Authigenic Clay Minerals from Interface Reactions of Concrete-Clay Engineered Barriers: A New Perspective on Mg-Clays Formation in Alkaline Environments
Abstract
1. Introduction
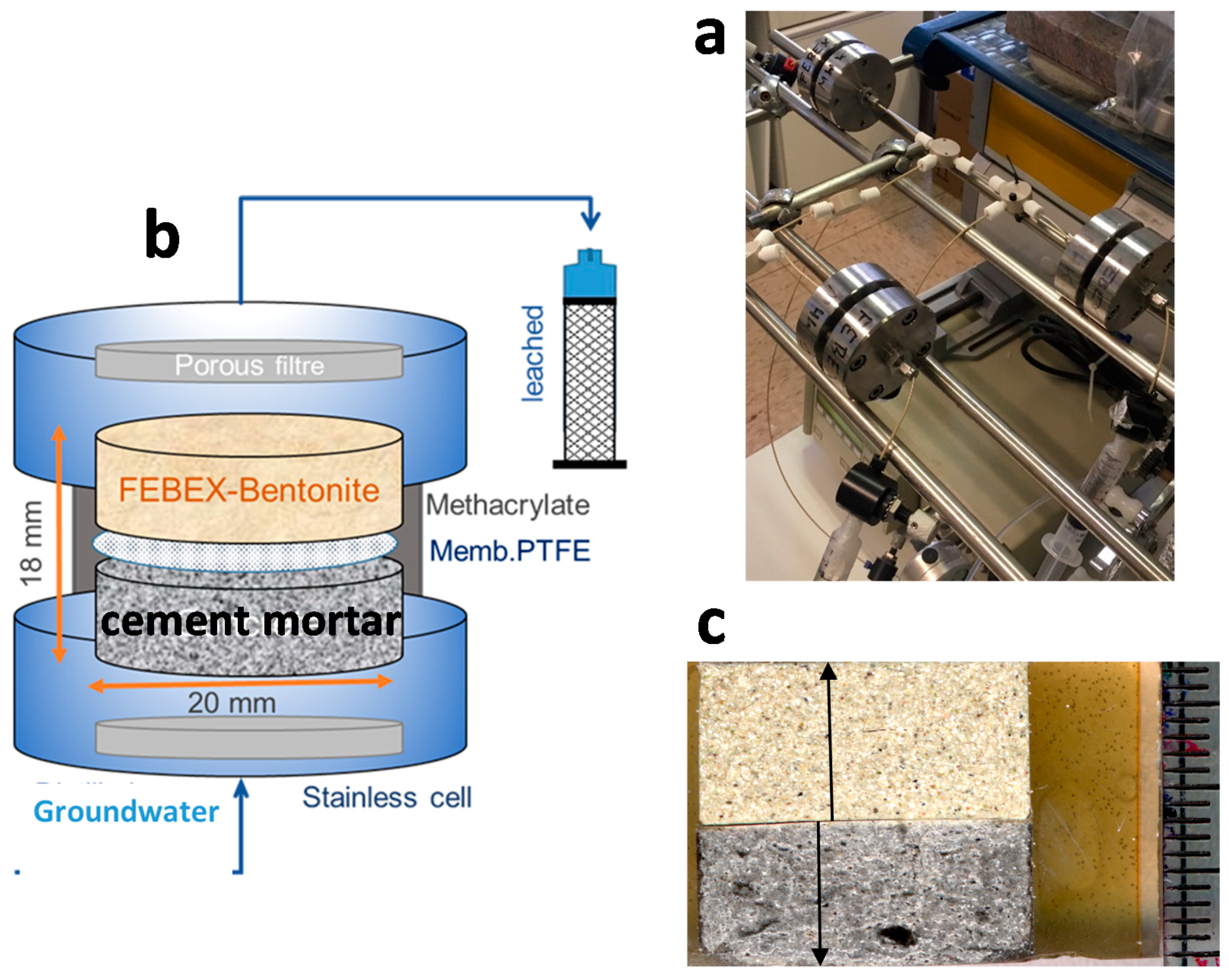
2. Materials and Methods
3. Results
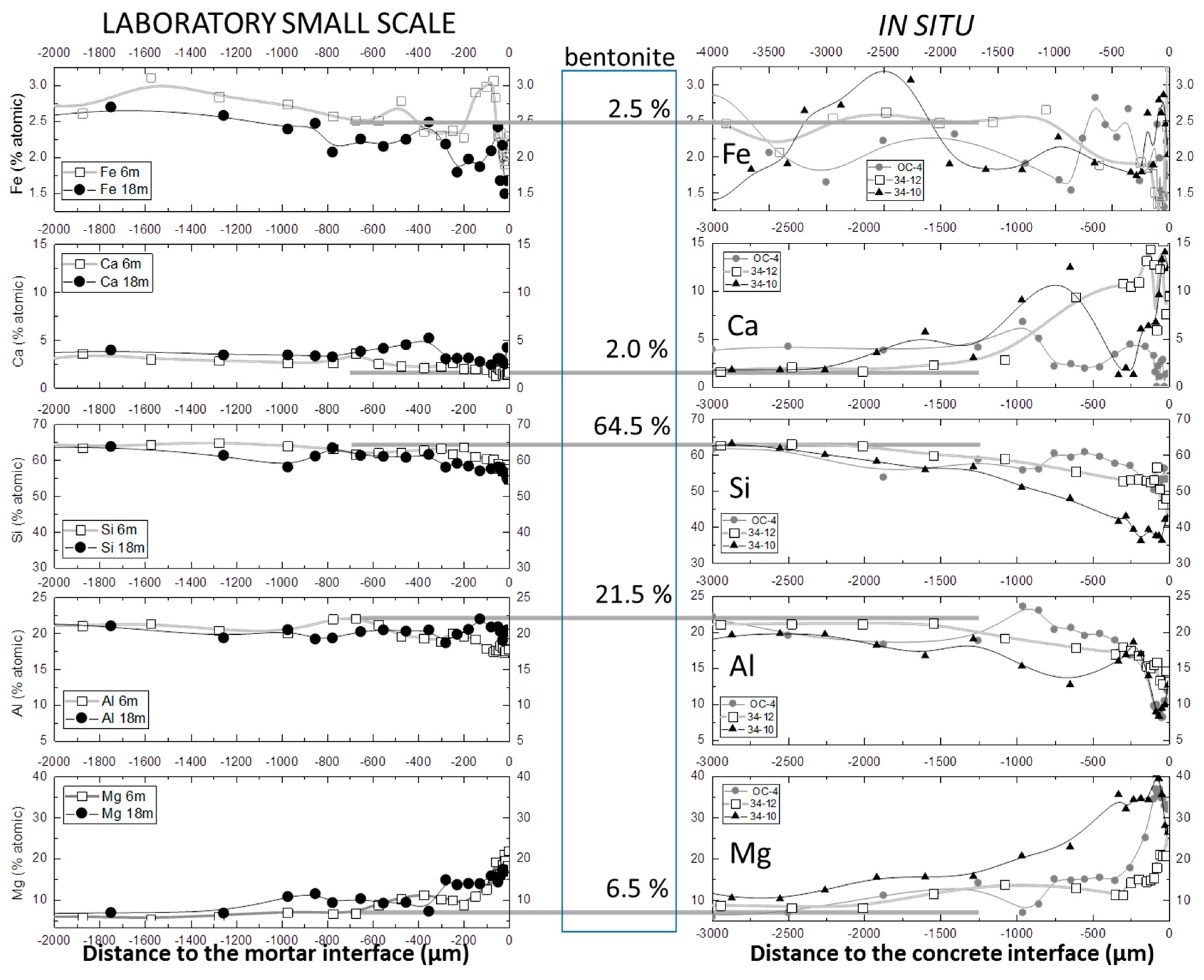
3.1. SEM-EDX Chemical Profiles
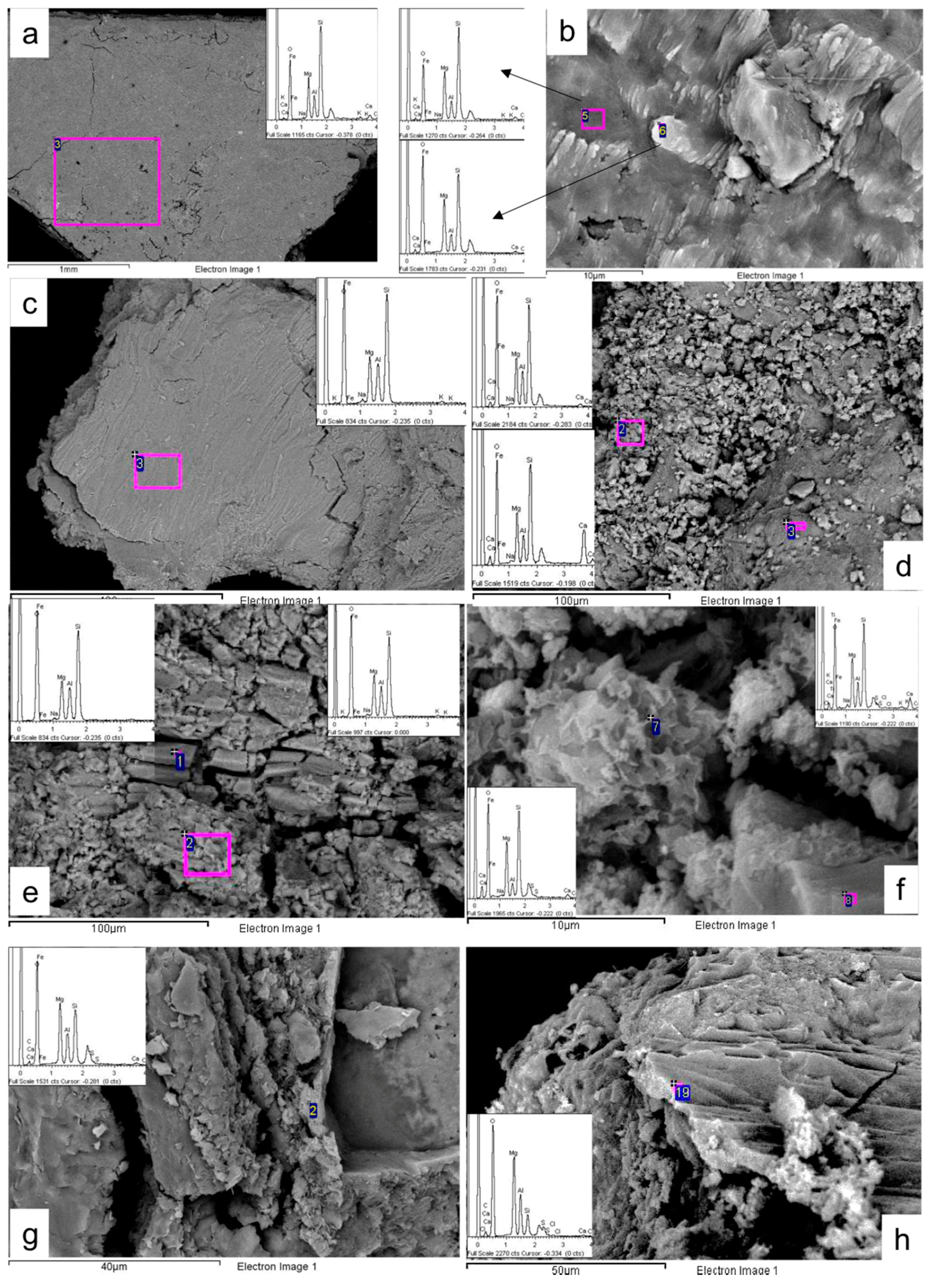
3.2. Textural Arrangements, Morphology and Chemistry
3.3. XRD and FTIR Data
3.4. Thermochemical Approach
4. Discussion
4.1. Geochemical Perturbation
4.2. The Nature of Alkaline Alteration: Connections with Natural Authigenesis in Alkaline Lakes.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- U.S. DOE. Evaluation of options for permanent geologic disposal of spent nuclear fuel and high-level radioactive waste in support of a comprehensive national nuclear fuel cycle strategy. In Prepared for U.S. Department of Energy Used Fuel Disposition Campaign by Sandia National Laboratories; U.S. DOE: Washington, DC, USA, 2014; Volume I, p. 155. [Google Scholar]
- Fernández, A.M.; Baeyens, B.; Bradbury, M.; Rivas, P. Analysis of the porewater chemical composition of a spanish compacted bentonite used in an engineered barrier. Phys. Chem. Earth Parts A/B/C 2004, 29, 105–118. [Google Scholar] [CrossRef]
- Bullard, J.W.; Jennings, H.M.; Livingston, R.A.; Nonat, A.; Scherer, G.W.; Schweitzer, J.S.; Scrivener, K.L.; Thomas, J.J. Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- International Atomic Energy Agency (IAEA). The Behaviours of Cementitious Materials in Long Term Storage and Disposal of Radioactive Waste; IAEA: Vienna, Austria, 2013; p. 61. [Google Scholar]
- Glasser, F.P.; Matschei, T. Interactions between portland cement and carbon dioxide. In Proceedings of the International Congress on the Chemistry of Cement-Cd-Rom Edition, Montréal, QC, Canada, 8–13 July 2007; p. TH3-13.4. [Google Scholar]
- Dauzeres, A.; Le Bescop, P.; Sardini, P.; Cau Dit Coumes, C. Physico-chemical investigation of clayey/cement-based materials interaction in the context of geological waste disposal: Experimental approach and results. Cem. Concr. Res. 2010, 40, 1327–1340. [Google Scholar] [CrossRef]
- Gaucher, E.C.; Blanc, P. Cement/clay interactions—A review: Experiments, natural analogues, and modeling. Waste Manag. 2006, 26, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Dauzeres, A.; Achiedo, G.; Nied, D.; Bernard, E.; Alahrache, S.; Lothenbach, B. Magnesium perturbation in low-ph concretes placed in clayey environment—Solid characterizations and modeling. Cem. Concr. Res. 2016, 79, 137–150. [Google Scholar] [CrossRef]
- Jenni, A.; Mäder, U.; Lerouge, C.; Gaboreau, S.; Schwyn, B. In situ interaction between different concretes and opalinus clay. Phys. Chem. Earth Parts A/B/C 2014, 70–71, 71–83. [Google Scholar] [CrossRef]
- Bildstein, O.; Claret, F. Stability of clay barriers under chemical perturbations. In Natural and Engineered Clay Barriers; Tournasat, C., Steefel, C.I., Bourg, I.C., Bergaya, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 6, pp. 155–226. [Google Scholar]
- Dauzères, A.; Le Bescop, P.; Cau-Dit-Coumes, C.; Brunet, F.; Bourbon, X.; Timonen, J.; Voutilainen, M.; Chomat, L.; Sardini, P. On the physico-chemical evolution of low-pH and CEM I cement pastes interacting with Callovo-Oxfordian pore water under its in situ CO2 partial pressure. Cem. Concr. Res. 2014, 58, 76–88. [Google Scholar] [CrossRef]
- Fernández, R.; González-Santamaría, D.; Angulo, M.; Torres, E.; Ruiz, A.I.; Turrero, M.J.; Cuevas, J. Geochemical conditions for the formation of Mg silicates phases in bentonite and implications for radioactive waste disposal. Appl. Geochem. 2018, 93, 1–9. [Google Scholar] [CrossRef]
- Fernández, R.; Torres, E.; Ruiz, A.I.; Cuevas, J.; Alonso, M.C.; García Calvo, J.L.; Rodríguez, E.; Turrero, M.J. Interaction processes at the concrete-bentonite interface after 13 years of febex-plug operation. Part II: Bentonite contact. Phys. Chem. Earth Parts A/B/C 2017, 99, 49–63. [Google Scholar] [CrossRef]
- Lerouge, C.; Gaboreau, S.; Grangeon, S.; Claret, F.; Warmont, F.; Jenni, A.; Cloet, V.; Mäder, U. In situ interactions between opalinus clay and low alkali concrete. Phys. Chem. Earth Parts A/B/C 2017, 99, 3–21. [Google Scholar] [CrossRef]
- Nied, D.; Enemark-Rasmussen, K.; L’Hopital, E.; Skibsted, J.; Lothenbach, B. Properties of magnesium silicate hydrates (M-S-H). Cem. Concr. Res. 2016, 79, 323–332. [Google Scholar] [CrossRef]
- Roosz, C.; Grangeon, S.; Blanc, P.; Montouillout, V.; Lothenbach, B.; Henocq, P.; Giffaut, E.; Vieillard, P.; Gaboreau, S. Crystal structure of magnesium silicate hydrates (M-S-H): The relation with 2:1 Mg–Si phyllosilicates. Cem. Concr. Res. 2015, 73, 228–237. [Google Scholar] [CrossRef]
- Bernard, E.; Dauzères, A.; Lothenbach, B. Magnesium and calcium silicate hydrates, part II: Mg-exchange at the interface “low-pH” cement and magnesium environment studied in a C-S-H and M-S-H model system. Appl. Geochem. 2018, 89, 210–218. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Cau-Dit-Coumes, C.; Chlique, C.; Dauzères, A.; Pochard, I. Magnesium and calcium silicate hydrates, part I: Investigation of the possible magnesium incorporation in calcium silicate hydrate (C-S-H) and of the calcium in magnesium silicate hydrate (M-S-H). Appl. Geochem. 2018, 89, 229–242. [Google Scholar] [CrossRef]
- Lothenbach, B.; Nied, D.; L’Hôpital, E.; Achiedo, G.; Dauzères, A. Magnesium and calcium silicate hydrates. Cem. Concr. Res. 2015, 77, 60–68. [Google Scholar] [CrossRef]
- Savage, D.; Benbow, S.; Watson, C.; Takase, H.; Ono, K.; Oda, C.; Honda, A. Natural systems evidence for the alteration of clay under alkaline conditions: An example from searles lake, california. Appl. Clay Sci. 2010, 47, 72–81. [Google Scholar] [CrossRef]
- Deocampo, D.M. Authigenic clay minerals in lacustrine mudstones. In Paying Attention to Mudrocks: Priceless! Larsen, D., Egenhoff, S.O., Fishman, N.S., Eds.; Geological Society of America Special Paper; The Geological Society of America: Boulder, CO, USA, 2015; Volume 515, pp. 49–64. [Google Scholar]
- Khoury, H.N.; Eberl, D.D.; Jones, B.F. Origin of magnesium clays from the amargosa desert, Nevada. Clays Clay Min. 1982, 30, 327–336. [Google Scholar] [CrossRef]
- Meunier, A. Clays in sedimentary environments. In Clays; Meunier, A., Ed.; Springer: New York, NY, USA, 2005; pp. 295–325. [Google Scholar]
- Pozo, M.; Casas, J. Origin of kerolite and associated Mg clays in palustrine-lacustrine environments. The esquivias deposit (neogene Madrid basin, Spain). Clay Min. 1999, 34, 395–418. [Google Scholar] [CrossRef]
- Bristow, T.F.; Milliken, R.E. Terrestrial perspective on authigenic clay mineral production in ancient martian lakes. Clays Clay Miner. 2011, 59, 339–358. [Google Scholar] [CrossRef]
- Shimbashi, M.; Sato, T.; Yamakawa, M.; Fujii, N.; Otake, T. Formation of Fe- and Mg-rich smectite under hyperalkaline conditions at Narra in Palawan, The Philippines. Minerals 2018, 8, 155. [Google Scholar] [CrossRef]
- Jones, B.F.; Galán, E. Sepiolite and palygorskite. In Hydrous Phyllosilicates (Exclusive of Micas); Bayley, S.W., Ed.; Geochemical Society; Mineralogical Society of America: Washington, DC, USA, 1988; Volume 19, pp. 631–674. [Google Scholar]
- Eberl, D.D.; Jones, B.F.; Khoury, H.N. Mixed-layer kerolite/stevensite from the Amargosa desert, Nevada. Clays Clay Miner. 1982, 30, 321–326. [Google Scholar] [CrossRef]
- Tosca, N.J.; Masterson, A.L. Chemical controls on incipient Mg-silicate crystallization at 25 °C: Implications for early and late diagenesis. Clay Miner. 2018, 49, 165–194. [Google Scholar] [CrossRef]
- Weaver, C.E. Clays, Muds, and Shales; Elsevier: Amsterdam, The Netherlands, 1989; p. 820. [Google Scholar]
- Huertas, F.; de la Cruz, B.; Fuentes-Cantillana, J.-L.; Alonso, E.; Linares, J.; Samper, J.; Elorza, F.J.; Svemar, C.; Salo, J.-P.; Muurinen, A.; et al. Full-Scale Engineered Barriers Experiment for a Deep Geological Repository for High-Level Waste in Crystalline Host Rock—Phase II Febex II; The European Commission: Brussels, Belgium, 2005; p. 52. [Google Scholar]
- González-Santamaría, D.; Angulo, M.; Ruiz, A.I.; Fernández, R.; Ortega, A.; Cuevas, J. Low-pH cement mortar-bentonite perturbations in a small-scale pilot laboratory experiment. Clay Miner. 2018, 1–32. [Google Scholar] [CrossRef]
- Villar, M.V.; Martín, P.L.; Bárcena, I.; García-Siñeriz, J.L.; Gómez-Espina, R.; Lloret, A. Long-term experimental evidences of saturation of compacted bentonite under repository conditions. Eng. Geol. 2012, 149–150, 57–69. [Google Scholar] [CrossRef]
- Ramírez, S.; Cuevas, J.; Petit, S.; Righi, D.; Meunier, A. Smectite reactivity in alkaline solutions. Geol. Carphatica 2002, 53, 87–92. [Google Scholar]
- Bárcena, I.; Fuentes-Cantillana, J.L.; García-Siñeriz, J.-L. Dismantling of Heater no 1 at the Febex “In Situ” Test: Description of Operations; IAEA: Vienna, Austria, 2003; p. 124. [Google Scholar]
- Parkhurst, D.L.; Appelo, C.A.J. User’s Guide to Phreeqc (Version 2)—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; USGS: Reston, VA, USA, 1999; p. 310.
- Blanc, P.; Lassin, A.; Piantone, P.; Azaroual, M.; Jacquemet, N.; Fabbri, A.; Gaucher, E.C. Thermoddem: A geochemical database focused on low temperature water/rock interactions and waste materials. Appl. Geochem. 2012, 27, 2107–2116. [Google Scholar] [CrossRef]
- Fernández, R.; Ruiz, A.I.; Cuevas, J. The role of smectite composition on the hyperalkaline alteration of bentonite. Appl. Clay Sci. 2014, 95, 83–94. [Google Scholar] [CrossRef]
- Velde, B. Clay Minerals—A Physico-Chemical Explanation of theIr Occurrence; Elsevier: Amsterdam, The Netherlands, 1985; Volume 40, p. 443. [Google Scholar]
- Fernández, R.; Vigil de la Villa, R.; Ruiz, A.I.; García, R.; Cuevas, J. Precipitation of chlorite-like structures during opc porewater diffusion through compacted bentonite at 90 °C. Appl. Clay Sci. 2013, 83–84, 357–367. [Google Scholar] [CrossRef]
- Frost, R.L.; Kloprogge, J.T. Infrared emission spectroscopic study of brucite. Spectrochim. Acta Part A 1999, 55, 2195–2205. [Google Scholar] [CrossRef]
- Madejova, J.; Gates, W.P.; Petit, S. Ir spectra of clay minerals. In Infrared and Raman Spectroscopies of Clay Minerals; Gates, W.P., Kloprogge, J.T., Madejova, J., Bergaya, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 8, pp. 107–149. [Google Scholar]
- Gaboreau, S.; Lerouge, C.; Dewonck, S.; Linard, Y.; Bourbon, X.; Fialips, C.I.; Mazurier, A.; Pret, D.; Borschneck, D.; Montouillout, V.; et al. In-situ interaction of cement paste and shotcrete with claystones in a deep disposal context. Am. J. Sci. 2012, 312, 314–356. [Google Scholar] [CrossRef]
- Zajac, M.; Rossberg, A.; Le Saout, G.; Lothenbach, B. Influence of limestone and anhydrite on the hydration of portland cements. Cem. Concr. Compos. 2014, 46, 99–108. [Google Scholar] [CrossRef]
- Alonso, M.C.; García Calvo, J.L.; Cuevas, J.; Turrero, M.J.; Fernández, R.; Torres, E.; Ruiz, A.I. Interaction processes at the concrete-bentonite interface after 13 years of febex-plug operation. Part I: Concrete alteration. Phys. Chem. Earth Parts A/B/C 2017, 99, 38–48. [Google Scholar] [CrossRef]
- Brindley, G.W.; Kao, C.-C. Formation, compositions, and properties of hydroxy-Al- and hydroxy-Mg-montmorillonite. Clays Clay Miner. 1980, 28, 435–443. [Google Scholar] [CrossRef]
- Soler, J.M. Reactive transport modeling of concrete-clay interaction during 15 years at the Tournemire underground rock laboratory. Eur. J. Min. 2013, 25, 639–654. [Google Scholar] [CrossRef]
- Fernández, R.; Rodríguez, M.; Villa, R.V.d.l.; Cuevas, J. Geochemical constraints on the stability of zeolites and C–S–H in the high pH reaction of bentonite. Geochim. Cosmochim. Acta 2010, 74, 890–906. [Google Scholar] [CrossRef]
- Siegel, M.D.; Bryan, C. Geochemistry of radioactive environmental contamination. In Environmemtal Gechemistry; Sherwood Lollar, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 9, pp. 205–262. [Google Scholar]
- Hay, R.L.; Hughes, R.E.; Kyser, T.K.; Glass, H.D.; Liu, J. Magnesium-rich clays of the Meerschaum mines in the Amboseli basin, Tanzania and Kenya. Clays Clay Miner. 1995, 43, 455–466. [Google Scholar] [CrossRef]
- Deocampo, D.M.; Jones, B.F. Geochemistry of saline lakes. In Surface and Groundwater, Weathering and Soils, 2nd ed.; Drever, J.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 7, pp. 437–469. [Google Scholar]
- Birsoy, R. Formation of sepiolite-palygorskite and related minerals from solution. Clays Clay Min. 2002, 50, 736–745. [Google Scholar] [CrossRef]
- Calvo, J.P.; Jones, B.J.; Bustillo, M.A.; Fort, R.; Alonso-Zarza, A.M.; Kendall, C. Sedimentology and geochemistry of carbonates from lacustrine secuences in the Madrid basin, central Spain. Chem. Geol. 1995, 123, 173–191. [Google Scholar] [CrossRef]
- Leguey, S.; Ruiz de León, D.; Ruiz, A.I.; Cuevas, J. The role of biomineralization in the origin of sepiolita and dolomite. Am. J. Sci. 2010, 310, 165–193. [Google Scholar] [CrossRef]
- Larsen, D. Revisiting silicate authigenesis in the pliocene–pleistocene lake Tecopa beds, southeastern California: Depositional and hydrological controls. Geosphere 2008, 4, 612–639. [Google Scholar] [CrossRef]

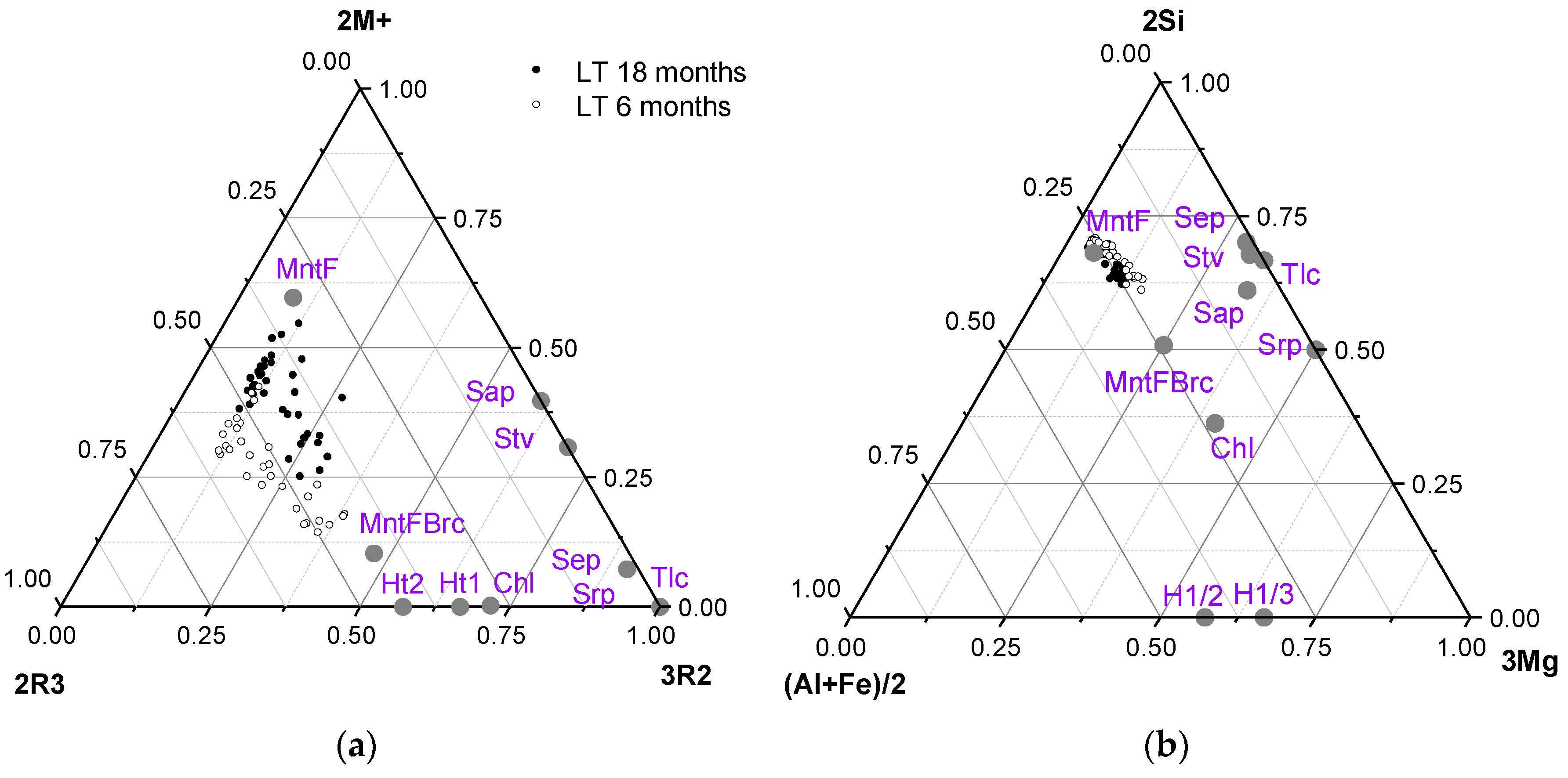
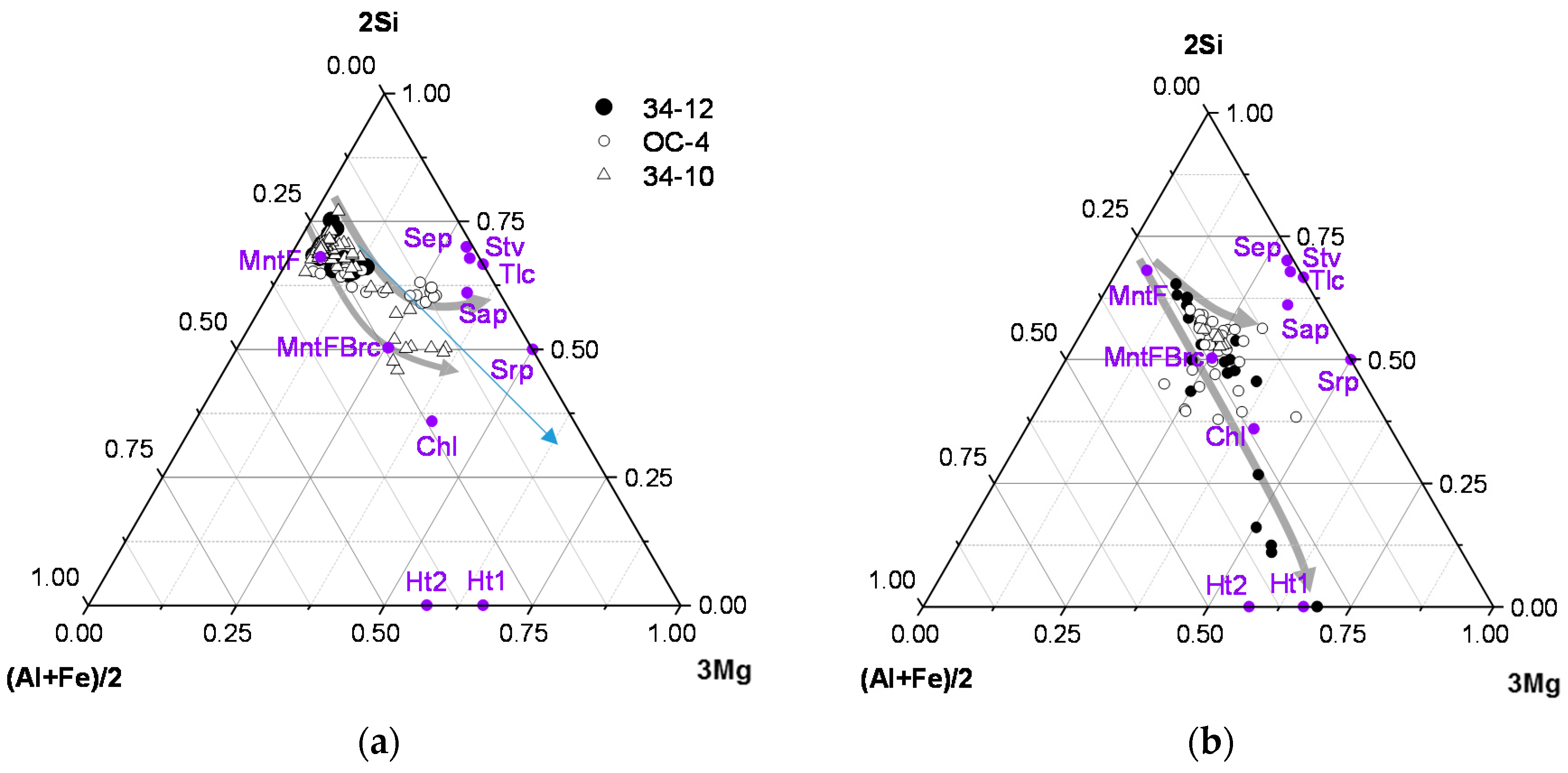
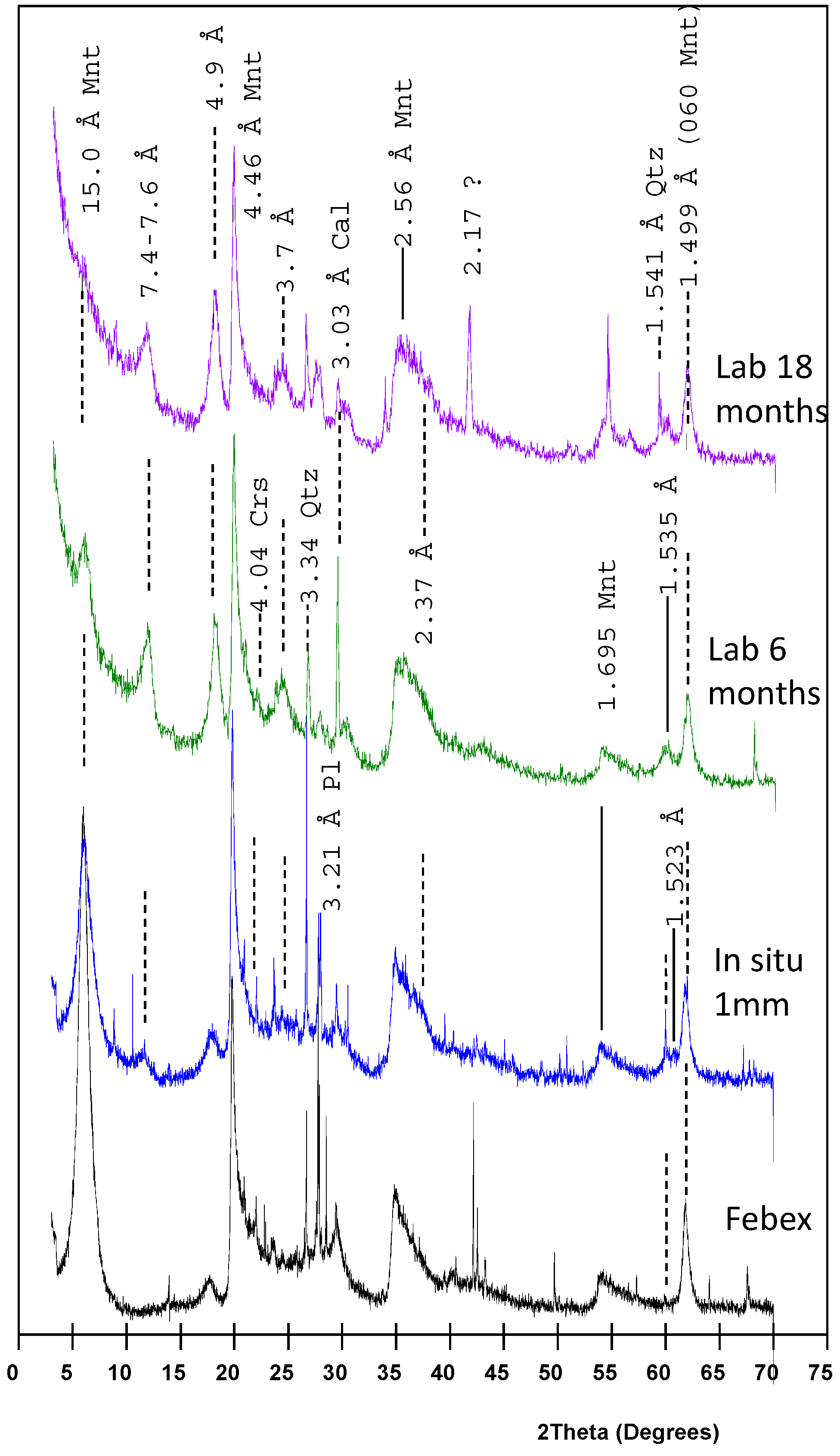
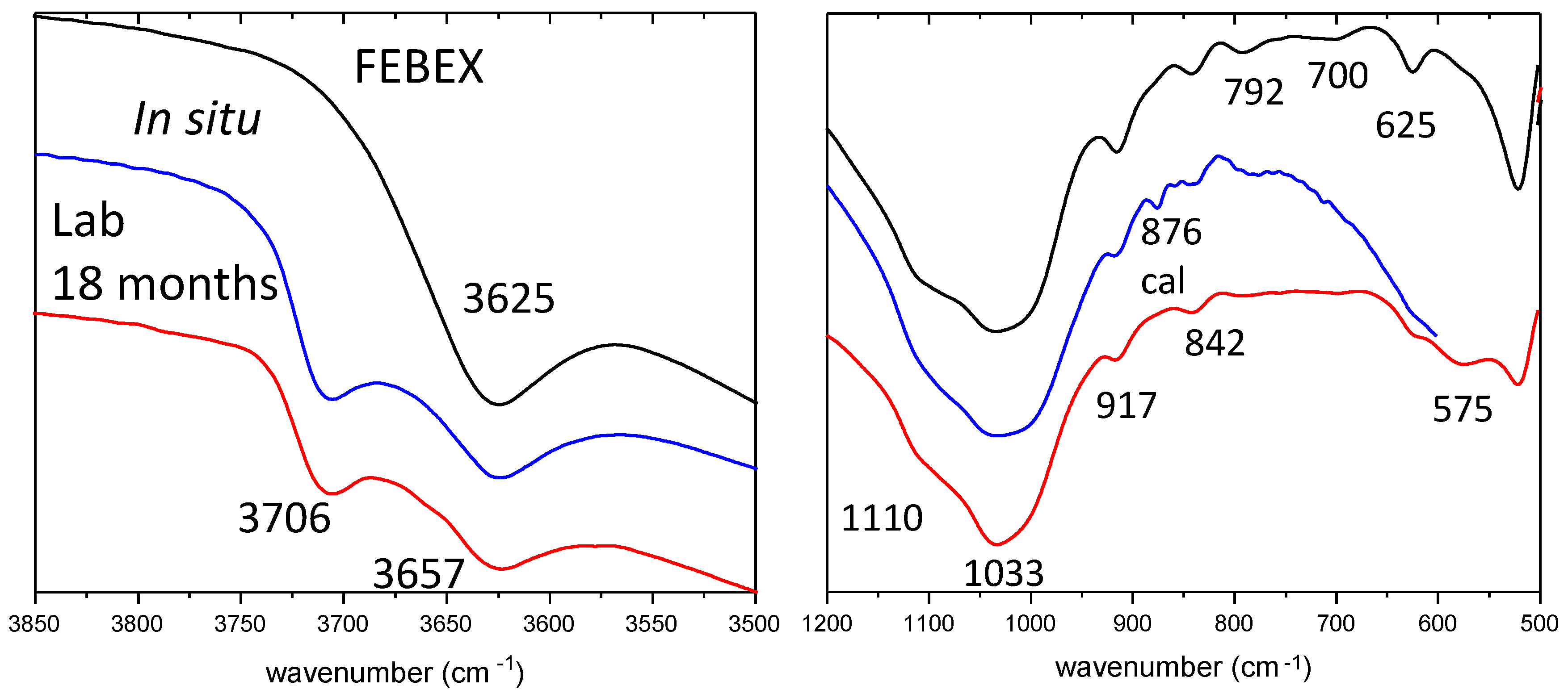
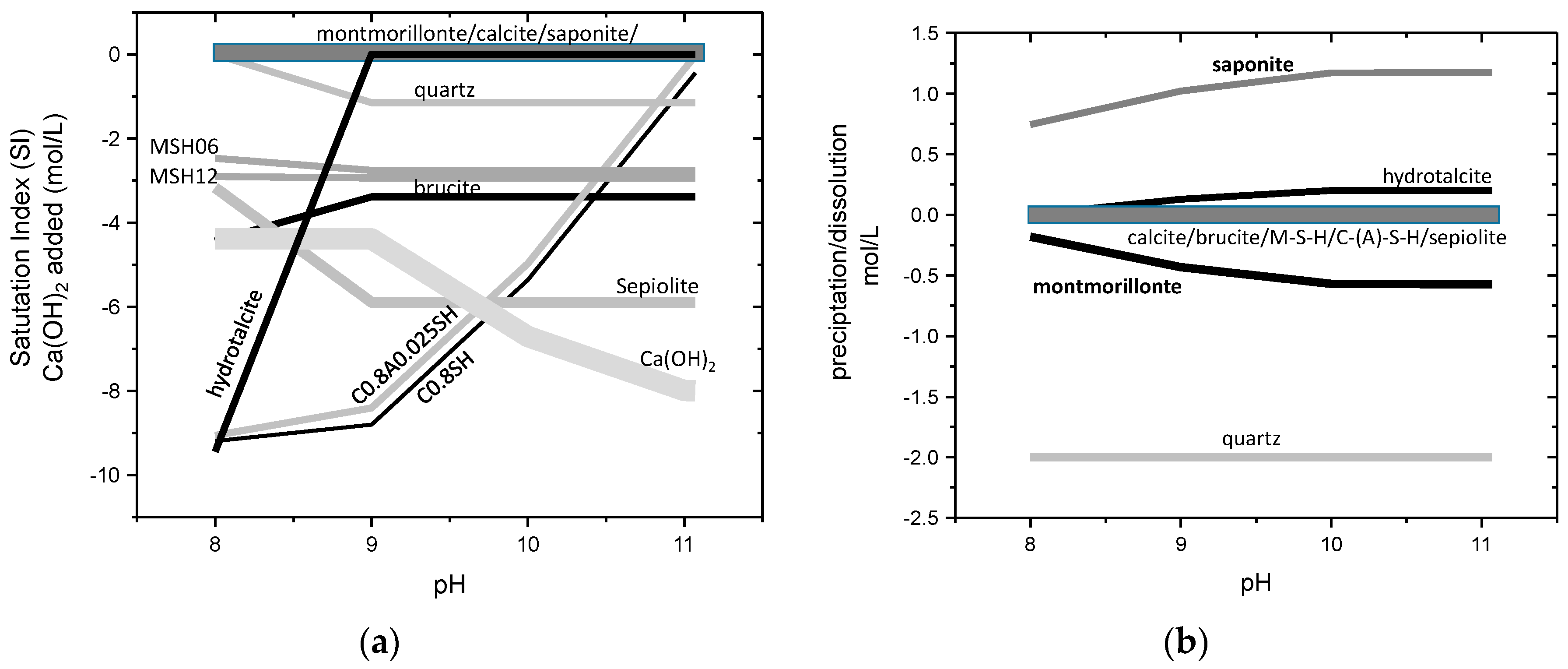
| Mineral Cation | MntF FEBEX | MntF Brc | Srp | Stv | Sap | Tlc | Sep | Chl | Ht1 | Ht2 |
|---|---|---|---|---|---|---|---|---|---|---|
| M+ | 0.5 | 0.1 | 0 | 0.2 | 0.35 | 0 | 0.033 | 0 | 0 | 0 |
| Al + Fe(3+) | 1.55 | 1.9 | 0 | 0.1 | 0.35 | 0 | 0.066 | 2 | 1 | 1 |
| Mg(2+) | 0.45 | 3.0 | 3 | 2.7 | 3 | 3 | 2.466 | 5 | 3 | 2 |
| Si(4+) 1 | 3.95 | 3.95 | 2 | 4 | 3.65 | 4 | 4.0 | 3 | 0 | 0 |
| Equilibrium Phases (Thermoddem) 1 | Initial Amount (mol/L) | Formula |
|---|---|---|
| Brucite | 0 | Mg(OH)2 |
| C0.8SH (Ca/Si 0.8 C-S-H) | 0 | Ca1.6 Si2O6.1698H1.1396:1.6122H2O |
| Calcite | 1 | CaCO3 |
| Hydrotalcite | 0 | Mg4Al2O7:10H2O |
| Montmorillonite, (High charge Mg Mnt) | 8 | Mg0.3Mg0.6Al1.4Si4O10(OH)2 |
| Quartz(alpha) | 2 | SiO2 |
| Saponite (Mg-saponite) | 0 | Mg0.17Mg3Al0.34Si3.66 O10(OH)2 |
| C0.8A0.025SH | 0 | Ca1.6Al0.04Si2O6.2343H1.1486:1.63H2O |
| MSH06; (Mg/Si 0.6 M-S-H) 2:1 sheet | 0 | Mg0.82SiO2.385(OH)0.87 |
| MSH12; (Mg/Si 1.2 M-S-H) ≈ 1:1 sheet | 0 | Mg1.07SiO2.075(OH)1.99 |
| Sepiolite | 0 | Mg4Si6O15(OH)2:6H2O |
| MgX2 2 | 4 | |
| pH_FIX 3 | 10 | Ca(OH)2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuevas, J.; Ruiz, A.I.; Fernández, R.; González-Santamaría, D.; Angulo, M.; Ortega, A.; Torres, E.; Turrero, M.J. Authigenic Clay Minerals from Interface Reactions of Concrete-Clay Engineered Barriers: A New Perspective on Mg-Clays Formation in Alkaline Environments. Minerals 2018, 8, 362. https://doi.org/10.3390/min8090362
Cuevas J, Ruiz AI, Fernández R, González-Santamaría D, Angulo M, Ortega A, Torres E, Turrero MJ. Authigenic Clay Minerals from Interface Reactions of Concrete-Clay Engineered Barriers: A New Perspective on Mg-Clays Formation in Alkaline Environments. Minerals. 2018; 8(9):362. https://doi.org/10.3390/min8090362
Chicago/Turabian StyleCuevas, Jaime, Ana Isabel Ruiz, Raúl Fernández, Daniel González-Santamaría, María Angulo, Almudena Ortega, Elena Torres, and María Jesús Turrero. 2018. "Authigenic Clay Minerals from Interface Reactions of Concrete-Clay Engineered Barriers: A New Perspective on Mg-Clays Formation in Alkaline Environments" Minerals 8, no. 9: 362. https://doi.org/10.3390/min8090362
APA StyleCuevas, J., Ruiz, A. I., Fernández, R., González-Santamaría, D., Angulo, M., Ortega, A., Torres, E., & Turrero, M. J. (2018). Authigenic Clay Minerals from Interface Reactions of Concrete-Clay Engineered Barriers: A New Perspective on Mg-Clays Formation in Alkaline Environments. Minerals, 8(9), 362. https://doi.org/10.3390/min8090362









