Estimating Bubble Loading in Industrial Flotation Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Conditions
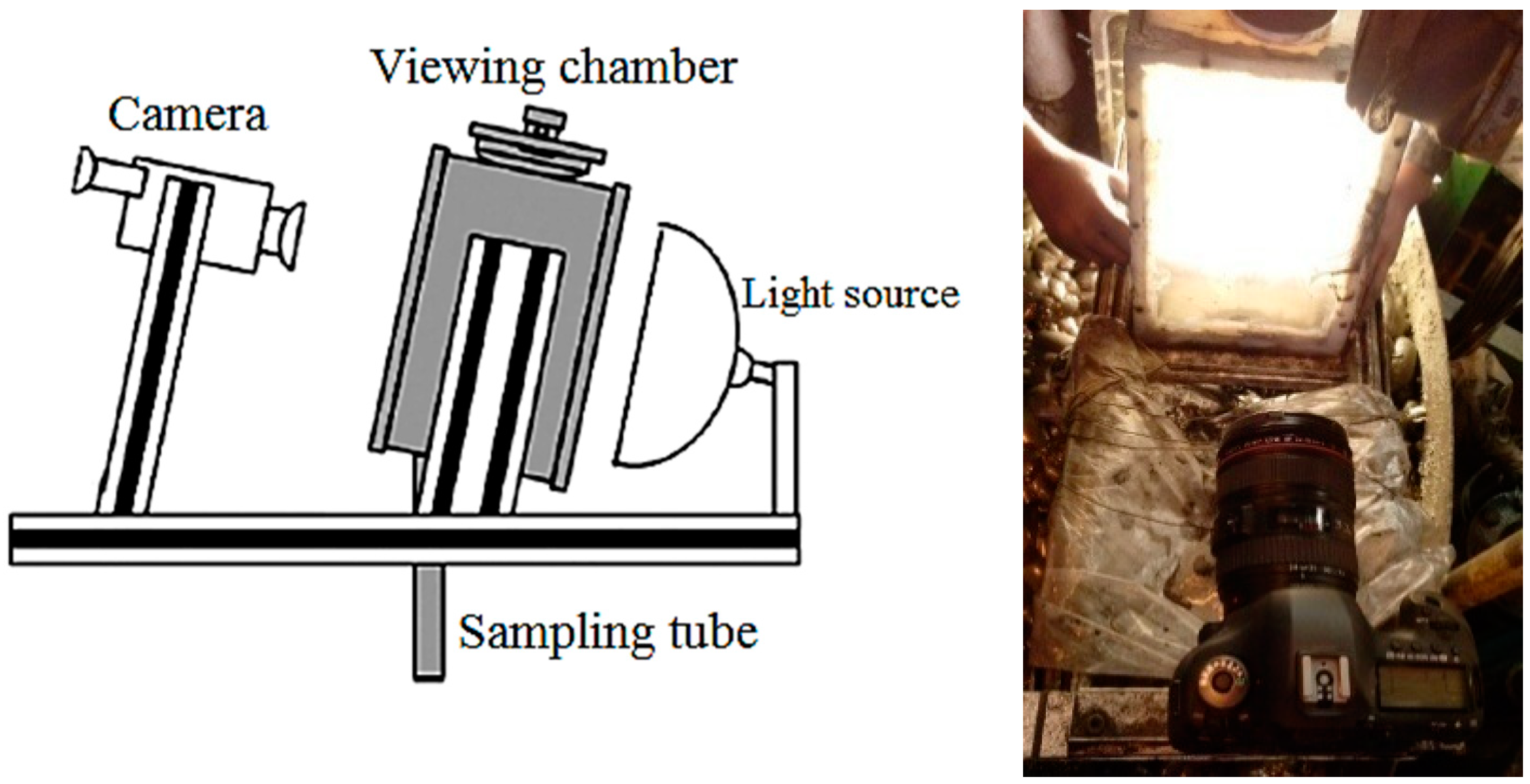
2.2. Measuring the Bubble Diameter
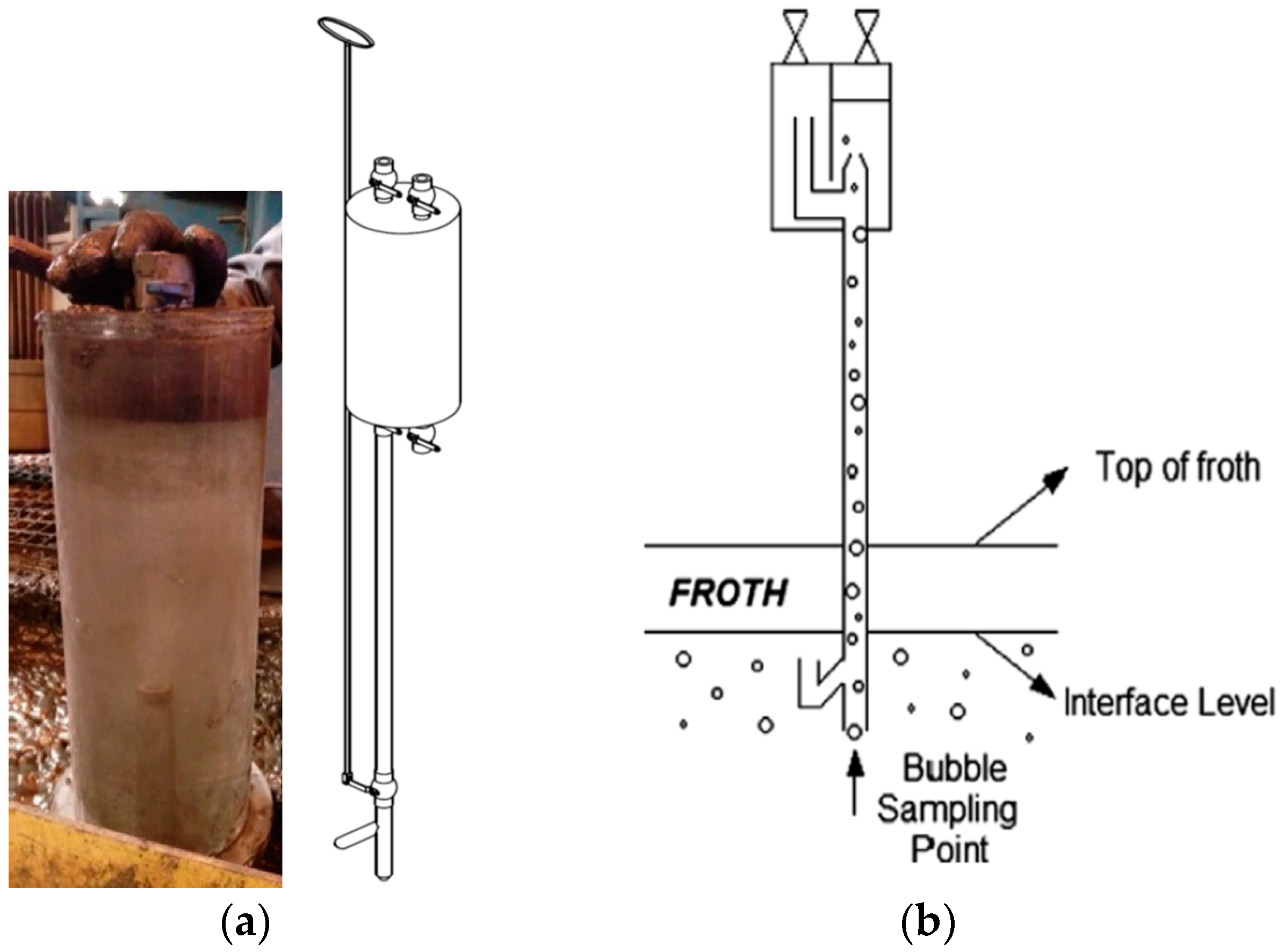
2.3. Bubble Loading
2.4. Measurement of the Diameter and Density of Particles
2.5. Rate of Mass Transfer of Particles to the Froth Phase
2.6. Statistical Analysis
3. Results and Discussion
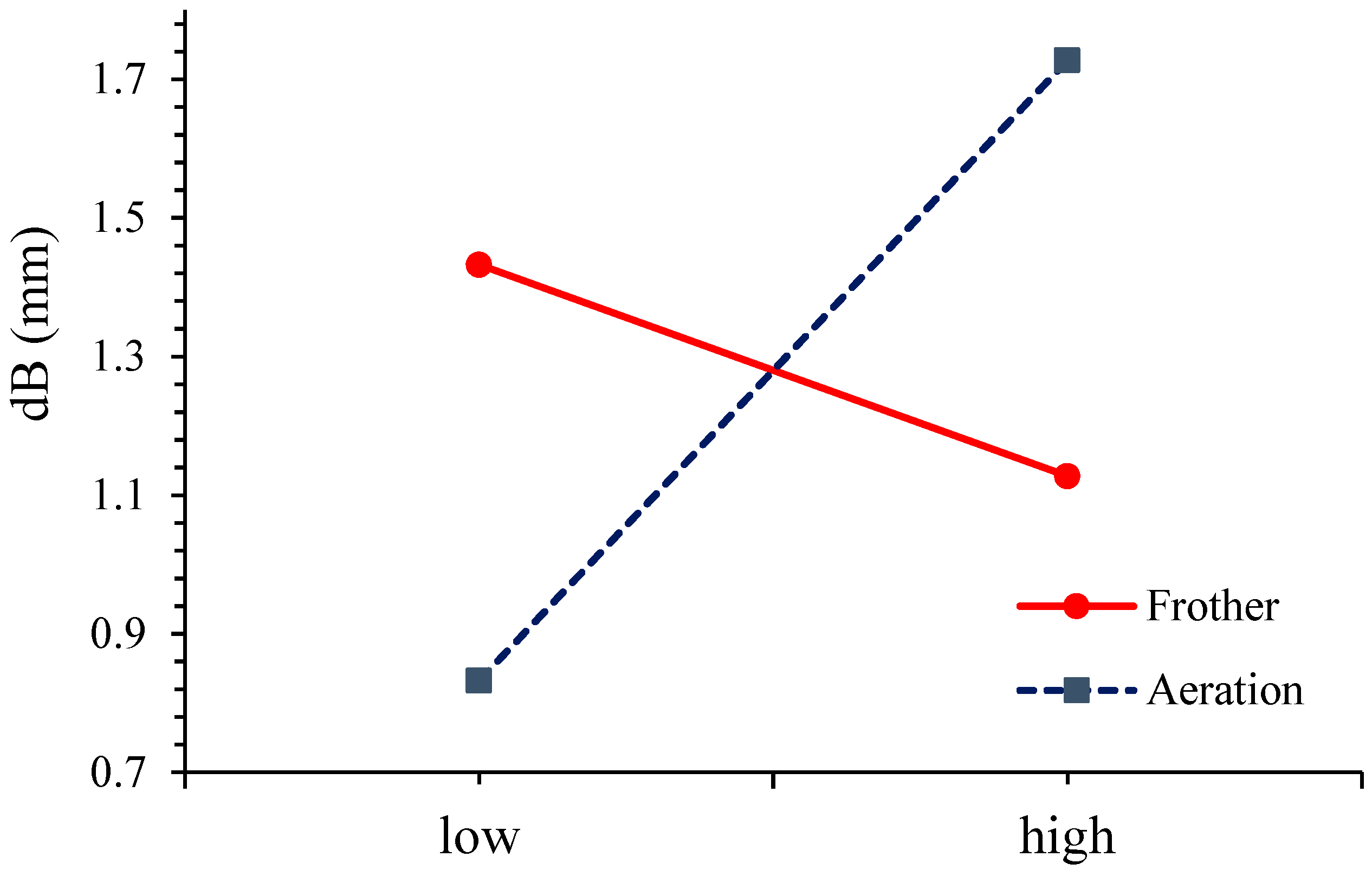
3.1. The Effect of Parameters on the Bubble Diameter
3.2. Effect of Operational Parameters on the Bubble Superficial Surface
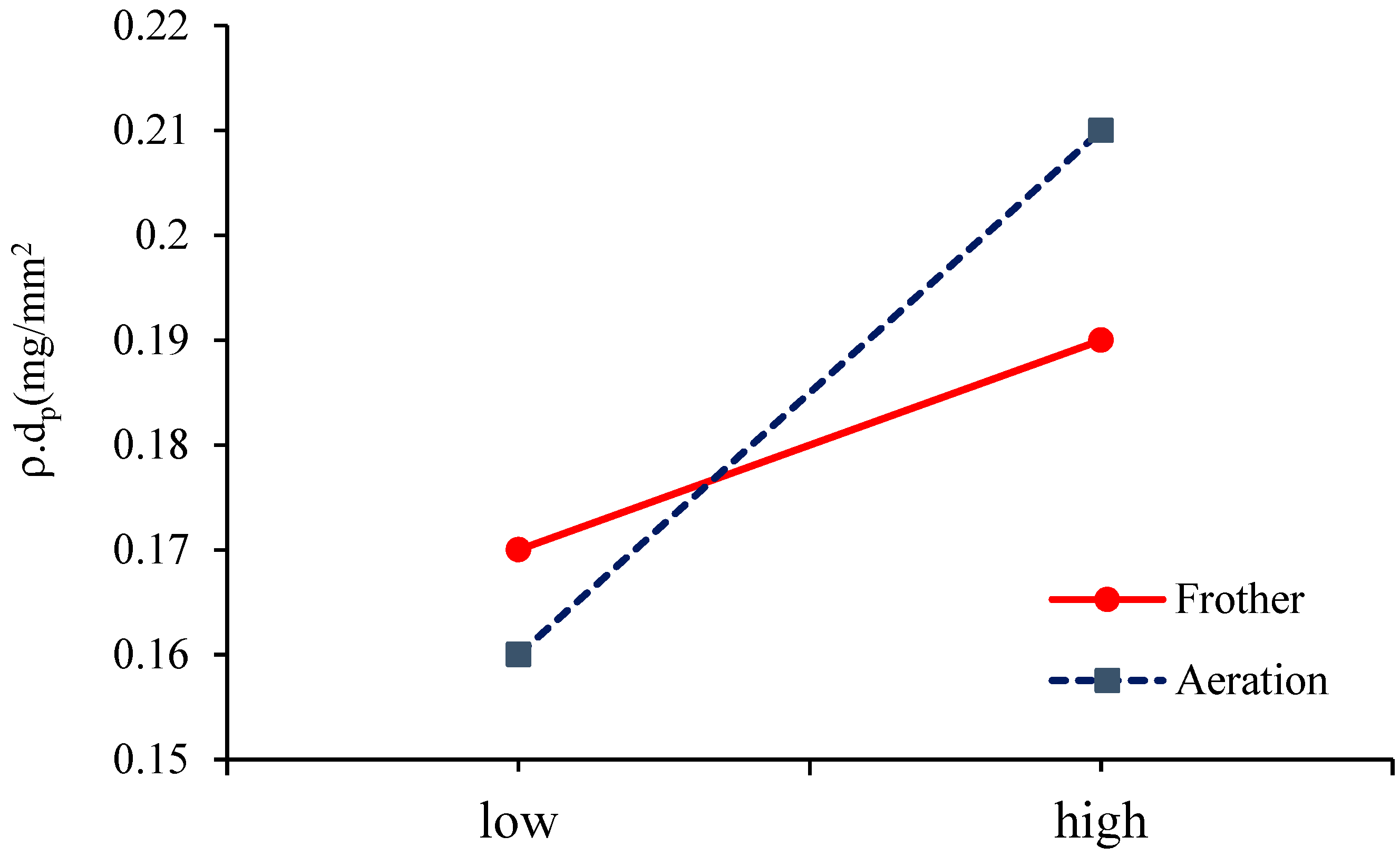
3.3. The Diameter and Density of the Solid Particles
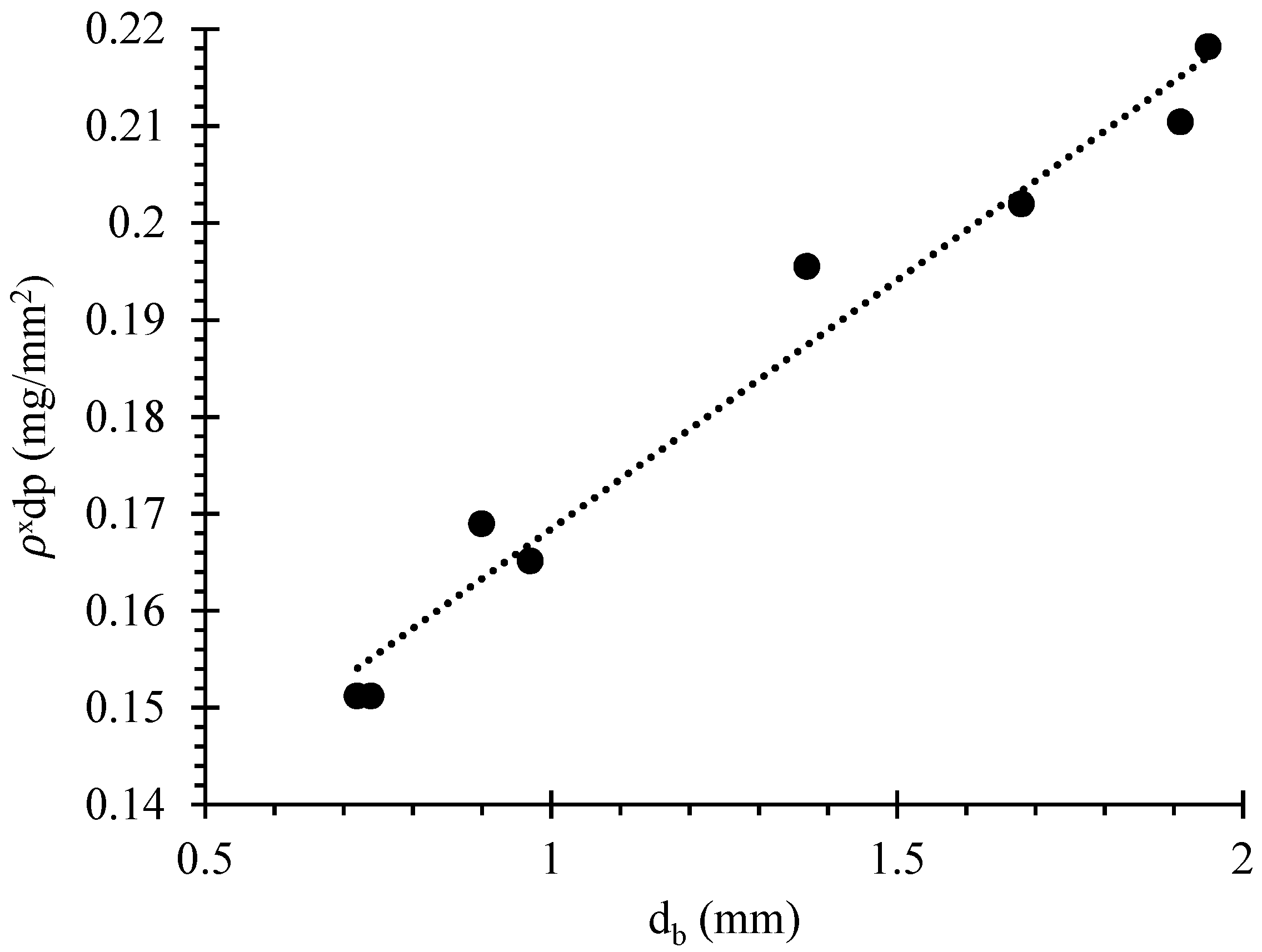
3.4. Bubble Loading
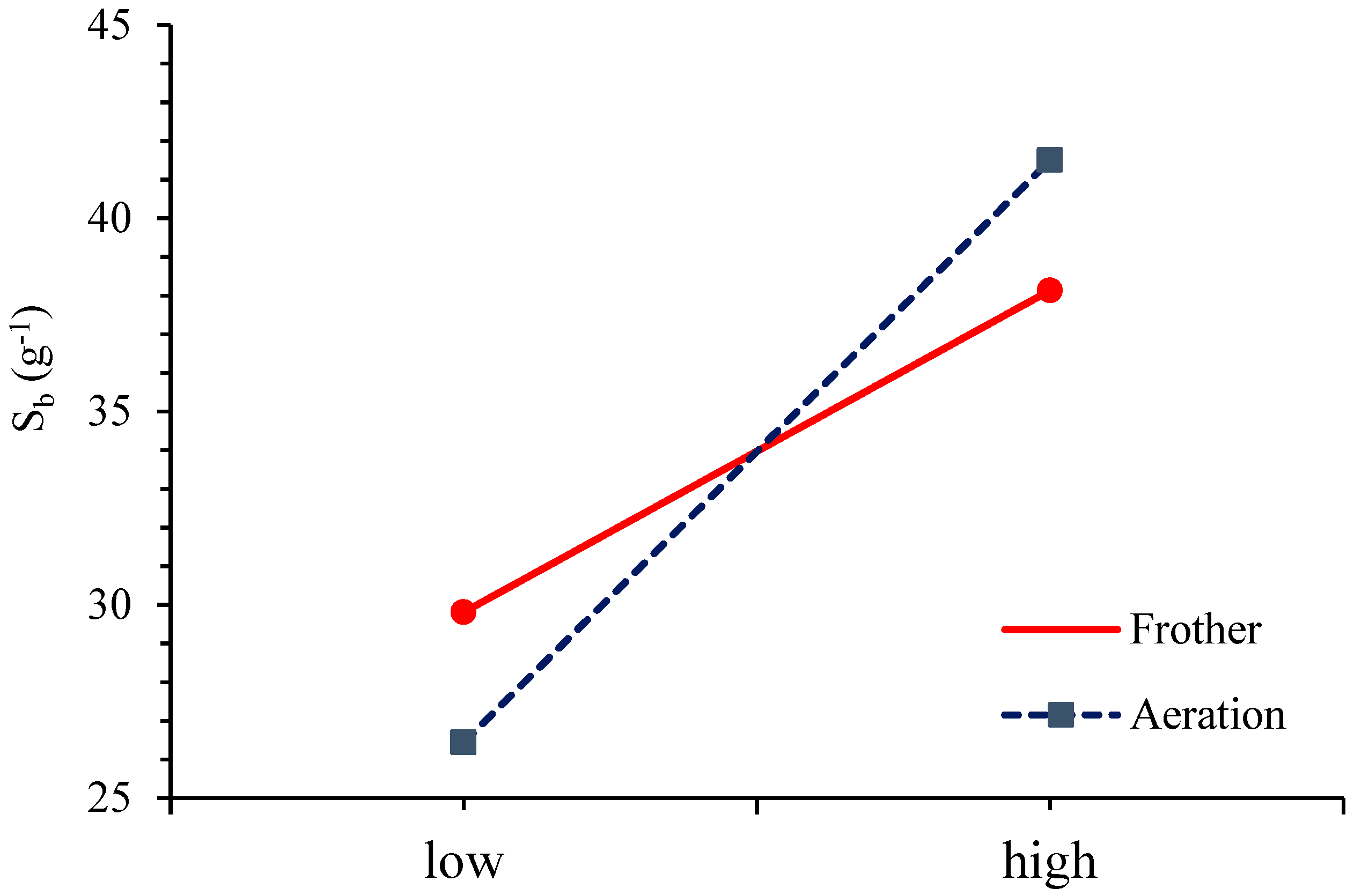
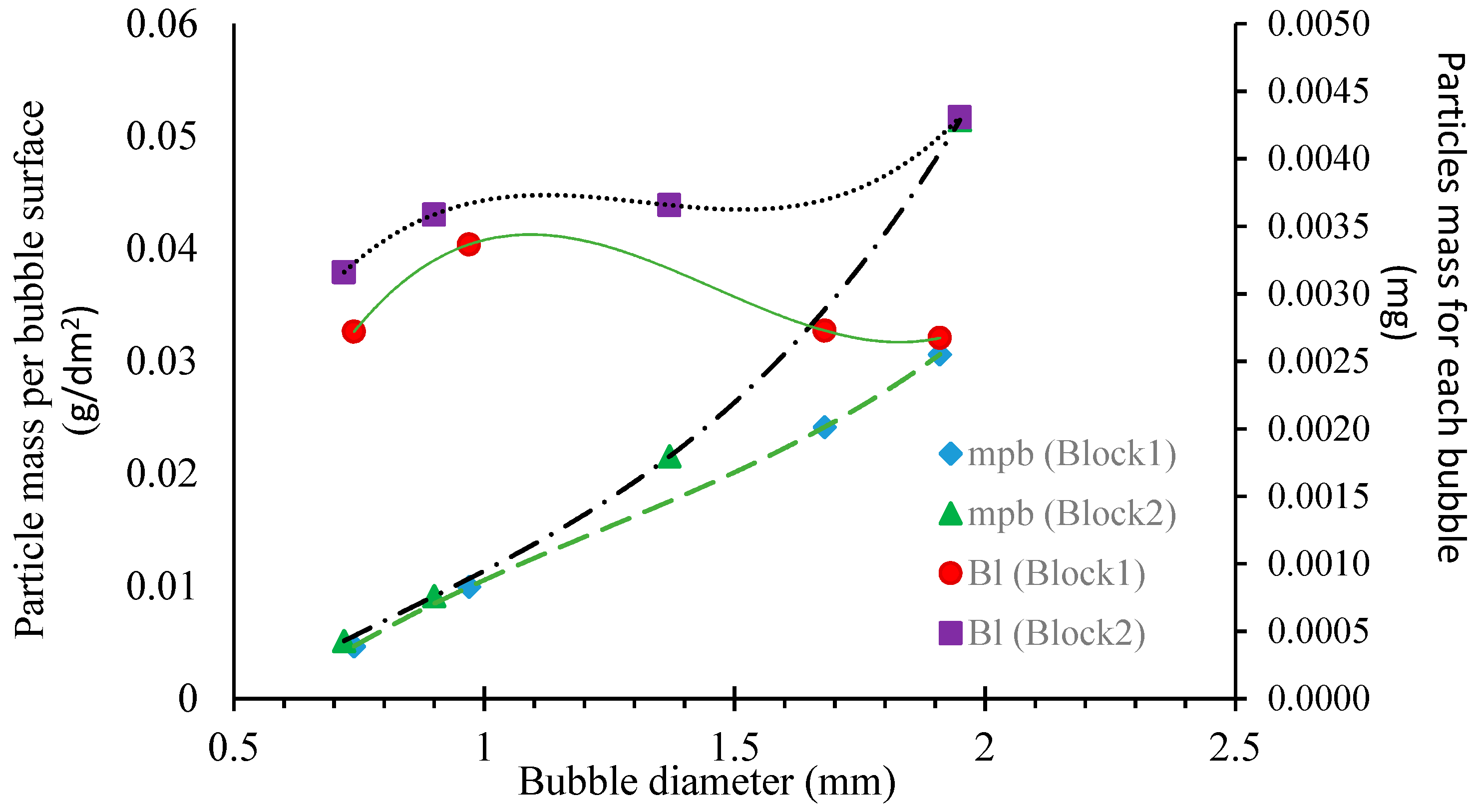
3.5. Mass Transfer Rate of Particles Attached to the Froth Phase
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Farrokhpay, S. The significance of froth stability in mineral flotation—A review. Adv. Colloid Interface Sci. 2001, 166, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Farrokhpay, S.; Zanin, M. An investigation into the effect of water quality on froth stability. Adv. Powder Technol. 2012, 23, 493–497. [Google Scholar] [CrossRef]
- Ata, S. Phenomena in the froth phase of flotation—A review. Int. J. Miner. Process. 2012, 102–103, 1–12. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Cao, Y.; Wang, Y.; Xu, M.; Li, C. Effect of compound collector and blending frother on froth stability and flotation performance of oxidized coal. Powder Technol. 2017, 305, 166–173. [Google Scholar] [CrossRef]
- Norori-McCormac, A.; Brito-Parada, P.R.; Hadler, K.; Cole, K.; Cilliers, J.J. The effect of particle size distribution on froth stability in flotation. Sep. Purif. Technol. 2017, 184, 240–247. [Google Scholar] [CrossRef]
- Achaye, I. Effect of Particle Properties on Froth Stability. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 2017. [Google Scholar]
- Cilek, E.C.; Uysal, K. Froth stabilization using nanoparticles in mineral flotation. Physicochem. Probl. Miner. Process. 2018, 54, 878–889. [Google Scholar]
- Ata, S. The role of frother on the detachment of particles from bubbles. Miner. Eng. 2011, 24, 476–478. [Google Scholar] [CrossRef]
- King, R.P.; Hatton, T.A.; Hulbert, D.G. Bubble loading during flotation. Trans. Inst. Mining Metall. 1974, 83, 112–115. [Google Scholar]
- Gallegos-Acevedo, P.M.; Pérez-Garibay, R.; Uribe-Salas, A. Maximum bubble loads: Experimental measurement vs. analytical estimation. Miner. Eng. 2006, 19, 12–18. [Google Scholar] [CrossRef]
- Yianatos, J.B.; Moys, M.H.; Contreras, F.; Villanueva, A. Froth recovery of industrial flotation cells. Miner. Eng. 2008, 21, 817–825. [Google Scholar] [CrossRef]
- Bradshaw, D.J.; Connor, C.T. Measurement of the sub-process of bubble loading in flotation. Miner. Eng. 1996, 9, 443–448. [Google Scholar] [CrossRef]
- Alexander, D.J.; Franzidis, J.P.; Manlapig, E.V. Froth recovery measurement in plant scale flotation cells. Miner. Eng. 2003, 16, 1197–1203. [Google Scholar] [CrossRef]
- Moys, M.H.; Yianatos, J.; Larenas, J. Measurement of particle loading on bubbles in the flotation process. Miner. Eng. 2010, 23, 131–136. [Google Scholar] [CrossRef]
- Seaman, D.R.; Franzidis, J.P.; Manlapig, E.V. Bubble load measurement in the pulp zone of industrial flotation machines—A new device for determining the froth recovery of attached particles. Int. J. Miner. Process. 2004, 74, 1–13. [Google Scholar] [CrossRef]
- Hemmati, C.M.; Abdollahy, M.; Khalesi, M.R. Bubble loading measurement in a continuous flotation column. Miner. Eng. 2016, 85, 49–54. [Google Scholar] [CrossRef]
- Eskanlou, A.; Khalesi, M.R.; Abdollahy, M.; Hemmati, C.M. Interactional effects of bubble size, particle size, and collector dosage on bubble loading in column flotation. J. Min. Environ. 2018, 9, 107–116. [Google Scholar]
- Gorain, B.; Franzidis, J.P.; Manlapig, E.V. Studies on impeller type, impeller speed and air flow rate in an industrial scale flotation cell. Part 1: Effect on bubble size distribution. Miner. Eng. 1995, 8, 615–635. [Google Scholar] [CrossRef]
- Fuerstenau, M.C.; Jameson, G.J.; Yoon, R.-H. Froth Flotation: A Century of Innovation; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2007. [Google Scholar]
- Vinnett, L.; Yianatos, J.; Alvarez, M. Gas dispersion measurements in mechanical flotation cells-Industrial experience in Chilean concentrators. Miner. Eng. 2014, 57, 12–15. [Google Scholar] [CrossRef]
- Kaartinen, J.; Hätönen, J.; Hyötyniemi, H.; Miettunen, J. Machine-vision-based control of zinc flotation—A case study. Control Eng. Pract. 2006, 14, 1455–1466. [Google Scholar] [CrossRef]
- Grau, R.A.; Heiskanen, K. Visual technique for measuring bubble size in flotation machines. Miner. Eng. 2002, 15, 507–513. [Google Scholar] [CrossRef]
- Morar, S.H. The Use of Machine Vision to Describe and Evaluate Froth Phase Behaviour and Performance in Mineral Flotation Systems. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 2010. [Google Scholar]
- Nissinen, N.; Lehikoinen, A.; Mononen, M.; Lähteenmäki, S.; Vauhkonen, M. Estimation of the bubble size and bubble loading in a flotation froth using electrical resistance tomography. Miner. Eng. 2014, 69, 1–12. [Google Scholar] [CrossRef]
- Gorain, B.; Oravainen, H.; Allenius, H.; Peaker, R.; Weber, A.; Tracyzk, F. Mechanical froth flotation cells. In Froth Flotation a Century of Innovation; Fuerstenau, M.C., Jameson, G.J., Yoon, Eds.; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2007; pp. 637–779. [Google Scholar]
- Gallegos-Acevedo, P.M.; Pérez-Garibay, R.; Uribe-Salas, A.; Nava-Alonso, F. Bubble load estimation in the froth zone to predict the concentrate mass flow rate of solids in column flotation. Miner. Eng. 2007, 20, 1210–1217. [Google Scholar] [CrossRef]
- Bhondayi, C.; Moys, M.H. Determination of sampling pipe (riser) diameter for a flotation bubble load measuring device. Miner. Eng. 2011, 24, 1664–1676. [Google Scholar] [CrossRef]
- Yianatos, J.; Vinnett, L.; Carrasco, C.; Alvarez-Silva, M. Effect of entrainment in bubble load measurement on froth recovery estimation at industrial scale. Miner. Eng. 2015, 72, 31–35. [Google Scholar] [CrossRef]
- Napier-Munn, T. Statistical Methods for Mineral Engineers: How to Design Experiments and Analyse Data; JKMRC: Indooroopilly, Australia, 2014. [Google Scholar]
- Li, C.; Runge, K.; Shi, F.; Farrokhpay, S. Effect of flotation conditions on froth rheology. Powder Technol. 2018, 340, 537–542. [Google Scholar] [CrossRef]
- Gupta, A.; Yan, D.S. Mineral Processing Design and Operations; Elsevier Science: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Ralston, J.; Fornasiero, D.; Hayes, R. Bubble- particle attachment and detachment in flotation. Int. J. Miner. Process. 1999, 56, 133–164. [Google Scholar] [CrossRef]




| No. | Block No. | Frother Dosage mL/min | Aeration Rate m3/h | ton/h | Feed | |
|---|---|---|---|---|---|---|
| % Fe | % S | |||||
| 1 | 1 | 70 | 45 | 108 | 65.7 | 1.09 |
| 2 | 150 | 45 | 110 | 65.02 | 1.09 | |
| 3 | 70 | 146 | 104 | 66.6 | 0.83 | |
| 4 | 150 | 146 | 115 | 65.75 | 1.06 | |
| 5 | 2 | 70 | 45 | 108 | 66.15 | 1.04 |
| 6 | 150 | 45 | 98 | 65.71 | 1.19 | |
| 7 | 70 | 146 | 105 | 65.42 | 0.93 | |
| 8 | 150 | 146 | 116 | 66.2 | 0.9 | |
| No. | db (mm) | dp (μm) | ρp (g/cm3) | λB (g/L) | Bl (mg/mm2) | K (%) | mpb (mg) | Sb (s−1) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.97 | 43 | 3.84 | 20.82 | 0.0034 | 3.61 | 0.012 | 22.33 |
| 2 | 0.74 | 40 | 3.78 | 22.05 | 0.0027 | 2.49 | 0.005 | 29.28 |
| 3 | 1.91 | 53 | 3.97 | 8.4 | 0.0027 | 3.02 | 0.02 | 36.8 |
| 4 | 1.68 | 51 | 3.96 | 9.74 | 0.0027 | 2.48 | 0.019 | 41.84 |
| 5 | 0.9 | 44 | 3.84 | 23.92 | 0.0036 | 3.61 | 0.012 | 24.07 |
| 6 | 0.72 | 40 | 3.78 | 26.34 | 0.0032 | 2.49 | 0.006 | 30.09 |
| 7 | 1.95 | 54 | 4.04 | 13.26 | 0.0043 | 4.52 | 0.045 | 36.05 |
| 8 | 1.37 | 50 | 3.91 | 16.02 | 0.0037 | 3.08 | 0.02 | 51.31 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostadrahimi, M.; Gharibi, K.; Dehghani, A.; Farrokhpay, S. Estimating Bubble Loading in Industrial Flotation Cells. Minerals 2019, 9, 222. https://doi.org/10.3390/min9040222
Ostadrahimi M, Gharibi K, Dehghani A, Farrokhpay S. Estimating Bubble Loading in Industrial Flotation Cells. Minerals. 2019; 9(4):222. https://doi.org/10.3390/min9040222
Chicago/Turabian StyleOstadrahimi, Mahdi, Khodakaram Gharibi, Ali Dehghani, and Saeed Farrokhpay. 2019. "Estimating Bubble Loading in Industrial Flotation Cells" Minerals 9, no. 4: 222. https://doi.org/10.3390/min9040222
APA StyleOstadrahimi, M., Gharibi, K., Dehghani, A., & Farrokhpay, S. (2019). Estimating Bubble Loading in Industrial Flotation Cells. Minerals, 9(4), 222. https://doi.org/10.3390/min9040222






