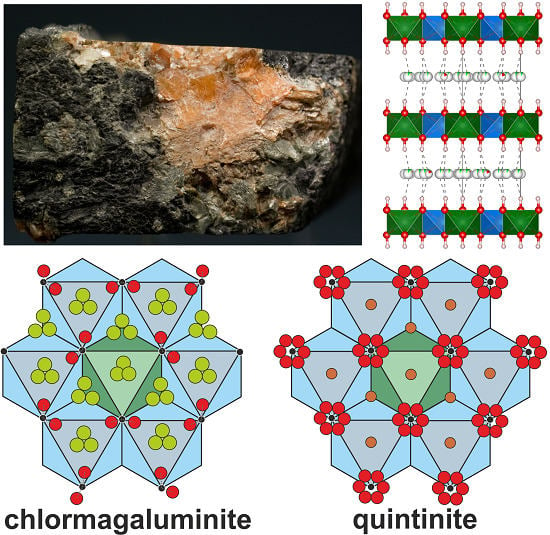
Crystal Chemistry of Chlormagaluminite, Mg4Al2(OH)12Cl2(H2O)2, a Natural Layered Double Hydroxide
Abstract
:1. Introduction
Previous Crystal Structure Determinations
2. Materials and Methods
2.1. Sample Description
2.2. Chemical Composition
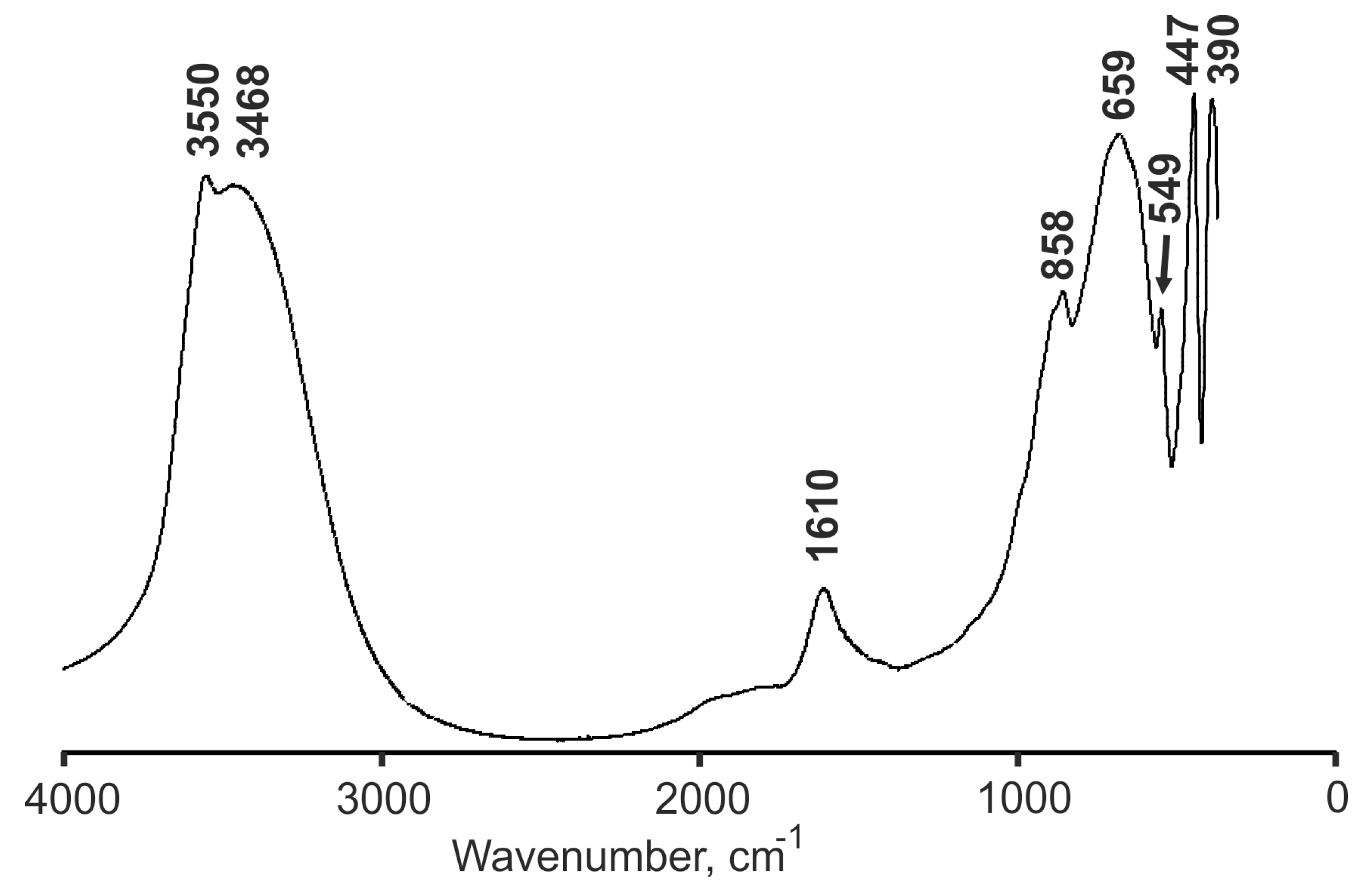
2.3. Infrared Spectroscopy (IR)
2.4. Single-Crystal X-ray Diffraction
2.5. Powder X-ray Diffraction
3. Results
3.1. Chemical Composition
3.2. Infrared Spectroscopy (IR)
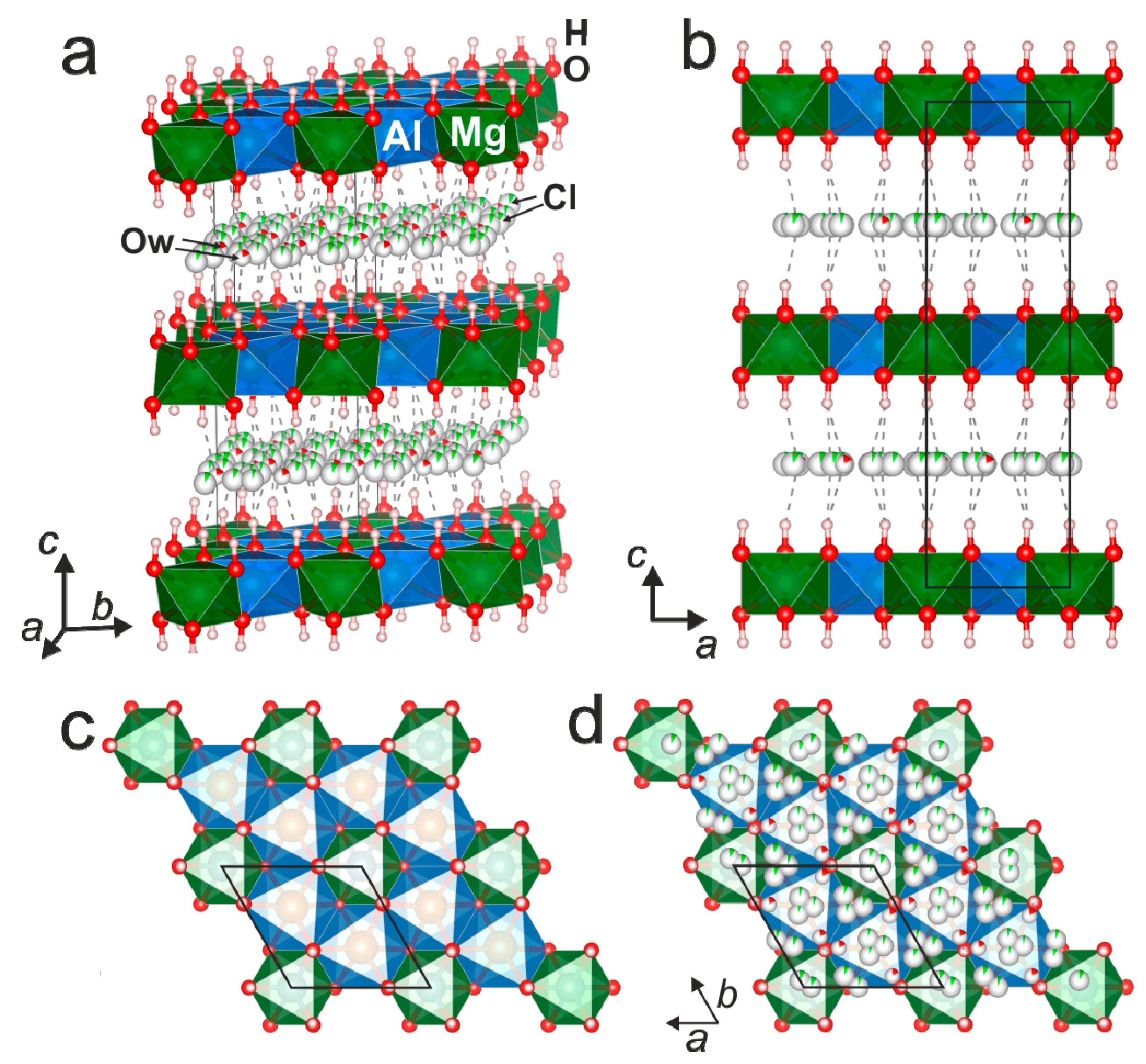
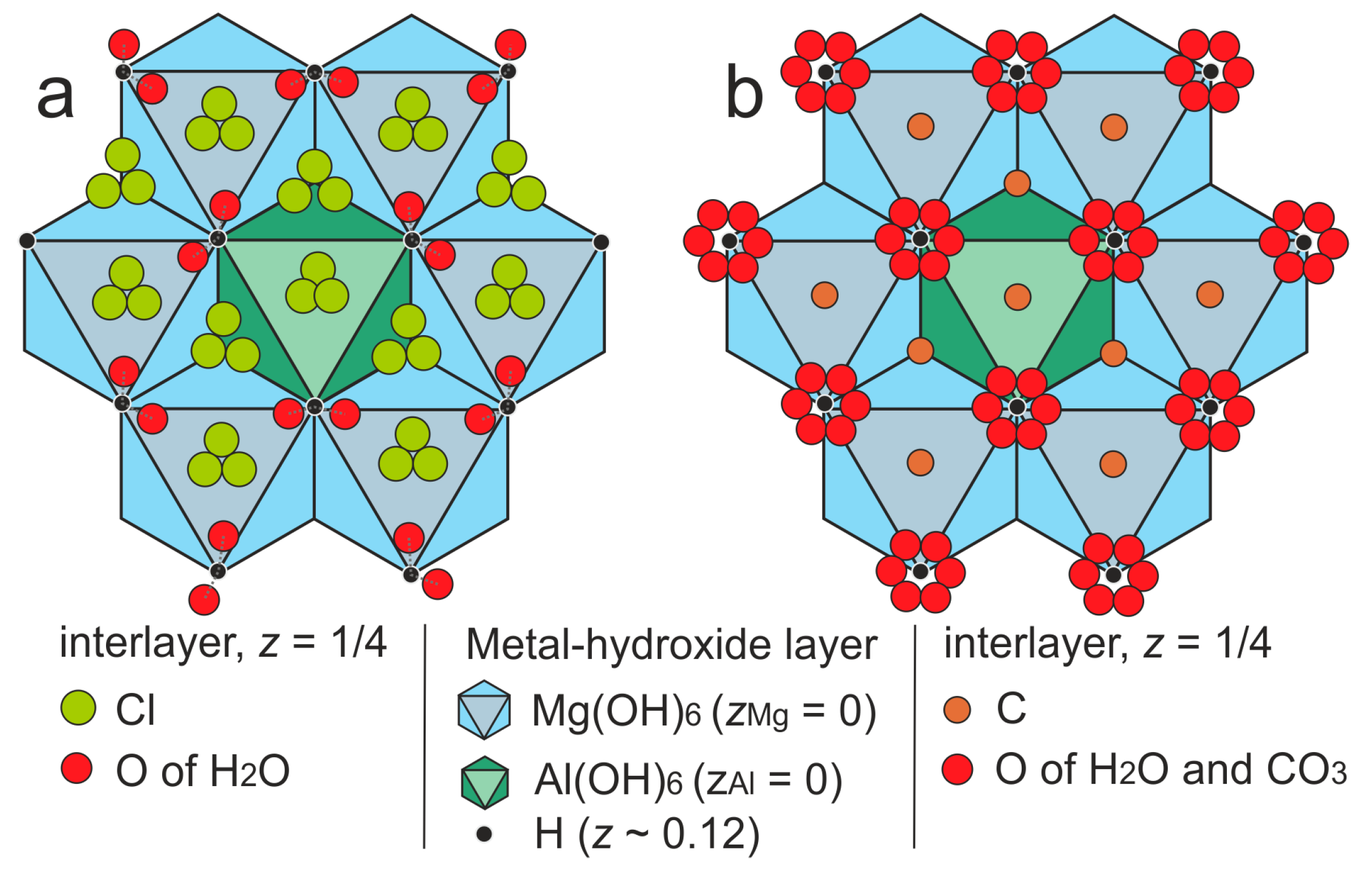
3.3. Single-Crystal X-ray Diffraction
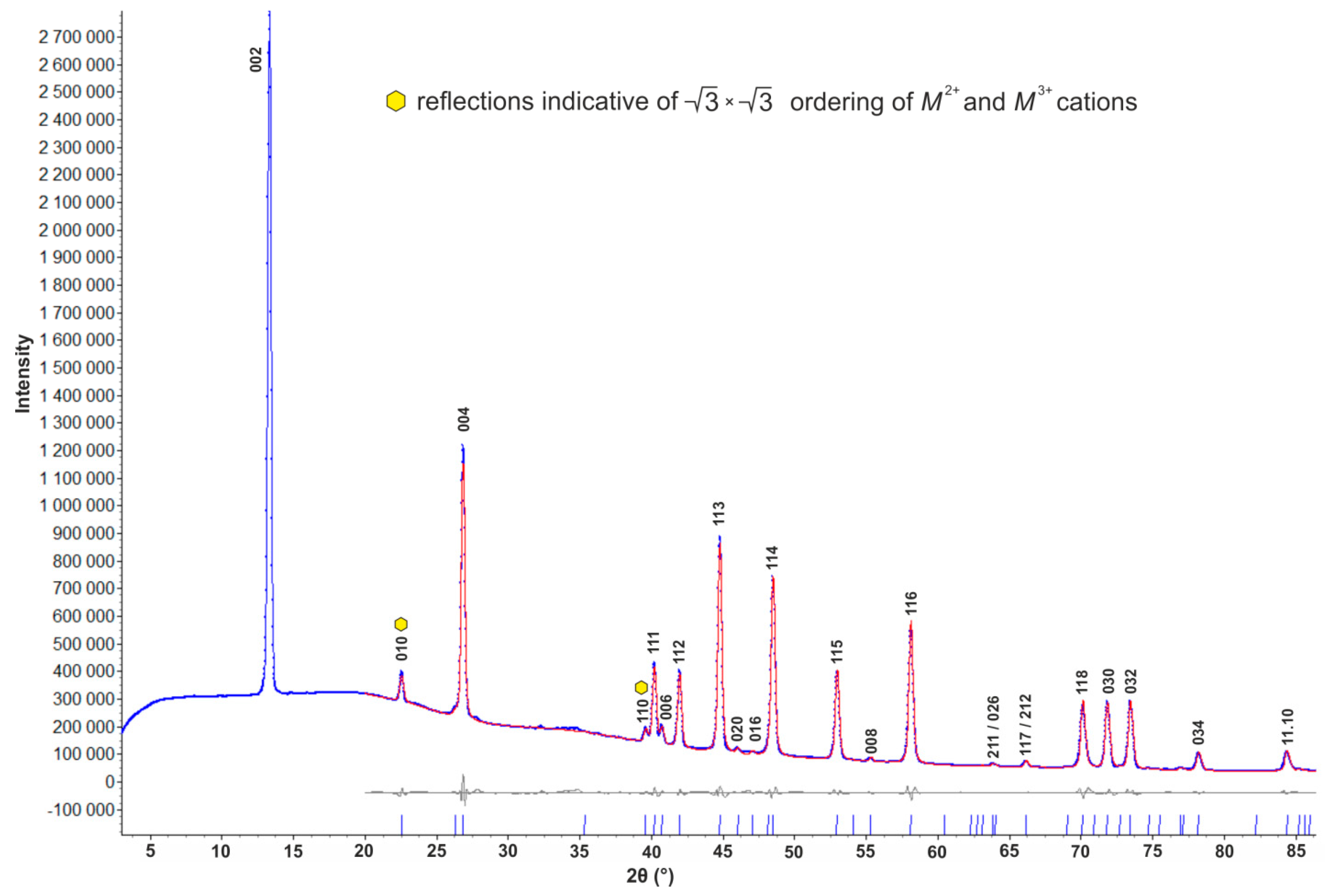
3.4. Powder X-ray Diffraction
4. Discussion
4.1. Chemical Composition
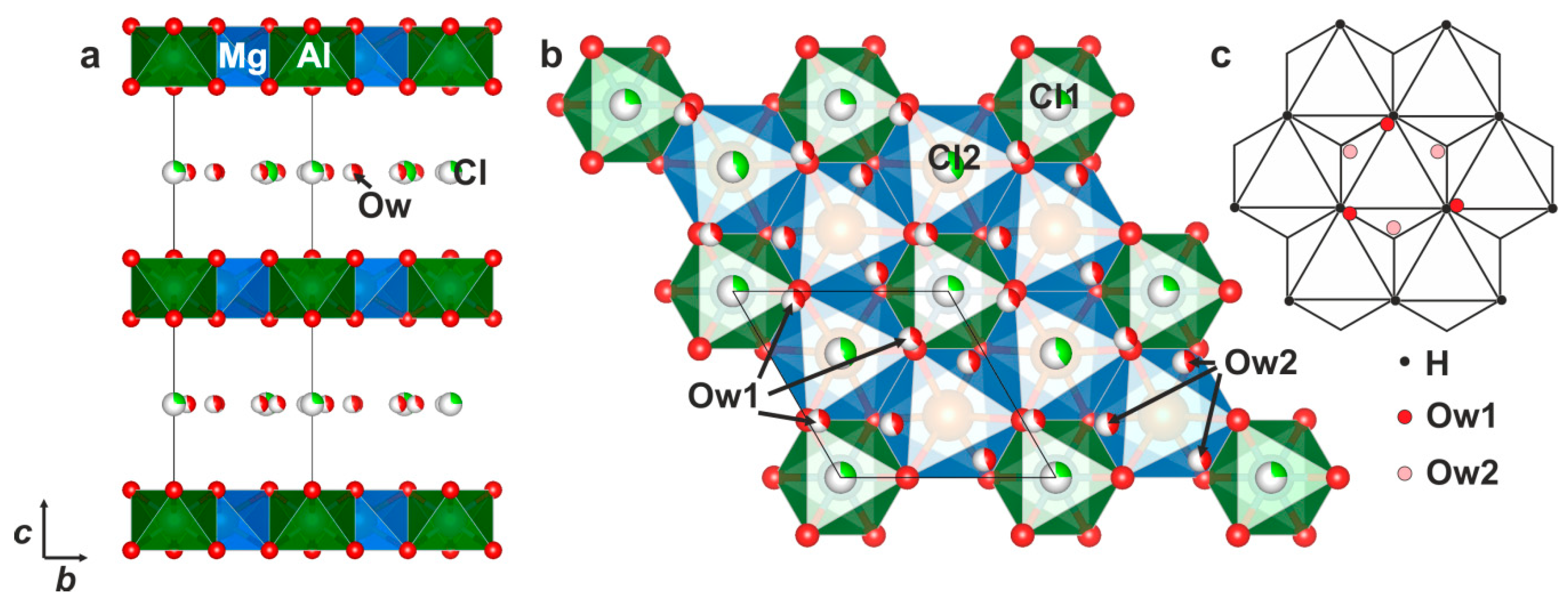
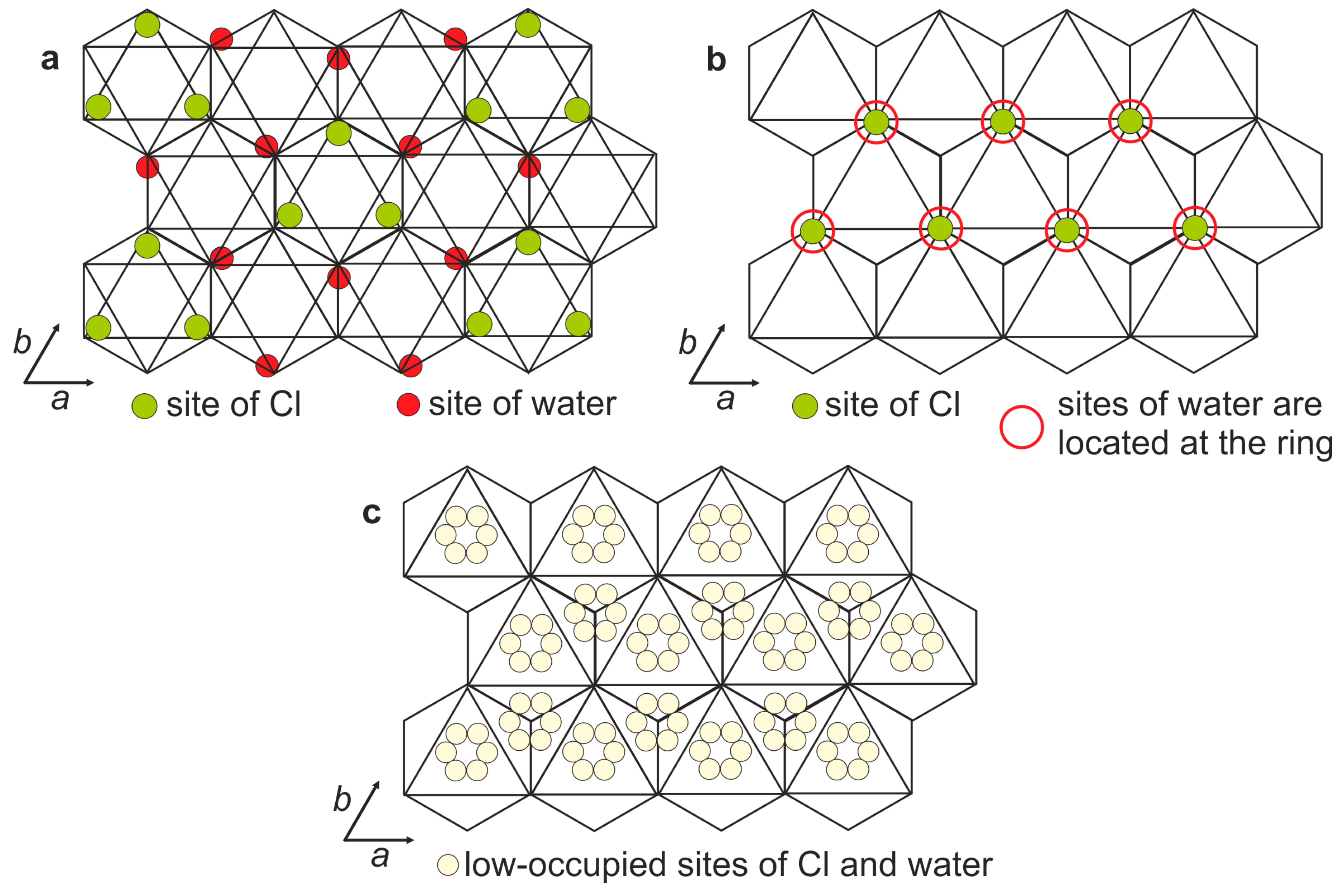
4.2. X-ray Crystallography and Crystal Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mills, S.J.; Christy, A.G.; Génin, J.-M.R.; Kameda, T.; Colombo, F. Nomenclature of the hydrotalcite supergroup: Natural layered double hydroxides. Mineral. Mag. 2012, 76, 1289–1336. [Google Scholar] [CrossRef]
- Aşçı, Y.S. Removal of textile dye mixtures by using modified Mg-Al-Cl layered double hydroxide (LDH). J. Dispers. Sci. Technol. 2017, 38, 923–929. [Google Scholar] [CrossRef]
- Hongo, T.; Wakasa, H.; Yamazaki, A. Synthesis and adsorption properties of nanosized Mg-Al layered double hydroxides with Cl−, NO3− or SO42− as interlayer anion. Mater. Sci. Pol. 2011, 29, 86–91. [Google Scholar] [CrossRef]
- Huang, P.-P.; Cao, C.-Y.; Wei, F.; Sun, Y.-B.; Song, W.-G. MgAl layered double hydroxides with chloride and carbonate ions as interlayer anions for removal of arsenic and fluoride ions in water. RSC Adv. 2015, 5, 10412–10417. [Google Scholar] [CrossRef]
- Islam, M.; Patel, R. Synthesis and physicochemical characterization of Zn/Al chloride layered double hydroxide and evaluation of its nitrate removal efficiency. Desalination 2010, 256, 120–128. [Google Scholar] [CrossRef]
- Mahjoubi, F.Z.; Khalidi, A.; Cherkaoui, O.; Elmoubarki, R.; Abdennouri, M.; Barka, N. Treatment of textile effluents by chloride-intercalated Zn-, Mg- and Ni-Al layered double hydroxides. J. Water Reuse Desalin. 2017, 7, 307–318. [Google Scholar] [CrossRef]
- Yue, X.; Liu, W.; Chen, Z.; Lin, Z. Simultaneous removal of Cu(II) and Cr(VI) by Mg-Al-Cl layered double hydroxide and mechanism insight. J. Environ. Sci. 2017, 53, 16–26. [Google Scholar] [CrossRef]
- Curtius, H.; Kattilparampil, Z. Sorption of iodine on Mg-Al-layered double hydroxide. Clay Miner. 2005, 40, 455–461. [Google Scholar] [CrossRef]
- Curtius, H.; Kaiser, G.; Rozov, K.; Neumann, A.; Dardenne, K.; Bosbach, D. Preparation and characterization of Fe-, Co-, and Ni-containing Mg-Al-Layered double hydroxides. Clays Clay Miner. 2013, 61, 424–439. [Google Scholar] [CrossRef]
- Curtius, H.; Ufer, K. Eu incorporation behavior of a Mg-Al-Cl layered double hydroxide. Clays Clay Miner. 2007, 55, 354–360. [Google Scholar] [CrossRef]
- Shan, D.; Cosnier, S.; Mousty, C. Layered double hydroxides: An attractive material for electrochemical biosensor design. J. Anal. Chem. 2003, 75, 3872–3879. [Google Scholar] [CrossRef]
- Polese, D.; Mattoccia, A.; Giorgi, F.; Pazzini, L.; Ferronea, A.; Di Giamberardino, L.; Maiolo, L.; Pecora, A.; Convertino, A.; Fortunato, G.; et al. Layered double hydroxides intercalated with chlorine used as low temperature gas sensors. Procedia Eng. 2015, 120, 1175–1178. [Google Scholar] [CrossRef]
- Cunha, V.R.; de Souza, R.B.; da Fonseca Martins, A.M.; Koh, I.H.; Constantino, V.R. Accessing the biocompatibility of layered double hydroxide by intramuscular implantation: Histological and microcirculation evaluation. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Conterosito, E.; Gianotti, V.; Palin, L.; Boccaleri, E.; Viterbo, D.; Milanesio, M. Facile preparation methods of hydrotalcite layered materials and their structural characterization by combined techniques. Inorg. Chim. Acta 2018, 470, 36–50. [Google Scholar] [CrossRef]
- Hu, G.; Wang, N.; O’Hare, D.; Davisa, J. Synthesis of magnesium aluminium layered double hydroxides in reverse microemulsions. J. Mater. Chem. 2017, 17, 2257–2266. [Google Scholar] [CrossRef]
- Isupov, V.P.; Chupakhina, L.E.; Mitrofanova, R.P. Mechanochemical Synthesis of Double Hydroxides. J. Mater. Synth. Process. 2000, 8, 251–253. [Google Scholar] [CrossRef]
- Oestreicher, V.; Jobbágy, M. One pot synthesis of Mg2Al(OH)6Cl·1.5H2O layered double hydroxides: The epoxide route. Langmuir 2013, 29, 12104–12109. [Google Scholar] [CrossRef]
- Poonoosamy, J.; Brandt, F.; Stekiel, M.; Kegler, P.; Klinkenberg, M.; Winkler, B.; Vinograd, V.; Bosbach, D.; Deissmann, G. Zr-containing layered double hydroxides: Synthesis, characterization and evaluation of thermodynamic properties. Appl. Clay Sci. 2018, 151, 54–65. [Google Scholar] [CrossRef]
- Xu, Z.P.; Lu, G.Q. Hydrothermal synthesis of layered double hydroxides (LDHs) from mixed MgO and Al2O3: LDH formation mechanism. Chem. Mater. 2005, 17, 1055–1062. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, L.; Li, D.; Zhao, J.; Hou, W. Synthesis of Mg2Al-Cl layered double hydroxide nanosheets in a surfactant-free reverse microemulsion. Colloid Polym. Sci. 2013, 291, 2515–2521. [Google Scholar] [CrossRef]
- Feoktistov, G.D.; Ivanov, S.I.; Kashaev, A.A.; Klyuchnskii, L.N.; Taskina, N.G.; Ushchapovskaya, Z.F. Occurrence of chlormanasseite in the USSR. Int. Geol. Rev. 1979, 21, 1229–1232. [Google Scholar] [CrossRef]
- Kashaev, A.A.; Feoktistov, G.D.; Petrova, S.V. Chlormagaluminite, (Mg,Fe2+)4Al2(OH)12(Cl,1/2CO3)2(H2O)2, a new mineral of the manasseite-sjögrenite group. Zapiski VMO (Proc. Soviet Miner. Soc.) 1982, 111, 121–127. (In Russian) [Google Scholar]
- Kashaev, A.A.; Feoktistov, G.D.; Petrova, S.V. Chlormagaluminite, (Mg,Fe2+)4Al2(OH)12(Cl,1/2CO3)2(H2O)2, a new mineral of the manasseite-sjögrenite group. Int. Geol. Rev. 1983, 25, 848–853. [Google Scholar] [CrossRef]
- Kashaev, A.A.; Kanashenok, S.V.; Rozhdestevenskaya, I.V. Structure Refinement of Chlormagaluminite. Crystal Chemistry and X-ray Diffraction of Minerals; Frank-Kamenetsky, V.A., Ed.; Nauka: Leningrad (USSR), Russia, 1987; pp. 101–105. (In Russian) [Google Scholar]
- Ennadi, A.; Legrouri, A.; De Roy, A.; Besse, J.P. X-Ray diffraction pattern simulation for thermally treated [Zn-Al-Cl] layered double hydroxide. Solid State Chem. 2000, 152, 568–572. [Google Scholar] [CrossRef]
- Lombardo, G.M.; Pappalardo, G.C.; Punzo, F.; Costantino, F.; Costantino, U.; Sisani, M. A novel integrated X-ray powder diffraction (XRPD) and molecular dynamics (MD) approach for modeling Mixed-Metal (Zn, Al) layered double hydroxides (LDHs). Eur. J. Inorg. Chem. 2005, 2005, 5026–5034. [Google Scholar] [CrossRef]
- Bruker AXS. APEX2; Version 2014.11-0; Bruker AXS: Madison, WI, USA, 2014. [Google Scholar]
- CrysAlis, PRO; Agilent Technologies Ltd.: Yarnton, UK, 2014.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Krivovichev, S.V.; Pekov, I.V.; Greenwell, H.C. Crystal chemistry of natural layered double hydroxides. 5. Single-crystal structure refinement of hydrotalcite, [Mg6Al2(OH)16](CO3)(H2O)4. Mineral. Mag. 2019. [Google Scholar] [CrossRef]
- Britvin, S.N.; Dolivo-Dobrovolsky, D.V.; Krzhizhanovskaya, M.G. Software for processing of X-ray powder diffraction data obtained from the curved image plate detector of Rigaku RAXIS Rapid II diffractometer. Zapiski RMO (Proc. Russian Miner. Soc.) 2017, 146, 104–107. (In Russian) [Google Scholar]
- Bruker AXS. TopasV4.2: General Profile and Structure Analysis Software for Powder Diffraction Data; Bruker AXS: Karlsruhe, Germany, 2009. [Google Scholar]
- Zhitova, E.S.; Krivovichev, S.V.; Yakovenchuk, V.N.; Ivanyuk, G.Y.; Pakhomovsky, Y.A.; Mikhailova, J.A. Crystal chemistry of natural layered double hydroxides: 4. Crystal structures and evolution of structural complexity of quintinite polytypes from the Kovdor alkaline-ultrabasic massif, Kola peninsula, Russia. Mineral. Mag. 2018, 82, 329–346. [Google Scholar] [CrossRef]
- Theiss, F.; López, A.; Frost, R.L.; Scholz, R. Spectroscopic characterisation of the LDH mineral quintinite Mg4Al2(OH)12CO3∙3H2O. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 150, 758–764. [Google Scholar] [CrossRef]
- Speck, A. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Pekov, I.V.; Chukanov, N.V.; Yapaskurt, V.O.; Bocharov, V.N. Minerals of the stichtite-pyroaurite-iowaite-woodallite system from serpentinites of Terektinsky range, Altay Mountains, Russia. Russ. Geol. Geoph. 2019, in press. [Google Scholar]
- Braithwaite, R.S.W.; Dunn, P.J.; Pritchard, R.G.; Paar, W.H. Iowaite, a re-investigation. Mineral. Mag. 1994, 58, 79–85. [Google Scholar] [CrossRef]
- Kohls, D.W.; Rodda, J.L. Iowaite, a new hydrous magnesium hydroxide-ferric oxychloride from the Precambrian of Iowa. Am. Mineral. 1967, 52, 1261–1271. [Google Scholar]
- Grguric, B.A.; Madsen, I.C.; Pring, A. Woodallite, a new chromium analog of iowaite from the Mount Keith nickel deposit, Western Australia. Mineral. Mag. 2001, 65, 427–435. [Google Scholar] [CrossRef]
- Mikhailenko, D.S.; Korsakov, A.V.; Rashchenko, S.V.; Seryotkin, Y.V.; Belakovskiy, D.I.; Golovin, A.V. Kuliginite, a new hydroxychloride mineral from the Udachnaya kimberlite pipe, Yakutia: Implications for low-temperature hydrothermal alteration of the kimberlites. Am. Mineral. 2018, 103, 1435–1444. [Google Scholar] [CrossRef]
- Mazurov, M.P.; Korneva, T.A.; Zhitova, L.M.; Istomin, V.E.; Palchik, N.A.; Stopovskaya, V.N.; Titov, A.T. Iowaite from Korshunovskoe deposit (the Siberian platform). Zapiski RMO (Proc. Russian Miner. Soc.) 2000, 129, 80–85. [Google Scholar]
- Evseev, A.A. Siberia’s Crystals and Symmetry in the Distribution of Occurrences of Minerals. World Stones 1973, 1, 11–20. [Google Scholar]
- Melchiorre, E.B.; Bottrill, R.; Huss, G.R.; Lopez, A. Conditions of stichtite (Mg6Cr2(OH)16[CO3]·4H2O) formation and its geochemical and isotope record of early phanerozoic serpentinizing environments. Geochim. Cosmochim. Acta 2017, 197, 43–61. [Google Scholar] [CrossRef]
- Guinier, A.; Bokij, G.B.; Boll-Dornberger, K.; Cowley, J.M.; Durovic, S.; Jagodzinski, H.; Krishna, P.; DeWolff, P.M.; Zvyagin, B.B.; Cox, D.E.; et al. Nomenclature of polytype structures. Report of the International Union of Crystallography ad-hoc committee on the Nomenclature of disordered, modulated and polytype structures. Acta Crystallogr. 1984, A40, 399–404. [Google Scholar] [CrossRef]
- Bookin, A.S.; Drits, V.A. Polytype diversity of the hydrotalcite-like minerals. I. Possible polytypes and their diffraction patterns. Clays Clay Miner. 1993, 41, 551–557. [Google Scholar] [CrossRef]
- Britto, S.; Kamath, P.V. Structure of bayerite-based lithium-aluminum layered double hydroxides (LDHs): Observation of monoclinic symmetry. Inorg. Chem. 2009, 48, 11646–11654. [Google Scholar] [CrossRef]
- Britto, S.; Kamath, P.V. Polytypism in the lithium-aluminum layered double hydroxides: The [LiAl2(OH)6]+ layer as a structural synthon. Inorg. Chem. 2011, 50, 5619–5627. [Google Scholar] [CrossRef] [PubMed]
- Britto, S.; Thomas, G.S.; Kamath, P.V.; Kannan, S. Polymorphism and structural disorder in the carbonate containing layered double hydroxide of Li with Al. J. Phys. Chem. 2008, 112, 9510–9515. [Google Scholar] [CrossRef]
- Sissoko, I.; Iyagba, E.T.; Sahai, I.; Biloen, P. Anion intercalation and exchange in Al(OH)3-derived compounds. Solid State Chem. 1985, 60, 283–288. [Google Scholar] [CrossRef]
- Allmann, R. The crystal structure of pyroaurite. Acta Crystallogr. 1968, B24, 972–977. [Google Scholar] [CrossRef]
- Ingram, L.; Taylor, H.F.W. The crystal structures of sjoegrenite and pyroaurite. Mineral. Mag. 1967, 36, 465–479. [Google Scholar]
- Zhitova, E.S.; Ivanyuk, G.Y.; Krivovichev, S.V.; Yakovenchuk, V.N.; Pakhomovsky, Y.A.; Mikhailova, Y.A. Crystal Chemistry of Pyroaurite from the Kovdor Pluton, Kola Peninsula, Russia, and the Långban Fe-Mn deposit, Värmland, Sweden. Geol. Ore Deposits. 2017, 59, 652–661. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Yakovenchuk, V.N.; Zhitova, E.S.; Zolotarev, A.A.; Pakhomovsky, Y.A.; Ivanyuk, G.Y. Crystal chemistry of natural layered double hydroxides. 1. Quintinite-2H-3c from the Kovdor alkaline massif, Kola peninsula, Russia. Mineral. Mag. 2010, 74, 821–832. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Yakovenchuk, V.N.; Zhitova, E.S.; Zolotarev, A.A.; Pakhomovsky, Y.A.; Ivanyuk, G.Y. Crystal chemistry of natural layered double hydroxides. 2. Quintinite-1M: First evidence of a monoclinic polytype in M2+-M3+ layered double hydroxides. Mineral. Mag. 2010, 74, 833–840. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Yakovenchuk, V.N.; Zhitova, E.S. Natural double layered hydroxides: Structure, chemistry, and information storage capacity. In Minerals as Advanced Materials II; Krivovichev, S.V., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 87–102. [Google Scholar]
- Zhitova, E.S.; Yakovenchuk, V.N.; Krivovichev, S.V.; Zolotarev, A.A.; Pakhomovsky, Y.A.; Ivanyuk, G.Y. Crystal chemistry of natural layered double hydroxides. 3. The crystal structure of Mg, Al-disordered quintinite-2H. Mineral. Mag. 2010, 74, 841–848. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Popov, M.P.; Krivovichev, S.V.; Zaitsev, A.N.; Vlasenko, N.S. Quintinite-1M from the Mariinskoe deposit, Ural Emerald Mines, Southern Urals, Russia. Geol. Ore Deposits. 2017, 59, 745–751. [Google Scholar] [CrossRef]
- Mills, S.J.; Whitfield, P.S.; Kampf, A.R.; Wilson, S.A.; Dipple, G.M.; Raudsepp, M.; Favreau, G. Contribution to the crystallography of hydrotalcites: The crystal structures of woodallite and takovite. J. Geosci. 2012, 58, 273–279. [Google Scholar] [CrossRef]
- Lozano, R.P.; Rossi, C.; La Iglesia, A.; Matesanz, E. Zaccagnaite-3R, a new Zn-Al hydrotalcite polytype from El Soplao cave (Cantabria, Spain). Am. Mineral. 2012, 97, 513–523. [Google Scholar] [CrossRef]

| Species | Chlormagaluminite | Synthetic Zn-analogs of Chlormagaluminite | Synthetic Al-analog of Iowaite | |
|---|---|---|---|---|
| Chemical formula | [Mg4Al2(OH)12] [Cl2(H2O)2] | [Zn4Al2(OH12] [Cl2(H2O)4] | [Zn3.9Al2.1(OH)12] [Cl2.1(H2O)2.1] | [Mg6Al2(OH)16] [Cl1.76(CO3)0.12(H2O)4.8] |
| Refinement | Single-crystal XRD data, direct method | Rietveld-Le Bail refinement | Rietveld refinement and MD-simulation | Rietveld refinement |
| Symmetry | Hexagonal | Trigonal | Trigonal | Trigonal |
| Space group | P-6c2 3 | R-3m | R-m4 | R-3m |
| a, Å | 5.280(2) | 3.083 | 3.07497(6) | 3.0649(7) |
| b, Å | =a | =a | =a | =a |
| c, Å | 15.344(6) | 23.47 | 23.1524(5) | 23.913(9) |
| V, Å3 | 374.67 | 193.19 5 | 189.588(6) | 194.53 5 |
| Z | 1 | 1 | 1 | 1 |
| Density, g/cm3 | 2.06 5/2.067 6 | 2.848 6 | 2.759 5/2.751 6 | 1.881 5/2.07 6 |
| Number of reflections | 121 | n.g. | n.g. | n.g. |
| R1 | 0.083 | 4.95 | 0.076 | n.g. |
| Rw | 0.088 | 13.25 | 0.119 | n.g. |
| Polytype | 2H | 3R | 3R | 3R (3R1) |
| a′, Å 1 | 3.05 | 3.09 | 3.08 | 3.06 |
| d, Å 2 | 7.73 | 7.85 | 7.72 | 7.97 |
| Source | [24] | [25] | [26] | [9] |
| Constituent | Wt.% | Range (Stand. Dev.) | Constituent | apfu | Range |
|---|---|---|---|---|---|
| Formula calculated on the basis of Mg + Al + Fe = 6 apfu | |||||
| MgO | 33.85 | 33.07–34.76 (0.32) | Mg | 3.91 | 3.87–3.96 |
| FeO | 1.09 | 0.55–1.96 (0.38) | Fe | 0.07 | 0.04–0.12 |
| ΣM2+ | 4.98 | ||||
| Al2O3 | 22.07 | 21.16–22.83 (0.24) | Al | 2.02 | 2.00–2.01 |
| ΣM3+ | 2.02 | ||||
| H2Otot(1),(2) | 30.96 | OH 1 | 12 | ||
| H2O2 | 2.0 | ||||
| Cl | 14.72 | 11.95–16.25 (1.32) | Cl | 1.97 | 1.70–2.14 |
| -Cl=O | −3.39 | ||||
| Total | 99.30 | M2+/M3+ | 2.0 | ||
| Chlormagaluminite | Quintinite [34] | Quintinite [35] | Assignment |
|---|---|---|---|
| 3550 | - | - | O–H stretching |
| 3432 | 3437 | 3388 | |
| - | ~3100–2700 sh | 3184, 3029 | interaction of interlayer water and carbonate-ion |
| 1800 sh | 1750–1500 sh | 1571 | H‒O‒H bending |
| 1610 | |||
| - | ~1400 sh | 1407 | Antisymmetric stretching of carbonate |
| - | 1355 | 1350 | |
| - | 960–940 sh | 949 | v2 bending of carbonate |
| 858 | 880–860 sh | 866, 841 | Al···O–H, Mg···O–H bending vibrations involving OH groups forming strong hydrogen bonds with O atoms |
| - | 783 | 776 | v4 bending of carbonate |
| 679 | 679 | 673 | Lattice modes |
| 549 | 552 | n.r. | |
| 447 | 449 | n.r. | |
| 390 | 393 | n.r. |
| Crystal Chemical Data | |
| Crystal system | Hexagonal |
| Space group | P63/mcm |
| a, (Å) | 5.268(3) |
| c, (Å) | 15.297(8) |
| Unit-cell volume (Å3) | 367.6(4) |
| Z | 1 |
| Calculated density (g/cm3) | 2.07 |
| Absorption coefficient | 0.871 |
| Data Collection | |
| Diffractometer | Bruker APEX II DUO |
| Temperature (K) | 293(2) |
| Radiation, wavelengths (Å) | Mo Kα, 0.71073 |
| Range for data collection, θ (°) | 2.66‒33.35 |
| h, k, l ranges | −8→6, −7→5, −23→19 |
| Total reflections collected | 277 |
| Unique reflections (Rint) | 0.0701 |
| Number of unique reflections F > 2σ(F) | 152 |
| Data completeness (%) | 95.5 |
| Structure Refinement | |
| Refinement method | Full-matrix least-squares on F2 |
| Weighting coefficients a, b | 0.117800, 0 |
| Data/ restraints/ parameters | 277/0/33 |
| R1 [F > 2σ(F)], wR2 [F > 2σ(F)] | 0.0826, 0.1954 |
| R1 all, wR2 all | 0.1207, 0.2181 |
| Goodness-of-fit on F2 | 1.093 |
| Largest diff. peak and hole (ēÅ-3) | 0.53, 0.29 |
| Atom | W.P. | x | y | z | Ueq | s.o.f | s.s. ref (ē) | Assigned Site Population |
|---|---|---|---|---|---|---|---|---|
| Octahedral layer | ||||||||
| Mg1 | 4d | 1/3 | 2/3 | 0 | 0.0167(8) | 1 * | 48 | Mg4 |
| Al1 | 2b | 0 | 0 | 0 | 0.0161(9) | 1 * | 26 | Al2 |
| O1 | 12k | 0.3094(5) | 0.3094(5) | 0.0644(2) | 0.0154(8) | 1 * | 96 | (OH)12 |
| H1 | 12k | 0.294(8) | 0.294(8) | 0.124(4) | 0.02 * | 1 * | 12 | |
| Interlayer gallery | ||||||||
| Cl1 | 12j | 0.739(8) | 0.338(12) | 1/4 | 0.022(3) | 0.044(6) | 9(1) | Cl2.2(3) |
| Cl2 | 6g | 0 | 0.054(5) | 1/4 | 0.020(1) | 0.080(5) | 8.2(7) | |
| Cl31 | 12j | 0.335(5) | 0.089(5) | 1/4 | 0.040(8) | 0.077(12) | 16(2) | |
| Cl32 | 6g | 0.39(2) | 0 | 1/4 | 0.06(3) | 0.05(2) | 5(2) | |
| O2w | 12j | 0.408(6) | 0.319(6) | 1/4 | 0.07(2) | 0.18(2) | 16(2) | (H2O)2.0(2) |
| Octahedral layer | |||||
| Mg‒O | 2.071(2) × 6 | Al‒O | 1.904(3) × 6 | ||
| Interlayer gallery | |||||
| Interlayer prism 1 | Interlayer prism 2 | Interlayer prism 3 | |||
| Cl1‒H1(a) | 2.59(6) × 4 | Cl2‒H1(a) | 2.42(5) × 4 | Cl31‒H1(a) | 2.28(5) × 2 |
| Cl1‒H1(b) | 2.96(6) × 2 | Cl2‒H1(b) | 2.68(5) × 2 | Cl31‒H1(b) | 2.73(5) × 2 |
| <Cl1‒H1> | 2.71 | <Cl2‒H1> | 2.51 | Cl31‒H1(c) | 2.95(5) × 2 |
| Cl32‒H1(a) | 2.55(8) × 2 | ||||
| Cl32‒H1(b) | 2.69(7) × 4 | ||||
| <Cl3‒H1> | 2.64 | ||||
| Hydrogen bonding scheme | |||||
| D‒H | d(D‒H) | d(H..A) | <DHA | d(D..A) | A |
| O1‒H1 | 0.917 | 2.952 | 127.76 | 3.587 | Cl1 |
| O1‒H1 | 0.917 | 2.570 | 136.27 | 3.294 | Cl1 |
| O1‒H1 | 0.917 | 2.585 | 134.47 | 3.293 | Cl1 |
| O1‒H1 | 0.917 | 2.952 | 127.76 | 3.587 | Cl1 |
| O1‒H1 | 0.917 | 2.399 | 148.30 | 3.215 | Cl2 |
| O1‒H1 | 0.917 | 2.659 | 141.39 | 3.424 | Cl2 |
| O1‒H1 | 0.917 | 2.271 | 149.48 | 3.096 | Cl31 |
| O1‒H1 | 0.917 | 2.943 | 126.00 | 3.560 | Cl31 |
| O1‒H1 | 0.917 | 2.709 | 138.15 | 3.446 | Cl31 |
| O1‒H1 | 0.917 | 2.678 | 137.18 | 3.408 | Cl32 |
| O1‒H1 | 0.917 | 2.540 | 134.37 | 3.247 | Cl32 |
| O1‒H1 | 0.917 | 2.003 | 160.49 | 2.883 | O2W |
| This Work | JCPDS-ICDD Card # 00-038-0446 [24] | ||||||
|---|---|---|---|---|---|---|---|
| Irel (%) | dmeas (Å) | dcalc (Å) | h | k | l | dmeas (Å) | Irel (%) |
| 100 | 7.72 | 7.72 | 0 | 0 | 2 | 7.67 | 100 |
| 5 | 4.574 | 4.577 | 0 | 1 | 0 | 4.55 | 20 |
| 5 | 3.891 | 3.937 | 0 | 1 | 2 | n.r. | n.r. |
| 38 | 3.856 | 3.858 | 0 | 0 | 4 | 3.86 | 80 |
| 2 | 2.988 | 2.950 | 0 | 1 | 4 | n.r. | n.r. |
| 2 | 2.642 | 2.643 | 1 | 1 | 0 | n.r. | n.r. |
| 12 | 2.603 | 2.605 | 1 | 1 | 1 | 2.60 | 80 |
| 3 | 2.571 | 2.572 | 0 | 0 | 6 | n.r. | n.r. |
| 12 | 2.499 | 2.500 | 1 | 1 | 2 | 2.49 | 70 |
| 38 | 2.350 | 2.351 | 1 | 1 | 3 | 2.34 | 90 |
| 1 | 2.287 | 2.289 | 0 | 2 | 0 | n.r. | n.r. |
| 1 | 2.240 | 2.242 | 0 | 1 | 6 | n.r. | n.r. |
| 34 | 2.179 | 2.180 | 1 | 1 | 4 | 2.17 | 90 |
| 17 | 2.007 | 2.007 | 1 | 1 | 5 | 2.00 | 60 |
| 1 | 1.930 | 1.929 | 0 | 0 | 8 | n.r. | n.r. |
| 29 | 1.843 | 1.843 | 1 | 1 | 6 | 1.839 | 100 |
| 1 | 1.716 | 1.719 | 2 | 1 | 1 | n.r. | n.r. |
| 1.710 | 0 | 2 | 6 | ||||
| 1 | 1.693 | 1.693 | 1 | 1 | 7 | n.r. | n.r. |
| 1.688 | 2 | 1 | 2 | ||||
| 2 | 1.639 | 1.640 | 2 | 1 | 3 | n.r. | n.r. |
| 16 | 1.558 | 1.558 | 1 | 1 | 8 | 1.555 | 80 |
| 13 | 1.525 | 1.526 | 0 | 3 | 0 | 1.526 | 90 |
| 2 | 1.500 | 1.509 | 2 | 1 | 5 | n.r. | n.r. |
| 13 | 1.496 | 1.497 | 0 | 3 | 2 | 1.496 | 90 |
| <1 | 1.475 | 1.475 | 0 | 2 | 8 | n.r. | n.r. |
| 1 | 1.439 | 1.438 | 1 | 1 | 9 | n.r. | n.r. |
| 1.435 | 2 | 1 | 6 | ||||
| 4 | 1.419 | 1.419 | 0 | 3 | 4 | 1.417 | 40 |
| 5 | 1.332 | 1.332 | 1 | 1 | 10 | 1.330 | 40 |
| 2 | 1.321 | 1.321 | 2 | 2 | 0 | n.r. | n.r. |
| <1 | 1.315 | 1.316 | 2 | 2 | 1 | n.r. | n.r. |
| 1.312 | 0 | 3 | 6 | ||||
| 1 | 1.302 | 1.302 | 2 | 2 | 2 | 1.302 | 30 |
| 1 | 1.286 | 1.288 | 2 | 1 | 8 | n.r. | n.r. |
| 1.286 | 0 | 0 | 12 | ||||
| 1 | 1.279 | 1.280 | 2 | 2 | 3 | 1.281 | 30 |
| 1.279 | 0 | 2 | 10 | ||||
| <1 | 1.269 | 1.269 | 3 | 1 | 0 | n.r. | n.r. |
| 1.265 | 3 | 1 | 1 | ||||
| 3 | 1.250 | 1.253 | 3 | 1 | 2 | 1.250 | 40 |
| 1.250 | 2 | 2 | 4 | ||||
| 1 | 1.239 | 1.239 | 1 | 1 | 11 | n.r. | n.r. |
| 1.238 | 0 | 1 | 12 | ||||
| 1 | 1.233 | 1.232 | 3 | 1 | 3 | 2.237 | 20 |
| 1 | 1.214 | 1.218 | 2 | 1 | 9 | 2.217 | 10 |
| 1.215 | 2 | 2 | 5 | ||||
| <1 | 1.200 | 1.200 | 0 | 3 | 8 | n.r. | n.r. |
| 4 | 1.175 | 1.175 | 2 | 2 | 6 | 1.175 | 60 |
| 1.174 | 3 | 1 | 5 | ||||
| 2 | 1.156 | 1.156 | 1 | 1 | 12 | 1.155 | 50 |
| <1 | 1.150 | 1.152 | 2 | 1 | 10 | n.r. | n.r. |
| <1 | 1.143 | 1.144 | 0 | 4 | 0 | n.r. | n.r. |
| <1 | 1.137 | 1.138 | 3 | 1 | 6 | n.r. | n.r. |
| <1 | 1.133 | 1.133 | 2 | 2 | 7 | n.r. | n.r. |
| 1.132 | 0 | 4 | 2 | ||||
| <1 | 1.102 | 1.102 | 0 | 0 | 14 | n.r. | n.r. |
| 1.100 | 3 | 1 | 7 | ||||
| 5 | 1.090 | 1.090 | 2 | 2 | 8 | 1.091 | 60 |
| 1.090 | 2 | 1 | 11 | ||||
| 1 | 1.083 | 1.085 | 0 | 3 | 10 | n.r. | n.r. |
| 1.083 | 1 | 1 | 13 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhitova, E.S.; Krivovichev, S.V.; Pekov, I.V.; Yapaskurt, V.O. Crystal Chemistry of Chlormagaluminite, Mg4Al2(OH)12Cl2(H2O)2, a Natural Layered Double Hydroxide. Minerals 2019, 9, 221. https://doi.org/10.3390/min9040221
Zhitova ES, Krivovichev SV, Pekov IV, Yapaskurt VO. Crystal Chemistry of Chlormagaluminite, Mg4Al2(OH)12Cl2(H2O)2, a Natural Layered Double Hydroxide. Minerals. 2019; 9(4):221. https://doi.org/10.3390/min9040221
Chicago/Turabian StyleZhitova, Elena S., Sergey V. Krivovichev, Igor V. Pekov, and Vasiliy O. Yapaskurt. 2019. "Crystal Chemistry of Chlormagaluminite, Mg4Al2(OH)12Cl2(H2O)2, a Natural Layered Double Hydroxide" Minerals 9, no. 4: 221. https://doi.org/10.3390/min9040221
APA StyleZhitova, E. S., Krivovichev, S. V., Pekov, I. V., & Yapaskurt, V. O. (2019). Crystal Chemistry of Chlormagaluminite, Mg4Al2(OH)12Cl2(H2O)2, a Natural Layered Double Hydroxide. Minerals, 9(4), 221. https://doi.org/10.3390/min9040221