Fingerprinting Paranesti Rubies through Oxygen Isotopes
Abstract
1. Introduction
1.1. Oxygen Isotopic Studies in Corundums
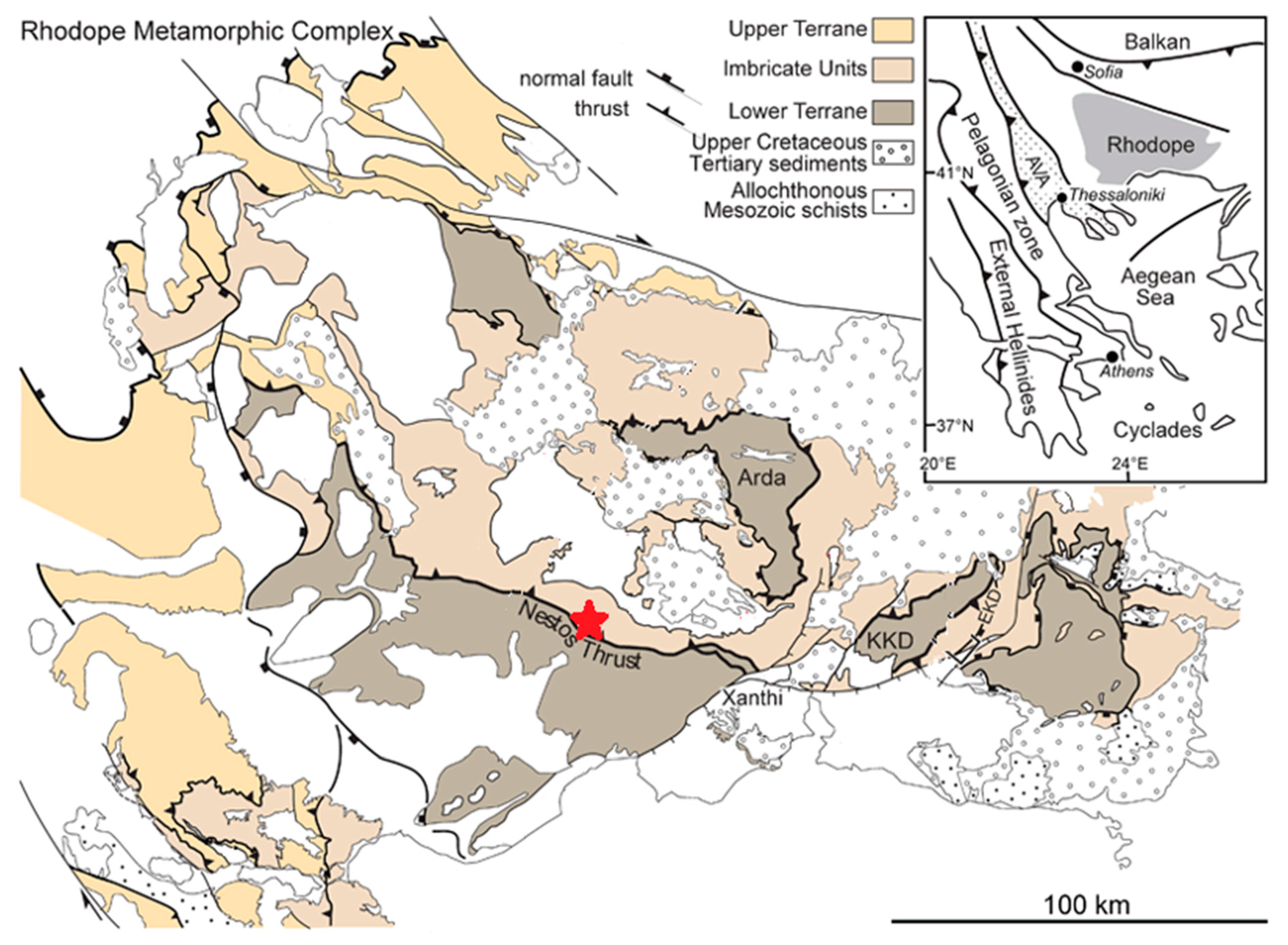
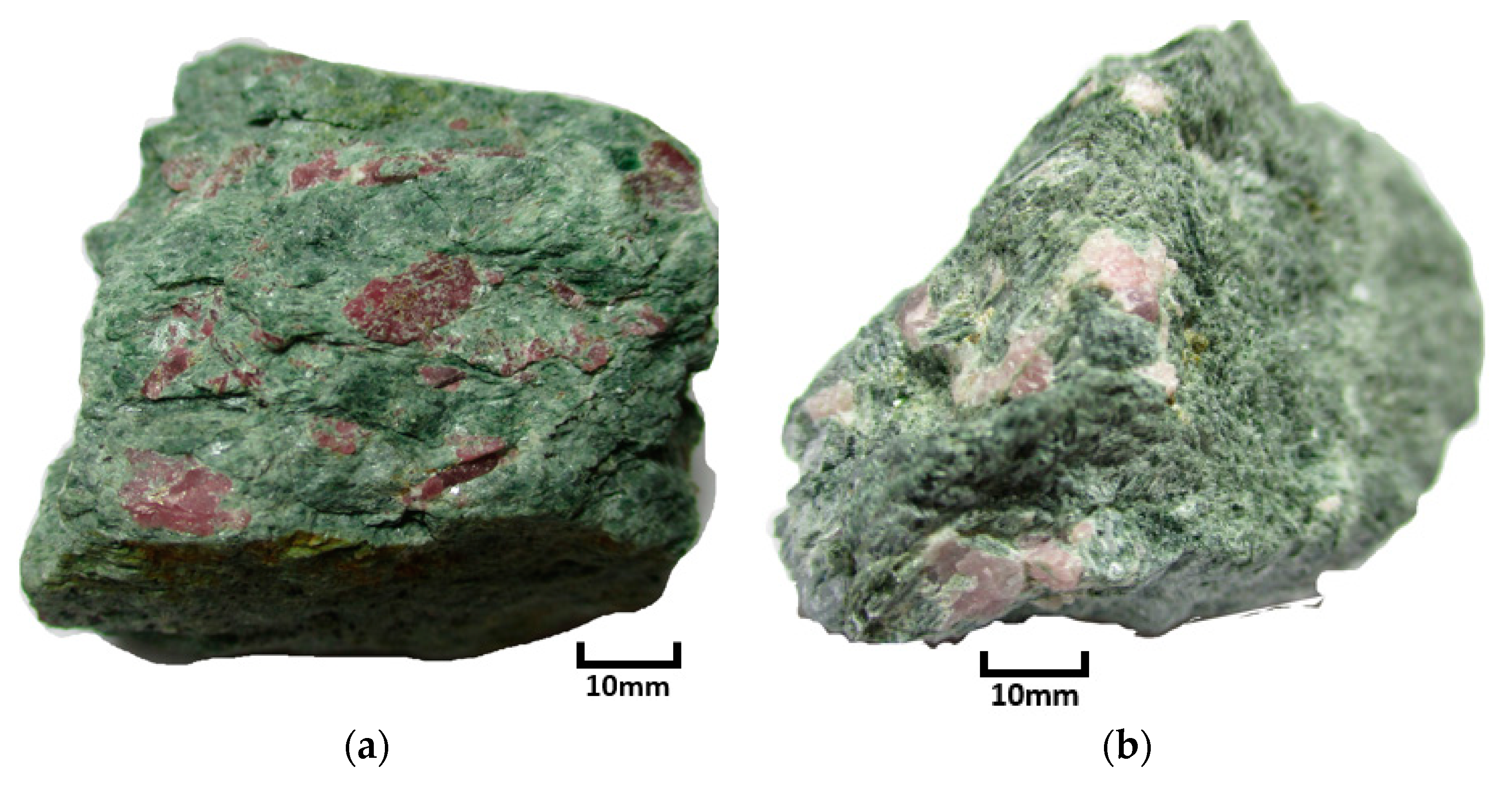
1.2. Geological Setting and Sample Background
2. Materials and Methods
2.1. Laser-Fluorination Method (2009)
2.2. Secondary Ion Mass Spectrometry (SIMS) Method (2017)
3. Results
3.1. Laser-Fluorination Results
3.2. Secondary Ionisation Mass Spectrometry (SIMS) Results
4. Discussion
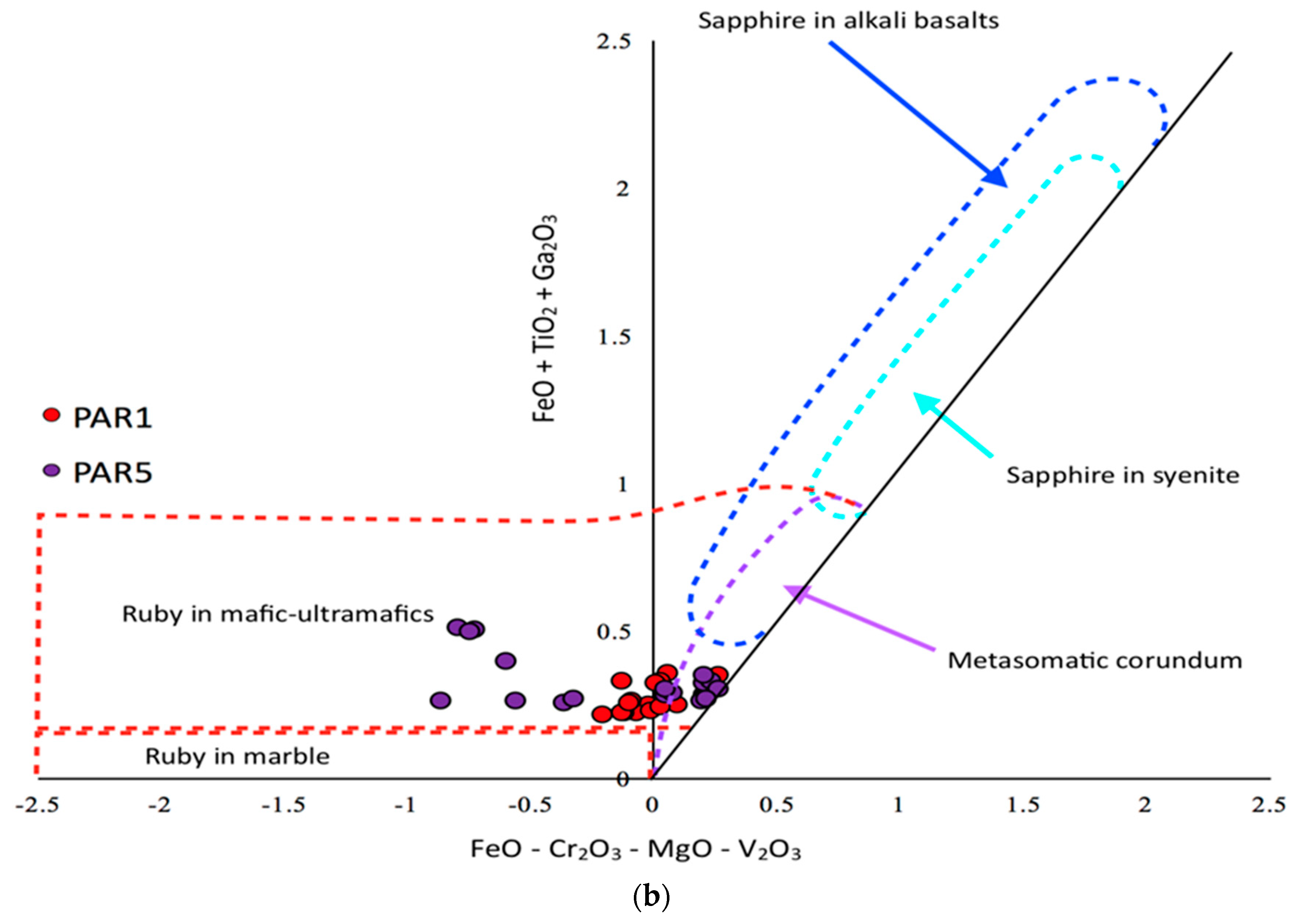
4.1. Corundum Oxygen Isotopes as An Identifier for Geological Origin
- Mafic gneiss hosted from 2.9‰ to 3.8‰;
- Mafic-ultramafic rocks (amphibolite, serpentinite) from 3.2‰ to 6.8‰;
- Desilicated pegmatites from 4.2‰ to 7.5‰;
- Shear zones cross-cutting ultramafic lenses and pegmatites within sillimanite gneisses 11.9‰–13.1‰;
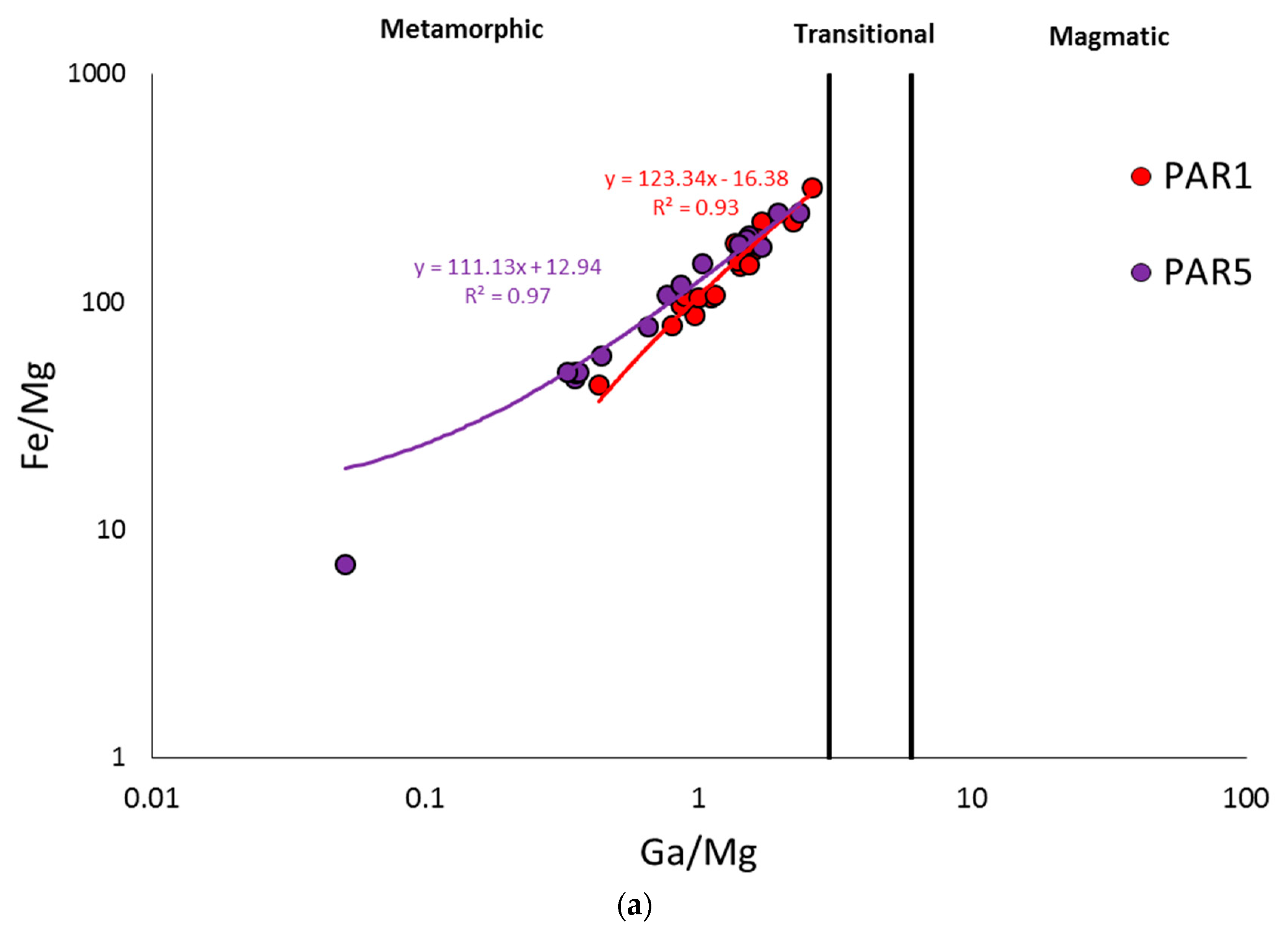
4.2. PAR-1 vs. PAR-5 Variations
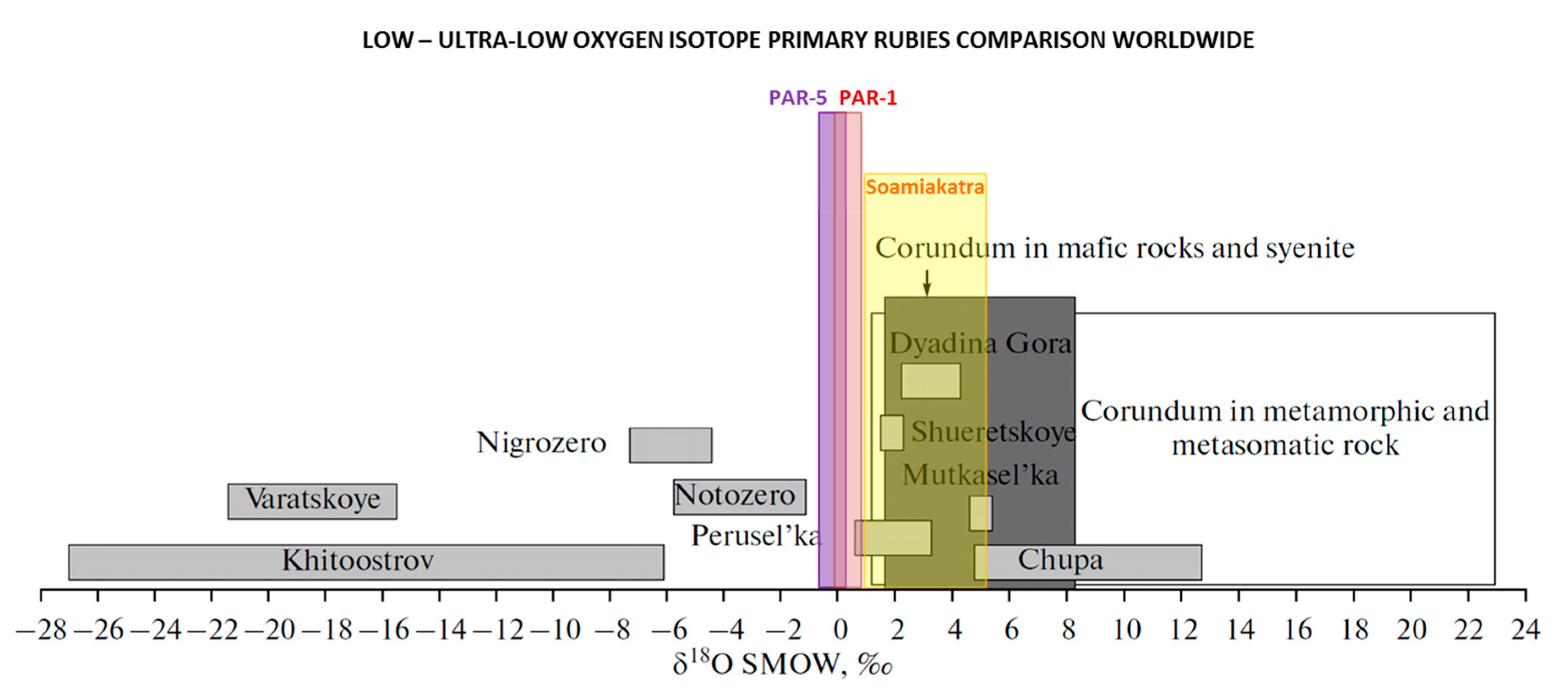
4.3. Global Low to Ultra-Low Oxygen Isotope Corundum Comparison
4.4. Possible Causes for Low Oxygen Isotope Corundum Formation
4.4.1. Kinetic Isotope Fractionation
4.4.2. Thermal Diffusion
4.4.3. Other Ultra-Low δ18O Protoliths
4.4.4. Hydrothermal Alteration Model
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoefs, J. Stable Isotope Geochemistry; Springer: Berlin/Heidelberg, Germany, 1997; ISBN 9783662033791. [Google Scholar]
- Bowen, G.J.; Wassenaar, L.I.; Hobson, K.A. Global application of stable hydrogen and oxygen isotopes to wildlife forensics. Oecologia 2005, 143, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.G.; Fairbanks, R.G.; Mountain, G.S. Tertiary oxygen isotope synthesis, sea level history and continental margin erosion. Paleoceanography 1987, 2, 1–19. [Google Scholar] [CrossRef]
- Criss, R.E.; Taylor, H.P., Jr. Meteoric-hydrothermal systems. Rev. Mineral. 1986, 16, 373–424. [Google Scholar]
- Valley, J.W. Stable Isotope Thermometry at High Temperatures. Rev. Mineral. Geochem. 2001, 43, 365–413. [Google Scholar] [CrossRef]
- Bindeman, I. Oxygen Isotopes in Mantle and Crustal Magmas as Revealed by Single Crystal Analysis. Rev. Mineral. Geochem. 2008, 69, 445–478. [Google Scholar] [CrossRef]
- Bindeman, I.N.; Schmitt, A.K.; Evans, D.A.D. Limits of hydrosphere-lithosphere interaction: Origin of the lowest-known δ18O silicate rock on Earth in the Paleoproterozoic Karelian rift. Geology 2010, 38, 631–634. [Google Scholar] [CrossRef]
- Giuliani, G.; Ohnenstetter, D.; Garnier, V.; Fallick, A.E.; Rakotondrazafy, M.; Schwarz, D. The geology and genesis of gem corundum deposits. In Geology of Gem Deposits; Groat, L.A., Ed.; Mineralogical Association of Canada: Québec, QC, Canada, 2007; Volume 37, pp. 23–78. ISBN 9780921294375. [Google Scholar]
- Yui, T.-F.; Khin, Z.; Limtrakun, P. Oxygen isotope composition of the Denchai sapphire, Thailand: A clue to its enigmatic origin. Lithos 2003, 67, 153–161. [Google Scholar] [CrossRef]
- Krylov, D.P.; Glebovitsky, V.A. Oxygen isotopic composition and nature of fluid during the formation of high-Al corundum-bearing rocks of Mt. Dyadina, northern Karelia. Doklady Earth Sci. 2007, 413, 210–212. [Google Scholar] [CrossRef]
- Giuliani, G.; Fallick, A.E.; Garnier, V.; France-Lanord, C.; Ohnenstetter, D.; Schwarz, D. Oxygen isotope composition as a tracer for the origins of rubies and sapphires. Geology 2005, 33, 249. [Google Scholar] [CrossRef]
- Vysotskiy, S.V.; Ignat’ev, A.V.; Levitskii, V.I.; Nechaev, V.P.; Velivetskaya, T.A.; Yakovenko, V.V. Geochemistry of stable oxygen and hydrogen isotopes in minerals and corundum-bearing rocks in northern Karelia as an indicator of their unusual genesis. Geochem. Int. 2014, 52, 773–782. [Google Scholar] [CrossRef]
- Bonev, N.; Burg, J.-P.; Ivanov, Z. Mesozoic–Tertiary structural evolution of an extensional gneiss dome—The Kesebir–Kardamos dome, eastern Rhodope (Bulgaria–Greece). Int. J. Earth Sci. 2006, 95, 318–340. [Google Scholar] [CrossRef]
- Krenn, K.; Bauer, C.; Proyer, A.; Mposkos, E.; Hoinkes, G. Fluid entrapment and reequilibration during subduction and exhumation: A case study from the high-grade Nestos shear zone, Central Rhodope, Greece. Lithos 2008, 104, 33–53. [Google Scholar] [CrossRef]
- Barr, S.R.; Temperley, S.; Tarney, J. Lateral growth of the continental crust through deep level subduction–accretion: A re-evaluation of central Greek Rhodope. Lithos 1999, 46, 69–94. [Google Scholar] [CrossRef]
- Nagel, T.J.; Schmidt, S.; Janák, M.; Froitzheim, N.; Jahn-Awe, S.; Georgiev, N. The exposed base of a collapsing wedge: The Nestos Shear Zone (Rhodope Metamorphic Province, Greece): The Nestos Shear Zone. Tectonics 2011, 30. [Google Scholar] [CrossRef]
- Moulas, E.; Schenker, F.L.; Burg, J.-P.; Kostopoulos, D. Metamorphic conditions and structural evolution of the Kesebir-Kardamos dome: Rhodope metamorphic complex (Greece-Bulgaria). Int. J. Earth Sci. 2017, 106, 2667–2685. [Google Scholar] [CrossRef]
- Wang, K.K.; Graham, I.T.; Lay, A.; Harris, S.J.; Cohen, D.R.; Voudouris, P.; Belousova, E.; Giuliani, G.; Fallick, A.E.; Greig, A. The Origin of a New Pargasite-Schist Hosted Ruby Deposit from Paranesti, Northern Greece. Can. Mineral. 2017, 55, 535–560. [Google Scholar] [CrossRef]
- Sutherland, F.; Zaw, K.; Meffre, S.; Yui, T.-F.; Thu, K. Advances in Trace Element “Fingerprinting” of Gem Corundum, Ruby and Sapphire, Mogok Area, Myanmar. Minerals 2014, 5, 61–79. [Google Scholar] [CrossRef]
- Giuliani, G.; Ohnenstetter, D.; Fallick, A.E. The geology and genesis of gem corundum deposits. In Geology of Gem Deposits. Series: Mineralogical Association of Canada Short Course Series, 44; Groat, L.A., Ed.; Mineralogical Association of Canada: Québec, QC, Canada, 2014; pp. 35–112. ISBN 9780921294542. [Google Scholar]
- Sharp, Z.D. In situ laser microprobe techniques for stable isotope analysis. Chem. Geol. 1992, 101, 3–19. [Google Scholar] [CrossRef]
- Garnier, V.; Giuliani, G.; Ohnenstetter, D.; Fallick, A.E.; Dubessy, J.; Banks, D.; Vinh, H.Q.; Lhomme, T.; Maluski, H.; Pêcher, A.; et al. Marble-hosted ruby deposits from Central and Southeast Asia: Towards a new genetic model. Ore Geol. Rev. 2008, 34, 169–191. [Google Scholar] [CrossRef]
- Zaw, K.; Sutherland, L.; Yui, T.-F.; Meffre, S.; Thu, K. Vanadium-rich ruby and sapphire within Mogok Gemfield, Myanmar: Implications for gem color and genesis. Miner. Deposita 2015, 50, 25–39. [Google Scholar] [CrossRef]
- Graham, I.; Sutherland, L.; Zaw, K.; Nechaev, V.; Khanchuk, A. Advances in our understanding of the gem corundum deposits of the West Pacific continental margins intraplate basaltic fields. Ore Geol. Rev. 2008, 34, 200–215. [Google Scholar] [CrossRef]
- Sutherland, F.L.; Duroc-Danner, J.M.; Meffre, S. Age and origin of gem corundum and zircon megacrysts from the Mercaderes–Rio Mayo area, South-west Colombia, South America. Ore Geol. Rev. 2008, 34, 155–168. [Google Scholar] [CrossRef]
- Giuliani, G.; Fallick, A.; Rakotondrazafy, M.; Ohnenstetter, D.; Andriamamonjy, A.; Ralantoarison, T.; Rakotosamizanany, S.; Razanatseheno, M.; Offant, Y.; Garnier, V.; et al. Oxygen isotope systematics of gem corundum deposits in Madagascar: Relevance for their geological origin. Miner. Deposita 2007, 42, 251–270. [Google Scholar] [CrossRef]
- Vysotskiy, S.V.; Nechaev, V.P.; Kissin, A.Y.; Yakovenko, V.V.; Ignat’ev, A.V.; Velivetskaya, T.A.; Sutherland, F.L.; Agoshkov, A.I. Oxygen isotopic composition as an indicator of ruby and sapphire origin: A review of Russian occurrences. Ore Geol. Rev. 2015, 68, 164–170. [Google Scholar] [CrossRef]
- Bindeman, I.N.; Serebryakov, N.S. Geology, Petrology and O and H isotope geochemistry of remarkably 18O depleted Paleoproterozoic rocks of the Belomorian Belt, Karelia, Russia, attributed to global glaciation 2.4 Ga. Earth Planet. Sci. Lett. 2011, 306, 163–174. [Google Scholar] [CrossRef]
- Yui, T.-F.; Wu, C.-M.; Limtrakun, P.; Sricharn, W.; Boonsoong, A. Oxygen isotope studies on placer sapphire and ruby in the Chanthaburi-Trat alkali basaltic gemfield, Thailand. Lithos 2006, 86, 197–211. [Google Scholar] [CrossRef]
- Krylov, D.P. Anomalous 18O/16O ratios in the corundum-bearing rocks of Khitostrov, northern Karelia. Doklady Earth Sci. 2008, 419, 453–456. [Google Scholar] [CrossRef]
- Bindeman, I.N.; Lundstrom, C.C.; Bopp, C.; Huang, F. Stable isotope fractionation by thermal diffusion through partially molten wet and dry silicate rocks. Earth Planet. Sci. Lett. 2013, 365, 51–62. [Google Scholar] [CrossRef]
- Akimova, E.Y.; Lokhov, K.I. Ultralight Oxygen in Corundum-Bearing Rocks of North Karelia, Russia, as a Result of Isotope Separation by Thermal Diffusion (Soret Effect) in Endogenous Fluid Flow. J. Mater. Sci. Chem. Eng. 2015, 3, 42–47. [Google Scholar] [CrossRef]
- Clayton, R.N.; Mayeda, T.K. Kinetic Isotope Effects in Oxygen in the Laboratory Dehydration of Magnesian Minerals. J. Phys. Chem. 2009, 113, 2212–2217. [Google Scholar] [CrossRef]
- Mendybayev, R.A.; Richter, F.M.; Spicuzza, M.J.; Davis, A.M. Oxygen isotope fractionation during evaporation of Mg- and Si- rich CMAS-liquid in vacuum. Lunar Planet. Inst. Sci. Conf. Abstr. 2010, 41, 2725. [Google Scholar]
- Bindeman, I.N.; Serebryakov, N.S.; Schmitt, A.K.; Vazquez, J.A.; Guan, Y.; Azimov, P.Y.; Astafiev, B.Y.; Palandri, J.; Dobrzhinetskaya, L. Field and microanalytical isotopic investigation of ultradepleted in 18O Paleoproterozoic “Slushball Earth” rocks from Karelia, Russia. Geosphere 2014, 10, 308–339. [Google Scholar] [CrossRef]
- Wilson, A.F.; Baksi, A.K. Widespread 18O depletion in some precambrian granulites of Australia. Precambrian Res. 1983, 23, 33–56. [Google Scholar] [CrossRef]
- Menant, A.; Jolivet, L.; Vrielynck, B. Kinematic reconstructions and magmatic evolution illuminating crustal and mantle dynamics of the eastern Mediterranean region since the late Cretaceous. Tectonophysics 2016, 675, 103–140. [Google Scholar] [CrossRef]

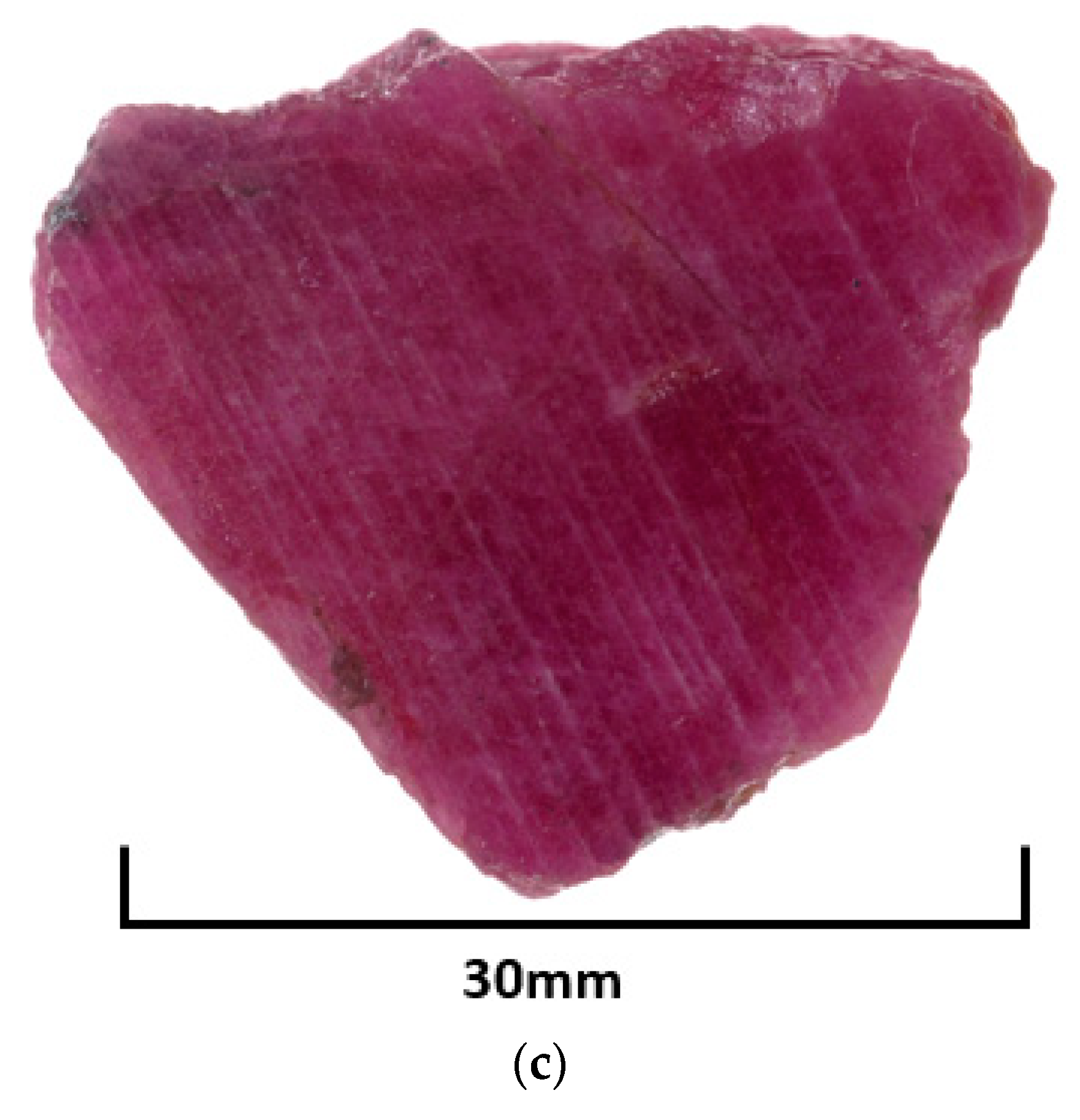
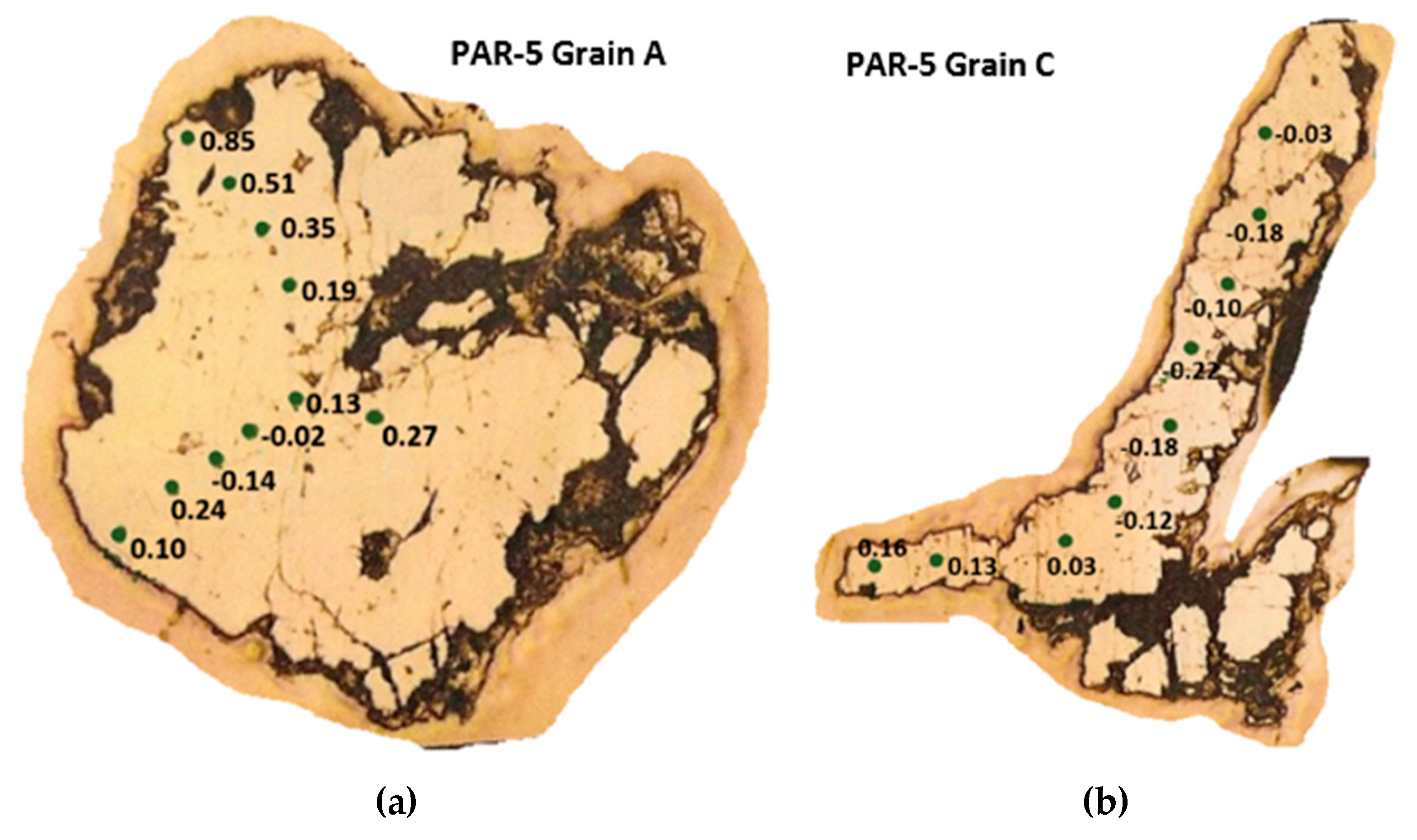
| Attributes | PAR-1 | PAR-5 |
|---|---|---|
| Physical Characteristics | ||
| Site | Hillside surface outcrop | Roadside surface outcrop—500 m east of PAR1 |
| Grain-size | 10 mm–20 mm | 5 mm–10 mm |
| Colour | Deeper red than PAR-5 (generally) | Medium red |
| Inclusions | Spinels | None |
| Microscope view | More fractured, finer-grained | - |
| Host rock | Pargasite schist | Pargasite schist |
| EMPA Analyses (wt. %) | ||
| Cr2O3 | 0.11–1.68 | 0.13–0.29 |
| FeO | 0.19–0.73 | 0.18–0.36 |
| TiO2 | 0–0.01 | 0–0.06 |
| Ga2O3 | 0–0.04 | 0–0.04 |
| LA-ICP-MS: Trace Element Analysis (ppm) | ||
| Cr | 360–2856 | 4–8627 |
| Fe | 1572–2664 | 1833–3822 |
| V | 1–3 | 2–5 |
| Mg | 7–42 | 8–376 |
| Ti | 6–184 | 10–190 |
| Ga | 14-23 | 13–29 |
| Si | 781–2456 | 837–2123 |
| Ca | 769–2119 | 653–1903 |
| Grain | δ18O Min | δ18O Max | δ18O Mean | Number of Analyses |
|---|---|---|---|---|
| PAR-1a | 0.64 | 1.62 | 1.00 ± 0.42 | 31 |
| PAR-1b | 0.44 | 1.17 | 0.67 ± 0.37 | 13 |
| PAR-1c | 0.77 | 1.68 | 1.27 ± 0.47 | 13 |
| PAR-1 Total | 0.44 | 1.68 | 1.00 ± 0.42 | 57 |
| PAR-5central | −0.04 | 0.51 | 0.27 | 12 |
| PAR-5a | −0.14 | 0.85 | 0.25 | 10 |
| PAR-5b | −0.31 | 0.42 | 0.03 | 8 |
| PAR-5c | −0.22 | 0.16 | −0.06 | 9 |
| PAR-5d | 0.08 | 0.27 | 0.17 | 5 |
| PAR-5 Total | −0.31 | 0.85 | 0.14 ± 0.24 | 44 |
| Combined PAR-1 and PAR-5 | −0.31 | 1.31 | 0.60 | 93 |
| Sample | Location | Sample Type | Deposit Type | δ18O |
|---|---|---|---|---|
| NAX2 | Naxos, Greece | Colourless sapphire | Desilicified pegmatite | 4.80 |
| NAX3 | Naxos, Greece | Colourless to blue sapphire | Desilicified pegmatite | 5.05 |
| PAR-1 | Paranesti, Greece | Red ruby | Pargasite schist | 1.00 |
| KIM2 | Kimi, Greece | Pink ruby | Marble-hosted | 20.50 |
| Xanthi | Xanthi, Greece | Purple-pink ruby | Marble-hosted | 22.09 |
| Country | District | δ18O‰ (Min) | δ18O‰ (Max) | Host Rock | Primary vs. Secondary | Corundum Type |
|---|---|---|---|---|---|---|
| Greece 1 Greece 1 | Paranesti-1 * Paranesti-5 * | 0.65 −0.31 | 1.31 0.85 | Pargasite schist Pargasite schist | Primary Primary | Ruby Ruby |
| Madagascar 2 | Soamiakatra * | 1.25 | 4.70 | Pyroxenitic enclaves in basalt | Primary | Ruby |
| Madagascar 2 | Ilakaka * | −0.30 | 16.5 | Placer in sandstone | Secondary | Sapphire |
| Madagascar 2 | Andilamena * | 0.50 | 3.9 | Placer in basalt | Secondary | Ruby |
| Russia 3,4,7 | Khitostrov ^ | −26.3 | −17.7 | plagiogneiss | Primary | Corundum |
| Russia 4,7 | Khitostrov * | −26 | - | Crn-St-Gt-Bi-Prg-Pl rocks with coarse grained Crn | Primary | Corundum |
| Russia 7 | Khitostrov * | −18.6 | - | Ky-Crn-Pl, leucocratic | Primary | Corundum |
| Russia 3 | Varastskoye ^ | −19.2 | −11.3 | plagiogneiss | Primary | Corundum |
| Russia 4 | Varastskoye # | −17.3 | - | Crn-Cam rock, coarse grained | Primary | Corundum |
| Varastskoye # | −19.2 | - | Crn and Crn-St-Pl substituting Ky | Primary | Corundum | |
| Russia 4 | Dyadina # | 0.49 | - | Inclusion of Cam-Crn in giant Gt | Inclusion | Corundum |
| Russia 4 | Dyadina # | 0.10 | - | Crn-Cam rock, coarse grained | Primary | Corundum |
| Russia 5 | Dyadina * | 0.4 | 0.8 | Corundum amphibolite | Primary | Corundum |
| Russia 4 | Kulezhma # | 0.31 | - | Cam-Crn rock | Primary | Corundum |
| Russia 4 | Pulonga # | 0.67 | - | Crn-Gt-Ged rock | Primary | Corundum |
| Russia 4 | Perusel’ka * | 0.26 | 3.45 | Crn-Cam rock, coarse grained | Primary | Corundum |
| Russia 5 | Perusel’ka # | 0.6 | - | Corundum-kyanite amphibolite | Primary | Corundum |
| Russia 5 | Perusel’ka # | 1.5 | - | Corundum amphibolite | Primary | Corundum |
| Russia 5 | Notozero * | −1.7 | −1.5 | Ged-Gt rocks with Crn and St | Primary | Corundum |
| Russia 4 | Mironova Guba ^ | (2.34) | - | Cam-Crn rock | Primary | Corundum |
| Thailand 6 | Bo Rai * | 1.30 | 4.20 | Placer in basalt | Secondary | Ruby |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.K.; Graham, I.T.; Martin, L.; Voudouris, P.; Giuliani, G.; Lay, A.; Harris, S.J.; Fallick, A. Fingerprinting Paranesti Rubies through Oxygen Isotopes. Minerals 2019, 9, 91. https://doi.org/10.3390/min9020091
Wang KK, Graham IT, Martin L, Voudouris P, Giuliani G, Lay A, Harris SJ, Fallick A. Fingerprinting Paranesti Rubies through Oxygen Isotopes. Minerals. 2019; 9(2):91. https://doi.org/10.3390/min9020091
Chicago/Turabian StyleWang, Kandy K., Ian T. Graham, Laure Martin, Panagiotis Voudouris, Gaston Giuliani, Angela Lay, Stephen J. Harris, and Anthony Fallick. 2019. "Fingerprinting Paranesti Rubies through Oxygen Isotopes" Minerals 9, no. 2: 91. https://doi.org/10.3390/min9020091
APA StyleWang, K. K., Graham, I. T., Martin, L., Voudouris, P., Giuliani, G., Lay, A., Harris, S. J., & Fallick, A. (2019). Fingerprinting Paranesti Rubies through Oxygen Isotopes. Minerals, 9(2), 91. https://doi.org/10.3390/min9020091







