Modeling the Crystallization and Emplacement Conditions of a Basaltic Trachyandesitic Sill at Mt. Etna Volcano
Abstract
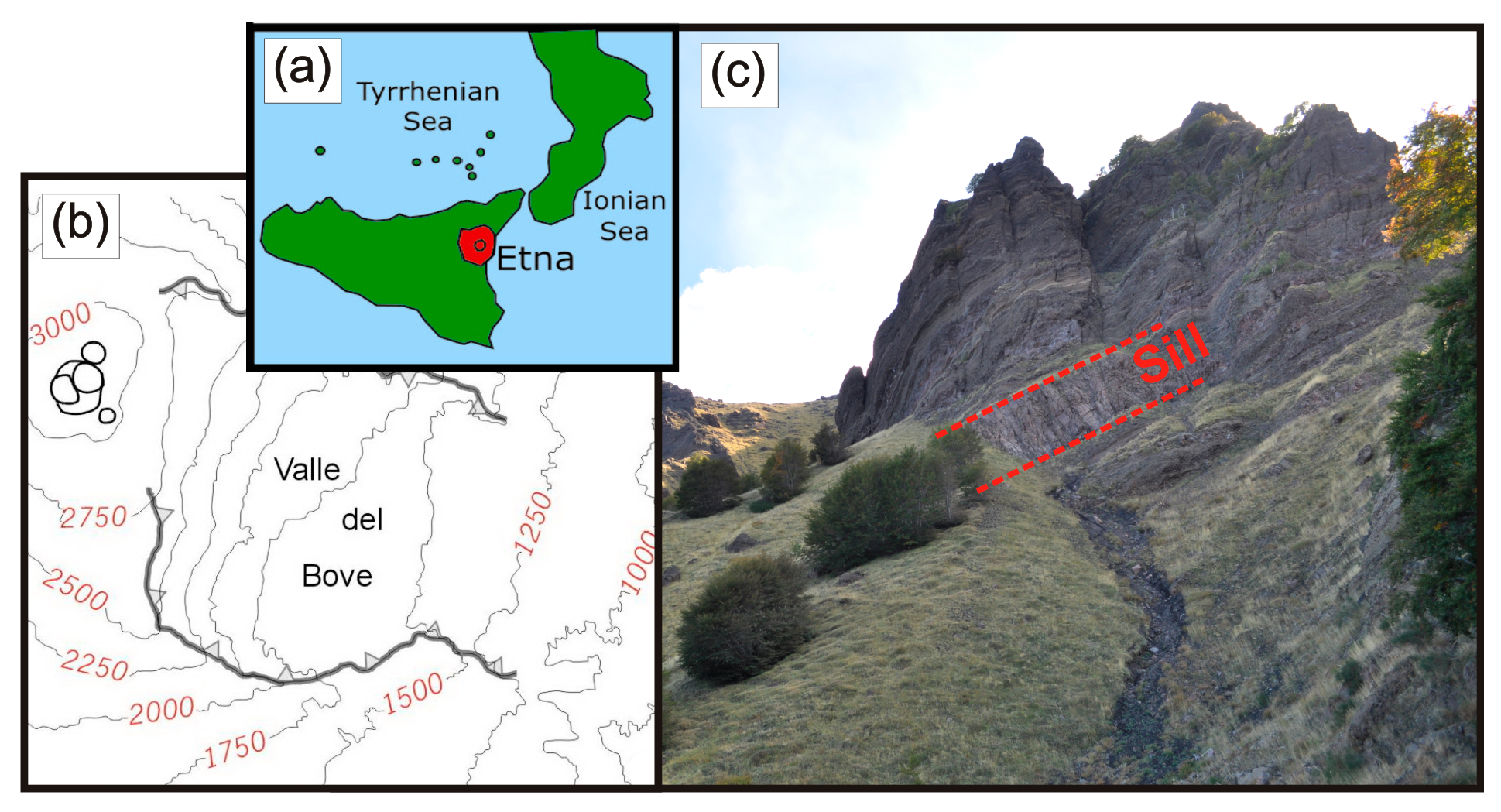
:1. Introduction
2. Methods
3. Results
3.1. Texture
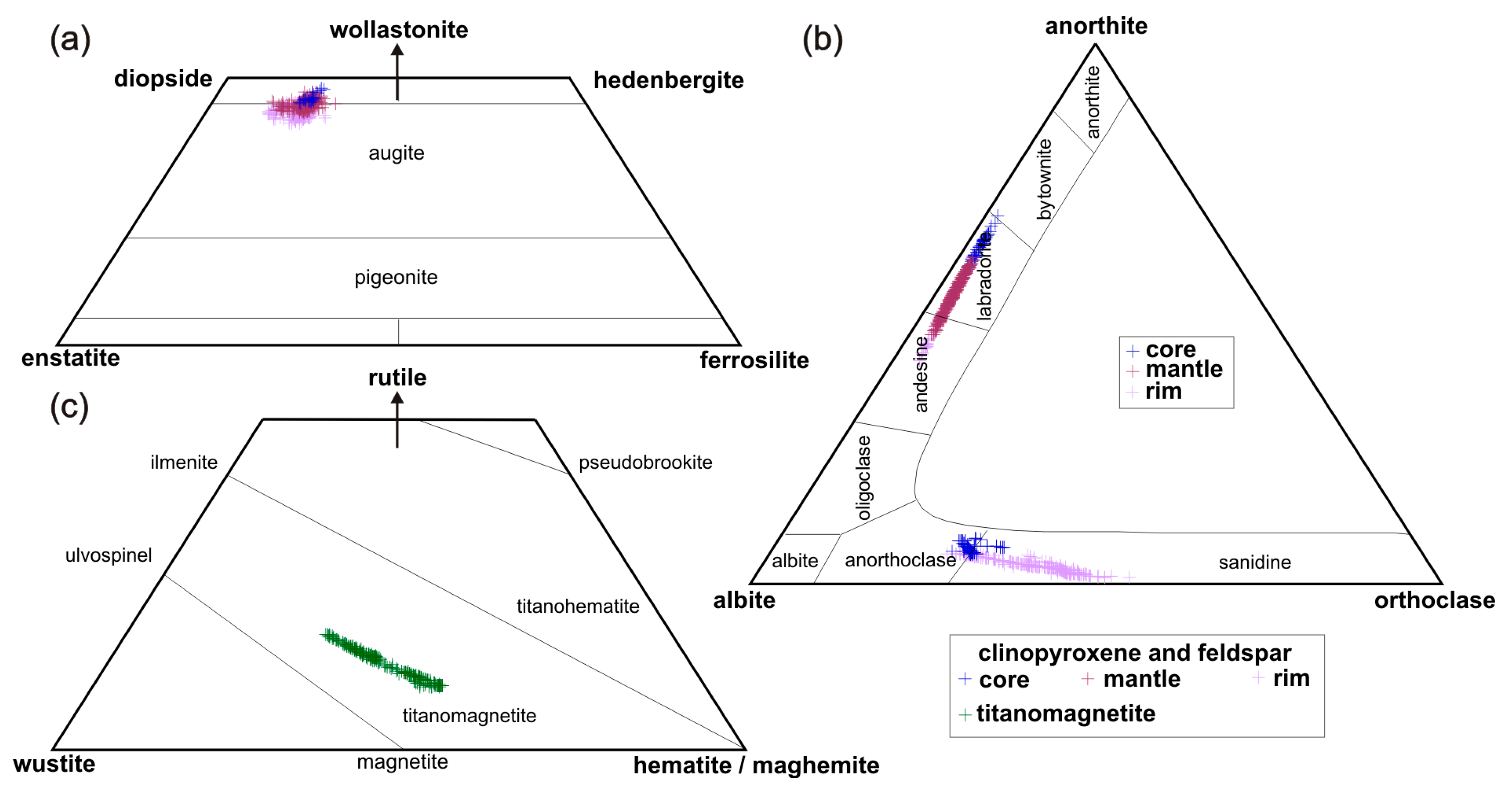
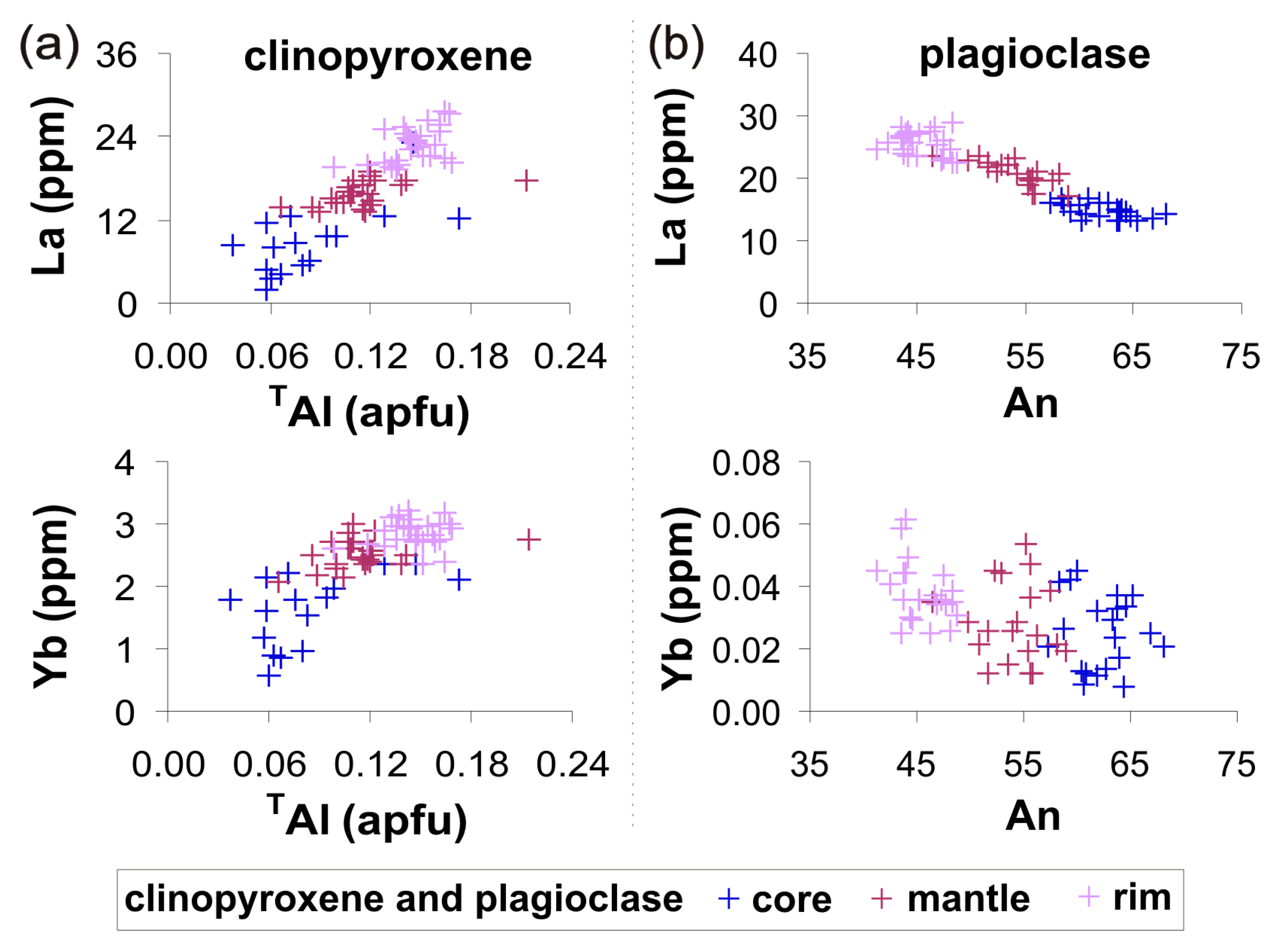
3.2. Mineral Chemistry
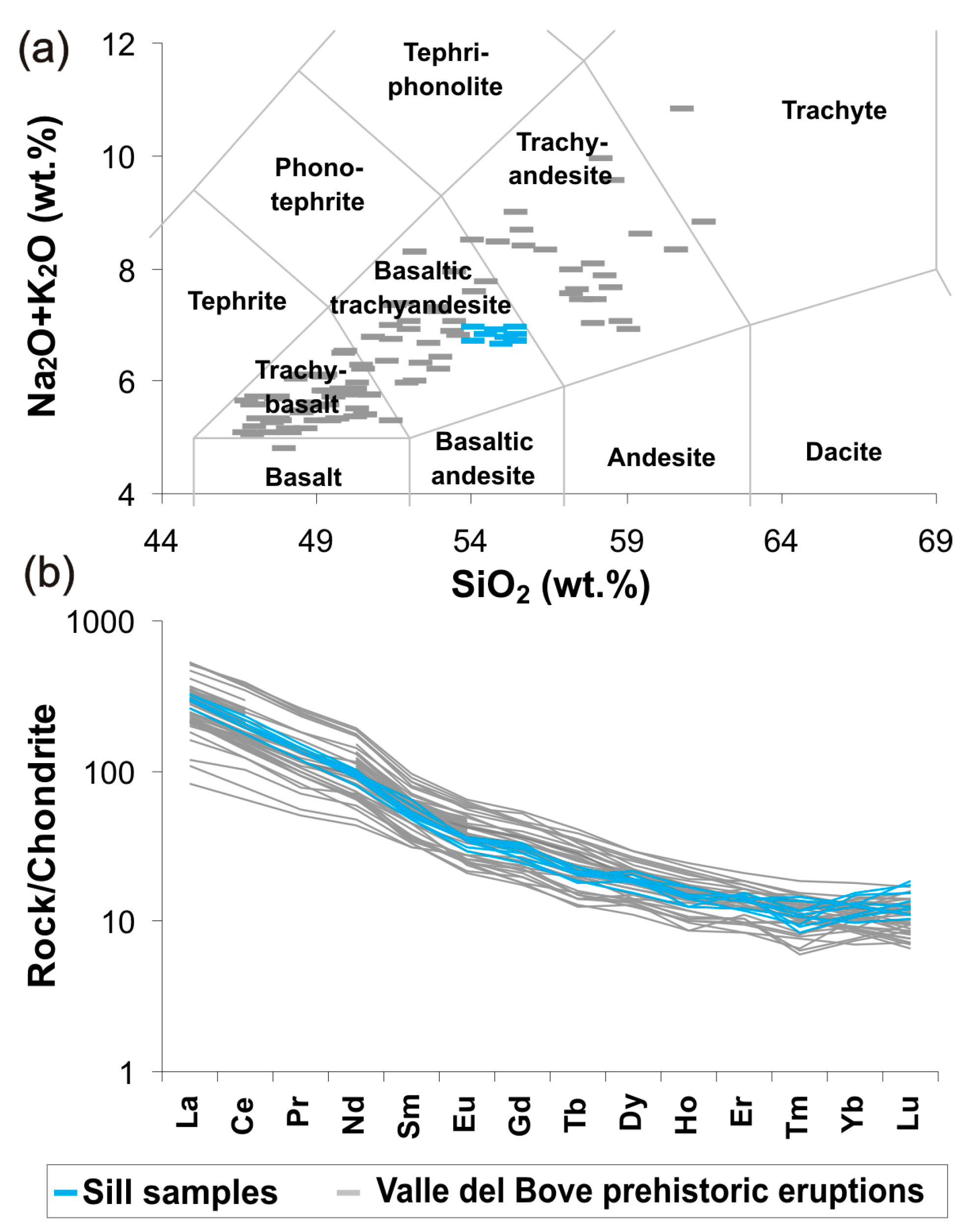
3.3. Bulk Rock Geochemistry
4. Discussion
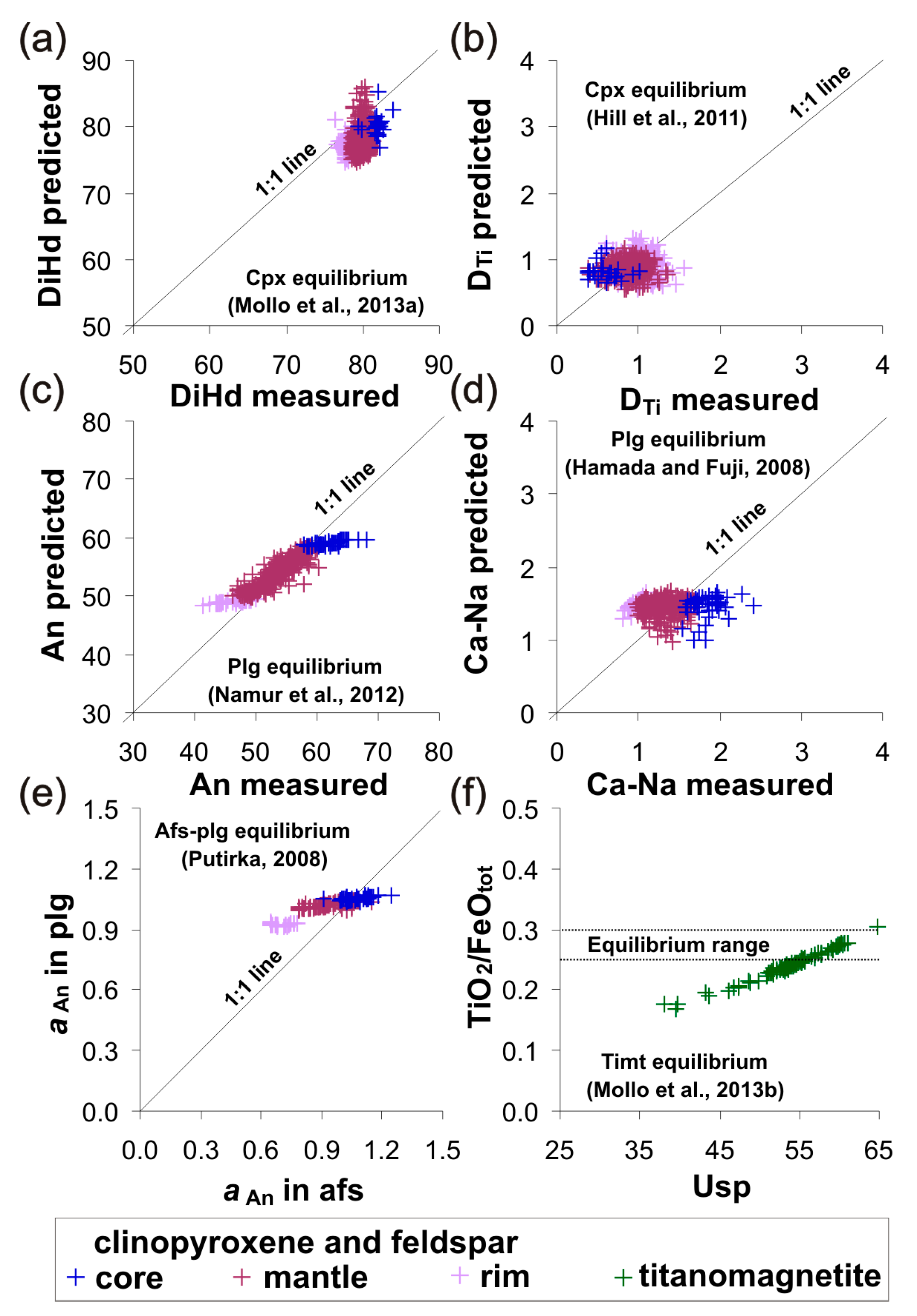
4.1. Identifying the Equilibrium Mineral Compositions
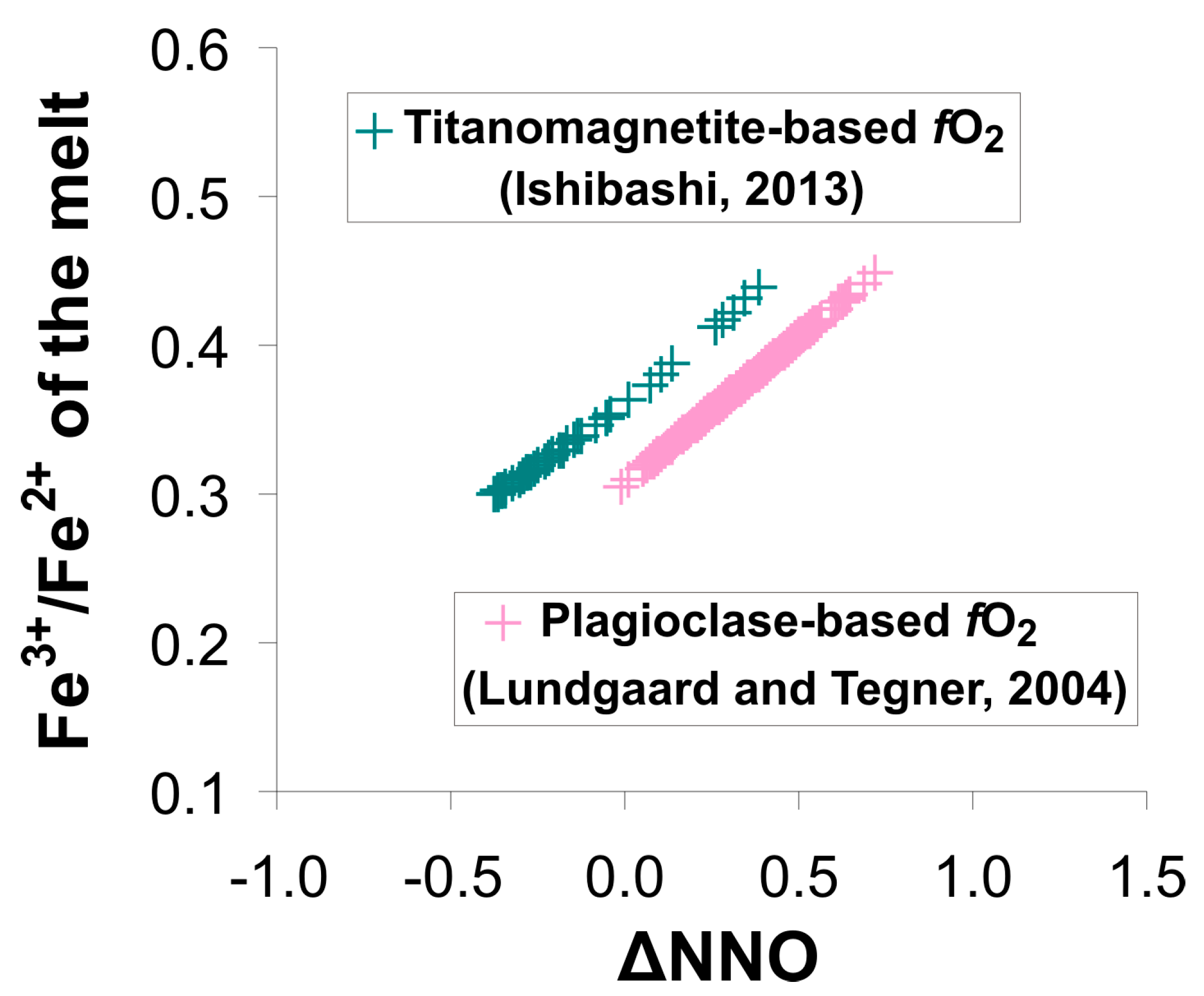
4.2. Retrieving the Crystallization Conditions of Magma
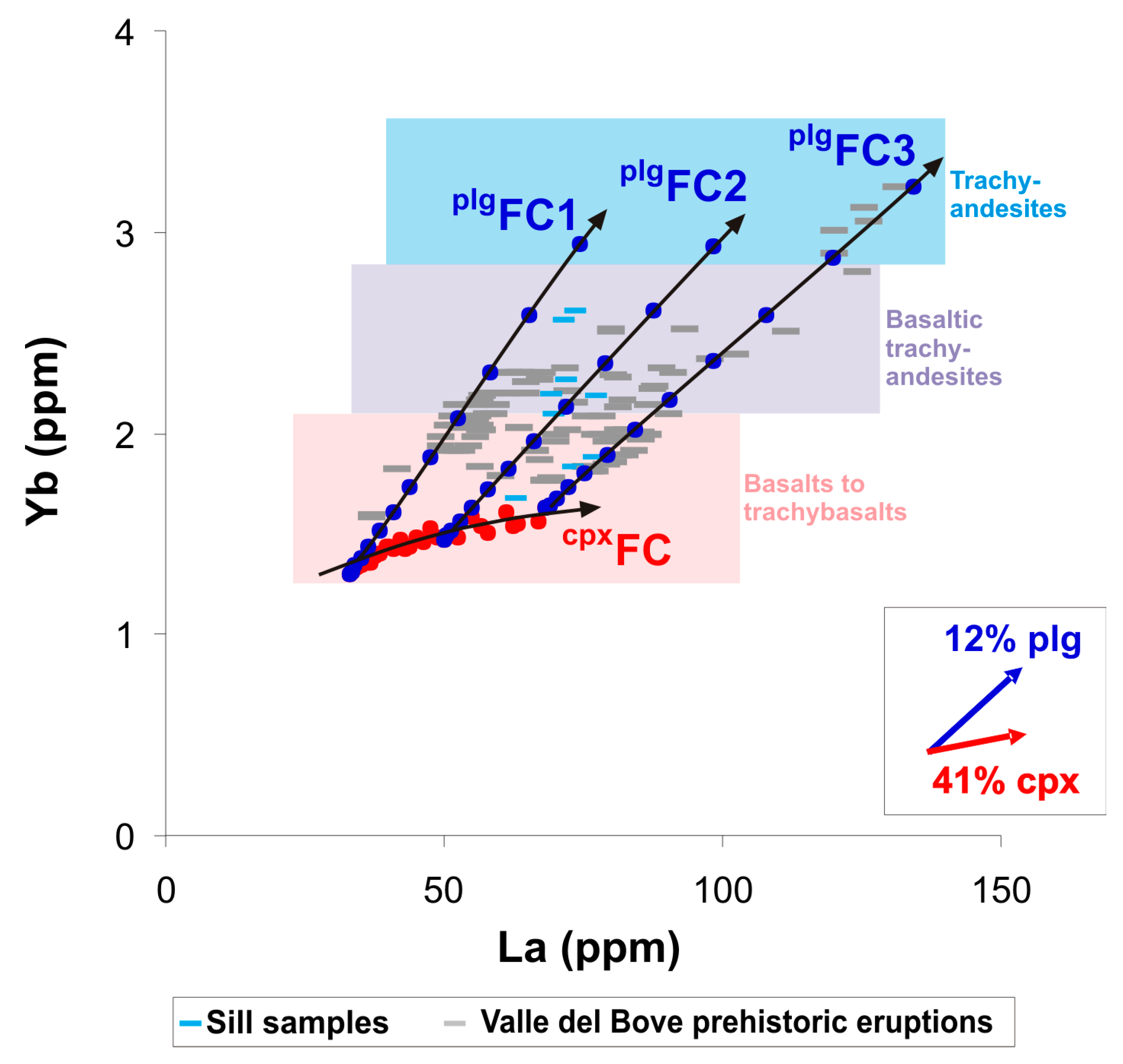
4.3. Modeling the Geochemical Evolution of Magma
5. Conclusions
- The overall decompression and cooling path of magma is constrained by pressure and temperature changes of 0.1–200 MPa and 1050–870 °C, respectively;
- The ascent of magma is accompanied by H2O exsolution up to ~2.2 wt. %, causing magma acceleration during ascent to the surface and degassing-induced undercooling phenomena;
- The early formation of clinopyroxene at depth is the main controlling factor for the REE signature of primitive basalts at Valle del Bove;
- In contrast, late degassing at low pressure conditions favors plagioclase crystallization and trace element enrichments in the more evolved trachyandesitic eruptions.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Branca, S.; Coltelli, M.; De Beni, E.; Wijbrans, J. Geological evolution of Mount Etna volcano (Italy) from earliest products until the first central volcanism (between 500 and 100 ka ago) inferred from geochronological and stratigraphic data. Int. J. Earth Sci. 2008, 97, 135–152. [Google Scholar] [CrossRef]
- Collins, S.J.; Pyle, D.M.; Maclennan, J. Melt inclusions track pre-eruption storage and dehydration of magmas at Etna. Geology 2009, 37, 571–574. [Google Scholar] [CrossRef]
- Ferlito, C.; Coltorti, M.; Lanzafame, G.; Giacomoni, P.P. The volatile flushing triggers eruptions at open conduit volcanoes: Evidence from Mount Etna volcano (Italy). Lithos 2014, 184–187, 447–455. [Google Scholar] [CrossRef]
- Ferlito, C. Mount Etna volcano (Italy). Just a giant hot spring! Earth-Sci. Rev. 2017, 177, 14–23. [Google Scholar] [CrossRef]
- Mollo, S.; Vetere, F.; Beherens, H.; Tecchiato, V.; Langone, A.; Scarlato, P.; Perugini, D. The effect of degassing and volatile exsolution on the composition of a trachybasaltic melt decompressed at slow and fast rates. Per. Min. 2017, 86, 185–197. [Google Scholar] [CrossRef]
- Perinelli, C.; Mollo, S.; Gaeta, M.; De Cristofaro, S.P.; Palladino, D.M.; Scarlato, P. Impulsive Supply of Volatile-Rich Magmas in the Shallow Plumbing System of Mt. Etna Volcano. Minerals 2018, 8, 482. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Pompilio, M.; Métrich, N.; Sobolev, A.V.; Kuzmin, D.V.; Thomas, R. Arrival of extremely volatile-rich high-Mg magmas changes explosivity of Mount Etna. Geology 2007, 35, 255–258. [Google Scholar] [CrossRef]
- Ferlito, C.; Coltorti, M.; Cristofolini, R.; Giacomoni, P.P. The contemporaneous emission of low-K and high-K trachybasalts and the role of the NE Rift during the 2002 eruptive event, Mt. Etna, Italy. Bull. Volcanol. 2009, 71, 575–587. [Google Scholar] [CrossRef]
- Ferlito, C.; Viccaro, M.; Cristofolini, R. Volatile-induced magma differentiation in the plumbing system of Mt. Etna volcano (Italy): Evidence from glass in tephra of the 2001 eruption. Bull. Volcanol. 2008, 70, 455–473. [Google Scholar] [CrossRef]
- Ferlito, C.; Lanzafame, G. The role of supercritical fluids in the potassium enrichment of magmas at Mount Etna volcano (Italy). Lithos 2010, 119, 642–650. [Google Scholar] [CrossRef]
- Giacomoni, P.P.; Ferlito, C.; Coltorti, M.; Bonadiman, C.; Lanzafame, G. Plagioclase as archive of magma ascent dynamics on “open conduit” volcanoes: The 2001-2006 eruptive period at Mt. Etna. Earth-Sci. Rev. 2014, 138, 371–393. [Google Scholar] [CrossRef]
- Kahl, M.; Chakraborty, S.; Pompilio, M.; Costa, F. Constraints on the nature and evolution of the magma plumbing system of Mt. Etna volcano (1991-2008) from a combined thermodynamic and kinetic modelling of the compositional record of minerals. J. Petrol. 2015, 56, 2025–2068. [Google Scholar] [CrossRef]
- Mollo, S.; Giacomoni, P.P.; Andronico, D.; Scarlato, P. Clinopyroxene and titanomagnetite cation redistributions at Mt. Etna volcano (Sicily, Italy): Footprints of the final solidification history of lava fountains and lava flows. Chem. Geol. 2015, 406, 45–54. [Google Scholar] [CrossRef]
- Giacomoni, P.P.; Coltorti, M.; Mollo, S.; Ferlito, C.; Braiato, M.; Scarlato, P. The 2011–2012 paroxysmal eruptions at Mt. Etna volcano: Insights on the vertically zoned plumbing system. J. Volcanol. Geotherm. Res. 2018, 349, 370–391. [Google Scholar] [CrossRef]
- Ubide, T.; Kamber, B.S. Volcanic crystals as time capsules of eruption history. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Armienti, P.; Tonarini, S.; Innocenzi, F.; D’Orazio, M. Mount Etna pyroxene as tracer of petrogenetic processes and Dynamics of the Feeding System. Geol. Soc. Am. 2007, 418, 265–276. [Google Scholar] [CrossRef]
- Armienti, P.; Perinelli, C.; Putirka, K.D. A new model to estimate deep-level magma ascent rates, with applications to Mt. Etna (Sicily, Italy). J. Petrol. 2013, 54, 795–813. [Google Scholar] [CrossRef]
- Corsaro, R.A.; Di Renzo, V.; Distefano, S.; Miraglia, L.; Civetta, L. Relationship between petrologic processes in the plumbing system of Mt. Etna and the dynamics of the eastern flank from 1995 to 2005. J. Volcanol. Geotherm. Res. 2013, 251, 75–89. [Google Scholar] [CrossRef]
- Lanzafame, G.; Mollo, S.; Iezzi, G.; Ferlito, C.; Ventura, G. Unraveling the solidification path of a pahoehoe “cicirara” lava from Mount Etna volcano. Bull. Volcanol. 2013, 75, 1–16. [Google Scholar] [CrossRef]
- Mollo, S.; Giacomoni, P.P.; Coltorti, M.; Ferlito, C.; Iezzi, G.; Scarlato, P. Reconstruction of magmatic variables governing recent Etnean eruptions: Constraints from mineral chemistry and P–T–fO2–H2O modeling. Lithos 2015, 215, 311–320. [Google Scholar] [CrossRef]
- Corsaro, R.A.; Civetta, L.; Di Renzo, V.; Miraglia, L. Petrology of lavas from the 2004-2005 flank eruption of Mt. Etna, Italy: Inferences on the dynamics of magma in the shallow plumbing system. Bull. Volcanol. 2009, 71, 781–793. [Google Scholar] [CrossRef]
- D’Orazio, M.; Tonarini, S.; Innocenti, F.; Pompilio, M. The northern Valle del Bove volcanic succession (Mt. Etna, Sicily): Petrography, geochemistry and Sr-Nd isotope data. Acta Vulcanol. 1997, 9, 69–79. [Google Scholar]
- Tanguy, J.; Condomines, M.; Kieffer, G.; Sevier, E.L.; Tanguy, J.; Condomines, M.; Kieffer, G. Evolution of the Mount Etna magma: Constraints on the present feeding system and eruptive mechanism. J. Volcanol. Geotherm. Res. 1997, 75, 221–250. [Google Scholar] [CrossRef]
- Corsaro, R.A.; Cristofolini, R.; Patanè, L. The 1669 eruption at Mount Etna: Chronology, petrology and geochemistry, with inferences on the magma sources and ascent mechanisms. Bull Volcanol 1996, 58, 348–358. [Google Scholar] [CrossRef]
- Armienti, P.; Tonarini, S.; D’Orazio, M.; Innocenti, F. Genesis and evolution of Mt. Etna alkaline lavas: Petrological and Sr-Nd-B isotope constraints. Period. Mineral. 2004, 73, 29–52. [Google Scholar]
- Ferlito, C.; Nicotra, E. The dyke swarm of Mount Calanna (Etna, Italy): An example of the uppermost portion of a volcanic plumbing system. Bull. Volcanol. 2010, 72, 1191–1207. [Google Scholar] [CrossRef]
- Patanè, D.; Barberi, G.; Cocina, O.; De Gori, P.; Chiarabba, C. Time-resolved seismic tomography detects magma intrusions at mount etna. Science 2006, 313, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Palano, M.; Puglisi, G.; Gresta, S. Ground deformation patterns at Mt. Etna from 1993 to 2000 from joint use of InSAR and GPS techniques. J. Volcanol. Geotherm. Res. 2008, 169, 99–120. [Google Scholar] [CrossRef]
- Bonforte, A.; Bonaccorso, A.; Guglielmino, F.; Palano, M.; Puglisi, G. Feeding system and magma storage beneath Mt. Etna as revealed by recent inflation/deflation cycles. JGR Solid Earth 2008, 113, 1–13. [Google Scholar] [CrossRef]
- Bonaccorso, A.; Bonforte, A.; Calvari, S.; Del Negro, C.; Di Grazia, G.; Ganci, G.; Neri, M.; Vicari, A.; Boschi, E. The initial phases of the 2008-2009 Mount Etna eruption: A multidisciplinary approach for hazard assessment. J. Geophys. Res. Solid Earth 2011, 116, 1–19. [Google Scholar] [CrossRef]
- Guillong, M.; Meier, D.L.; Allan, M.M.; Heinrich, C.A.; Yardley, B.W. Appendix A6: SILLS: A MATLAB-based program for the reduction of laser ablation ICP-MS data of homogeneous materials and inclusions. In Laser Ablation–ICP–MS in the Earth Sciences: Current Practices and Outstanding Issues; Sylvester, P., Ed.; Mineralogical Association of Canada Short Course Series Volume 40; Mineralogical Association of Canada: Québec, QC. Canada, 2008; pp. 328–333. [Google Scholar]
- Corsaro, R.A.; Cristofolini, R. Nuovi dati petrochimici ed isotopici sulla successione del Mongibello Recente. Boll. Acc. Gioenia. Sci. Nat. 1993, 341, 185–225. [Google Scholar]
- Armienti, P.; Barberi, F.; Innocenti, F. A model of the Phlegraean Fields magma chamber in the last 10,500 years. Bull. Volcanol. 1984, 47, 349–358. [Google Scholar] [CrossRef]
- Murphy, M.D.; Sparks, R.S.J.; Barclay, J.; Carroll, M.R.; Brewer, T.S. Remobilization of andesite magma by intrusion of mafic magma at the Soufriere Hills Volcano, Montserrat, West Indies. J. Petrol. 2000, 41, 21–42. [Google Scholar] [CrossRef]
- Mollo, S.; Putirka, K.; Misiti, V.; Soligo, M.; Scarlato, P. A new test for equilibrium based on clinopyroxene-melt pairs: clues on the solidification temperatures of Etnean alkaline melts at post-eruptive conditions. Chem. Geol. 2013, 352, 92–100. [Google Scholar] [CrossRef]
- Mollo, S.; Del, P.; Ventura, G.; Iezzi, G.; Scarlato, P. Dependence of clinopyroxene composition on cooling rate in basaltic magmas: Implications for thermobarometry. Lithos 2010, 118, 302–312. [Google Scholar] [CrossRef]
- Mollo, S.; Blundy, J.D.; Scarlato, P.; Iezzi, G.; Langone, A. The partitioning of trace elements between clinopyroxene and trachybasaltic melt during rapid cooling and crystal growth. Contrib. Mineral. Petrol. 2013, 166, 1633–1654. [Google Scholar] [CrossRef]
- Lindsley, D.H. Pyroxene thermometry. Am Mineral. 1983, 68, 477–493. [Google Scholar]
- Putirka, K.D.; Johnson, M.; Kinzler, R.; Longhi, J.; Walker, D. Thermobarometry of mafic igneous rocks based on clinopyroxene-liquid equilibria, 0–30 kbar. Contrib. Mineral. Petrol. 1996, 123, 92–108. [Google Scholar] [CrossRef]
- Morimoto, N.; Fabries, J.; Ferguson, A.K.; Ginzburg, I.V.; Ross, M.; Seifert, F.A.; Zussman, J.; Aoki, K.; Gottardi, G. Nomenclature of pyroxenes. Mineral. Mag. 1988, 52, 535–550. [Google Scholar] [CrossRef]
- Muñoz, M.; Sagredo, J. Clinopyroxenes as geobarometric indicators in mafic and ultramafic rocks from Canary Islands. Contrib. Mineral. Petrol. 1974, 44, 139–147. [Google Scholar] [CrossRef]
- Mollo, S.; Lanzafame, G.; Masotta, M.; Iezzi, G.; Ferlito, C.; Scarlato, P. Cooling history of a dike as revealed by mineral chemistry: a case study from Mt. Etna volcano. Chem. Geol. 2011, 288, 39–52. [Google Scholar] [CrossRef]
- Mollo, S.; Blundy, J.D.; Giacomoni, P.P.; Nazzari, M.; Scarlato, P.; Coltorti, M.; Langone, A.; Andronico, D. Clinopyroxene-melt element partitioning during interaction between trachybasaltic magma and siliceous crust: clues from quartzite enclaves at Mt. Etna volcano. Lithos 2017, 284–285, 447–461. [Google Scholar] [CrossRef]
- Sun, S.-S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. In Magmatism in the Ocean Basins; Saunders, A.D., Norry, M.J., Eds.; Geological Society, London, Special Publication; Geological Society of London: London, UK, 1989; pp. 13–345. [Google Scholar]
- Scarlato, P.; Mollo, S.; Blundy, J.D.; Iezzi, G.; Tiepolo, M. The role of natural solidification paths on REE partitioning between clinopyroxene and melt. Bull. Volcanol. 2014, 76, 1–4. [Google Scholar] [CrossRef]
- Giacomoni, P.P.; Coltorti, M.; Bryce, J.G.; Fahnestock, M.F.; Guitreau, M. Mt. Etna plumbing system revealed by combined textural, compositional, and thermobarometric studies in clinopyroxenes. Contrib. Mineral. Petrol. 2016, 171, 1–15. [Google Scholar] [CrossRef]
- Smith, C.B. Pb, Sr and Nd isotopic evidence for sources of Southern African Cretaceous kimberlites. Nature 1983, 30, 51–54. [Google Scholar] [CrossRef]
- Smith, J.V.; Brown, W.L. Feldspar Minerals; Volume I; Springer-Verlag: Berlin/Heidelberg, Germany, 1988. [Google Scholar]
- Loomis, T.P. An investigation of disequilibrium growth processes of plagioclase in the system anorthite-albite-water by methods of numerical simulation. Contrib. Mineral. Petrol. 1981, 76, 196–205. [Google Scholar] [CrossRef]
- Loomis, T.P.; Welber, P.W. Crystallization processes in the Rocky Hill Sanodiorite pluton, California: An interpretation based on compositional zoning of plagioclase. Contrib. Mineral. Petrol. 1982, 81, 230–239. [Google Scholar] [CrossRef]
- Tegner, C. Iron in plagioclase as a monitor of the differentiation of the Skaergaard intrusion. Contrib. Mineral. Petrol. 1997, 128, 45–51. [Google Scholar] [CrossRef]
- Pietranik, A.; Koepke, J.; Puziewicz, J. Crystallization and resorption in plutonic plagioclase: Implications on the evolution of granodiorite magma (Gesiniec granodiorite, Strzelin Crystalline Massif, SW Poland). Lithos 2006, 86, 260–280. [Google Scholar] [CrossRef]
- Mollo, S.; Putirka, K.; Iezzi, G.; Del Gaudio, P.; Scarlato, P. Plagioclase–melt (dis)equilibrium due to cooling dynamics: Implications for thermometry, barometry and hygrometry. Lithos 2011, 125, 221–235. [Google Scholar] [CrossRef]
- Viccaro, M.; Ferlito, C.; Cortesogno, L.; Cristofolini, R.; Gaggero, L. Magma mixing during the 2001 event at Mount Etna (Italy): Effects on the eruptive dynamics. J. Volcanol. Geotherm. Res. 2006, 149, 139–159. [Google Scholar] [CrossRef]
- Stormer, J.C. The effects of recalculation on estimates of temperature and oxygen fugacity from analyses of multicomponent iron-titanium oxides. Am. Mineral. 1983, 68, 586–594. [Google Scholar]
- Zhou, W.; Van Der Voo, R.; Peacor, D.R.; Zhang, Y. Variable Ti-content and grain size of titanomagnetite as a function of cooling rate in very young MORB. Earth Planet. Sci. Lett. 2000, 179, 9–20. [Google Scholar] [CrossRef]
- Le Bas, M.J.; Le Maitre, R.W.; Streckeisen, A.; Zanettin, B. A chemical classification of volcanic rocks on the total alkali-silica diagram. J. Petrol. 1986, 27, 745–750. [Google Scholar] [CrossRef]
- D’Orazio, M.; Armienti, P.; Cerretini, S. Petrology Phenocryst/matrix trace-element partition coefficients for hawaiite-trachyte lavas from the Ellittico volcanic sequence (Mt. Etna, Sicily, Italy). Mineral. Petrol. 1998, 64, 65–88. [Google Scholar] [CrossRef]
- Putirka, K.D. Thermometers and Barometers for Volcanic Systems. Rev. Mineral. Geochem. 2008, 69, 61–120. [Google Scholar] [CrossRef]
- Lanzafame, G.; Iezzi, G.; Mancini, L.; Lezzi, F.; Mollo, S.; Ferlito, C. Solidification and Turbulence (Non-laminar) during Magma Ascent: Insights from 2D and 3D Analyses of Bubbles and Minerals in an Etnean Dyke. J. Petrol. 2017, 58, 1511–1533. [Google Scholar] [CrossRef]
- Vetere, F.; Mollo, S.; Giacomoni, P.P.; Iezzi, G.; Coltorti, M.; Ferlito, C.; Holtz, F.; Perugini, D.; Scarlato, P. Experimental constraints on the origin of pahoehoe “cicirara” lavas at Mt. Etna Volcano (Sicily, Italy). Bull. Volcanol. 2015, 77. [Google Scholar] [CrossRef]
- Mollo, S.; Blundy, J.D.; Scarlato, P.; De Cristofaro, S.P.; Tecchiato, V.; Di Stefano, F.; Vetere, F.; Holtz, F.; Bachmann, O. An integrated P-T-H2O-lattice strain model to quantify the role of clinopyroxene fractionation on REE+Y and HFSE patterns of mafic alkaline magmas: Application to eruptions at Mt. Etna. Earth-Sci. Rev. 2018, 185, 32–56. [Google Scholar] [CrossRef]
- Mollo, S.; Hammer, J.E. Dynamic crystallization in magmas. In Mineral Reaction Kinetics: Microstructures, Textures, Chemical and Isotopic Signatures; Heinrich, W., Abart, R., Eds.; Mineralogical Society: Chantilly, VA, USA, 2017; pp. 378–418. [Google Scholar]
- Mollo, S.; Masotta, M. Optimizing pre-eruptive temperature estimates in thermally and chemically zoned magma chambers. Chem. Geol. 2014, 368, 97–103. [Google Scholar] [CrossRef]
- Hill, E.; Blundy, J.D.; Wood, B.J. Clinopyroxene–melt trace element partitioning and the development of a predictive model for HFSE and Sc. Contrib. Mineral. Petrol. 2011, 161, 423–438. [Google Scholar] [CrossRef]
- Wood, B.J.; Trigila, R. Experimental determination of aluminous clinopyroxene-melt partition coefficients for potassic liquids, with application to the evolution of the Roman province potassic magmas. Chem. Geol. 2001, 172, 213–223. [Google Scholar] [CrossRef]
- Mollo, S.; Misiti, V.; Scarlato, P.; Soligo, M. The role of cooling rate in the origin of high temperature phases at the chilled margin of magmatic intrusions. Chem. Geol. 2012, 322–323, 28–46. [Google Scholar] [CrossRef]
- Mollo, S.; Putirka, K.; Iezzi, G.; Scarlato, P. The control of cooling rate on titanomagnetite composition: implications for a geospeedometry model applicable to alkaline rocks from Mt. Etna volcano. Contrib. Mineral. Petrol. 2013, 165, 457–475. [Google Scholar] [CrossRef]
- Putirka, K.D.; Mikaelian, H.; Ryerson, F.; Shaw, H. New clinopyroxene-liquid thermobarometers for mafic, evolved, and volatile-bearing lava compositions, with applications to lavas from Tibet and the Snake River Plain, Idaho. Am. Mineral. 2003, 88, 1542–1554. [Google Scholar] [CrossRef]
- Namur, O.; Charlier, B.; Toplis, M.J.; Vander Auwera, J. Prediction of plagioclase-melt equilibria in anhydrous silicate melts at 1-atm. Contrib. Mineral. Petrol. 2012, 163, 133–150. [Google Scholar] [CrossRef]
- Hamada, M.; Fujii, T. Experimental constraints on the effects of pressure and H2O on the fractional crystallization of high-Mg island arc basalt. Contrib. Mineral. Petrol. 2008, 155, 767–790. [Google Scholar] [CrossRef]
- Ushioda, M.; Takahashi, E.; Hamada, M.; Suzuki, T. Water content in arc basaltic magma in the Northeast Japan and Izu arcs: An estimate from Ca/Na partitioning between plagioclase and melt. Earth Planets Space 2014, 66, 127. [Google Scholar] [CrossRef]
- Sisson, T.W.; Grove, T.L. Temperatures and H2O contents of low-MgO high-alumina basalts. Contrib. Mineral. Petrol. 1993, 113, 167–184. [Google Scholar] [CrossRef]
- Applegarth, L.J.; Tuffen, H.; James, M.R.; Pinkerton, H.; Cashman, K.V. Direct observations of degassing-induced crystallization in basalts. Geology 2013, 41, 243–246. [Google Scholar] [CrossRef]
- Mollo, S.; Masotta, M.; Forni, F.; Bachmann, O.; Astis, D.; Moore, G.; Scarlato, P. A K-feldspar–liquid hygrometer specific to alkaline differentiated magmas. Chem. Geol. 2015, 392, 1–8. [Google Scholar] [CrossRef]
- Ishibashi, H. Spinel-melt oxygen barometry; a method and application to Cenozoic alkali basaltic magmas from the Higashi-Matsuura District, NW Kyushu, Japan. Geosci. Rep. Shizuoka Univ. 2013, 40, 21–32. [Google Scholar]
- Ariskin, A.A.; Nikolaev, G.S. An empirical model for the calculation of spinel-melt equilibria in mafic igneous systems at atmospheric pressure: 1. Chromian spinels. Contrib. Mineral. Petrol. 1996, 123, 282–292. [Google Scholar] [CrossRef]
- Sack, R.O. Some constraints on the thermodynamic mixing properties of Fe–Mg orthopyroxenes and olivines. Contrib. Mineral. Petrol. 1980, 71, 257–269. [Google Scholar] [CrossRef]
- Armienti, P.; Pareschi, M.T.; Innocenti, F.; Pompilio, M. Effects of magma storage and ascent on the kinetics of crystal growth—The case of the 1991-93 Mt. Etna eruption. Contrib. Mineral. Petrol. 1994, 115, 402–414. [Google Scholar] [CrossRef]
- Lundgaard, K.L.; Tegner, C. Partitioning of ferric and ferrous iron between plagioclase and silicate melt. Contrib. Mineral. Petrol. 2004, 147, 470–483. [Google Scholar] [CrossRef]
- Tanguy, J.C.; Clocchiatti, R. The Etnean Lavas, 1977-1983: Petrology and mineralogy. Bull. Volcanol. 1984, 47, 879–894. [Google Scholar] [CrossRef]
- Dolfi, D.; Trigila, R. Clinopyroxene solid solutions and water in magmas: Results in the system phonolitic tephrite-H2O. Mineral. Mag. 1983, 47, 347–351. [Google Scholar] [CrossRef]
- Métrich, N.; Rutherford, M.J. Low pressure crystallization paths of H2O-saturated basaltic-hawaiitic melts from Mt Etna: Implications for open-system degassing of basaltic volcanoes. Geochim. Cosmochim. Acta 1998, 62, 1195–1205. [Google Scholar] [CrossRef]
- Perinelli, C.; Mollo, S.; Gaeta, M.; De Cristofaro, S.P.; Palladino, D.M.; Armienti, P.; Scarlato, P.; Putirka, K.D. An improved clinopyroxene-based hygrometer for Etnean magmas and implications for eruption triggering mechanisms. Am. Mineral. 2016, 101, 2774–2777. [Google Scholar] [CrossRef] [Green Version]
- Métrich, N.; Allard, P.; Spilliaert, N.; Andronico, D.; Burton, M. 2001 flank eruption of the alkali- and volatile-rich primitive basalt responsible for Mount Etna’s evolution in the last three decades. Earth Planet. Sci. Lett. 2004, 228, 1–17. [Google Scholar] [CrossRef]
- Spilliaert, N.; Métrich, N.; Allard, P. S-Cl-F degassing pattern of water-rich alkali basalt: Modelling and relationship with eruption styles on Mount Etna volcano. Earth Planet. Sci. Lett. 2006, 248, 772–786. [Google Scholar] [CrossRef]
- Witham, F.; Blundy, J.D.; Kohn, S.C.; Lesne, P.; Dixon, J.; Churakov, S.V.; Botcharnikov, R. SolEx: A model for mixed COHSCl-volatile solubilities and exsolved gas compositions in basalt. Comp. Geosci. 2012, 45, 87–97. [Google Scholar] [CrossRef]
- Mollo, S.; Scarlato, P.; Lanzafame, G.; Ferlito, C. Deciphering lava flow posteruption differentiation processes by means of geochemical and isotopic variations: A case study from Mt. Etna volcano. Lithos 2013, 162–163, 115–127. [Google Scholar] [CrossRef]
- Gardner, J.E.; Rutherford, M.; Carey, S.; Sigurdsson, H. Experimental constraints on pre-eruptive water contents and changing magma storage prior to explosive eruptions of Mount St. Helens volcano. Bull. Volcanol. 1995, 57, 1–17. [Google Scholar] [CrossRef]
- Frey, H.M.; Lange, R.A. Phenocryst complexity in andesites and dacites from the Tequila volcanic field, Mexico: Resolving the effects of degassing vs. magma mixing. Contrib. Mineral. Petrol. 2011, 162, 415–445. [Google Scholar] [CrossRef]
- Médard, E.; Grove, T.L. The effect of H2O on the olivine liquidus of basaltic melts: Experiments and thermodynamic models. Contrib. Mineral. Petrol. 2008, 155, 417–432. [Google Scholar] [CrossRef]
- Del Bello, E.; Mollo, S.; Scarlato, P.; von Quadt, A.; Forni, F.; Bachmann, O. New petrological constraints on the last eruptive phase of the Sabatini Volcanic District (central Italy): Clues from mineralogy, geochemistry, and Sr-Nd isotopes. Lithos 2014, 205, 28–38. [Google Scholar] [CrossRef]
- Moore, G.; Righter, K.; Carmichael, I.S.E. The effect of dissolved water on the oxidation state of iron in natural silicate liquids. Contrib. Mineral. Petrol. 1995, 120, 170–179. [Google Scholar] [CrossRef]
- Mathez, E.A. Influence of degassing on oxidation states of basaltic magmas. Nature 1984, 310, 371–375. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Özgür Yazaydin, A.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh porosity in metal–organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, R.A.; Pompilio, M. Magma dynamics in the shallow plumbing system of Mt. Etna as recorded by compositional variations in the volcanics of recent summit activity (1995–1999). J. Volc. Geotherm. Res. 2004, 137, 55–71. [Google Scholar] [CrossRef]
- Aloisi, M.; Bonaccorso, A.; Gambino, S. Imaging composite dike propagation (Etna, 2002 case). J. Geophys. Res. Solid Earth 2006, 111, 1–13. [Google Scholar] [CrossRef]
- Rutherford, M.J.; Hill, P.M. Magma ascent rates from amphibole breakdown: experiments and the 1980–1986 Mount St. Helens eruptions. J. Geophys. Res. Solid Earth 1993, 98, 19667–19685. [Google Scholar] [CrossRef]
- Rutherford, M.J. Magma ascent rates. In Minerals, Inclusions and Volcanic Processes; Putirka, K.D., Tepley III, F.J., Eds.; Reviews in Mineralogy and Geochemistry; Mineralogical Society of America and Geochemical Society: Chantilly, VA, USA, 2008; Volume 69, pp. 241–271. [Google Scholar]
- Toramaru, A.; Noguchi, S.; Oyoshihara, S.; Tsune, A. MND (microlite number density) water exsolution rate meter. J. Volcanol. Geotherm. Res. 2008, 175, 156–167. [Google Scholar] [CrossRef]
- Gonnermann, H.M.; Manga, M. Magma ascent in the volcanic conduit. In Modeling Volcanic Processes: The Physics and Mathematics of Volcanism.; Fagents, S.A., Gregg, T.K.P., Lopez, R.C., Eds.; Cambridge University Press: Cambridge, UK, 2013; pp. 55–84. [Google Scholar]
- Lindstrom, D.P. Experimental Study of the Partitioning of the Transition Metals Between Clinopyroxene and Coexisting Silicate Liquids. Ph.D. Thesis, University of Oregon, Eugene, OR, USA, 1976; p. 188. [Google Scholar]
- Ray, G.L.; Shimizu, N.; Hart, S.R. An ion microprobe study of the partitioning of trace elements between clinopyroxene and liquid in the system diopside-albite-anorthite. Geochim. Cosmochim. Acta 1983, 47, 2131–2140. [Google Scholar] [CrossRef]
- Hart, S.R.; Dunn, T. Experimental cpx/melt partitioning of 24 trace elements. Contrib. Mineral. Petrol. 1993, 113, 1–8. [Google Scholar] [CrossRef]
- Forsythe, L.M.; Nielsen, R.L.; Fisk, M.R. High-field-strength element partitioning between pyroxene and basaltic to dacitic magmas. Chem. Geol. 1994, 117, 107–125. [Google Scholar] [CrossRef]
- Gaetani, G.A.; Grove, T.L. Partitioning of rare earth elements between clinopyroxene and silicate melt Crystal-chemical controls. Geochim. Cosmochim. Acta 1995, 59, 1951–1962. [Google Scholar] [CrossRef]
- Lundstrom, C.C.; Shaw, H.F.; Ryerson, F.J.; Phinney, D.L.; Gill, J.B.; Williams, Q. Compositional controls on the partitioning of U, Th, Ba, Pb, Sr and Zr between clinopyroxene and haplobasaltic melts: implications for uranium series disequilibria in basalts. Earth Planet. Sci. Lett. 1994, 128, 407–423. [Google Scholar] [CrossRef]
- Lundstrom, C.C.; Shaw, H.F.; Ryerson, F.J.; Williams, Q.; Gill, J. Crystal chemical control of clinopyroxene-melt partitioning in the Di-Ab-An system: Implications for elemental fractionations in the depleted mantle. Geochim. Cosmochim. Acta 1998, 62, 2849–2862. [Google Scholar] [CrossRef]
- Skulski, T.; Minarik, W.; Watson, E.B. High-pressure experimental trace-element partitioning between clinopyroxene and basaltic melts. Chem. Geol. 1994, 117, 127–147. [Google Scholar] [CrossRef]
- Sun, C.; Liang, Y. Distribution of REE between clinopyroxene and basaltic melt along a mantle adiabat: Effects of major element composition, water, and temperature. Contrib. Mineral. Petrol. 2012, 163, 807–823. [Google Scholar] [CrossRef]
- Yao, L.; Sun, C.; Liang, Y. A parameterized model for REE distribution between low-Ca pyroxene and basaltic melts with applications to REE partitioning in low-Ca pyroxene along a mantle adiabat and during pyroxenite-derived melt and peridotite interaction. Contrib. Mineral. Petrol. 2012, 164, 261–280. [Google Scholar] [CrossRef]
- Blundy, J.D.; Robinson, J.A.C.; Wood, B.J. Heavy REE are compatible in clinopyroxene on the spinel lherzolite solidus. Earth Planet. Sci. Lett. 1998, 160, 493–504. [Google Scholar] [CrossRef]
- Schosnig, M.; Hoffer, E. Compositional dependence of REE partitioning between diopside and melt at 1 atmosphere. Contrib. Mineral. Petrol. 1998, 133, 205–216. [Google Scholar] [CrossRef]
- Bennett, S.L.; Blundy, J.; Elliott, T. The effect of sodium and titanium on crystal-melt partitioning of trace elements. Geochim. Cosmochim. Acta 2004, 68, 2335–2347. [Google Scholar] [CrossRef]
- Marks, M.; Halama, R.; Wenzel, T.; Markl, G. Trace element variations in clinopyroxene and amphibole from alkaline to peralkaline syenites and granites: Implications for mineral-melt trace-element partitioning. Chem. Geol. 2004, 211, 185–215. [Google Scholar] [CrossRef]
- Adam, J.; Green, T. Trace element partitioning between mica-and amphibole-bearing garnet lherzolite and hydrous basanitic melt: 1. Experimental results and the investigation of controls on partitioning behaviour. Contrib. Mineral. Petrol. 2006, 152, 1–17. [Google Scholar] [CrossRef]
- Tuff, J.; Gibson, S.A. Trace-element partitioning between garnet, clinopyroxene and Fe-rich picritic melts at 3 to 7 GPa. Contrib. Mineral. Petrol. 2007, 153, 369–387. [Google Scholar] [CrossRef]
- Francis, D.; Minarik, W. Aluminum-dependent trace element partitioning in clinopyroxene. Contrib. Mineral. Petrol. 2008, 156, 439–451. [Google Scholar] [CrossRef]
- Mollo, S.; Forni, F.; Bachmann, O.; Blundy, J.D.; De Astis, G.; Scarlato, P. Trace element partitioning between clinopyroxene and trachy-phonolitic melts: A case study from the Campanian ignimbrite (Campi Flegrei, Italy). Lithos 2016, 252–253, 160–172. [Google Scholar] [CrossRef]
- Hill, E.; Wood, B.J.; Blundy, J.D. The effect of Ca-Tschermaks component on trace element partitioning between clinopyroxene and silicate melt. Lithos 2000, 53, 203–215. [Google Scholar] [CrossRef]
- Dohmen, R.; Blundy, J. A predictive thermodynamic model for element partitioning between plagioclase and melt as a function of pressure, temperature and composition. Am. J. Sci. 2014, 314, 1319–1372. [Google Scholar] [CrossRef]
- Kneip, H.J.; Liebau, F. Feldspars with trivalent non-tetrahedral cations: Experimental studies in the system NaAlSi3O8-CaAl2Si2O8-LaAl3SiO8. Eur. J. Min. 1994, 6, 87–98. [Google Scholar] [CrossRef]
- Blundy, J.; Wood, B. Crystal-chemical controls on the partitioning of Sr and Ba between plagioclase feldspar, silicate melts, and hydrothermal solutions. Geochim. Cosmochim. Acta 1991, 55, 193–209. [Google Scholar] [CrossRef]
- Bindeman, I.N.; Davis, A.M.; Drake, M.J. Ion microprobe study of plagioclase-basalt partition experiments at natural concentration levels of trace elements. Geochim. Cosmochim. Acta 1998, 62, 1175–1193. [Google Scholar] [CrossRef]
- Bindeman, I.N.; Davis, A.M. Trace element partitioning between plagioclase and melt: Investigation of dopant influence on partition behavior. Geochim. Cosmochim. Acta 2000, 64, 2863–2878. [Google Scholar] [CrossRef]
- Wood, B.J.; Blundy, J.D. The effect of cation charge on crystal–melt partitioning of trace elements. Earth Planet. Sci. Lett. 2001, 188, 59–71. [Google Scholar] [CrossRef]
- Bédard, J.H. Trace element partitioning in plagioclase feldspar. Geochim. Cosmochim. Acta 2006, 70, 3717–3742. [Google Scholar] [CrossRef]
- Tepley, F.J.; Lundstrom, C.C.; McDonough, W.F.; Thompson, A. Trace element partitioning between high-An plagioclase and basaltic to basaltic andesite melt at 1 atmosphere pressure. Lithos 2010, 118, 82–94. [Google Scholar] [CrossRef]
- Hui, H.; Oshrin, J.G.; Neal, C.R. Investigation into the petrogenesis of Apollo 14 high-Al basaltic melts through crystal stratigraphy of plagioclase. Geochim. Cosmochim. Acta 2011, 75, 6439–6460. [Google Scholar] [CrossRef]
- Sun, C.; Graff, M.; Liang, Y. Trace element partitioning between plagioclase and silicate melt: The importance of temperature and plagioclase composition, with implications for terrestrial and lunar magmatism. Geochim. Cosmochim. Acta 2017, 206, 273–295. [Google Scholar] [CrossRef]
- Blundy, J.; Wood, B. Prediction of crystal-melt partition coefficients from elastic moduli. Nature 1994, 372, 452–454. [Google Scholar] [CrossRef]
- Wood, B.J.; Blundy, J.D. A predictive model for rare earth element partitioning between clinopyroxene and anhydrous silicate melt. Contrib. Mineral. Petrol. 1997, 129, 166–181. [Google Scholar] [CrossRef]
- Forni, F.; Petricca, E.; Bachmann, O.; Mollo, S.; De Astis, G.; Piochi, M. The role of magma mixing/mingling and cumulate melting in the Neapolitan Yellow Tuff caldera-forming eruption (Campi Flegrei, Southern Italy). Contrib. Mineral. Petrol. 2018, 173, 45. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazzari, M.; Di Stefano, F.; Mollo, S.; Scarlato, P.; Tecchiato, V.; Ellis, B.; Bachmann, O.; Ferlito, C. Modeling the Crystallization and Emplacement Conditions of a Basaltic Trachyandesitic Sill at Mt. Etna Volcano. Minerals 2019, 9, 126. https://doi.org/10.3390/min9020126
Nazzari M, Di Stefano F, Mollo S, Scarlato P, Tecchiato V, Ellis B, Bachmann O, Ferlito C. Modeling the Crystallization and Emplacement Conditions of a Basaltic Trachyandesitic Sill at Mt. Etna Volcano. Minerals. 2019; 9(2):126. https://doi.org/10.3390/min9020126
Chicago/Turabian StyleNazzari, Manuela, Flavio Di Stefano, Silvio Mollo, Piergiorgio Scarlato, Vanni Tecchiato, Ben Ellis, Olivier Bachmann, and Carmelo Ferlito. 2019. "Modeling the Crystallization and Emplacement Conditions of a Basaltic Trachyandesitic Sill at Mt. Etna Volcano" Minerals 9, no. 2: 126. https://doi.org/10.3390/min9020126
APA StyleNazzari, M., Di Stefano, F., Mollo, S., Scarlato, P., Tecchiato, V., Ellis, B., Bachmann, O., & Ferlito, C. (2019). Modeling the Crystallization and Emplacement Conditions of a Basaltic Trachyandesitic Sill at Mt. Etna Volcano. Minerals, 9(2), 126. https://doi.org/10.3390/min9020126




