1. Introduction
Basalt rocks are widely used nowadays to obtain different types of materials: thermal insulation, continuous fibers, cast stone material etc. Unlike construction materials, whose operating temperature is limited within the range between minus 50 °C and 70 °C, technical insulation can be used at temperatures above 500 °C, for example, for heat protection of pipes with superheated steam at a power plants. At this temperature, the immiscibility process with the subsequent crystallization begins in basalt and mineral glass fibers. Crystals act as a stress raiser that leads to mechanical properties degradation, embrittlement and material destruction [
1,
2]. The high vibration level accelerates this process [
3].
Different approaches, such as increasing the density of the materials, preliminary heat treatment, and the chemical composition change are used to increase the operating temperature of mineral and basalt fibers [
4,
5]. The easiest way is to add refractory oxides to the composition. The silicon oxide addition leads to an increase in the glass transition temperature and the application temperature. The iron oxides ratio in the basalt and mineral fibers composition has a distinctive influence on the crystallization process and thermal stability. It was shown in [
6,
7] that the preliminary reduction of Fe
3+ to Fe
2+ or even to metallic iron leads to an improvement in thermal properties.
Phosphorous glass and fiberglass materials are widely used in biomedical, optical and composite applications [
8,
9]. Phosphorus is an element incompatible with ferriferous silica-alumina systems. Crystallization of the phosphorous minerals, such as apatite or whitlockite, begins when the phosphorus concentration in the glasses reaches a few mass percent [
10]. In natural basalt magmas phosphorus content up to 2% is detected [
11].
Complex structural interactions between dissolved phosphorus and silicate components have a multidirectional effect on the viscosity of aluminosilicate glasses. In highly polymerized melt in the system KAlSi
3O
8–NaAlSi
3O
8–SiO
2, the viscosity decreases with an increase in the content of P
2O
5 [
12]. In basaltic melts, the opposite effect was observed [
10].
One of the first roles of phosphorus in silicate glass-forming systems was studied by Hess [
13], who found out that the binding phosphorus was energetically more beneficial to divalent metals in a melt than the formation of M–O–Si bonds. Based on the obtained results, a mechanism of interaction of phosphorus with a silicate network in melts was proposed: 1.5Si–O–M–O–Si + PO
2.5 ↔ 1.5Si–O–Si + M
1.5PO
4.
The addition of phosphorus oxide leads to decrease of the aluminosilicate melts density and the compressibility modulus [
13]. Phosphorus forms complexes that are supposed to be outside the silicate network. A large increase in compressibility, however, shows that the presence of incompressible tetrahedral phosphorus polyhedrons is responsible for further complex structural changes in glass structure [
12,
13].
In works [
14,
15], the authors studied the direct effect of phosphorus on the crystallization processes in basalt glasses. Various small concentrations of P
2O
5 were introduced into the basalt composition. The results confirmed the solubility of phosphorus in the basalt melt and showed that at a fixed temperature, the gradual addition of phosphorus caused: (1) the disappearance of olivine under reducing conditions, (2) the disappearance of magnetite under oxidizing conditions, (3) the stabilization of pigeonite in the entire fO
2 range studied, (4) an increase in the plagioclase/pyroxene ratio and (5) large changes in the composition of coexisting melts, in particular, the content of SiO
2. The destabilization of magnetite can be associated with the formation of Fe
3+(PO
4)
3− complexes.
Many authors [
14,
16] show that 8–9 mass %. phosphorus oxide content in aluminosilicate glasses does not lead to significant structural changes. Moreover, an increase in the phosphorus oxide content in basalt glasses leads to a change in the structural role of iron oxides. High phosphorus oxide addition triggers a structural collapse related to the immiscibility in a silicate melts with the formation of a Fe
3+Fe
2+-containing disordered/nano-sized oxide phase [
17,
18].
The research of phosphorus-containing silicate glass-forming systems is hampered by the strong dependence of the crystallization properties of glasses on their structure. A sufficiently large effect can be observed even at low concentrations of phosphorus oxide [
11].
While P
2O
5 is a low-melting oxide the increase in the silicate glasses refractoriness is often observed. The authors of work [
19] studying the properties of glasses in the Na
2O–CaO–SiO
2 system argue that the introduction of P
2O
5 additives leads to an increase in the polymerization of the structure, which is accompanied by an increase in high-temperature viscosity and a decrease in the glass transition temperature. These results are interpreted on the basis of the structural model of the mono- and diphosphate complexes formation and their incorporation into the vacancy of the silicate network.
The doping of the silicon-oxygen matrix with small P2O5 additives leads to steric distortions in glass structure and a decrease in its density. With a significant content of P2O5, aggregation of phosphate structures together with some matrix modifiers (primarily with Ca2+) can occur and the separation of the phosphate glass phase from the silicate (segregation) becomes preferable.
It was found [
20] that small additives of P
2O
5 (0.3 wt %) act as an inhibitor of crystallization of industrial silicate glasses. They can change the upper limit and the tendency of the glass to crystallize, without significantly changing the liquidus temperature, i.e., phase equilibrium in the glass. Probably, the effect of these additives is related to the effect on the kinetics of the crystallization process. At the same time, the conditions of crystallization of glasses depend on the content of P
2O
5 and not on the raw material that was used as a precursor.
Iron phosphate glasses are commonly characterized by O/P ratio indicating whether orthophosphate, pyrophosphate or metaphosphate units prevail in the structure [
21,
22]. Authors studied the mutual influence of the components in the P
2O
5–SiO
2–K
2O–MgO–CaO–Fe
2O
3 system in detail. The results obtained in this study confirmed that materials with lower iron oxide addition are more polymerized at room temperature. It was shown that with a rather significant variation in composition (from 2 to 30 mol % Fe
2O
3) the thermal properties detected in glasses are considerably minor.
The effect of phosphorus oxide on the structure and properties of aluminosilicate systems strongly depends on their composition, which makes it very difficult to study the same issue for basalt systems. The peculiarity of this work is not only the study of the influence of phosphorus oxide additives for the basalt systems suitable for the fibers production, but also the preparation of the basalt glasses in the form of fibers, i.e., at very high quenching speed, the study of their structure, crystallization capacity and thermal stability.
2. Materials and Methods
2.1. Preparation of Glass Fibers
Basaltic glass fibers with various P2O5 contents were produced in two stages. The first stage included obtaining bulk glasses. The bulk glasses were prepared by adding (NH4)4P2O7 to milled andesitic basalt from the Sil’tsevskoe deposit (Carpathians, Ukraine).
Batch of mixture placed in a platinum crucible was heated in a high-temperature furnace at a rate of 250 °C/h to 1200 °C and at 50 °C/h in a range 1200–1600 °C and then held at 1600 °C for 20 h. The bulk glass was quenched from 1873 to 298 K by rapid pouring the melt into water. Mixtures of basalt with 3.4, 6.7 and 10.0 wt %, (NH4)4P2O7, respectively, were prepared to obtain glasses containing 2, 4, and 6 wt % P2O5 (P-BS2, P-BS4, P-BS6).
Basalt continuous fibers (BCF) were produced using prepared bulk glass and a laboratory-scale system [
7]. BCF were drawn from melts and wound onto a take up reel. A fiber diameter was controlled by varying the rotation rate of the reel. In subsequent investigation, we used fibers that are 13 μm in diameter. The fiber drawing speed was about 800 m/min, the diameter of the nozzle was 2.2 mm.
2.2. X-ray Fluorescence Analysis
X-ray fluorescence analysis of the specimens was performed on a PAN Analytical Axios Advanced spectrometer (PANalytical B.V., Almelo, The Netherlands). Characteristic X-rays were excited using a 4 kW Rh-anode X-ray tube. The excited radiation was recorded by a wavelength dispersive spectrometer (PANalytical B.V., Almelo, The Netherlands) with five exchangeable analyzer crystals (PE_002_C, PX_1, GEIII_C, LIF_200, LIF_220) and a detector (flow proportional and scintillation). Measurements were made in transmission geometry in vacuum.
The concentration of elements was expressed as weight percentage (wt %) of the corresponding oxides. The accuracy of XRF determinations of elements was controlled by analyses of standard samples OOKO 301 and OOKO 201 (State certified reference material 5358-90/5367-90) and was within 0.6% for SiO2, within 0.4% for Al2O3, within 0.2% for Fe2O3, within 0.3% for CaO, within 0.1% for MgO, within 0.1% for K2O, within 0.2% for Na2O, within 0.1% for TiO2, within 0.3% for P2O5.
2.3. X-ray Diffraction
X-ray diffraction (XRD) was performed at room temperature on Thermo ARL X’TRA powder diffractometer (CuKα1 radiation, λ = 1.54060 Å). XRD patterns were collected in an angular range 2θ = 10°–60° at a scan step 2θ = 0.02° and a scan rate of 1°(2θ)/min. The fiber samples were ground into powder by means of an agate mortar. The powder X-ray diffraction patterns of the prepared samples were verified using the JCPDS PDF-2 database.
2.4. Thermal Analysis
Coupled DSC–TG measurements were carried out using NETZSCH STA Jupiter 449 C (NETZSCH-Geratebau GmbH, Selb, Germany). The samples of fiber with a mass of 30–40 mg were twisted and placed in a platinum crucible. After that, the samples were additionally tamped in a crucible. Measurements were provided at heating rate 10 °C/min within the temperature range of 40–1100 °C in air with a constant flow of 50 mL/min. The temperature and the sensitivity of the STA were calibrated against a Netzsch standard kit. Recording of a baseline with empty crucibles was performed for each heating rate. DSC curves were processed using NETZSCH Proteus Analysis software (7th generation, NETZSCH-Geratebau GmbH, Selb, Germany). The glass transition temperature was determined as a midpoint temperature according to ASTM E 1356.
2.5. Raman Analysis
Raman spectra were obtained from the surface of fibers (micro Raman system, Renishaw 1000, Renishaw plc, Wotton-under-Edge, UK) using the 514 nm line Ar laser at about 40 mW for excitation. Raman scatter was collected by means of a microscope (Leica Microsystems Wetzlar GmbH, Wetzlar, Germany). Each spectrum was recorded with a resolution of 1 cm−1. Focal length was 250 mm. Focusing was carried out on the surface of the fibers. Calibration was carried out using a standard sample—single-crystal silicon (520 cm–1).
2.6. Density Measurement
The hydrostatic weighing was used for the determination of glasses density at room temperature. A few fragments of each glass sample were weighed in air and water. The density values were determined using the following Equation:
where
Wa is the weight of the sample in air,
Wb is the weight of the sample in water, and
ρb is the density of water. The density of water was 999.5 kg/m
3. Measurements were taken five times to obtain the most precise density values. The overall accuracy of these measurements was ±0.5 kg/m
3 and the error in measurement was ±0.05%.
3. Results
3.1. Preliminary Characterization of Basaltic Glass Fibers
The criteria for choosing a source of phosphorus in basalt glasses was high chemical purity, relatively low hygroscopicity and its complete decomposition, which would not lead to a change in the ratio of the cations composition in glass as a result of glass melting. Therefore, anhydrous ammonium pyrophosphate was chosen as a precursor. The X-ray pattern of the reagent is shown in
Figure 1. All reflexes correspond to the composition (NH
4)
4P
2O
7 (ICDD No. 11-634).
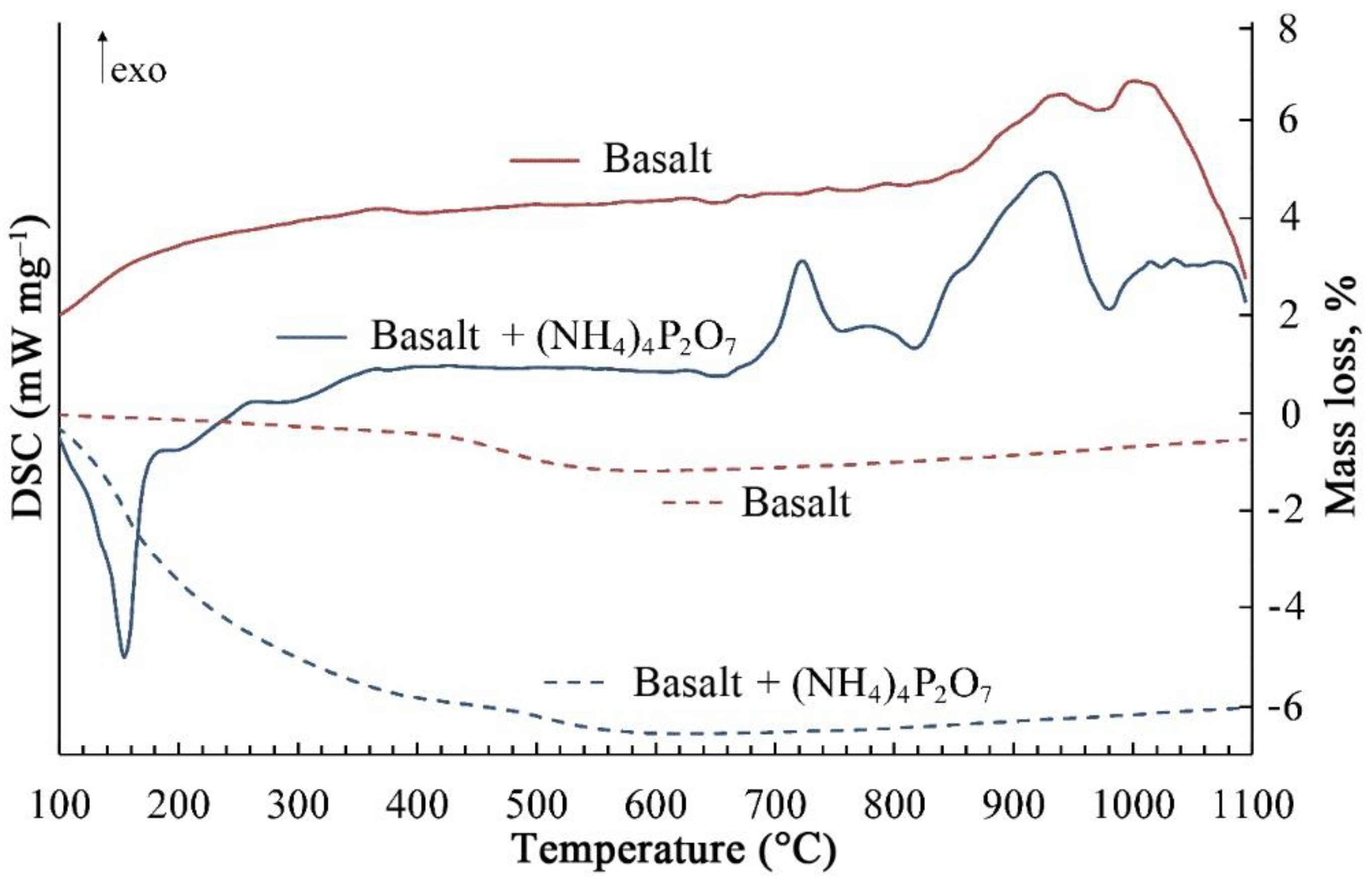
In order to study the decomposition of ammonium pyrophosphate upon heating and its interaction with basalt rock, we performed differential thermal analysis of basalt mixture with 16.1 wt % (NH
4)
4P
2O
7 (which corresponds to 10 wt % in terms of phosphorus pentoxide). The DSC and TG curves of the mixture in comparison with pure basalt are presented in
Figure 2.
The endothermic effect on the DSC curve at 150 °C corresponds to the ammonium pyrophosphate decomposition by the reaction:
As a result, the weight loss of the mixture sample was 5.4%, which corresponds to the value calculated theoretically (5.6%). Further heating leads to an increase in the degree of polymerization of metaphosphoric acid.
At temperatures above 700 °C, it interacts with the basalt mixture, as evidenced by exothermic effects in the temperature range of 700–1000 °C, which are not observed when heating pure basalt rock. The chemical compositions of natural basalt glass fiber (BS) and of the phosphorous-containing glass fibers (P-BS2, P-BS4, and P-BS6) were determined by X-ray fluorescence analysis, and data are shown in
Table 1. All iron is reported as Fe
2O
3tot.
The fibers were also investigated by XRD analysis. The obtained X-ray diffraction patterns (
Figure 3) indicate that all samples are X-ray amorphous
3.2. The Structure of Phosphorus-Containing Glass Fibers
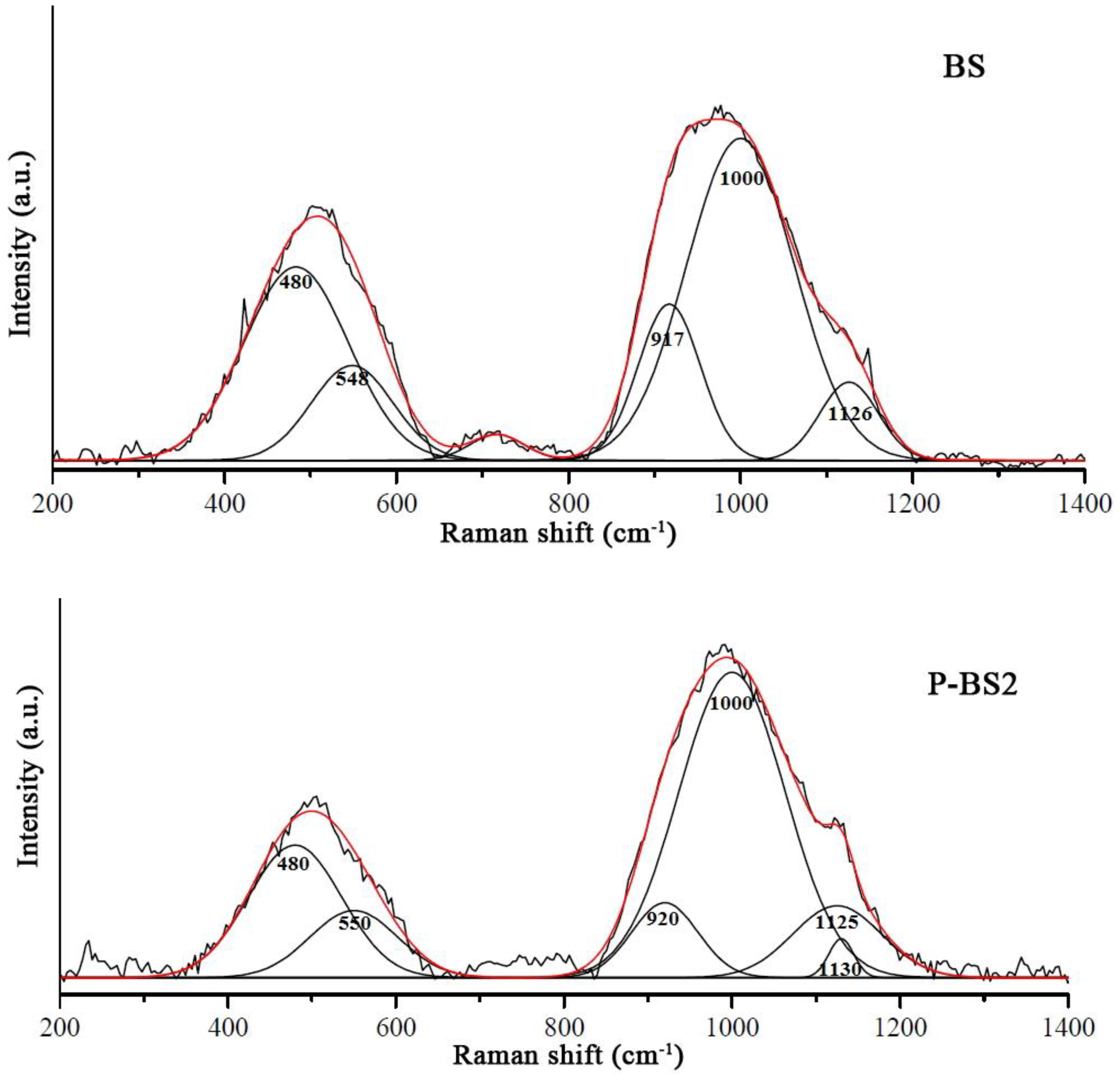
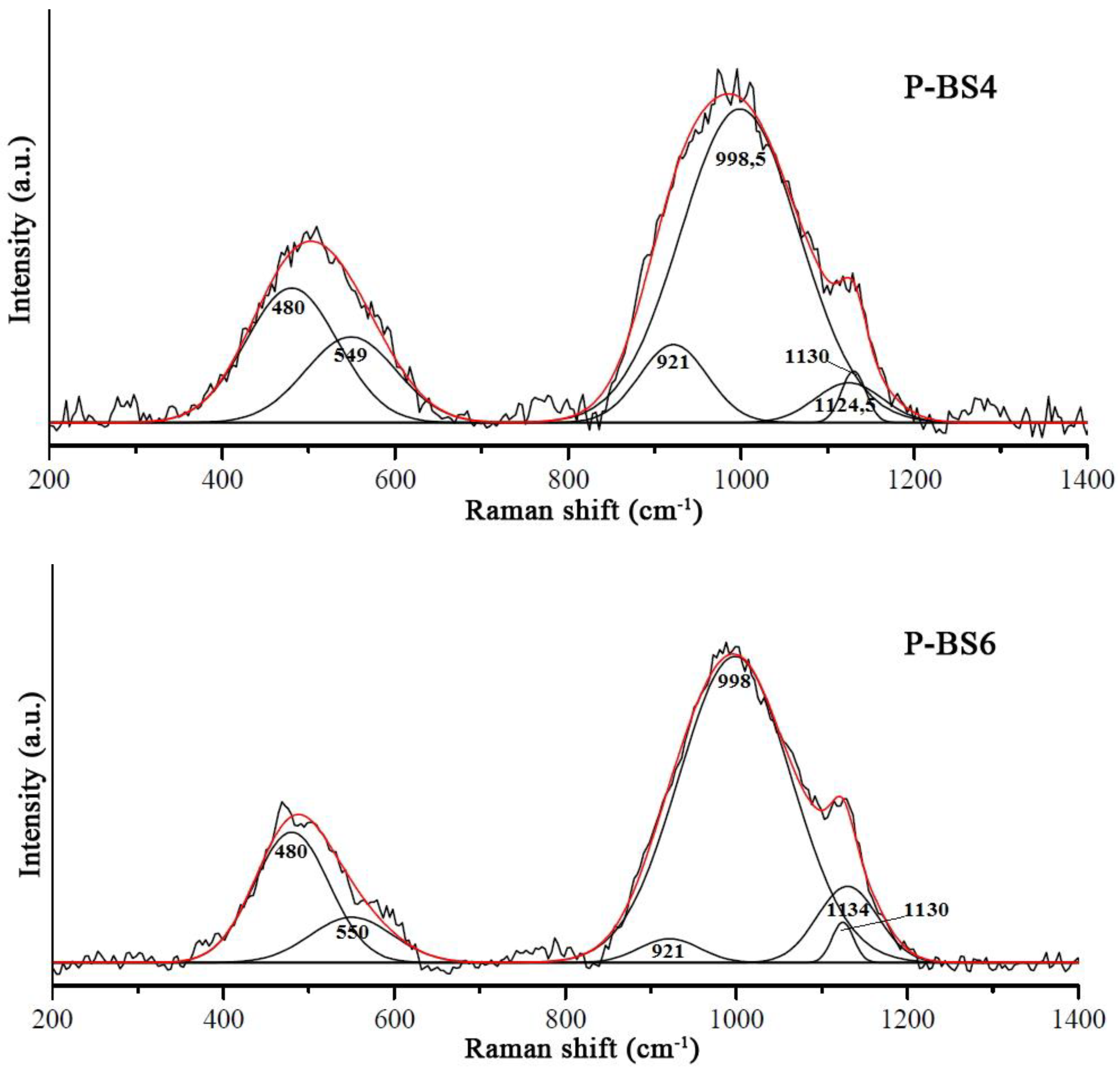
The structure of glasses doped with phosphorus oxide was studied by Raman spectroscopy. The obtained Raman spectra are shown in
Figure 4. The decomposition of the spectra into Gaussian was carried out based on the published data [
23,
24]. During the decomposition, the position of the bands was fixed, varying in the half-width and peak amplitudes. Two regions can be distinguished in all spectra: low-frequency (350–650 cm
−1) and high-frequency (800–1200 cm
−1).
The low-frequency region of the Raman spectrum is a superposition of several types of oscillations. The most characteristic band in this region is the symmetric stretching vibrations of O–T–O bonds corresponding to T cations (in this case T=P or Si) in a tetrahedral oxygen environment. Less often, the bands in this region are attributed to oscillation in Q
4 structural units: vibrations of the T atom in the tetrahedral environment with a small oxygen displacement, T–O–T bending in structural units with non-bridge oxygen (O
–), and also breathing modes of four- and three-membered rings of TO
4 tetrahedra [
14,
25].
For basalt glass structure, there is a wide band of about 480–540 cm−1 due to symmetric stretching and partially bending vibrations of the Si–O–Si (Si–O0) bridges. Most bands in the high-frequency region can be attributed to Si–O– and Si–O0 vibrations for various structural units in basalt glass. The intensity of the bands responsible for Si–O– systematically decreases with the increasing degree of polymerization.
The band at 1125 cm
–1 is associated with vibrations of non-bridging Si–O
– bonds in Q
3 structural units, which are fragments of silicon-oxygen sheet structures and are SiO
4 tetrahedra with three bridging and one non-bridging oxygen atoms (Si
2O
52–) [
23,
24]. In addition, a band is present in the region of 920 cm
−1 due to the presence of relatively depolymerized structural units Q
2 with two non-bridging oxygen atoms (SiO
32−).
The band with a maximum near 800 cm–1 is associated with deformation vibrations of Si–O–Si bonds in the glass local regions, whose structure is close to the structure of glassy SiО2 and is constructed from Q4 units. These fully polymerized units with all bridging bonds do not have characteristic bands in obtained spectra. Also, for all structural units containing bridge oxygen, a band of about 1000 cm–1 appears.
3.3. Crystallization Properties of Phosphorus-Containing Glasses
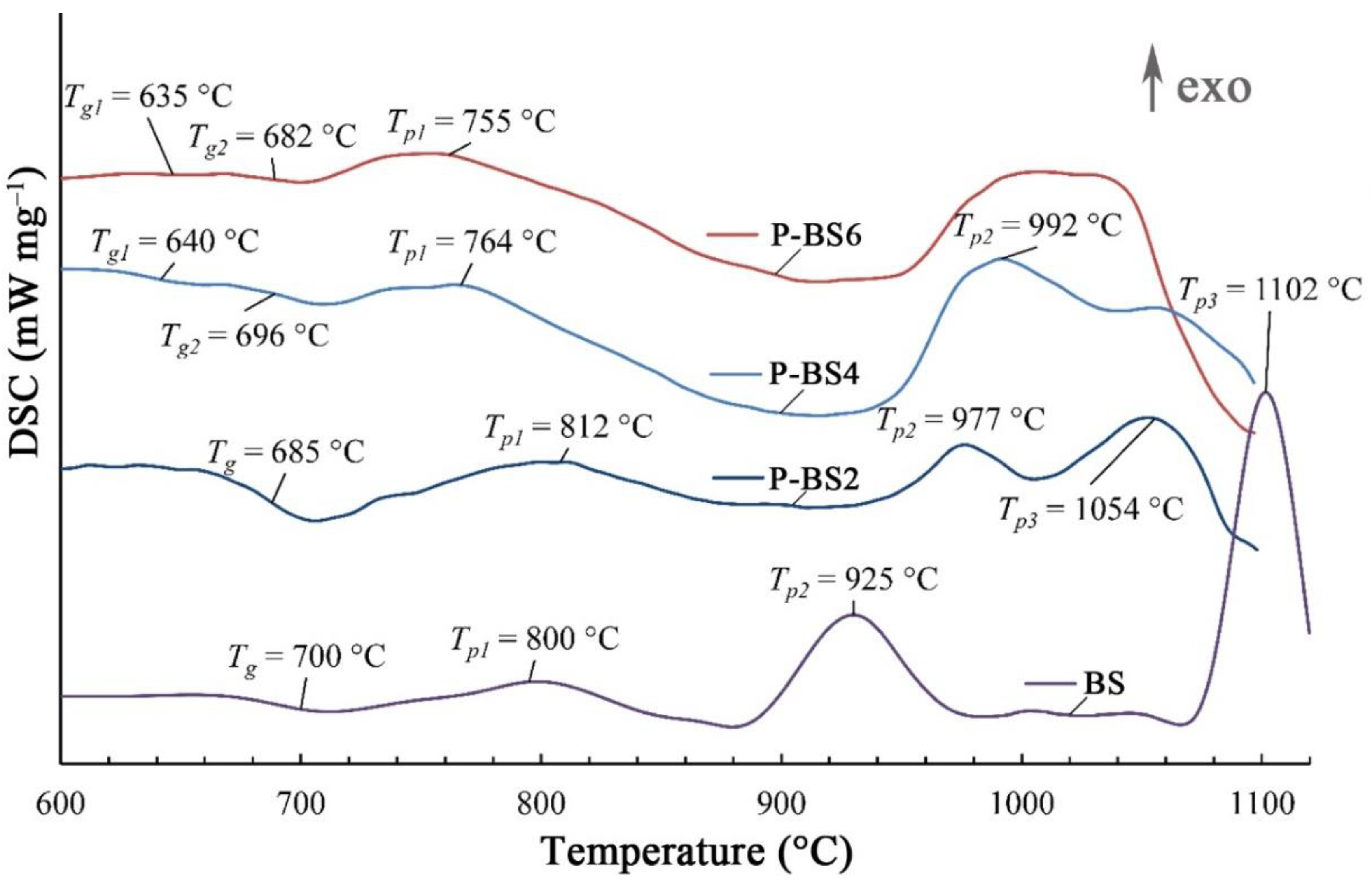
The thermal properties of phosphorus-containing glasses were investigated by differential scanning calorimetry. Thermoanalytic curves are shown in
Figure 5.
The introduction of 2 wt % of P
2O
5 decreases the glass transition temperature from 700 °C to 685 °C compared to the initial composition (BS). The exothermic effects of
Tp1 and
Tp2 for the P-BS2 glass are shifted towards higher temperatures. An increase in the content of P
2O
5 from 2 to 6 wt % leads to a shift in the exothermic effects (
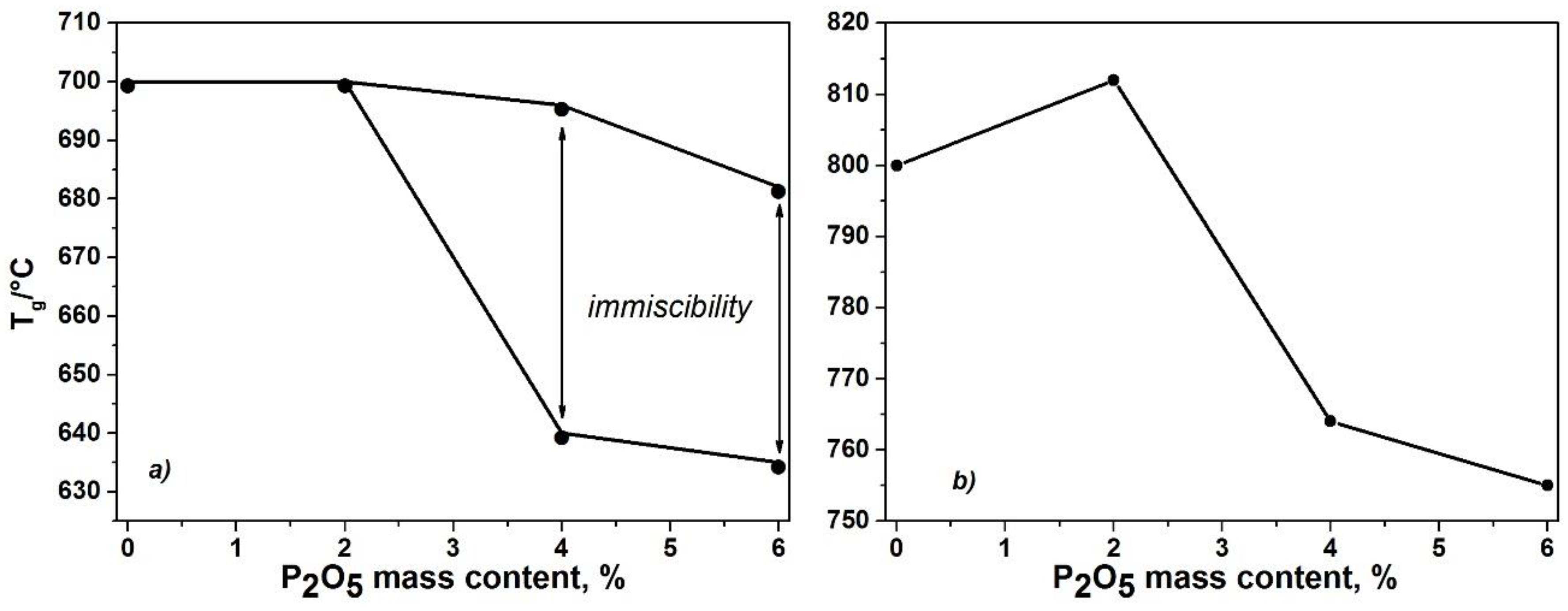
Tp1) to lower temperatures which is due to crystallization of the nucleating phase. For the samples P-BS4 and P-BS6, the double endothermic effect on the DSC curves can be interpreted as two glass transition temperature (
Tg1 and
Tg2), that appear due to liquid-phase separation (
Figure 6).
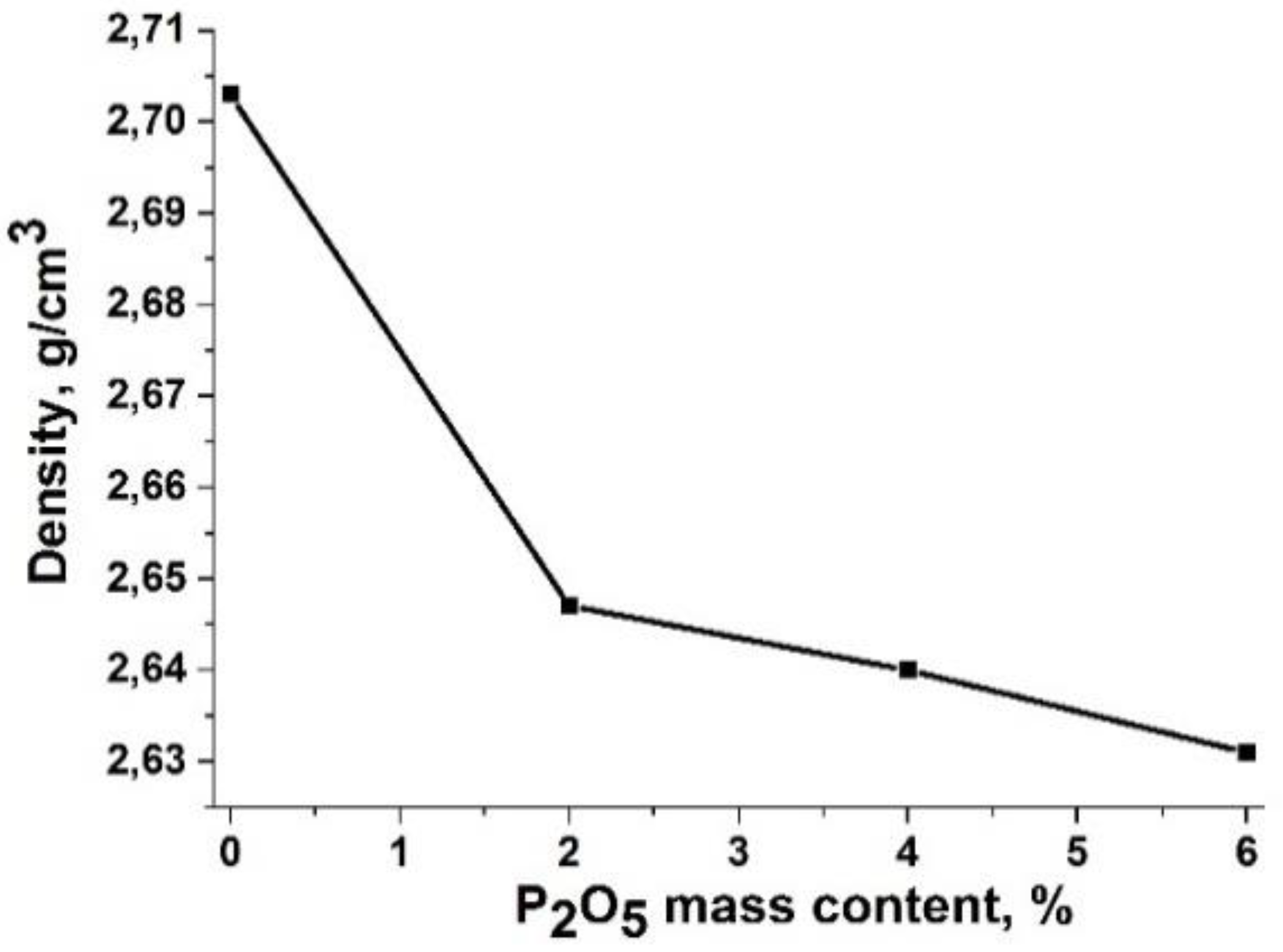
An increase in the content of P
2O
5 to 6 wt % leads to a decrease in the density of glasses (
Figure 7), which may be due to the polymerizing effect of phosphorus oxide, which binds a certain amount of divalent modifier ions into M
3(PO
4)
2 complexes and is integrated into the aluminosilicate framework. This preparation is confirmed by the literature data obtained for similar compositions [
15,
21].
An XRD analysis of glasses thermally treated in air at temperatures of 700, 800, 900 and 1000 °C for 24 h was carried out to determine the sequence of phase transformations. The diffraction patterns of the annealed samples are shown in
Figure 8,
Figure 9,
Figure 10 and
Figure 11.
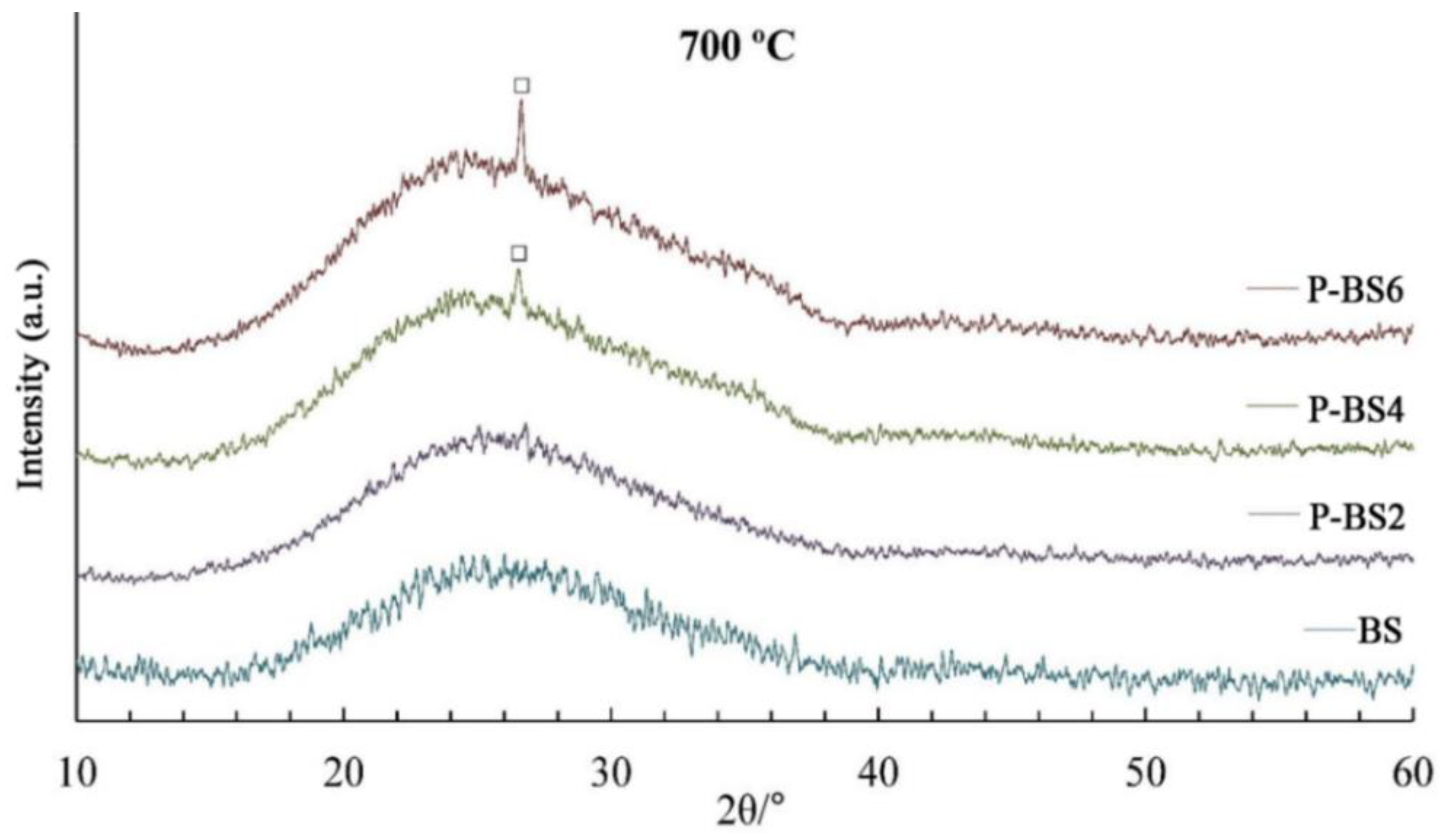
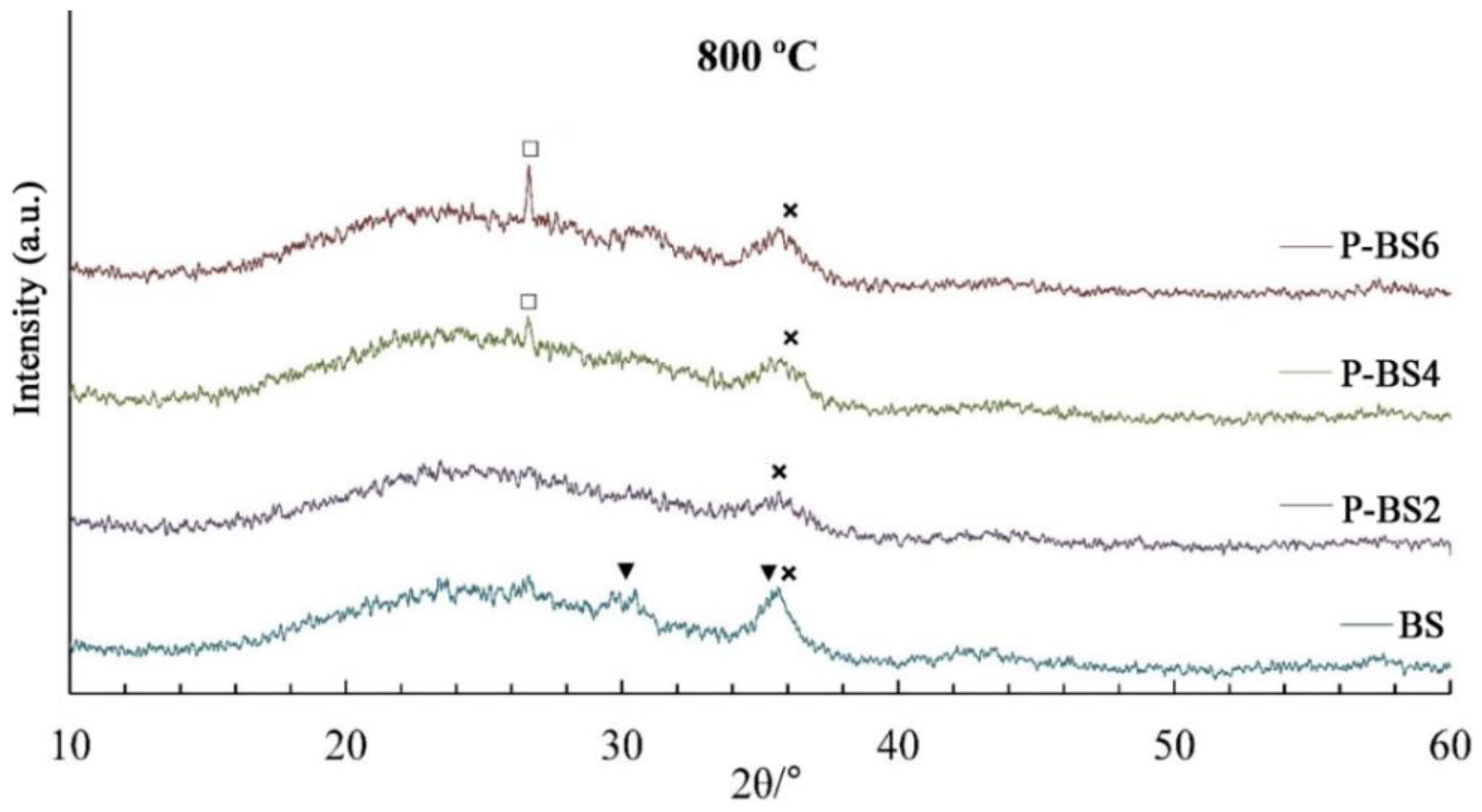
Samples P-BS2 and P-BS6 remain amorphous at 700 °C, while a single peak of quartz appears for basalt glass fibers with 4 and 6 wt % P
2O
5 (ICDD No. 85-798). Based on literature data [
13], it was suggested that, due to its high electronegativity, phosphorus replaces silicon in bonds with divalent metals:
Heat treatment at 800 °С leads to the appearance of spinel reflexes in the diffraction patterns of samples with 0–6 wt % P2O5. It is worth noting that for P-BS2, the formation of the crystalline phase is manifested to a lesser extent, which may indicate a better high-temperature stability of this composition.
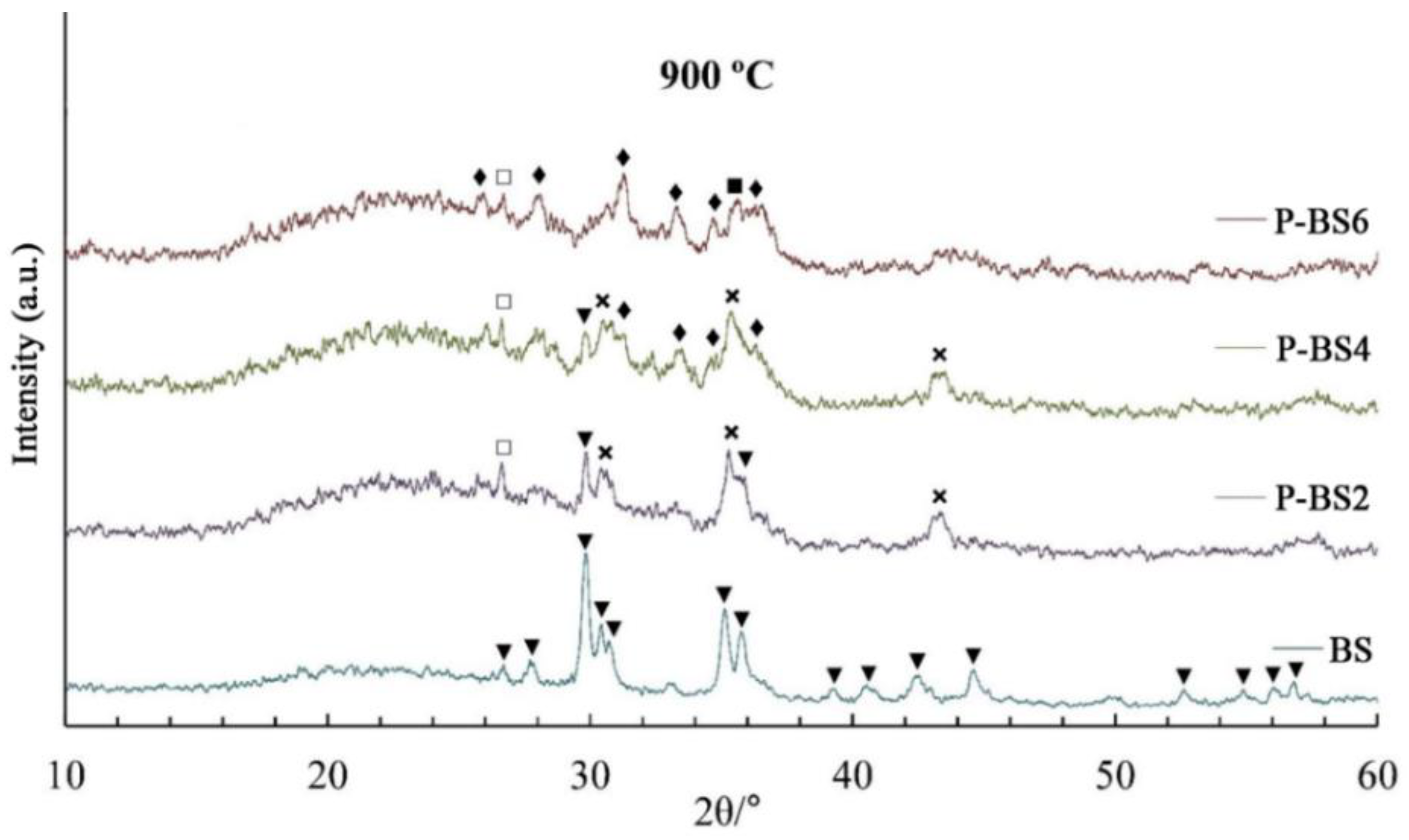
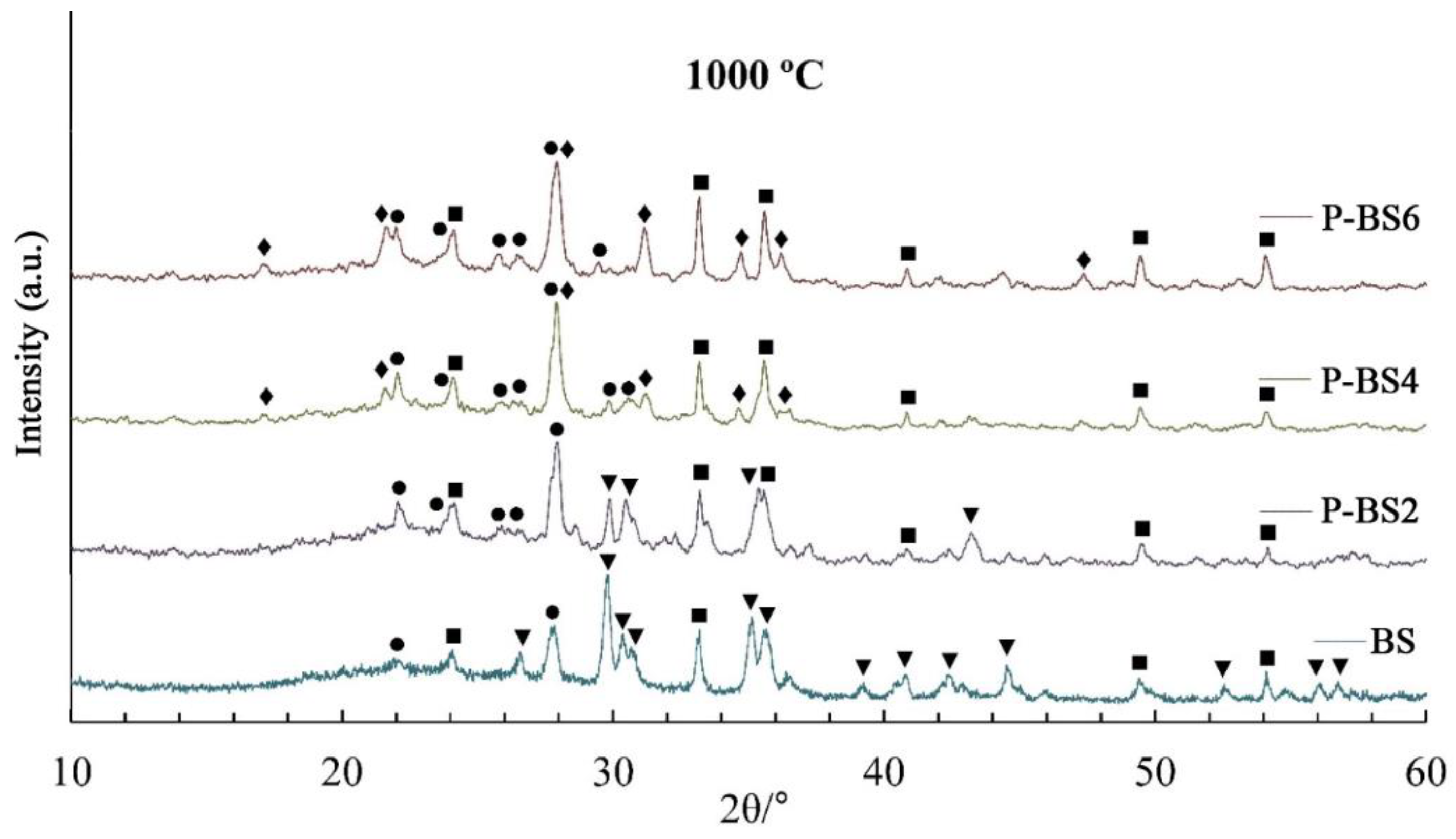
In natural basalt glass fiber annealed at 900 °C, the main crystalline phase is common pyroxene mineral with C2/c space group ((Mg, Fe, Al, Ti) (Ca, Mg, Fe, Na) (Si, Al)2O6, ICDD No. 88-856). The content of pyroxene phase in the annealed fiber decreases with an increase in the concentration of phosphorus oxide. In the diffraction pattern of the P-BS6 chain silicate reflexes are completely absent. At the same time, in the samples P-BS4–P-BS6 there is crystallization of the whitlockite phase (Ca3(PO4)2, ICDD No. 9-169) and hematite (α-Fe2O3 ICDD No. 89-598). High hematite content in annealed fibers may allude to pyroxene destabilization with the introduction of phosphorus oxide. As a result of heat treatment at 1000 °С in P-BS2–P-BS4 fibers, the main crystalline phases are plagioclase ((Na, Ca) Al (Si, Al)3O8, ICDD No. 41-1480) and hematite.
4. Discussion
There are several types of silicate-phosphate interactions in silicate glasses. For peralkaline melts, the phosphate species become more polymerized and the silicate species become less polymerized. The following interactions occur in phosphate aluminosilicate glasses with increasing temperature: 2PO
4 + Q
4Si ↔ P
2O
7 + Q
3Si, P
2O
7 + 5Q
4Si↔ 2Q
1P + 3Q
3Si, where Q
nSi denotes speciation of silicate and Q
nP speciation of phosphate [
14]. In the Raman spectrum, the Raman bands assigned to P-O stretch vibrations are distinct because of mass differences between Si and P and the P-O force constant depends on the next-nearest cation Si or P [
16].
Despite the basalt glasses are multicomponent aluminosilicate systems, we believe that similar processes are observed for the studied compositions.
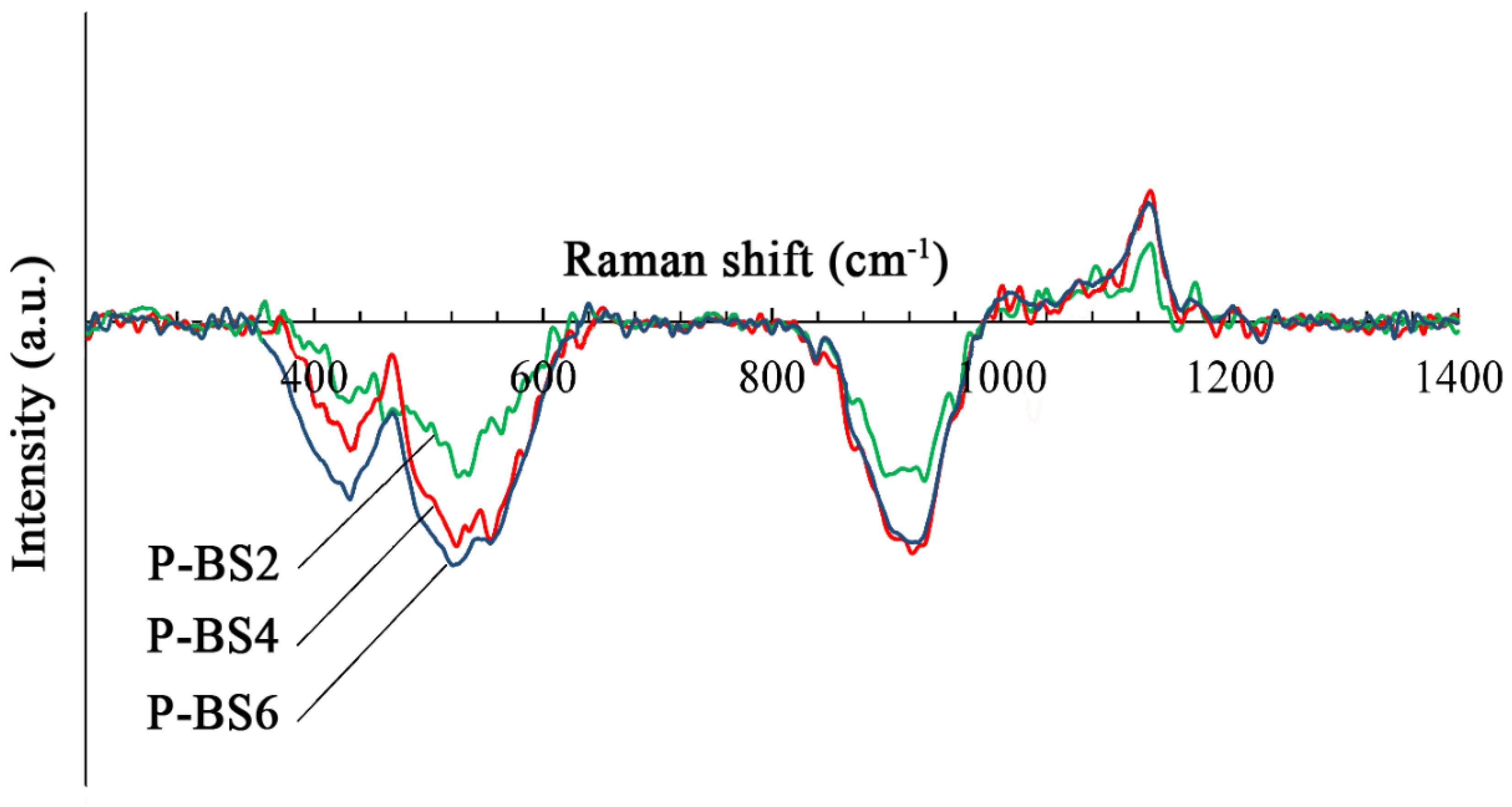
Figure 12 shows curves obtained by subtracting the spectrum of the natural basalt glass fiber (BS) from the Raman spectra of phosphorus-containing glass fibers (P-BS2, P-BS4, P-BS6).
The band intensity of about 920 cm
−1 decreases with increasing phosphorus oxide content, while the band intensity of about 1140 cm
−1 increases. This indicates an increase in the number of structural units Q
3 in comparison with Q
2 whence it follows that the degree of structure polymerization increases. The band observed at 920–930 cm
−1 is attributed to the symmetric vibrations in P–O links of orthophosphate groups (PO
4) [
8,
15]. The band around 1140 cm
−1 is attributed to the symmetric stretching mode of O–P–O non- bridging oxygen, (PO
2)
sym., in phosphate tetrahedral [
8].
With the introduction of P
2O
5, a new band appears in the region of 1130 cm
−1 and its intensity increases with an increase in the concentration of P
2O
5 in glasses. This is probably due to the imposition of a new band on the characteristic region of structural units Q
3, which can be attributed to symmetric stretching vibrations of non-bridging oxygen atoms in the orthophosphate tetrahedra PO
43− [
13]. The band in this region is characteristic, for example, for apatite containing the PO
43− group [
26], and its appearance indicates the crystallization of calcium orthophosphate in glass.
As was noted in the literature [
10], phosphorus oxide added in small concentrations to iron-containing aluminosilicate glasses leads to an increase in the degree of polymerization of their structure and can act as a crystallization inhibitor by binding divalent modifier ions. Based on literature data [
13,
27], we planned that the addition of small amounts of phosphorus oxide to the natural basalt glasses composition will inhibit the crystallization process, which would increase the basalt fibers application temperature.
The obtained results of DSC and XRD analysis confirmed the prospect of our approach. Earlier in our works, we studied in detail as the sequence of phase transformations for basalt glasses suitable for continuous fiber production [
1], as the kinetics of the crystallization process [
28]. It is important to note the special role of iron cations in the initialization of this process [
7,
29].
The thermal analysis data allow us to make the assumption that the most thermally stable sample is a sample containing 2 wt % phosphorus oxide. The glass transition temperature of P-BS2 varies slightly, which corresponds to literature data [
21,
22]. At the same time, the position of the exothermal peak corresponding to the crystallization process shifts to the higher temperature (
Figure 6). With an increase in the content of phosphorus oxide in the samples, the ability to crystallize increases, which, in our opinion, can be related to immiscibility in glasses [
30].
The data of XRD of the annealed samples confirm this assumption (
Table 2). The crystallization in natural basalt glass and fibers begins with spontaneous spinel-like phase formation, which is favored by the initial liquid-liquid separation; the spinel-like crystals become nucleation sites for the precipitation of a major phase with pyroxene structure. The kinetics of crystallization in bulk glasses was interpreted as a three-dimensional diffusion-controlled pyroxene-like formation on a fixed number of nuclei [
1,
31]. The quantitative ratio of plagioclase/hematite decreases with an increase in the content of phosphorus oxide.
As noted in [
16] the effect of phosphate doping silicate glasses on the crystallization processes that occur during heat treatment depends on both the concentration of P
2O
5 and the chemical composition of the glass, and therefore is a complex subject of study. Indeed, the interpretation of the diffraction patterns of annealed phosphorus-containing fibers is very difficult due to the large number of reflections of possible crystallization products.
As a result of annealing fibers containing up to 6 wt % P
2O
5 at 800 °С, the spinel phase crystallizes in all samples, which contradicts the results of [
10], according to which the formation of iron phosphate complexes FePO
4 upon the introduction of phosphorus oxide into glasses prevents the formation of spinel-like phases. With an increase in the P
2O
5 content of more than 4 wt %, there is a tendency to a decrease in the proportion of pyroxene in samples P-BS4 and P-BS6 after thermal treatment at temperatures higher than 900 °C for 24 h. The iron-containing phase (hematite) crystallizes in P-BS6 sample even after treatment at 900 °C. Thus, the introduction of large quantities of P
2O
5 leads to a strong change in the crystallization process of the fibers.
This effect is accompanied by a change in the ratio of Fe
3+/Fe
2+ cations in basalt glasses with the addition of phosphorus. The coordination of iron cations is also changed [
18]. At P
2O
5 contents lower than 8% iron is located in the glass structure and the Fe
3+/Fe
2+ ratio is about 80/20. An increase in the content of phosphorus oxide leads to a change in the structure of the glass, associated with a phase change, which causes crystallization of the more reduced iron-containing phosphate phase with a crystalline structure similar to that of Ca
4Mg
5(PO
4)
6.
The addition of P2O5 from 4 to 6 wt % activates the microsegregation processes in the glass, accompanied by the formation of amorphous phases with different viscosities. This is also evidenced by double endothermic effects on thermoanalytical curves. The increase in the immiscibility of phases with an increase in the content of phosphorus oxide can be the cause of an increase in the defectiveness of the structure of the fibers during their heat treatment, which leads to a deterioration in their thermal stability.
5. Conclusions
The target of the research was the effect of phosphorus oxide on the structure and properties of basaltic glass fibers. The basaltic glass fibers with a different P2O5 content (within the range of 2 and 6 mass %) were obtained using a laboratory-scale system. The obtained X-ray diffraction patterns indicate all samples are X-ray amorphous.
The introduction of 2 mass % of P2O5 decreases the glass transition temperature from 700 to 685 °C compared to the initial composition (BS). An increase in the content of P2O5 from 2 to 6 wt % leads to a shift in the exothermic effects to lower temperatures which is due to crystallization of the nucleating phase.
In the Raman spectra of obtained basaltic glass fibers, the decrease in the intensity of the line corresponding to vibrations of the structural units Q2 (about 920 cm–1) with respect to the line corresponding to Q3 (about 1125 cm–1) is related to an increase in P2O5 contents and indicates also an increase in the polymerization degree of the glass structure. This is also confirmed by a decrease in the density of the resulting basaltic glass fibers upon the addition of phosphorus oxide.
The crystallization in natural basalt glass and fibers begins with spontaneous spinel-like phase formation, which is favored by the initial liquid-liquid separation. With an increase in the P2O5 content of more than 4 wt% the pyroxene and hematite crystallization phase content is enhanced. The quantitative ratio of plagioclase/hematite decreases with an increase in the content of phosphorus oxide.
Thus, the totality of the data on the structure of glasses obtained by Raman spectroscopy and DSC-TG analyses indicates the prospect of using the proposed approach to obtain basalt glass fibers with enhanced thermal and mechanical stability.