The Distribution of Precious Metals in High-Grade Banded Quartz Veins from Low-Sulfidation Epithermal Deposits: Constraints from µXRF Mapping
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Locations
2.2. Analytical Methods
3. Results
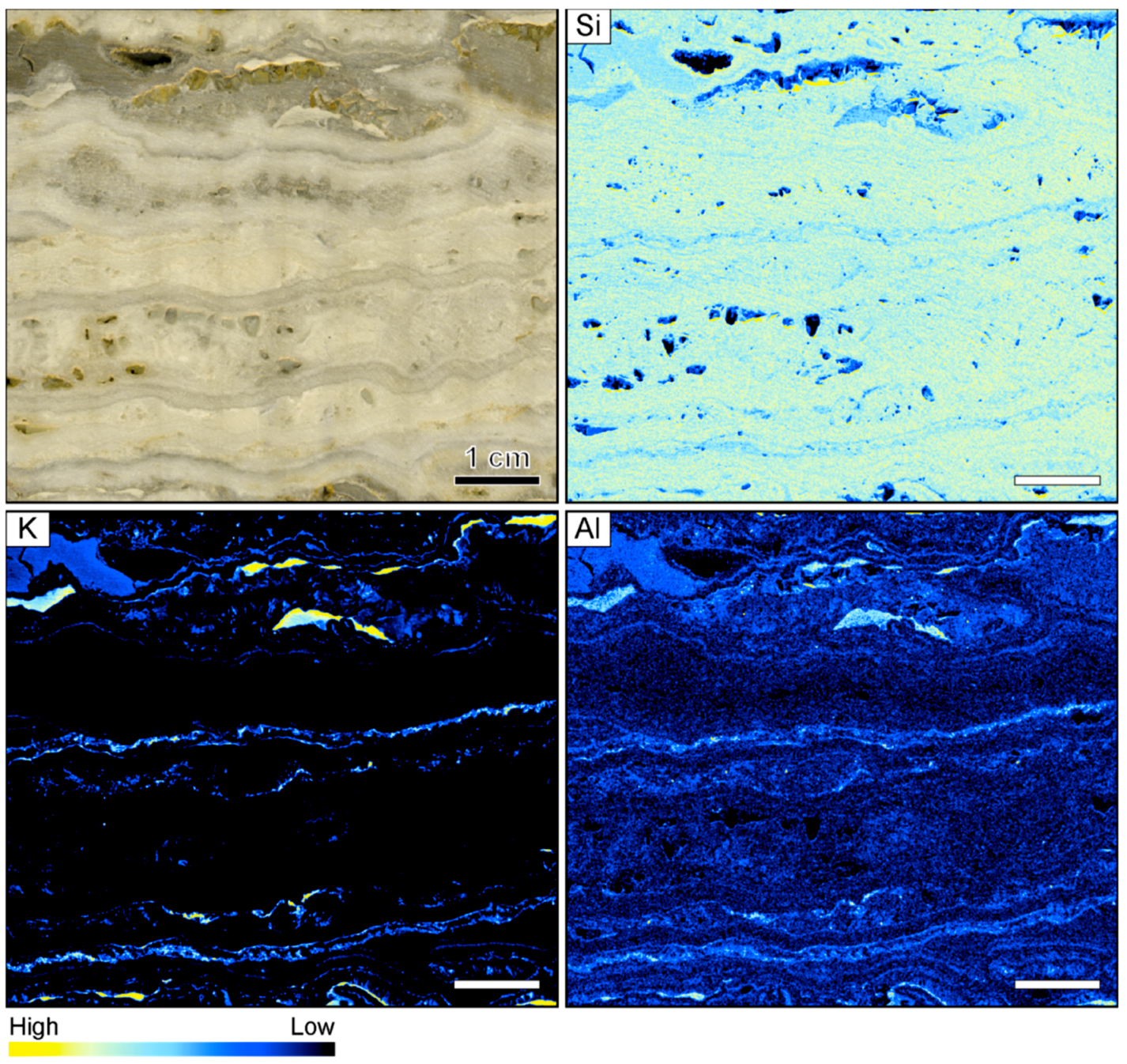
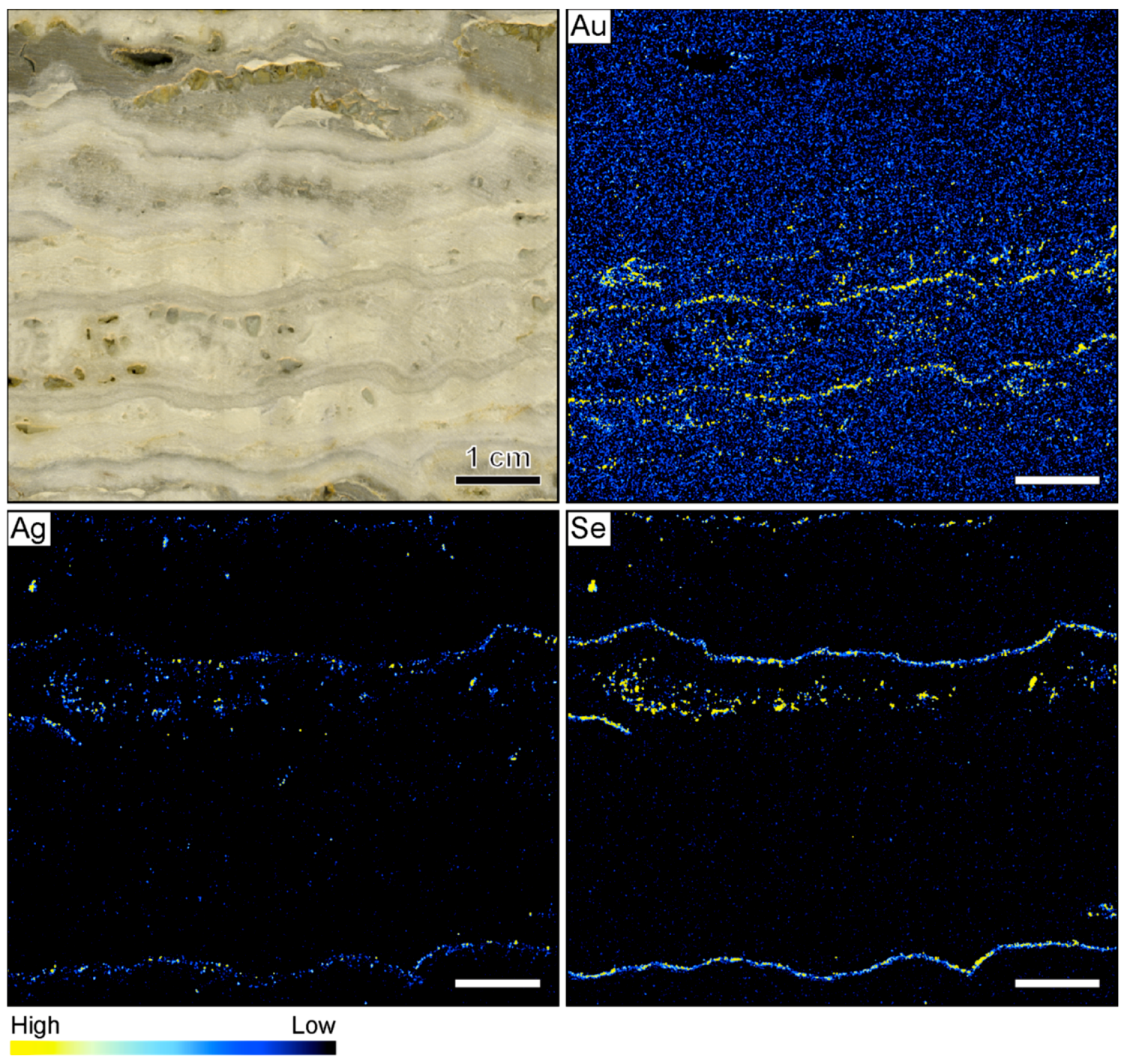
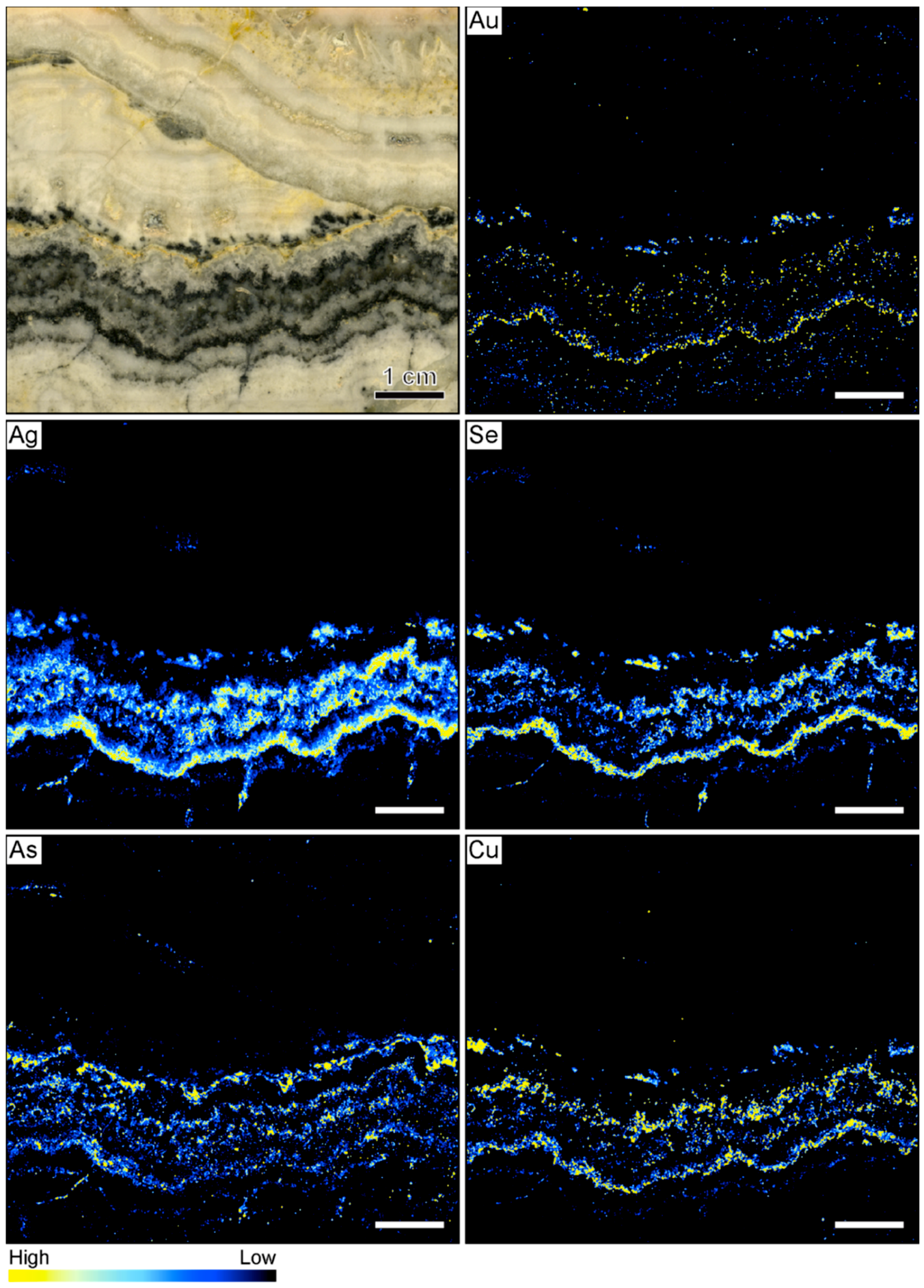
3.1. Banded Vein from Hokuryu in Japan
3.2. Banded Vein from Midas in Nevada
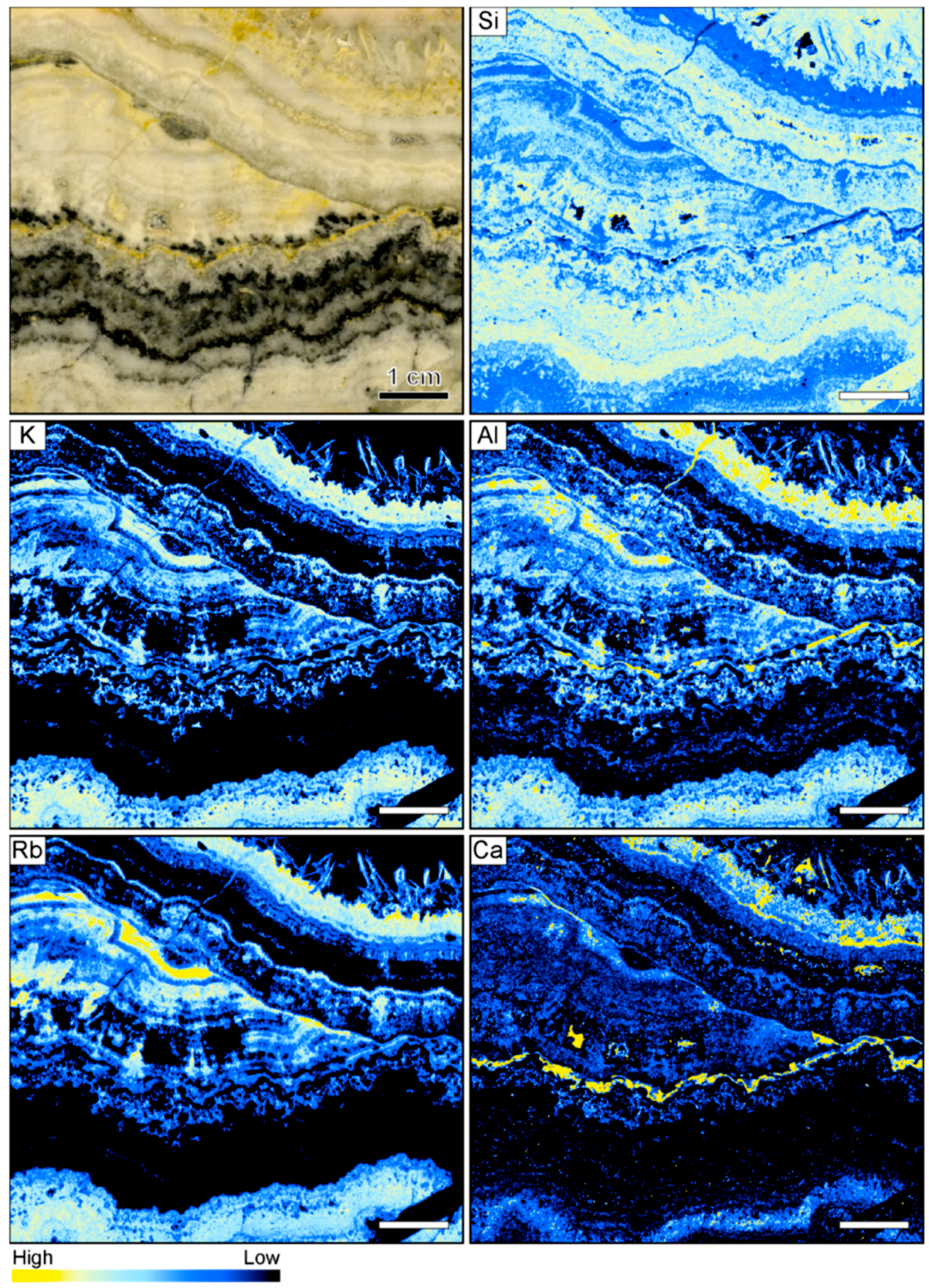
3.3. Banded Vein from Sado Kinzan in Japan
4. Discussion
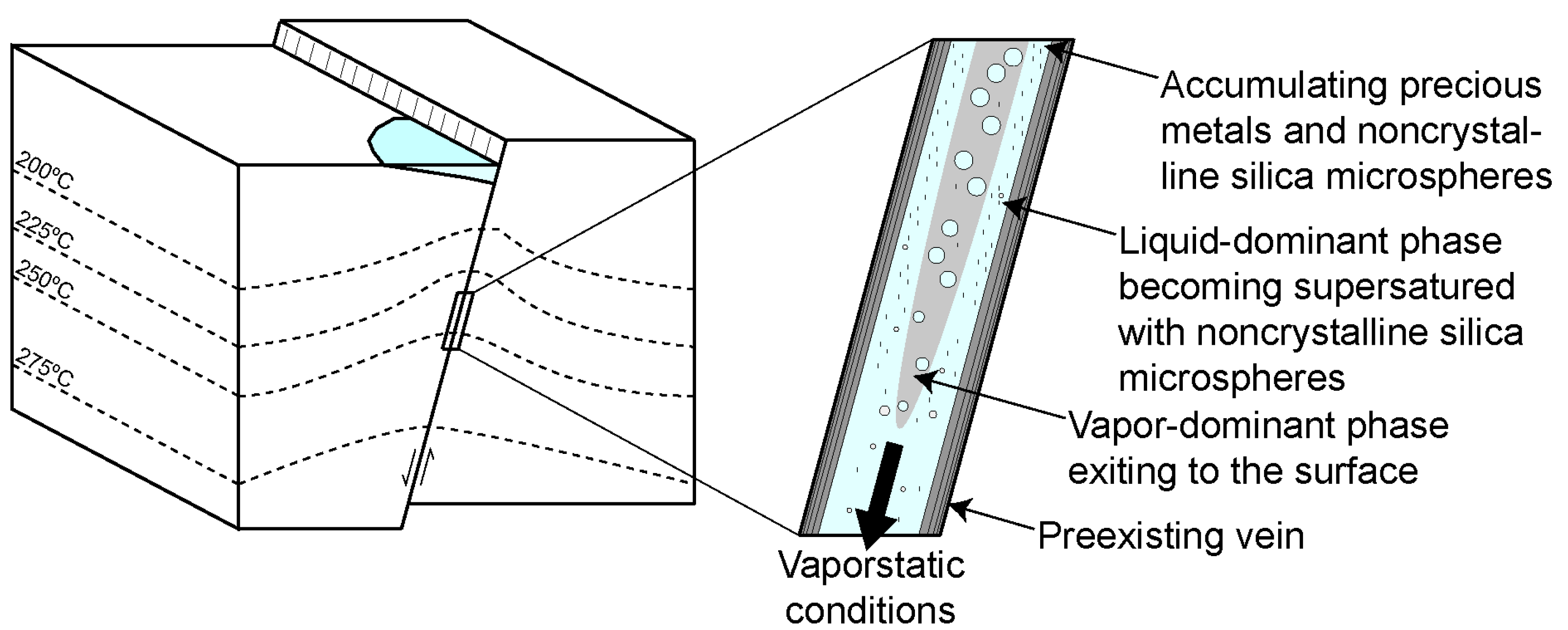
4.1. Silica Deposition in Ore-Bearing Bands
4.2. Compositional Variations in Ore-Bearing Bands
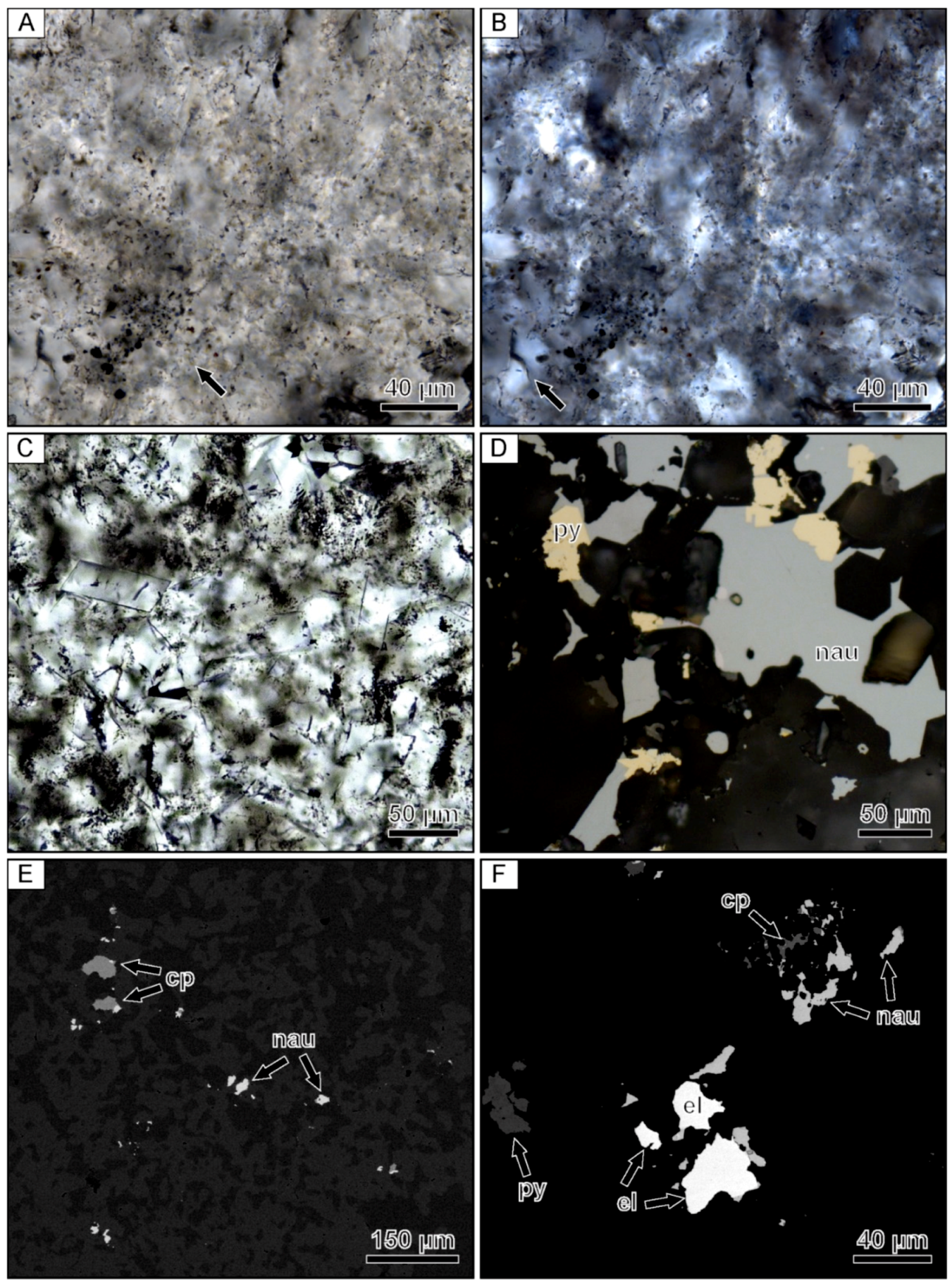
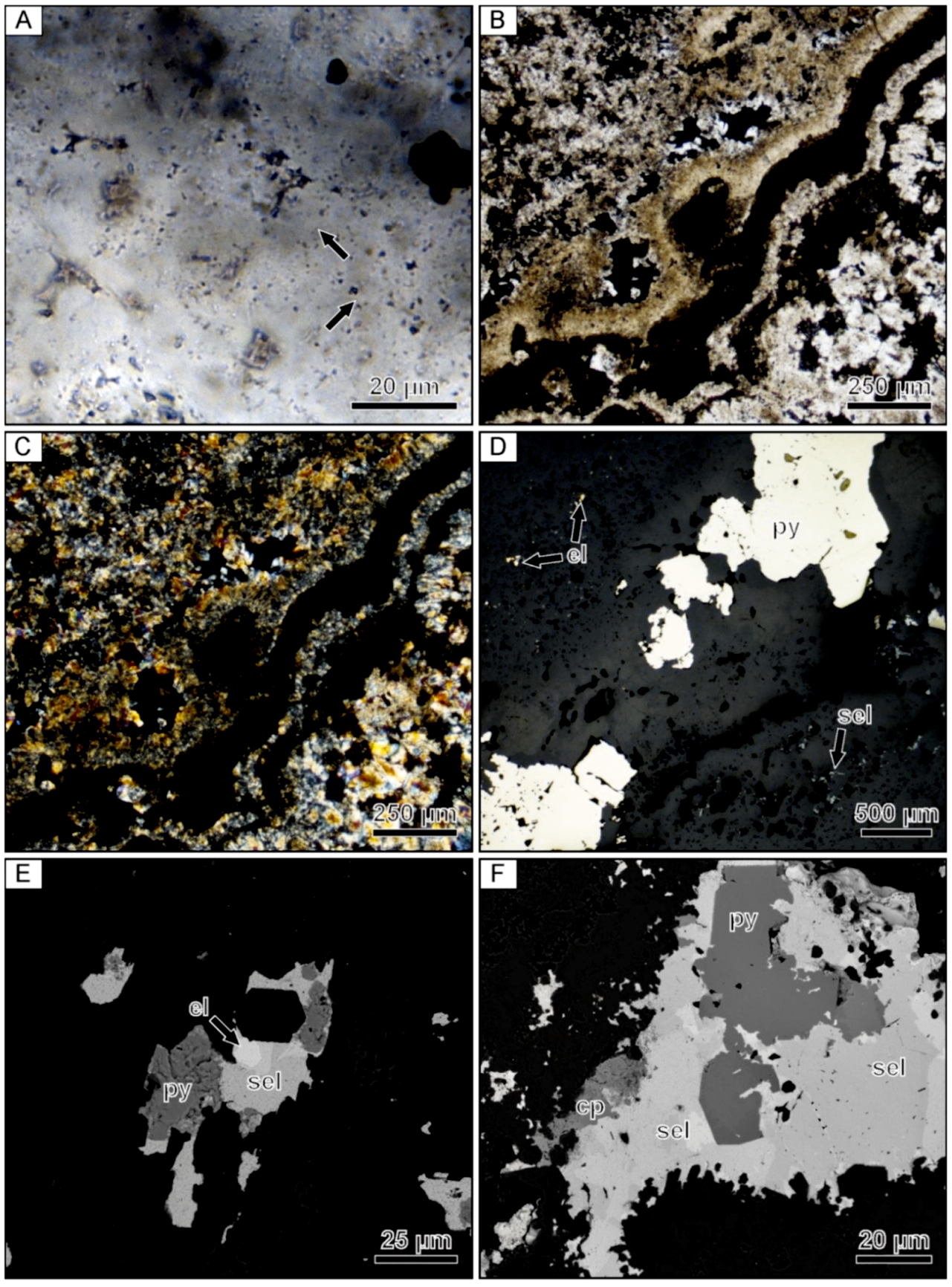
4.3. Formation of Ore Minerals in Ore-Bearing Bands
4.4. Design of Textural and Microanalytical Studies
4.5. Exploration Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lipson, R. The promise and perils of porphyry deposits in the future of gold production. SEG Newsl. 2014, 98, 14–21. [Google Scholar]
- Hedenquist, J.W.; Arribas, A.; Gonzalez-Urien, E. Exploration for epithermal gold deposits. Rev. Econ. Geol. 2000, 13, 245–277. [Google Scholar]
- Simmons, S.F.; White, N.C.; John, D.A. Geological characteristics of epithermal precious and base metal deposits. In Economic Geology 100th Anniversary Volume; Hedenquist, J.W., Thompson, J.F.H., Goldfarb, R.J., Richards, J.P., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2005; pp. 485–522. [Google Scholar]
- Bodnar, R.J.; Lecumberri-Sanchez, P.; Moncada, D.; Steele-MacInnis, M. Fluid inclusions in hydrothermal ore deposits. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; pp. 119–142. [Google Scholar]
- Takeuchi, K.; Shikazono, N. Mineralization of the Arakawa No. 4 vein of the Kushikino mine, Kagoshima Prefecture, Japan. Mining Geol. 1984, 34, 187–195. [Google Scholar]
- Matsuhisa, Y.; Aoki, M. Temperature and oxygen isotope variations during formation of the Hishikari epithermal gold-silver veins, southern Kyushu, Japan. Econ. Geol. 1994, 89, 1608–1613. [Google Scholar] [CrossRef]
- Shimizu, T.; Matsueda, H.; Ishiyama, D.; Matsubaya, O. Genesis of epithermal Au–Ag mineralization of the Koryu mine, Hokkaido, Japan. Econ. Geol. 1998, 93, 303–325. [Google Scholar] [CrossRef]
- Faure, K.; Matsuhisa, Y.; Metsugi, H.; Mizota, C.; Hayashi, S. The Hishikari Au–Ag epithermal deposit, Japan: Oxygen and hydrogen isotope evidence in determining the source of paleohydrothermal fluids. Econ. Geol. 2002, 97, 481–498. [Google Scholar] [CrossRef]
- Leavitt, E.D.; Spell, T.L.; Goldstrand, P.M.; Arehart, G.B. Geochronology of the Midas low-sulfidation epithermal gold-silver deposit, Elko County, Nevada. Econ. Geol. 2004, 99, 1665–1686. [Google Scholar] [CrossRef]
- Sanematsu, K.; Watanabe, K.; Duncan, R.A.; Izawa, E. The history of vein formation determined by 40Ar/39Ar dating of adularia in the Hosen-1 vein at the Hishikari epithermal gold deposit, Japan. Econ. Geol. 2006, 101, 685–698. [Google Scholar] [CrossRef]
- Camprubí, A.; Albinson, T. Epithermal deposits in México—Update of current knowledge, and an empirical reclassification. Geol. Soc. Am. Spec. Pap. 2007, 422, 377–415. [Google Scholar]
- Shimizu, T. Reinterpretation of quartz textures in terms of hydrothermal fluid evolution at the Koryu Au-Ag deposit, Japan. Econ. Geol. 2014, 109, 2051–2065. [Google Scholar] [CrossRef]
- Mukaiyama, H. On some gold-silver ores from the Sado mine, Sado Island, Niigata Prefecture, Japan. J. Geol. Soc. Jpn. 1950, 56, 181–187. [Google Scholar] [CrossRef]
- Saunders, J.A.; Unger, D.L.; Kamenov, G.D.; Fayek, M.; Hames, W.E.; Utterback, W.C. Genesis of Middle Miocene Yellowstone hotspot-related bonanza epithermal Au–Ag deposits, northern Great Basin, USA. Miner. Depos. 2008, 43, 715–734. [Google Scholar] [CrossRef]
- Zeeck, L. The Role of Flashing in the Formation of High-Grade, Low-Sulfidation Epithermal Deposits: A Case Study from the Omu Camp in Hokkaido, Japan. Master’s Thesis, Colorado School of Mines, Golden, CO, USA, August 2018. [Google Scholar]
- Izawa, E.; Urashima, Y.; Ibaraki, K.; Suzuki, R.; Yokoyama, T.; Kawasaki, K.; Koga, A.; Taguchi, S. The Hishikari gold deposit: High-grade epithermal veins in Quaternary volcanics of southern Kyushu, Japan. J. Geochem. Explor. 1990, 36, 1–56. [Google Scholar] [CrossRef]
- Shimada, N.; Nakamura, T.; Morinaga, Y.; Shikama, Y. Invisible gold from the Hishikari epithermal gold deposit, Japan: Implication for gold distribution and deposition. Resour. Geol. 2005, 55, 91–100. [Google Scholar] [CrossRef]
- Suzuki, M.; Konoya, M.; Fujiwara, T. Explanatory text of the Geological Map of Japan (Scale 1:50,000): Omu (Abashiri-6); Geological Survey of Hokkaido: Sapporo, Japan, 1966. [Google Scholar]
- Watanabe, Y. A tectonic model for epithermal Au mineralization in NE Hokkaido, Japan. Resour. Geol. Spec. Issue 1995, 18, 257–269. [Google Scholar]
- Goldstrand, P.M.; Schmidt, K.W. Geology, mineralization, and ore controls at the Ken Snyder gold-silver mine, Elko County, Nevada. In Geology and Ore Deposits 2000: The Great Basin and Beyond; Cluer, J.K., Price, J.G., Struhsacker, E.M., Hardyman, R.F., Morris, C.L., Eds.; Geological Society of Nevada: Reno, NV, USA, 2000; pp. 265–287. [Google Scholar]
- John, D.A.; Vikre, P.G.; du Bray, E.A.; Blakely, R.J.; Fey, D.L.; Rockwell, B.W.; Mauk, J.L.; Anderson, E.D.; Graybeal, F.T. Descriptive Models for Epithermal Gold-Silver Deposits; USGS Scientific Investigation Report 2010-5070-Q; United States Government Publishing Office: Washington, DC, USA, 2018; p. 247.
- Shikazono, N.; Tsunakawa, H. K-Ar ages of Hosokura Pb-Zn and Sado Au-Ag vein-type deposits, north eastern part of Japan. Mining Geol. 1982, 32, 479–482. [Google Scholar]
- Bunno, M. Hessite from the Sado mine, Niigata Prefecture, Japan. Min. Geol. 1971, 21, 301–305. [Google Scholar]
- Shikazono, N.; Nakata, M.; Shimizu, M. Geochemical, mineralogic and geologic characteristics of Se- and Te-bearing epithermal gold deposits in Japan. Min. Geol. 1990, 40, 337–352. [Google Scholar]
- Sherlock, R.L.; Lehrman, N.J. Occurrences of dendritic gold at the McLaughlin mine hot-spring gold deposit. Miner. Depos. 1995, 30, 323–327. [Google Scholar] [CrossRef]
- Taksavasu, T.; Monecke, T.; Reynolds, T.J. Textural characteristics of noncrystalline silica in sinters and quartz veins: Implications for the formation of bonanza veins in low-sulfidation epithermal deposits. Minerals 2018, 8, 331. [Google Scholar] [CrossRef]
- Jones, B.; Renaut, R.W.; Rosen, M.R. Biogenicity of silica precipitation around geysers and hot-spring vents, North Island, New Zealand. J. Sediment. Res. 1997, 67, 88–104. [Google Scholar]
- Herdianita, N.R.; Browne, P.R.L.; Rodgers, K.A.; Campbell, K.A. Mineralogical and textural changes accompanying ageing of silica sinter. Miner. Depos. 2000, 35, 48–62. [Google Scholar] [CrossRef]
- Campbell, K.A.; Rodgers, K.A.; Brotheridge, J.M.A.; Browne, P.R.L. An unusual modern silica-carbonate sinter from Pavlova spring, Ngatamariki, New Zealand. Sedimentology 2002, 49, 835–854. [Google Scholar] [CrossRef]
- Guidry, S.A.; Chafetz, H.S. Anatomy of siliceous hot springs: Examples from Yellowstone National Park, Wyoming, USA. Sediment. Geol. 2003, 157, 71–106. [Google Scholar] [CrossRef]
- Lynne, B.Y.; Campbell, K.A. Morphologic and mineralogic transitions from opal-A to opal-CT in low-temperature siliceous sinter diagenesis, Taupo Volcanic Zone, New Zealand. J. Sediment. Res. 2004, 74, 561–579. [Google Scholar] [CrossRef]
- Rodgers, K.A.; Browne, P.R.L.; Buddle, T.F.; Cook, K.L.; Greatrex, R.A.; Hampton, W.A.; Herdianita, N.R.; Holland, G.R.; Lynne, B.Y.; Martin, R.; et al. Silica phases in sinters and residues from geothermal fields of New Zealand. Earth Sci. Rev. 2004, 66, 1–61. [Google Scholar] [CrossRef]
- Fernandez-Turiel, J.L.; Garcia-Valles, M.; Gimeno-Torrente, D.; Saavedra-Alonso, J.; Martinez-Manent, S. The hot spring and geyser sinters of El Tatio, northern Chile. Sediment. Geol. 2005, 180, 125–147. [Google Scholar] [CrossRef]
- Skinner, B.J.; White, D.E.; Rose, H.J.; Mays, R.E. Sulfides associated with the Salton Sea geothermal brine. Econ. Geol. 1967, 62, 316–330. [Google Scholar] [CrossRef]
- Karabelas, A.J.; Andritsos, N.; Mouza, A.; Mitrakas, M.; Vrouzi, F.; Christanis, K. Characteristics of scales from the Milos geothermal plant. Geothermics 1989, 18, 169–174. [Google Scholar] [CrossRef]
- Reyes, A.G.; Trompetter, W.J.; Britten, K.; Searle, J. Mineral deposits in the Rotokawa geothermal pipelines, New Zealand. J. Volcanol. Geotherm. Res. 2002, 119, 215–239. [Google Scholar] [CrossRef]
- Saunders, J.A. Colloidal transport of gold and silica in epithermal precious-metal systems: Evidence from the Sleeper deposit, Nevada. Geology 1990, 18, 757–760. [Google Scholar] [CrossRef]
- Saunders, J.A. Silica and gold textures in bonanza ores of the Sleeper deposit, Humboldt County, Nevada: Evidence for colloids and implications for epithermal ore-forming processes. Econ. Geol. 1994, 89, 628–638. [Google Scholar] [CrossRef]
- Saunders, J.A.; Beasley, L.; Vikre, P.; Unger, D.L. Colloidal and physical transport textures exhibited by electrum and naumannite in bonanza epithermal veins from western USA, and their significance. In Great Basin Evolution and Metallogeny, Proceedings of Geological Society of Nevada 2010 Symposium, Reno-Sparks, NV, USA, 14–22 May 2010; Steininger, R.C., Pennell, B., Eds.; Geological Society of Nevada: Reno-Sparks, NV, USA, 2011; pp. 825–831. [Google Scholar]
- Unger, D.L. Geochronology and geochemistry of mid-Miocene bonanza low-sulfidation epithermal ores of the northern Great Basin, USA. Master’s Thesis, Auburn University, Auburn, AL, USA, May 2008. [Google Scholar]
- Aseto, C.O. Geology, geochemistry and geochronology of the mid-Miocene, low-sulfidation epithermal gold-silver ores on War Eagle Mountain, Silver City district, Idaho. Master’s Thesis, Auburn University, Auburn, AL, USA, August 2012. [Google Scholar]
- Campbell, K.A.; Sannazzaro, K.; Rodgers, K.A.; Herdianita, N.R.; Browne, P.R.L. Sedimentary facies and mineralogy of the late Pleistocene Umukuri silica sinter, Taupo Volcanic Zone, New Zealand. J. Sediment. Res. 2001, 71, 727–746. [Google Scholar] [CrossRef]
- Lynne, B.Y.; Campbell, K.A.; Moore, J.N.; Browne, P.R.L. Diagenesis of 1900-year-old siliceous sinter (opal-A to quartz) at Opal Mound, Roosevelt Hot Springs, Utah, USA. Sediment. Geol. 2005, 179, 249–278. [Google Scholar] [CrossRef]
- Lynne, B.Y.; Campbell, K.A.; James, B.J.; Browne, P.R.L.; Moore, J. Tracking crystallinity in siliceous hot-spring deposits. Am. J. Sci. 2007, 307, 612–641. [Google Scholar] [CrossRef]
- Mitzutani, S. Silica minerals in the early stage of diagenesis. Sedimentology 1970, 15, 419–436. [Google Scholar] [CrossRef]
- Murata, K.J.; Nakata, J.K. Cristobalitic stage in the diagenesis of diatomaceous shale. Science 1974, 184, 567–568. [Google Scholar] [CrossRef]
- Murata, K.J.; Friedman, I.; Gleason, J.D. Oxygen isotope relations between diagenetic silica minerals in Monterey Shale, Temblor Range, California. Am. J. Sci. 1977, 277, 259–272. [Google Scholar] [CrossRef]
- Mustoe, G.E. Diatomaceous origin of siliceous shale in Eocene lake beds of central British Columbia. Can. J. Earth Sci. 2005, 42, 231–241. [Google Scholar] [CrossRef]
- Lovering, T.G. Jasperoid in the United States—Its Characteristics, Origin, and Economic Significance; USGS Professional Paper 710; United States Government Publishing Office: Washington, DC, USA, 1972; p. 164.
- Ernst, W.G.; Calvert, S.E. An experimental study of the recrystallization of porcelanite and its bearing on the origin of some bedded cherts. Am. J. Sci. 1969, 267-A, 114–133. [Google Scholar]
- Bettermann, P.; Liebau, F. The transformation of amorphous silica to crystalline silica under hydrothermal conditions. Contrib. Mineral. Petr. 1975, 53, 25–36. [Google Scholar] [CrossRef]
- Oehler, J.H. Hydrothermal crystallization of silica gel. Geol. Soc. Am. Bull. 1976, 87, 1143–1152. [Google Scholar] [CrossRef]
- Moncada, D.; Mutchler, S.; Nieto, A.; Reynolds, T.J.; Rimstidt, J.D.; Bodnar, R.J. Mineral textures and fluid inclusion petrography of the epithermal Ag–Au deposits at Guanajuato, Mexico: Application to exploration. J. Geochem. Explor. 2012, 114, 20–35. [Google Scholar] [CrossRef]
- Saunders, J.A.; Burke, M. Formation and aggregation of gold (electrum) nanoparticles in epithermal ores. Minerals 2017, 7, 163. [Google Scholar] [CrossRef]
- Rowland, J.V.; Simmons, S.F. Hydrologic, magmatic, and tectonic controls on hydrothermal flow, Taupo Volcanic Zone, New Zealand: Implications for the formation of epithermal vein deposits. Econ. Geol. 2012, 107, 427–457. [Google Scholar] [CrossRef]
- Muffler, L.J.P.; White, D.E.; Truesdell, A.H. Hydrothermal explosion craters in Yellowstone National Park. Geol. Soc. Am. Bull. 1971, 82, 723–740. [Google Scholar] [CrossRef]
- Nairn, I.A.; Wiradiradja, S. Late Quaternary hydrothermal explosion breccias at Kawerau geothermal field, New Zealand. Bull. Volcanol. 1980, 43, 1–13. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Henley, R.W. Hydrothermal eruptions in the Waiotapu geothermal system, New Zealand: Their origin, associated breccias, and relation to precious metal mineralization. Econ. Geol. 1985, 80, 1640–1668. [Google Scholar] [CrossRef]
- Marini, L.; Principe, C.; Chiodini, G.; Cioni, R.; Fytikas, M.; Marinelli, G. Hydrothermal eruptions of Nisyros (Dodecanese, Greece). Past events and present hazard. J. Volcanol. Geotherm. Res. 1993, 56, 71–94. [Google Scholar] [CrossRef]
- Weissberg, B.G. Gold-silver ore-grade precipitates from New Zealand thermal waters. Econ. Geol. 1969, 64, 95–108. [Google Scholar] [CrossRef]
- Brown, K.L. Gold deposition from geothermal discharges in New Zealand. Econ. Geol. 1986, 81, 979–983. [Google Scholar] [CrossRef]
- Krupp, R.E.; Seward, T.M. The Rotokawa geothermal system, New Zealand: An active epithermal gold-depositing environment. Econ. Geol. 1987, 82, 1109–1129. [Google Scholar] [CrossRef]
- Christenson, B.W.; Hayba, D.O. Hydrothermal eruptions in ore forming reservoirs: Analogues and models. In Exploring the Rim, Proceedings of the PACRIM Congress, Auckland, New Zealand, 19–22 November 1995; Australasian Institute of Mining and Metallurgy: Melbourne, Australia, 1995; pp. 119–124. [Google Scholar]
- Simmons, S.F.; Browne, P.R.L. Hydrothermal minerals and precious metals in the Broadlands-Ohaaki geothermal system: Implications for understanding low-sulfidation epithermal environments. Econ. Geol. 2000, 95, 971–999. [Google Scholar] [CrossRef]
- Hardardóttir, V.; Hannington, M.; Hedenquist, J.; Kjarsgaard, I.; Hoal, K. Cu-rich scales in the Reykjanes geothermal system, Iceland. Econ. Geol. 2010, 105, 1143–1155. [Google Scholar] [CrossRef]
- Sanchez-Alfaro, P.; Reich, M.; Driesner, T.; Cembrano, J.; Arancibia, G.; Pérez-Flores, P.; Heinrich, C.A.; Rowland, J.; Tardani, D.; Lange, D.; et al. The optimal windows for seismically-enhanced gold precipitation in the epithermal environment. Ore Geol. Rev. 2016, 79, 463–473. [Google Scholar] [CrossRef]
- Browne, P.R.L.; Ellis, A.J. The Ohaki-Broadlands hydrothermal area, New Zealand: Mineralogy and related geochemistry. Am. J. Sci. 1970, 269, 97–131. [Google Scholar] [CrossRef]
- Hedenquist, J.W. The thermal and geochemical structure of the Broadlands-Ohaaki geothermal system, New Zealand. Geothermics 1990, 19, 151–185. [Google Scholar] [CrossRef]
- Dong, G.; Morrison, G.W. Adularia in epithermal veins, Queensland: Morphology, structural state and origin. Miner. Depos. 1995, 30, 11–19. [Google Scholar] [CrossRef]
- Shimizu, T. Elemental analysis of bonanza ores of the Ryosen veins, Hishikari epithermal Au-Ag deposit, Japan, using micro X-ray fluorescence (µ-XRF). Bull. Geol. Surv. Jpn. 2015, 66, 1–14. [Google Scholar] [CrossRef][Green Version]
- Moncada, D.; Baker, D.; Bodnar, R.J. Mineralogical, petrographic and fluid inclusion evidence for the link between boiling and epithermal Ag-Au mineralization in the La Luz area, Guanajuato mining district, México. Ore Geol. Rev. 2017, 89, 143–170. [Google Scholar] [CrossRef]
- Sander, M.V.; Black, J.E. Crystallization and recrystallization of growth-zoned vein quartz crystals from epithermal systems—Implications for fluid inclusion studies. Econ. Geol. 1988, 83, 1052–1060. [Google Scholar] [CrossRef]
- Bobis, R.E. A review of the description, classification and origin of quartz textures in low sulphidation epithermal veins. J. Geol. Soc. Philipp. 1994, 49, 15–39. [Google Scholar]
- Dong, G.; Morrison, G.; Jaireth, S. Quartz textures in epithermal veins, Queensland–Classification, origin, and implication. Econ. Geol. 1995, 90, 1841–1856. [Google Scholar] [CrossRef]
- Corbett, G. Epithermal gold for explorationists. AIG News 2002, 67, 1–26. [Google Scholar]
- Fournier, R.O. The behavior of silica in hydrothermal solutions. Rev. Econ. Geol. 1985, 2, 45–61. [Google Scholar]
- Browne, P.R.L. Minimum age of the Kawerau geothermal field, North Island, New Zealand. J. Volcanol. Geotherm. Res. 1979, 6, 213–215. [Google Scholar] [CrossRef]
- Grimes, S.; Rickard, D.; Hawkesworth, C.; van Calsteren, P.; Browne, P. A U-Th calcite isochron age from an active geothermal field in New Zealand. J. Volcanol. Geotherm. Res. 1998, 81, 327–333. [Google Scholar] [CrossRef]
- Arehart, G.B.; Christenson, B.W.; Wood, C.P.; Foland, K.A.; Browne, P.R.L. Timing of volcanic, plutonic and geothermal activity at Ngatamariki, New Zealand. J. Volcanol. Geotherm. Res. 2002, 116, 201–214. [Google Scholar] [CrossRef]
- Simmons, S.F.; Brown, K.L.; Tutolo, B.M. Hydrothermal transport of Ag, Au, Cu, Pb, Te, Zn, and other metals and metalloids in New Zealand geothermal systems: Spatial patterns, fluid-mineral equilibria, and implications for epithermal mineralization. Econ. Geol. 2016, 111, 589–618. [Google Scholar] [CrossRef]
- Giggenbach, W.F. Magma degassing and mineral deposition in hydrothermal systems along convergent plate boundaries. Econ. Geol. 1992, 87, 1927–1944. [Google Scholar]
- Brown, K.L.; Simmons, S.F. Precious metals in high-temperature geothermal systems in New Zealand. Geothermics 2003, 32, 619–625. [Google Scholar] [CrossRef]
- Simmons, S.F.; Brown, K.L. The flux of gold and related metals through a volcanic arc, Taupo Volcanic Zone, New Zealand. Geology 2007, 35, 1099–1102. [Google Scholar] [CrossRef]
- Seward, T.M. Thio complexes of gold and the transport of gold in hydrothermal ore solutions. Geochim. Cosmochim. Acta 1973, 37, 379–399. [Google Scholar] [CrossRef]
- Seward, T.M. The hydrothermal chemistry of gold and its implications for ore formation: Boiling and conductive cooling as examples. Econ. Geol. Monogr. 1989, 6, 398–404. [Google Scholar]
- Stefánsson, A.; Seward, T.M. Experimental determination of the stability and stoichiometry of sulphide complexes of silver(I) in hydrothermal solutions to 400 °C. Geochim. Cosmochim. Acta 2003, 67, 1395–1413. [Google Scholar] [CrossRef]
- Stefánsson, A.; Seward, T.M. Gold(I) complexing in aqueous sulphide solutions to 500 °C at 500 bar. Geochim. Cosmochim. Acta 2004, 68, 4121–4143. [Google Scholar] [CrossRef]
- Taguchi, S.; Nakamura, M. Subsurface thermal structure of the Hatchobaru geothermal system, Japan, determined by fluid inclusion study. Geochem. J. 1991, 25, 301–314. [Google Scholar] [CrossRef]
- Hannington, M.; Hardardóttir, V.; Garbe-Schönberg, D.; Brown, K.L. Gold enrichment in active geothermal systems by accumulating colloidal suspensions. Nat. Geosci. 2016, 9, 299–302. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tharalson, E.R.; Monecke, T.; Reynolds, T.J.; Zeeck, L.; Pfaff, K.; Kelly, N.M. The Distribution of Precious Metals in High-Grade Banded Quartz Veins from Low-Sulfidation Epithermal Deposits: Constraints from µXRF Mapping. Minerals 2019, 9, 740. https://doi.org/10.3390/min9120740
Tharalson ER, Monecke T, Reynolds TJ, Zeeck L, Pfaff K, Kelly NM. The Distribution of Precious Metals in High-Grade Banded Quartz Veins from Low-Sulfidation Epithermal Deposits: Constraints from µXRF Mapping. Minerals. 2019; 9(12):740. https://doi.org/10.3390/min9120740
Chicago/Turabian StyleTharalson, Erik R., Thomas Monecke, T. James Reynolds, Lauren Zeeck, Katharina Pfaff, and Nigel M. Kelly. 2019. "The Distribution of Precious Metals in High-Grade Banded Quartz Veins from Low-Sulfidation Epithermal Deposits: Constraints from µXRF Mapping" Minerals 9, no. 12: 740. https://doi.org/10.3390/min9120740
APA StyleTharalson, E. R., Monecke, T., Reynolds, T. J., Zeeck, L., Pfaff, K., & Kelly, N. M. (2019). The Distribution of Precious Metals in High-Grade Banded Quartz Veins from Low-Sulfidation Epithermal Deposits: Constraints from µXRF Mapping. Minerals, 9(12), 740. https://doi.org/10.3390/min9120740



