1. Introduction
Due to the high demand for iron and steel from various industries, even iron ores which have exhibited a complicated mineralogy can be considered as potential resources and can be processed by different beneficiation methods [
1,
2,
3]. Magnetic separation and reverse flotation are the most efficient techniques that have been widely used for the separation and removal of gangue phases in the iron oxide ores [
1,
2,
4]. Reverse flotation of iron oxide ores can be performed by anionic and cationic collectors, while iron oxides are depressed by various chemicals (starch, water glass, sodium humate, dextrin, sodium carboxymethyl, etc.) [
5,
6,
7,
8]. It was understood that depressants are typically coating the surface of minerals by inducing a hydrophilic film and prevent bubble-particle attachment [
7]. However, due to various interactions with the flotation slurry, depressants can simultaneously act as pH modifiers or flocculants. It was reported that starch in the industrial reverse hematite flotation separation from quartz can be used as a depressant and flocculant [
5,
6].
Among the gangue minerals found in iron oxide ores, phosphorous minerals (mainly in the form of apatite group) are the most unwanted associated minerals for the steelmaking process. Phosphor decreases the ductilibility of steels by increasing its brittleness [
9]. Where there are many high-phosphorus iron mines around the world (Iran, Sweden, and Mexico) [
3,
10,
11], reverse anionic flotation, floating phosphorous minerals and depressing iron oxides using sodium silicate (SS), is the main traditional method for separation of iron minerals and removing phosphorus content. However, it was reported that, in some cases, SS is not an efficient depressant for iron oxides when phosphorous minerals are among the associated gangue minerals [
3]. Thus, further investigations on synthesizing depressants for a selective reverse flotation of iron oxide ores are essential.
The main aim of this study is introducing a newly synthesized depressant “sodium co-silicate (SCS)” for a reverse anionic flotation separation of hematite from fluorapatite at different scales (micro, batch, and industrial). Micro-flotation experiments are conducted by using pure minerals in order to compare the performance of SCS and SS. Batch and industrial scale experiments are performed by using samples from the Iranian Chadormalu iron ore processing plant and considering the plant operating conditions. For comparison purposes, experiments are carried out by using both SS and SCS with the same conditions. The outcomes of this investigation can lead to higher efficiency and lower reagent consumptions in the plant by using SCS.
2. Materials and Methods
2.1. Materials
The XRD analysis of the feed from Chadormalu iron ore processing plant showed that the main minerals in the feed are hematite, magnetite and fluorapatite (small amount of quartz and ankerite). To simulate the feed, pure fluorapatite and hematite were prepared and used in micro-flotation tests. Energy dispersive X-ray spectroscopy (EDS) (Model Vegato, RONTEC’s EDX Model, Tescan, Brno, Czech Republic) and potassium dichromate titration were used to analyze these two pure minerals (
Table 1). The pure minerals were freshly crushed and pulverized by a hammer and ground in a porcelain mortar. The −75 + 25 µm fraction was used for micro-flotation tests. For bench-scale flotation tests, samples were collected from the feed stream to the flotation circuit in the plant. The grades of Fe and P in the batch flotation feed samples were 59.11% and 0.79%, respectively. The d80 of the feed sample was –45 µm. In the plant, Alke 742 FL (type of carboxylic acids which contains an ester agent group) is used as a collector (produced at the Isfahan Copolymer Chemical Co. Isfahan, Iran), together with a mixture of NaOH:Na
2CO
3 as a pH modifier and sodium silicate (SS) as the hematite depressant. Collector consumption in the plant is around 350 g/t.
The sodium co-silicate “SCS” (Na
4SiCO
6) is a modified depressant and, unlike the SS gel, is a solid white powder. SCS is obtained from the synthesis of sodium metasilicate with sodium carbonate and caustic soda. Based on the synthesizing conditions, SCS may have different properties (
Table 2). SCS can modify the pH of a slurry solution and set it in the range of 9 to 10. In this study, SCS with the “PS-SCS 23” code was used. For comparison purposes, all micro, batch, and industrial flotation tests were performed with the plant conditioning in the presence of different depressants (SS vs. SCS).
2.2. Micro and Batch Flotation Experiments
Micro-flotation tests were performed in a 150 mL modified Hallimond tube flotation cell, and by using 1.5 g each of pure minerals at a pH of 9.5. The samples were conditioned for 3 min under constant agitation then depressant was added and agitated for 3 min. The pH was adjusted and then the collector solution (1 wt %) was added and conditioned for another 3 min. After that, flotation tests were carried out for 3 min and the floated particles were collected and dried. The main aim of conducting micro-flotation experiments was to explore the potential of using SCS instead of SS.
For the batch flotation tests, samples from the plant feed (dephosphorization unit) are subjected to a Denver flotation machine (D12, Metso, Helsinki, Finland) with a 2 L cell capacity. In this stage, the main aim for conducting experiments was optimizing the SCS dosage. The solid percentage in the batch process was set to 30% and the agitation rate was 1200 rpm. The airflow rate was 3.8 L/min. First, conditioning time for the slurry was 4 min without adding reagent. Then, the pulp with pH ~9.5 was conditioned by SCS as a depressant for 3 min. In the next step, similar to the conditioning in the plant, the collector was added and conditioned for three more minutes.
2.3. Industrial Flotation Experiments
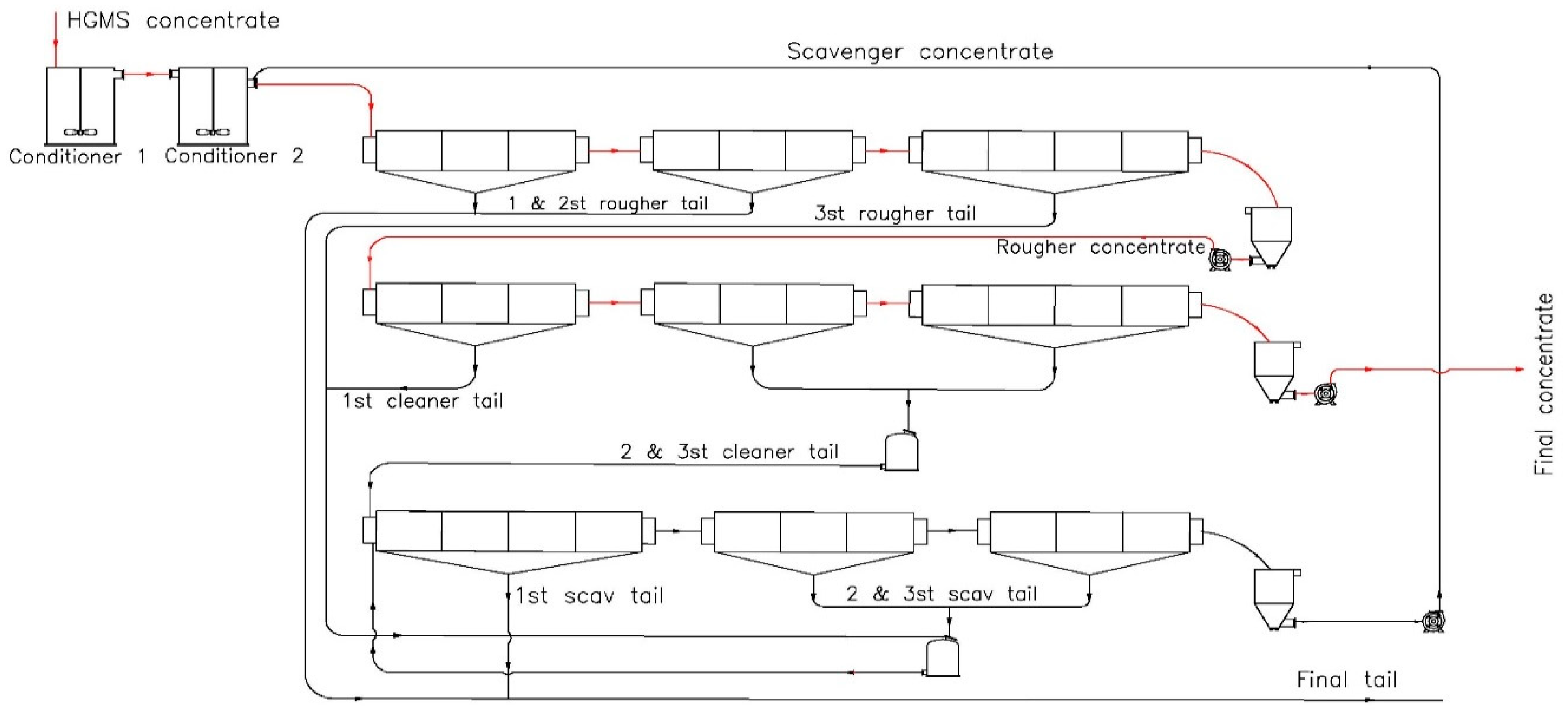
In the flotation circuit of the Chadormalu plant, SS is used as the iron oxides depressant. The dephosphorization flotation circuit consists of rougher, cleaner, and scavenger circuits (each having three stages) (
Figure 1). Flotation feed is the concentrate which has been produced from a high-gradient magnetic separator (HGMS). The rougher and cleaner stages consist of 10 cells with 11 m
3 capacity. The scavenger stage consists of 10 cells with 3.3 m
3 capacity. To compare SS vs. SCS at the industrial scale, three parallel lines (out of five lines) were used based on different conditions (
Table 3). During the tests, a homogeneous stockpile was prepared and fed to the plant. The feed rate for the experiments was close to the common plant feed rate. The flotation tests were run on two consecutive days. In the first 24 hours the SS, and in the second 24 h the SCS depressant were examined (
Table 3).
3. Results and Discussions.
3.1. Micro-Flotation Experiments
Micro-flotation outcomes (
Figure 2) indicate that hematite recovery in the absence of depressants is about 74%, while by adding depressants it increases significantly (the iron recovery increases by more than 20%). Adding depressants slightly decreases the flourapatite recovery in the froth phase. This slight drop in fluorapatite floatability is probably due to the interactions between the collector and the depressants [
12]. The depression of hematite by SCS is 3.5% higher than SS (
Figure 2). Moreover, in the presence of SCS, the fluorapatite recovery in froth phase is higher than SS. These results demonstrate that (i) the presence of depressant generally has no significant effect on the fluorapatite floatability, and (ii) the SCS is more efficient than SS.
3.2. Batch Flotation Experiments
In a reverse flotation of iron oxides, the depressant dosage plays an essential role and significantly can affect the iron recovery [
13,
14,
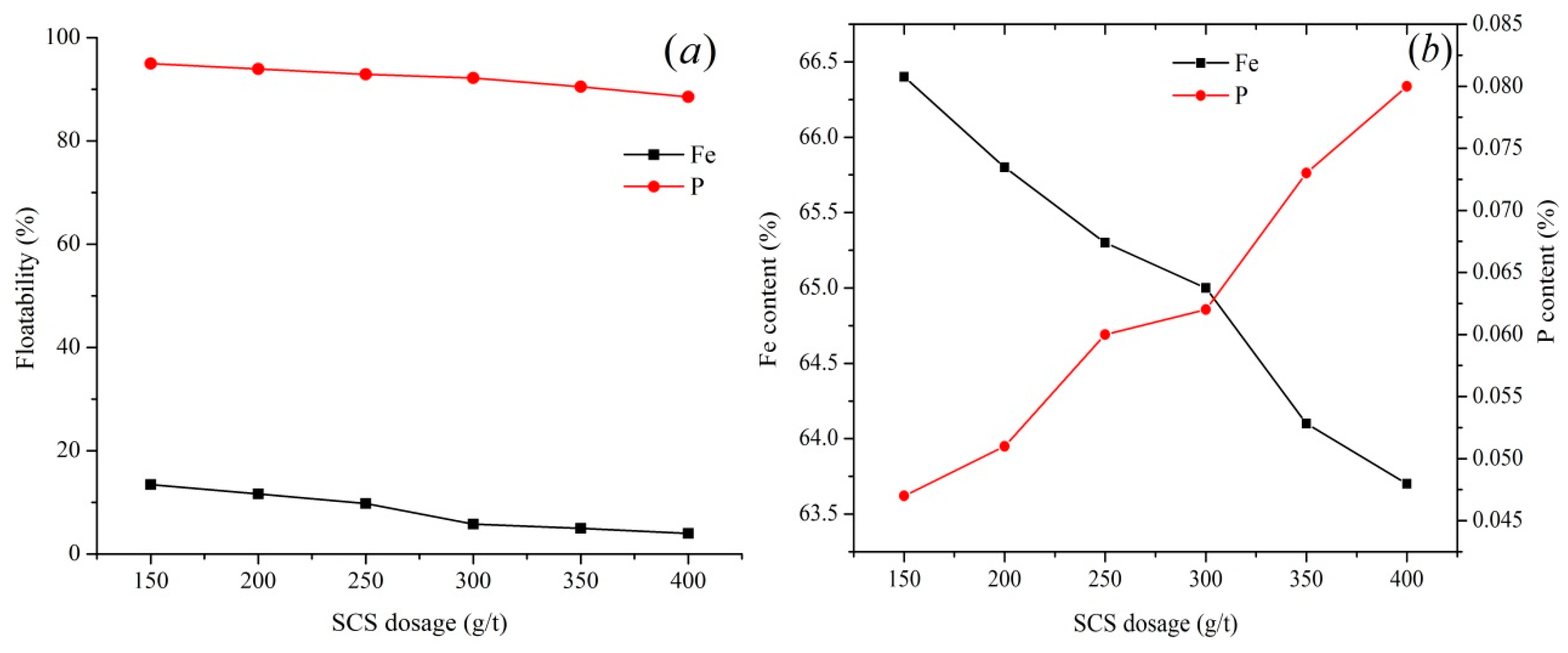
15]. Exploring the effect of SCS dosage on the recovery and grade of hematite (depression) and fluorapatite (floatability) from Chadormalu samples indicates that the presence of 150 g/t SCS hematite depression is not adequate while increasing the SCS concentration to 300 g/t can improve the iron recovery to 94.2% (
Figure 3). A further increase in the SCS concentration (>300 g/t) may increase the phosphorus content in the iron concentrate (0.082%). It was documented that since the silicate group in SCS is dominating, a high concentration of SCS may increase the precipitation of calcium silicate on the fluorapatite surfaces. Calcium silicate precipitation can decrease the collector adsorption on the apatite surface [
12,
14]. The SCS dosage of 400 g/t decreases the iron loss in the tails; however, it increases the phosphorus content of the concentrate (i.e., it reduces the iron grade) (
Figure 3). 300 g/t SCS can adjust the pH value of the pulp to around 9.5. Thus, in order to keep the pH at ~9.5 and hinder the decrease of fluorapatite floatability, a dosage of 300 g/t SCS was selected for the industrial-scale experiments.
3.3. Industrial Scale Experiments
Various conditions (
Table 4) are used in the three parallel circuits (lines 1, 2, and 3) in the Chadormalu iron ore processing plant. During two different days the influence of SCS vs. SS on the plant efficiency are compared. Based on the industrial experiment results (
Table 5) in all the examined processing lines, when SCS is used, the metallurgical results are better than conditioning using Na
2CO
3 + SS + NaOH (by using SCS the recovery value is increased by about 3.3%). When conditioning by SCS, the iron content of the concentrate is increased by about 0.5% while the phosphorus content is decreased by about 0.011% in the iron concentrate compared to the Na
2CO
3 + NaOH + SS conditioning. Since the acceptable phosphorus content in the iron concentrate for steelmaking units should be around 0.047% [
2], it can be concluded that the metallurgical results by using SCS (
Table 5) are desirable for steelmaking.
Based on these results (
Table 4), with a mean consumption of 293.33 g/t SCS, the pH value can be approximately 9.51. Meanwhile, a mean pH of 9.61 was reported with a consumption of 400 g/t SS, 33.67 g/t NaOH, and 156 g/t Na
2CO
3, collectively. Therefore, the metallurgical results that are obtained from using SCS generally show superior depression performance even at the industrial scale.
4. Conclusions
In this study, a new synthetic depressant “sodium co-silicate” (SCS) for reverse flotation separation of hematite from fluorapatite was introduced. SCS is obtained from the synthesis of sodium metasilicate with sodium carbonate and caustic soda. SCS can provide a slurry solution in the range of pH 9 to 10 (thus, in the presence of SCS, there is no need for a pH modifier). Various flotation experiments were conducted at different scales with similar conditioning: micro-flotation, and batch flotation at the laboratory- and industrial-scale. Based on the experimental results, the following conclusions can be drawn:
Results of micro-flotation tests indicated that SCS could effectively depress hematite particles whereas it did not show a significant impact on the fluorapatite flotation. Furthermore, results showed that the SCS had a higher depression impact on hematite compared to sodium silicate (SS) as the conventional depressant.
Outcomes of batch flotation experiments showed that a selective flotation separation of phosphorus from hematite for the Chadormalu iron ore samples could be achieved by using 300 g/t SCS dosage as the depressant.
Industrial-scale test results revealed that, in the case of reverse flotation of the Chadormalu iron ore, the three different type of reagents (including: NaOH, Na2CO3, and SS gel) can be replaced by just SCS while the efficiency of the plant would be increased.
Author Contributions
A.T. and R.D. conceived and designed the experiments; A.T. and O.A.R. performed the experiments; C.C.T. and J.R. analyzed the data and provided some advice; A.T. and R.D. contributed analyses and assessments; and C.C.T. and J.R. wrote the paper.
Funding
This research received no external funding.
Acknowledgments
The first and fifth authors would like to thank the management and personnel of the Chadormalu Mining and Industrial Complex and Parsulfite Chemical Company for supporting this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Farahat, M.; Hirajima, T.; Sasaki, K.; Doi, K. Adhesion of Escherichia coli onto quartz, hematite and corundum: Extended DLVO theory and flotation behavior. Colloids. Surf. B Bio. 2009, 74, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Tohry, A.; Dehghani, A. Effect of sodium silicate on the reverse anionic flotation of a siliceous–phosphorus iron ore. Sep. Purif. Technol. 2016, 164, 28–33. [Google Scholar] [CrossRef]
- Nunes, A.P.L.; Pinto, C.L.L.; Valadão, G.E.S.; de Magalhães Viana, P.R. Floatability studies of wavellite and preliminary results on phosphorus removal from a Brazilian iron ore by froth flotation. Miner. Eng. 2012, 39, 206–212. [Google Scholar] [CrossRef]
- Ma, X.; Marques, M.; Gontijo, C. Comparative studies of reverse cationic/anionic flotation of Vale iron ore. Int. J. Miner. Process. 2011, 100, 179–183. [Google Scholar] [CrossRef]
- Iwasaki, I. Iron ore flotation, theory and practice. Min. Eng. 1983, 35, 622–631. [Google Scholar]
- Iwasaki, I. Bridging theory and practice in iron ore flotation. In Advances in Coal and Mineral Processing Using Flotation; Chander, C., Klimpel, R.R., Eds.; SME: Littleton, CO, USA, 1989; pp. 177–190. [Google Scholar]
- Pavlovic, S.; Brandão, P.R.G. Adsorption of starch, amylose, amylopectin and glucose monomer and their effect on the flotation of hematite and quartz. Miner. Eng. 2003, 16, 1117–1122. [Google Scholar] [CrossRef]
- Dos Santos, I.D.; Oliveira, J.F. Utilization of humic acid as a depressant for hematite in the reverse flotation of iron ore. Miner. Eng. 2007, 20, 1003–1007. [Google Scholar] [CrossRef]
- Chiaverini, V. Açose. Ferros Fundidos: Características Gerais, Tratamentos Térmicos, Principais Tipos, 7th edição; ABM: São Paulo, Brazil, 2008; pp. 177–178. (In Portuguese) [Google Scholar]
- Yu, K.P.; Yu, Y.F.; Xu, X.Y. Separation behavior and mechanism of hematite and collophane in the presence of collector RFP-138. Trans. Nonfer. Met. Soc. China 2013, 23, 501–507. [Google Scholar]
- Tohry, A.; Dehghani, A.; Hosseini-Nasab, M. Removal of fine gangue minerals from Chadormalu iron concentrate using hydroseparator. Physicochem. Probl. Mineral Pro. 2017, 53, 259–263. [Google Scholar]
- Potapova, E.; Yang, X.; Grahn, M.; Holmgren, A.; Forsmo, S.P.E.; Fredriksson, A.; Hedlund, J. The effect of calcium ions, sodium silicate and surfactant on charge and wettability of magnetite. Colloids Surf. A Physicochem. Eng. Asp. 2011, 386, 79–86. [Google Scholar] [CrossRef]
- Feng, D.; Aldrich, C. Influence of operating parameters on the flotation of apatite. Miner. Eng. 2004, 17, 453–455. [Google Scholar] [CrossRef]
- Qi, G.W.; Klauber, C.; Warren, L.J. Mechanism of action of sodium silicate in the flotation of apatite from hematite. Int. J. Miner. Process. 1993, 39, 251–273. [Google Scholar] [CrossRef]
- Dho, H.; Iwasaki, I. Role of sodium silicate in phosphate flotation. Miner Metall. Process. 1990, 7, 215–221. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).