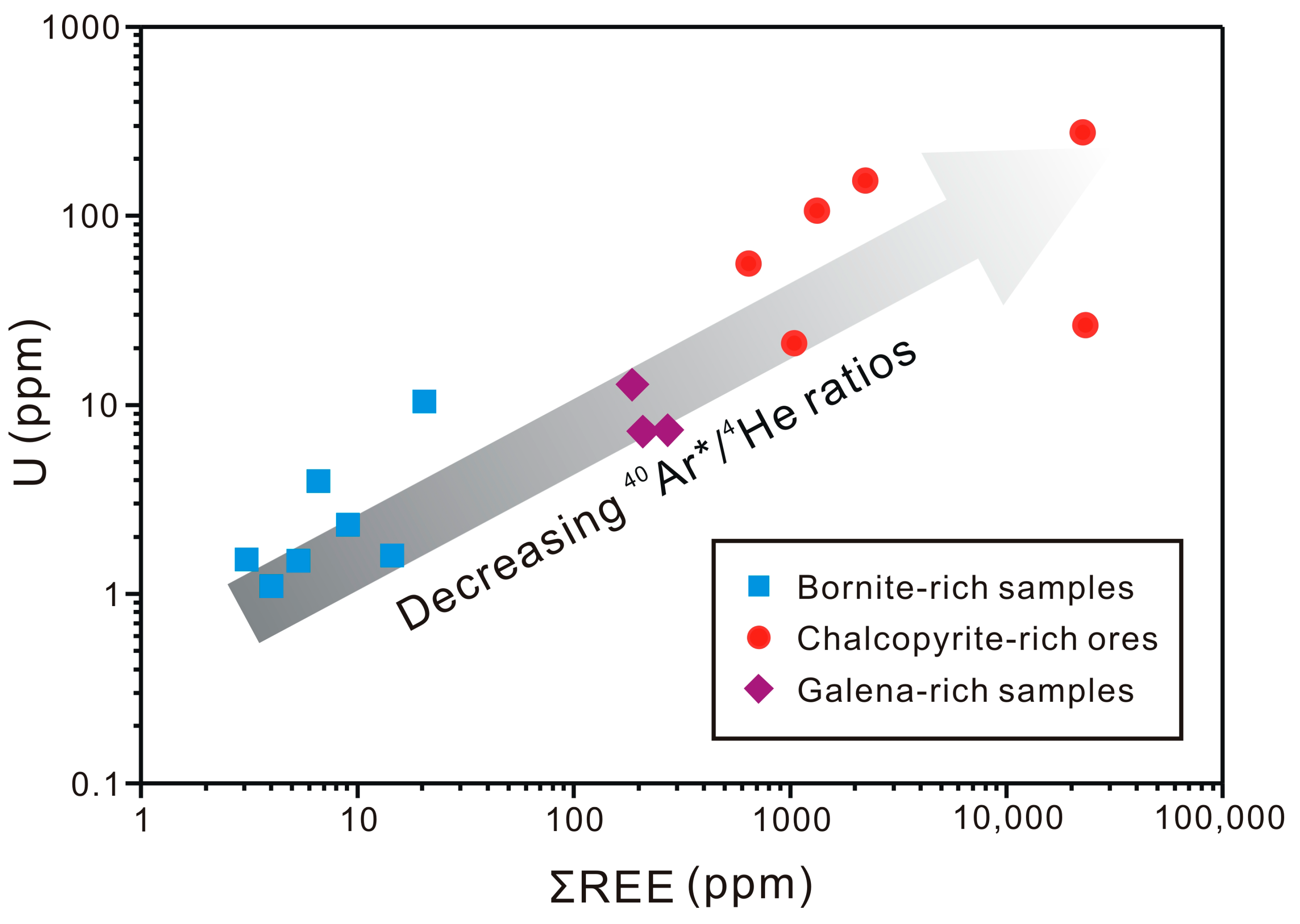
5.1. Helium and Argon Sources
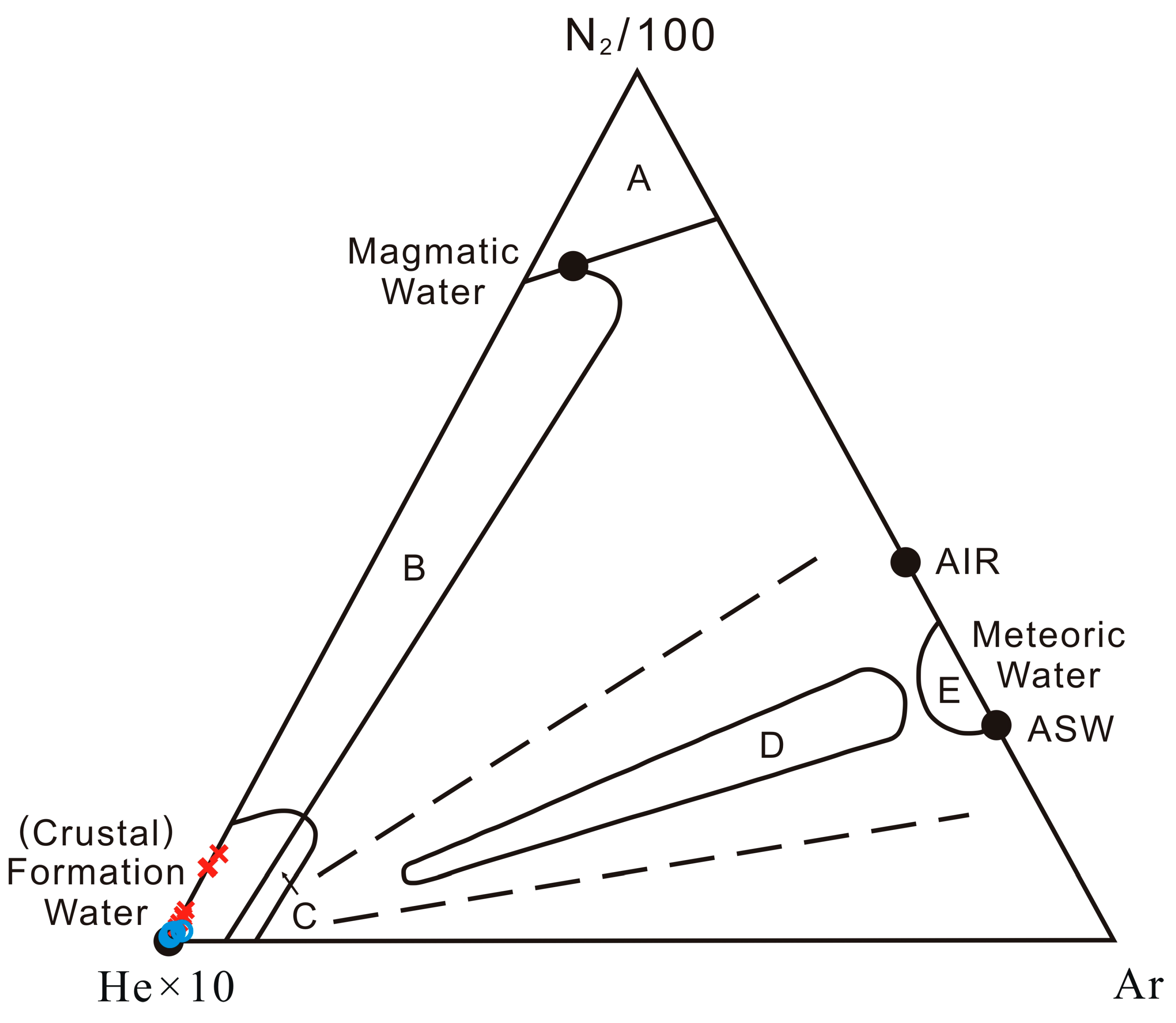
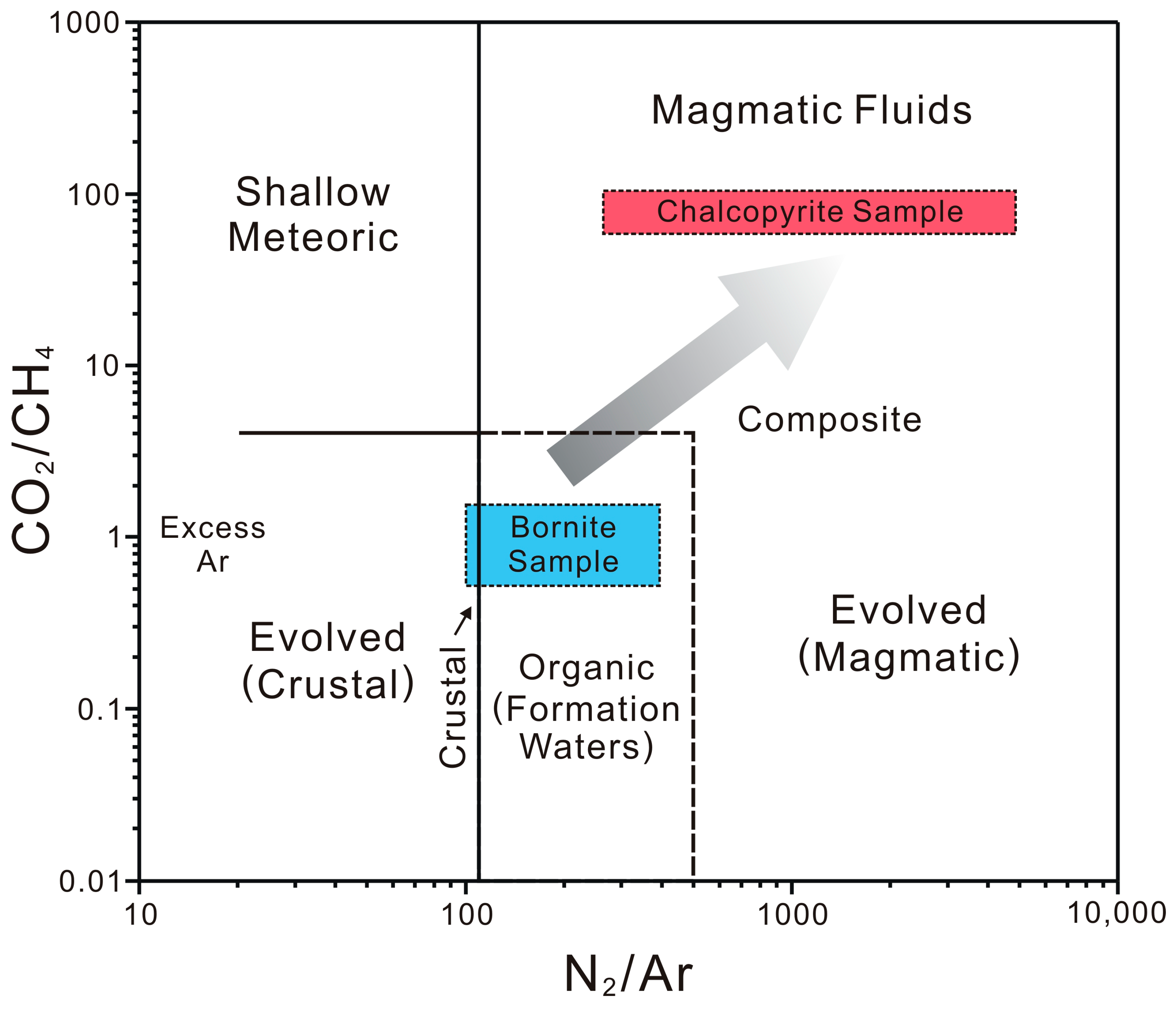
5.2. The N2–Ar–He Systematics
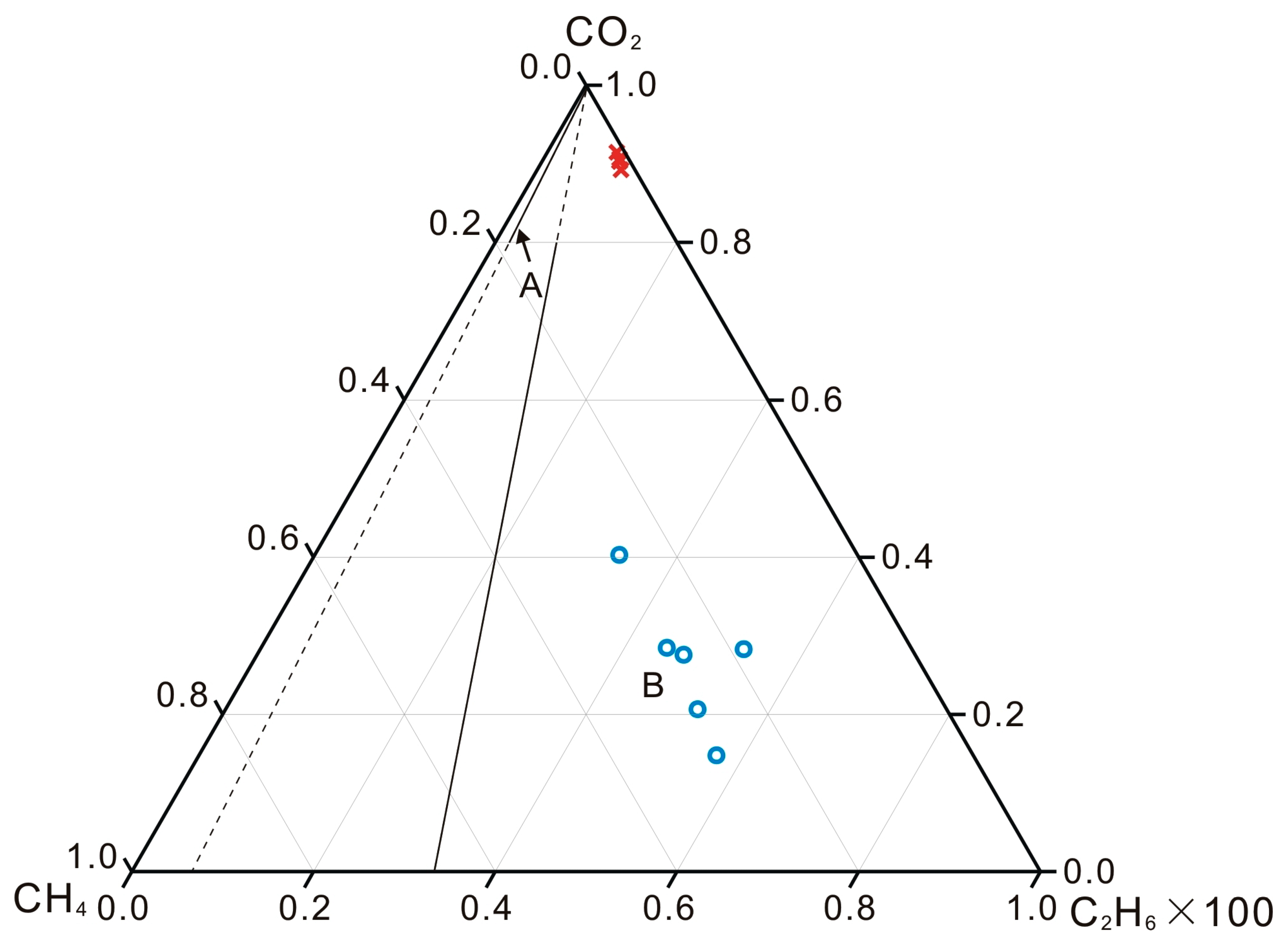
5.3. Compositions of Light Hydrocarbon Gases
5.4. Possible Mechanisms for the Oxidation of Hydrocarbons to CO2
5.5. Constraints on the Genesis of Yushui Ore Deposit
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gu, L.; Zaw, K.; Hu, W.; Zhang, K.; Ni, P.; He, J.; Xu, Y.; Lu, J.; Lin, C. Distinctive features of Late Palaeozoic massive sulphide deposits in South China. Ore Geol. Rev. 2007, 31, 107–138. [Google Scholar] [CrossRef]
- He, Y. Metallogenic-geologic characteristics of Yushui hydrothermal-sedimentary polymetallic deposit in Meixian County, Guangdong Province. Guangdong Geol. 1990, 5, 1–13. (In Chinese) [Google Scholar]
- Chen, B.; Chen, J.; Guo, R.; Yu, S. The genesis of Yushui copper-polymetallic deposit in Meixian County, Guangdong Province. Guangdong Geol. 1992, 7, 59–69. (In Chinese) [Google Scholar]
- He, Y. Geology of Yushui copper-lead-zinc (silver) deposit, Meixian. Guangdong Geol. 1997, 12, 41–48. (In Chinese) [Google Scholar]
- Wang, L.; Yang, M.; Peng, S. On the genesis of the Yushui polymetallic deposit in Meixian County, Guangdong Province. Geotecton. Metall. 1999, 23, 345–352. (In Chinese) [Google Scholar]
- Chen, H. A study on the isotopic geochemical exploration in the Yushui-Yinshi copper and polymetallic mineralization belt, Guangdong. Acta Geosci. Sin. 1994, 15, 152–156. (In Chinese) [Google Scholar]
- Cai, J.; Liu, J.; Chen, Y.; Qiu, Q. Metallogenetic epoch of Yushui copper polymetallic deposit of Meixian, Guangdong. Guangdong Geol. 1996, 11, 55–58. (In Chinese) [Google Scholar]
- Liu, J. Thermobarogeochemistry, ore-forming age and genesis study for the Yushui copper-rich multimetal deposit in Meixian County, Guangdong Province, southern China. Geol. Miner. Resour. South China 1997, 1, 37–50. (In Chinese) [Google Scholar]
- Guo, R.; Chen, B.; Yu, S. Mineralogy of the Yushui Cu-polymetallic deposit in Meixian, Guangdong. Geol. Explor. Nonferrous Met. 1999, 8, 428–431. (In Chinese) [Google Scholar]
- Chen, W. The ore-forming geology condition and origin of Yushui copper-polymetallic deposit. Nonferrous Met. 2007, 59, 18–19. (In Chinese) [Google Scholar]
- Huang, Y.; Sun, X.; Shi, G.; Sa, R.; Guan, Y.; Jiang, X.; Que, H. Re-Os dating of sulphides from the Yushui Cu-polymetallic deposit in eastern Guangdong Province, South China. Ore Geol. Rev. 2015, 70, 281–289. [Google Scholar] [CrossRef]
- Jiang, B.; Zhu, X.; Cheng, X.; Wang, H. Characteristics and geological significance of fluid inclusions in the Yushui copper polymetallic deposit, Guangdong Province. Geol. China 2016, 43, 2163–2172. (In Chinese) [Google Scholar]
- He, Y. Silver in Yushui copper-lead-zinc deposit, Meixian. Guangdong Geol. 1997, 12, 33–36. (In Chinese) [Google Scholar]
- Liu, X. Copper resources in China: Today and tomorrow. Northwest. Geol. 2007, 40, 83–88. (In Chinese) [Google Scholar]
- Gu, L.; Hu, W.; Ni, P.; He, J.; Xu, Y.; Lu, J.; Lin, C.; Li, W. New discussion on the South China-type massive sulphide deposits formed on continental crust. Geol. J. China Univ. 2003, 9, 592–608. (In Chinese) [Google Scholar]
- Lupton, J.E.; Craig, H. A major helium-3 source at 15°S on the East Pacific Rise. Science 1981, 214, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Simmons, S.F.; Sawkins, F.J.; Schlutter, D.J. Mantle-derived helium in two Peruvian hydrothermal ore deposits. Nature 1987, 329, 429–432. [Google Scholar] [CrossRef]
- Lupton, J.E.; Baker, E.T.; Massoth, G.J. Variable 3He/heat ratios in submarine hydrothermal systems: Evidence from two plumes over the Juan de Fuca ridge. Nature 1989, 337, 161–164. [Google Scholar] [CrossRef]
- Baker, E.T.; Lupton, J.E. Changes in submarine hydrothermal 3He/heat ratios as an indicator of magmatic/tectonic activity. Nature 1990, 346, 556–558. [Google Scholar] [CrossRef]
- Stuart, F.M.; Turner, G.; Duckworth, R.C.; Fallick, A.E. Helium isotopes as tracers of trapped hydrothermal fluids in ocean-floor sulfides. Geology 1994, 22, 823–826. [Google Scholar] [CrossRef]
- Stuart, F.M.; Burnard, P.G.; Taylor, R.P.; Turner, G. Resolving mantle and crustal contributions to ancient hydrothermal fluids: He-Ar isotopes in fluid inclusions from Dae Hwa W-Mo mineralization, South Korea. Geochim. Cosmochim. Acta 1995, 59, 4663–4673. [Google Scholar] [CrossRef]
- Jean-Baptiste, P.; Fouquet, Y. Abundance and isotopic composition of helium in hydrothermal sulfides from the East Pacific Rise at 13°N. Geochim. Cosmochim. Acta 1996, 60, 87–93. [Google Scholar] [CrossRef]
- Hu, R.; Burnard, P.G.; Turner, G.; Bi, X. Helium and argon isotope systematics in fluid inclusions of Machangqing copper deposit in west Yunnan Province, China. Chem. Geol. 1998, 146, 55–63. [Google Scholar] [CrossRef]
- Burnard, P.G.; Hu, R.; Turner, G.; Bi, X. Mantle, crustal and atmospheric noble gases in Ailaoshan gold deposits, Yunnan Province, China. Geochim. Cosmochim. Acta 1999, 63, 1595–1604. [Google Scholar] [CrossRef]
- Hou, Z.; Li, Y.; Ai, Y.; Tang, S.; Zhang, Q. Helium isotopic compositions of the active hydrothermal system in the Okinawa trough: Evidence for the mantle-derived helium. Sci. China Ser. D Earth Sci. 1999, 29, 155–162. (In Chinese) [Google Scholar]
- Winckler, G.; Aeschbach-Hertig, W.; Kipfer, R.; Botz, R.; Rübel, A.P.; Bayer, R.; Stoffers, P. Constraint on origin and evolution of Red Sea brines from helium and argon isotopes. Earth Planet. Sci. Lett. 2001, 184, 671–683. [Google Scholar] [CrossRef]
- Zeng, Z.; Qin, Y.; Zhai, S. He, Ne and Ar isotope compositions of fluid inclusions in hydrothermal sulfides from the TAG hydrothermal field, Mid-Atlantic Ridge. Sci. China Ser. D Earth Sci. 2001, 44, 221–228. [Google Scholar] [CrossRef]
- Ballentine, C.J.; Burnard, P.G. Production, release and transport of noble gases in the continental crust. Rev. Mineral. Geochem. 2002, 47, 481–538. [Google Scholar] [CrossRef]
- Graham, D.W. Noble gas isotope geochemistry of Mid-Ocean Ridge and Ocean Island Basalts: Characterization of mantle source reservoirs. Rev. Mineral. Geochem. 2002, 47, 247–317. [Google Scholar] [CrossRef]
- Hou, Z.; Zaw, K.; Li, Y.; Zhang, Q.; Zeng, Z.; Tetsuro, U. Contribution of magmatic fluid to the active hydrothermal system in the JADE field, Okinawa Trough: Evidence from fluid inclusions, oxygen and helium isotopes. Int. Geol. Rev. 2005, 47, 420–437. [Google Scholar]
- Lüders, V.; Niedermann, S. Helium isotope composition of fluid inclusions hosted in massive sulfides from modern submarine hydrothermal systems. Econ. Geol. 2010, 105, 443–449. [Google Scholar] [CrossRef]
- Zeng, Z.; Niedermann, S.; Chen, S.; Wang, X.; Li, Z. Noble gases in sulfide deposits of modern deep-sea hydrothermal systems: Implications for heat fluxes and hydrothermal fluid processes. Chem. Geol. 2015, 409, 1–11. [Google Scholar] [CrossRef]
- Guan, Y.; Ren, Y.; Sun, X.; Xiao, Z.; Guo, Z. Helium and argon isotopes in the Fe-Mn polymetallic crusts and nodules from the South China Sea: Constraints on their genetic sources and origins. Minerals 2018, 8, 471. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Z.; Sun, X.; Deng, X.; Guan, Y.; Xu, L.; Huang, Y.; Cao, K. He-Ar-S isotopic compositions of polymetallic sulphides from hydrothermal vent fields along the ultraslow-spreading Southwest Indian Ridge and their geological implications. Minerals 2018, 8, 512. [Google Scholar] [CrossRef]
- Stuart, F.M.; Turner, G. The abundance and isotopic composition of the noble gases in ancient fluids. Chem. Geol. 1992, 101, 97–109. [Google Scholar] [CrossRef]
- Hu, R.; Turner, G.; Burnard, P.G.; Zhong, H.; Ye, Z.; Bi, X. Helium and argon isotopic geochemistry of Jinding superlarge Pb-Zn deposit. Sci. China Ser. D Earth Sci. 1998, 41, 442–448. [Google Scholar] [CrossRef]
- Kendrick, M.A.; Burgess, R.; Pattrick, R.A.D.; Turner, G. Fluid inclusion noble gas and halogen evidence on the origin of Cu-Porphyry mineralising fluids. Geochim. Cosmochim. Acta 2001, 65, 2651–2668. [Google Scholar] [CrossRef]
- Kendrick, M.A.; Burgess, R.; Leach, D.; Pattrick, R.A.D. Hydrothermal fluid origins in Mississippi valley-type ore districts: combined noble gas (He, Ar, Kr) and halogen (Cl, Br, I) analysis of fluid inclusions from the Illinois-Kentucky fluorspar district, Viburnum Trend, and Tri-State districts, midcontinent United States. Econ. Geol. 2002, 97, 453–469. [Google Scholar]
- Kendrick, M.A.; Burgess, R.; Pattrick, R.A.D.; Turner, G. Hydrothermal fluid origins in a fluorite-rich Mississippi valley-type district: combined noble gas (He, Ar, Kr) and halogen (Cl, Br, I) analysis of fluid inclusions from the South Pennine ore field, United Kingdom. Econ. Geol. 2002, 97, 435–451. [Google Scholar] [CrossRef]
- Kendrick, M.A.; Burgess, R.; Harrison, D.; Bjørlykke, A. Noble gas and halogen evidence for the origin of Scandinavian sandstone-hosted Pb-Zn deposits. Geochim. Cosmochim. Acta 2005, 69, 109–129. [Google Scholar] [CrossRef]
- Zhao, K.; Jiang, S.; Xiao, H.; Ni, P. Origin of ore-forming fluids of the Dachang Sn-polymetallic ore deposit: Evidence from helium isotopes. Chin. Sci. Bull. 2002, 47, 1041–1045. [Google Scholar] [CrossRef]
- Xue, C.; Chen, Y.; Wang, D.; Yang, J.; Yang, W.; Zeng, R. Geology and isotopic composition of helium, neon, xenon and metallogenic age of the Jinding and Baiyangping ore deposits, northwest Yunnan, China. Sci. China Ser. D Earth Sci. 2003, 46, 789–800. [Google Scholar] [CrossRef]
- Hu, R.; Burnard, P.G.; Bi, X.; Zhou, M.; Peng, J.; Su, W.; Wu, K. Helium and argon isotope geochemistry of alkaline intrusion-associated gold and copper deposits along the Red River-Jinshajiang fault belt, SW China. Chem. Geol. 2004, 203, 305–317. [Google Scholar] [CrossRef]
- Sun, X.; Wang, M.; Xue, T.; Ma, M.; Li, Y. He-Ar isotopic systematics of fluid inclusions in pyrites from PGE-polymetallic deposits in Lower Cambrian black rock series, South China. Acta Geol. Sin. 2004, 78, 471–475. [Google Scholar]
- Sun, X.; Zhang, Y.; Xiong, D.; Sun, W.; Shi, G.; Zhai, W.; Wang, S. Crust and mantle contributions to gold-forming process at the Daping deposit, Ailaoshan gold belt, Yunnan, China. Ore Geol. Rev. 2009, 36, 235–249. [Google Scholar] [CrossRef]
- Zhai, W.; Sun, X.; Wu, Y.; Sun, Y.; Hua, R.; Ye, X. He-Ar isotope geochemistry of the Yaoling-Meiziwo tungsten deposit, North Guangdong Province: Constraints on Yanshanian crust-mantle interaction and metallogenesis in SE China. Chin. Sci. Bull. 2012, 57, 1150–1159. [Google Scholar] [CrossRef]
- Yang, F.; Li, Q.; Yang, C.; Zhang, Z. A combined fluid inclusion and S-H-O-He-Ar isotope study of the Devonian Ashele VMS-type copper-zinc deposit in the Altay orogenic belt, northwest China. J. Asian Earth Sci. 2018, 161, 139–163. [Google Scholar] [CrossRef]
- Norman, D.I.; Sawkins, I.J. Analysis of volatiles in fluid inclusions by mass spectrometer. Chem. Geol. 1987, 61, 1–10. [Google Scholar] [CrossRef]
- Norman, D.I.; Musgrave, J.A. N2-Ar-He compositions in fluid inclusions: Indicators of fluid source. Geochim. Cosmochim. Acta 1994, 58, 1119–1131. [Google Scholar] [CrossRef]
- Graney, J.R.; Kesler, S.E. Factors affecting gas analysis of inclusion fluid by quadrupole mass spectrometry. Geochim. Cosmochim. Acta 1995, 59, 3977–3986. [Google Scholar] [CrossRef]
- Sun, X.; Norman, D.I.; Sun, K.; Chen, B.; Chen, J. N2-Ar-He systematics and source of ore-forming fluid in Changkeng Au-Ag deposit, central Guangdong, China. Sci. China Ser. D Earth Sci. 1999, 42, 475–481. [Google Scholar] [CrossRef]
- Sun, X.; Norman, D.I.; Sun, K.; Chen, J.; Chen, B. A new indicator of ore-forming fluid sources: N2-Ar-He compositions in fluid inclusions. Geol. Rev. 2000, 46, 99–104. (In Chinese) [Google Scholar]
- Moore, J.N.; Norman, D.I.; Kennedy, B.M. Fluid inclusion gas compositions from an active magmatic-hydrothermal system: A case study of the Geysers geothermal field, USA. Chem. Geol. 2001, 173, 3–30. [Google Scholar] [CrossRef]
- Sun, X.; Norman, D.I.; Sun, K.; Chen, J.; Chen, B. N2-Ar-He tracing systematics of ore-forming fluids: A case study from the Songxi large-scale Ag (Sb) deposit, eastern Guangdong Province, China. Prog. Nat. Sci. 1999, 9, 1364–1367. (In Chinese) [Google Scholar]
- Sun, X.; Wang, M.; Xue, T.; Sun, K. New advances in research of trace gaseous compositions in fluid inclusions and their applications as indicators of ore-forming fluid sources and processes. Earth Sci. Front. 2004, 11, 471–478. (In Chinese) [Google Scholar]
- Giggenbach, W.F.; Sano, Y.; Wakita, H. Isotopic composition of helium, and CO2 and CH4 contents in gases produced along the New Zealand part of a convergent plate boundary. Geochim. Cosmochim. Acta 1993, 57, 3427–3455. [Google Scholar] [CrossRef]
- Giggenbach, W.F.; Sheppard, D.S.; Robinson, B.W.; Stewart, M.K.; Lyon, G.L. Geochemical structure and position of the Waiotapu geothermal field, New Zealand. Geothermics 1994, 23, 599–644. [Google Scholar] [CrossRef]
- Giggenbach, W.F. Variations in the chemical and isotopic composition of fluids discharged from the Taupo Volcanic Zone, New Zealand. J. Volcanol. Geotherm. Res. 1995, 68, 89–116. [Google Scholar] [CrossRef]
- Giggenbach, W.F. Relative importance of thermodynamic and kinetic processes in governing the chemical and isotopic composition of carbon gases in high-heatflow sedimentary basins. Geochim. Cosmochim. Acta 1997, 61, 3763–3785. [Google Scholar] [CrossRef]
- Zhuang, H.; Lu, J.; Fu, J.; Liu, J.; Shi, J. Preliminary study on light hydrocarbons in ore-forming fluids of gold and antimony deposits in southwestern Guizhou, China. Chin. Sci. Bull. 1997, 42, 1708–1711. [Google Scholar] [CrossRef]
- Sun, X.; Norman, D.I.; Sun, K.; Chen, B.; Chen, J. Compositions of light hydrocarbons in fluid inclusions and its significance for ore genesis—A case study of Songxi Ag (Sb) deposit, eastern Guangdong. Geol. Rev. 1999, 45, 817–821. (In Chinese) [Google Scholar]
- Sun, X.; Norman, D.I.; Sun, K.; Chen, B.; Chen, J. Organic gases in fluid inclusions of ore minerals and their constraints on ore genesis: A case study of the Changkeng Au-Ag deposit, Guangdong, China. Acta Geol. Sin. 2003, 77, 86–94. [Google Scholar]
- Sun, X.; Norman, D.I.; Sun, K.; Wang, M.; Chen, B.; Chen, J.; Yu, S. Light hydrocarbons in fluid inclusions and their constraints on ore genesis: A case study of the Songxi Ag (Sb) deposit, Eastern Guangdong, China. Acta Geol. Sin. 2003, 77, 227–236. [Google Scholar]
- Kendrick, M.A.; Honda, M.; Oliver, N.H.S.; Phillips, D. The noble gas systematics of late-orogenic H2O–CO2 fluids, Mt Isa, Australia. Geochim. Cosmochim. Acta 2011, 75, 1428–1450. [Google Scholar] [CrossRef]
- Kendrick, M.A.; Honda, M.; Walshe, J.; Petersen, K. Fluid sources and the role of abiogenic-CH4 in Archean gold mineralization: Constraints from noble gases and halogens. Precambrian Res. 2011, 189, 313–327. [Google Scholar] [CrossRef]
- Blamey, N.J.F. Composition and evolution of crustal, geothermal and hydrothermal fluids interpreted using quantitative fluid inclusion gas analysis. J. Geochem. Explor. 2012, 116–117, 17–27. [Google Scholar] [CrossRef]
- Cawood, P.A.; Zhao, G.; Yao, J.; Wang, W.; Xu, Y.; Wang, Y. Reconstructing South China in Phanerozoic and Precambrian supercontinents. Earth-Sci. Rev. 2018, 186, 173–194. [Google Scholar] [CrossRef]
- Shu, L. Predevonian tectonic evolution of South China: From Cathaysian Block to Caledonian period folded orogenic belt. Geol. J. China Univ. 2006, 12, 418–431. (In Chinese) [Google Scholar]
- Li, S.; Zhao, S.; Liu, X.; Cao, H.; Yu, S.; Li, X.; Somerville, I.; Yu, S.; Suo, Y. Closure of the Proto-Tethys Ocean and Early Paleozoic amalgamation of microcontinental blocks in East Asia. Earth-Sci. Rev. 2018, 186, 37–75. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, Y.; Huang, B.; Dong, Y.; Li, S.; Zhang, G.; Yu, S. Geological reconstructions of the East Asian blocks: From the breakup of Rodinia to the assembly of Pangea. Earth-Sci. Rev. 2018, 186, 262–286. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Wartho, J.A.; Clark, C.; Li, W.; Zhang, C.; Bao, C. Magmatic and metamorphic events during the early Paleozoic Wuyi-Yunkai orogeny, southeastern South China: New age constraints and pressure-temperature conditions. Geol. Soc. Am. Bull. 2010, 122, 772–793. [Google Scholar] [CrossRef]
- Guo, R.; Peng, E. Regional geological background, ore-forming characters and metallogeny of Songxi silver deposit, east Guagndong. Geotect. Metall. 2009, 33, 567–572. (In Chinese) [Google Scholar]
- Chen, Y.; Liang, B.; Liao, L. Major characteristics of carboniferous sedimentary facies of Yushui-Yinshi district in Meixian County, Guangdong Province. Guangdong Geol. 1992, 7, 27–39. (In Chinese) [Google Scholar]
- Leach, D.L.; Sangster, D.F.; Kelley, K.D.; Large, R.R.; Garven, G.; Allen, C.R.; Gutzmer, J.; Walters, S. Sediment-hosted lead-zinc deposits: A global perspective. Econ. Geol. 2005, 100, 561–607. [Google Scholar]
- Leach, D.L.; Bradley, D.C.; Huston, D.; Pisarevsky, S.A.; Taylor, R.D.; Gardoll, S.J. Sediment-hosted lead-zinc deposits in Earth History. Econ. Geol. 2010, 105, 593–625. [Google Scholar] [CrossRef]
- Hu, R.; Bi, X.; Jiang, G.; Chen, H.; Peng, J.; Qi, Y.; Wu, L.; Wei, W. Mantle-derived noble gases in ore-forming fluids of the granite-related Yaogangxian tungsten deposit, Southeastern China. Miner. Depos. 2012, 47, 623–632. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Liu, H.; Zhang, J.; Jin, G.; Zhang, J.; Han, J. Helium isotope composition of inclusions in mineral grains using Helix SFT noble gas mass spectrometer. Acta Geol. Sin. 2015, 89, 1826–1831. (In Chinese) [Google Scholar]
- Xie, G.; Mao, J.; Li, W.; Zhu, Q.; Liu, H.; Jia, G.; Li, Y.; Li, J.; Zhang, J. Different proportion of mantle-derived noble gases in the Cu-Fe and Fe skarn deposits: He-Ar isotopic constraint in the Edong district, Eastern China. Ore Geol. Rev. 2016, 72, 343–354. [Google Scholar] [CrossRef]
- Allègre, C.J.; Staudacher, T.; Sarda, P. Rare gas systematics: Formation of the atmosphere, evolution and structure of the earth’s mantle. Earth Planet. Sci. Lett. 1987, 81, 127–150. [Google Scholar] [CrossRef]
- Andrews, J.N. The isotopic composition of radiogenic helium and its use to study groundwater movement in confined aquifers. Chem. Geol. 1985, 49, 339–351. [Google Scholar] [CrossRef]
- Patterson, D.B.; Honda, M.; McDougall, I. Noble gases in mafic phenocrysts and xenoliths from New Zealand. Geochim. Cosmochim. Acta 1994, 58, 4411–4427. [Google Scholar] [CrossRef]
- Dunai, T.J.; Baur, H. Helium, neon and argon systematics of the European subcontinental mantle: Implications for its geochemical evolution. Geochim. Cosmochim. Acta 1995, 59, 2767–2783. [Google Scholar] [CrossRef]
- Reid, M.R.; Graham, D.W. Resolving lithospheric and sub-lithospheric contributions to helium isotope variations in basalts from the southwestern US. Earth Planet. Sci. Lett. 1996, 144, 213–222. [Google Scholar] [CrossRef]
- Ballentine, C.J.; Burgess, R.; Marty, B. Tracing fluid origin, transport and interaction in the crust. Rev. Mineral. Geochem. 2002, 47, 539–614. [Google Scholar] [CrossRef]
- Torgersen, T.; Kennedy, B.M.; Hiyagon, H.; Chiou, K.Y.; Reynolds, J.H.; Clarke, W.B. Argon accumulation and the crustal degassing flux of 40Ar in the Great Artesian Basin, Australia. Earth Planet. Sci. Lett. 1989, 92, 43–56. [Google Scholar] [CrossRef]
- Huizenga, J.-M. Thermodynamic modelling of C–O–H fluids. Lithos 2001, 55, 101–114. [Google Scholar] [CrossRef]
- Potter, J.; Konnerup-Madsen, J. A review of the occurrence and origin of abiogenic hydrocarbons in igneous rocks. Geol. Soc. London Spec. Publ. 2003, 214, 151–173. [Google Scholar] [CrossRef]
- Konnerup-Madsen, J.; Rose-Hansen, J. Volatiles associated with alkaline igneous rift activity: Fluid inclusions in the Ilímaussaq Intrusion and the Gardar granitic complexes (south Greenland). Chem. Geol. 1982, 37, 79–93. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, Q. CO2-Hydrocarbon fluids of the Jade hydrothermal field in the Okinawa Trough: Fluid inclusion evidence. Sci. China Ser. D Earth Sci. 1998, 41, 408–415. [Google Scholar] [CrossRef]
- Wilkinson, J.J. Fluid inclusions in hydrothermal ore deposits. Lithos 2001, 55, 229–272. [Google Scholar] [CrossRef]
- Kesler, S.E. Ore-forming fluids. Elements 2005, 1, 13–18. [Google Scholar] [CrossRef]
- Norman, D.I.; Moore, J.N. Methane and excess N2 and Ar in geothermal fluid inclusions. In Proceedings of the Twenty-Third Workshop on Geothermal Reservoir Engineering, Stanford University, Stanford, CA, USA, 25–27 January 1999. [Google Scholar]
- Barré, G.; Truche, L.; Bazarkina, E.F.; Michels, R.; Dubessy, J. First evidence of the trisulfur radical ion S3− and other sulfur polymers in natural fluid inclusions. Chem. Geol. 2017, 462, 1–14. [Google Scholar] [CrossRef]
- Goldstein, T.P.; Aizenshtat, Z. Thermochemical sulfate reduction: A review. J. Therm. Anal. 1994, 42, 241–290. [Google Scholar] [CrossRef]
- Machel, H.G.; Krouse, H.R.; Sassen, R. Products and distinguishing criteria of bacterial and thermochemical sulfate reduction. Appl. Geochem. 1995, 10, 373–389. [Google Scholar] [CrossRef]
- Machel, H.G. Bacterial and thermochemical sulfate reduction in diagenetic settings—Old and new insights. Sediment. Geol. 2001, 140, 143–175. [Google Scholar] [CrossRef]
- Basuki, N.; Taylor, B.E.; Spooner, E.T.C. Sulfur isotope evidence for thermochemical reduction of dissolved sulfate in Mississippi Valley-type zinc-lead mineralization, Bongara Area, Northern Peru. Econ. Geol. 2008, 103, 783–799. [Google Scholar] [CrossRef]
- Thom, J.; Anderson, G.M. The role of thermochemical sulfate reduction in the origin of Mississippi Valley-type deposits. I. Experimental results. Geofluids 2008, 8, 16–26. [Google Scholar] [CrossRef]
- Gadd, M.G.; Layton-Matthews, D.; Peter, J.M.; Paradis, S.; Jonasson, I.R. The world-class Howard’s Pass SEDEX Zn-Pb district, Selwyn Basin, Yukon. Part II: the roles of thermochemical and bacterial sulfate reduction in metal fixation. Miner. Depos. 2017, 52, 405–419. [Google Scholar] [CrossRef]
- Kerrick, D.M.; Connolly, J.A.D. Metamorphic devolatilization of subducted marine sediments and the transport of volatiles into the Earth’s mantle. Nature 2010, 411, 293–296. [Google Scholar] [CrossRef]
- Hu, W.; Kang, X.; Cao, J.; Wang, X.; Fu, B.; Wu, H. Thermochemical oxidation of methane induced by high-valence metal oxides in a sedimentary basin. Nat. Commun. 2018, 9, 5131. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Worden, R.H.; Jin, Z.J.; Liu, W.H.; Li, J.; Gao, B.; Zhang, D.W.; Hu, A.P.; Yang, C. TSR versus non-TSR processes and their impact on gas geochemistry and carbon stable isotopes in Carboniferous, Permian and Lower Triassic marine carbonate gas reservoirs in the Eastern Sichuan Basin, China. Geochim. Cosmochim. Acta 2013, 100, 96–115. [Google Scholar] [CrossRef]
- Tarantola, A.; Mullis, J.; Vennemann, T.; Dubessy, J.; de Capitani, C. Oxidation of methane at the CH4/H2O–(CO2) transition zone in the external part of the Central Alps, Switzerland: Evidence from stable isotope investigations. Chem. Geol. 2007, 237, 329–357. [Google Scholar] [CrossRef]
- Tarantola, A.; Mullis, J.; Guillaume, D.; Dubessy, J.; de Capitani, C.; Abdelmoula, M. Oxidation of CH4 to CO2 and H2O by chloritization of detrital biotite at 270 ± 5 °C in the external part of the Central Alps, Switzerland. Lithos 2009, 112, 497–510. [Google Scholar] [CrossRef]
- Lu, H.; Liu, C. Formation of strata-bound ore deposits in China: Studies on fluid inclusions. Chin. J. Geochem. 1990, 9, 347–360. (In Chinese) [Google Scholar]
- Roedder, E. Fluid inclusions. Rev. Mineral. 1984, 12, 1–644. [Google Scholar]
- Cai, J.; Liu, J. Characteristics of structures and prospecting targets in the metallogenic province at the south sector of Yongmei depression, Guangdong. Geotect. Metall. 1996, 20, 333–339. (In Chinese) [Google Scholar]
- Essarraj, S.; Boiron, M.-C.; Cathelineau, M.; Tarantola, A.; Leisen, M.; Boulvais, P.; Maacha, L. Basinal brines at the origin of the Imiter Ag-Hg deposit (Anti-Atlas, Morocco): Evidence from LA-ICP-MS data on fluid inclusions, halogen signatures, and stable isotopes (H, C, O). Econ. Geol. 2016, 111, 1753–1781. [Google Scholar] [CrossRef]

| Sample No. | YS-035 | YS-038 | YS-040 | YS-041 | YS-042 | YS-044 | YS-008 | YS-020 |
|---|---|---|---|---|---|---|---|---|
| Minerals | Chalcopyrite | Bornite | Bornite | Bornite | Bornite | Chalcopyrite | Galena | Chalcopyrite |
| 3He (10−12) | 1.99 ± 0.26 | 2.66 ± 0.08 | 1.66 ± 0.05 | 0.53 ± 0.04 | 4.06 ± 0.16 | 2.98 ± 0.18 | 2.01 | 34.88 |
| 4He (10−5) | 22.41 ± 0.34 | 3.43 ± 0.05 | 4.27 ± 0.07 | 2.27 ± 0.03 | 7.07 ± 0.11 | 20.80 ± 0.32 | 4.80 | 160.00 |
| 40Ar (10−7) | 6.28 ± 0.04 | 27.63 ± 0.16 | 37.34 ± 0.22 | 8.57 ± 0.05 | 37.82 ± 0.22 | 20.23 ± 0.12 | 13.96 | 12.96 |
| 36Ar (10−9) | 1.25 ± 0.03 | 8.25 ± 0.06 | 10.40 ± 0.06 | 1.97 ± 0.03 | 8.82 ± 0.12 | 6.06 ± 0.07 | 3.62 | 3.22 |
| 3He/4He (Ra) | 0.006 ± 0.001 | 0.056 ± 0.002 | 0.028 ± 0.001 | 0.017 ± 0.001 | 0.041 ± 0.002 | 0.010 ± 0.001 | 0.030 | 0.016 |
| 40Ar/36Ar | 501.68 ± 12.32 | 334.87 ± 3.02 | 359.06 ± 3.06 | 434.28 ± 7.88 | 428.62 ± 6.23 | 333.76 ± 4.12 | 385.70 | 402.30 |
| 40Ar*/4He | 0.0012 | 0.0095 | 0.0155 | 0.0121 | 0.0166 | 0.0011 | 0.0068 | 0.0002 |
| F4He | 1,081,365 | 25,149 | 24,793 | 69,407 | 48,398 | 207,394 | 80,149 | 3,000,697 |
| Mineral | Description | Crush No. | N2 | Ar | He | N2/Ar | Ar/He |
|---|---|---|---|---|---|---|---|
| bornite | Hand-picked separates of fine-grained aggregates from the Cu bonanza ores | 1 | 0.3782 | 0.0014 | 0.0461 | 270.1 | 0.030 |
| 2 | 0.0405 | <0.0001 | 0.0054 | - | - | ||
| 3 | 0.0302 | <0.0001 | 0.0098 | - | - | ||
| 4 | 0.4806 | 0.0048 | 0.0519 | 100.1 | 0.092 | ||
| 5 | 0.0280 | <0.0001 | 0.0087 | - | - | ||
| 6 | 0.0395 | 0.0001 | 0.0065 | 395.0 | 0.015 | ||
| chalcopyrite | Hand-picked separates of fine-grained aggregates from massive sulphide ores | 1 | 1.4548 | 0.0003 | 0.0164 | 4849.3 | 0.018 |
| 2 | 0.9537 | 0.0003 | 0.0088 | 3179.0 | 0.034 | ||
| 3 | 0.8076 | 0.0003 | 0.0073 | 2692.0 | 0.041 | ||
| 4 | 1.9852 | 0.0007 | 0.0237 | 2836.0 | 0.030 | ||
| 5 | 0.4037 | 0.0004 | 0.0127 | 1009.3 | 0.031 | ||
| 6 | 0.1917 | 0.0004 | 0.0084 | 479.3 | 0.048 | ||
| 7 | 0.2421 | 0.0003 | 0.0096 | 807.0 | 0.031 | ||
| 8 | 0.1415 | 0.0003 | 0.0084 | 471.7 | 0.036 | ||
| 9 | 0.0797 | 0.0003 | 0.0078 | 265.7 | 0.038 |
| Sampling Location | Selected from Some of the Richest Bonanza Ore Bodies at the −100 m Level of No. 2 Stope in the Yushui Copper-Polymetallic Deposit | Selected from Typical Stratiform Massive Sulphide Ore Bodies at the −100 m Level of No. 2 Stope in the Yushui Deposit | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mineral | Hand-Picked Separates of Fine-Grained Bornite Aggregates | Hand-Picked Separates of Fine-Grained Chalcopyrite Aggregates | ||||||||||
| Crush No. | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 |
| CH4 | 55.31 | 38.85 | 47.53 | 63.43 | 47.42 | 38.91 | 1.204 | 1.638 | 1.073 | 1.259 | 1.266 | 0.968 |
| C2H4 | 0.292 | 0.211 | 0.218 | 0.288 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| C2H6 | 1.049 | 0.497 | 0.786 | 1.275 | 0.870 | 1.114 | 0.093 | 0.101 | 0.090 | 0.093 | 0.092 | 0.082 |
| C3H6 | 0.104 | 0.064 | 0.104 | 0.066 | 0.057 | 0.041 | 0.064 | 0.063 | 0.062 | 0.062 | 0.065 | 0.052 |
| C3H8 | 0.750 | 0.451 | 0.619 | 0.787 | 0.330 | 0.441 | 0.142 | 0.150 | 0.132 | 0.138 | 0.150 | 0.128 |
| C4H8 | 0.209 | 0.062 | 0.128 | 0.275 | 0.026 | 0.086 | 0.003 | 0.005 | 0.005 | 0.004 | 0.003 | 0.004 |
| C4H10 | 0.341 | 0.094 | 0.218 | 0.467 | 0.033 | 0.109 | 0.002 | 0.002 | 0.003 | 0.003 | 0.002 | 0.003 |
| C6H6 | 0.142 | 0.065 | 0.105 | 0.164 | 0.014 | 0.010 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| C7H8 | 0.032 | 0.009 | 0.020 | 0.049 | 0.001 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| CO2 | 41.77 | 59.70 | 50.27 | 33.20 | 51.25 | 59.29 | 98.49 | 98.04 | 98.64 | 98.44 | 98.42 | 98.76 |
| CO2/CH4 | 0.76 | 1.54 | 1.06 | 0.52 | 1.08 | 1.52 | 81.78 | 59.86 | 91.92 | 78.21 | 77.73 | 102.00 |
| CH4/C2H6 | 52.72 | 78.23 | 60.46 | 49.75 | 54.48 | 34.92 | 12.99 | 16.23 | 11.98 | 13.52 | 13.76 | 11.81 |
| C2H6/C3H8 | 1.40 | 1.10 | 1.27 | 1.62 | 2.64 | 2.52 | 0.65 | 0.67 | 0.68 | 0.67 | 0.61 | 0.64 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Wu, Z.; Sun, X.; Wang, Y.; Shi, G.; Zhai, W.; Guan, Y. He-Ar Isotopes and Trace Gas Compositions of Fluid Inclusions in Massive Sulphides from the Yushui Copper-Polymetallic Deposit, South China: Metallogenic Implications. Minerals 2019, 9, 258. https://doi.org/10.3390/min9050258
Huang Y, Wu Z, Sun X, Wang Y, Shi G, Zhai W, Guan Y. He-Ar Isotopes and Trace Gas Compositions of Fluid Inclusions in Massive Sulphides from the Yushui Copper-Polymetallic Deposit, South China: Metallogenic Implications. Minerals. 2019; 9(5):258. https://doi.org/10.3390/min9050258
Chicago/Turabian StyleHuang, Yi, Zhongwei Wu, Xiaoming Sun, Yan Wang, Guiyong Shi, Wei Zhai, and Yao Guan. 2019. "He-Ar Isotopes and Trace Gas Compositions of Fluid Inclusions in Massive Sulphides from the Yushui Copper-Polymetallic Deposit, South China: Metallogenic Implications" Minerals 9, no. 5: 258. https://doi.org/10.3390/min9050258
APA StyleHuang, Y., Wu, Z., Sun, X., Wang, Y., Shi, G., Zhai, W., & Guan, Y. (2019). He-Ar Isotopes and Trace Gas Compositions of Fluid Inclusions in Massive Sulphides from the Yushui Copper-Polymetallic Deposit, South China: Metallogenic Implications. Minerals, 9(5), 258. https://doi.org/10.3390/min9050258





