Influence of High Conductive Magnetite Impurity on the Electrical Conductivity of Dry Olivine Aggregates at High Temperature and High Pressure
Abstract
1. Introduction
2. Experimental Procedures
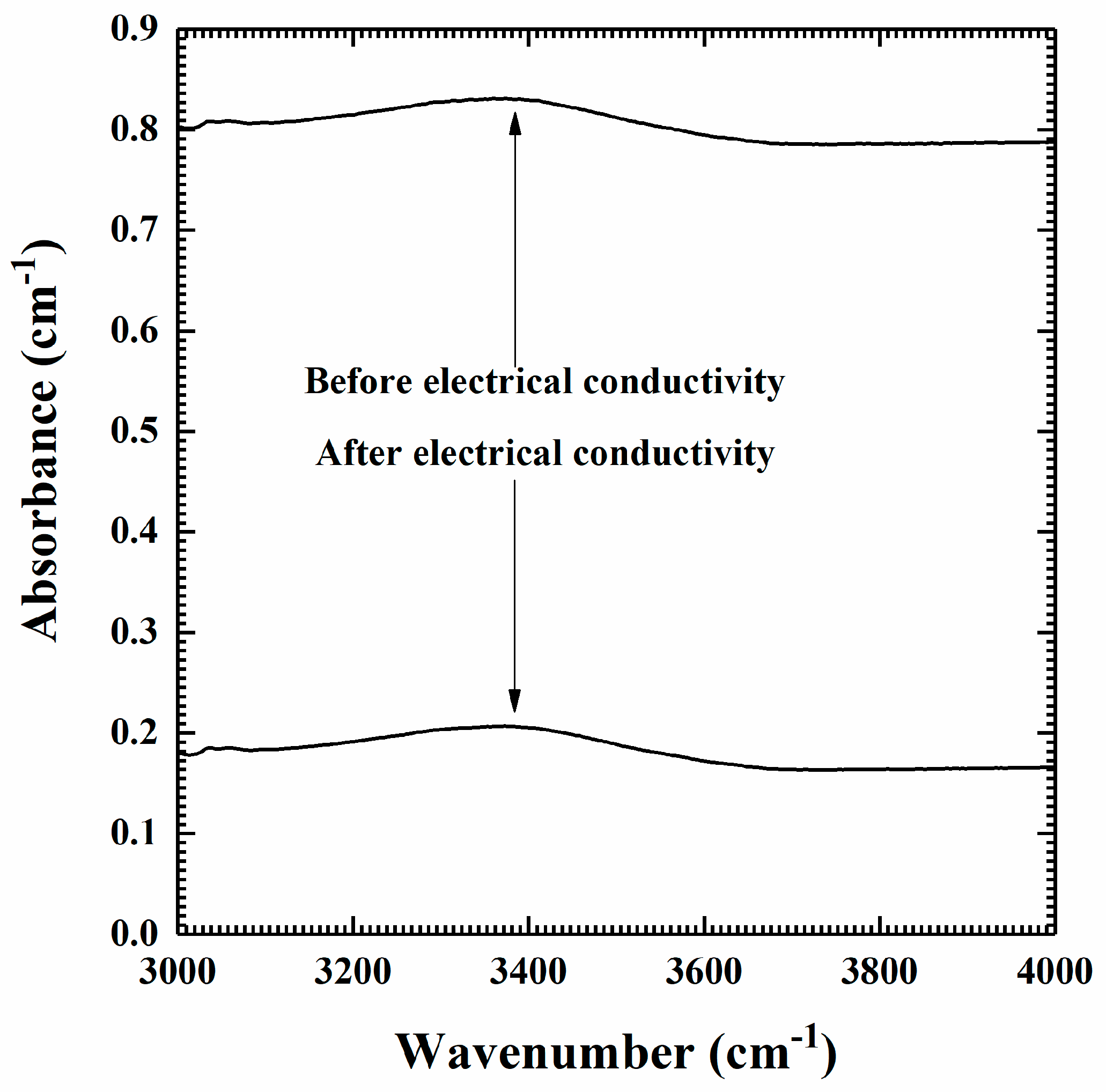
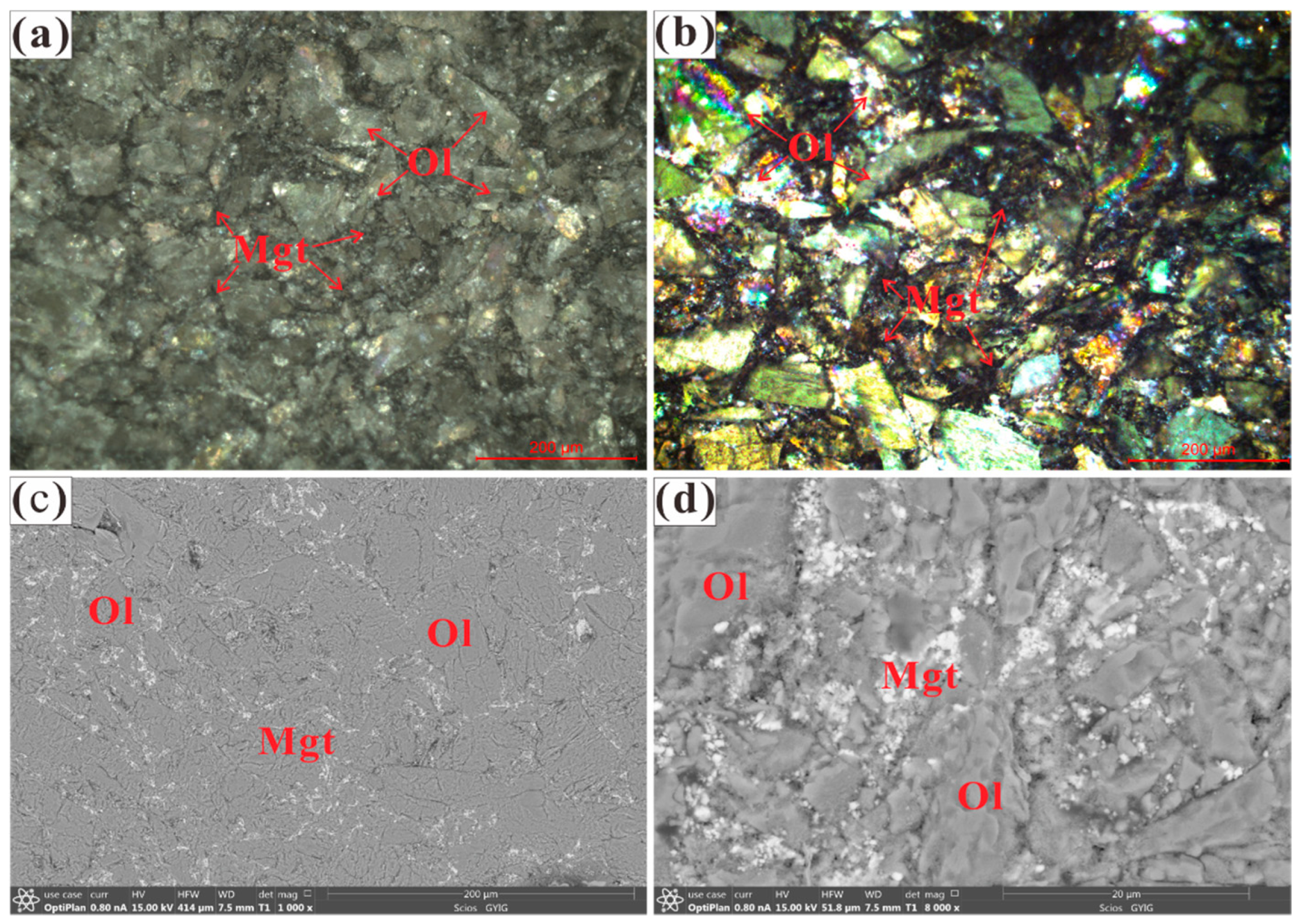
2.1. Sample Preparation and Characterization
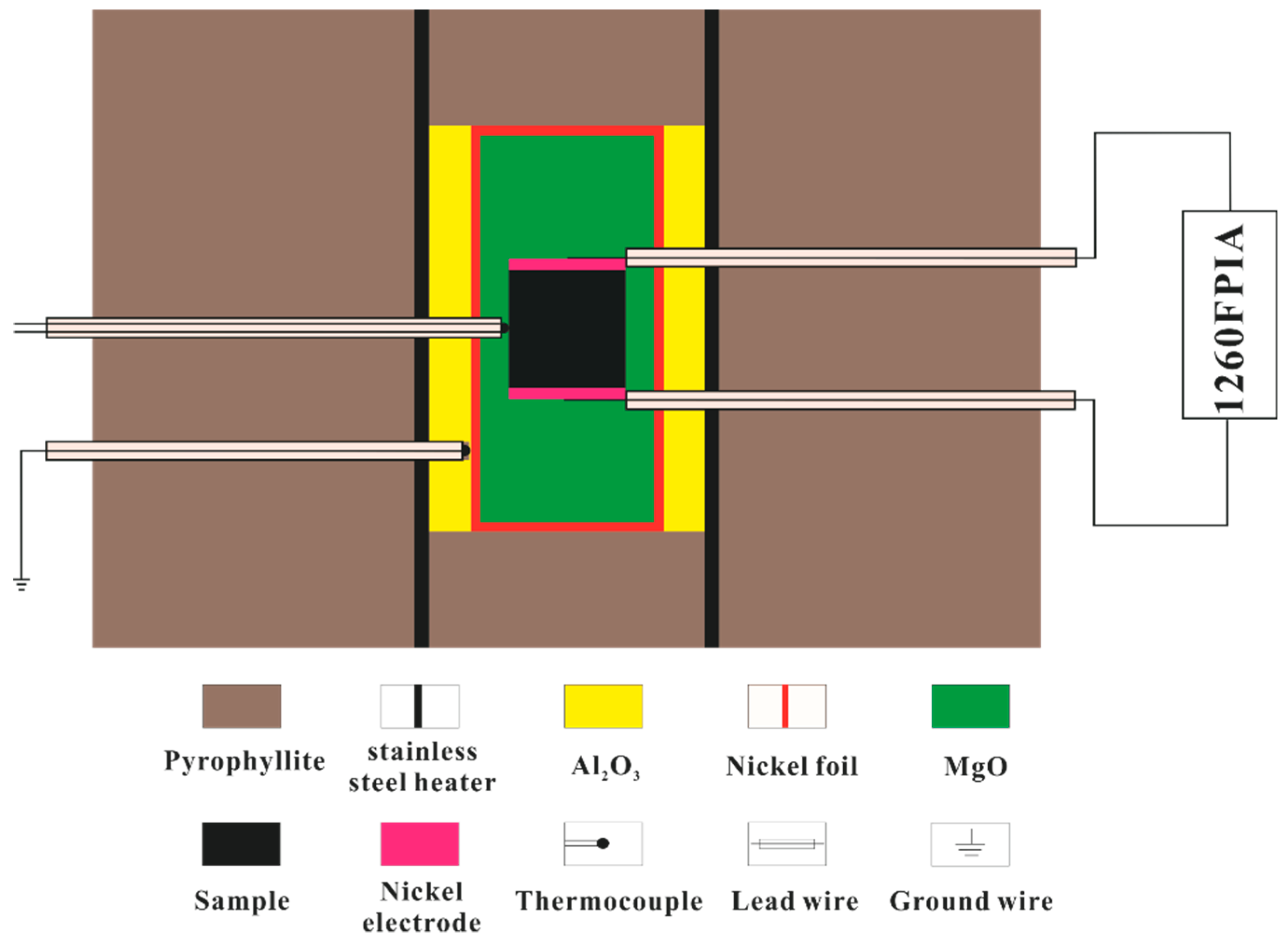
2.2. High-Pressure Cell and Impedance Measurements
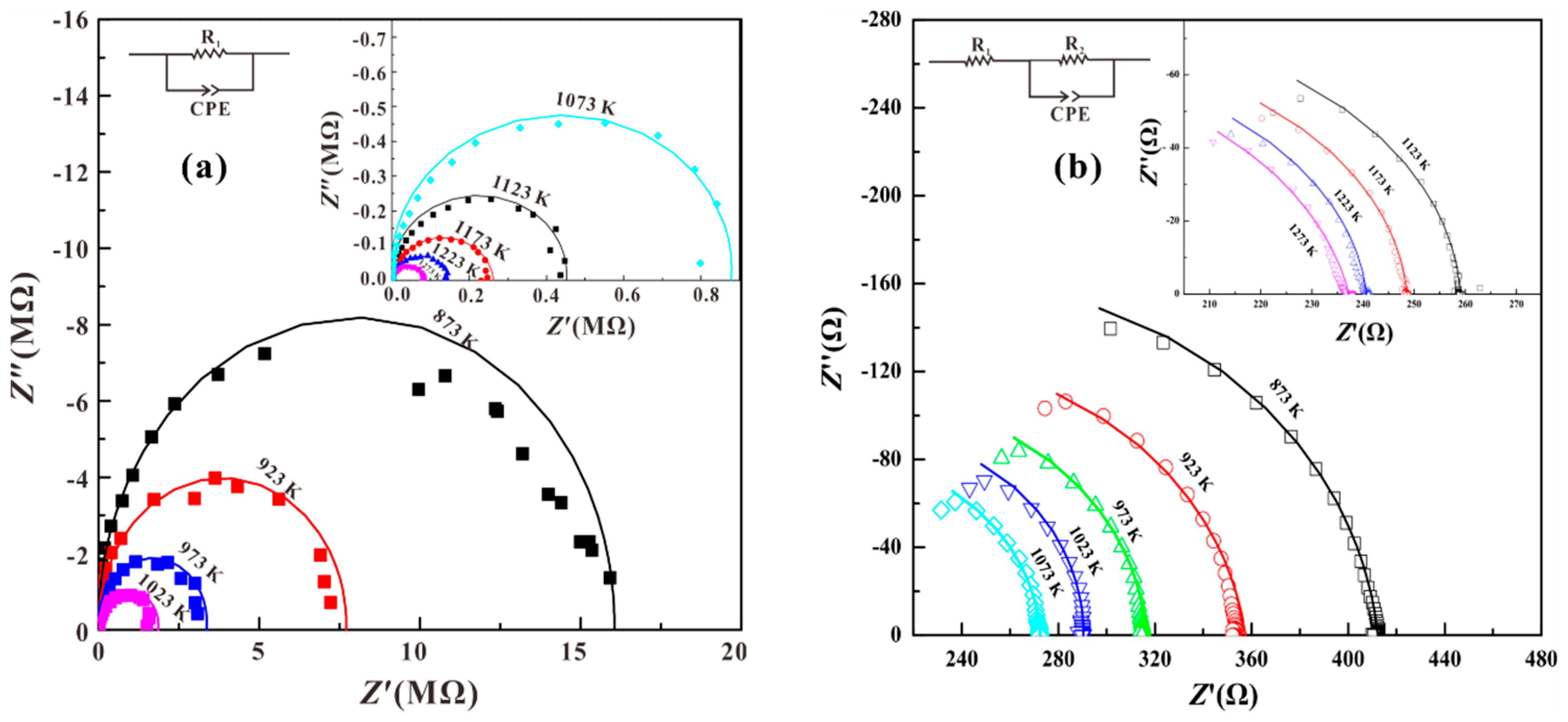
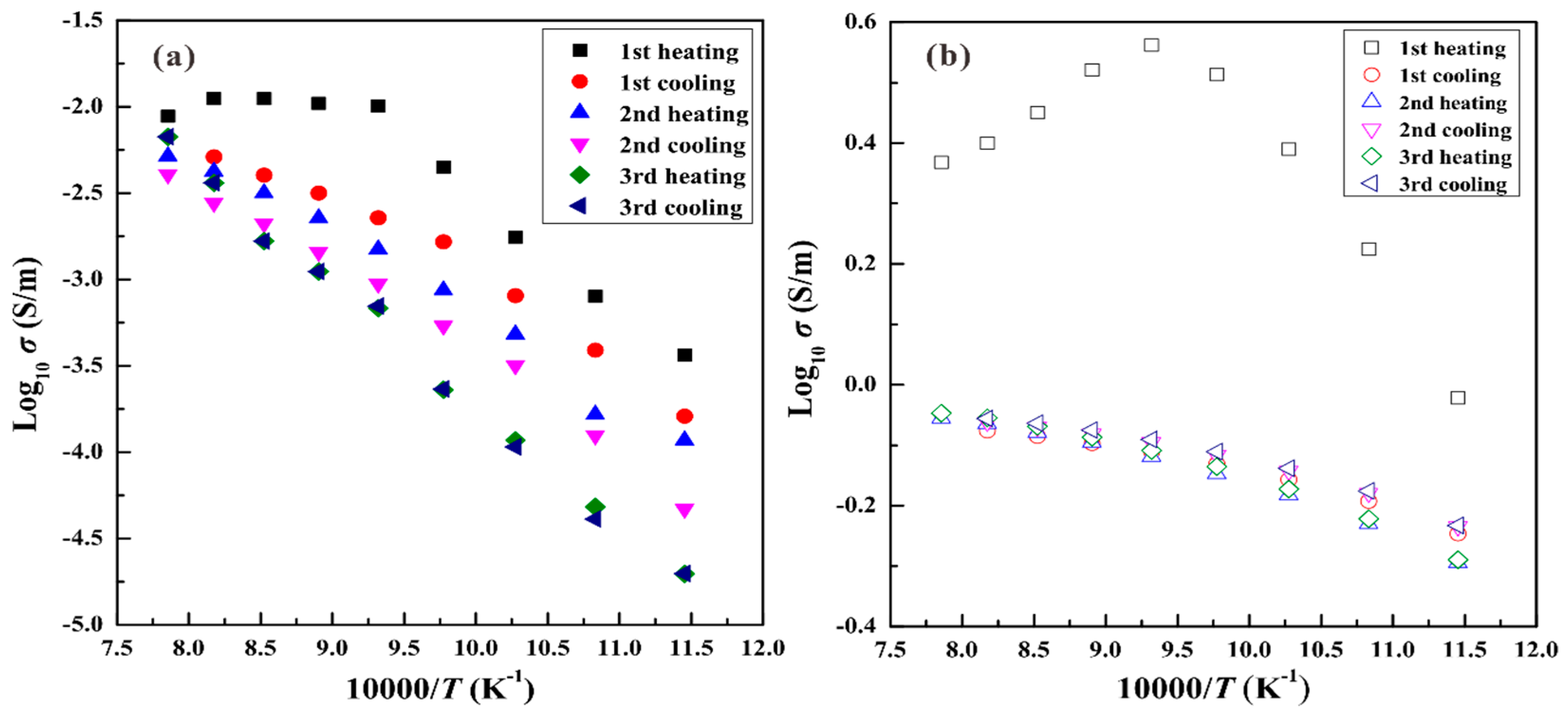
3. Results
4. Discussion
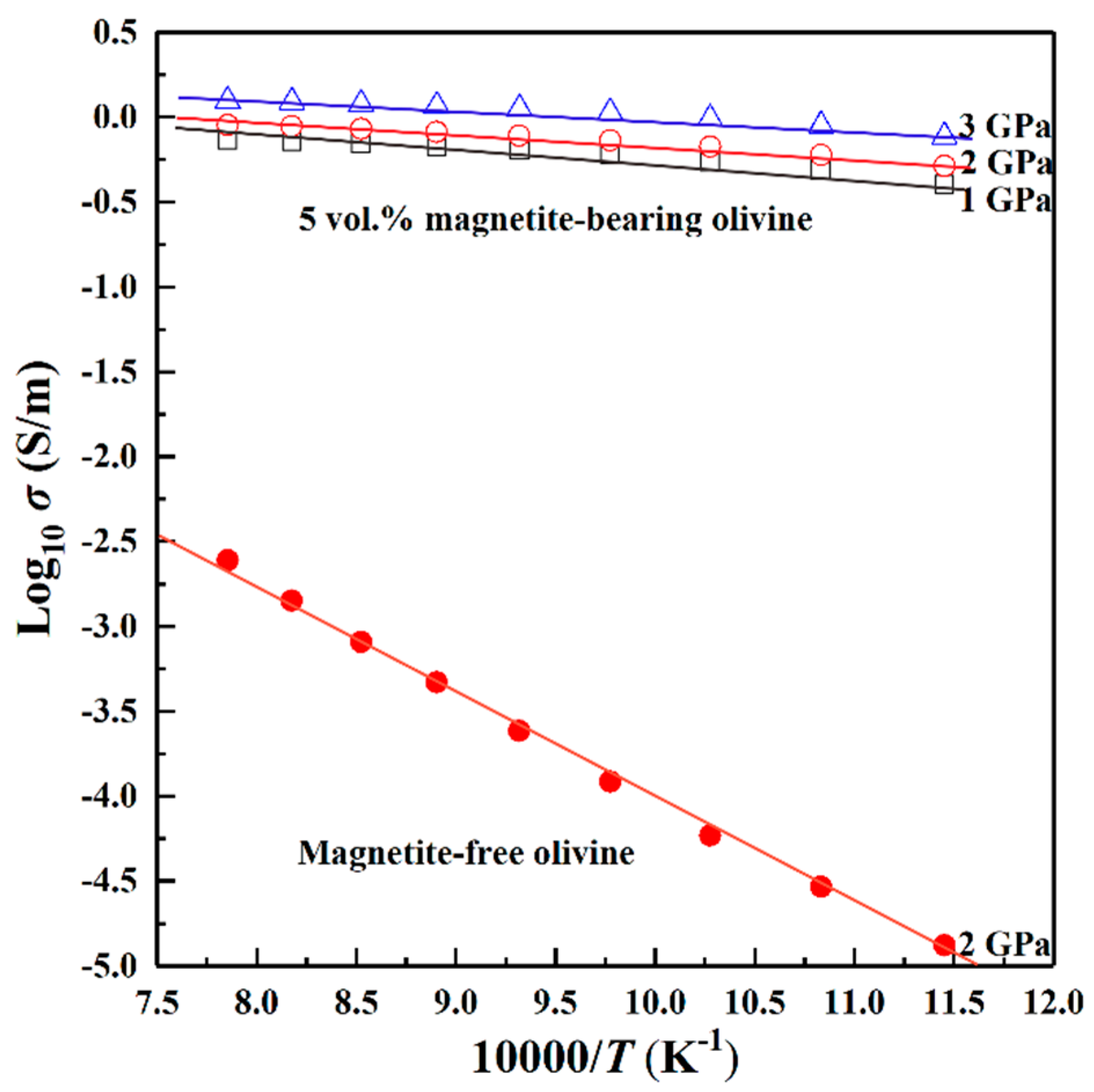
4.1. Influence of Pressure on the Electrical Conductivity
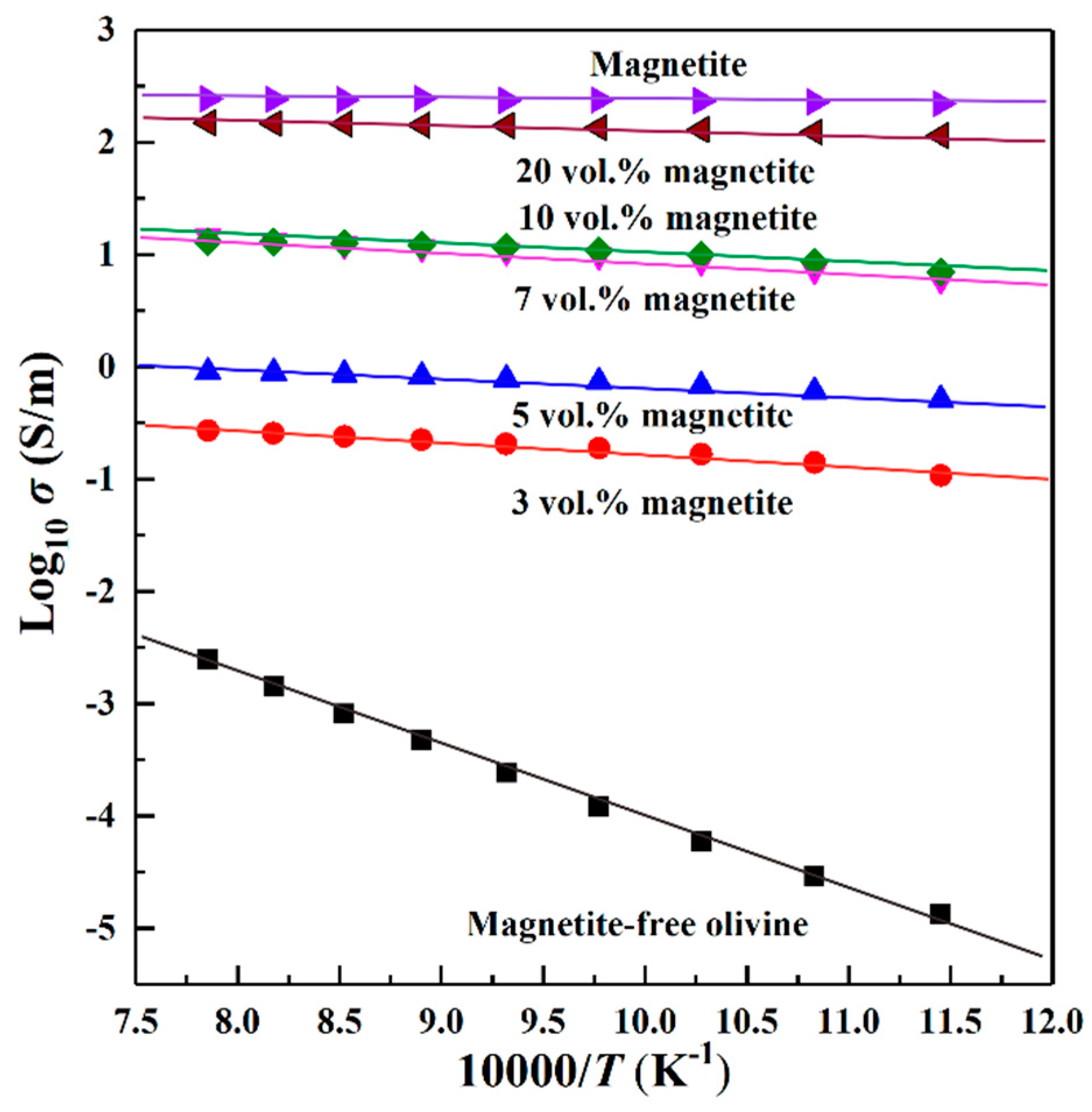
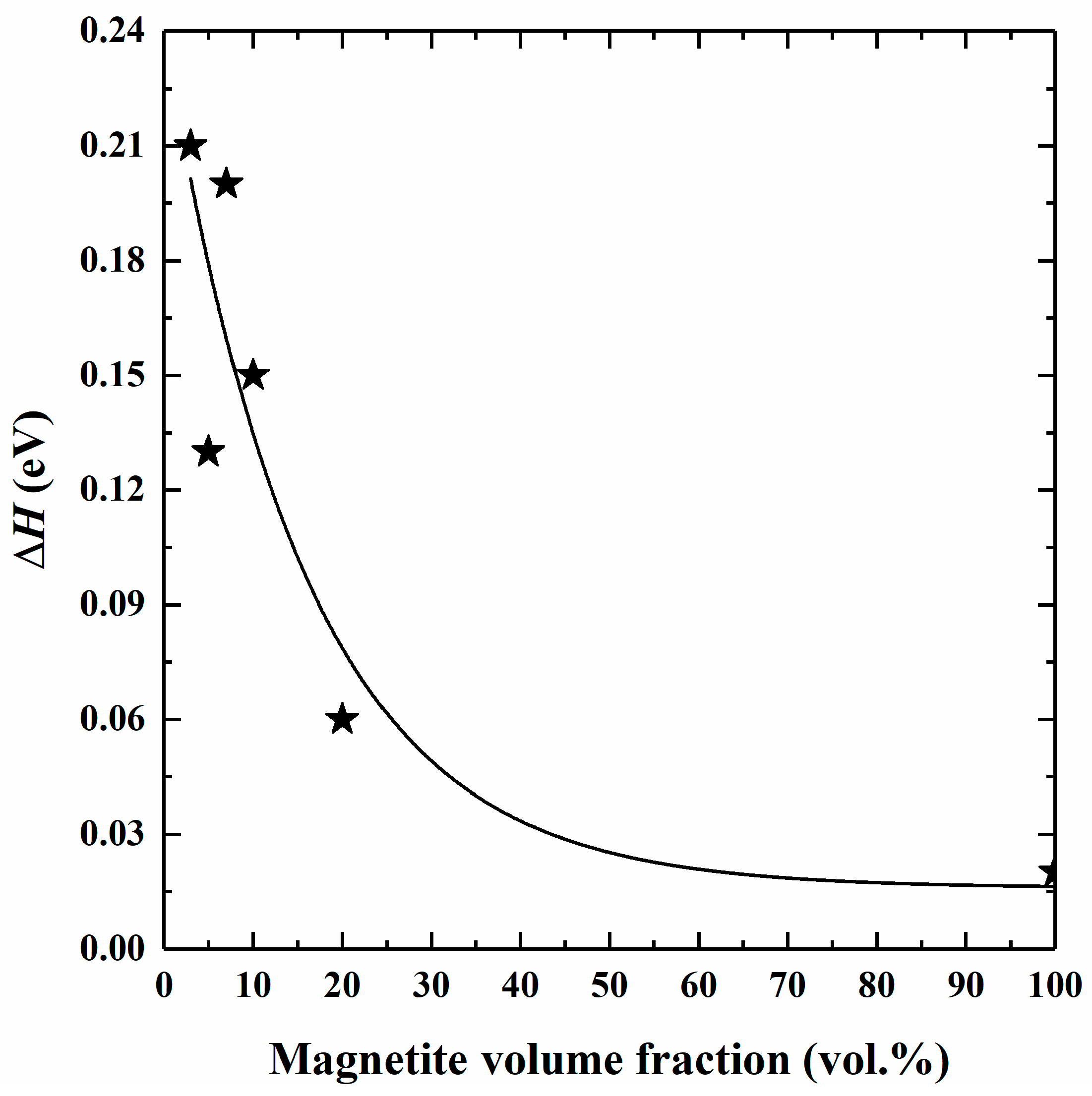
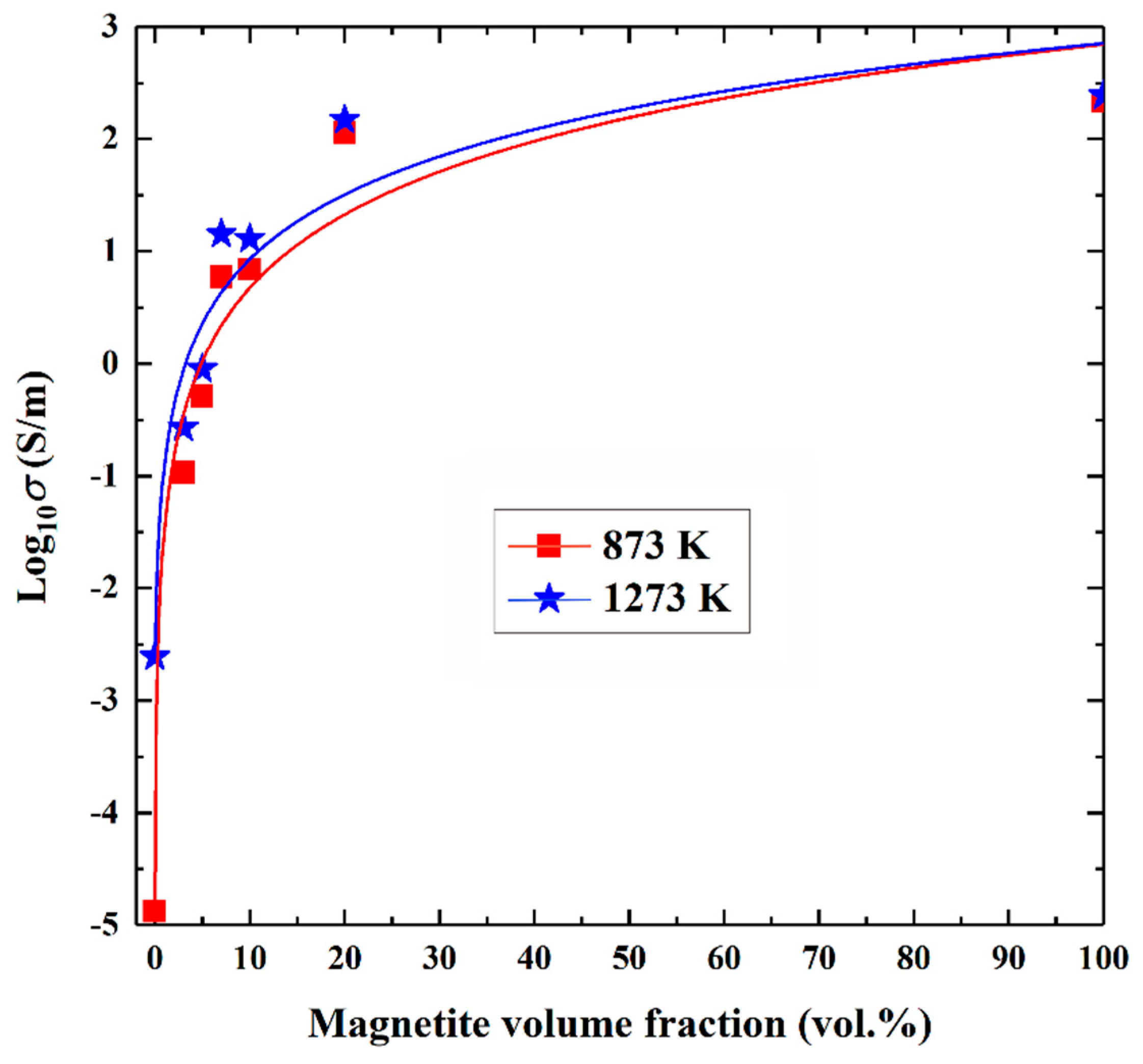
4.2. Influence of Magnetite Volume Fraction on Electrical Conductivity
4.3. Conduction Mechanism
5. Implications
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, X.; Xu, Y.; Karato, S. Water content in the transition zone from electrical conductivity of wadsleyite and ringwoodite. Nature 2005, 434, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Mookherjee, M.; Xu, Y.; Karato, S. The effect of water on the electrical conductivity of olivine. Nature 2006, 443, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Manthilake, G.; Bolfan-Casanova, N.; Novella, D.; Mookherjee, M.; Andrault, D. Dehydration of chlorite explains anomalously high electrical conductivity in the mantle wedges. Sci. Adv. 2016, 2, e1501631. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, X.; Yoshino, T.; Jin, Z.; Li, P. Dehydration of phengite inferred by electrical conductivity measurements: Implication for the high conductivity anomalies relevant to the subduction zones. Geology 2018, 46, 11–14. [Google Scholar] [CrossRef]
- Gaillard, F.; Malki, M.; Iacono-Marziano, G.; Pichavant, M.; Scaillet, B. Carbonatite melts and electrical conductivity in the asthenosphere. Science 2008, 322, 1363–1365. [Google Scholar] [CrossRef] [PubMed]
- Laumonier, M.; Farla, R.; Frost, D.; Katsura, T.; Marquardt, K.; Bouvier, A.; Baumgartner, L. Experimental determination of melt interconnectivity and electrical conductivity in the upper mantle. Earth Planet. Sci. Lett. 2017, 463, 286–297. [Google Scholar] [CrossRef]
- Hu, H.; Dai, L.; Li, H.; Sun, W.; Li, B. Effect of dehydrogenation on the electrical conductivity of Fe-bearing amphibole: Implications for high conductivity anomalies in subduction zones and continental crust. Earth Planet. Sci. Lett. 2018, 498, 27–37. [Google Scholar] [CrossRef]
- Dai, L.; Karato, S. High and highly anisotropic electrical conductivity of the asthenosphere due to hydrogen diffusion in olivine. Earth Planet. Sci. Lett. 2014, 408, 79–86. [Google Scholar] [CrossRef]
- Yoshino, T.; Walter, M.; Katsura, T. Core formation in planetesimals triggered by permeable flow. Nature 2003, 422, 154–157. [Google Scholar] [CrossRef]
- Watson, H.; Roberts, J.; Tyburczy, J. Effect of conductive impurities on electrical conductivity in polycrystalline olivine. Geophys. Res. Lett. 2010, 37, L02302. [Google Scholar] [CrossRef]
- Wang, D.; Karato, S.; Jiang, Z. An experimental study of the influence of graphite on the electrical conductivity of olivine aggregates. Geophys. Res. Lett. 2013, 40, 2028–2032. [Google Scholar] [CrossRef]
- Zhang, Z.; Pommier, A. Electrical investigation of metal-olivine systems and application to the deep interior of Mercury. J. Geophys. Res. Planets 2017, 122, 2702–2718. [Google Scholar] [CrossRef]
- Yamanaka, T.; Shimazu, H.; Ota, K. Electric conductivity of Fe2SiO4–Fe3O4 spinel solid solutions. Phys. Chem. Miner. 2001, 28, 110–118. [Google Scholar] [CrossRef]
- Zhang, R.; Shu, J.; Mao, H.; Liou, J. Magnetite lamellae in olivine and clinohumite from Dabie UHP ultramafic rocks, central China. Am. Mineral. 1999, 84, 564–569. [Google Scholar] [CrossRef]
- Hu, J.; Mao, S.; Du, G.; Wu, Y.; Zhang, P. A new thermodynamic analysis of the intergrowth of hedenbergite and magnetite with Ca-Fe-rich olivine. Am. Mineral. 2001, 96, 599–608. [Google Scholar] [CrossRef]
- Sun, W.; Dai, L.; Li, H.; Hu, H.; Wu, L.; Jiang, J. Electrical conductivity of mudstone (before and after dehydration at high PT) and a test of high conductivity layers in the crust. Am. Mineral. 2017, 102, 2450–2456. [Google Scholar] [CrossRef]
- Dai, L.; Karato, S. The effect of pressure on the electrical conductivity of olivine under the hydrogen-rich conditions. Phys. Earth Planet. Inter. 2014, 232, 51–56. [Google Scholar] [CrossRef]
- Dai, L.; Karato, S. Influence of FeO and H on the electrical conductivity of olivine. Phys. Earth Planet. Inter. 2014, 237, 73–79. [Google Scholar] [CrossRef]
- Paterson, M. The determination of hydroxyl by infrared absorption in quartz, silicate glasses, and similar materials. Bull. Mineral. 1982, 105, 20–29. [Google Scholar]
- Dai, L.; Li, H.; Hu, H.; Shan, S.; Jiang, J.; Hui, K. The effect of chemical composition and oxygen fugacity on the electrical conductivity of dry and hydrous garnet at high temperatures and pressures. Contrib. Mineral. Petrol. 2012, 163, 689–700. [Google Scholar] [CrossRef]
- Hu, H.; Dai, L.; Li, H.; Hui, K.; Sun, W. Influence of dehydration on the electrical conductivity of epidote and implications for high-conductivity anomalies in subduction zones. J. Geophys. Res. Solid Earth 2017, 122, 2751–2762. [Google Scholar] [CrossRef]
- Xu, Y.; Shankland, T.; Duba, A. Pressure effect on electrical conductivity of mantle olivine. Phys. Earth Planet. Inter. 2000, 118, 149–161. [Google Scholar] [CrossRef]
- Dai, L.; Li, H.; Li, C.; Hu, H.; Shan, S. The electrical conductivity of dry polycrystalline olivine compacts at high temperatures and pressures. Mineral. Mag. 2010, 74, 849–857. [Google Scholar] [CrossRef]
- Poe, B.; Romano, C.; Nestola, F.; Smyth, J.R. Electrical conductivity anisotropy of dry and hydrous olivine at 8 GPa. Phys. Earth Planet. Inter. 2010, 181, 103–111. [Google Scholar] [CrossRef]
- Yang, X. Orientation-related electrical conductivity of hydrous olivine, clinopyroxene and plagioclase and implications for the structure of the lower continental crust and uppermost mantle. Earth Planet. Sci. Lett. 2012, 317, 241–250. [Google Scholar] [CrossRef]
- Dai, L.; Karato, S. Influence of oxygen fugacity on the electrical conductivity of hydrous olivine: Implications for the mechanism of conduction. Phys. Earth Planet. Inter. 2014, 232, 57–60. [Google Scholar] [CrossRef]
- Dai, L.; Hu, H.; Li, H.; Wu, L.; Hui, K.; Jiang, J.; Sun, W. Influence of temperature, pressure, and oxygen fugacity on the electrical conductivity of dry eclogite, and geophysical implications. Geochem. Geophys. Geosyst. 2016, 17, 2394–2407. [Google Scholar] [CrossRef]
- Dobson, D.; Richmond, N.; Brodholt, J. A high-temperature electrical conduction mechanism in the lower mantle phase (Mg,Fe)1−xO. Science 1997, 275, 1779–1781. [Google Scholar] [CrossRef]
- Dobson, D.; Brodholt, J. The electrical conductivity of the lower mantle phase magnesiowustite at high temperatures and pressures. J. Geophys. Res. Solid Earth 2000, 105, 531–538. [Google Scholar] [CrossRef]
- Goddat, A.; Peyronneau, J.; Poirier, J. Dependence on pressure of conduction by hopping of small polarons in minerals of the Earth’s lower mantle. Phys. Chem. Minerals 1999, 27, 81–87. [Google Scholar] [CrossRef]
- Karato, S. Theory of isotope diffusion in a material with multiple species and its implications for hydrogen-enhanced electrical conductivity in olivine. Phys. Earth Planet. Inter. 2014, 219, 49–54. [Google Scholar] [CrossRef]
- Yoshino, T.; Zhang, B.; Rhymer, B.; Zhao, C.; Fei, H. Pressure dependence of electrical conductivity in forsterite. J. Geophys. Res. Solid Earth 2017, 122, 158–171. [Google Scholar] [CrossRef]
- Dai, L.; Karato, S. Electrical conductivity of wadsleyite at high temperatures and high pressures. Earth Planet. Sci. Lett. 2009, 287, 277–283. [Google Scholar] [CrossRef]
- Yoshino, T.; Matsuzaki, T.; Yamashita, S.; Katsura, T. Hydrous olivine unable to account for conductivity anomaly at the top of the asthenosphere. Nature 2006, 443, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Omura, K.; Kurita, K.; Kumazawa, M. Experimental study of pressure dependence of electrical conductivity of olivine at high temperatures. Phys. Earth Planet. Inter. 1989, 57, 291–303. [Google Scholar] [CrossRef]
- Schock, R.; Duba, A.; Shankland, T. Electrical conduction in olivine. J. Geophys. Res. 1989, 94, 5829–5839. [Google Scholar] [CrossRef]
- Morris, E.; Williams, Q. Electrical resistivity of Fe3O4 to 48 GPa: Compression-induced changes in electron hopping at mantle pressures. J. Geophys. Res. Solid Earth 1997, 102, 18139–18148. [Google Scholar] [CrossRef]
- Schollenbruch, K.; Woodland, A.; Frost, D.; Langenhorst, F. Detecting the spinel–post-spinel transition in Fe3O4 by in situ electrical resistivity measurements. High Press. Res. 2009, 29, 520–524. [Google Scholar] [CrossRef]
- Naemura, K.; Hirajima, T.; Svojtka, M. The pressure–temperature path and the origin of phlogopite in spinel–garnet peridotites from the Blanský les Massif of the Moldanubian zone, Czech Republic. J. Petrol. 2009, 50, 1795–1827. [Google Scholar] [CrossRef]
- Khedr, M.; Arai, S. Composite origin of magnetite deposits hosted in Oman peridotites: Evidence for iron mobility during serpentinization. Ore Geol. Rev. 2018, 101, 180–198. [Google Scholar] [CrossRef]
- Evans, R.; Hirth, G.; Baba, K.; Forsyth, D.; Chave, A.; Makie, R. Geophysical evidence from the MELT area for compositional control on oceanic plates. Nature 2005, 437, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; McCammon, C. Fe3+-rich augite and high electrical conductivity in the deep lithosphere. Geology 2012, 40, 131–134. [Google Scholar] [CrossRef]
- Naif, S.; Key, K.; Constable, S.; Evans, R. Melt-rich channel observed at the lithosphere–asthenosphere boundary. Nature 2013, 495, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yoshino, T.; Shimojuku, A. Electrical conductivity of albite–(quartz)–water and albite–water–NaCl systems and its implication to the high conductivity anomalies in the continental crust. Earth Planet. Sci. Lett. 2015, 412, 1–9. [Google Scholar] [CrossRef]
- Wannamaker, P.; Caldwell, T.; Jiracek, G.; Maris, V.; Hill, G.; Ogawa, Y.; Bibby, H.; Bennie, S.; Heise, W. Fluid and deformation regime of an advancing subduction system at Marlborough, New Zealand. Nature 2009, 460, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yoshino, T.; Katayama, I. Electrical conductivity anisotropy of deformed talc rocks and serpentinites at 3 GPa. Phys. Earth Planet. Inter. 2011, 188, 69–81. [Google Scholar] [CrossRef]
- Worzewski, T.; Jegen, M.; Kopp, H.; Brasse, H.; Castillo, W. Magnetotelluric image of the fluid cycle in the Costa Rican subduction zone. Nat. Geosci. 2011, 4, 108–111. [Google Scholar] [CrossRef]
- Hasegawa, A. Seismic imaging of mantle wedge corner flow and arc magmatism. Proc. Jpn. Acad. 2018, 94, 217–234. [Google Scholar] [CrossRef]
- Kawano, S.; Yoshino, T.; Katayama, I. Electrical conductivity of magnetite-bearing serpentinite during shear deformation. Geophys. Res. Lett. 2012, 39, L20313. [Google Scholar] [CrossRef]
- Liu, X.; Xiong, Z.; Shieh, S.; He, Q.; Deng, L.; Zhang, Y.; Chang, L.; Wang, F.; Hong, X.; Chen, Z. Non-monotonic compositional dependence of isothermal bulk modulus of the (Mg1−x Mnx) Cr2O4 spinel solid solutions, and its origin and implication. Solid Earth Sci. 2016, 1, 89–100. [Google Scholar] [CrossRef]
- Liu, L.; Liu, X.; Bao, X.; He, Q.; Yan, W.; Ma, Y.; He, M.; Tao, R.; Zou, R. Si-disordering in MgAl2O4-spinel under high PT conditions, with implications for Si-Mg disorder in Mg2SiO4-Ringwoodite. Minerals 2018, 8, 210. [Google Scholar] [CrossRef]
- Mi, Z.; Shi, W.; Zhang, L.; Shieh, S.; Liu, X. Equation of state of a natural chromian spinel at ambient temperature. Minerals 2018, 8, 591. [Google Scholar] [CrossRef]
- Woodland, A.; Angel, R. Crystal structure of a new spinelloid with the wadsleyite structure in the system Fe2SiO4-Fe3O4 and implications for the Earth’s mantle. Am. Mineral. 1998, 83, 404–408. [Google Scholar] [CrossRef]
| Run No. | P (GPa) | ϕmgt (vol. %) a | Log σ0 | ΔH (eV) | γ2 (b) |
|---|---|---|---|---|---|
| DL1807 | 2.0 | 0 | 1.27 ± 0.03 | 1.27 ± 0.01 | 99.76 |
| DL1816 | 2.0 | 3 | 0.28 ± 0.03 | 0.21 ± 0.02 | 96.92 |
| DL1820 | 1.0 | 5 | 0.44 ± 0.02 | 0.14 ± 0.02 | 98.49 |
| DL1824 | 2.0 | 5 | 0.49 ± 0.02 | 0.13 ± 0.01 | 99.21 |
| DL1826 | 3.0 | 5 | 0.56 ± 0.01 | 0.11 ± 0.01 | 99.36 |
| DL1829 | 2.0 | 7 | 1.97 ± 0.01 | 0.20 ± 0.02 | 99.41 |
| DL1831 | 2.0 | 10 | 1.72 ± 0.03 | 0.15 ± 0.01 | 97.94 |
| DL1832 | 2.0 | 20 | 2.40 ± 0.02 | 0.06 ± 0.01 | 98.92 |
| DL1836 | 2.0 | 100 | 2.47 ± 0.02 | 0.02 ± 0.01 | 96.21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, L.; Hu, H.; Sun, W.; Li, H.; Liu, C.; Wang, M. Influence of High Conductive Magnetite Impurity on the Electrical Conductivity of Dry Olivine Aggregates at High Temperature and High Pressure. Minerals 2019, 9, 44. https://doi.org/10.3390/min9010044
Dai L, Hu H, Sun W, Li H, Liu C, Wang M. Influence of High Conductive Magnetite Impurity on the Electrical Conductivity of Dry Olivine Aggregates at High Temperature and High Pressure. Minerals. 2019; 9(1):44. https://doi.org/10.3390/min9010044
Chicago/Turabian StyleDai, Lidong, Haiying Hu, Wenqing Sun, Heping Li, Changcai Liu, and Mengqi Wang. 2019. "Influence of High Conductive Magnetite Impurity on the Electrical Conductivity of Dry Olivine Aggregates at High Temperature and High Pressure" Minerals 9, no. 1: 44. https://doi.org/10.3390/min9010044
APA StyleDai, L., Hu, H., Sun, W., Li, H., Liu, C., & Wang, M. (2019). Influence of High Conductive Magnetite Impurity on the Electrical Conductivity of Dry Olivine Aggregates at High Temperature and High Pressure. Minerals, 9(1), 44. https://doi.org/10.3390/min9010044







