The Effect of Grinding on Tremolite Asbestos and Anthophyllite Asbestos
Abstract
1. Introduction
2. Materials and Methods
3. Results
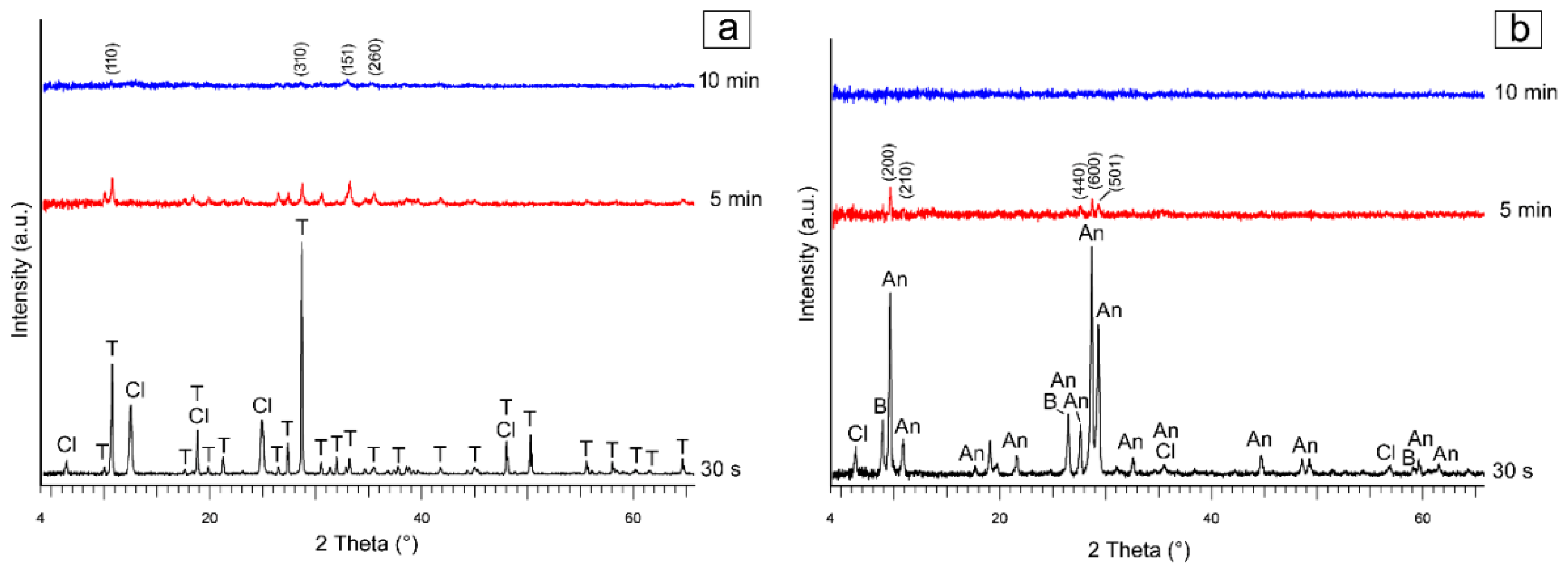
3.1. X-ray Diffraction Characterization
3.1.1. Tremolite
3.1.2. Anthophyllite
3.2. Thermal Analysis Characterization
3.2.1. Tremolite
3.2.2. Anthophyllite
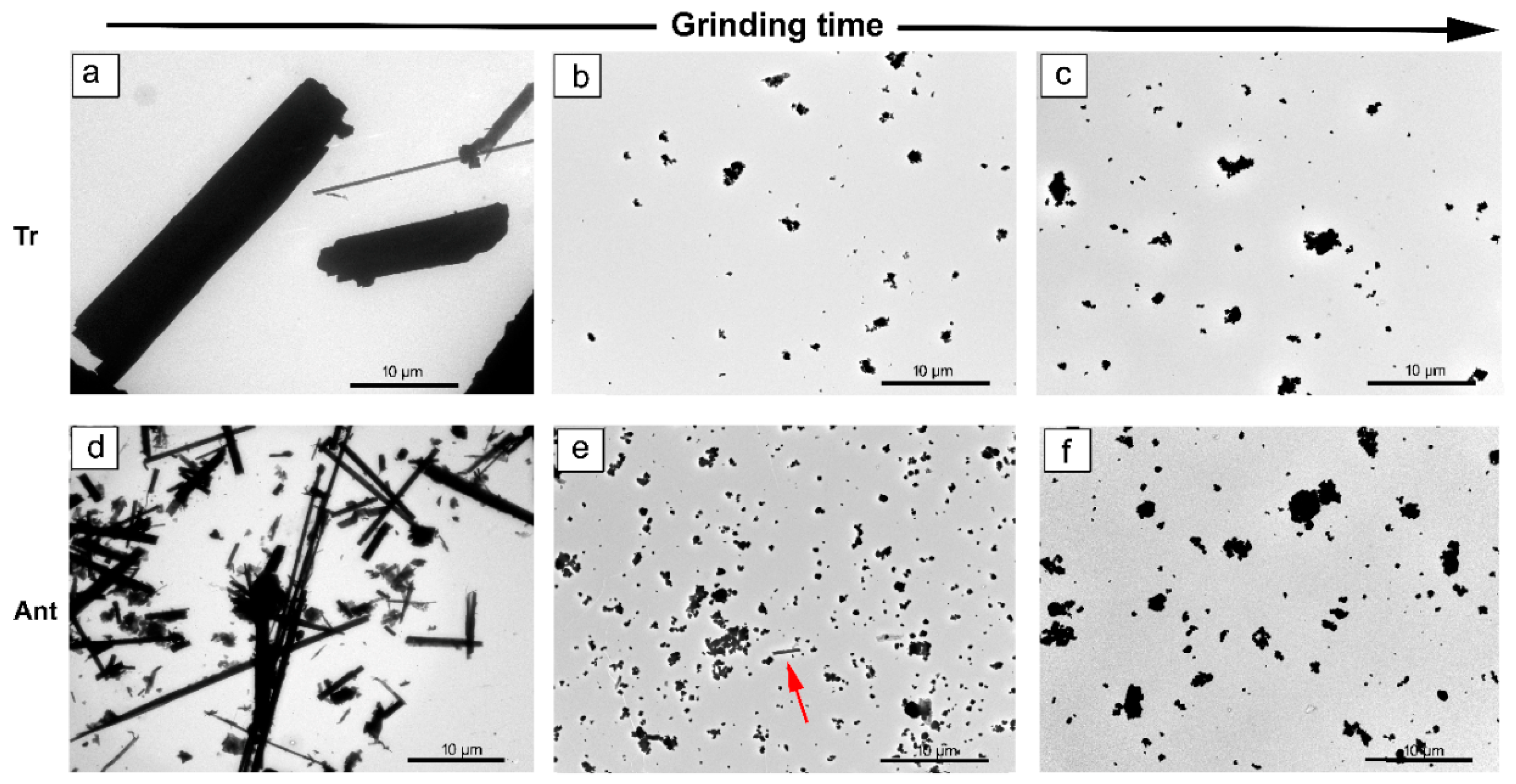
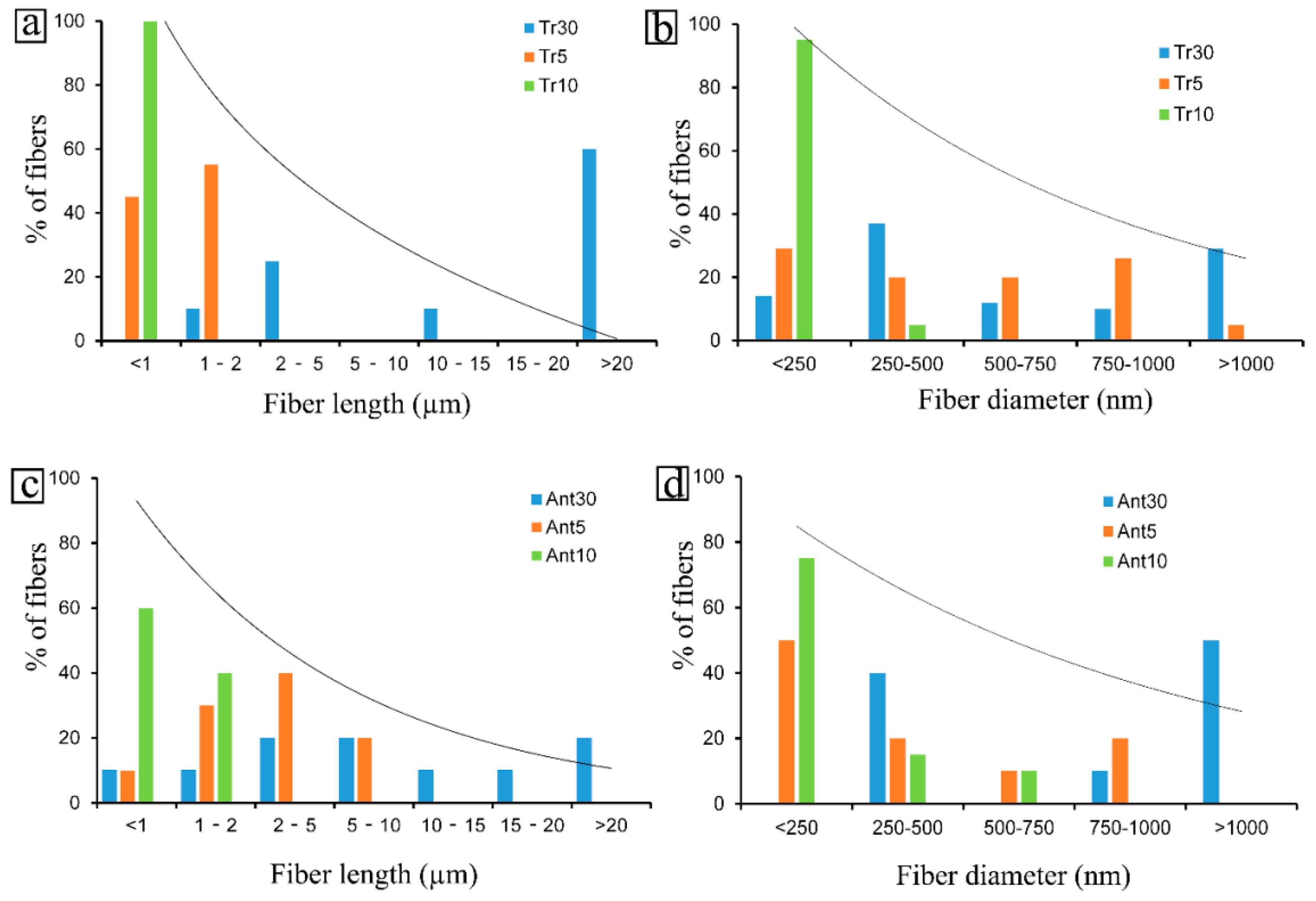
3.3. TEM Characterization
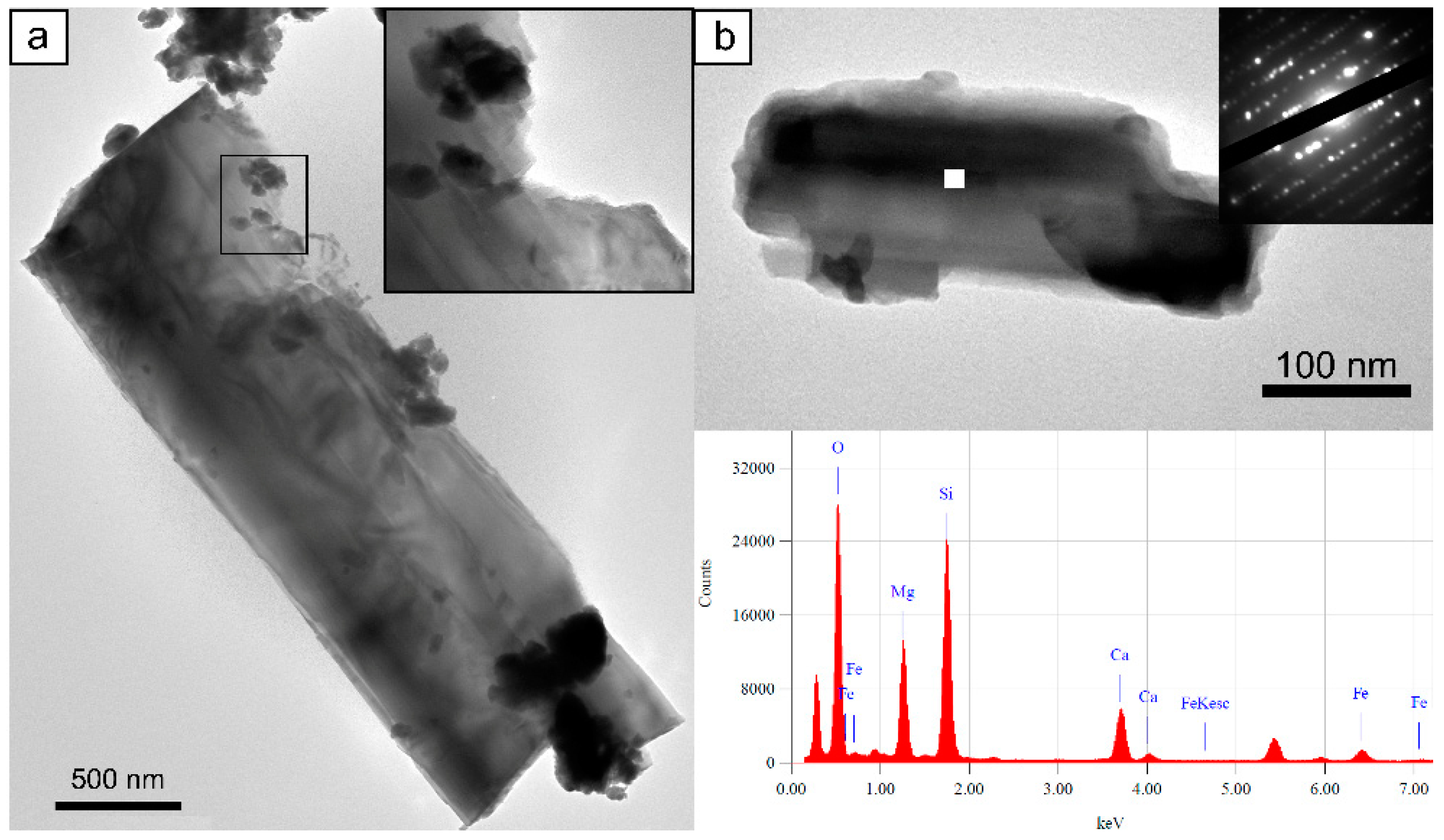
3.3.1. Tremolite
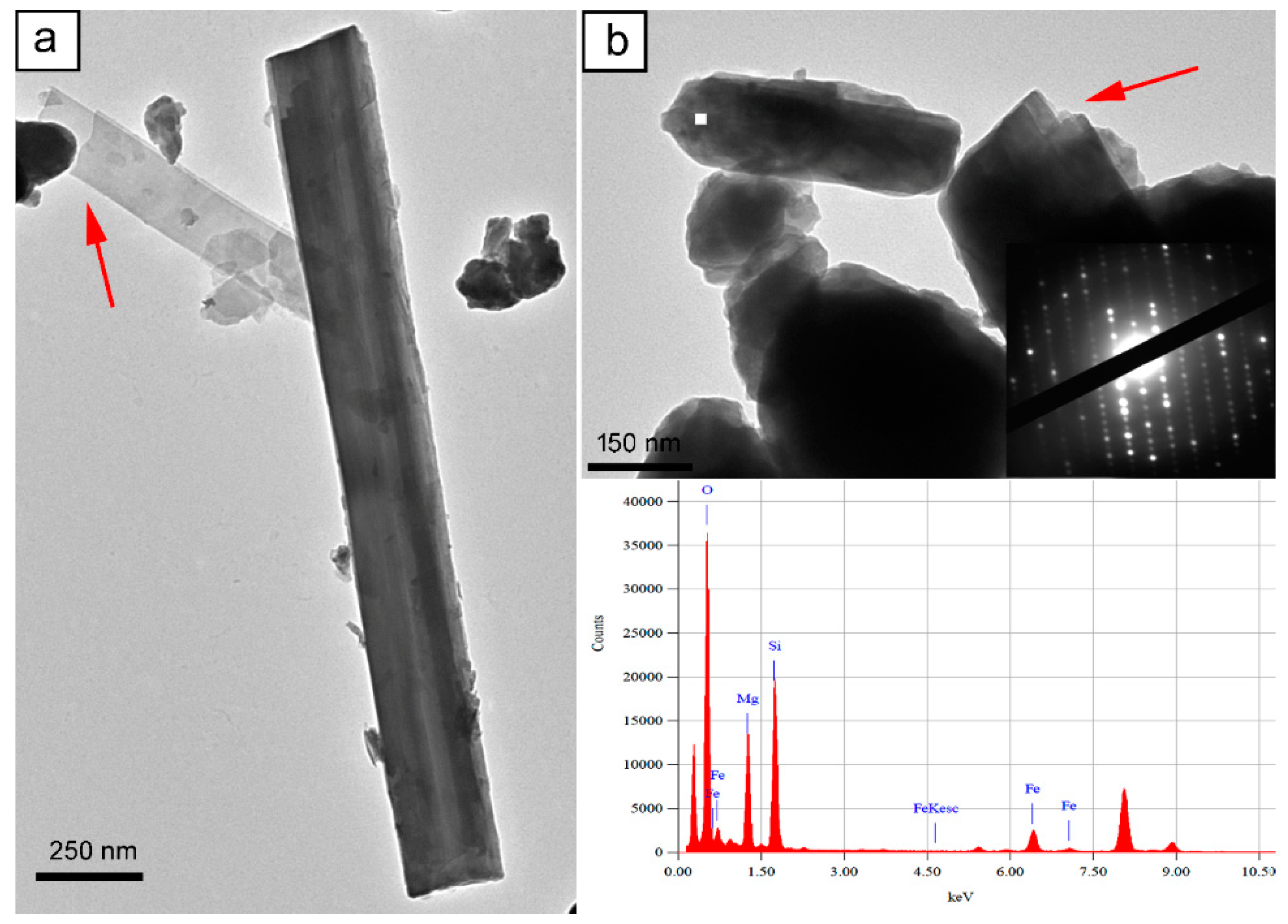
3.3.2. Anthophyllite
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer (IARC). Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42; International Agency for Research on Cancer: Lyon, French, 1987; p. 440. [Google Scholar]
- World Health Organization (WHO). Asbestos and Other Natural Mineral Fibres. Environmental Health Criteria, 53; World Health Organization: Geneva, Switzerland, 1986; p. 194. [Google Scholar]
- Meurman, L.O.; Pukkala, E.; Hakama, M. Incidence of cancer among anthophyllite asbestos miners in Finland. Occup. Environ. Med. 1994, 51, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Karjalainen, A.; Meurman, L.O.; Pukkala, E. Four cases of mesothelioma among Finnish anthophyllite miners. Occup. Environ. Med. 1994, 51, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Luce, D.; Bugel, I.; Goldberg, P.; Goldberg, M.; Salomon, C.; Billon-Galland, M.A.; Nicolau, J.; Quénel, P.; Fevotte, J.; Brochard, P. Environmental exposure to tremolite and respiratory cancer in New Caledonia: A case-control study. Am. J. Epidemiol. 2000, 151, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Favero-Longo, S.E.; Turci, F.; Tomatis, M.; Compagnoni, R.; Piervittori, R.; Fubini, B. The effect of weathering on ecopersistence, reactivity, and potential toxicity of naturally occurring asbestos and asbestiform minerals. J. Toxicol. Environ. Health A 2009, 72, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Pugnaloni, A.; Giantomassi, F.; Lucarini, G.; Capella, S.; Bloise, A.; Di Primio, R.; Belluso, E. Cytotoxicity induced by exposure to natural and synthetic tremolite asbestos: An in vitro pilot study. Acta Histochem. 2013, 115, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, S.H.; Grespin, M.; Garnick, L.; Drechsel, D.A.; Hazan, R.; Paustenbach, D.J.; Simmons, B.D. Anthophyllite asbestos: State of the science review. J. Appl. Toxicol. 2017, 37, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Medici, J.C. Minerals of the Fairfax quarry, Centreville, Virginia. Mineral. Rec. 1972, 3, 173–179. [Google Scholar]
- Geyer, A.R.; Smith, R.C.I.I.; Barnes, J.H. Mineral Collecting in Pennsylvania; Department of Environmental Resources, Topographic and Geologic Survey, General Geology Report: Pennsylvania, PA, USA, 1976; p. 260.
- Bernstein, L.R. Minerals of the Washington, D.C. Area; Maryland Geological Survey: Baltimore, MD, USA, 1980; p. 148.
- Van Gosen, B.S.; Lowers, H.A.; Sutley, S.J.; Gent, C.A. Using the geologic setting of talc deposits as an indicator of amphibole asbestos content. Environ. Geol. 2004, 45, 920–939. [Google Scholar] [CrossRef]
- Bloise, A.; Critelli, T.; Catalano, M.; Apollaro, C.; Miriello, D.; Croce, A.; Barrese, E.; Liberi, F.; Piluso, E.; Rinaudo, C.; et al. Asbestos and other fibrous minerals contained in the serpentinites of the Gimigliano-Mount Reventino unit (Calabria, S-Italy). Environ. Earth Sci. 2014, 71, 3773–3786. [Google Scholar] [CrossRef]
- Addison, J.; McConnell, E.E. A review of carcinogenicity studies of asbestos and non-asbestos tremolite and other amphiboles. Regul. Toxicol. Pharmacol. 2008, 52, 1871S–S1899. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.; Virta, R.L. Occurrence, production and uses of asbestos. In The Health Effects of Chrysotile Asbestos—Contribution of Science to Risk-Management Decisions; Nolan, R.P., Langer, A.M., Ross, M., Wicks, F.J., Martin, R.F., Eds.; The Canadian Mineralogist, Special Publication: Ottawa, ON, Canada, 2001; Volume 5, pp. 79–88. [Google Scholar]
- Baris, Y.I.; Bilir, N.; Artvinli, M.; Sahin, A.A.; Kalyoncu, F.; Sebastien, P. An epidemiological study in an Anatolian village environmentally exposed to tremolite asbestos. Br. J. Ind. Med. 1988, 45, 838–840. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.C.; Chamberlain, M.; Brown, R.C.; Berry, G.; Pooley, F.D.; Davies, R.; Griffiths, D.M. Biological effects of tremolite. Br. J. Cancer 1982, 45, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Kogel, J.E.; Trivedi, N.; Barker, J.M.; Krukowski, S.T. Industrial Minerals & Rocks: Commodities, Markets, and Uses; Society for Mining, Metallurgy, and Exploration: Littleton, CO, USA, 2006; p. 1568. [Google Scholar]
- Bowles, O. Asbestos Industry; U.S. Government Publishing Office, Bureau of Mines Bulletin: Washington, DC, USA, 1955; Volume 552, pp. 1–122.
- Virta, R.L. Mineral Commodity Profiles—Asbestos; U.S. Geological Survey Circular 1255–KK: Washington, DC, USA, 2005; p. 56.
- Gualtieri, A.F. Introduction. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Gualtieri, A.F., Ed.; European Mineralogical Union: London, UK, 2017; Volume 18, pp. 1–15. [Google Scholar]
- Huuskonen, M.S.; Ahlman, K.; Mattsson, T.; Tossavainen, A. Asbestos disease in Finland. J. Occup. Med. 1980, 22, 751–755. [Google Scholar] [PubMed]
- Gualtieri, A.F. Mineral fibre-based building materials and their health hazards. In Toxicity of Building Materials; Pacheco-Torgal, F., Jalali, S., Fucic, A., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 166–195. [Google Scholar]
- Directive 2009/148/EC of the European Parliament and of the Council of 30 November 2009 on the protection of workers from the risks related to exposure to asbestos at work. Off. J. Eur. Union 2009, 330, 28–36.
- Roggli, V.L.; Coin, P. Mineralogy of asbestos. In Pathology of Asbestos Associated Diseases; Roggli, V., Oury, T.D., Eds.; Springer Verlag: New York, NY, USA, 2004; pp. 1–17. [Google Scholar]
- Spasiano, D.; Pirozzi, F. Treatments of asbestos containing wastes. J. Environ. Manag. 2017, 204, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M. Asbestos Exposure in India; Anchor Academic Publishing: Hamburg, Germany, 2016; p. 72. [Google Scholar]
- Broughton, E. The Bhopal disaster and its aftermath: A review. Environ. Health 2005, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.; Smith, W.L.; Ashton, W.H. Triclinic talc and associated amphiboles from Gouverneur mining district New York. Am. Mineral. 1968, 53, 751–769. [Google Scholar]
- Kleinfeld, M.M.D.; Messite, J.M.D.; Zaki, M.H.M.D. Mortality experiences among talc workers: A follow-up study. J. Occup. Med. 1974, 16, 345–349. [Google Scholar] [PubMed]
- Virta, R.L. The Talc Industry-An Overview; U.S. Department of the Interior, Bureau of Mines: Washington, DC, USA, 1989; p. 30.
- CNN News. Available online: https://edition.cnn.com/2018/05/24/health/johnson--johnson-talc-asbestos-verdict-california/index.html (accessed on 25 May 2018).
- Gualtieri, A.F.; Pollastri, S.; Gandolfi, N.B.; Ronchetti, F.; Albonico, C.; Cavallo, A.; Zanetti, G.; Marini, P.; Sala, O. Determination of the concentration of asbestos minerals in highly contaminated mine tailings: An example from inactive mine waste of Cre’taz and E’marese (Valle d’Aosta, Italy). Am. Mineral. 2014, 99, 1233–1247. [Google Scholar] [CrossRef]
- Bloise, A.; Belluso, E.; Critelli, T.; Catalano, M.; Apollaro, C.; Miriello, D.; Barrese, E. Amphibole asbestos and other fibrous minerals in the meta-basalt of the Gimigliano-Mount Reventino Unit (Calabria, South-Italy). Rend Online Soc. Geol. Ital. 2012, 21, 847–848. [Google Scholar]
- Bloise, A.; Miriello, D. Multi-analytical approach for identifying asbestos minerals in situ. Geosciences 2018, 8, 133. [Google Scholar] [CrossRef]
- Bloise, A.; Catalano, M.; Critelli, T.; Apollaro, C.; Miriello, D. Naturally occurring asbestos: Potential for human exposure, San Severino Lucano (Basilicata, Southern Italy). Environ. Earth Sci. 2017, 76, 648. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Giacobbe, C.; Sardisco, L.; Saraceno, M.; Gualtieri, M.L.; Lusvardi, G.; Cavenati, C.; Zanatto, I. Recycling of the product of thermal inertization of cement-asbestos for various industrial applications. Waste Manag. 2011, 31, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Kusiorowski, R.; Zaremba, T.; Piotrowski, J.; Podwórny, J. Utilisation of cement-asbestos wastes by thermal treatment and the potential possibility use of obtained product for the clinker bricks manufacture. J. Mater. Sci. 2015, 50, 6757–6767. [Google Scholar] [CrossRef]
- Kusiorowski, R.; Zaremba, T.; Piotrowski, J. Influence of the type of pre-calcined asbestos containing wastes on the properties of sintered ceramics. Constr. Build. Mater. 2016, 106, 422–429. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kida, A.; Noma, Y.; Terazono, A.; Sakai, S. Evaluation of thermally treated asbestos based on fiber number concentration determined by transmission electron microscopy. J. Mater. Cycles Waste Manag. 2016, 20, 214–222. [Google Scholar] [CrossRef]
- Witek, J.; Kusiorowski, R. Neutralization of cement-asbestos waste by melting in an arc-resistance furnace. Waste Manag. 2017, 69, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Spasiano, D. Dark fermentation process as pretreatment for a sustainable denaturation of asbestos containing wastes. J. Hazard Mater. 2018, 349, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Kusiorowski, R.; Zaremba, T. The use of asbestos wastes as a fillers on Sorel cement. Ceram. Silik. 2018, 62, 31–40. [Google Scholar] [CrossRef]
- Plescia, P.; Gizzi, D.; Benedetti, S.; Camilucci, L.; Fanizza, C.; De Simone, P.; Paglietti, F. Mechanochemical treatment to recycling asbestos-containing waste. Waste Manag. 2003, 23, 209–218. [Google Scholar] [CrossRef]
- Inoue, R.; Kano, J.; Shimme, K.; Saito, F. Safe decomposition of asbestos by mechano-chemical reaction. Mater. Sci. Forum 2007, 561, 2257–2260. [Google Scholar] [CrossRef]
- Hashimoto, S.; Takeda, H.; Okuda, A.; Kambayashi, A.; Honda, S.; Iwamoto, Y.; Fukuda, K. Detoxification of industrial asbestos waste by low-temperature heating in a vacuum. J. Ceram. Soc. Jpn. 2008, 116, 242–246. [Google Scholar] [CrossRef]
- Colangelo, F.; Cioffi, R.; Lavorgna, M.; Verdolotti, L.; De Stefano, L. Treatment and recycling of asbestos-cement containing waste. J. Hazard. Mater. 2011, 195, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Bloise, A.; Catalano, M.; Gualtieri, A.F. Effect of grinding on chrysotile, amosite and crocidolite and implications for thermal treatment. Minerals 2018, 8, 135. [Google Scholar] [CrossRef]
- Bhattacherjee, S.; Paul, A. Correlation between chemical corrosion and structural variations in fibrous tremolite. J. Mater. Sci. 1992, 27, 704–710. [Google Scholar] [CrossRef]
- Bloise, A.; Catalano, M.; Barrese, E.; Gualtieri, A.F.; Gandolfi, N.B.; Capella, S.; Belluso, E. TG/DSC study of the thermal behaviour of hazardous mineral fibres. J. Therm. Anal. Calorim. 2016, 123, 2225–2239. [Google Scholar] [CrossRef]
- Bloise, A.; Barca, D.; Gualtieri, A.F.; Pollastri, S.; Belluso, E. Trace elements in hazardous mineral fibres. Environ. Pollut. 2016, 216, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Ballirano, P.; Bloise, A.; Gualtieri, A.F.; Lezzerini, M.; Pacella, A.; Perchiazzi, N.; Dogan, M.; Dogan, A.U. The Crystal Structure of Mineral Fibres. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Gualtieri, A.F., Ed.; European Mineralogical Union: London, UK, 2017; Volume 18, pp. 17–53. [Google Scholar]
- Luckewicz, W. Differential thermal analysis of chrysotile asbestos in pure talc and talc containing other minerals. J. Soc. Cosmet. Chem. 1975, 26, 431–437. [Google Scholar]
- Bloise, A.; Fornero, E.; Belluso, E.; Barrese, E.; Rinaudo, C. Synthesis and characterization of tremolite asbestos fibres. Eur. J. Mineral. 2008, 20, 1027–1033. [Google Scholar] [CrossRef]
- Bloise, A.; Kusiorowski, R.; Lassinantti Gualtieri, M.; Gualtieri, A.F. Thermal behaviour of mineral fibres. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Gualtieri, A.F., Ed.; European Mineralogical Union: London, UK, 2017; Volume 18, pp. 215–252. [Google Scholar]
- Wittels, M.C. The structural disintegration of some amphiboles. Am. Mineral. 1952, 37, 28–36. [Google Scholar]
- Miller, J.G.; Oulton, T.D. Prototropy in kaolinite during percussive grinding. Clays Clay Miner. 1970, 18, 313–323. [Google Scholar] [CrossRef]
- Kusiorowski, R.; Zaremba, T.; Gerle, A.; Piotrowski, J.; Simka, W.; Adamek, J. Study on the thermal decomposition of crocidolite asbestos. J. Therm. Anal. Calorim. 2015, 120, 1585–1595. [Google Scholar] [CrossRef]
- Freeman, A.G. The dehydroxylation behaviour of amphiboles. Min. Mag. 1966, 35, 953–957. [Google Scholar] [CrossRef]
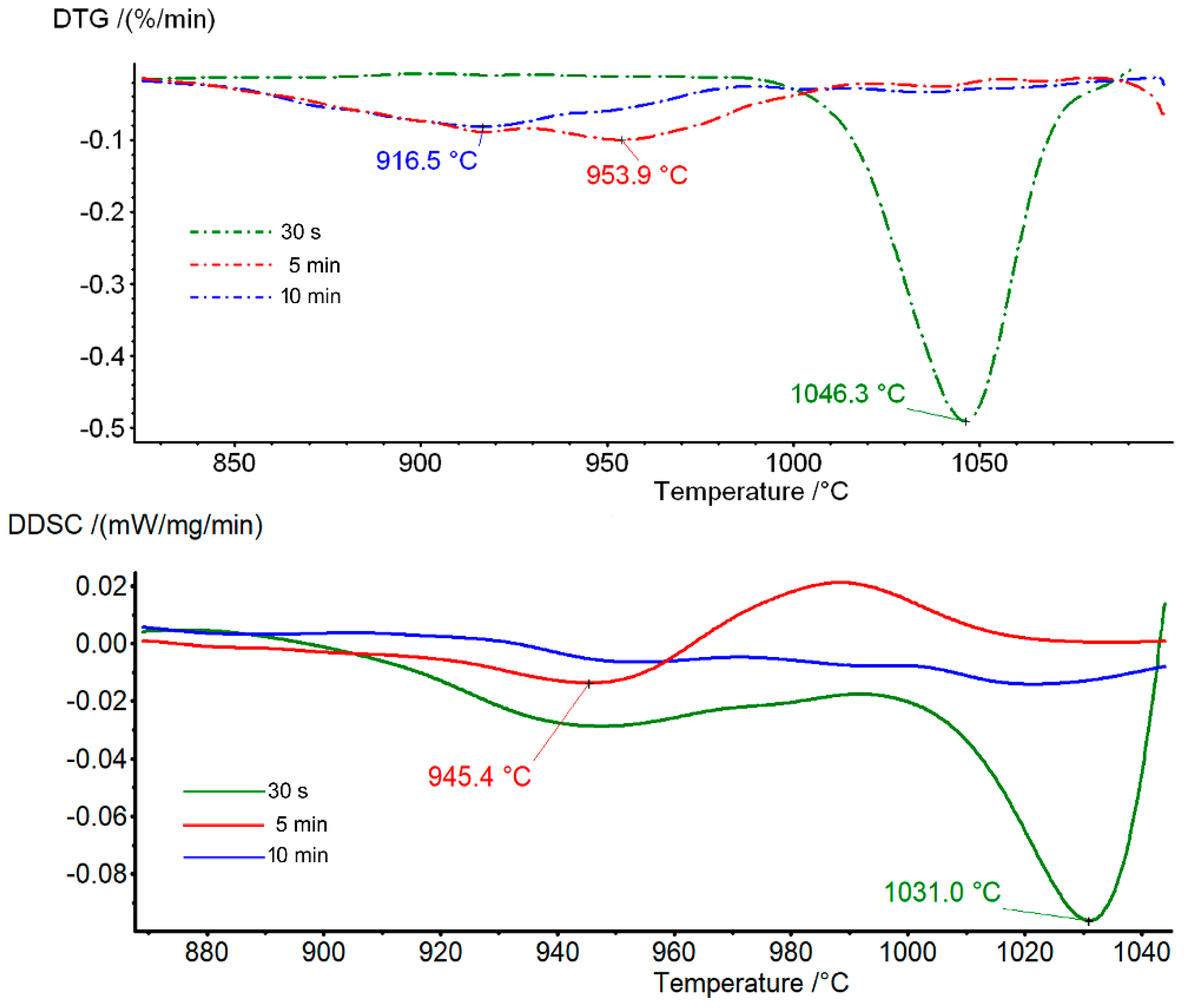
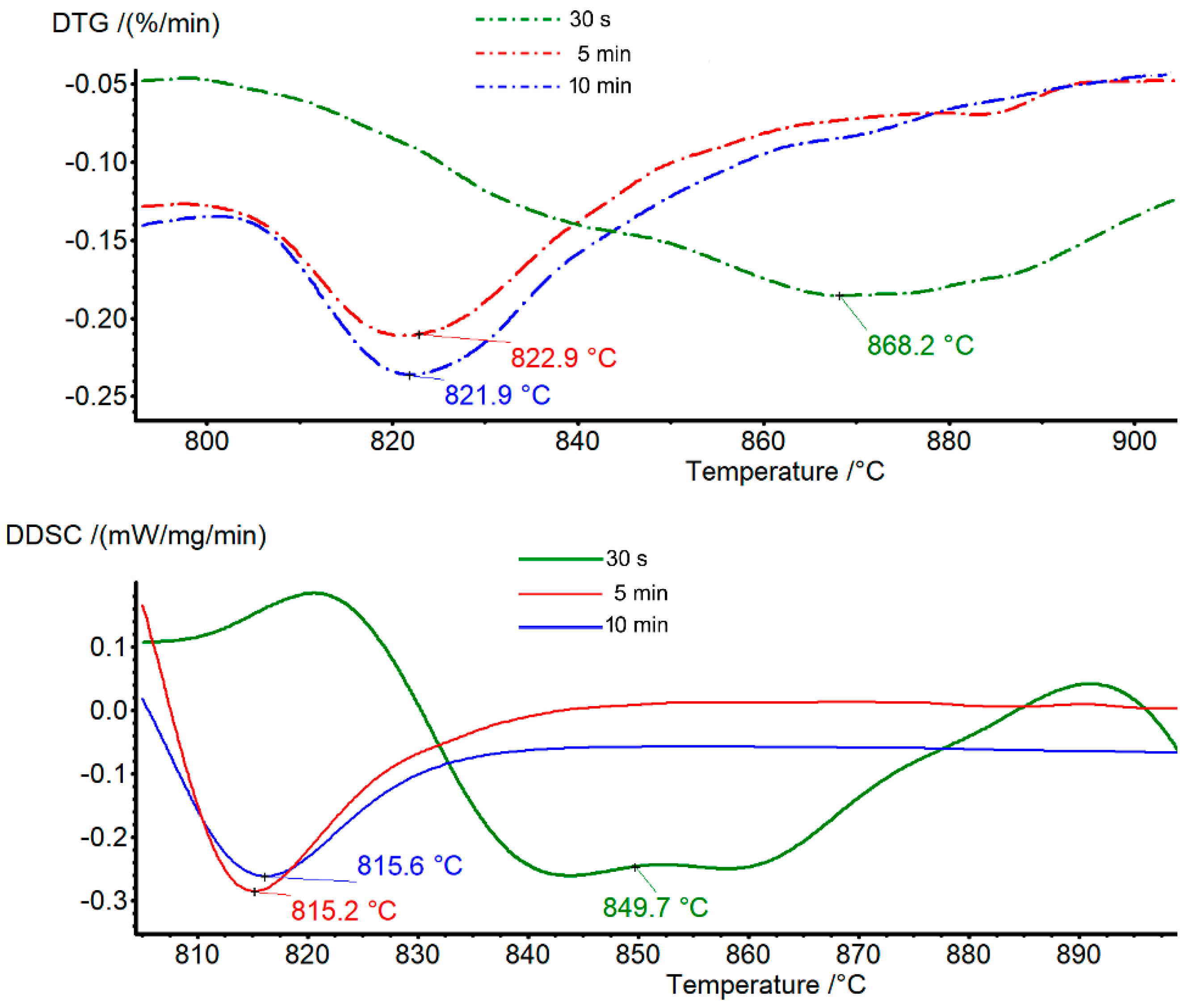
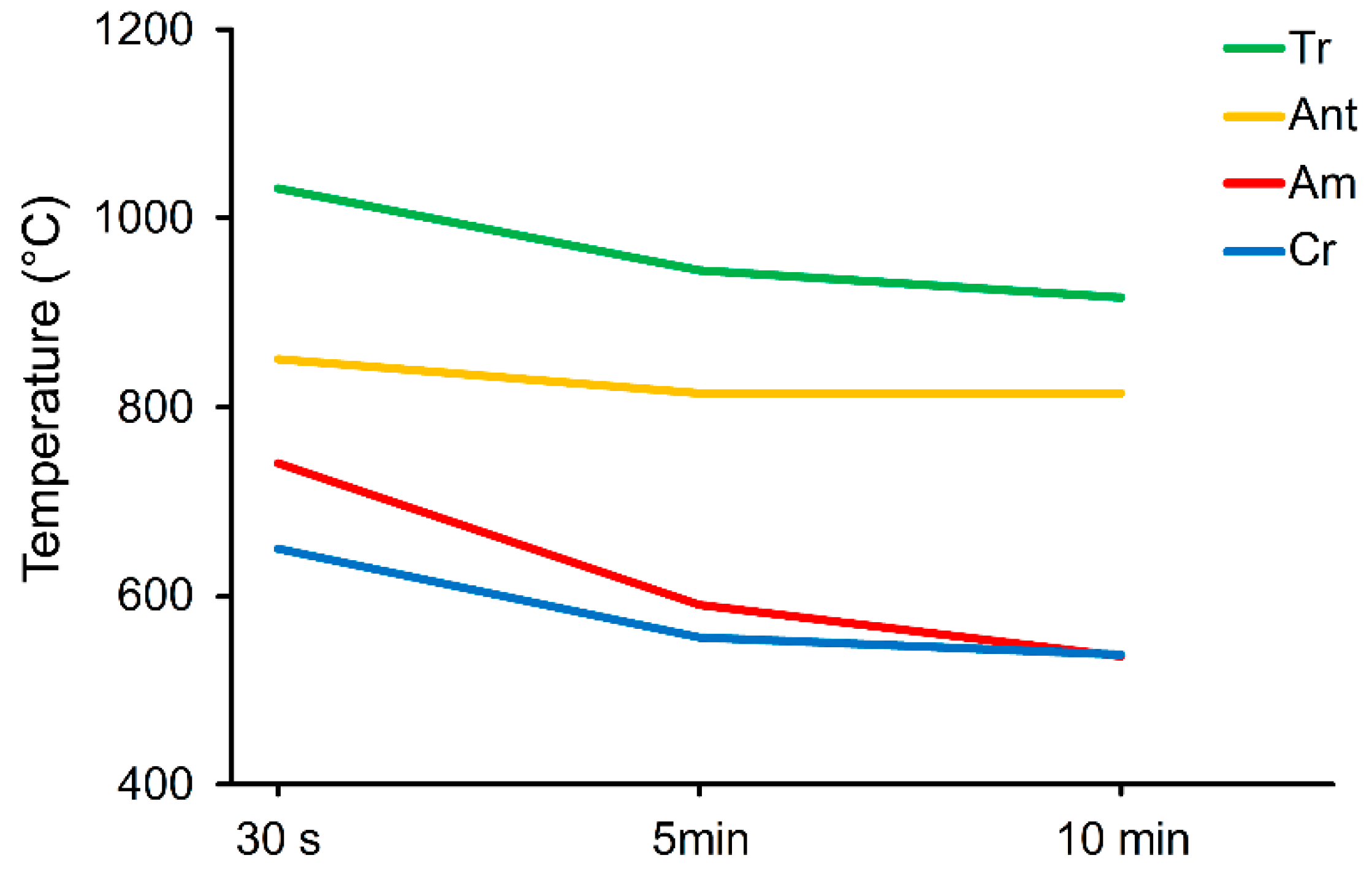
| Sample (Grinding Time) | Tr 30 s | Tr 5 min | Tr 10 min |
|---|---|---|---|
| DTG (°C) | 1046 endo s | 954 endo w | 916 endo vw |
| (start–end) (°C) | (1000–1090) | (930–1000) | (830–940) |
| DDSC (°C) | 1031 endo s | 945 endo w | -- |
| Sample (Grinding Time) | Ant 30 s | Ant 5 min | Ant 10 min |
|---|---|---|---|
| DTG (°C) | 868 endo b | 823 endo w | 822 endo w |
| (start-end) (°C) | (810–900) | (810–850) | (810–850) |
| DDSC (°C) | 850 endo b | 815 endo w | 815 endo w |
| Grinding Time | 30 s | 5 min | 10 min |
|---|---|---|---|
| Tr | 1031 endo s | 945 endo w | # 916 endo v w |
| Ant | 850 endo b | 815 endo w | 815 endo w |
| * Am | 740 endo | 591 endo | 536 endo |
| * Cr | 650 endo | 555 endo w | 538 endo w |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bloise, A.; Kusiorowski, R.; Gualtieri, A.F. The Effect of Grinding on Tremolite Asbestos and Anthophyllite Asbestos. Minerals 2018, 8, 274. https://doi.org/10.3390/min8070274
Bloise A, Kusiorowski R, Gualtieri AF. The Effect of Grinding on Tremolite Asbestos and Anthophyllite Asbestos. Minerals. 2018; 8(7):274. https://doi.org/10.3390/min8070274
Chicago/Turabian StyleBloise, Andrea, Robert Kusiorowski, and Alessandro F. Gualtieri. 2018. "The Effect of Grinding on Tremolite Asbestos and Anthophyllite Asbestos" Minerals 8, no. 7: 274. https://doi.org/10.3390/min8070274
APA StyleBloise, A., Kusiorowski, R., & Gualtieri, A. F. (2018). The Effect of Grinding on Tremolite Asbestos and Anthophyllite Asbestos. Minerals, 8(7), 274. https://doi.org/10.3390/min8070274