Abstract
A series of thermodynamic calculations are performed for the roasting of pyrite in changing temperatures and atmospheres. The relationship between ΔrGθ and temperature in the range of T = 300–1200 K shows that, depending on the atmosphere it is in, reactions of pyrolysis, oxidation or reduction can occur. Both the pyrolysis of pyrite in an inert atmosphere and its oxidation by oxygen can form pyrrhotite (mainly Fe0.875S and FeS), but the temperature required for oxidation is much lower than that for pyrolysis. In an oxygen-containing atmosphere, the isothermal predominance areas for the Fe–S–O system indicate that a change in temperature and oxygen partial pressure can lead the pyrite to undergo desulphurization to pyrrhotite (FeS2 → Fe0.875S/FeS) or iron oxides (FeS2 → Fe3O4/Fe2O3), or sulphation to iron sulphates (FeS2 → FeSO4/Fe2(SO4)3). The presence of carbon is beneficial to the desulphurization of pyrite under an oxidizing atmosphere since iron sulphates can be converted to iron oxides at very low levels of PCO/PCO2. Results presented in this paper offer theoretical guidance for the optimization of roasting of pyrite for different purposes.
1. Introduction
Pyrite (FeS2) is one of the most common and widely distributed sulphide minerals [1]. In industry, pyrite is a chief raw material to produce sulphur, sulphur dioxide and sulphuric acid. Also, pyrite is usually found in association with valuable metallic elements such as Au, Ag and Cu that can be recovered in a comprehensive utilization of resources [2]. In terms of the auriferous pyrite (i.e., sulfidic gold ore), gold often occurs as submicroscopic particles that are easily enclosed in crystal lattices of pyrite [3]. Consequently, gold is difficult to be exposed unless undergoing ultrafine grinding, resulting in high energy consumption [4]. Gold is also sometimes associated with pyrite and preg-robbing carbonaceous matters (mainly carbon) that readily adsorb gold complexes from the leach solution. Under such a circumstance, carbonaceous sulphide gold ores that are regarded as the most refractory ores [5] emerge and render their gold extraction challenging. Pretreatments are thus necessary for improving the gold extraction from this type of refractory gold ores.
Oxidative roasting of carbonaceous sulphide gold ores has currently been one of the most widespread and effective pretreating methods [6,7,8,9]. The effect of oxidative roasting is mainly twofold. On the one hand, as a result of the oxidation of sulphur in pyrite to form sulphur dioxide (SO2), porous iron oxides (i.e., Fe2O3 and Fe3O4) are formed that expose the gold particles locked in pyrite. On the other hand, the oxidation of carbonaceous matter eliminates its preg-robbing effect on leached gold. It is clear that the formation of SO2, hence the subsequent production of sulphuric acid, and the comprehensive recovery of associated valuable metals from pyrite are closely related to the roasting behaviour of pyrite. If carbonaceous matters are also present, the possible impacts of carbon (C) or its oxides (CO and CO2) on the roasting of pyrite should also be taken into consideration.
Experimentally, the roasting behaviours of pyrite have been studied by a number of researchers. Dunn and De [10,11] investigated the effect of temperature and atmosphere on the oxidation of pyrite in different particle size ranges by differential thermal analysis (DTA) and thermogravimetric analysis (TGA). It was observed that pyrite less than 0.045 mm in size could be directly and completely oxidized to hematite at 776 K in an air atmosphere. In the size range of 0.09–0.125 mm and under an air atmosphere, hematite formed at temperatures lower than 788 K whilst pyrrhotite formed at temperatures higher than 788 K. With increasing partial oxygen pressure in the atmosphere, the oxidation of pyrite was enhanced significantly even at relatively low temperatures and iron sulphates (Fe2(SO4)3 and FeSO4) were easily produced. Similarly, by means of DTA and TGA, Jorgensen and Moyle [12] studied the phase transformation of pyrite for its oxidation in air in the particle size 0.053–0.074 mm. It was found that the pyrite surface was transformed into hematite at 702 K and pyrrhotite at 850 K, but with the temperature increasing to 881 K and 942 K, the resultant species were ferrous sulphate and ferric sulphate, respectively. The X-ray diffraction (XRD) analysis conducted by Schorr and Everhart [13] proved that the oxidation of pyrite in air with a low heating rate up to a temperature of 753 K in a furnace took place in a direct oxidation way to form iron oxides. The technique of Mössbauer spectroscopy used in the work of Prasad et al. [14] showed that pyrrhotite was detected after roasting pyrite in air at a temperature of 883 K. The roasting of pyrite was further investigated in a gas mixture of CO2 and O2 by Hong and Fegley [15], revealing that both pyrrhotite and hematite were formed at a temperature range 665–733 K while only pyrrhotite was found when the temperature was controlled in the range 757–811 K.
The roasting of pyrite is intimately associated with the process variables such as temperature, atmosphere and mineral particle size. In different research, various roasting reactions and phase transformations of pyrite occur under different reaction conditions. Distinctly, no comprehensive and definite information on the possible reactions with the corresponding conditions has been offered for the roasting of pyrite.
Thermodynamic analysis can provide significant information on the possibility of chemical reactions that may occur, pyrometallurgical conditions relevant to the predominance area of mineral phase, and phase transformations of mineral during the roasting process. Few endeavours have recently focused on studying the roasting behaviour of pyrite from systematic thermodynamic calculations. The thermodynamic modelling of Fe–S system was studied by Waldner and Pelton [16], but little information was involved for the roasting of pyrite. The effects of CO, mixture of CO and CO2, and solid C on the thermodynamic behaviour of arsenopyrite (FeAsS) were researched by Chakraborti and Lynch [17]. The roasting of pyrite in the presence of C or CO, however, has seldom been researched by thermodynamic analysis.
This paper uses thermodynamic calculations to analyse the roasting behaviour of pyrite. The possible involved chemical reactions are discussed. The effect of roasting temperature and atmosphere on the pyrolysis and oxidation of pyrite as well as that of carbon on the roasting of pyrite are also studied. It can provide a theoretical basis to better understand and guide the optimization of the roasting of pyrite for a specific purpose.
2. A Preliminary Analysis of Possible Chemical Reactions during the Roasting of Pyrite
Under different roasting conditions, pyrite can be transformed to a variety of solid phases such as pyrrhotite (Fe1−xS, mainly Fe0.875S or FeS which is the commonest form [16,18]), magnetite (Fe3O4), hematite (Fe2O3), ferrous sulphate (FeSO4) and ferric sulphate (Fe2(SO4)3), and gas phases such as sulphur vapour (S2) and sulphur dioxide (SO2). In the presence of C or other phases such as CaO, MgO and Al2O3, various reduction reactions by C/CO or sulphur-fixation reactions can also take place. According to the relevant species of reactants and resultants, 45 possible chemical reactions can be deduced as listed in Table 1. They can be divided into mainly three categories: (i) pyrolysis in an inert atmosphere (Equations (1),(2)), (ii) oxidation by O2 (Equations (3)–(30)) and (iii) reduction by C or CO (Equations (31)–(45)). In addition, based on the standard Gibbs free energies of formation for species (ΔfGθ, kJ·mol−1) at different temperatures (T = 300–1200 K), the corresponding ΔrGθ for each reaction can be obtained as a function of ΔrGθ and T (listed in Table 1). The variation of ΔrGθ with T for the possible reactions is also clearly depicted in Figure 1. Thermodynamically, ΔrGθ > 0 means that a chemical reaction cannot occur; on the contrary (ΔrGθ < 0), the reaction will spontaneously occur, and the more negative the ΔrGθ value is, the more easily the reaction takes place.

Table 1.
Possible chemical reactions and corresponding ΔrGθ at temperatures of 300–1200 K *.
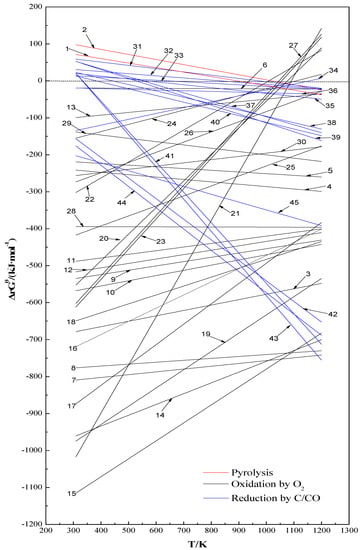
Figure 1.
ΔrGθ–T (300–1200 K) relationship for possible reactions during the roasting of pyrite.
As shown in Table 1 and Figure 1, the pyrolysis of pyrite to sulphur vapour (S2) and pyrrhotite Fe0.875S (Equation (1)) or FeS (Equation (2)) can proceed spontaneously only when the temperature exceeds around 900–1000 K (ΔrGθ < 0). However, their ΔrGθ values are slightly negative even at temperatures >1000 K, indicating that the pyrite pyrolysis is thermodynamically difficult to occur. The kinetic observations from Lambert et al. [20] and Boyabat et al. [21] suggested that the rate-controlling step of pyrite pyrolysis in an inert atmosphere was the desorption of S2 from the pyrite surface. However, under an oxygen-containing atmosphere, the oxidation of S2 by O2 to volatile SO2 (Equation (3)) is apt to take place due to its rather negative ΔrGθ as presented in Figure 1. Not surprisingly, with the formation of SO2, pyrite is readily oxidized by O2 to FeS (Equation (4)) or Fe0.875S (Equation (5)). Research also found that the oxidation rate of pyrite core to pyrrhotite (FeS) was relatively fast at moderate oxygen concentration levels (e.g., 5 vol % of O2) [22]. It can thus be considered that, in the presence of O2, pyrite firstly undergoes partial desulphurization to produce pyrrhotite and S2 (Equations (1) and (2)), and then the easy oxidation of S2 (Equation (3)) occurs with S2 acting as an intermediate in Equations (4) and (5). In addition, Fe0.875S can be further oxidized by O2 to FeS and SO2 (Equation (6)), although the corresponding ΔrGθ is much less negative than that from the oxidation of FeS2 to Fe0.875S/FeS.
Table 1 and Figure 1 also show that pyrite and its pyrolysis product (FeS or Fe0.875S) are readily oxidized by O2 to iron oxides (mainly Fe3O4 and Fe2O3) (Equations (7)–(12)) with rather negative ΔrGθ values in the order of FeS2 << FeS < Fe0.875S << 0. The formed Fe3O4 can be further oxidized to Fe2O3 (Equation (13)). In addition, the iron sulphates of Fe2(SO4)3 and FeSO4 can be generated directly from FeS2 (Equations (14) and (15)) or indirectly from the intermediates such as FeS/Fe0.875S (Equations (16)–(19)) and Fe3O4/Fe2O3 (Equations (20)–(23)). Most of ΔrGθ for these sulphation reactions are rather negative. Thermodynamically, the formation of iron sulphates from the iron sulphides (Equations (14)–(19)) tends to be easier than from the iron oxides (Equations (20)–(23)). When the temperature is overhigh (>1000–1100 K), the sulphation of Fe3O4/Fe2O3 cannot occur spontaneously due to ΔrGθ > 0. Moreover, the formation of Fe2(SO4)3 is thermodynamically easier than that of FeSO4 from the sulphating roasting of pyrite, and thus FeSO4 can be further oxidized to Fe2(SO4)3 as shown in Equation (24). In the presence of some common gangue phases such as CaO, MgO and Al2O3, they are also shown to readily react with SO2 to form sulphates (Equations (25)–(27)), capturing SO2 during the pyrite roasting and thus preventing its release into the atmosphere.
When there are carbonaceous matters, the existence of C further complicates the conditions of pyrite roasting. C can be easily oxidized by O2 to CO and/or CO2 (Equations (28)–(30)), and at temperatures >1000 K, C can also react with CO2 to form CO (Equation (31)). Thus, various reduction reactions involved with C or CO may occur during the roasting of pyrite. As shown by Equations (32) and (33), pyrite can be reduced by CO to pyrrhotite and oxysulphide (COS) at relatively high temperatures (>650–850 K) with mildly negative ΔrGθ values, and an increasing temperature is shown to favour the occurrence of these reduction reactions. S2, apart from being oxidized by O2 (Equation (3)), can also be readily reduced by CO to COS (Equation (34)). Similar with the oxidation of pyrite (Equations (4) and (5)), S2 is also likely an intermediate during the reduction of pyrite by CO. However, COS has been shown to be unstable in the presence of O2, and easily oxidized by O2 to CO2 and SO2 [23,24]. It is therefore not difficult to consider that the formation of COS and its effects are possibly negligible during the pyrite roasting in an O2-containing atmosphere. In addition, as the temperature increases, the presence of C or CO is conducive to the reduction of Fe2O3 to Fe3O4 (Equations (35) and (36)), iron sulphates to iron oxides (Equations (37)–(44)), and Fe2(SO4)3 to FeSO4 (Equation (45)).
Therefore, thermodynamically, various reactions may occur during the roasting of pyrite under different temperatures and atmospheres. The pyrolysis of pyrite is retarded unless at high temperatures (>900–1000 K). In contrast, most reactions of oxidation by O2 and reduction by C/CO can proceed spontaneously. With respect to the roasting of an auriferous pyrite to expose gold, the S is normally expected to be oxidized as SO2 with the formation of porous and insoluble iron oxides instead of soluble iron sulphates. The presence of carbonaceous matters may be advantageous to the formation of iron oxides due to the reduction of iron sulphates by C or CO, which will be discussed later.
3. Thermodynamic Behaviours for the Roasting of Pyrite
Based on the preliminary analysis of chemical reactions that may occur during the pyrite roasting, a better understanding is allowed by a further thermodynamic analysis for the processes of pyrolysis, oxidation by O2 and reduction by C/CO.
3.1. Pyrolysis of Pyrite
A number of studies on the pyrolysis of pyrite [21,25] have demonstrated that the resultants are pyrrhotite (Fe1−xS) and sulphur vapour (S2) as shown in Equation (46):
2(1 − x)·FeS2 = 2 Fe1−xS + (1 − 2x)·S2 (g); (0 ≤ x ≤ 0.223)
There are various allotropes of elemental sulphur that can be represented by Sm with m varying from 1 to 8 or higher. Hu et al. [26] have pointed out that the sulphur vapour from the thermal decomposition of pyrite mainly occurs as S2. Similarly, pyrrhotite Fe1−xS can be FeS, Fe11S12, Fe10S11, Fe9S10 or Fe7.016S8 (i.e., Fe0.875S), but a wide range of studies [16,18] have suggested that the most common forms of Fe1−xS are Fe0.875S and FeS. So Fe0.875S/FeS and S2 were considered as the main resultants for the pyrite pyrolysis, which had also been adopted as discussed in Section 2. Based on the above, the relevant mechanism for the pyrolysis of pyrite was analysed in detail.
According to the pyrolysis reactions (Equations (1) and (2)), the equilibrium constant (lnKθ) could be obtained, that is, lnKθ = ln{[PS2/Pθ]ν} (ν is the stoichiometric ratio of gaseous S2). After taking lnKθ into the Van’t Hoff equation of ΔrGθ = −RTlnKθ, the relationship between PS2 and temperature was presented as PS2 = Pθ{exp[ΔrGθ/(−νRT)]}.
The variation of PS2 with T for the pyrite pyrolysis is clearly shown in Figure 2. With the formation of S2 and pyrrhotite, the thermal decomposition of pyrite occurs only at relatively high temperatures. Thermodynamically, the formation of Fe0.875S (>~800 K) is easier than that of FeS (>~900 K). As the temperature is higher than 895 K and 1020 K, with a pronounced increase of PS2 (≥100 kPa) the pyrite decomposes markedly to Fe0.875S and FeS, respectively. This is consistent with the analysis in Section 2 and previous experimental observations [11,12,27]. In addition, the formed FeS and Fe0.875S may further decompose to Fe and S2 as shown by Equations (47) and (48) (Table 2). The relationship formulas between PS2 and T are also listed in Table 2. The further pyrolysis of pyrrhotite is, however, very difficult since the calculated PS2 for the pyrolysis of Fe0.875S and FeS is separately as low as 5.554 × 10−7 kPa and 4.9383 × 10−4 kPa even at a high temperature of 1200 K.
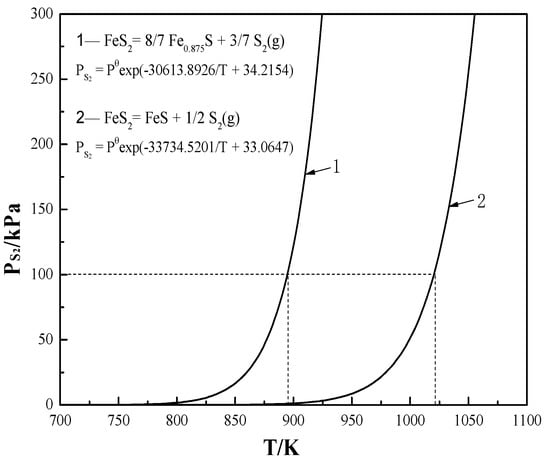
Figure 2.
Relationship of PS2 and T during the pyrolysis of pyrite.

Table 2.
Pyrolysis of FeS and Fe0.875S and the corresponding relationship between PS2 and T.
Pyrrhotite, as a typical pyrolysis product from pyrite, is also often found from the oxidative roasting of pyrite. Its formation is largely affected by the heterogeneous atmosphere, the heating effect of reactions and the particle size of pyrite. This can be illuminated from the aspects of thermodynamics and kinetics as follows:
(i) A partial inert atmosphere may be formed due to the restricted mass transfer of O2, so pyrrhotite can be generated from the pyrolysis of pyrite (Equations (1) and (2)). In an oxidizing atmosphere where O2 is freely accessible, pyrite can also be oxidized to pyrrhotite as shown by Equations (4) and (5). As mentioned in Section 2, with S2 being an intermediate, pyrrhotite can be easily formed from the oxidation of pyrite by O2 at much lower temperatures compared to the pyrolysis of pyrite.
(ii) The thermal decomposition of pyrite is endothermic whilst the oxidation of pyrite by O2 is exothermic. In particular, the oxidation of intermediate S2 by O2 (Equation (3)) is typically accompanied with the release of a large amount of heat. The exothermic effect may cause partial overhigh temperatures that favour the pyrite pyrolysis under a partial inert atmosphere.
(iii) During the roasting of pyrite particles, the S2 desorption from the pyrite surface has been suggested to be the rate-controlling step for pyrite pyrolysis [20]. In an oxidative roasting process, the formation of pyrrhotite likely conforms to a shrinking-core reaction model with pyrite as the core and pyrrhotite as the shell [22]. In addition, the rate of pyrrhotite formation from the pyrite oxidation by O2 is two orders of magnitude larger than that from the pyrite pyrolysis [22]. This is possibly due to the fact that in an O2-containing atmosphere, once the intermediate S2 makes contact with O2, it is easily oxidized as volatile SO2, which will rapidly decrease the S2 concentration in the reaction interface of pyrite and thus improve the formation of pyrrhotite. At moderate O2 concentrations, the produced pyrrhotite was found to be porous, which is beneficial to the diffusion of O2 and SO2 [21].
Oxygen can expedite the formation of pyrrhotite, but under relatively high O2 concentrations, the nonoxidized pyrrhotite continues to oxidize or the pyrite is oxidized by O2 without forming pyrrhotite as an intermediate. As described in Section 2, the oxidation products may be iron oxides or iron sulphates and SO2. The oxidation of pyrite by O2 was further discussed in detail as will be shown in the following section.
3.2. Oxidation of Pyrite by Oxygen
3.2.1. Phase Transformation of Pyrite Roasting
During the pyrite oxidation by O2, FeS2 may be converted to various iron phases that include sulphides (Fe0.875S/FeS), oxides (Fe3O4/Fe2O3) and sulphates (FeSO4/Fe2(SO4)3) as mentioned in Section 2 (Equations (4)–(24)). In addition, the produced SO2 changes the roasting atmosphere and hence has a great impact on the phase transformations for pyrite roasting.
The equilibrium constant (lnKθ) from the relevant oxidation reactions could be attained, that is, lnKθ = ln{[PSO2/Pθ](±ν1)/[PO2/Pθ]ν2}, where ±ν1 (−ν1 for the reactant and +ν1 for the resultant) and ν2 are the stoichiometric ratio of SO2 and O2, respectively. Based on ΔrGθ = −RTlnKθ, the relationship between PSO2/Pθ and PO2/Pθ was rearranged as lg[PSO2/Pθ] = [ν2/(±ν1)]{lg[PO2/Pθ]} + [ΔrGθ/(−RT)]/[(±ν1)ln10]. At a constant temperature, the isothermal predominance areas for the Fe–S–O system (Figure 3) were determined as a function of lg[PSO2/Pθ] and lg[PO2/Pθ].
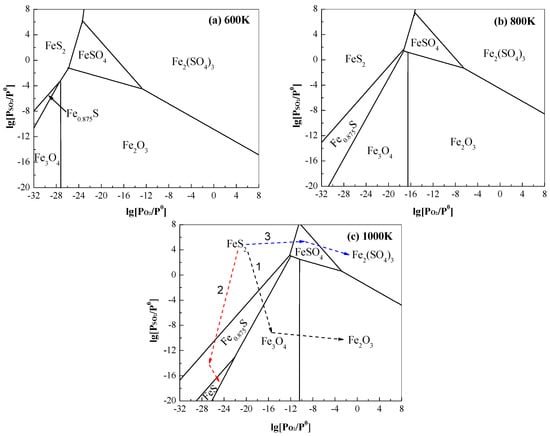
Figure 3.
Isothermal predominance area of the Fe–S–O system as a function of lg[PSO2/Pθ] and lg[PO2/Pθ] at a temperature of (a) 600 K, (b) 800 K and (c) 1000 K.
Figure 3 shows that, in a wide range of lg[PSO2/Pθ] (= −20–8) and lg[PO2/Pθ] (= −32–8), an increasing temperature from 600 K to 1000 K observably enlarges the stability regions of FeS2, Fe0.875S/FeS and Fe3O4 but shrinks those of Fe2O3, FeSO4 and Fe2(SO4)3. The stability area of FeS appears only as the temperature increases to 1000 K (Figure 3c). At a constant temperature, low PO2 is shown to benefit the stability of iron sulphides while iron oxides and sulphates tend to be stable under relatively high PO2. Under low PO2, pyrite is stable at relatively high PSO2; the decrease of PSO2 favours the existence of pyrrhotite. When PO2 is relatively high, a high PSO2 obviously benefits the occurrence of iron sulphates. On the contrary, low PSO2 is evidently advantageous to stabilise the iron oxides.
Depending upon the reaction conditions, thermodynamically, pyrite may experience three routes (1–3 marked in Figure 3c) of phase transformation during its roasting process. (i) Under insufficient SO2, pyrite can be directly oxidized with enough O2 to iron oxides (Equations (7) and (8)) via Route 1. This is consistent with the research results [10,12,13,27] showing that only hematite is observed during the roasting of pyrite in an air atmosphere. (ii) When SO2 and O2 are both inadequate, pyrite is oxidized to pyrrhotite (Equations (4) and (5)) by Route 2 as discussed in Section 3.1. (iii) In the presence of sufficient SO2 and O2, pyrite can be directly transformed to iron sulphates (Equations (14) and (15)) through Route 3, which is also supported by previous experimental studies [10,11,12].
The practical roasting process of pyrite is complex due mainly to the influence of mineral particle size and heterogeneous atmosphere. Taking the most common roasting of pyrite in excess of air/oxygen for an example, O2 is easily accessible to the surface of the pyrite particle, so iron oxides can be produced via Route 1. The diffusion of O2 into the interior of pyrite, however, is not easy due to the resistance from the outer layer of the particle. Consequently, an inert or weak O2-containing atmosphere is formed, and hence the particle nucleus tends to decompose as pyrrhotite via Route 2. When the generated pyrrhotite contacts sufficient O2, it can be further oxidized to iron oxides. Thus, a complex route of FeS2 → Fe0.875S/FeS (intermediates) → Fe3O4/Fe2O3 occurs during the pyrite roasting, which is consistent with a number of studies [10,11,14]. Similarly, during the sulphating roasting of pyrite, the pyrrhotite and/or iron oxides can also be produced as intermediates.
3.2.2. Desulphurization of Pyrite to Iron Oxides
Refractory auriferous pyrites have been extensively roasted to porous calcines (iron oxides) in order to expose the enclosed gold [9]. This roasting process is often accompanied by sintering and some side-reactions of the sulphation of iron oxides (Equations (20)–(23)). It has been suggested from Section 3.2.1 and many other studies [28,29,30,31,32,33,34,35] that the desulphurization of pyrite and sulphation of iron oxides are largely determined by the roasting temperature and atmosphere. As shown in Figure 3a–c, under a certain range of lg[PSO2/Pθ] and lg[PO2/Pθ], the increase of temperature (600–1000 K) destabilises the iron sulphates by significantly reducing their stability areas, but high temperatures also easily cause sintering and hence the secondary encapsulation of gold. Assuming that PSO2/Pθ was constant, the effects of temperature and oxygen on the roasting of pyrite were further investigated.
We could also attain a relationship formula of lg[PO2/Pθ] = ΔrGθ/[(ν2ln10)RT] + [(±ν1)/ν2]lg[PSO2/Pθ] for the desulphurization reactions of iron sulphides (Equations (7)–(12)) and sulphation reactions of iron oxides (Equations (20)–(23)) according to the equilibrium constant lnKθ = ln{[ PSO2/Pθ](±ν1)/[ PO2/Pθ]ν2} and ΔrGθ = −RTlnKθ. At a constant of PSO2/Pθ (0.05 or 0.5), the effects of T and O2 on the pyrite roasting as a function of lg[PO2/Pθ] and T are shown in Figure 4.
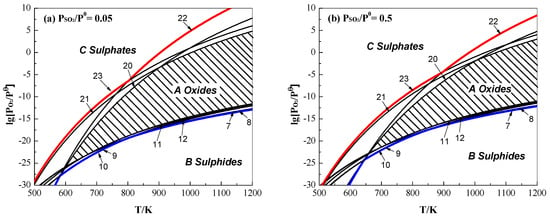
Figure 4.
Effects of O2 and T on the roasting of pyrite as a function of lg[PO2/Pθ] and T under (a) PSO2/Pθ = 0.05 and (b) PSO2/Pθ = 0.5.
As seen in Figure 4, iron oxides are produced from the oxidation of pyrite and its pyrolysis product, that is, pyrrhotite in the areas above Lines 7–12 (i.e., Equations (7)–(12)) and also from the decomposition of iron sulphates in the areas below Lines 20–23 (i.e., Equations (20)–(23)). As a result, an intersected area (i.e., shaded Area A) was obtained that represents the stability area of iron oxides. Similarly, iron sulphides and sulphates are thermodynamically stable in Area B and Area C, respectively. Comparing Figure 4a with Figure 4b, the decrease of PSO2/Pθ enlarges Area A and hence improves the thermodynamical stability of iron oxides, which is consistent with the results in Figure 3. As seen from Area A, an increasing T and O2 partial pressure appears to favour the formation of iron oxides. Thermodynamically, the reaction conditions of O2 partial pressure (or concentration) and T should be controlled within Area A to ensure the roasting of pyrite to iron oxides. In practice, besides minimizing the pressure or concentration of SO2, the temperature should be not too high in order to avoid the occurrence of sintering during roasting.
3.3. Effect of Carbon on Pyrite Roasting
As analysed in Section 2, carbon can impact the roasting of pyrite by the reduction from C/CO (Equations (35)–(44)). The reduction reactions may proceed by the direct reduction of C or the indirect reduction of CO produced from the gasification of C (Equation (31)). It is assumed that the direct reduction by C during the roasting process was negligible due mainly to the limited solid–solid reaction interfaces. Therefore, C influences the pyrite roasting mainly in a two-step way of firstly the gasification of C to CO and then the reducing action of CO.
Using the same calculation method as mentioned before, based on lnKθ = ln{[PSO2/Pθ]ν1 [PCO2/PCO]ν2} (ν1 and ν2 are the stoichiometric ratios of SO2 and CO2/CO, respectively) and ΔrGθ = −RTlnKθ, the relationship formula of lg[PCO/PCO2] = ΔrGθ/[(ν2ln10)RT] + (ν1/ν2)lg[PSO2/Pθ] was obtained for the relevant reduction reactions. Under a constant PSO2/Pθ (= 0.05), the effect of C on the pyrite roasting as a function of lg[PCO/PCO2] and T is shown in Figure 5.
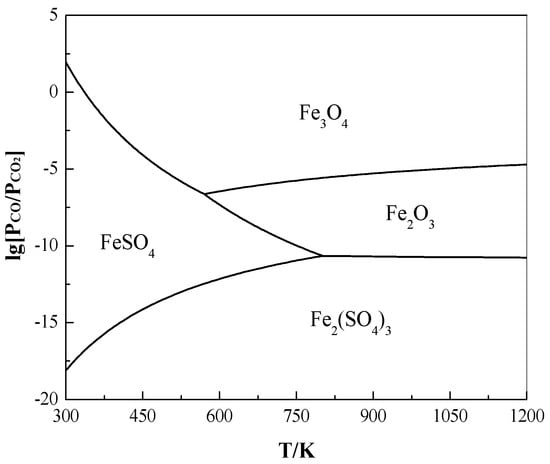
Figure 5.
Effect of C on the roasting of pyrite as a function of lg[PCO/PCO2] and T under PSO2/Pθ = 0.05.
It is clearly shown in Figure 5 that the iron sulphates are readily transformed to the iron oxides due to the reduction of CO at very low levels of PCO/PCO2. When T is lower than ~800 K, FeSO4 easily changes to Fe3O4 or Fe2O3 with an increasing T. The required PCO/PCO2 for this transformation is reduced from 10−6 at 500 K to 10−10.6 at 800 K. In addition, CO is liable to reduce Fe2(SO4)3 to FeSO4 once PCO/PCO2 is higher than 10−18–10−10.6 and then further reduce from FeSO4 to Fe3O4/Fe2O3. As T exceeds ~800 K, Fe2(SO4)3 tends to be more thermodynamically stable than FeSO4, but it is apt to be directly oxidized to Fe2O3 at PCO/PCO2 > ~10−10. Therefore, during the desulphurizing roasting of pyrite to iron oxides, the presence of a certain amount of carbon is likely conducive to prevent the formation of the by-products of iron sulphates, which is preliminarily verified by a recent research on the roasting of a refractory carbonaceous sulphide gold concentrate [36].
4. Conclusions
The roasting behaviour of pyrite under different temperatures and atmospheres is analysed by a series of thermodynamic calculations. The ΔrGθ-T (300–1200 K) relationship suggests that the pyrite roasting can include pyrolysis, oxidation, sulphation and reduction reactions under different atmospheres. In an inert atmosphere, the pyrolysis of pyrite to pyrrhotite spontaneously proceeds only at a relatively high T (>900–1000 K). Pyrrhotite can also be formed in an O2-containing atmosphere. However, comparing with the pyrite pyrolysis, the formation of pyrrhotite from oxidation can occur at much lower temperatures due mainly to the easy oxidation of S2 by O2 to SO2. The isothermal predominance areas for the Fe–S–O system indicate that pyrite may experience three routes of the phase transformation during its roasting. Firstly, pyrite is directly oxidized with sufficient O2 to iron oxides under low levels of PSO2/Pθ (i.e., Route 1: FeS2 → Fe3O4/Fe2O3). Secondly, pyrite is oxidized to pyrrhotite under low levels of PO2/Pθ and PSO2/Pθ (i.e., Route 2: FeS2 → Fe0.875S/FeS). Thirdly, pyrite is oxidized to iron sulphates under high levels of PO2/Pθ and PSO2/Pθ (i.e., Route 3: FeS2 → FeSO4/Fe2(SO4)3). The reaction conditions of PO2/Pθ and T for stabilising iron oxides (Area A), sulphides (Area B) and sulphates (Area C) could also be obtained for the roasting of pyrite. In terms of the desulphurization of pyrite to porous iron oxides, theoretically, an appropriate range of PO2/Pθ and not too high T can be chosen from Area A in order to avoid or minimize sintering, which is beneficial to expose the common associated valuable metals such as Au and Ag, and hence their subsequent leaching. In addition, C is shown to favour the formation of iron oxides. This is largely attributed to the fact that iron sulphates can be reduced by CO to iron oxides at very low levels of PCO/PCO2 such as ~10−10.6 at T > 800 K. Therefore, the presence of carbon is likely advantageous to the desulphurizing roasting of carbonaceous pyrite with O2 to iron oxides.
The actual roasting of pyrite is complicated due mainly to the particle size of pyrite and/or the heterogeneous atmosphere. During the roasting of pyrite in an O2-containing atmosphere, different reactions involving a number of intermediates may take place, which is also well explained from the thermodynamic analysis in this paper with published kinetic research. The thermodynamic results presented in this paper provide a theoretical basis and a tool for the optimization of a specific roasting behaviour of pyrite.
Author Contributions
Y.Z. collected the thermodynamic data; Y.Z. and Q.L. performed the calculations; Y.Z. and X.L. wrote the paper; B.X., Y.Y. and T.J. reviewed it before submission.
Funding
This research was funded by National Natural Science Foundation of China (Grant Nos. 51574284 and 51504293), the Fundamental Research Funds for the Central Universities of Central South University (No. 2017zzts194) and the China Scholarship Council (Grant Nos. 201706370222 and 201606370128).
Acknowledgments
Financial supports from the National Natural Science Foundation of China, the Fundamental Research Funds for the Central Universities of Central South University and the China Scholarship Council are all gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, X.; Xu, B.; Min, X.; Li, Q.; Yang, Y.; Jiang, T.; He, Y.; Zhang, X. Effect of pyrite on thiosulfate leaching of gold and the role of ammonium alcohol polyvinyl phosphate (AAPP). Metals 2017, 7, 278. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Liu, X.; Xu, B.; Yang, Y.; Jiang, T. Improvement of gold leaching from a refractory gold concentrate calcine by separate pretreatment of coarse and fine size fractions. Minerals 2017, 7, 80. [Google Scholar]
- Liu, X.; Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; Zhang, X.; Zhang, Y. Effect of galena on thiosulfate leaching of gold. Hydrometallurgy 2017, 171, 157–164. [Google Scholar] [CrossRef]
- Frser, K.S.; Whlton, R.H.; Wells, J.A. Processing of refractory gold ores. Miner. Eng. 1991, 4, 1029–1041. [Google Scholar]
- Zhou, J.; Gu, Y. Chapter 6: Geometallurgical Characterization and Automated Mineralogy of Gold Ores. In Gold Ore Processing: Project Development and Operations, 2nd ed.; Adams, M.D., Ed.; Elsevier: Oxford, UK, 2016; pp. 95–111. [Google Scholar]
- Afenya, P.M. Treatment of carbonaceous refractory gold ores. Miner. Eng. 1991, 4, 1043–1055. [Google Scholar]
- De Michelis, I.; Olivieri, A.; Ubaldini, S.; Ferella, F.; Beolchini, F.; Vegli, F. Roasting and chlorine leaching of gold-bearing refractory concentrate: Experimental and process analysis. Int. J. Min. Sci. Technol. 2013, 23, 709–715. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Q.; Song, X.; Dong, J. Research status of carbonaceous matter in carbonaceous gold ores and bio-oxidation pretreatment. Trans. Nonferrous Met. Soc. China. 2013, 23, 3405–3411. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Zhang, Y.; Jiang, T.; Yang, Y.; Xu, B.; He, Y. Improving gold recovery from a refractory ore via Na2SO4 assisted roasting and alkaline Na2S leaching. Hydrometallurgy 2019, 185, 133–141. [Google Scholar] [CrossRef]
- Dunn, J.G.; De, G.C. The effect of experimental variables on the mechanism of the oxidation of pyrite. Part 1. Oxidation of particles less than 45 μm in size. Thermochim. Acta 1989, 145, 115–130. [Google Scholar] [CrossRef]
- Dunn, J.G.; De, G.C. The effect of experimental variables on the mechanism of the oxidation of pyrite. Part 2. Oxidation of particles of size 90–125 um. Thermochim. Acta 1989, 155, 135–149. [Google Scholar] [CrossRef]
- Jorgensen, F.R.A.; Moyle, F.J. Phases formed during the thermal analysis of pyrite in air. J. Therm. Anal. 1982, 25, 473–485. [Google Scholar] [CrossRef]
- Schorr, J.R.; Everhart, J.O. Thermal behavior of pyrite and its relation to carbon and sulfur oxidation in clays. J. Am. Ceram. Soc. 1969, 52, 351–354. [Google Scholar] [CrossRef]
- Prasad, A.; Singru, R.M.; Biswa, A.K. Study of the roasting of pyrite minerals by Mössbauer spectroscopy. Phys. Status Solidi A 1985, 87, 267–271. [Google Scholar] [CrossRef]
- Hong, Y.; Fegley, B. The kinetics and mechanism of pyrite thermal decomposition. Berichte der Bunsengesellschaft für physikalische Chemie 1997, 101, 1870–1881. [Google Scholar] [CrossRef]
- Waldner, P.; Pelton, A.D. Thermodynamic modeling of the Fe-S system. J. Phase Equilib. Diffus. 2005, 26, 23–38. [Google Scholar] [CrossRef]
- Chakraborti, N.; Lynch, D.C. Thermodynamics of roasting arsenopyrite. Metall. Trans. B 1983, 14, 239–251. [Google Scholar] [CrossRef]
- Hoare, I.C.; Hurst, H.J.; Stuart, W.I. Thermal decomposition of pyrite: Kinetic analysis of thermogravimetric data by predictor-corrector numerical methods. J. Chem. Soc., Faraday Trans. 1 1988, 84, 3071–3077. [Google Scholar] [CrossRef]
- Barin, I. Thermochemical Data of Pure Substances, 3rd ed.; VCH Verlagsgesellschaft mbH: Weinheim, Germany, 1995. [Google Scholar]
- Lambert, J.M.; Simkovich, G.; Walker, P.L. The kinetics and mechanism of the pyrite-to-pyrrhotite Transformation. Metall. Mater. Trans. B 1998, 29, 385–396. [Google Scholar] [CrossRef]
- Boyabat, N.; Özer, A.K.; Bayrakceken, S.; Glaboğlu, M.Ş. Thermal decomposition of pyrite in the nitrogen atmosphere. Fuel Process. Technol. 2003, 85, 179–188. [Google Scholar] [CrossRef]
- Hansen, J.P.; Jensen, L.S.; Wedel, S.; Dam-Johansen, K. Decomposition and oxidation of pyrite in a fixed-bed reactor. Ind. Eng. Chem. Res. 2003, 42, 4290–4295. [Google Scholar] [CrossRef]
- Lv, W.; Yu, D.; Wu, J.; Zhang, L.; Xu, M. The chemical role of CO2 in pyrite thermal decomposition. Proc. Combust. Inst. 2015, 35, 3637–3644. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Lincoln, F.J. Mechanochemical milling-induced reactions between gases and sulfide minerals: II. Reactions of CO2 with arsenopyrite, pyrrhotite and pyrite. J. Alloys Compd. 2001, 314, 103–113. [Google Scholar] [CrossRef]
- Hu, H.; Chen, Q.; Yin, Z.; Zhang, P. Thermal behaviors of mechanically activated pyrites by thermogravimetry (TG). Thermochim. Acta 2003, 398, 233–240. [Google Scholar] [CrossRef]
- Hu, G.; Dam-Johansen, K.; Wedel, S.; Hansen, J.P. Decomposition and oxidation of pyrite. Prog. Energy Combust. Sci. 2006, 32, 295–314. [Google Scholar] [CrossRef]
- Bhargava, S.K.; Garg, A.; Subasinghe, N.D. In situ high-temperature phase transformation studies on pyrite. Fuel 2009, 88, 988–993. [Google Scholar] [CrossRef]
- Schwab, G.M.; Philinis, J. Reactions of iron pyrite: Its thermal decomposition, reduction by hydrogen and air oxidation. J. Am. Chem. Soc. 1947, 69, 2588–2596. [Google Scholar] [CrossRef]
- Eneroth, E.; Koch, C.B. Crystallite size of haematite from thermal oxidation of pyrite and marcasite—Effects of grain size and iron disulphide polymorph. Miner. Eng. 2003, 16, 1257–1267. [Google Scholar] [CrossRef]
- Eymery, J.P. On a phase transformation produced by mechanical activation in iron pyrite. Eur. Phys. J. Appl. Phys. 1999, 5, 115–121. [Google Scholar] [CrossRef]
- Dunn, J.G.; Gong, W.; Shi, D. A Fourier transform infrared study of the oxidation of pyrite. Thermochim. Acta 1992, 208, 293–303. [Google Scholar] [CrossRef]
- Dunn, J.G.; Gong, W.; Shi, D. A Fourier transform infrared study of the oxidation of pyrite. The influences of experimental variables. Thermochim. Acta 1993, 215, 247–254. [Google Scholar] [CrossRef]
- Komraus, J.; Popiel, E.; Mocek, R. Chemical transformations of ferruginous minerals during the process of oxidation of hard coal. Hyperfine Interact 1990, 58, 2589–2592. [Google Scholar] [CrossRef]
- Allen, G.C.; Paul, M. Chemical characterization of transition metal spinel-type oxides by infrared spectroscopy. Appl. Spectrosc. 1995, 49, 451–458. [Google Scholar] [CrossRef]
- Eymery, J.P.; Ylli, F. Study of a mechanochemical transformation in iron pyrite. J. Alloys Compd. 2000, 298, 306–309. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Zhang, Y.; Jiang, T.; Yang, Y.; Xu, B.; He, Y. Simultaneous removal of S and As from a refractory gold ore in a single stage O2-enriched roasting process. Metall. Mater. Trans. B 2019, in press. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).