First Detection of Methane within Chromitites of an Archean-Paleoproterozoic Greenstone Belt in Brazil
Abstract
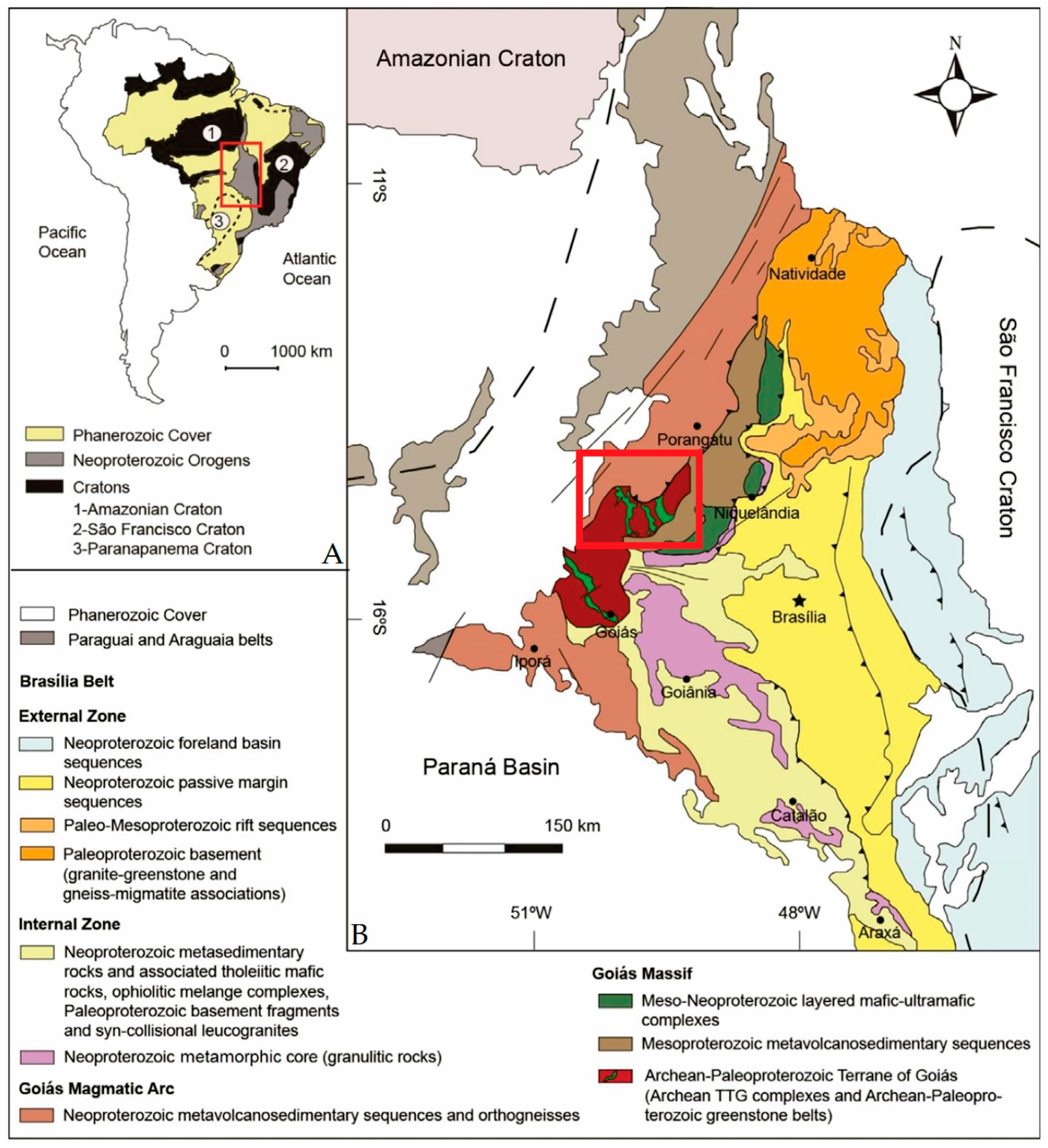
1. Introduction
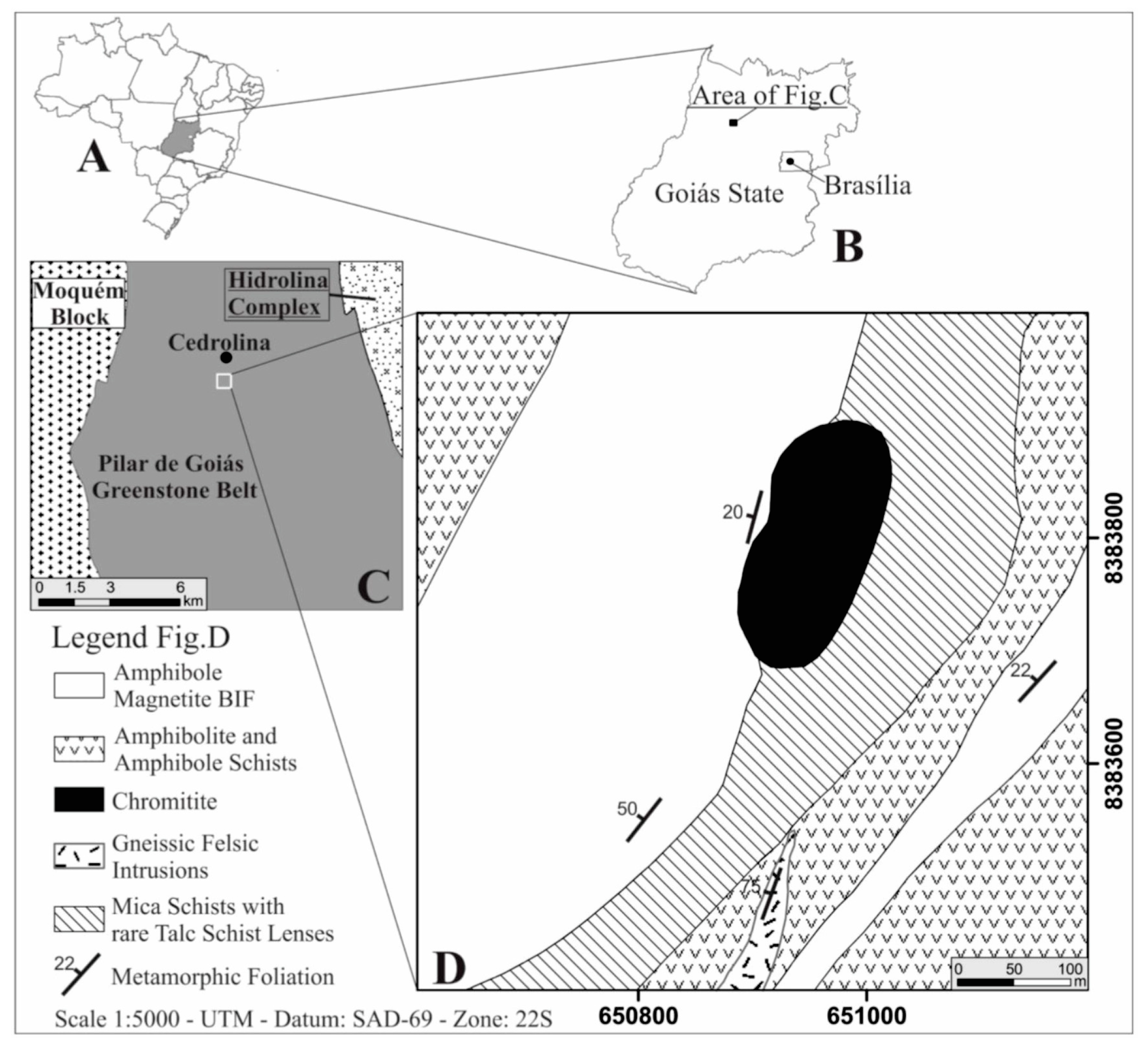
2. General Geology
The Cedrolina Chromitite
3. Materials and Methods
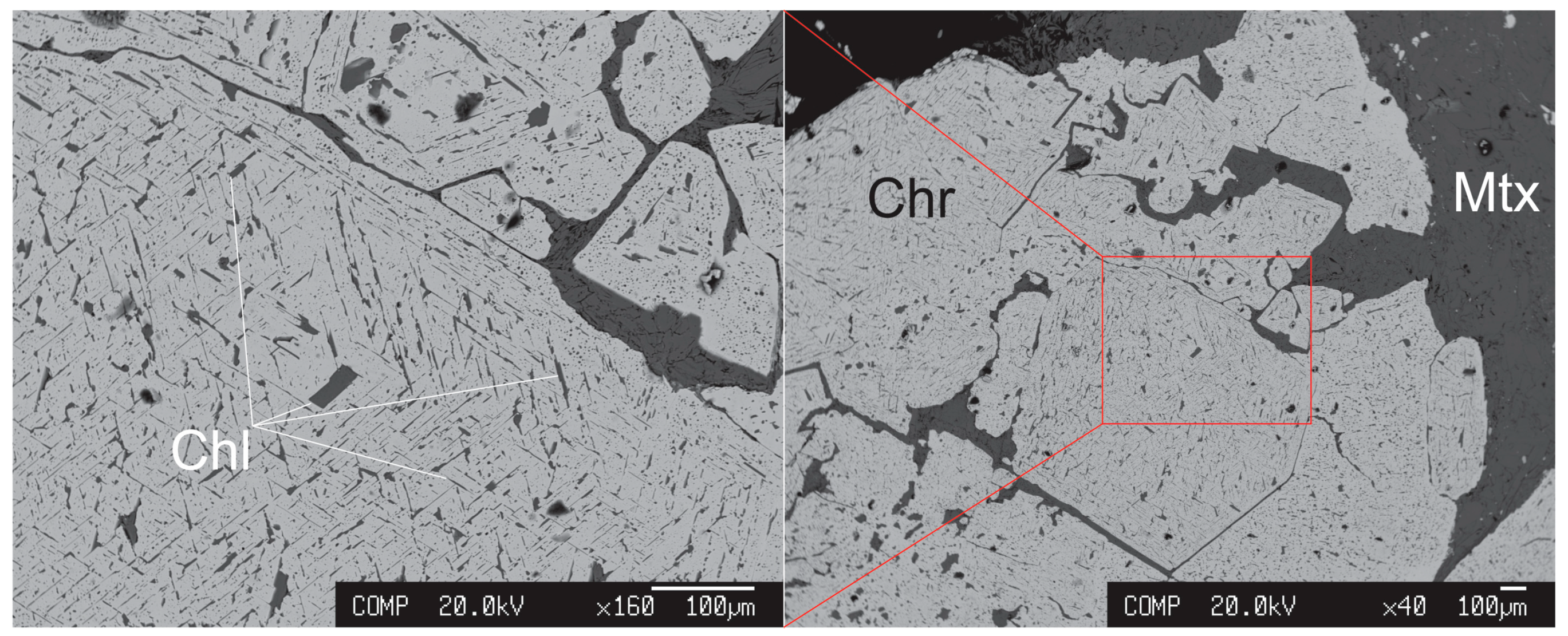
4. Results
Chlorite Microanalyses and Geothermometry
5. Discussion
Chlorite Thermometry, Metamorphism and H2 Generation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thayer, T.P. Serpentinization considered as a constant-volume metasomatic process. Am. Miner. 1966, 51, 685–710. [Google Scholar]
- Etiope, G.; Whiticar, M.J. Abiotic methane in continental ultramafic rock systems: Towards a genetic model. Appl. Geochem. 2019, 102, 139–152. [Google Scholar]
- Wang, D.T.; Reeves, E.P.; McDermott, J.M.; Seewald, J.S.; Ono, S. Clumped isotopologue constraints on the origin of methane at seafloor hot springs. Geochim. Cosmochim. Acta 2018, 223, 141–158. [Google Scholar] [CrossRef]
- McCollom, T.M.; Seewald, J.S. Abiotic synthesis of organic compounds in deep-sea hydrothermal environments. Chem. Rev. 2007, 107, 382–401. [Google Scholar] [CrossRef]
- Marques, J.M.; Etiope, G.; Neves, M.O.; Carreira, P.M.; Rocha, C.; Vance, S.D.; Christensen, L.; Miller, A.Z.; Suzuki, S. Linking serpentinization, hyperalkaline mineral waters and abiotic methane production in continental peridotites: An integrated hydrogeological-bio-geochemical model from the Cabeço de Vide CH4-rich aquifer (Portugal). Appl. Geochem. 2018, 96, 287–301. [Google Scholar]
- Cipolli, F.; Gambardella, B.; Marini, L.; Ottonello, G.; Vetuschi Zuccolini, M. Geochemistry of high-pH waters from serpentinites of the Gruppo di Voltri (Genova, Italy) and reaction path modeling of CO2 sequestration in serpentinite aquifers. Appl. Geochem. 2004, 19, 787–802. [Google Scholar] [CrossRef]
- Sherwood Lollar, B.; Westgate, T.D.; Ward, J.A.; Slater, G.F.; Lacrampe-Couloume, G. Abiogenic formation of alkanes in the Earth’s crust as a minor source for global hydrocarbon reservoirs. Nature 2002, 416, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.J.; Hall, A.J.; Martin, W. Serpentinization as a source of energy at the origin of life. Geobiology 2010, 8, 355–371. [Google Scholar] [CrossRef]
- Tobie, G.; Lunine, J.I.; Sotin, C. Episodic outgassing as the origin of atmospheric methane on Titan. Nature 2006, 440, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Atreya, S.K.; Mahaffy, P.R.; Wong, A.-S. Methane and related trace species on Mars: Origin, loss, implications for life, and habitability. Planet. Space Sci. 2007, 55, 358–369. [Google Scholar] [CrossRef]
- Oehler, D.Z.; Etiope, G. Methane Seepage on Mars: Where to Look and Why. Astrobiology 2017, 17, 1233–1264. [Google Scholar] [CrossRef] [PubMed]
- Szatmari, P. Petroleum Formation by Fischer-Tropsch Synthesis in Plate-Tectonics. Aapg Bull. Assoc. Pet. Geol. 1989, 73, 989–998. [Google Scholar]
- Szatmari, P.; da Fonseca, T.C.O.; Miekeley, N.F. Mantle-like trace element composition of petroleum—Contributions from serpentinizing peridotites. In Tectonics; Closson, D., Ed.; InTech: Rijeka, Croatia, 2011; pp. 332–358. [Google Scholar]
- Etiope, G.; Schoell, M. Abiotic gas: Atypical, but not rare. Elements 2014, 10, 291–296. [Google Scholar] [CrossRef]
- Etiope, G.; Ionescu, A. Low-temperature catalytic CO2 hydrogenation with geological quantities of ruthenium: A possible abiotic CH4 source in chromitite-rich serpentinized rocks. Geofluids 2015, 15, 438–452. [Google Scholar] [CrossRef]
- Etiope, G.; Sherwood Lollar, B. Abiotic methane on earth. Rev. Geophys. 2013, 51, 276–299. [Google Scholar] [CrossRef]
- Klein, F.; Grozeva, N.G.; Seewald, J.S.; McCollom, T.M.; Humphris, S.E.; Moskowitz, B.; Berquó, T.S.; Kahl, W.-A. Fluids in the Crust. Experimental constraints on fluid-rock reactions during incipient serpentinization of harzburgite. Am. Miner. 2015, 100, 991–1002. [Google Scholar] [CrossRef]
- McCollom, T.M.; Klein, F.; Robbins, M.; Moskowitz, B.; Berquó, T.S.; Jöns, N.; Bach, W.; Templeton, A. Temperature trends for reaction rates, hydrogen generation, and partitioning of iron during experimental serpentinization of olivine. Geochim. Cosmochim. Acta 2016, 181, 175–200. [Google Scholar] [CrossRef]
- Mayhew, L.E.; Ellison, E.T.; McCollom, T.M.; Trainor, T.P.; Templeton, A.S. Hydrogen generation from low-temperature water–rock reactions. Nat. Geosci. 2013, 6, 478–484. [Google Scholar] [CrossRef]
- Früh-Green, G.L.; Connolly, J.A.D.; Plas, A.; Kelley, D.S.; Grobéty, B. Serpentinization of Oceanic Peridotites: Implications for Geochemical cycles and Biological Activity. Subseafloor Biosph. Mid-Ocean Ridges, AGU Geophys. Monogr. Ser. 2004, 144, 119–136. [Google Scholar]
- Lazar, C.; McCollom, T.M.; Manning, C.E. Abiogenic methanogenesis during experimental komatiite serpentinization: Implications for the evolution of the early Precambrian atmosphere. Chem. Geol. 2012, 326–327, 102–112. [Google Scholar] [CrossRef]
- Etiope, G.; Ifandi, E.; Nazzari, M.; Procesi, M.; Tsikouras, B.; Ventura, G.; Steele, A.; Tardini, R.; Szatmari, P. Widespread abiotic methane in chromitites. Sci. Rep. 2018, 8, 8728. [Google Scholar] [CrossRef]
- Portella, Y.D.M.; Zaccarini, F.; Luvizotto, G.L.; Garuti, G.; Bakker, R.J.; Angeli, N.; Thalhammer, O. The Cedrolina Chromitite, Goiás State, Brazil: A Metamorphic Puzzle. Minerals 2016, 6, 91. [Google Scholar] [CrossRef]
- Pimentel, M.M.; Fuck, R.A.; Jost, H.; Ferreira Filho, C.F.; Araújo, S.M. The basement of the Brasília Fold Belt and the Goiás Magmatic Arc. In Proceedings of the Tectonic Evolution of South America, 31st International Geological Congress, Rio de Janeiro, Brazil, 6–17 August 2000; pp. 195–229. [Google Scholar]
- Jost, H.; Junior, F.C.; Fuck, R.A.; Dussin, I.A.Ô. Uvá Complex, The Oldest Orthogneisses of the Archean-Paleoproterozoic Terrane of Central Brazil. J. South Am. Earth Sci. 2013, 47, 201–212. [Google Scholar] [CrossRef]
- Borges, C.C.A.; Toledo, C.L.B.; Silva, A.M.; Chemale, F.; Jost, H.; de Carvalho Lana, C. Geochemistry and isotopic signatures of metavolcanic and metaplutonic rocks of the Faina and Serra de Santa Rita greenstone belts, Central Brazil: Evidences for a Mesoarchean intraoceanic arc. Precambrian Res. 2017, 292, 350–377. [Google Scholar] [CrossRef]
- Pimentel, M.M.; Jost, H.; Fuck, R.A. O embasamento da Faixa Brasília e o Arco Magmático de Goiás. In Geologia do Continente Sul-Americano: Evolução da obra de Fernando Fávio Marques de Almeida; Mantesso-Neto, V., Bartorelli, A., Carneiro, C.D.R., Neves, B.B.B., Eds.; Beca Produções Culturais Ltda: São Paulo, Brazil, 2004; pp. 356–368. (In Portuguese) [Google Scholar]
- Jost, H.; Fuck, R.; Brod, J.A.; Dantas, E.L.; Meneses, P.R.; Assad, M.L.; Pimentel, M.M.; Blum, M.L.B.; Silva, A.; Spigolon, A.L.D. Geologia de terrenos Arqueanos e Proterozóicos da região de Crixás-Cedrolina, Goiás. Rev. Bras. Geociên. 2001, 31, 315–328. (In Portuguese) [Google Scholar] [CrossRef]
- Ribeiro Filho, W. Reavaliação da geologia de Pilar-Mara Rosa. In Proceedings of the Anais do I Simpósio de Geologia do Centro-Oeste, Goiânia-GO, Brazil, 25–31 Outubro de 1981; pp. 281–296. (In Portuguese). [Google Scholar]
- Jost, H.; De Oliveira, A.M. Stratigraphy of the greenstone belts, Crixás region, Goiás, central Brazil. J. South Am. Earth Sci. 1991, 4, 201–214. [Google Scholar] [CrossRef]
- Jost, H.; Scandolara, J.E. Structural, Petrographic and Geochemical Characteristics of Mafic Dikes Intrusive in Metasedimentary Rocks of the Crixás Greenstone Belt, Goiás. Geol. USP, Sér. cient. 2010, 10, 119–134. (In Portuguese) [Google Scholar] [CrossRef]
- Portella, Y.D.M. Mapeamento Geológico, Caracterização Litoquímica e Potencial Econômico do Corpo de Cromitito de Cedrolina, Santa Terezinha de Goiás-GO. Unpublished Bachelor’s Thesis, São Paulo State University (UNESP), Rio Claro, Brazil, 2011. Available online: https://repositorio.unesp.br/handle/11449/120644?locale-attribute=en (accessed on 1 April 2019). (In Portuguese).
- Lacerda, H. Mapa geológico 1/100.000 do distrito mineiro do Greenstone-Belt de Crixás-Guarinos-Pilar de Goiás (GO). In Proceedings of the Anais do IV Simpósio de Geologia do Centro-Oeste, Cuiabá-MT, Brazil, 26–31 Outubro de 1997; Departamento Nacional de Produção Mineral: Goiânia, Brazil, 1997. (In Portuguese) [Google Scholar]
- Zaccarini, F.; Portella, Y.D.M.; Bakker, R.J.; Angeli, N.; Garuti, G.; Thalhammer, O.A. Electron microprobe and raman spectroscopic investigation of monazite from chromitites of Cedrolina (Goiás State, Brazil). Neues Jahrb. fur Mineral. Abhandlungen 2012, 189, 207–215. [Google Scholar] [CrossRef]
- Cathelineau, M.; Nieva, D. A chlorite solid solution geothermometer the Los Azufres (Mexico) geothermal system. Contrib. Miner. Petrol. 1985, 91, 235–244. [Google Scholar] [CrossRef]
- Kranidiotis, P.; MacLean, W.H. Systematics of chlorite alteration at the Phelps Dodge massive sulfide deposit, Matagami, Quebec. Econ. Geol. 1987, 82, 1898–1911. [Google Scholar] [CrossRef]
- Cathelineau, M. Cation site occupancy in chlorites and illites as a function of temperature. Clay Miner. 1988, 23, 471–485. [Google Scholar] [CrossRef]
- Zang, W.; Fyfe, W.S. Chloritization of the hydrothermally altered bedrock at the Igarapé Bahia gold deposit, Carajás, Brazil. Miner. Depos. 1995, 30, 30–38. [Google Scholar] [CrossRef]
- Frimmel, H.E. Chlorite Thermometry in the Witwatersrand Basin: Constraints on the Paleoproterozoic Geotherm in the Kaapvaal Craton, South Africa. J. Geol. 1997, 105, 601–616. [Google Scholar] [CrossRef]
- Zwicker, J.; Birgel, D.; Bach, W.; Richoz, S.; Smrzka, D.; Grasemann, B.; Gier, S.; Schleper, C.; Rittmann, S.K.-M.R.; Koşun, E.; et al. Evidence for archaeal methanogenesis within veins at the onshore serpentinite-hosted Chimaera seeps, Turkey. Chem. Geol. 2018, 483, 567–580. [Google Scholar] [CrossRef]
- Caritat, P.D.E.; Hutcheon, I.A.N.; Walshe, J.L. Chlorite geothermometry: A review. Clays Clay Miner. 1993, 41, 219–239. [Google Scholar] [CrossRef]
- Eckstrand, O.R. The Dumont Serpentinite: A Model for Control of Nickel-iferous Opaque Mineral Assemblages by Alteration Reactions in Ultramafic Rocks. Econ. Geol. 1975, 70, 183–201. [Google Scholar] [CrossRef]
- Young, E.D.; Kohl, I.E.; Sherwood Lollar, B.; Etiope, G.; Rumble III, D.; Li, S.; Haghnegahdar, M.A.; Schauble, E.A.; McCain, K.A.; Foustoukos, D.I.; et al. The relative abundances of resolved 12CH2D2 and 13CH3D and mechanisms controlling isotopic bond ordering in abiotic and biotic methane gases. Geochim. Cosmochim. Acta 2017, 203, 235–264. [Google Scholar] [CrossRef]


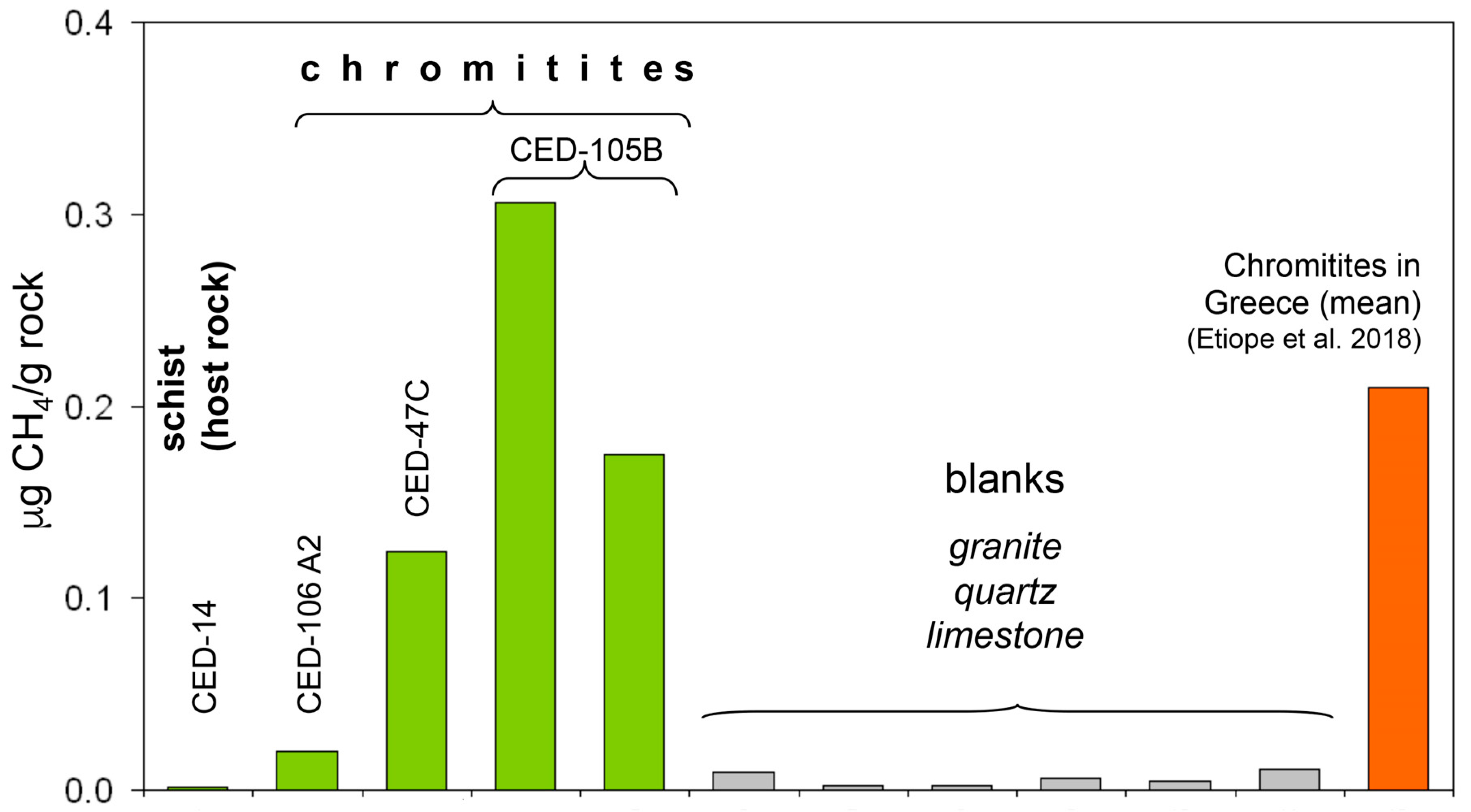
| Sample (CED) | 14 | 106A2 | 47C | 105B | 105B |
|---|---|---|---|---|---|
| Rock Type | talc schist * | chromitite | chromitite | chromitite | chromitite |
| Weathering Stage | mild | low | mild | strong | strong |
| Chromite’s Modal Content in the rock | <5% | 20–30% | 50–60% | 40–50% | 40–50% |
| Accessory Minerals | Mag | Apy; Ccp; Gn; Pn; Pyr; | Apy; Sp; Pyr; AsS; Hw; Arg; Mag | Apy; Hw; Aw | Apy; Hw; Aw |
| Weight (g) | 140 | 160 | 270 | 270 | 190 |
| CH4 ppmv | 2 | 32 | 434 | 610 | 612 |
| CH4 (µg/grock) | 0.002 | 0.020 | 0.124 | 0.175 | 0.306 |
| H2 ppmv | 5 | 12 | 2830 | >4500 | 50 |
| δ13C-CH4 (‰ VPDB) | −39.2 | −30 |
| (wt%) | Sample (CED) | SiO2 | TiO2 | Al2O3 | FeO | MnO | MgO | CaO | Na2O | K2O | Cr2O3 | NiO | Total | |||||
| Matrix | 105C.10 | 28.60 | 0.02 | 18.26 | 1.79 | 0.07 | 29.95 | 0.01 | 0.00 | 0.03 | 4.33 | 0.32 | 83.37 | |||||
| 105C.2 | 29.52 | 0.02 | 17.06 | 2.26 | 0.10 | 31.21 | 0.00 | 0.02 | 0.00 | 3.30 | 0.31 | 83.80 | ||||||
| 105C.4 | 28.62 | 0.04 | 18.87 | 2.30 | 0.05 | 30.22 | 0.01 | 0.07 | 0.04 | 3.40 | 0.38 | 83.99 | ||||||
| 105C.5 | 29.93 | 0.04 | 16.43 | 1.84 | 0.00 | 30.10 | 0.02 | 0.05 | 0.05 | 3.87 | 0.31 | 82.63 | ||||||
| 105C.7 | 29.25 | 0.03 | 18.03 | 2.54 | 0.04 | 29.40 | 0.01 | 0.08 | 0.03 | 3.96 | 0.28 | 83.65 | ||||||
| 105C.8 | 30.02 | 0.02 | 15.98 | 2.17 | 0.02 | 30.90 | 0.04 | 0.04 | 0.04 | 3.95 | 0.34 | 83.52 | ||||||
| 105C.9 | 30.26 | 0.02 | 16.29 | 2.11 | 0.04 | 31.87 | 0.00 | 0.03 | 0.03 | 3.62 | 0.32 | 84.59 | ||||||
| 106D.1 | 31.70 | 0.05 | 17.60 | 4.10 | 0.11 | 27.99 | 0.12 | 0.04 | 0.16 | 2.13 | 0.22 | 84.23 | ||||||
| 106D.10 | 31.15 | 0.07 | 17.35 | 3.78 | 0.00 | 29.79 | 0.05 | 0.03 | 0.18 | 2.10 | 0.08 | 84.56 | ||||||
| 106D.3 | 33.26 | 0.04 | 20.68 | 3.02 | 0.07 | 23.89 | 0.07 | 0.02 | 0.13 | 1.97 | 0.22 | 83.38 | ||||||
| 106D.6 | 32.10 | 0.07 | 17.92 | 3.94 | 0.01 | 29.25 | 0.14 | 0.03 | 0.19 | 1.99 | 0.22 | 85.85 | ||||||
| 106D.7 | 32.06 | 0.04 | 19.22 | 3.51 | 0.01 | 28.19 | 0.02 | 0.01 | 0.09 | 2.19 | 0.25 | 85.57 | ||||||
| 106D.8 | 29.86 | 0.01 | 20.30 | 4.02 | 0.06 | 27.43 | 0.07 | 0.01 | 0.02 | 2.47 | 0.22 | 84.48 | ||||||
| Included in Chr | 105Cin2 | 29.81 | 0.02 | 17.35 | 1.81 | 0.01 | 29.51 | 0.02 | 0.08 | 0.04 | 4.43 | 0.31 | 83.38 | |||||
| 105Cin3 | 31.80 | 0.02 | 16.58 | 1.62 | 0.01 | 30.73 | 0.05 | 0.01 | 0.03 | 3.83 | 0.30 | 84.95 | ||||||
| 105Cin4 | 30.67 | 0.04 | 17.66 | 1.76 | 0.00 | 30.22 | 0.01 | 0.05 | 0.07 | 4.05 | 0.30 | 84.83 | ||||||
| 105Cin5 | 29.63 | 0.03 | 16.89 | 1.57 | 0.02 | 30.60 | 0.04 | 0.04 | 0.02 | 4.08 | 0.30 | 83.22 | ||||||
| 105Cin6 | 30.54 | 0.01 | 16.71 | 1.53 | 0.05 | 31.49 | 0.01 | 0.01 | 0.01 | 3.88 | 0.31 | 84.57 | ||||||
| 105Cin8 | 28.65 | 0.04 | 18.87 | 1.92 | 0.08 | 30.02 | 0.06 | 0.03 | 0.02 | 3.20 | 0.37 | 83.26 | ||||||
| 105Cin9 | 31.04 | 0.04 | 17.63 | 1.63 | 0.05 | 31.17 | 0.02 | 0.00 | 0.03 | 3.88 | 0.38 | 85.86 | ||||||
| 106A1in1 | 29.63 | 0.05 | 19.69 | 6.97 | 0.03 | 28.71 | 0.04 | 0.00 | 0.06 | 1.90 | 0.18 | 87.26 | ||||||
| 106Din1 | 30.76 | 0.03 | 19.22 | 3.91 | 0.12 | 30.07 | 0.00 | 0.02 | 0.11 | 3.02 | 0.19 | 87.44 | ||||||
| 106Din2 | 29.92 | 0.04 | 17.83 | 4.03 | 0.09 | 31.13 | 0.04 | 0.07 | 0.04 | 2.92 | 0.13 | 86.24 | ||||||
| 106Din4 | 30.56 | 0.04 | 17.31 | 3.43 | 0.04 | 28.09 | 0.21 | 0.13 | 0.04 | 2.68 | 0.21 | 82.72 | ||||||
| Calculated T (°C) | ||||||||||||||||||
| (at%) | Si | Ti | Al(IV) | Al(VI) | Fe | Mn | Mg | Ca | Na | K | Cr | Ni | Fe# | 1 | 2 | 3 | 4 | |
| Matrix | 105C.10 | 5.65 | 0.00 | 2.35 | 1.91 | 0.30 | 0.01 | 8.83 | 0.00 | 0.00 | 0.01 | 0.68 | 0.10 | 0.03 | 267 | 316 | 269 | 295 |
| 105C.2 | 5.80 | 0.00 | 2.20 | 1.76 | 0.37 | 0.02 | 9.15 | 0.00 | 0.01 | 0.00 | 0.51 | 0.10 | 0.04 | 251 | 292 | 254 | 279 | |
| 105C.4 | 5.62 | 0.01 | 2.38 | 1.98 | 0.38 | 0.01 | 8.84 | 0.00 | 0.03 | 0.01 | 0.53 | 0.12 | 0.04 | 271 | 322 | 274 | 299 | |
| 105C.5 | 5.95 | 0.01 | 2.05 | 1.80 | 0.31 | 0.00 | 8.92 | 0.00 | 0.02 | 0.01 | 0.61 | 0.10 | 0.03 | 235 | 268 | 238 | 264 | |
| 105C.7 | 5.77 | 0.00 | 2.23 | 1.97 | 0.42 | 0.01 | 8.65 | 0.00 | 0.03 | 0.01 | 0.62 | 0.09 | 0.05 | 254 | 297 | 258 | 281 | |
| 105C.8 | 5.93 | 0.00 | 2.07 | 1.64 | 0.36 | 0.00 | 9.10 | 0.01 | 0.02 | 0.01 | 0.62 | 0.11 | 0.04 | 238 | 272 | 241 | 266 | |
| 105C.9 | 5.89 | 0.00 | 2.11 | 1.63 | 0.34 | 0.01 | 9.25 | 0.00 | 0.01 | 0.01 | 0.56 | 0.10 | 0.04 | 241 | 277 | 244 | 270 | |
| 106D.1 | 6.20 | 0.01 | 1.80 | 2.26 | 0.67 | 0.02 | 8.16 | 0.03 | 0.02 | 0.04 | 0.33 | 0.07 | 0.08 | 209 | 228 | 215 | 233 | |
| 106D.10 | 6.08 | 0.01 | 1.92 | 2.07 | 0.62 | 0.00 | 8.67 | 0.01 | 0.01 | 0.04 | 0.32 | 0.02 | 0.07 | 222 | 248 | 227 | 247 | |
| 106D.3 | 6.45 | 0.01 | 1.55 | 3.18 | 0.49 | 0.01 | 6.91 | 0.01 | 0.01 | 0.03 | 0.30 | 0.07 | 0.07 | 182 | 188 | 187 | 208 | |
| 106D.6 | 6.15 | 0.01 | 1.85 | 2.20 | 0.63 | 0.00 | 8.36 | 0.03 | 0.01 | 0.05 | 0.30 | 0.07 | 0.07 | 214 | 235 | 219 | 239 | |
| 106D.7 | 6.13 | 0.01 | 1.87 | 2.46 | 0.56 | 0.00 | 8.04 | 0.00 | 0.00 | 0.02 | 0.33 | 0.08 | 0.07 | 216 | 239 | 221 | 242 | |
| 106D.8 | 5.83 | 0.00 | 2.17 | 2.50 | 0.66 | 0.01 | 7.98 | 0.01 | 0.00 | 0.00 | 0.38 | 0.07 | 0.08 | 248 | 288 | 254 | 273 | |
| Included in Chr | 105Cin2 | 5.80 | 0.00 | 2.20 | 1.76 | 0.37 | 0.02 | 9.15 | 0.00 | 0.01 | 0.00 | 0.51 | 0.10 | 0.04 | 251 | 292 | 254 | 279 |
| 105Cin3 | 6.12 | 0.00 | 1.88 | 1.88 | 0.26 | 0.00 | 8.82 | 0.01 | 0.00 | 0.01 | 0.58 | 0.09 | 0.03 | 217 | 240 | 219 | 246 | |
| 105Cin4 | 5.93 | 0.01 | 2.07 | 1.96 | 0.28 | 0.00 | 8.71 | 0.00 | 0.02 | 0.02 | 0.62 | 0.09 | 0.03 | 237 | 271 | 240 | 266 | |
| 105Cin5 | 5.85 | 0.00 | 2.15 | 1.78 | 0.26 | 0.00 | 9.01 | 0.01 | 0.02 | 0.01 | 0.64 | 0.09 | 0.03 | 246 | 284 | 248 | 275 | |
| 105Cin6 | 5.93 | 0.00 | 2.07 | 1.75 | 0.25 | 0.01 | 9.11 | 0.00 | 0.00 | 0.00 | 0.60 | 0.10 | 0.03 | 238 | 272 | 240 | 267 | |
| 105Cin8 | 5.65 | 0.01 | 2.35 | 2.04 | 0.32 | 0.01 | 8.83 | 0.01 | 0.01 | 0.00 | 0.50 | 0.12 | 0.03 | 267 | 316 | 269 | 295 | |
| 105Cin9 | 5.92 | 0.01 | 2.08 | 1.89 | 0.26 | 0.01 | 8.87 | 0.00 | 0.00 | 0.01 | 0.58 | 0.12 | 0.03 | 238 | 272 | 240 | 267 | |
| 106A1in1 | 5.70 | 0.01 | 2.30 | 2.16 | 1.12 | 0.00 | 8.23 | 0.01 | 0.00 | 0.01 | 0.29 | 0.05 | 0.12 | 262 | 308 | 271 | 282 | |
| 106Din1 | 5.82 | 0.00 | 2.18 | 2.11 | 0.62 | 0.02 | 8.49 | 0.00 | 0.01 | 0.03 | 0.45 | 0.06 | 0.07 | 248 | 288 | 254 | 274 | |
| 106Din2 | 5.77 | 0.01 | 2.23 | 1.82 | 0.65 | 0.01 | 8.95 | 0.01 | 0.03 | 0.01 | 0.44 | 0.04 | 0.07 | 254 | 297 | 259 | 280 | |
| 106Din4 | 6.09 | 0.01 | 1.91 | 2.15 | 0.57 | 0.01 | 8.34 | 0.05 | 0.05 | 0.01 | 0.42 | 0.07 | 0.06 | 221 | 246 | 226 | 247 | |
| Mean | 238 | 273 | 242 | 266 | ||||||||||||||
| Median | 240 | 275 | 242 | 268 | ||||||||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portella, Y.d.M.; Zaccarini, F.; Etiope, G. First Detection of Methane within Chromitites of an Archean-Paleoproterozoic Greenstone Belt in Brazil. Minerals 2019, 9, 256. https://doi.org/10.3390/min9050256
Portella YdM, Zaccarini F, Etiope G. First Detection of Methane within Chromitites of an Archean-Paleoproterozoic Greenstone Belt in Brazil. Minerals. 2019; 9(5):256. https://doi.org/10.3390/min9050256
Chicago/Turabian StylePortella, Yuri de Melo, Federica Zaccarini, and Giuseppe Etiope. 2019. "First Detection of Methane within Chromitites of an Archean-Paleoproterozoic Greenstone Belt in Brazil" Minerals 9, no. 5: 256. https://doi.org/10.3390/min9050256
APA StylePortella, Y. d. M., Zaccarini, F., & Etiope, G. (2019). First Detection of Methane within Chromitites of an Archean-Paleoproterozoic Greenstone Belt in Brazil. Minerals, 9(5), 256. https://doi.org/10.3390/min9050256





