Barren and Li–Sn–Ta Mineralized Pegmatites from NW Spain (Central Galicia): A Comparative Study of Their Mineralogy, Geochemistry, and Wallrock Metasomatism
Abstract
:1. Introduction
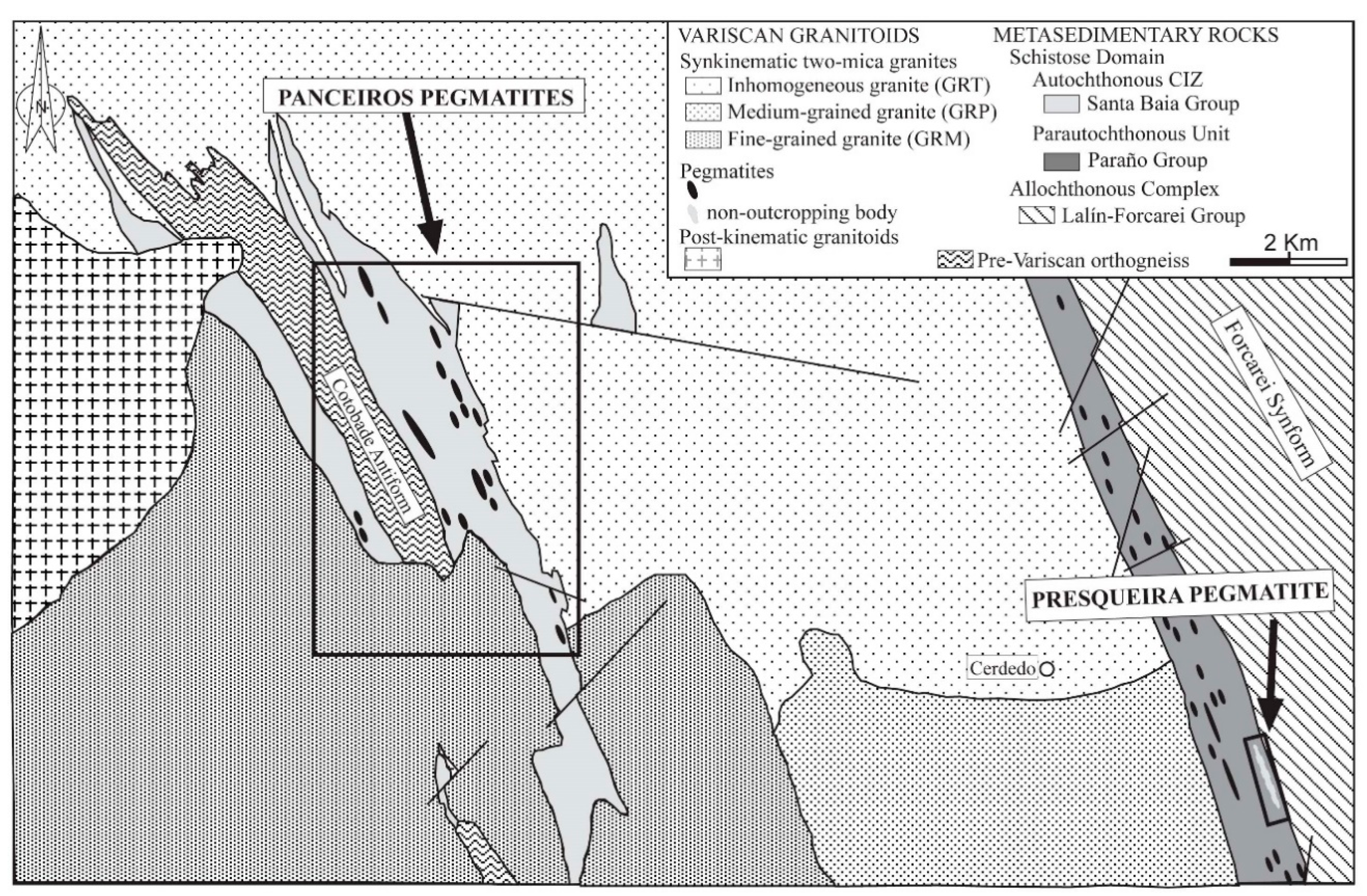
2. Geological Setting
3. Analytical Techniques
4. The Panceiros Pegmatites
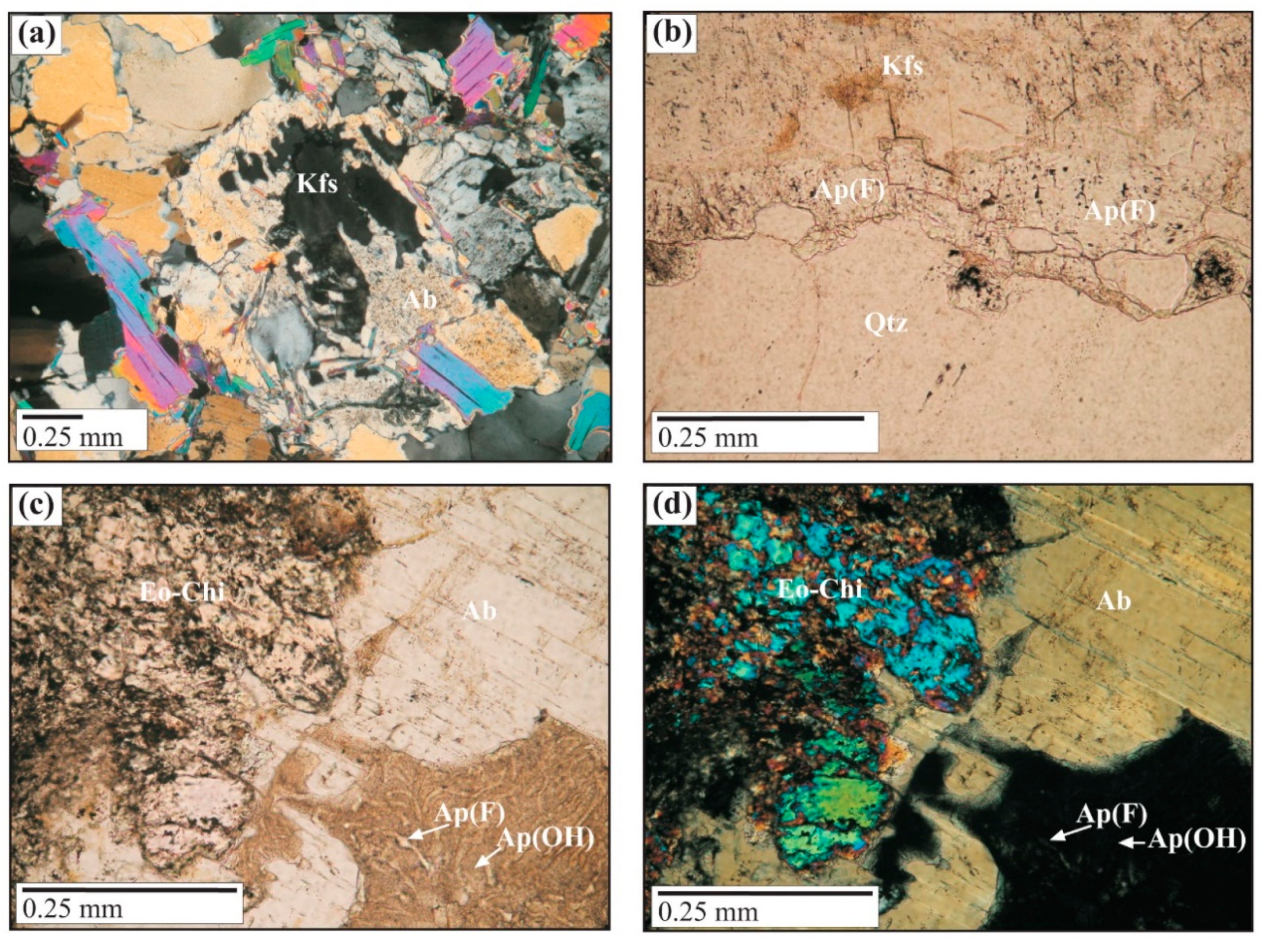
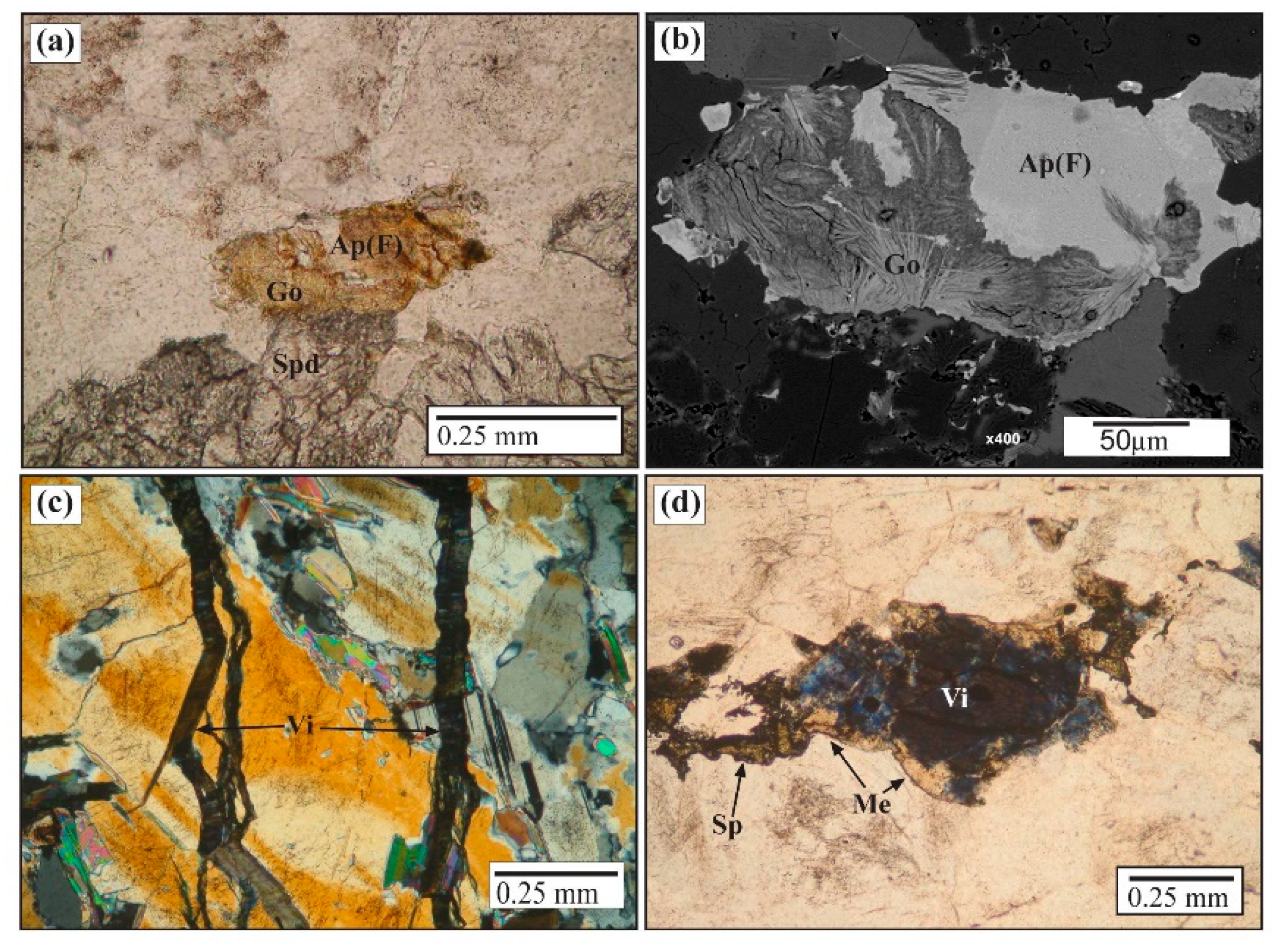
Mineralogy and Geochemistry
5. The Presqueira Pegmatite
Mineralogy and Geochemistry
6. Whole Rock Geochemistry
6.1. Igneous Rock Geochemistry: Pegmatites and Granites
6.2. Pegmatite–Wallrock Interaction
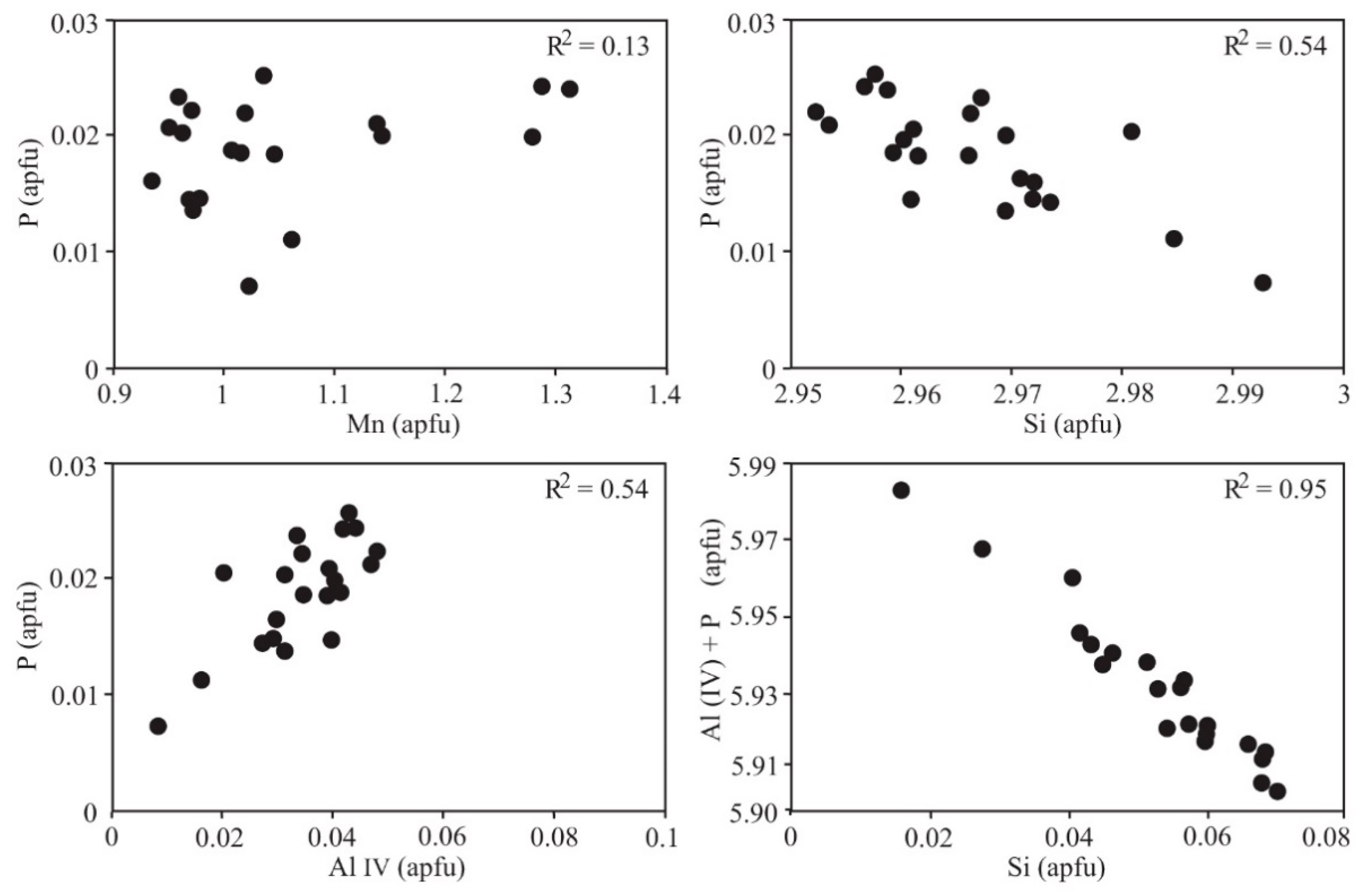
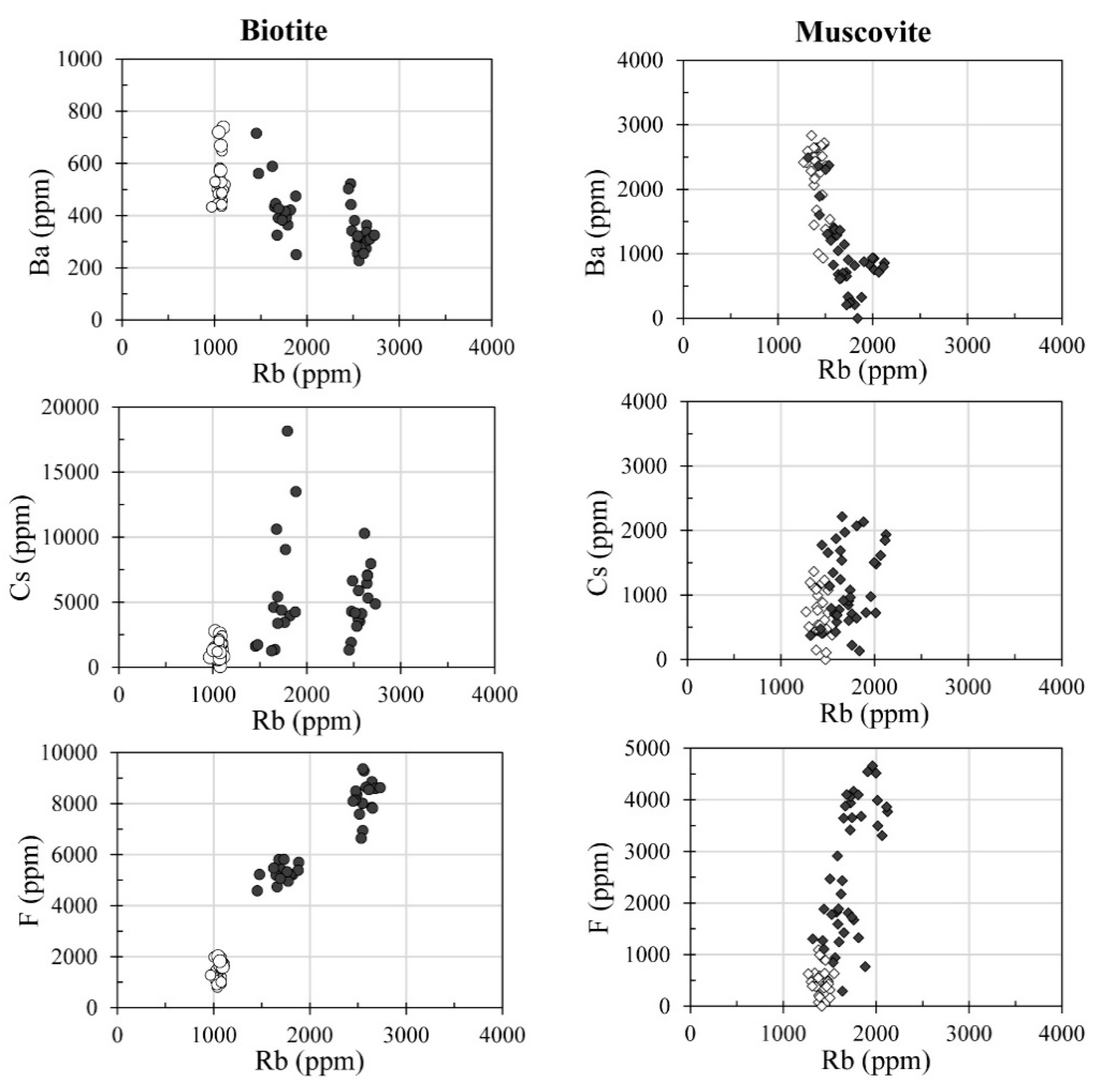
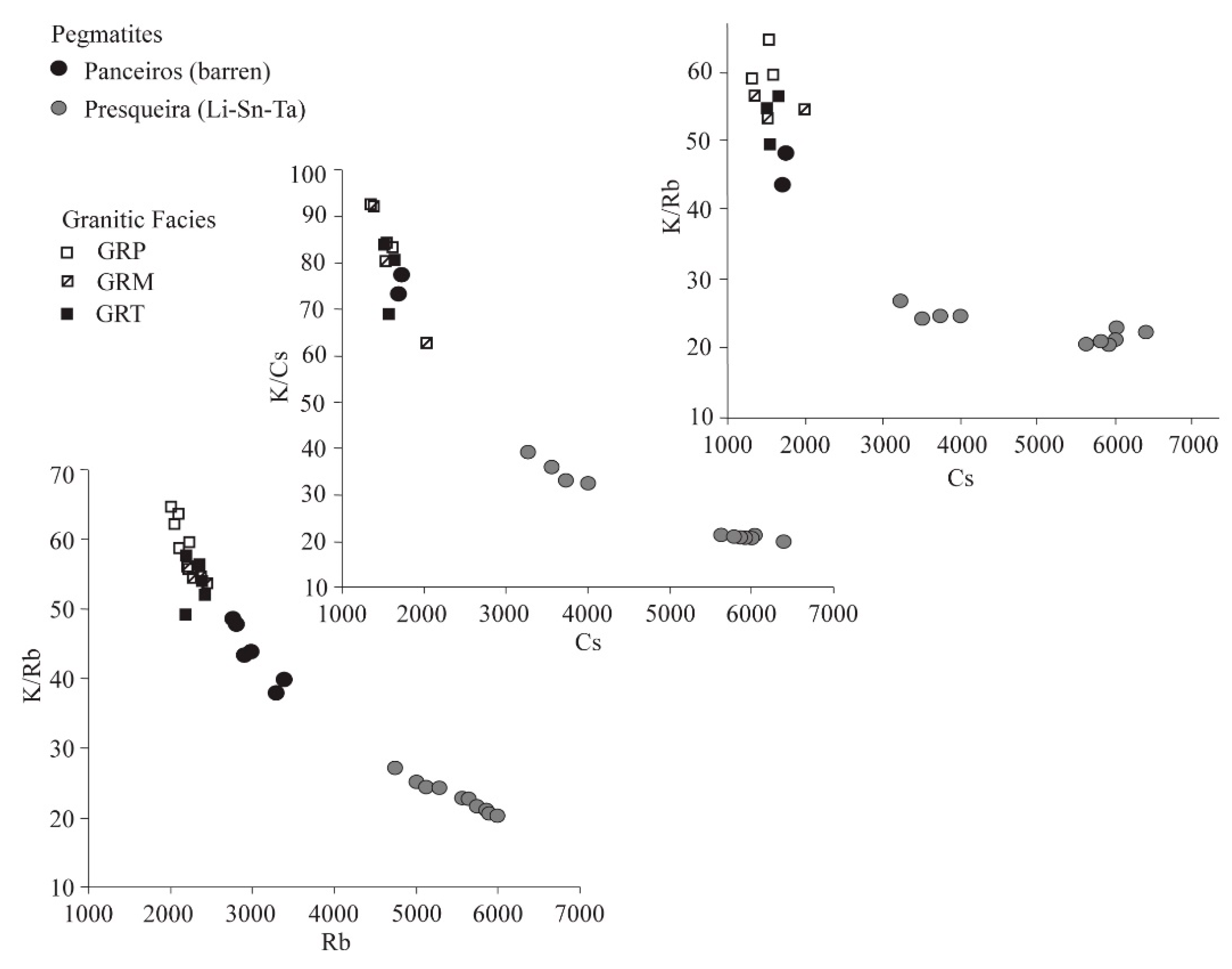
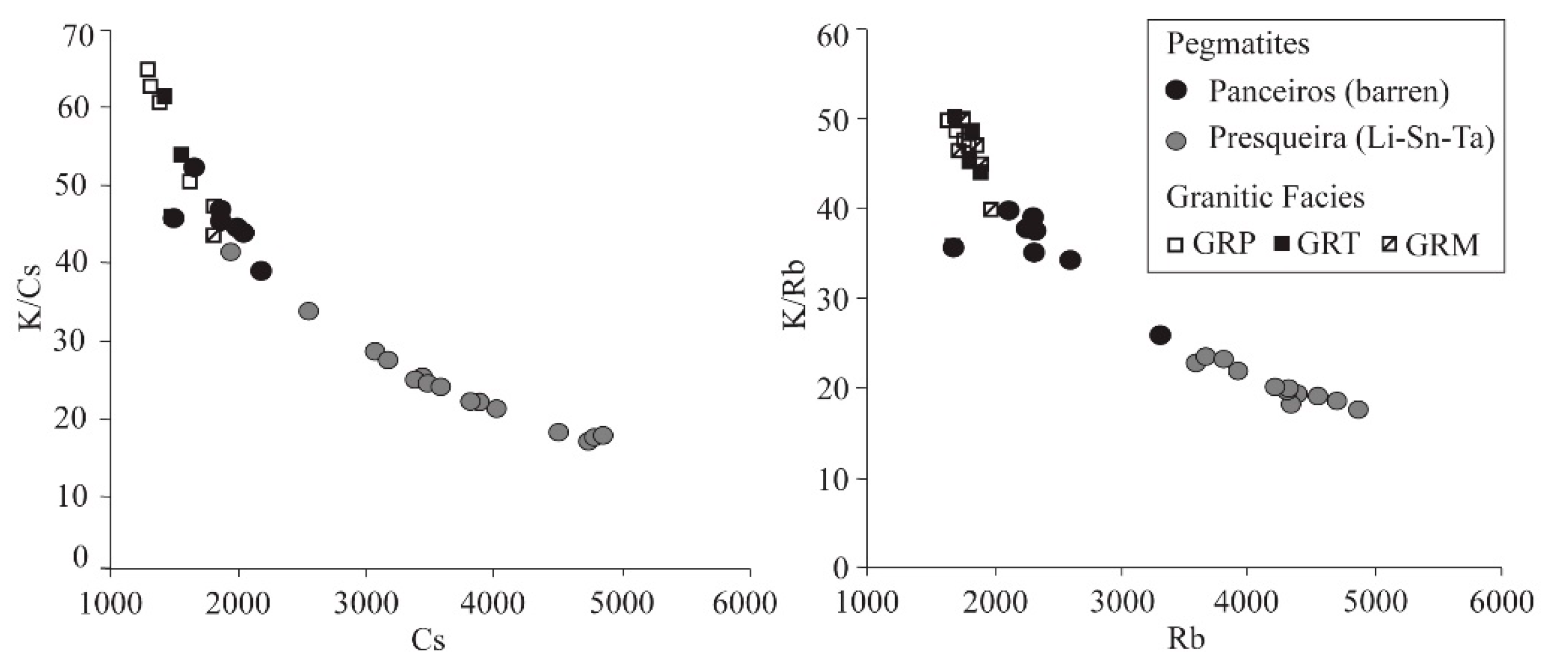
7. Mineral Geochemistry: Fractionation Trends
8. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Barren Pegmatites (Panceiros) | Li–Sn–Ta Mineralized Pegmatite (Presqueira) | ||||||
|---|---|---|---|---|---|---|---|
| wt % Oxide | (a) | (b) | (c) | (a) | (b) | (c) | (d) |
| SiO2 | 64.38 | 63.71 | 63.62 | 63.47 | 64.38 | 63.17 | 63.68 |
| Al2O3 | 18.88 | 19.38 | 18.94 | 18.55 | 18.65 | 18.30 | 18.29 |
| Na2O | 0.48 | 1.03 | 1.52 | 0.90 | 0.41 | 0.59 | 0.99 |
| K2O | 16.04 | 15.24 | 15.13 | 14.85 | 15.41 | 15.49 | 14.95 |
| Rb2O | 0.37 | 0.36 | 0.32 | 0.64 | 0.62 | 0.52 | 0.63 |
| Cs2O | - | - | 0.18 | 0.62 | 0.68 | 0.35 | 0.64 |
| BaO | 0.01 | - | - | - | - | - | - |
| ZnO | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | - | 0.01 |
| P2O5 | 0.59 | 0.58 | 0.52 | 0.42 | 0.63 | 0.65 | 0.54 |
| Total | 100.77 | 100.32 | 100.24 | 99.47 | 100.8 | 99.07 | 99.73 |
| Cations based on 8 Oxygens | |||||||
| Si | 2.96 | 2.93 | 2.94 | 2.96 | 2.97 | 2.96 | 2.97 |
| Al | 1.02 | 1.05 | 1.03 | 1.02 | 1.01 | 1.01 | 1.00 |
| P | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 |
| Sum Z | 4.00 | 4.01 | 3.99 | 4.00 | 4.00 | 4.00 | 3.99 |
| Na | 0.04 | 0.09 | 0.14 | 0.08 | 0.04 | 0.05 | 0.09 |
| K | 0.94 | 0.89 | 0.89 | 0.88 | 0.91 | 0.93 | 0.89 |
| Rb | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 |
| Cs | - | - | - | 0.01 | 0.01 | 0.01 | 0.01 |
| Sum X | 1.00 | 1.00 | 1.04 | 0.99 | 0.98 | 1.01 | 1.01 |
| Trace elements (ppm) | |||||||
| Rb | 3384 | 3283 | 2893 | 5861 | 5645 | 4726 | 5738 |
| P | 2595 | 2524 | 2256 | 1842 | 2759 | 2831 | 2357 |
| Cs | - | - | 1704 | 5824 | 6424 | 3291 | 5999 |
| Zn | 146 | 139 | 91 | 134 | 141 | - | 112 |
| Ba | 124 | - | - | - | - | - | - |
| K/Rb | 39.36 | 38.52 | 43.40 | 21.03 | 22.66 | 27.21 | 21.63 |
| K/Cs | - | - | 73.69 | 21.16 | 19.91 | 39.07 | 20.69 |
| Barren Pegmatites (Panceiros) | Li–Sn–Ta Mineralized Pegmatite (Presqueira) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| wt % Oxide | (a) | (b) | (c) | (d) | (a) | (b) | (c) | (d) | (e) |
| SiO2 | 66.44 | 67.01 | 68.08 | 67.53 | 67.20 | 68.49 | 68.54 | 68.36 | 67.58 |
| Al2O3 | 21.13 | 19.97 | 19.57 | 19.78 | 20.10 | 19.61 | 19.18 | 19.60 | 19.88 |
| FeO | 0.27 | - | - | - | - | - | - | - | - |
| CaO | 0.15 | 0.26 | 0.13 | 0.07 | 0.09 | - | - | - | 0.08 |
| Na2O | 11.22 | 11.95 | 12.15 | 12.25 | 12.28 | 12.12 | 12.06 | 12.09 | 12.31 |
| K2O | 0.17 | 0.15 | 0.15 | 0.10 | 0.07 | 0.07 | 0.12 | 0.09 | 0.09 |
| Rb2O | 0.23 | 0.25 | 0.26 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.23 |
| BaO | - | - | - | 0.02 | 0.01 | - | 0.03 | 0.02 | - |
| P2O5 | 0.80 | 0.54 | 0.13 | 0.50 | 0.65 | 0.04 | 0.15 | 0.08 | 0.56 |
| Total | 100.41 | 100.13 | 100.47 | 100.50 | 100.65 | 100.58 | 100.33 | 100.49 | 100.73 |
| Cations based on 32 Oxygens | |||||||||
| Si | 11.60 | 11.75 | 11.90 | 11.80 | 11.73 | 11.94 | 11.98 | 11.93 | 11.78 |
| Al | 4.35 | 4.13 | 4.03 | 4.08 | 4.13 | 4.03 | 3.95 | 4.03 | 4.08 |
| P | 0.12 | 0.08 | 0.02 | 0.07 | 0.10 | 0.01 | 0.02 | 0.01 | 0.08 |
| Sum Z | 16.06 | 15.96 | 15.95 | 15.95 | 15.96 | 15.98 | 15.95 | 15.97 | 15.94 |
| Fe | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ca | 0.03 | 0.05 | 0.02 | 0.01 | 0.02 | 0.00 | 0.00 | 0.00 | 0.01 |
| Na | 3.80 | 4.06 | 4.12 | 4.15 | 4.16 | 4.10 | 4.09 | 4.09 | 4.16 |
| K | 0.04 | 0.03 | 0.03 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 |
| Rb | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Sum X | 3.90 | 4.15 | 4.17 | 4.18 | 4.20 | 4.11 | 4.11 | 4.11 | 4.20 |
| Ab | 99.26 | 98.80 | 99.40 | 99.70 | 99.59 | 100.00 | 100.00 | 100.00 | 99.64 |
| An | 0.74 | 1.20 | 0.60 | 0.30 | 0.41 | 0.00 | 0.00 | 0.00 | 0.36 |
| Trace elements (ppm) | |||||||||
| P | 3502 | 2368 | 550 | 2181 | 2816 | 195 | 673 | 357 | 2458 |
| Rb | 2123 | 2242 | 2335 | 2274 | 2243 | 2274 | 2284 | 2245 | 2101 |
| Ba | - | - | - | 163 | - | - | 247 | 145 | - |
| Barren Pegmatites (Panceiros) | Li–Sn–Ta Mineralized Pegmatite (Presqueira) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| wt % Oxide | (a) | (b) | (c) | (d) | (e) | (a) | (b) | (c) | (d) | (e) |
| SiO2 | 45.83 | 46.05 | 45.98 | 46.09 | 46.48 | 45.8 | 45.16 | 45.19 | 45.43 | 45.34 |
| Al2O3 | 35.82 | 36.13 | 36.57 | 35.89 | 36.2 | 38.37 | 38.1 | 37.47 | 37.46 | 37.32 |
| FeO | 2.07 | 2.30 | 1.96 | 2.40 | 2.38 | 0.25 | 0.43 | 0.70 | 0.65 | 0.68 |
| TiO2 | 0.07 | - | 0.10 | - | - | - | - | - | - | - |
| MgO | 0.13 | 0.10 | 0.10 | 0.14 | 0.12 | - | - | - | - | - |
| CaO | 0.07 | - | - | - | - | - | - | - | - | - |
| Na2O | 0.77 | 0.47 | 0.67 | 0.58 | 0.28 | 0.45 | 0.56 | 0.62 | 0.55 | 0.54 |
| K2O | 9.92 | 10.12 | 10.21 | 10.3 | 10.09 | 10.56 | 9.78 | 10.21 | 10.24 | 10.26 |
| Rb2O | 0.25 | 0.25 | 0.25 | 0.36 | 0.23 | 0.42 | 0.39 | 0.53 | 0.50 | 0.47 |
| Cs2O | - | 0.23 | 0.18 | 0.20 | - | 0.33 | 0.21 | 0.41 | 0.51 | 0.37 |
| BaO | - | - | - | - | - | - | 0.01 | 0.01 | 0.02 | 0.02 |
| P2O5 | 0.07 | 0.05 | 0.05 | 0.05 | 0.01 | - | - | - | - | - |
| F | 0.43 | 0.52 | 0.5 | 0.42 | 0.21 | 0.17 | 0.22 | 0.33 | 0.135 | 0.25 |
| ZnO | 0.04 | 0.04 | 0.03 | 0.04 | 0.05 | 0.02 | 0.04 | 0.04 | 0.05 | 0.04 |
| LiO2 * | 0.13 | 0.16 | 0.16 | 0.12 | 0.05 | 0.04 | 0.05 | 0.09 | 0.03 | 0.06 |
| H2O * | 4.27 | 4.27 | 4.30 | 4.33 | 4.43 | 4.52 | 4.40 | 4.33 | 4.44 | 4.37 |
| O = F,Cl | 0.19 | 0.22 | 0.21 | 0.18 | 0.09 | 0.07 | 0.09 | 0.14 | 0.05 | 0.10 |
| Total | 100.06 | 100.91 | 101.27 | 101.10 | 100.62 | 101.00 | 99.44 | 100.07 | 100.07 | 99.82 |
| Cations based on 22 Oxygens | ||||||||||
| Si | 6.13 | 6.12 | 6.08 | 6.15 | 6.16 | 5.97 | 6.02 | 6.03 | 6.05 | 6.05 |
| AlIV | 1.87 | 1.88 | 1.92 | 1.85 | 1.84 | 2.03 | 1.98 | 1.97 | 1.95 | 1.95 |
| P | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | - | - | - | - | - |
| Sum(Z) | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.000 | 8.00 | 8.00 | 8.00 |
| AlVI | 3.77 | 3.77 | 3.78 | 3.75 | 3.81 | 4.01 | 4.00 | 3.93 | 3.94 | 3.93 |
| Ti | <0.01 | - | 0.01 | - | - | - | - | - | - | - |
| Fe | 0.23 | 0.25 | 0.23 | 0.26 | 0.26 | 0.03 | 0.05 | 0.08 | 0.07 | 0.08 |
| Mg | 0.03 | 0.02 | 0.02 | 0.03 | 0.02 | - | - | - | - | - |
| Zn | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Li * | 0.07 | 0.09 | 0.08 | 0.07 | 0.03 | 0.02 | 0.03 | 0.05 | 0.01 | 0.03 |
| Sum (Y) | 4.11 | 4.14 | 4.12 | 4.12 | 4.13 | 4.10 | 4.08 | 4.06 | 4.03 | 4.04 |
| Ca | 0.01 | - | - | - | - | - | - | - | - | - |
| Na | 0.20 | 0.12 | 0.17 | 0.15 | 0.07 | 0.11 | 0.15 | 0.16 | 0.14 | 0.14 |
| K | 1.69 | 1.75 | 1.76 | 1.74 | 1.70 | 1.76 | 1.66 | 1.74 | 1.74 | 1.75 |
| Ba | - | - | - | - | - | - | <0.01 | <0.01 | <0.01 | <0.01 |
| Rb | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 | 0.03 | 0.03 | 0.05 | 0.04 | 0.04 |
| Cs | - | 0.01 | 0.01 | 0.01 | - | 0.02 | 0.01 | 0.02 | 0.03 | 0.02 |
| Sum (X) | 1.92 | 1.90 | 1.96 | 1.93 | 1.80 | 1.92 | 1.85 | 1.97 | 1.95 | 1.95 |
| OH * | 3.81 | 3.78 | 3.79 | 3.82 | 3.91 | 3.93 | 3.91 | 3.86 | 3.94 | 3.90 |
| F | 0.18 | 0.23 | 0.21 | 0.17 | 0.09 | 0.07 | 0.09 | 0.14 | 0.05 | 0.10 |
| Trace elements (ppm) | ||||||||||
| Rb | 2321 | 2262 | 2312 | 3308 | 2133 | 3804 | 3602 | 4881 | 4572 | 4315 |
| Cs | - | 2204 | 1660 | 1884 | - | 3105 | 1963 | 3870 | 4829 | 3489 |
| Zn | 303 | 289 | 281 | 339 | 369 | 174 | 304 | 330 | 368 | 285 |
| Ba | - | - | - | - | - | - | - | 116 | 146 | 170 |
| P | 316 | 219 | 216 | 212 | 433 | - | - | - | - | - |
| K/Rb | 35.5 | 37.9 | 37.4 | 25.8 | 39.3 | 23.1 | 22.5 | 17.4 | 18.6 | 19.7 |
| wt % Oxide | (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) | (j) | (k) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 36.00 | 36.04 | 36.15 | 35.74 | 35.65 | 35.65 | 35.64 | 35.66 | 35.53 | 35.78 | 35.83 |
| Al2O3 | 19.94 | 19.87 | 19.88 | 19.91 | 19.78 | 19.80 | 19.73 | 19.82 | 19.68 | 19.93 | 19.78 |
| MgO | 0.14 | 0.15 | 0.16 | 0.10 | 0.11 | 0.11 | 0.13 | - | 0.04 | 0.09 | 0.07 |
| FeO | 30.03 | 30.44 | 30.09 | 29.24 | 29.10 | 29.94 | 30.40 | 25.90 | 25.23 | 28.04 | 27.63 |
| MnO | 13.99 | 13.76 | 13.77 | 14.76 | 14.84 | 13.75 | 13.26 | 18.21 | 18.66 | 16.28 | 16.30 |
| CaO | 0.09 | 0.09 | 0.08 | 0.08 | 0.14 | 0.07 | 0.09 | 0.11 | 0.13 | 0.13 | 0.15 |
| P2O5 | 0.21 | 0.34 | 0.29 | 0.36 | 0.26 | 0.31 | 0.23 | 0.28 | 0.34 | 0.30 | 0.29 |
| Total | 100.40 | 100.69 | 100.42 | 100.19 | 99.88 | 99.63 | 99.48 | 99.98 | 99.61 | 100.55 | 100.05 |
| Cations based on 12 Oxygens | |||||||||||
| Si | 2.97 | 2.97 | 2.96 | 2.96 | 2.96 | 2.97 | 2.97 | 2.96 | 2.96 | 2.95 | 2.97 |
| AlIV | 0.03 | 0.03 | 0.02 | 0.04 | 0.04 | 0.03 | 0.03 | 0.04 | 0.04 | 0.05 | 0.03 |
| P | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| AlVI | 1.92 | 1.90 | 1.92 | 1.90 | 1.90 | 1.91 | 1.91 | 1.90 | 1.90 | 1.90 | 1.91 |
| Fe3+ | 0.06 | 0.07 | 0.06 | 0.06 | 0.07 | 0.06 | 0.06 | 0.07 | 0.07 | 0.07 | 0.07 |
| Fe2+ | 2.01 | 2.03 | 2.02 | 1.96 | 1.95 | 2.02 | 2.06 | 1.73 | 1.69 | 1.86 | 1.85 |
| Mn | 0.98 | 0.96 | 0.96 | 1.03 | 1.04 | 0.97 | 0.94 | 1.28 | 1.32 | 1.14 | 1.14 |
| Mg | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 | - | 0.01 | 0.01 | 0.01 |
| Ca | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 |
| Total | 8.01 | 8.01 | 8.00 | 8.01 | 8.01 | 8.01 | 8.01 | 8.01 | 8.01 | 8.01 | 8.01 |
| Almandine | 65.64 | 66.00 | 65.97 | 63.90 | 63.29 | 66.10 | 67.06 | 55.79 | 54.16 | 60.26 | 59.92 |
| Espessartine | 33.51 | 33.08 | 33.12 | 35.42 | 35.78 | 33.21 | 32.11 | 43.88 | 45.24 | 38.97 | 39.31 |
| Andradite | 0.27 | 0.29 | 0.24 | 0.25 | 0.44 | 0.20 | 0.26 | 0.34 | 0.40 | 0.40 | 0.46 |
| Pyrope | 0.58 | 0.63 | 0.68 | 0.44 | 0.48 | 0.49 | 0.56 | 0.00 | 0.19 | 0.37 | 0.31 |
| Probable Hydroxylapatite | Mn-Rich Fluorapatite | Eosphorite–Childrenite | |||||
|---|---|---|---|---|---|---|---|
| wt % Oxide | (1) | (2) | (3) | (4) | (5) | (6) | (7) |
| P2O5 | 32.96 | 42.92 | 42.74 | 33.58 | 33.77 | 33.86 | 33.36 |
| Al2O3 | 1.77 | 0.00 | 0.00 | 22.70 | 22.81 | 22.37 | 22.71 |
| CaO | 34.53 | 47.73 | 47.92 | 0.36 | 0.48 | 0.49 | 0.40 |
| MnO | 3.73 | 5.50 | 5.15 | 14.37 | 13.93 | 28.11 | 27.27 |
| FeO | 1.17 | 1.14 | 1.25 | 15.46 | 15.22 | 1.69 | 3.11 |
| SrO | 0.17 | - | - | - | - | - | - |
| BaO | - | 0.02 | 0.02 | - | - | - | - |
| Rb2O | 0.05 | - | - | - | - | - | - |
| K2O | - | - | - | - | 0.06 | - | - |
| F | 0.74 | 3.48 | 3.61 | 0.53 | 0.49 | 0.59 | 0.53 |
| Cl | 0.85 | - | - | - | - | - | - |
| Total | 75.97 | 100.79 | 100.68 | 87.01 | 86.75 | 87.10 | 87.37 |
| O = F, Cl | 0.50 | 1.47 | 1.52 | 0.22 | 0.21 | 0.25 | 0.22 |
| Total | 75.46 | 99.32 | 99.16 | 86.79 | 86.55 | 86.85 | 87.15 |
| Cations based on 5 PO4 | Cations based on 1 PO4 | ||||||
| P | 5.00 | 5.00 | 5.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Al | 0.37 | - | - | 0.94 | 0.94 | 0.92 | 0.95 |
| Ca | 6.63 | 7.04 | 7.10 | 0.01 | 0.02 | 0.02 | 0.01 |
| Mn | 0.57 | 0.64 | 0.60 | 0.43 | 0.41 | 0.83 | 0.82 |
| Fe | 0.18 | 0.13 | 0.14 | 0.45 | 0.44 | 0.05 | 0.09 |
| Sr | 0.018 | - | - | - | - | - | - |
| K | - | - | - | - | 0.06 | - | - |
| Ba | - | <0.01 | <0.01 | - | - | - | - |
| Rb | <0.01 | - | - | - | - | - | - |
| F | 0.42 | 1.52 | 1.58 | 0.06 | 0.05 | 0.06 | 0.06 |
| Cl | 0.26 | - | - | - | - | - | - |
| Fe/(Fe + Mn) | 0.23 | 0.17 | 0.19 | 0.515 | 0.519 | 0.056 | 0.101 |
| Fluorapatite | Messelite | Goyazite | Vivianite | Montebrasite | |||
|---|---|---|---|---|---|---|---|
| wt % Oxide | (1) | (2) | (3) | (4) | (5) | (6) | (7) |
| P2O5 | 43.16 | 40.86 | 41.92 | 30.81 | 35.99 | 53.99 | 53.74 |
| Al2O3 | - | 0.07 | - | 28.34 | - | 36.32 | 36.37 |
| CaO | 50.61 | 30.34 | 29.82 | - | - | - | - |
| MgO | - | 0.35 | 0.36 | - | 0.13 | - | - |
| MnO | 2.11 | 6.00 | 14.83 | 0.03 | 0.89 | - | - |
| FeO | 0.21 | 13.26 | 4.76 | 3.40 | 43.55 | - | 0.09 |
| SrO | 0.05 | 0.09 | - | 15.95 | - | 0.02 | - |
| BaO | 0.05 | - | - | 0.02 | - | - | - |
| Rb2O | 0.01 | - | 0.01 | 0.02 | - | - | - |
| ZnO | - | - | - | 0.02 | - | - | - |
| F | 3.61 | 0.68 | 0.76 | 0.42 | 0.35 | 3.38 | 1.57 |
| Cl | - | - | - | - | - | 0.00 | 0.03 |
| Total | 99.80 | 91.65 | 92.45 | 79.00 | 80.91 | 93.71 | 91.79 |
| O = F, Cl | 1.52 | 0.29 | 0.32 | 0.18 | 0.15 | 1.42 | 0.67 |
| Total | 98.28 | 91.36 | 92.13 | 78.83 | 80.76 | 92.28 | 91.12 |
| Based on | 5 PO4 | 2 PO4 | 1 PO4 | ||||
| P | 5.00 | 2.00 | 2.00 | 2.00 | 2.00 | 1.00 | 1.00 |
| Al | - | <0.01 | - | 2.56 | - | 0.94 | 0.94 |
| Ca | 7.42 | 1.88 | 1.808 | - | - | - | - |
| Mg | - | 0.03 | 0.03 | - | 0.01 | - | - |
| Mn | 0.24 | 0.29 | 0.71 | <0.01 | 0.05 | - | - |
| Fe | 0.02 | 0.64 | 0.22 | 0.22 | 2.39 | - | <0.01 |
| Sr | <0.01 | <0.01 | - | 0.71 | - | - | - |
| Ba | <0.01 | - | - | <0.01 | - | - | - |
| Rb | <0.01 | - | - | <0.01 | - | - | - |
| Zn | - | - | - | <0.01 | - | - | |
| F | 1.56 | 0.12 | 0.14 | 0.10 | 0.07 | 0.23 | 0.11 |
| Cl | - | - | - | - | - | - | <0.01 |
| Li * | - | - | - | - | - | 1.000 | 1.000 |
| Fe/(Fe + Mn) | - | 0.69 | 0.24 | 0.99 | 0.98 | - | - |
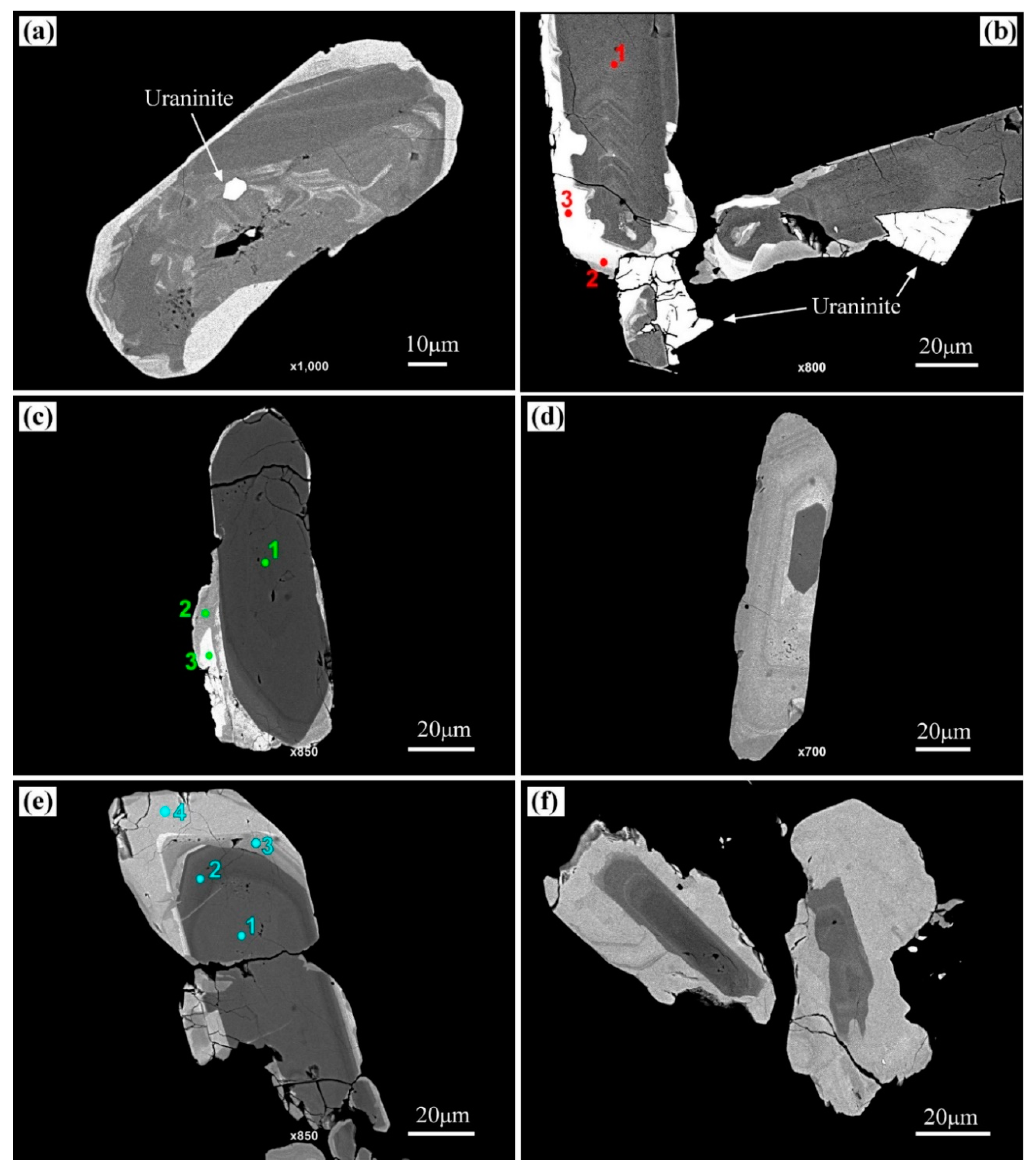
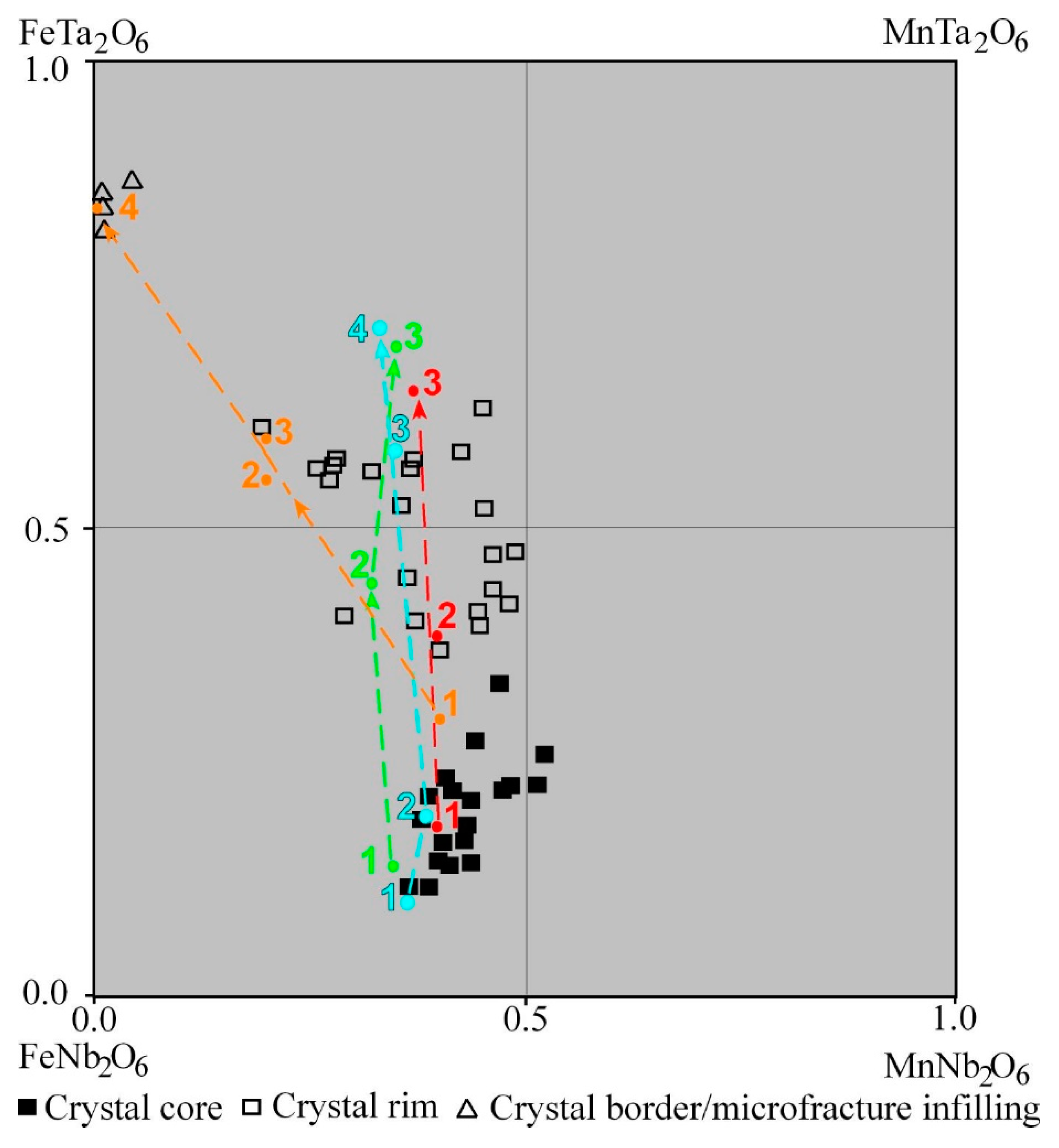
| Columbite–Tantalite | Tapiolite-(Fe) | Cassiterite | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| wt % Oxide | (a) | (b) | (c) | (d) | (a) | (a) | (b) | (c) | (d) | |
| SnO2 | 0.83 | 0.55 | 1.09 | 0.21 | 0.87 | 98.89 | 95.13 | 99.19 | 99.24 | |
| Ta2O5 | 19.52 | 27.19 | 45.59 | 58.17 | 77.54 | 0.87 | 3.40 | 1.28 | 0.59 | |
| Nb2O5 | 59.78 | 55.22 | 36.68 | 26.21 | 6.73 | 0.45 | 0.94 | - | 0.36 | |
| FeO | 10.82 | 8.88 | 8.38 | 9.60 | 13.49 | 0.29 | 0.74 | 0.13 | 0.35 | |
| MnO | 7.27 | 8.52 | 7.62 | 5.55 | 0.61 | - | - | - | - | |
| TiO2 | - | - | - | - | 1.41 | 0.10 | 0.17 | 0.08 | 0.18 | |
| UO2 | 1.25 | - | - | - | - | - | - | - | - | |
| ZnO | 0.12 | 0.12 | 0.06 | 0.11 | - | - | - | - | - | |
| In2O3 | - | - | - | - | - | 0.13 | 0.15 | 0.14 | 0.16 | |
| Total | 99.59 | 100.47 | 99.42 | 99.85 | 100.65 | 100.74 | 100.52 | 100.81 | 100.88 | |
| Cations based on 6 Oxygens | Cations based on 2 Oxygens | |||||||||
| Fe | 0.56 | 0.46 | 0.48 | 0.59 | 0.90 | 0.01 | 0.01 | <0.01 | 0.01 | |
| Mn | 0.38 | 0.45 | 0.45 | 0.34 | 0.04 | - | - | - | - | |
| Nb | 1.67 | 1.56 | 1.14 | 0.87 | 0.24 | <0.01 | 0.01 | - | <0.01 | |
| Ta | 0.33 | 0.46 | 0.86 | 1.15 | 1.69 | 0.01 | 0.02 | 0.01 | <0.01 | |
| Sn | 0.02 | 0.01 | 0.03 | 0.01 | 0.03 | 0.98 | 0.95 | 0.99 | 0.98 | |
| Ti | - | - | - | - | 0.08 | <0.01 | <0.01 | <0.01 | <0.01 | |
| U | 0.02 | - | - | - | - | - | - | - | - | |
| Zn | <0.01 | 0.01 | <0.01 | 0.01 | - | - | - | - | - | |
| In | - | - | - | - | - | <0.01 | <0.01 | <0.01 | <0.01 | |
| Mn/(Mn + Fe) | 0.40 | 0.49 | 0.48 | 0.37 | 0.04 | |||||
| Ta/(Ta + Nb) | 0.16 | 0.23 | 0.43 | 0.57 | 0.87 | |||||
| Panceiros Pegmatites (Barren) | Presqueira Peg. (Li–Sn–Ta) | Two-Mica Granitic Facies | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Samples * | PAN-1 | PAN-2 | PAN-3 | PAN-4 | PAN-5 | PR | GRT | GRM | GRP |
| wt % Oxide | |||||||||
| SiO2 | 74.19 | 74.34 | 73.69 | 75.62 | 74.41 | 73.73 | 72.31 | 71.97 | 73.77 |
| Al2O3 | 14.74 | 14.93 | 15.11 | 14.16 | 14.59 | 16.67 | 14.78 | 14.84 | 14.46 |
| Fe2O3 ** | 0.71 | 0.83 | 0.71 | 0.84 | 0.79 | 0.43 | 2.01 | 1.63 | 1.43 |
| MgO | 0.01 | 0.06 | 0.02 | 0.04 | 0.07 | 0.01 | 0.36 | 0.26 | 0.26 |
| CaO | 0.43 | 0.17 | 0.17 | 0.17 | 0.12 | 0.2 | 0.58 | 0.41 | 0.67 |
| Na2O | 4.92 | 4.38 | 4.24 | 3.97 | 4.17 | 4.95 | 3.13 | 3.03 | 3.26 |
| K2O | 3.32 | 3.51 | 4.71 | 3.56 | 4.47 | 2.46 | 5.33 | 5.16 | 4.81 |
| TiO2 | - | 0.02 | - | 0.01 | 0.03 | - | 0.23 | 0.15 | 0.15 |
| P2O5 | 0.68 | 0.33 | 0.44 | 0.36 | 0.24 | 0.52 | 0.28 | 0.35 | 0.23 |
| MnO | 0.09 | 0.08 | 0.13 | 0.08 | 0.02 | 0.03 | 0.04 | 0.03 | 0.02 |
| Cr2O3 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| L.O.I. | 0.8 | 1.3 | 0.7 | 1.1 | 1.0 | 0.8 | 0.8 | 2.1 | 0.8 |
| Total | 99.10 | 98.66 | 99.23 | 98.82 | 98.92 | 98.99 | 99.06 | 97.84 | 99.07 |
| Na2O/K2O | 1.48 | 1.25 | 0.90 | 1.12 | 0.93 | 2.01 | 0.59 | 0.59 | 0.68 |
| ISA | 1.26 | 1.36 | 1.25 | 1.36 | 1.25 | 1.54 | 1.35 | 1.40 | 1.37 |
| A/CNK | 1.18 | 1.32 | 1.22 | 1.32 | 1.22 | 1.49 | 1.23 | 1.31 | 1.23 |
| A | 55.15 | 69.22 | 52.51 | ||||||
| B | 39.98 | 28.74 | 26.23 | ||||||
| Trace elements (ppm) | |||||||||
| Li | 35 | 63 | 24 | 98 | 57 | >2000 | 157 | 225 | 119 |
| Be | 95 | 40 | 5 | 54 | 1 | 145 | 2 | 8 | 5 |
| Rb | 469 | 325 | 468 | 439 | 382 | 999 | 384 | 404 | 250 |
| Cs | 14 | 13 | 11 | 23 | 17 | 59 | 18 | 23 | 17 |
| Nb | 20 | 22 | 17 | 28 | 15 | 66 | 15 | 16 | 9 |
| Ta | 4 | 7 | 4 | 16 | 4 | 64 | 2 | 3 | 1 |
| Sn | 30 | 27 | 24 | 38 | 27 | 845 | 11 | 23 | 12 |
| W | 5 | 7 | 5 | 5 | 5 | 3 | 6 | 6 | 5 |
| Tl | 3 | 2 | 3 | 3 | 2 | 7 | 2 | 2 | 1 |
| Ba | 10 | 26 | 7 | 7 | 9 | 3 | 256 | 161 | 187 |
| Sr | 51 | 16 | 9 | 10 | 6 | 95 | 58 | 39 | 50 |
| U | 18 | 7 | 8 | 10 | 4 | 12 | 12 | 14 | 7 |
| Hf | 1 | 1 | 1 | 1 | 1 | 2 | 3 | 2 | 2 |
| Zr | 15 | 16 | 14 | 11 | 15 | 21 | 101 | 70 | 69 |
| Y | 1 | 1 | 1 | 2 | 1 | 0.4 | 9 | 5 | 7 |
| Zn | 49 | 23 | 21 | 33 | 26 | 99 | 69 | 58 | 37 |
| As | 26 | 14 | 593 | 44 | 21 | 2 | 4 | 3 | 5 |
| Panceiros Pegmatites (Barren) | Presqueira Peg. (Li–Sn–Ta) | Two-Mica Granitic Facies | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| REE (ppm) | LOD ** | PAN-1 | PAN-2 | PAN-3 | PAN-4 | PAN-5 | PR | GRT | GRM | GRP |
| La | 0.1 | 1.10 | 1.20 | 0.60 | 0.70 | 0.80 | 0.40 | 22.40 | 13.70 | 13.80 |
| Ce | 0.1 | 1.30 | 1.60 | 1.10 | 1.60 | 1.30 | 0.60 | 46.20 | 29.30 | 28.20 |
| Pr | 0.02 | 0.14 | 0.30 | 0.12 | 0.15 | 0.13 | 0.06 | 5.59 | 3.43 | 3.54 |
| Nd | 0.3 | 0.50 | 1.00 | - | 1.00 | 0.50 | - | 21.30 | 12.10 | 13.30 |
| Sm | 0.05 | 0.16 | 0.19 | 0.35 | 0.26 | 0.12 | 0.15 | 4.72 | 2.96 | 3.08 |
| Eu | 0.02 | - | 0.08 | - | 0.03 | - | - | 0.35 | 0.19 | 0.37 |
| Gd | 0.05 | 0.25 | 0.35 | 0.19 | 0.18 | 0.17 | 0.07 | 3.62 | 2.23 | 2.43 |
| Tb | 0.01 | 0.05 | 0.07 | 0.04 | 0.07 | 0.04 | 0.04 | 0.51 | 0.32 | 0.37 |
| Dy | 0.05 | 0.26 | 0.41 | 0.28 | 0.45 | 0.27 | 0.08 | 1.82 | 1.40 | 1.79 |
| Ho | 0.02 | 0.03 | 0.09 | 0.05 | 0.06 | 0.08 | - | 0.30 | 0.20 | 0.29 |
| Er | 0.03 | 0.10 | 0.16 | 0.11 | 0.18 | 0.15 | 0.03 | 0.83 | 0.48 | 0.66 |
| Tm | 0.01 | 0.01 | 0.03 | 0.01 | 0.02 | 0.02 | - | 0.10 | 0.04 | 0.08 |
| Yb | 0.05 | 0.15 | 0.20 | 0.10 | 0.28 | 0.19 | - | 0.68 | 0.41 | 0.45 |
| Lu | 0.01 | 0.01 | 0.03 | 0.02 | 0.04 | 0.02 | - | 0.07 | 0.03 | 0.07 |
| ∑ REE | 4.06 | 5.71 | 2.97 | 5.02 | 3.79 | 1.43 | 108.49 | 66.79 | 68.43 | |
| Eu/Eu * | - | 0.948 | - | 0.424 | - | - | 0.259 | 0.226 | 0.413 | |
| La/Yb | 7.33 | 6.00 | 6.00 | 2.50 | 4.21 | - | 32.94 | 33.41 | 30.66 | |
| La/Sm | 6.87 | 6.31 | 1.71 | 2.69 | 6.66 | 4.74 | 4.62 | 4.48 | ||
| Gd/Yb | 1.66 | 1.75 | 1.90 | 0.64 | 0.89 | 5.32 | 5.43 | 5.40 | ||
| Santa Baia Host-Schist of Panceiros Pegmatites (Barren) | Paraño Host-Schist of Presqueira Pegmatite (Li–Sn–Ta) | ||||||
|---|---|---|---|---|---|---|---|
| Schist | Wallrock Schist | Schist | Wallrock Schist | ||||
| Samples | ESQ-P | ESQ-STA | ESQ-C | ESQ-4 | ESQ-PA | ESQ-S | ESQ-PR |
| wt % Oxide | |||||||
| SiO2 | 58.01 | 56.37 | 62.36 | 61.42 | 62.73 | 58.84 | 53.27 |
| Al2O3 | 21.24 | 22.95 | 18.78 | 19.42 | 18.99 | 20.97 | 23.21 |
| Fe2O3 * | 7.26 | 8.19 | 6.53 | 6.63 | 7.11 | 7.59 | 9.01 |
| MgO | 1.91 | 2.03 | 1.81 | 1.87 | 0.63 | 1.65 | 2.59 |
| CaO | 0.03 | 0.04 | 0.08 | 0.44 | - | 0.01 | 0.12 |
| Na2O | 0.36 | 0.38 | 0.48 | 0.56 | 0.31 | 0.41 | 0.61 |
| K2O | 4.41 | 4.67 | 6.04 | 5.29 | 4.14 | 4.84 | 6.12 |
| TiO2 | 0.91 | 0.87 | 0.74 | 0.74 | 0.82 | 0.75 | 0.92 |
| P2O5 | 0.07 | 0.11 | 0.07 | 0.37 | 0.08 | 0.08 | 0.09 |
| MnO | 0.08 | 0.09 | 0.08 | 0.09 | 0.07 | 0.23 | 0.15 |
| Cr2O3 | 0.016 | 0.017 | 0.014 | 0.015 | 0.014 | 0.014 | 0.017 |
| L.O.I. | 5.5 | 4.1 | 2.8 | 2.9 | 4.9 | 4.4 | 3.6 |
| Total | 99.78 | 99.82 | 99.76 | 99.77 | 99.8 | 99.78 | 99.72 |
| Trace elements (ppm) | |||||||
| Li | 230.7 | 184.7 | 585.4 | 502.7 | 64.5 | 93.6 | 1365.3 |
| Be | 2 | 1 | 29 | 29 | 3 | 2 | 2 |
| Rb | 189.4 | 207 | 883.8 | 568.5 | 182.5 | 221.1 | 665.1 |
| Cs | 10.8 | 20.7 | 313 | 80.5 | 14.3 | 14.2 | 195.9 |
| Nb | 15.9 | 16.4 | 17.6 | 18 | 15 | 13.9 | 16.6 |
| Ta | 1.3 | 1.1 | 3.1 | 5 | 1.1 | 1.3 | 1.2 |
| Sn | 5 | 4 | 117 | 59 | 3 | 4 | 102 |
| W | 2.8 | 3.1 | 8.3 | 6.9 | 3.5 | 1.9 | 7.8 |
| Tl | 0.8 | 1.1 | 6.3 | 3.8 | 0.9 | 1.2 | 4.9 |
| Ga | 23.1 | 24.3 | 23.3 | 21.2 | 22.1 | 26.4 | 29.3 |
| Ba | 750 | 590 | 512 | 620 | 609 | 755 | 834 |
| Sr | 50.6 | 47.1 | 32.4 | 66.3 | 60.7 | 70.4 | 80.7 |
| U | 3.3 | 4.3 | 4.3 | 4.6 | 3.8 | 2.7 | 3.4 |
| Hf | 5.9 | 4.2 | 5.3 | 4.5 | 5.6 | 3.2 | 3.3 |
| Zr | 218.6 | 144.7 | 180.3 | 176.5 | 192.1 | 117.7 | 122.1 |
| Y | 22.6 | 33 | 19.6 | 21.3 | 21.7 | 26.2 | 27.1 |
| Zn | 85 | 102 | 106 | 97 | 44 | 108 | 148 |
| As | 5 | 7 | 48 | 29 | - | 8 | 52 |
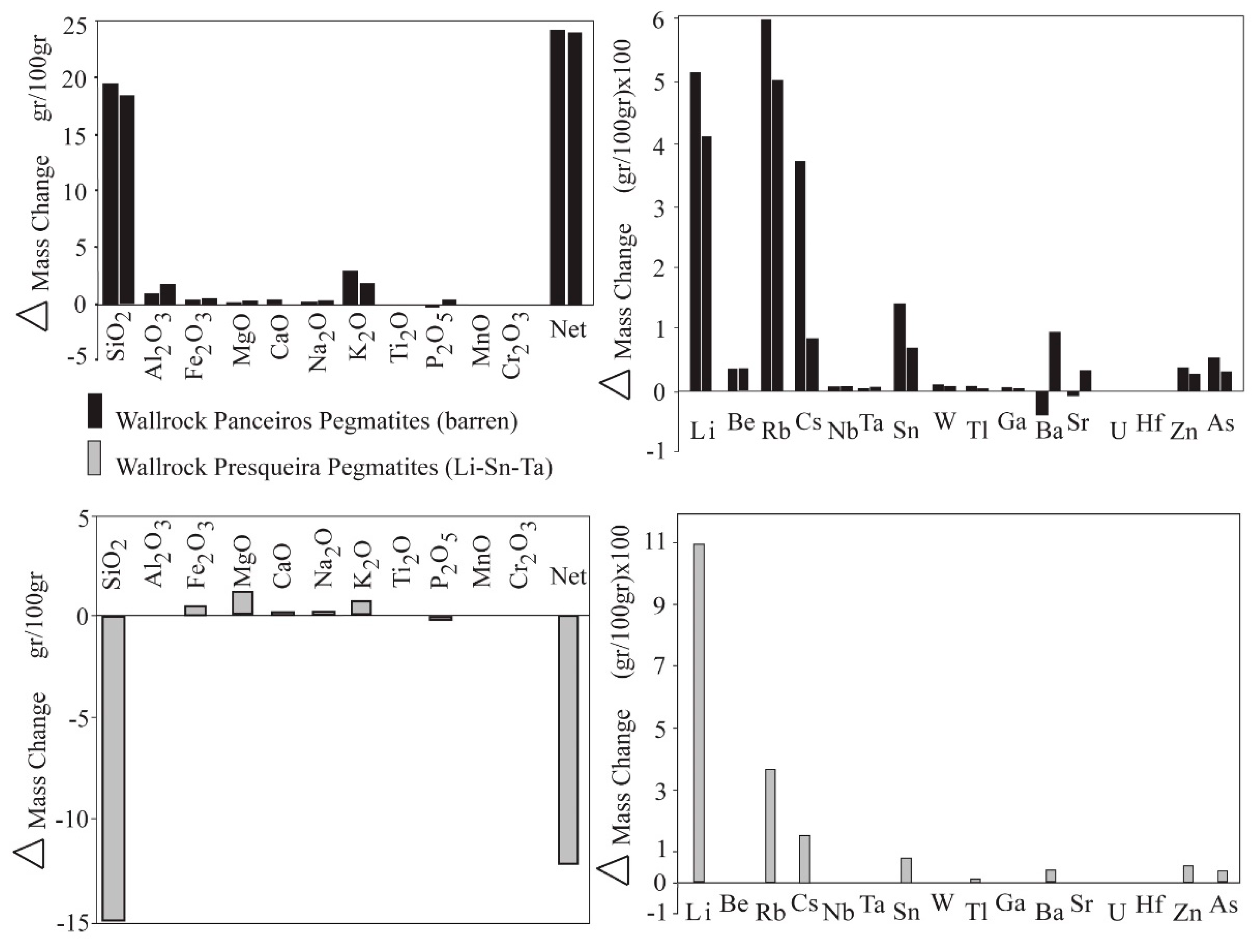
| Wallrock Schist of Pegmatites From | ||||||
|---|---|---|---|---|---|---|
| Pegmatite Locality | Panceiros (Barren) | Presqueira (Li–Sn–Ta) | ||||
| Samples | ESQ-C | ESQ-4 | ESQ-PR | |||
| Immobile Element | Ti | (Zr) * | Ti | (Zr )* | Al | (Y) * |
| Mass Change (gr/100gr) | ||||||
| ∆ SiO2 | 19.5 | (18.4) | 18.3 | (18.9) | −14.9 | (−13.9) |
| ∆ Al2O3 | 1.0 | (0.7) | 1.8 | (2.0) | 0.0 | (0.5) |
| ∆ Fe2O3 | 0.3 | (0.2) | 0.4 | (0.5) | 0.4 | (0.6) |
| ∆ MgO | 0.3 | (0.2) | 0.3 | (0.4) | 1.2 | (1.3) |
| ∆ CaO | 0.1 | (0.1) | 0.5 | (0.5) | 0.1 | (0.1) |
| ∆ Na2O | 0.2 | (0.2) | 0.3 | (0.3) | 0.2 | (0.2) |
| ∆ K2O | 2.9 | (2.8) | 2.0 | (2.0) | 0.8 | (0.9) |
| ∆ TiO2 | 0.0 | (0.0) | 0.0 | (0.0) | 0.0 | (0.0) |
| ∆ P2O5 | 0.0 | (0.0) | 0.4 | (0.4) | 0.0 | (0.0) |
| ∆ MnO | 0.0 | (0.0) | 0.0 | (0.0) | 0.0 | (0.0) |
| ∆ Cr2O3 | 0.0 | (0.0) | 0.0 | (0.0) | 0.0 | (0.0) |
| Net mass change | 24.3 | (22.6) | 24.1 | (24.9) | −12.3 | (−10.4) |
| Mass change (gr/100gr) × 100 | ||||||
| ∆ Li | 5.12 | (5.02) | 4.10 | (4.15) | 10.97 | (11.24) |
| ∆ Be | 0.34 | (0.34) | 0.34 | (0.34) | −0.01 | (−0.01) |
| ∆ Rb | 8.87 | (8.73) | 5.01 | (5.06) | 3.71 | (3.84) |
| ∆ Cs | 3.69 | (3.64) | 0.83 | (0.84) | 1.54 | (1.58) |
| ∆ Nb | 0.06 | (0.05) | 0.06 | (0.06) | <−0.01 | (<0.01) |
| ∆ Ta | 0.03 | (0.03) | 0.05 | (0.05) | <−0.01 | (<−0.01) |
| ∆ Sn | 1.39 | (1.37) | 0.68 | (0.69) | 0.84 | (0.86) |
| ∆ W | 0.07 | (0.07) | 0.05 | (0.06) | 0.04 | (0.04) |
| ∆ Tl | 0.07 | (0.07) | 0.04 | (0.04) | 0.03 | (0.03) |
| ∆ Ga | 0.05 | (0.04) | 0.02 | (0.03) | 0.02 | (0.02) |
| ∆ Ba | −0.40 | (−0.49) | 0.92 | (0.98) | 0.39 | (0.56) |
| ∆ Sr | −0.09 | (−0.09) | 0.33 | (0.33) | 0.04 | (0.06) |
| ∆ U | 0.01 | (0.01) | 0.02 | (0.02) | <−0.01 | (<−0.01) |
| ∆ Hf | 0.01 | (0.01) | <0.01 | (<0.01) | −0.01 | (−0.01) |
| ∆ Zn | 0.37 | (0.35) | 0.26 | (0.27) | 0.58 | (0.61) |
| ∆ As | 0.53 | (0.52) | 0.30 | (0.30) | 0.43 | (0.44) |
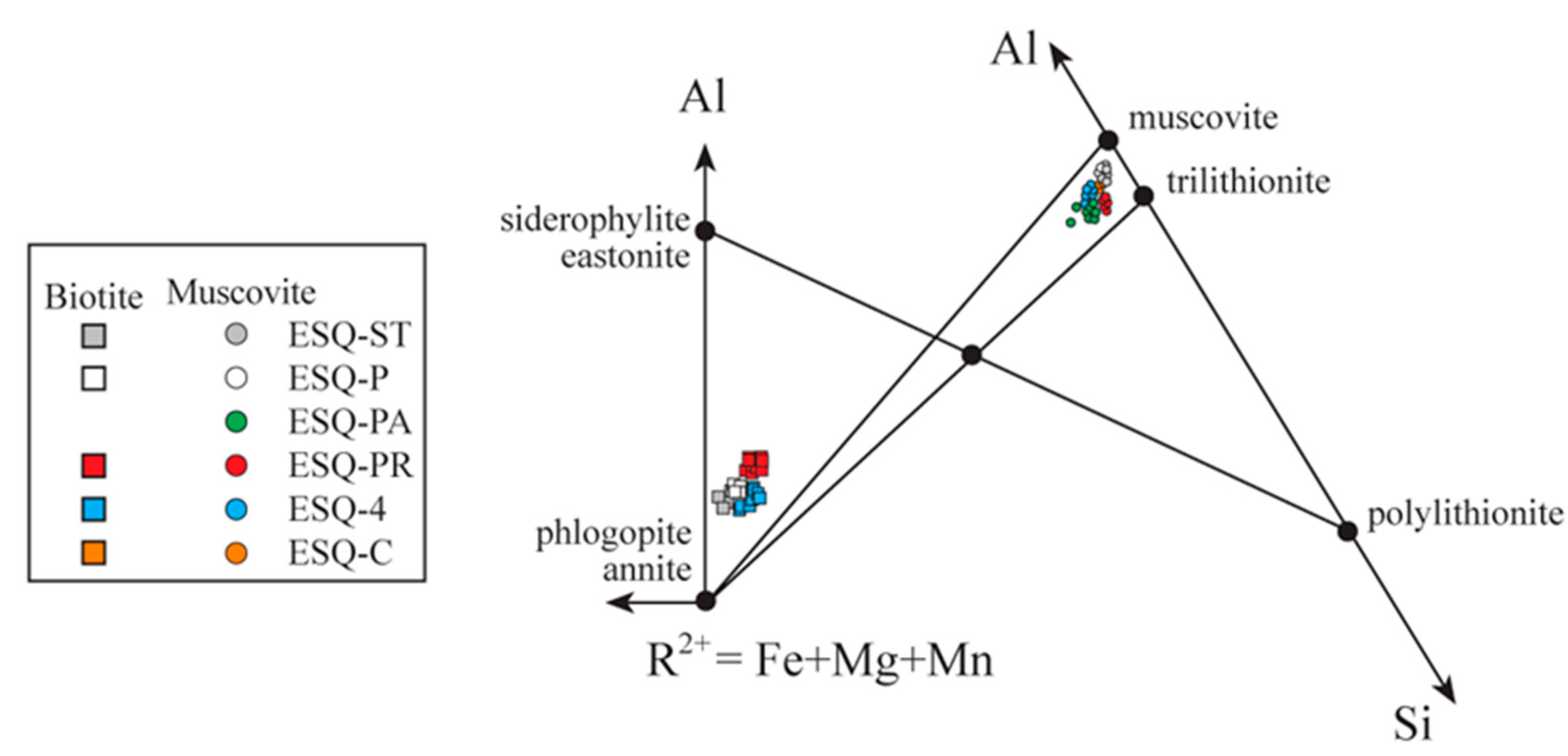
| Santa Baia Schist | Panceiros Pegmatite Wallrock Schist | Presqueiras Pegmatite Wallrock Schist | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESQ-ST | ESQ-P | ESQ-C | ESQ-4 | ESQ-PR | |||||||||
| 5 | 137 | 18 | 50 | 49 | 69 | 81 | 73 | 82 | 94 | 17 | 41 | 37 | |
| SiO2 | 34.23 | 33.49 | 34.17 | 34.26 | 34.05 | 34.94 | 34.59 | 35.18 | 35.42 | 35.18 | 34.79 | 35.02 | 34.87 |
| TiO2 | 2.70 | 2.57 | 2.39 | 2.13 | 2.06 | 2.04 | 2.48 | 2.10 | 2.25 | 1.75 | 1.37 | 1.36 | 1.36 |
| Al2O3 | 19.31 | 19.31 | 19.37 | 19.07 | 19.29 | 18.73 | 18.41 | 18.23 | 18.94 | 18.42 | 19.49 | 20.04 | 20.17 |
| FeO | 21.98 | 24.51 | 22.49 | 22.74 | 22.50 | 22.57 | 22.96 | 23.49 | 21.79 | 22.42 | 22.63 | 21.79 | 21.96 |
| MnO | 0.26 | 0.25 | 0.21 | 0.18 | 0.24 | 0.33 | 0.30 | 0.47 | 0.35 | 0.34 | 0.40 | 0.32 | 0.34 |
| MgO | 6.86 | 6.31 | 6.80 | 6.95 | 7.01 | 6.78 | 6.45 | 6.64 | 6.62 | 6.42 | 6.71 | 7.13 | 6.85 |
| CaO | 0.03 | - | 0.03 | - | - | 0.03 | - | - | 0.04 | - | - | - | - |
| Na2O | 0.34 | 0.21 | 0.31 | 0.23 | 0.27 | 0.10 | 0.21 | 0.15 | 0.27 | 0.24 | 0.12 | 0.18 | 0.19 |
| K2O | 8.77 | 8.43 | 8.49 | 8.77 | 8.49 | 8.99 | 8.82 | 8.50 | 8.92 | 8.82 | 7.87 | 7.69 | 7.45 |
| BaO | 0.06 | 0.05 | 0.06 | 0.08 | 0.08 | 0.03 | 0.05 | 0.04 | 0.04 | 0.03 | 0.04 | 0.04 | 0.05 |
| Rb2O | 0.12 | 0.11 | 0.11 | 0.12 | 0.12 | 0.21 | 0.20 | 0.27 | 0.28 | 0.29 | 0.21 | 0.20 | 0.20 |
| Cs2O | - | - | - | 0.23 | 0.26 | 1.43 | 0.42 | 0.71 | 0.44 | 1.09 | 0.55 | 1.33 | 1.42 |
| ZnO | 0.08 | 0.05 | 0.08 | 0.05 | 0.05 | 0.10 | 0.10 | 0.08 | 0.07 | 0.06 | 0.05 | 0.04 | 0.05 |
| F | 0.17 | 0.13 | 0.20 | 0.20 | 0.16 | 0.57 | 0.52 | 0.84 | 0.76 | 0.86 | 0.47 | 0.48 | 0.40 |
| Cl | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Li2O * | 0.27 | 0.06 | 0.25 | 0.28 | 0.22 | 0.48 | 0.37 | 0.55 | 0.62 | 0.55 | 0.38 | 0.50 | 0.45 |
| H2O * | 3.79 | 3.78 | 3.77 | 3.76 | 3.77 | 3.61 | 3.61 | 3.49 | 3.55 | 3.45 | 3.62 | 3.67 | 3.70 |
| Subtotal | 98.70 | 99.19 | 98.49 | 98.77 | 98.35 | 100.52 | 99.17 | 100.30 | 99.85 | 99.50 | 98.36 | 99.34 | 99.48 |
| O = F,Cl | 0.07 | 0.06 | 0.09 | 0.08 | 0.07 | 0.24 | 0.22 | 0.36 | 0.32 | 0.36 | 0.20 | 0.21 | 0.17 |
| Total | 98.63 | 99.14 | 98.41 | 98.69 | 98.28 | 100.28 | 98.95 | 99.95 | 99.52 | 99.14 | 98.16 | 99.13 | 99.31 |
| Cations based on 22 Oxygens | |||||||||||||
| Si | 5.30 | 5.23 | 5.30 | 5.33 | 5.31 | 5.39 | 5.38 | 5.43 | 5.43 | 5.47 | 5.39 | 5.39 | 5.38 |
| AlIV | 2.70 | 2.77 | 2.70 | 2.67 | 2.69 | 2.61 | 2.62 | 2.57 | 2.57 | 2.53 | 2.61 | 2.61 | 2.62 |
| AlVI | 0.82 | 0.78 | 0.85 | 0.82 | 0.86 | 0.80 | 0.76 | 0.74 | 0.85 | 0.84 | 0.98 | 1.02 | 1.05 |
| Ti | 0.31 | 0.30 | 0.28 | 0.25 | 0.24 | 0.24 | 0.29 | 0.24 | 0.26 | 0.20 | 0.16 | 0.16 | 0.16 |
| Fe | 2.85 | 3.20 | 2.92 | 2.96 | 2.93 | 2.91 | 2.99 | 3.03 | 2.79 | 2.91 | 2.95 | 2.80 | 2.83 |
| Mn | 0.03 | 0.03 | 0.03 | 0.02 | 0.03 | 0.04 | 0.04 | 0.06 | 0.04 | 0.04 | 0.05 | 0.04 | 0.04 |
| Mg | 1.58 | 1.47 | 1.57 | 1.61 | 1.63 | 1.56 | 1.50 | 1.53 | 1.51 | 1.49 | 1.56 | 1.64 | 1.57 |
| Zn | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 |
| Li * | 0.17 | 0.04 | 0.16 | 0.18 | 0.14 | 0.30 | 0.23 | 0.34 | 0.38 | 0.34 | 0.24 | 0.31 | 0.28 |
| Ca | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| Na | 0.10 | 0.06 | 0.09 | 0.07 | 0.08 | 0.03 | 0.06 | 0.05 | 0.08 | 0.07 | 0.04 | 0.05 | 0.06 |
| K | 1.73 | 1.68 | 1.68 | 1.74 | 1.69 | 1.77 | 1.75 | 1.67 | 1.74 | 1.75 | 1.56 | 1.45 | 1.47 |
| Ba | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Rb | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.03 | 0.03 | 0.03 | 0.02 | 0.02 | 0.02 |
| Cs | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.09 | 0.03 | 0.05 | 0.03 | 0.07 | 0.04 | 0.09 | 0.09 |
| OH * | 3.91 | 3.94 | 3.90 | 3.90 | 3.92 | 3.72 | 3.74 | 3.59 | 3.63 | 3.58 | 3.76 | 3.76 | 3.80 |
| F | 0.08 | 0.06 | 0.10 | 0.10 | 0.08 | 0.28 | 0.26 | 0.41 | 0.37 | 0.42 | 0.23 | 0.23 | 0.19 |
| Santa Baia Schist | Panceiros Pegmatite Wallrock Schist | Paraño Group Schist | Presqueiras Pegmatite Wallrock Schist | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ESQ-ST | ESQ-P | ESQ-C | ESQ-4 | ESQ-PA | ESQ-PR | |||||
| 12 | 141 | 32 | 87 | 86 | 91 | 106 | 115 | 6 | 26 | |
| SiO2 | 46.03 | 45.92 | 46.03 | 45.88 | 46.10 | 46.15 | 46.50 | 45.93 | 46.53 | 46.41 |
| TiO2 | 0.28 | 0.61 | 0.74 | 0.76 | 0.30 | 0.85 | 0.20 | 0.23 | 0.34 | 0.37 |
| Al2O3 | 36.16 | 36.04 | 36.14 | 35.17 | 35.32 | 34.53 | 34.30 | 35.30 | 34.78 | 35.63 |
| FeO | 0.86 | 0.91 | 1.07 | 1.01 | 1.42 | 1.37 | 2.72 | 1.50 | 1.14 | 1.03 |
| MnO | - | - | - | - | - | - | - | - | - | - |
| MgO | 0.41 | 0.42 | 0.43 | 0.58 | 0.79 | 0.84 | 0.78 | 0.40 | 0.65 | 0.58 |
| CaO | - | - | - | - | - | - | - | 0.03 | - | - |
| Na2O | 1.31 | 1.16 | 1.14 | 1.26 | 1.07 | 1.01 | 0.87 | 0.88 | 1.12 | 1.21 |
| K2O | 9.70 | 9.54 | 9.83 | 9.57 | 10.14 | 9.86 | 9.76 | 8.84 | 9.27 | 9.48 |
| BaO | 0.28 | 0.28 | 0.30 | 0.26 | 0.08 | 0.08 | 0.15 | 0.16 | 0.22 | 0.21 |
| Rb2O | 0.15 | 0.15 | 0.16 | 0.16 | 0.18 | 0.23 | 0.18 | 0.16 | 0.18 | 0.18 |
| Cs2O | - | 0.02 | - | 0.18 | 0.21 | 0.17 | 0.07 | 0.11 | - | - |
| ZnO | 0.02 | - | - | 0.03 | 0.02 | 0.07 | 0.05 | 0.01 | - | |
| F | - | - | - | 0.25 | 0.41 | 0.33 | 0.17 | - | 0.11 | 0.16 |
| Cl | - | - | - | - | - | - | - | - | - | - |
| Li2O * | - | - | - | 0.06 | 0.12 | 0.09 | 0.04 | - | 0.02 | 0.04 |
| H2O * | 4.48 | 4.48 | 4.50 | 4.37 | 4.31 | 4.33 | 4.41 | 4.41 | 4.42 | 4.43 |
| Subtotal | 99.66 | 99.52 | 100.36 | 99.53 | 100.44 | 99.86 | 100.21 | 97.99 | 98.81 | 99.72 |
| O = F,Cl | 0.03 | 0.02 | 0.02 | 0.10 | 0.17 | 0.14 | 0.07 | 0.03 | 0.05 | 0.07 |
| Total | 99.63 | 99.50 | 100.34 | 99.43 | 100.27 | 99.72 | 100.14 | 97.96 | 98.76 | 99.65 |
| Cations based on 22 Oxygens | ||||||||||
| Si | 6.12 | 6.11 | 6.09 | 6.14 | 6.13 | 6.17 | 6.21 | 6.19 | 6.23 | 6.17 |
| AlIV | 1.88 | 1.89 | 1.91 | 1.86 | 1.87 | 1.83 | 1.79 | 1.81 | 1.77 | 1.83 |
| AlVI | 3.79 | 3.77 | 3.73 | 3.68 | 3.67 | 3.60 | 3.61 | 3.80 | 3.72 | 3.75 |
| Ti | 0.03 | 0.06 | 0.07 | 0.08 | 0.03 | 0.09 | 0.02 | 0.02 | 0.03 | 0.04 |
| Fe | 0.10 | 0.10 | 0.12 | 0.11 | 0.16 | 0.15 | 0.30 | 0.17 | 0.13 | 0.11 |
| Mg | 0.08 | 0.08 | 0.09 | 0.12 | 0.16 | 0.17 | 0.15 | 0.08 | 0.13 | 0.11 |
| Zn | - | - | - | - | - | - | 0.01 | - | - | - |
| Li * | - | - | - | 0.03 | 0.06 | 0.05 | 0.02 | - | 0.01 | 0.02 |
| Na | 0.34 | 0.30 | 0.29 | 0.33 | 0.28 | 0.26 | 0.22 | 0.23 | 0.29 | 0.31 |
| K | 1.65 | 1.62 | 1.66 | 1.63 | 1.72 | 1.68 | 1.66 | 1.52 | 1.58 | 1.61 |
| Ba | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | ||
| Rb | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 |
| Cs | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 |
| OH * | 3.97 | 3.98 | 3.98 | 3.89 | 3.83 | 3.86 | 3.93 | 3.97 | 3.95 | 3.93 |
| F | 0.03 | 0.02 | 0.02 | 0.10 | 0.17 | 0.14 | 0.07 | 0.03 | 0.05 | 0.07 |
References
- Linnen, R.L.; Van Lichtervelde, M.; Černý, P. Granitic pegmatites as sources of strategic metals exploration and evaluation. Elements 2012, 8, 275–280. [Google Scholar] [CrossRef]
- Fuertes-Fuente, M.; Martin-Izard, A. The Forcarei Sur rare-element granitic pegmatite field and associated mineralization, Galicia, Spain. Can. Mineral. 1998, 36, 303–325. [Google Scholar]
- Fuertes-Fuente, M.; Martin-Izard, A.; Boiron, M.C.; Mangas, J. Fluid evolution of rare-element and muscovite granitic pegmatites from central Galicia, NW Spain. Miner. Depos. 2000, 35, 332–345. [Google Scholar] [CrossRef]
- Canosa, F.; Martin-Izard, A.; Fuertes-Fuente, M. Evolved granitic systems as a source of rare element deposits: The Ponte Segade case (Galicia, NW Spain). Lithos 2012, 153, 165–176. [Google Scholar] [CrossRef]
- Julivert, M.; Fontboté, J.M.; Ribeiro, A.; Nabais Conde, L.E. Mapa Tectónico de la Península Ibérica y Baleares, E 1:1000000; Memoria explicativa; Instituto Geológico y Minero de España: Madrid, Spain, 1972; 113p. [Google Scholar]
- Farias, P.; Gallastegui, G.; González Lodeiro, F.; Marquínez, J.; Mantín Parra, L.M.; De Pablo Macía, J.G.; Rodríguez Fernández, L.R. Aportaciones al conocimiento de la litoestratigrafia y estructura de Galicia Central. Mem. Fac. Ciências, Univ. Porto 1987, 1, 411–431. [Google Scholar]
- Farias, P.; Ordoñez-Casado, B.; Marcos, A.; Rubio-Ordoñez, A.; Fanning, C.M. U–Pb zircon SHRIMP evidence for Cambrian volcanism in the schistose domain within the Galicia-Trás-os-Montes zone (Variscan Orogen, NW Iberian Peninsula). Geol. Acta 2014, 12, 209–218. [Google Scholar]
- Pérez-Estaún, A.; Bastida, F.; Martínez Catalán, J.R.; Gutiérrez Marco, J.C.; Marcos, A.; Pulgar, J.A. West Asturian–Leonese zone, stratigraphy. In Pre-Mesozoic Geology of Iberia; Dallmeyer, R.D., Martínez-García, E., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 92–102. [Google Scholar]
- Martínez-Catalán, J.R.; Arenas, R.; Díaz García, F.; Rubio Pascual, F.J.; Abati, J.; Marquínez, J. Variscan exhumation of a subducted Palaeozoic continental margin: The basal units of the Órdenes Complex, Galicia, NW Spain. Tectonics 1996, 15, 106–121. [Google Scholar] [CrossRef]
- Díez Fernández, R.; Martínez Catalán, J.R.; Arenas, R.; Abati, J.; Gerdes, A.; Fernández-Suárez, J. U–Pb detrital zircon analysis of the lower allochthon of NW Iberia: Age constraints, provenance and links with the Variscan mobile belt and Gondwanan cratons. J. Geol. Soc. 2012, 169, 655–665. [Google Scholar] [CrossRef]
- Ribeiro, A.; Pereira, E.; Dias, R. Central–Iberian zone. Allochthonous sequences. Structure in the geology of Iberia. In Pre-Mesozoic Geology of Iberia; Dallmeyer, R.D., Martínez-García, E., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 237–246. [Google Scholar]
- Martínez Catalán, J.R.; Arenas, R.; Díaz García, F.; Abati, J. Allochthonous units in the Variscan Belt of NW Iberia. In Terranes and Accretionary History, Basement Tectonic; Sinha, A.K., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; Volume 13, pp. 65–84. [Google Scholar]
- Marcos, A.; Farias, P.; Galán, G.; Fernández, J.J.; Llana-Fúnez, S. Tectonic framework of the Cabo Ortegal Complex: A slab of lower crust exhumed in the Variscan orogeny (Northwestern Iberia Peninsula). In Variscan-Appalachian Dynamics: The Building of the Late Paleozoic Basement; Martínez Catalán, J.R., Hatcher, R.D., Arenas, R., Díaz García, F., Eds.; The Geological Society of America: Colorado, CO, USA, 2002; Volume 364, pp. 143–162. [Google Scholar]
- Murphy, J.B.; Gutiérrez Alonso, G.; Fernández Suárez, J.; Braid, J.A. Probing crustal and mantle lithosphere origin through Ordovician volcanic rocks along the Iberian passive margin of Gondwana. Tectonophysics 2008, 461, 166–180. [Google Scholar] [CrossRef]
- Barrera, J.L.; Farias, P.; González, F.; Marquínez, J.; Martín, L.M.; Martínez, J.R.; Del Olmo, A.; De Pablo, J.G. Mapa Geológico 1: 200,000 de Ourense/Verín; Memoria Explicativa; Publicación del Instituto Tecnológico Geominero de España: Madrid, Spain, 1989; 284p. [Google Scholar]
- Marcos, A.; Llana-Fúnez, S. Estratigrafía y estructura de la lámina tectónica del Para-autóctono y de su autóctono en el área de Chantada (Galicia, NO de España). Trab. de Geol. 2002, 23, 53–72. [Google Scholar]
- Farias, P.; Marcos, A. Dominio esquistoso de Galicia-tras-os-montes. In Geología de España; Vera, J.A., Ed.; SGE-IGME: Madrid, Spain, 2004; pp. 135–138. [Google Scholar]
- Van Calsteren, P.W.C.; Boelrijk, N.A.I.M.; Hebeda, E.H.; Priem, H.N.A.; Den Tex, E.; Verdurmen, E.A.T.H.; Verschure, R.H. Isotopic dating of older elements (including the Cabo Ortegal mafic-ultramafic complex) in the Hercynian Orogen of NW Spain: Manifestations of a presumed Early-Paleozoic mantle-plume. Chem. Geol. 1979, 24, 35–36. [Google Scholar] [CrossRef]
- Santos Zalduegui, J.F.; Schaérer, U.; Gil Ibarguchi, J.I. Isotope constraints on the age and origin of magmatism and metamorphism in the Malpica-Tuy allochton, Galicia, NW-Spain. Chem. Geol. 1995, 121, 91–103. [Google Scholar] [CrossRef]
- Gil Ibarguchi, J.I.; Arenas, R. Metamorphic evolution of the allochthonous complexes from the northwest of Iberian Peninsula. In Pre-Mesozoic Geology of Iberia; Dallmeyer, R.D., Martínez-García, E., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 237–246. [Google Scholar]
- Arenas, R. Evolución Petrológica y Geoquímica de la Unidad Alóctona Inferior del Complejo Metamórfico Básico-Ultrabásico de Cabo Ortegal (Unidad de Moeche) y del Silúrico Parautóctono, Cadena Hercínica Ibérica (NW de España). Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 1985; 543p. [Google Scholar]
- Ribeiro, A. Contribution a l’étude tectonique de Trás-Os-Montes Oriental. Mem. Serv. Geol. Port. 1974, 24, 1–168. [Google Scholar]
- Dallmeyer, R.D.; Martínez Catalán, J.R.; Arenas, R.; Gil Ibarguchi, J.I.; Gutiérrez-Alonso, G.; Farias, P.; Aller, J.; Bastida, F. Diachronous Variscan tectonothermal activity in the NW Iberian Massif: Evidence from 40Ar/39Ar dating of regional fabrics. Tectonophysics 1997, 277, 307–337. [Google Scholar] [CrossRef]
- Capdevila, R.; Vialette, Y. Estimation radiométrique de l’age de la deuxiéme phase tectonique hercynienne en Galice moyenne (Nord-Ouest de l’Espagne). C. R. Acad. Sci. 1970, 270, 2527–2530. [Google Scholar]
- Plümber, O.; Putnis, A. The complex hydrothermal history of granitic rocks: Multiple feldspar replacement reactions under subsolidus conditions. J. Petrol. 2009, 50, 967–987. [Google Scholar] [CrossRef]
- Monier, G.; Robert, J.L. Muscovite solid solutions in the system K2O–MgO–FeO–A12O3–SiO2–H2O: An experimental study at 2 kbar PH2O and comparison with natural Li-free white micas. Mineral. Mag. 1986, 50, 257–266. [Google Scholar] [CrossRef]
- Haggerty, S.E.; Fung, A.T.; Burt, D.M. Apatite, phosphorus and titanium in eclogitic garnet from the upper mantle. Geophys. Res. Lett. 1994, 21, 1699–1702. [Google Scholar] [CrossRef]
- Burt, D.M. Compositional limits of phosphorus substitution in garnet in pegmatites and in the mantle. Proc. Geol. Assoc. Can. Mineral. Assoc. Can. 1996, 2, A-15. [Google Scholar]
- Breiter, K.; Novák, M.; Koller, F.; Cempírek, J. Phosphorus—An omnipresent minor element in garnet of diverse textural types from leucocratic granitic rocks. Mineral. Petrol. 2005, 85, 205–221. [Google Scholar] [CrossRef]
- Taylor, L.S.; Wise, M.A. Geochemistry and mode of occurrence of phosphorus in spessartine. In Proceedings of the Geological Society of America Annual Meeting, New Orleans, LA, USA, 6–9 November 1995; Volume 27, p. 470A. [Google Scholar]
- Taylor, L.S.; Wise, M.A.; Simmons, W.B.; Falster, A.U. Occurrence of phosphorus in garnets from gem-bearing pegmatites. Rock Miner. 1997, 3, 189–190. [Google Scholar]
- Anderson, S.D.; Cerný, P.; Halden, N.M.; Chapman, R.; Uher, P. The Yitt-B pegmatite swarm at Bernic Lake, southeastern Manitoba: A geochemical and paragenetic anomaly. Can. Mineral. 1998, 36, 283–301. [Google Scholar]
- Pasero, M.; Kampf, A.R.; Ferraris, C.; Pekov, I.V.; Rakovan, J.; White, T.J. Nomenclature of the apatite supergroup minerals. Eur. J. Mineral. 2010, 22, 163–179. [Google Scholar] [CrossRef]
- Bartlett, S.C. Alberta-1 Rare Metals Project, Galicia, Spain, Mineral Resources, NI 43-101 Technical Report; Solid Resources Limited: Vancouver, BC, Canada, 2014; 148p. [Google Scholar]
- Černý, P.; Ferguson, R.B. The Tanco pegmatite at Bernic Lake, Manitoba; IV, Petalite and spodumene relations. Can. Mineral. 1972, 11, 660–678. [Google Scholar]
- Hensen, B.J. Mineralogy and petrography of some tin-lithium and beryllium bearing albite pegmatites near Doade, Galicia (Orense). Leidse Geol. Med. 1967, 39, 249–259. [Google Scholar]
- Feng, Y.; Liang, T.; Yang, X.; Zhang, Z.; Wang, Y. Chemical evolution of Nb–Ta oxides and cassiterite in phosphorus-rich albite–spodumene pegmatites in the Kangxiwa–Dahongliutan Pegmatite Field, Western Kunlun Orogen, China. Minerals 2019, 9, 166. [Google Scholar] [CrossRef]
- Lahti, S.I. Zoning in columbite—Tantalite crystals from the granitic pegmatites of the Eräjärvi area, southern Finland. Geochim. Cosmochim. Acta 1987, 51, 509–517. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Wang, R.C.; Marignac, C.; Cuney, M.; Mercadier, J.; Che, X.D.; Charles, N.; Lespinasse, M. Petrogenesis of Nb–(Ta) aplo-pegmatites and fine-grained granites from the Early Cretaceous Huangshan rare-metal granite suite, northeast Jiangxi Province, southeast China. Lithos 2019, 346–347, 105150. [Google Scholar] [CrossRef]
- Debon, F.; Le Fort, P. A cationic classification of common plutonic rocks and associations: Principles, method, application. Bull. Minéral. 1988, 111, 493–510. [Google Scholar] [CrossRef]
- Taylor, S.R.; Mclennan, S.H. The Continental Crust: Its Composition and Evolution; Blackwell: Oxford, UK, 1985; 312p. [Google Scholar]
- Fuertes-Fuente, M.; Cepedal, A.; Martin-Izard, A. Mineralogical study of the wallrock metasomatism in barren and Li–Sn–Ta mineralized pegmatites from NW Spain (Central Galicia). Can. Mineral. 2019, 57, 741–743. [Google Scholar] [CrossRef]
- Maclean, W.H.; Barrett, T.J. Lithogeochemical techniques using immobile elements. J. Geochem. Explor. 1993, 48, 109–133. [Google Scholar] [CrossRef]
- Finlow-Bates, T.; Stumpfl, E.F. The behaviour of the so-called immobile elements in hydrothermally altered rocks associated with volcanogenic submarine exhalative ore deposits. Miner. Deposita 1981, 16, 319–328. [Google Scholar] [CrossRef]
- Barret, T.J.; Mclean, W.H. Chemostratigraphy and hydrothermal alteration in exploration for VHMS deposits in greenstones and younger volcanic rocks. In Alteration and Alteration Processes Associated with Ore Forming Systems; Lentz, D.R., Ed.; Geological Association of Canada: St. John’s, NL, Canada, 1994; Volume 11, pp. 433–467. [Google Scholar]
- O’Hara, K. Fluid flow and Volume loss during mylonitization: An origin for phyllonite in an overthrust setting, North Carolina, USA. Tectonophysics 1988, 156, 21–36. [Google Scholar] [CrossRef]
- Selverstone, J.; Morteani, G.; Staude, J.M. Fluid channelling during ductile shearing: Transformation of granodiorite into aluminous schist in the Tauern Window, Eastern Alps. J. Metamorph. Geol. 1991, 9, 419–431. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Wang, R.C.; Che, X.D.; Zhu, J.C.; Wei, X.L.; Huang, X.E. Magmatic–Hydrothermal rare-element mineralization in the Songshugang granite (northeastern Jiangxi, China): Insights from an electron-microprobe study of Nb–Ta–Zr minerals. Ore Geol. Rev. 2015, 65, 749–760. [Google Scholar] [CrossRef]
- Tindle, A.G.; Webb, P.C. Estimation of lithium contents in trioctahedral micas using microprobe data: Application to micas from granitic rocks. Eur. J. Mineral. 1990, 2, 595–610. [Google Scholar] [CrossRef]
- Tischendorf, G.; Gottesmann, B.; Förster, H.J.; Trumbull, R.B. On Li-bearing micas: Estimating Li from electron microprobe analysis and an improved diagram for graphical representation. Mineral. Mag. 1997, 61, 809–834. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussmann, J. An Introduction to Rock-Forming Minerals; Longman Scientific and Technical: New York, NY, USA, 1992; 696p. [Google Scholar]
- Zack, T.; Kronz, A.; Foley, S.F.; Rivers, T. Trace element abundances in rutile from eclogites and associated garnet mica schists. Chem. Geol. 2002, 184, 97–122. [Google Scholar] [CrossRef]
- Müller, A.; Halls, C. Rutile-the tin-tungsten host in the intrusive tourmaline breccia at Wheal Remfry, SW England. In Mineral Deposit Research: Meeting the Global Challenge; Mao, J., Bierlein, P.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 441–444. [Google Scholar]
- Černý, P.; Trueman, D.L.; Ziehlke, D.V.; Goad, B.E.; Paul, B.J. The Cat Lake-Winnipeg River and the Wekusko Lake Pegmatite Fields; Manitoba Department of Energy and Mines, Mineral Resources Division: Manitoba, ON, Canada, 1981; 216p.
- Trueman, D.L.; Černý, P. Exploration for rare-element granitic pegmatites. In Granitic Pegmatites in Science and Industry; Černý, P., Ed.; Mineralogical Association of Canada: Québec, QC, Canada, 1982; Volume 8, pp. 463–494. [Google Scholar]
- Smeds, S.A. Trace elements in potassium-feldspar and muscovite as a guide in the prospecting for lithium- and tin-bearing pegmatites in Sweden. J. Geochem. Explor. 1992, 42, 351–369. [Google Scholar] [CrossRef]
- London, D. Pegmatites; The Canadian Mineralogist, Special Publication: Québec, QC, Canada, 2008; Volume 10, 340p. [Google Scholar]
- Černý, P.; Ercit, S. Some recent advances in the mineralogy and geochemistry of Nb and Ta in rare-element granitic pegmatites. Bull. Mineral. 1985, 108, 499–532. [Google Scholar] [CrossRef]
- Černý, P.; Ercit, S. Mineralogy of niobium and tantalum: Crystal chemical relationships, paragenetic aspects and their economic implications. In Lanthanides, Tantalum and Niobium; Möller, P., Černý, P., Saupé, F., Eds.; Springer: New York, NY, USA, 1989; pp. 27–29. [Google Scholar]
- Wise, M.A.; Meintzer, R.E.; Černý, P. The Yellowknife pegmatite field: Mineralogy and geochemistry of Nb-Ta-Sn oxide minerals. In Contribution to the Geology of the Northwest Territories; Geology Division, INAC: Yellowknife, NT, Canada, 1986; Volume 2, pp. 15–25. [Google Scholar]
- Černý, P. Geochemical and petrogenetic features of mineralization in rare-element granitic pegmatites in the light of current research. Appl. Geochem. 1992, 7, 393–416. [Google Scholar]
- Spilde, M.N.; Shearer, C.K. A comparison of tantalum-niobium oxide assemblages in two mineralogically distinct rare-element granitic pegmatites, Black Hills, South Dakota. Can. Mineral. 1992, 30, 719–737. [Google Scholar]
- Uher, P.; Černý, P.; Novák, M.; Siman, P. Niobium–tantalum minerals from granitic pegmatites in the Malé Karpaty, Považský Inovec and Žiar Mountains, Western Carpathians, Slovakia. Miner. Slovaca 1994, 26, 157–164. [Google Scholar]
- Abella, P.A.; Corbella, M.; Melgarejo, J.C. Nb–Ta- minerals from the Cap de Creus pegmatite field, eastern Pyrenees: Distribution and geochemical trends. Mineral. Petrol. 1995, 55, 53–69. [Google Scholar] [CrossRef]
- Tindle, A.G.; Breaks, F.W. Columbite–tantalite mineral chemistry from rare-element granitic pegmatites: Separation Lake area, N.W. Ontario, Canada. Mineral. Petrol. 2000, 70, 165–198. [Google Scholar] [CrossRef]
- London, D. Stability of spodumene in acidic and saline fluorine-rich environments. Carnegie Inst. Geophys. Lab. Annu. Rep. 1982, 81, 331–334. [Google Scholar]
- Canosa, F.; Fuertes-Fuente, M.; Martín-Izard, A. Mineralization of Sn–Ta–Nb oxides in Ponte Segade Deposit (North of Galicia, NW Spain). In Let’s Talk Ore Deposits; Barra, F., Reich, M., Campos, E., Tornos, F., Eds.; Ediciones Universidad Católica del Norte: Antofagasta, Chile, 2011; pp. 163–165. [Google Scholar]
- Chudík, P.; Uher, P.; Gadas, P.; Škoda, R.; Pršek, J. Niobium-tantalum oxide minerals in the Jezuitské Lesy granitic pegmatite, Bratislava Massif, Slovakia: Ta to Nb and Fe to Mn evolutionary trends in a narrow Be, Cs-rich and Li, B-poor dike. Mineral. Petrol. 2011, 102, 15–27. [Google Scholar] [CrossRef]
- Wood, S.A.; Williams-Jones, A.E. Theoretical studies of the alteration of spodumene, petalite, eucryptite and pollucite in granitic pegmatites: Exchange reactions with alkali feldspars. Contrib. Mineral. Petrol. 1993, 114, 255–263. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Vieira, R.; Pesquera, A.; Lima, A. Chemical variations and significance of phosphates from the Fregeneda-Almendra pegmatite field, Central Iberian Zone (Spain and Portugal). Mineral. Petrol. 2010, 100, 23–34. [Google Scholar] [CrossRef]
- Charoy, B.; Chaussidon, M.; Le Carlier De Veslud, C.; Duthou, J.L. Evidence of Sr mobility in and around the albite-lepidolite-topaz granite of Beauvoir (France): An in-situ ion and electron probe study of secondary Sr-rich phosphates. Contrib. Mineral. Petrol. 2003, 145, 673–690. [Google Scholar] [CrossRef]
- Charoy, B.; Noronha, F.; Lima, A. Spodumene–petalite–eucryptite: Mutual relationships and pattern of alteration in li-rich aplite–pegmatite dykes from northern Portugal. Can. Mineral. 2001, 39, 729–746. [Google Scholar] [CrossRef]
- Dias, F.; Lima, A.; Roda-Robles, E. Mutual relationships between spodumene and petalite from the Iberian Massif pegmatites: More than PT changes? Can. Mineral. 2019, 57, 731–732. [Google Scholar] [CrossRef]
- Černý, P. Petrogenesis of granitic pegmatites. In Granitic Pegmatites in Science and Industry; Černý, P., Ed.; Mineralogical Association of Canada: Québec, QC, Canada, 1982; Volume 8, pp. 405–461. [Google Scholar]
- Simmons, W.B.; Lee, M.T.; Brewster, R.H. Geochemistry and evolution of the South Platte granite–pegmatite system, Jefferson County, Colorado. Geochim. Cosmochim. Acta 1987, 51, 455–471. [Google Scholar] [CrossRef]
- Černý, P. REE trends in rare-element granitic pegmatites: Enrichment vs. depletion in granite-to-pegmatite sequences. J. Geosci. 1997, 42, 34. [Google Scholar]
- Wu, F.; Sun, D.Y.; Jahn, B.M.; Wilde, S. A Jurassic garnet-bearing granitic pluton from NE China showing tetrad REE patterns. J. Asian Earth Sci. 2004, 23, 731–744. [Google Scholar] [CrossRef]
- Černý, P.; Masau, M.; Goad, B.E.; Ferreira, K. The Greer Lake leucogranite Manitoba and the origin of lepidolite-subtype granitic pegmatites. Lithos 2005, 80, 305–321. [Google Scholar] [CrossRef]
- Lee, S.G.; Shin, S.C.; Jin, M.S.; Ogasawara, M.; Yang, M.K. Two Paleoproterozoic strongly peraluminous granitic plutons (Nonggeori and Naedeokri granites) at the northeastern part of Yeongnam Massif, Korea: Geochemical and isotopic constraints in East Asian crustal formation history. Precambrian Res. 2005, 139, 101–120. [Google Scholar] [CrossRef]
- Beurlen, H.; Rhede, D.; Da Silva, M.R.R.; Thomas, R.; Guimarães, I.P. Petrography, geochemistry and chemical electron microprobe U–Pb–Th dating of pegmatitic granites in Borborema Province, North-eastern Brazil: A possible source of the rare-element granitic pegmatites. Terrae 2008, 3, 65–74. [Google Scholar]
- London, D. Experimental synthesis and stability of tourmaline: A historical overview. Can. Mineral. 2011, 49, 117–136. [Google Scholar] [CrossRef]
- Shearer, C.K.; Papike, J.J.; Simon, S.B.; Laul, J.C. Pegmatite–wallrock interactions, Black Hills, South Dakota: Interaction between pegmatite-derived fluids and quartz-mica schist wallrock. Am. Mineral. 1986, 71, 518–539. [Google Scholar]
- London, D. Geochemistry of alkali and alkaline earth elements in ore-forming granites, pegmatites and rhyolites. In Rare Element Geochemistry and Mineral Deposits; Linnen, R.L., Samson, I.M., Eds.; Geological Association of Canada: St. John’s, NL, Canada, 2005; Volume 17, pp. 17–43. [Google Scholar]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roza Llera, A.; Fuertes-Fuente, M.; Cepedal, A.; Martin-Izard, A. Barren and Li–Sn–Ta Mineralized Pegmatites from NW Spain (Central Galicia): A Comparative Study of Their Mineralogy, Geochemistry, and Wallrock Metasomatism. Minerals 2019, 9, 739. https://doi.org/10.3390/min9120739
Roza Llera A, Fuertes-Fuente M, Cepedal A, Martin-Izard A. Barren and Li–Sn–Ta Mineralized Pegmatites from NW Spain (Central Galicia): A Comparative Study of Their Mineralogy, Geochemistry, and Wallrock Metasomatism. Minerals. 2019; 9(12):739. https://doi.org/10.3390/min9120739
Chicago/Turabian StyleRoza Llera, Ana, Mercedes Fuertes-Fuente, Antonia Cepedal, and Agustín Martin-Izard. 2019. "Barren and Li–Sn–Ta Mineralized Pegmatites from NW Spain (Central Galicia): A Comparative Study of Their Mineralogy, Geochemistry, and Wallrock Metasomatism" Minerals 9, no. 12: 739. https://doi.org/10.3390/min9120739
APA StyleRoza Llera, A., Fuertes-Fuente, M., Cepedal, A., & Martin-Izard, A. (2019). Barren and Li–Sn–Ta Mineralized Pegmatites from NW Spain (Central Galicia): A Comparative Study of Their Mineralogy, Geochemistry, and Wallrock Metasomatism. Minerals, 9(12), 739. https://doi.org/10.3390/min9120739





