The Effects of Al2O3 and SiO2 on the Formation Process of Silico-Ferrite of Calcium and Aluminum (SFCA) by Solid-State Reactions
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Preparation
2.2. Phase Determination (XRD, SEM and EDS)
3. Results and Discussion
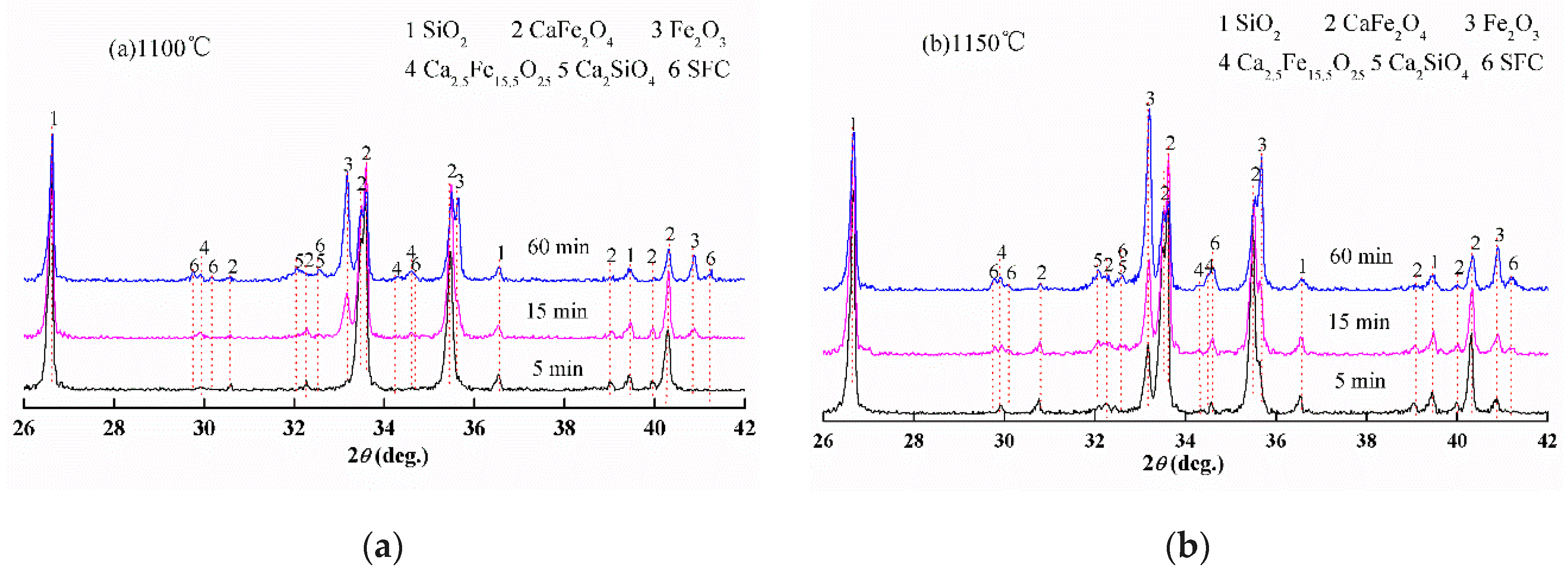
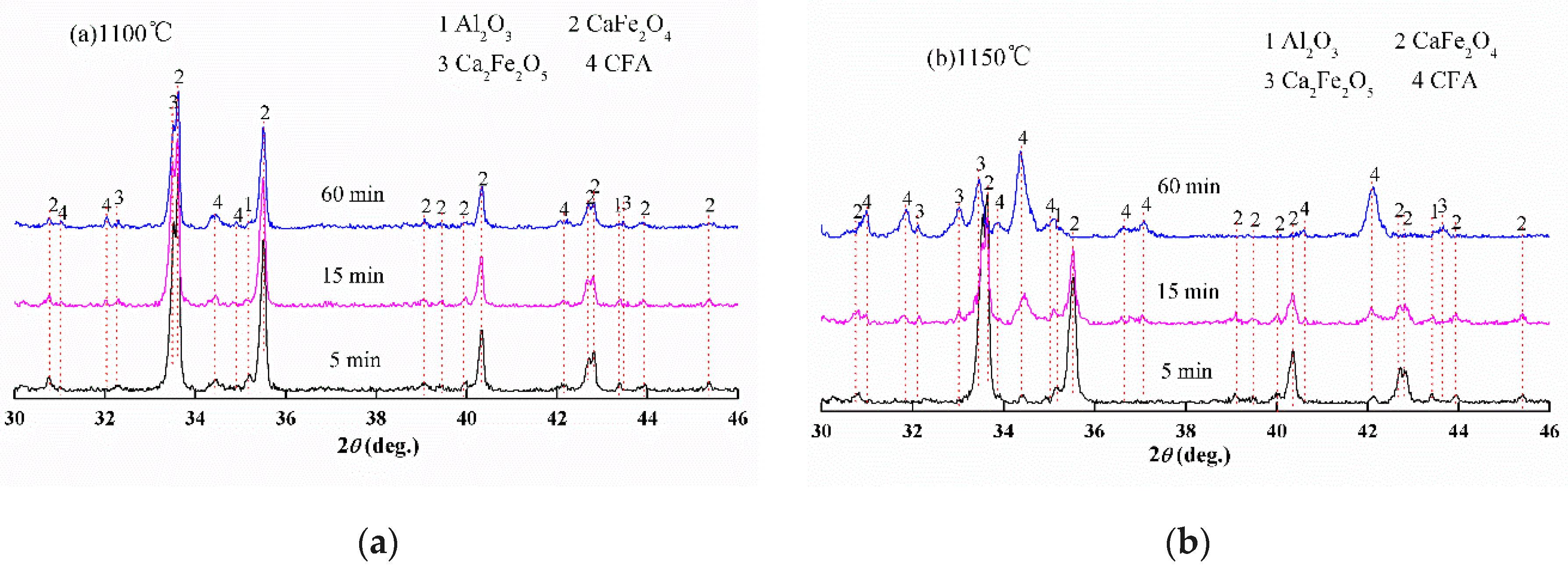
3.1. Reactions between CF and SiO2, CF and Al2O3
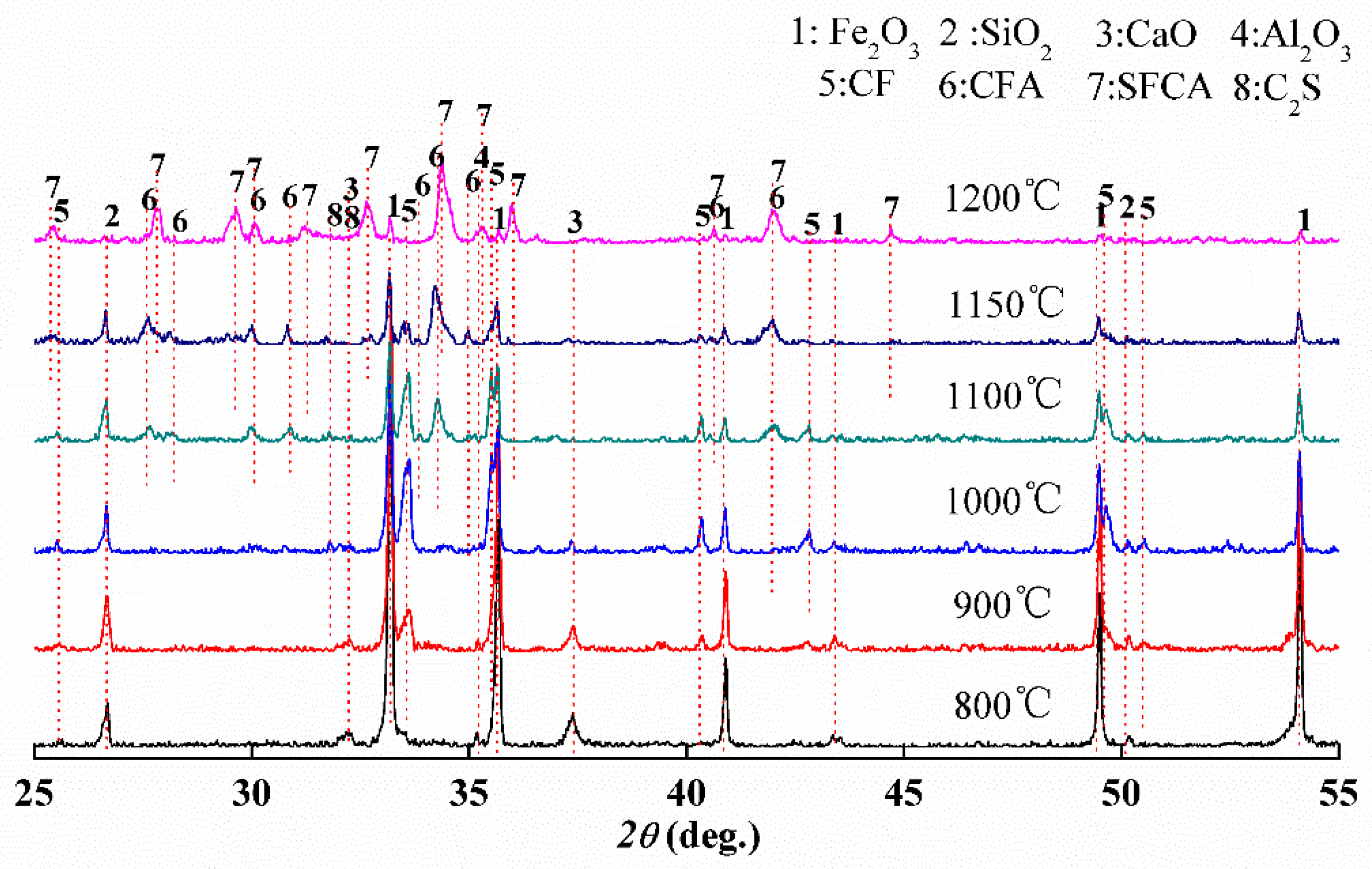
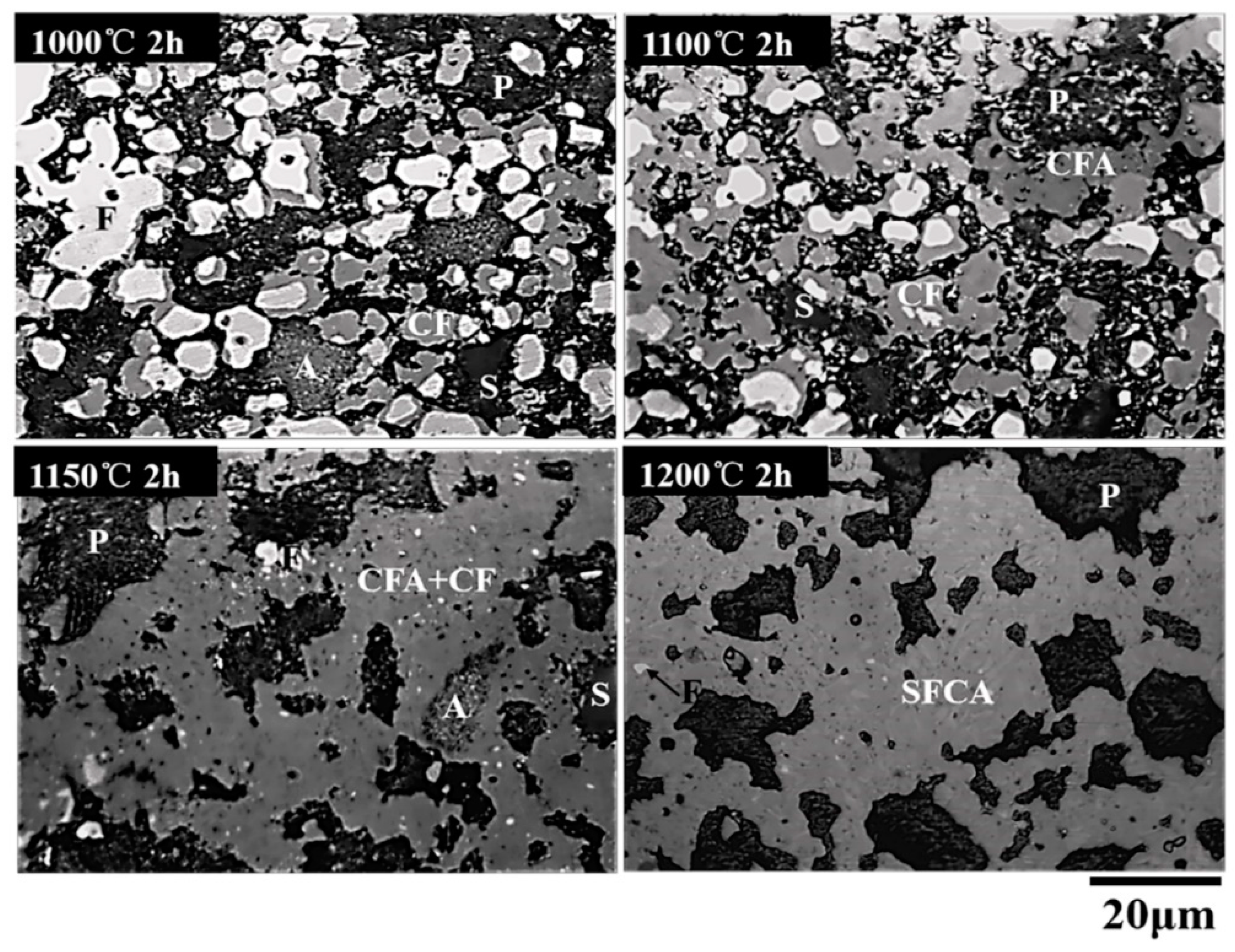
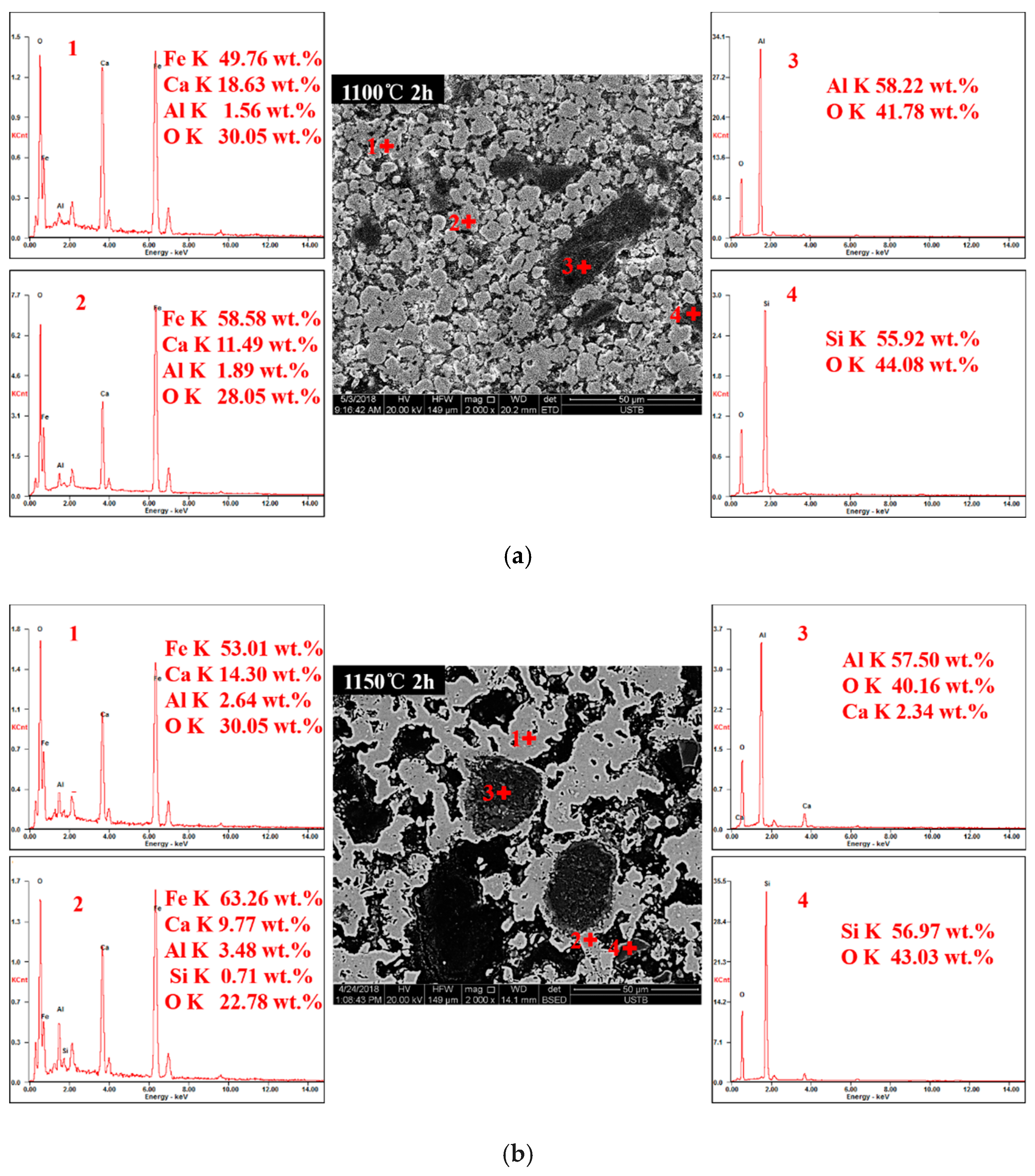
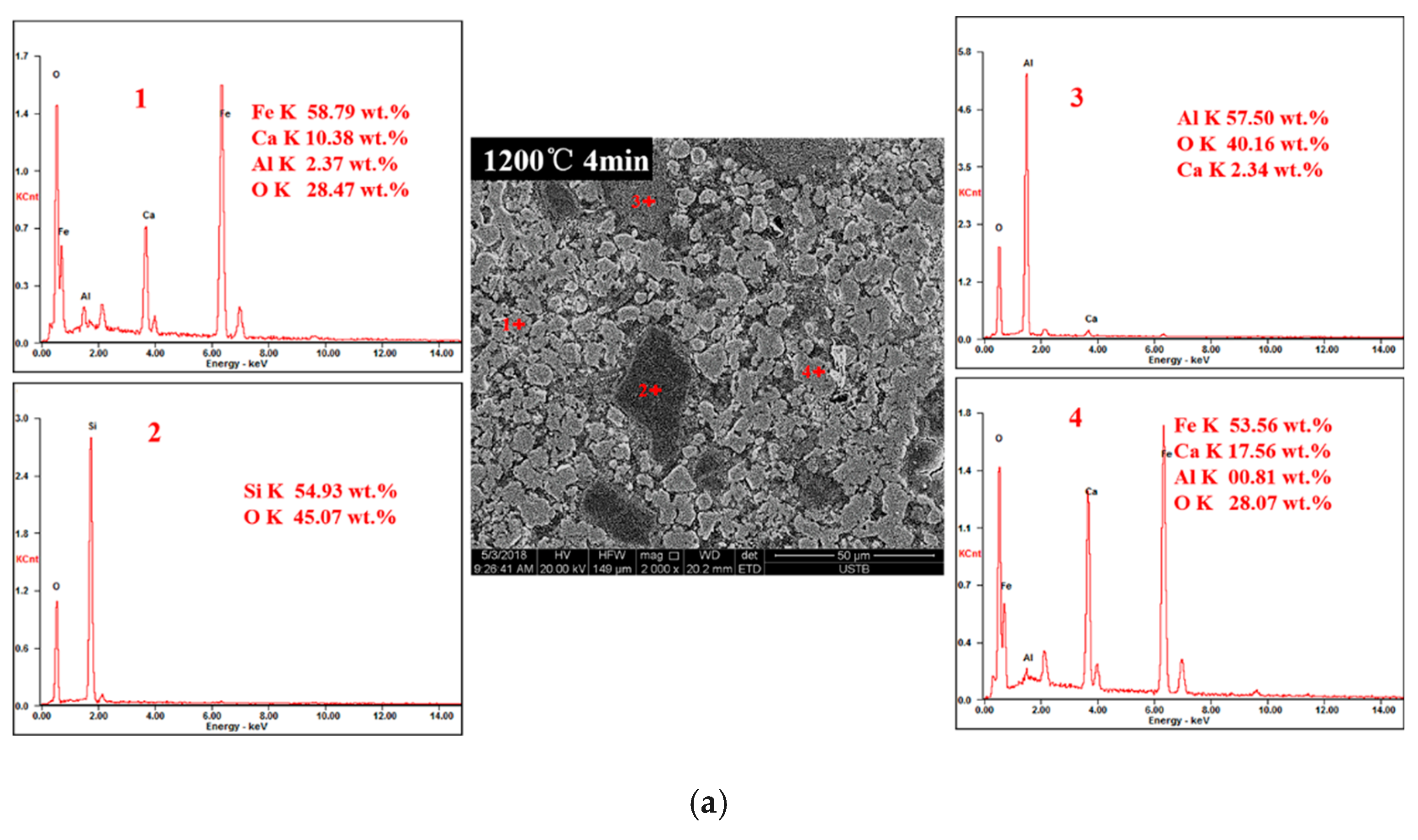
3.2. Phase Composition of Calcium Ferrites Formation with Temperature
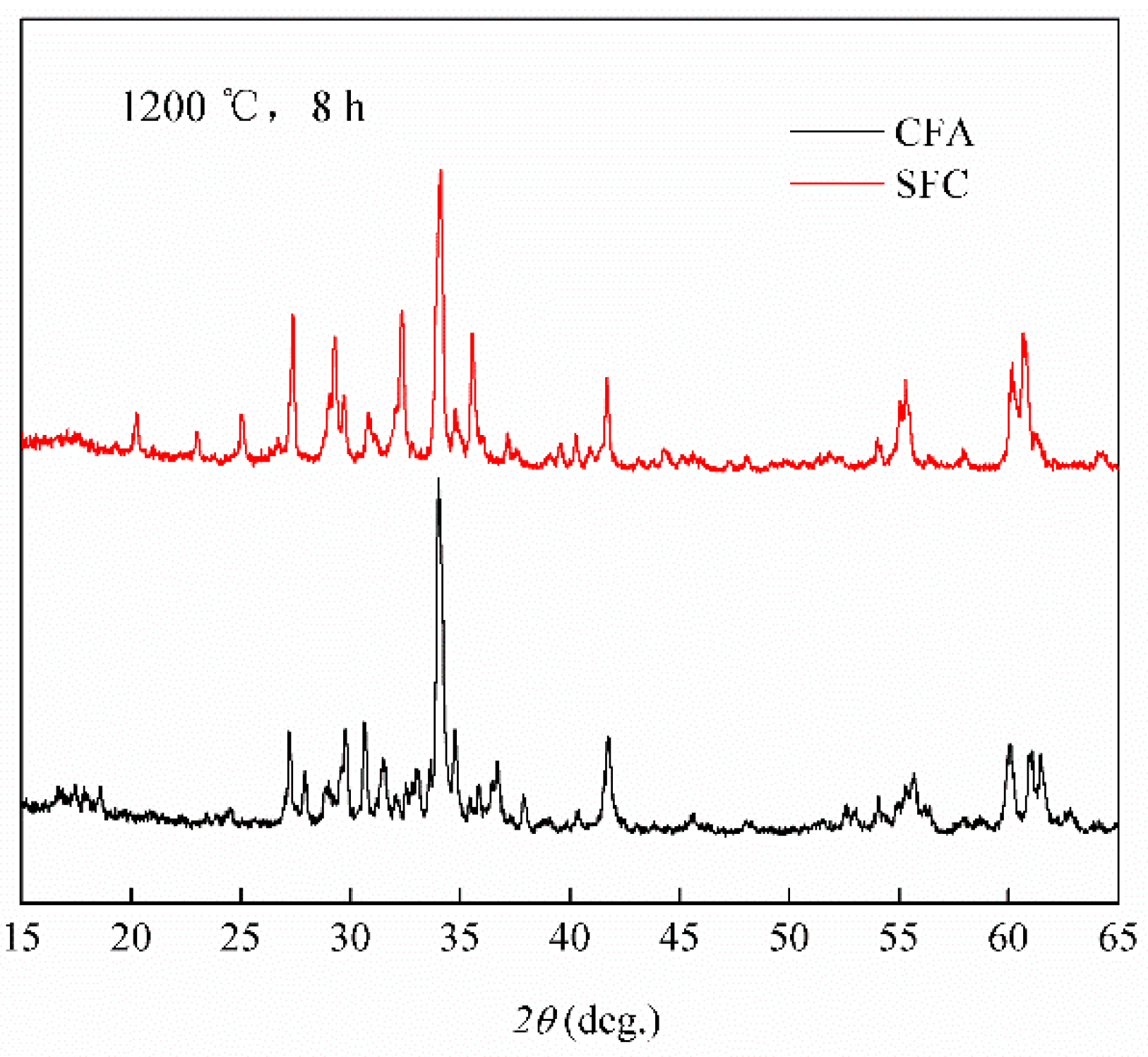
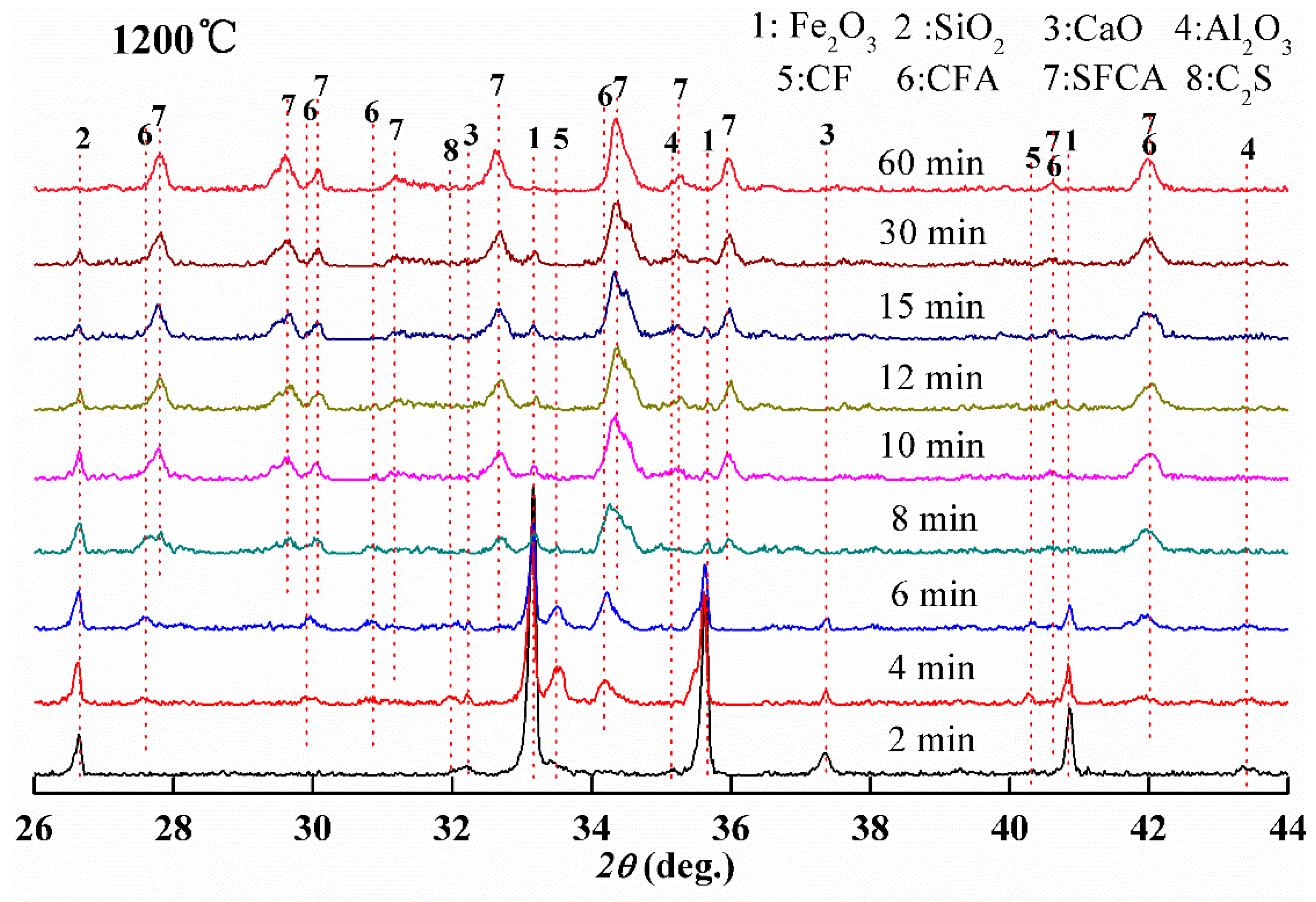
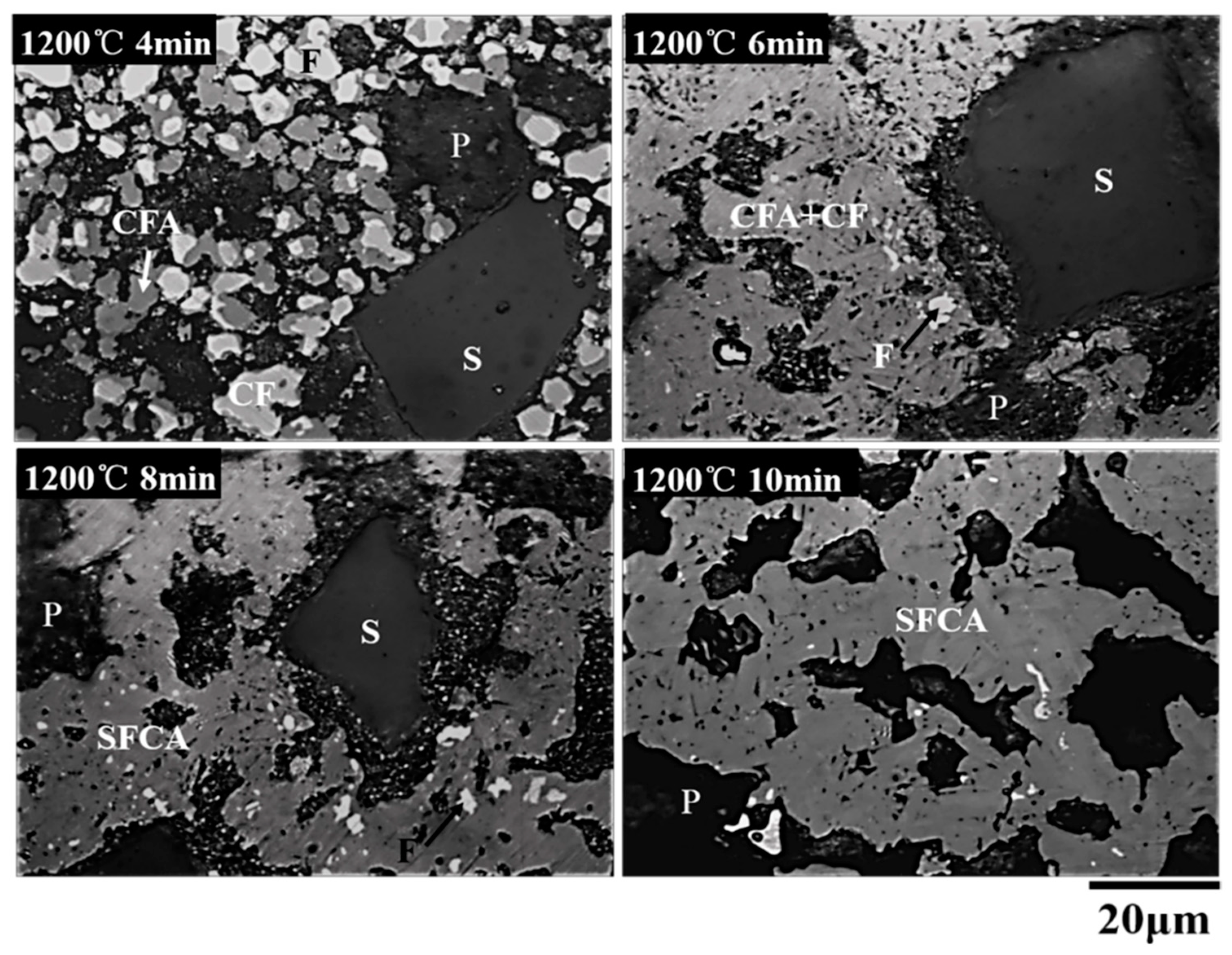
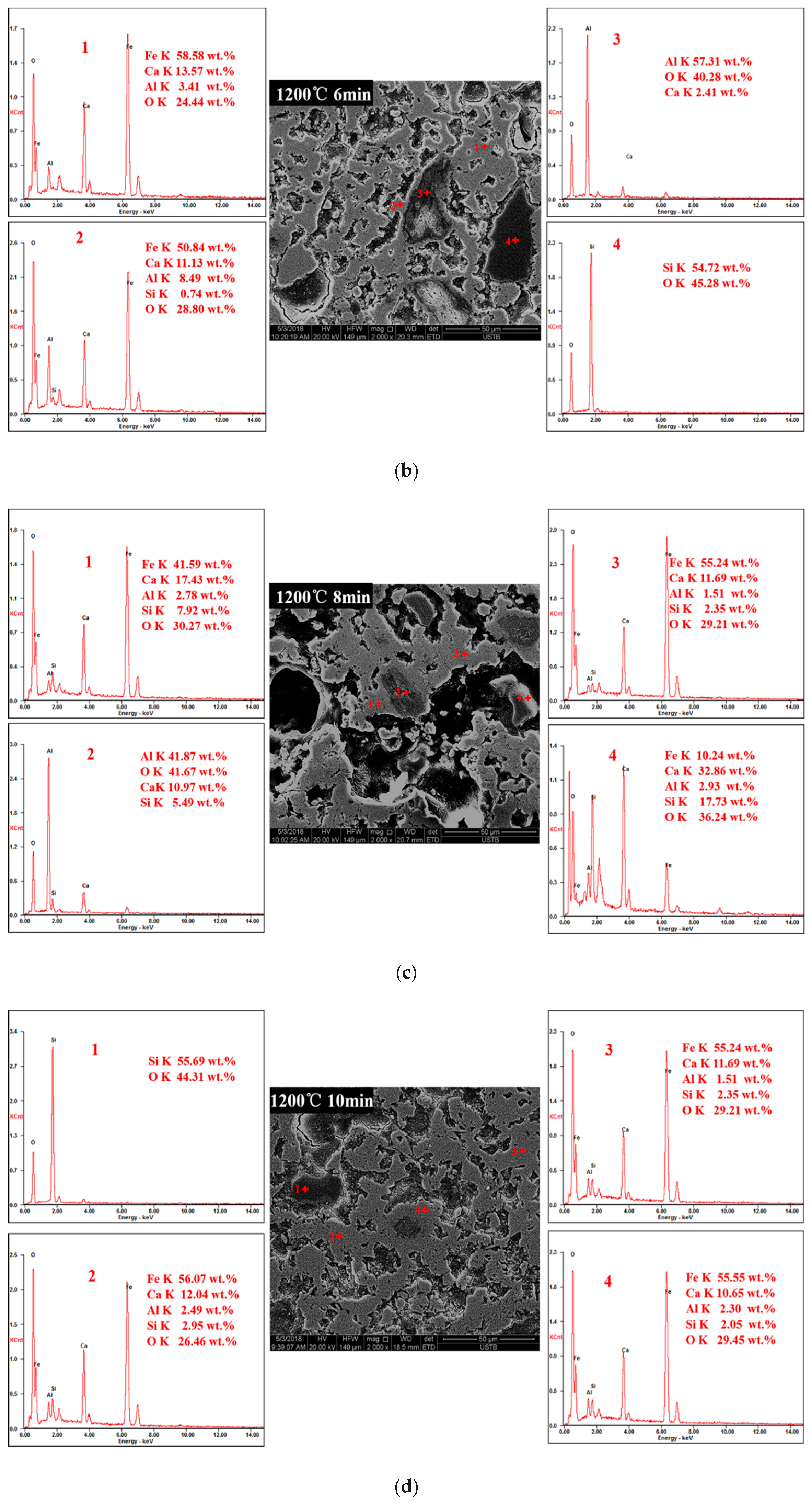
3.3. Order of Calcium Ferrite Formation with Time at 1200 °C
4. Conclusions
- (1)
- Al2O3 reacted with CF to form CFA and C2F, while SiO2 initially reacted with CF to form Ca2SiO4 and Fe2O3 before SFC; the reaction rate of CFA formation is faster than that of SFC under the same conditions.
- (2)
- The CFA as a precursor participated in the SFCA formation at 1100 °C, while SiO2 participated in the SFCA formation until the temperature reached 1150 °C.
- (3)
- Al2O3 participated in the formation of SFCA in the form of CFA, while SiO2 dissolved into CFA and promoted the formation of SFCA.
Author Contributions
Funding
Conflicts of Interest
References
- Frazer, F.W.; Westenberger, H.; Boss, K.H.; Thumm, W. The relationship between basicity and swelling on reduction of iron-ore pellets. Int. J. Miner. Process. 1975, 2, 353–365. [Google Scholar] [CrossRef]
- Hsieh, L.H.; Whiteman, J.A. Effect of oxygen potential on mineral formation in lime-fluxed iron ore sinter. ISIJ Int. 1989, 29, 625–634. [Google Scholar] [CrossRef]
- Patrick, T.R.C.; Lovel, R.R. Leaching dicalcium silicates from iron ore sinter to remove phosphorus and other contaminants. ISIJ Int. 2001, 41, 128–135. [Google Scholar] [CrossRef]
- Choudhary, M.K.; Bhattacharjee, D.; Bannerjee, P.S.; Lahiri, A.K. Effect of variation of alumina on development of phases during iron ore sintering. ISIJ Int. 2008, 48, 1804–1806. [Google Scholar] [CrossRef]
- Cores, A.; Babich, A.; Muñiz, M.; Ferreira, S.; Mochon, J. The influence of different iron ores mixtures composition on the quality of sinter. ISIJ Int. 2010, 50, 1089–1098. [Google Scholar] [CrossRef]
- Inoue, K.; Ikeda, T. The solid solution state and the crystal structure of calcium ferrite formed in lime-fluxed iron ores. Tetsu-To-Hagane 1982, 68, 2190–2199. [Google Scholar] [CrossRef]
- Patrick, T.R.C.; Pownceby, M.I. Stability of silico-ferrite of calcium and aluminum (SFCA) in air-solid solution limits between 1240 °C and 1390 °C and phase relationships within the Fe2O3–CaO–Al2O3–SiO2 (FCAS) system. Metall. Mater. Trans. B 2002, 33B, 79–89. [Google Scholar] [CrossRef]
- Mumme, W.G.; Gable, R.W. Crystal structures of monoclinic variants of two SFCA structure-types containing significant Fe2+: Crystal structure of monoclinic SFCA-II, Ca2.6 Fe3+8.0Fe2+3.4Al4O24; and proposed crystal structure of monoclinic SFCA-I, ideally, Ca 2Fe2+8Fe3+8Al4O28. Neues Jb. Miner. Abh. J. Min. Geochem. 2018, 195, 89–100. [Google Scholar] [CrossRef]
- Hamilton, J.D.G.; Hoskins, B.F.; Mumme, W.G.; Borbidge, W.E.; Montague, M.A. The crystal structure and crystal chemistry of Ca2.3Mg0.8Al1.5Si1.1Fe8.3O20 (SFCA): Solid solution limits and selected phase relationships of SFCA in the SiO2–Fe2O3–CaO(−Al2O3) system. Neues Jb. Miner. Abh. 1989, 161, 1–26. [Google Scholar]
- Ding, X.; Guo, X.M. The formation process of silico-ferrite of calcium (SFC) from binary calcium ferrite. Metall. Mater. Trans. B 2014, 45B, 1221–1231. [Google Scholar] [CrossRef]
- Ding, X.; Guo, X.M. Effect of SiO2 on the crystal structure stability of SFC at 1473 K (1200 C). Metall. Mater. Trans. B 2015, 46, 1146–1153. [Google Scholar] [CrossRef]
- Lister, D.H.; Glasser, F.P. Phase relations in the system CaO-Al2O3-iron oxide. Br. Ceram. Trans. J. 1967, 66, 293–305. [Google Scholar]
- Mumme, W.G. The crystal structure of SFCA-II, Ca5.1Al9.3Fe3+18.7Fe2+0.9O48 a new homologue of the aenigmatite structure-type, and structure refinement of SFCA-type, Ca2Al5Fe7O20. Implications for the nature of the “ternary-phase solid-solution” previously reported in the CaO–Al2O3–iron oxide system. Neues Jb. Miner. Abh. 2003, 178, 307–335. [Google Scholar]
- Guo, H.; Guo, X.M. Effect of Aluminum Dissolved in Hematite on Formation of Calcium Ferrites at 1473K. Metall. Mater. Trans. B 2018, 49, 1974–1984. [Google Scholar] [CrossRef]
- Sugiyama, K.; Monkawa, A.; Sugiyama, T. Crystal structure of the SFCAM phase Ca2(Ca, Fe, Mg, Al)6(Fe, Al, Si)6O20. ISIJ Int. 2005, 45, 560–568. [Google Scholar] [CrossRef]
- Scarlett, N.V.Y.; Madsen, I.C.; Pownceby, M.I.; Christensen, A.N. In situ X-ray diffraction analysis of iron ore sinter phases. J. Appl. Crystallogr. 2004, 37, 362–368. [Google Scholar] [CrossRef]
- Scarlett, N.V.Y.; Pownceby, M.I.; Madsen, I.C.; Christensen, A.N. Reaction sequences in the formation of silico-ferrites of calcium and aluminum in iron ore sinter. Metall. Mater. Trans. B 2004, 35B, 929–936. [Google Scholar] [CrossRef]
- Webster, N.A.S.; Pownceby, M.I.; Madsen, I.C.; Kimpton, J.A. Silico-ferrite of calcium and aluminum (SFCA) iron ore sinter bonding phases: New insights into their formation during heating and cooling. Metall. Mater. Trans. B 2012, 43B, 1344–1357. [Google Scholar] [CrossRef]
- Webster, N.A.S.; Pownceby, M.I.; Madsen, I.C.; Kimpton, J.A. Effect of oxygen partial pressure on the formation mechanisms of complex Ca-rich ferrites. ISIJ Int. 2013, 53, 774–781. [Google Scholar] [CrossRef]
- Webster, N.A.S.; Churchill, J.G.; Tufaile, F.; Pownceby, M.I.; Manuel, J.R.; Kimpton, J.A. Fundamentals of silico-ferrite of calcium and aluminium (SFCA) and SFCA-I iron ore sinter bonding phase formation: Effects of titanomagnetite-based ironsand and titanium addition. ISIJ Int. 2016, 56, 1715–1722. [Google Scholar] [CrossRef]
- Webster, N.A.S.; Pownceby, M.I.; Pattel, R. Fundamentals of silico-ferrite of calcium and aluminium (SFCA) and SFCA-I iron ore sinter bonding phase formation: Effects of mill scale addition. Powder Diffr. 2017, 32, S85–S89. [Google Scholar] [CrossRef]
- Liles, D.C.; Villiers, J.P.R.; Kahlenberg, V. Refinement of iron ore sinter phases: A silico-ferrite of calcium and aluminium (SFCA) and an Al-free SFC, and the effect on phase quantification by X-ray diffraction. Mineral. Petrol. 2016, 110, 141–147. [Google Scholar] [CrossRef]
- Hancart, J.; Leroy, V.; Bragard, A. A study of the phases present in blast furnace sinter. Some considerations on the mechanism of their formation. CNRM Metall. Rep. 1967, 14, 3–7. [Google Scholar]
- Ahsan, S.N.; Mukherjee, T.; Whiteman, J.A. Structure of fluxed sinter. Ironmak. Steelmak. 1983, 10, 54–64. [Google Scholar]
- Ding, X.; Guo, X.M. Study of SiO2 involved in the formation process of silico-ferrite of calcium (SFC) by solid-state reactions. Int. J. Min. Process. 2016, 149, 69–77. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]

| Sample | Fe2O3 | SiO2 | CaO | Al2O3 | CaFe2O4 |
|---|---|---|---|---|---|
| A | __ | 23.44 | __ | __ | 76.56 |
| B | __ | __ | __ | 20.65 | 79.35 |
| C | 70.34 | 6.60 | 17.45 | 5.61 | __ |
| Sample | Phase Fractions (Mass Percent) | Rp (%) | Rwp (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CFA | C2F | Al2O3 | SFC | CF | Fe2O3 | Ca2SiO4 | SiO2 | CFF | |||
| CF + SiO2 (5 min) | - | - | - | 2.7 | 63.0 | 6.8 | 3.3 | 22.6 | 1.6 | 10.3 | 13.5 |
| CF + SiO2 (15 min) | - | - | - | 4.9 | 50.2 | 14.1 | 7.1 | 21.5 | 2.2 | 9.5 | 12.2 |
| CF + SiO2 (60 min) | -- | - | - | 9.8 | 34.7 | 25.1 | 8.6 | 18.9 | 2.9 | 9.7 | 12.9 |
| CF + Al2O3 (5 min) | 7.5 | 5.5 | 18.2 | - | 68.8 | - | - | - | - | 8.9 | 11.2 |
| CF + Al2O3 (15 min) | 20.9 | 18.4 | 14.6 | - | 46.1 | - | - | - | - | 8.6 | 12.3 |
| CF + Al2O3 (60 min) | 57.7 | 36.8 | 5.5 | - | 0 | - | - | - | - | 8.3 | 11.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, F.; Guo, X.-M. The Effects of Al2O3 and SiO2 on the Formation Process of Silico-Ferrite of Calcium and Aluminum (SFCA) by Solid-State Reactions. Minerals 2019, 9, 101. https://doi.org/10.3390/min9020101
Liao F, Guo X-M. The Effects of Al2O3 and SiO2 on the Formation Process of Silico-Ferrite of Calcium and Aluminum (SFCA) by Solid-State Reactions. Minerals. 2019; 9(2):101. https://doi.org/10.3390/min9020101
Chicago/Turabian StyleLiao, Fei, and Xing-Min Guo. 2019. "The Effects of Al2O3 and SiO2 on the Formation Process of Silico-Ferrite of Calcium and Aluminum (SFCA) by Solid-State Reactions" Minerals 9, no. 2: 101. https://doi.org/10.3390/min9020101
APA StyleLiao, F., & Guo, X.-M. (2019). The Effects of Al2O3 and SiO2 on the Formation Process of Silico-Ferrite of Calcium and Aluminum (SFCA) by Solid-State Reactions. Minerals, 9(2), 101. https://doi.org/10.3390/min9020101





