Raman Micro-Spectroscopy Identifies Carbonaceous Particles Lying on the Surface of Crocidolite, Amosite, and Chrysotile Fibers
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer (IARC). Asbestos (chrysotile, amosite, crocidolite, tremolite, actinolite, and anthophyllite). In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2012; Volume 100C, pp. 219–309. ISBN 978 92 832 1320 8. [Google Scholar]
- Hawthorne, F.C.; Oberti, R.; Harlow, G.E.; Maresch, W.V.; Martin, R.F.; Schumacher, J.C.; Welch, M.D. Nomenclature of the amphibole supergroup. Am. Mineral. 2012, 97, 2031–2048. [Google Scholar] [CrossRef]
- Italian Government, Legislative Decree No. 277 of 15 August 1991, Implementing EU Directives No. 80/1107/EEC, No. 82/605/EEC, No. 83/477/EEC, No. 86/188/EEC, and No. 88/642/EEC, on the Protection of Workers from the Risks Related to Exposure to Chemical, Physical and Biological Agents at Work. Gazzetta Ufficiale Supplemento Ordinario no. 200, 27 August 1991.
- Leake, B.E.; Woolley, A.R.; Arps, C.E.S.; Birch, W.D.; Gilbert, M.C.; Grice, J.D.; Hawthorne, F.C.; Kato, A.; Kisch, H.J.; Krivovichev, V.G.; et al. Nomenclature of amphiboles: Report of the Subcommittee on amphiboles of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Can. Mineral. 1997, 35, 219–246. [Google Scholar]
- Rinaudo, C.; Belluso, E.; Gastaldi, D. Assessment of the use of Raman spectroscopy for the determination of amphibole asbestos. Mineral. Mag. 2004, 68, 455–465. [Google Scholar] [CrossRef]
- Rinaudo, C.; Gastaldi, D.; Belluso, E. Characterization of chrysotile, antigorite and lizardite by FT-Raman spectroscopy. Can. Mineral. 2003, 41, 883–890. [Google Scholar] [CrossRef]
- Cooke, W.E. Pulmonary asbestosis. Br. Med. J. 1927, 2, 1024–1025. [Google Scholar] [CrossRef] [PubMed]
- Cooke, W.E. Asbestos dust and the curious bodies found in pulmonary asbestosis. Br. Med. J. 1929, 2, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Seaton, A. A short history of the toxicology of inhaled particles. Part. Fibre Toxicol. 2012, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Wachowski, L.; Domka, L. Sources and effects of asbestos and other mineral fibres present in ambient air. Pol. J. Environ. Stud. 2000, 9, 443–454. [Google Scholar]
- Spasiano, D.; Pirozzi, F. Treatments of asbestos containing wastes. J. Environ. Manag. 2017, 204, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; Malvezzi, M.; La Vecchia, C.; Levi, F.; Decarli, A.; Negri, E. The mesothelioma epidemic in Western Europe: An update. Br. J. Cancer 2004, 90, 1022–1024. [Google Scholar] [CrossRef] [PubMed]
- Peto, J.; Decarli, A.; La Vecchia, C.; Levi, F.; Negri, E. The European mesothelioma epidemic. Br. J. Cancer 1999, 79, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Andolfi, L.; Trevisan, E.; Zweyer, M.; Prato, S.; Troian, B.; Vita, F.; Borelli, V.; Soranzo, M.R.; Melato, M.; Zabucchi, G. The crocidolite fiber interaction with human mesothelial cells as investigated by combining electron microscopy, atomic force and scanning near-field optical microscopy. J. Microsc. 2013, 249, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Aust, A.E.; Cook, P.M.; Dodson, R.F. Morphological and chemical mechanisms of elongated mineral particle toxicities. J. Toxicol. Environ. Health Part B 2011, 14, 40–75. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Ly, B.H.; Dodson, R.F.; Pagano, I.; Morris, P.T.; Dogan, U.A.; Gazdar, A.F.; Pass, H.I.; Yang, H. Malignant mesothelioma: Facts, myths, and hypotheses. J. Cell. Physiol. 2012, 227, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D. Electron microscopy applied to studies of the biological significance of defects in crocidolite asbestos. J. Microsc. 1980, 120, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Fubini, B.; Mollo, L. Role of iron in the reactivity of mineral fibers. Toxicol. Lett. 1995, 82–83, 951–960. [Google Scholar] [CrossRef]
- Goodglick, L.A.; Kane, A.B. Cytotoxicity of long and short crocidolite asbestos fibers in vitro and in vivo. Cancer Res. 1990, 50, 5153–5163. [Google Scholar] [PubMed]
- Hearne, G.R.; Kolk, B.; Pollak, H.; van Wyk, J.A.; Gulumian, M. Bulk and surface modifications in detoxified crocidolite. J. Inorg. Biochem. 1993, 50, 145–156. [Google Scholar] [CrossRef]
- Martra, G.; Chiardola, E.; Coluccia, S.; Marchese, L.; Tomatis, M.; Fubini, B. Reactive sites at the surface of crocidolite asbestos. Langmuir 1999, 15, 5742–5752. [Google Scholar] [CrossRef]
- Mossman, B.; Light, W.; Wei, E. Asbestos: Mechanisms of toxicity and carcinogenicity in the respiratory tract. Annu. Rev. Pharmacol. 1983, 23, 595–615. [Google Scholar] [CrossRef] [PubMed]
- Pacella, A.; Fantauzzi, M.; Turci, F.; Cremisini, C.; Montereali, M.R.; Nardi, E.; Atzei, D.; Rossi, A.; Andreozzi, G.B. Dissolution reaction and surface iron speciation of UICC crocidolite in buffered solution at pH 7.4: A combined ICP-OES, XPS and TEM investigation. Geochim. Cosmochim. Acta 2014, 127, 221–232. [Google Scholar] [CrossRef]
- Rihn, B.; Coulais, C.; Kauffer, E.; Bottin, M.C.; Martin, P.; Yvon, F.; Vigneron, J.C.; Binet, S.; Monhoven, N.; Steiblen, G.; et al. Inhaled crocidolite mutagenicity in lung DNA. Environ. Health Perspect. 2000, 108, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.C.; Berry, G.; Timbrell, V. Mesotheliomata in rats after inoculation with asbestos and other materials. Br. J. Cancer 1973, 28, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.C.; Griffiths, D.M.; Hill, R.J. The effect of fiber size on the in vivo activity of UICC crocidolite. Br. J. Cancer 1984, 49, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.J.; Hochella, M.F., Jr.; Guthrie, G.D.; Hardy, J.A.; Aust, A.E.; Rimstidt, J.D. Asbestiform riebeckite (crocidolite) dissolution in presence of Fe chelators: Implications for mineral-induced disease. Am. Mineral. 1995, 80, 1093–1103. [Google Scholar] [CrossRef]
- Yao, S.; Della Ventura, G.; Petibois, C. Analytical characterization of cell-asbestos fiber interactions in lung pathogenesis. Anal. Bioanal. Chem. 2010, 397, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- Zucali, P.A.; Ceresoli, G.L.; De Vincenzo, F.; Simonelli, M.; Lorenzi, E.; Gianoncelli, L.; Santoro, A. Advances in the biology of malignant pleural mesothelioma. Cancer Treat. Rev. 2011, 37, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.F.; Layard, M.; Tegeris, A.; Miller, E.; May, M.; Morgan, E.; Smith, A. Relation of particle dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. J. Natl. Cancer Inst. 1981, 67, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Occupational Safety and Health Administration (OSHA). Occupational Exposure to Asbestos, Tremolite, Anthophyllite and Actinolite; [Docket No. H-033-d]; 29 CFR Parts 1910 and 1926; Federal Register 1992; OSHA: Washington, DC, USA, 1992; Volume 57, pp. 24310–24331. ISSN USA-1986-R-2725.
- World Health Organization (WHO). Environmental Health Criteria 53—Asbestos and Other Natural Mineral Fibres; International Programme for Chemical Safety: Geneva, Switzerland, 1986; ISBN 92 4 154193 8. [Google Scholar]
- Bernstein, D.; Castranova, V.; Donaldson, K.; Fubini, B.; Hadley, J.; Hesterberg, T.; Kane, A.; Lai, D.; McConnell, E.E.; Muhle, H.; et al. Testing of fibrous particles: Short-term assays and strategies. Report of an ILSI Risk Science Institute Working Group. Inhal. Toxicol. 2005, 17, 497–537. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Okimoto, G.; Jube, S.; Napolitano, A.; Pass, H.I.; Laczko, R.; DeMay, R.M.; Khan, G.; Tiirikainen, M.; Rinaudo, C.; et al. Continuous exposure to chrysotile asbestos can cause transformation of human mesothelial cells via HMGB1 and TNF-α signaling. Am. J. Pathol. 2013, 183, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.G.; Aust, A.E. Iron mobilization from crocidolite asbestos greatly enhances crocidolite-dependent formation of DNA single-strand breaks in ϕ X174 RFI DNA. Carcinogenesis 1992, 13, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.G.; Williams, M.G.; Dodson, R.F.; Aust, A.E. Iron associated with asbestos bodies is responsible for the formation of single strand breaks in ϕ X174 RFI DNA. Occup. Environ. Med. 1994, 51, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Prandi, L.; Tomatis, M.; Penazzi, N.; Fubini, B. Iron cycling mechanisms and related modifications at the asbestos surface. Ann. Occup. Hyg. 2002, 46 (suppl. I), 140–143. [Google Scholar] [CrossRef]
- Rinaudo, C.; Gastaldi, D.; Belluso, E.; Capella, S. Application of Raman spectroscopy on asbestos fibre identification. Neues Jahrbuch Fur Mineralogie Monatshefte 2005, 182, 31–36. [Google Scholar] [CrossRef]
- Croce, A.; Allegrina, M.; Rinaudo, C.; Gaudino, G.; Yang, H.; Carbone, M. Numerous iron-rich particles lie on the surface of erionite fibers from Rome (Oregon, USA) and Karlik (Cappadocia, Turkey). Microsc. Microanal. 2015, 21, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.J.; Edwards, R.E.; Carthew, P. Early changes in the pleural mesothelium following intrapleural inoculation of the mineral fibre erionite and the subsequent development of mesotheliomas. J. Exp. Pathol. 1990, 71, 105–118. [Google Scholar]
- International Agency for Research on Cancer (IARC). Erionite. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2012; Volume 100C, pp. 311–316. ISBN 978 92 832 1320 8. [Google Scholar]
- DiPaolo, J.A.; DeMarinis, A.J.; Doniger, J. Asbestos and benzo(a)pyrene synergism in the transformation of Syrian hamster embryo cells. Pharmacology 1983, 27, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Eastman, A.; Mossman, B.T.; Bresnick, E. Influence of asbestos on the uptake of benzo(a)pyrene and DNA alkylation in hamster tracheal epithelial cells. Cancer Res. 1983, 43, 1251–1255. [Google Scholar] [PubMed]
- Kimizuka, G.; Azuma, M.; Ishibashi, M.; Shinozaki, K.; Hayashi, Y. Co-carcinogenic effect of chrysotile and amosite asbestos with benzo(a) pyrene in the lung of hamsters. Pathol. Int. 1993, 43, 149–153. [Google Scholar] [CrossRef]
- Croce, A.; Musa, M.; Allegrina, M.; Rinaudo, C.; Baris, Y.I.; Dogan, A.U.; Powers, A.; Rivera, Z.; Bertino, P.; Yang, H.; et al. Micro-Raman spectroscopy identifies crocidolite and erionite fibers in tissue sections. J. Raman Spectrosc. 2013, 44, 1440–1445. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Giacobbe, C.; Rinaudo, C.; Croce, A.; Allegrina, M.; Gaudino, G.; Yang, H.; Carbone, M. Preliminary results of the spectroscopic and structural characterization of mesothelioma inducing crocidolite fibers injected in mice. Period. Mineral. 2013, 82, 299–312. [Google Scholar] [CrossRef]
- Pollastri, S.; Perchiazzi, N.; Lezzerini, M.; Plaisier, J.R.; Cavallo, A.; Dalconi, M.C.; Bursi Gandolfi, N.; Gualtieri, A.F. The crystal structure of mineral fibres 1. Chrysotile. Period. Mineral. 2016, 85, 249–259. [Google Scholar] [CrossRef]
- Beyssac, O.; Goffé, B.; Chopin, C.; Rouzaud, J.N. Raman spectra of carbonaceous material in metasediments: A new geothermometer. J. Metamorph. Geol. 2002, 20, 859–871. [Google Scholar] [CrossRef]
- Beyssac, O.; Goffé, B.; Petitet, J.P.; Froigneux, E.; Moreau, M.; Rouzaud, J.N. On the characterization of disordered and heterogeneous carbonaceous materials by Raman spectroscopy. Spectrochim. Acta Part A 2003, 59, 2267–2276. [Google Scholar] [CrossRef]
- Boccaleri, E.; Arrais, A.; Frache, A.; Gianelli, W.; Fino, P.; Camino, G. Comprehensive spectral and instrumental approaches for the easy monitoring of features and purity of different carbon nanostructures for nanocomposite applications. Mater. Sci. Eng. B Solid 2006, 131, 72–82. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.L.; Couzi, M. Raman spectra of Carbon-based materials (from graphite to carbon black) and of some silicone composites. C 2015, 1, 77–94. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 95–107. [Google Scholar] [CrossRef]
- Jehlička, J.; Beny, C. First and second order Raman spectra of natural highly carbonified organic compounds from metamorphic rocks. J. Mol. Struct. 1999, 480–481, 541–545. [Google Scholar] [CrossRef]
- Lespade, P.; Al-Jishi, R.; Dresselhaus, M.S. Model for Raman scattering from incompletely graphitized carbons. Carbon 1982, 20, 427–431. [Google Scholar] [CrossRef]
- Lespade, P.; Marchand, A.; Couzi, M.; Cruege, F. Caracterisation de materiaux carbones par microspectrometrie Raman. Carbon 1984, 22, 375–385. [Google Scholar] [CrossRef]
- Nakamizo, M. Raman spectra of iron-containing glassy carbons. Carbon 1991, 29, 757–761. [Google Scholar] [CrossRef]
- Groppo, C.; Rinaudo, C.; Cairo, S.; Gastaldi, D.; Compagnoni, R. Micro-Raman spectroscopy for a quick and reliable identification of serpentine minerals from ultramafics. Eur. J. Mineral. 2006, 18, 319–329. [Google Scholar] [CrossRef]
- Arrais, A.; Diana, E.; Boccaleri, E. A study on the carbon soot derived from the wood combustion and on the relative alkali-extractable fraction. J. Mater. Sci. 2006, 41, 6035–6045. [Google Scholar] [CrossRef]
- Marshall, C.P.; Love, G.D.; Snape, C.E.; Hill, A.C.; Allwood, A.C.; Walter, M.R.; Van Kranendonk, M.J.; Bowden, S.A.; Sylva, S.P.; Summons, R.E. Structural characterization of kerogen in 3.4 Ga Archaean cherts from the Pilbara Craton, Western Australia. Precambrian Res. 2007, 155, 1–23. [Google Scholar] [CrossRef]
- Tuschel, D. The effect of microscope objectives on the Raman spectra of crystals. Spectroscopy 2017, 32, 14–23. [Google Scholar]
- Lewis, I.R.; Chaffin, N.C.; Gunter, M.E.; Griffiths, P.R. Vibrational spectroscopic studies of asbestos and comparison of suitability for remote analysis. Spectrochim. Acta A 1996, 52, 315–328. [Google Scholar] [CrossRef]
- Bard, D.; Yarwood, J.; Tylee, B. Asbestos fibre identification by Raman microspectrometry. J. Raman Spectrosc. 1997, 28, 803–809. [Google Scholar] [CrossRef]
- Miyano, T.; Beukes, N.J. Mineralogy and petrology of the contact metamorphosed amphibole asbestos-bearing Penge iron formation, Eastern Transvaal, South Africa. J. Petrol. 1997, 38, 651–676. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; John Wiley and Sons: Chichester, NY, USA, 2001; ISBN 0-471-85298-8. [Google Scholar]
- Gibbs, G.W. The organic geochemistry of chrysotile asbestos from the Eastern Township, Quebec. Geochim. Cosmochim. Acta 1971, 35, 485–502. [Google Scholar] [CrossRef]
- Hilborn, J.J.; Thomas, R.S.; Lao, R.C. The organic content of the international reference samples of asbestos. Sci. Total Environ. 1974, 3, 129–140. [Google Scholar] [CrossRef]
- Bowes, D.R.; Farrow, C.M. Major and trace element composition of the UICC standard asbestos samples. Am. J. Ind. Med. 1997, 32, 592–594. [Google Scholar] [CrossRef]
- Commins, B.T.; Gibbs, G.W. Contaminating organic material in asbestos. Br. J. Cancer 1969, 23, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Harington, J.S. Chemical studies of asbestos. Ann. N. Y. Acad. Sci. 1965, 132, 31–47. [Google Scholar] [CrossRef] [PubMed]
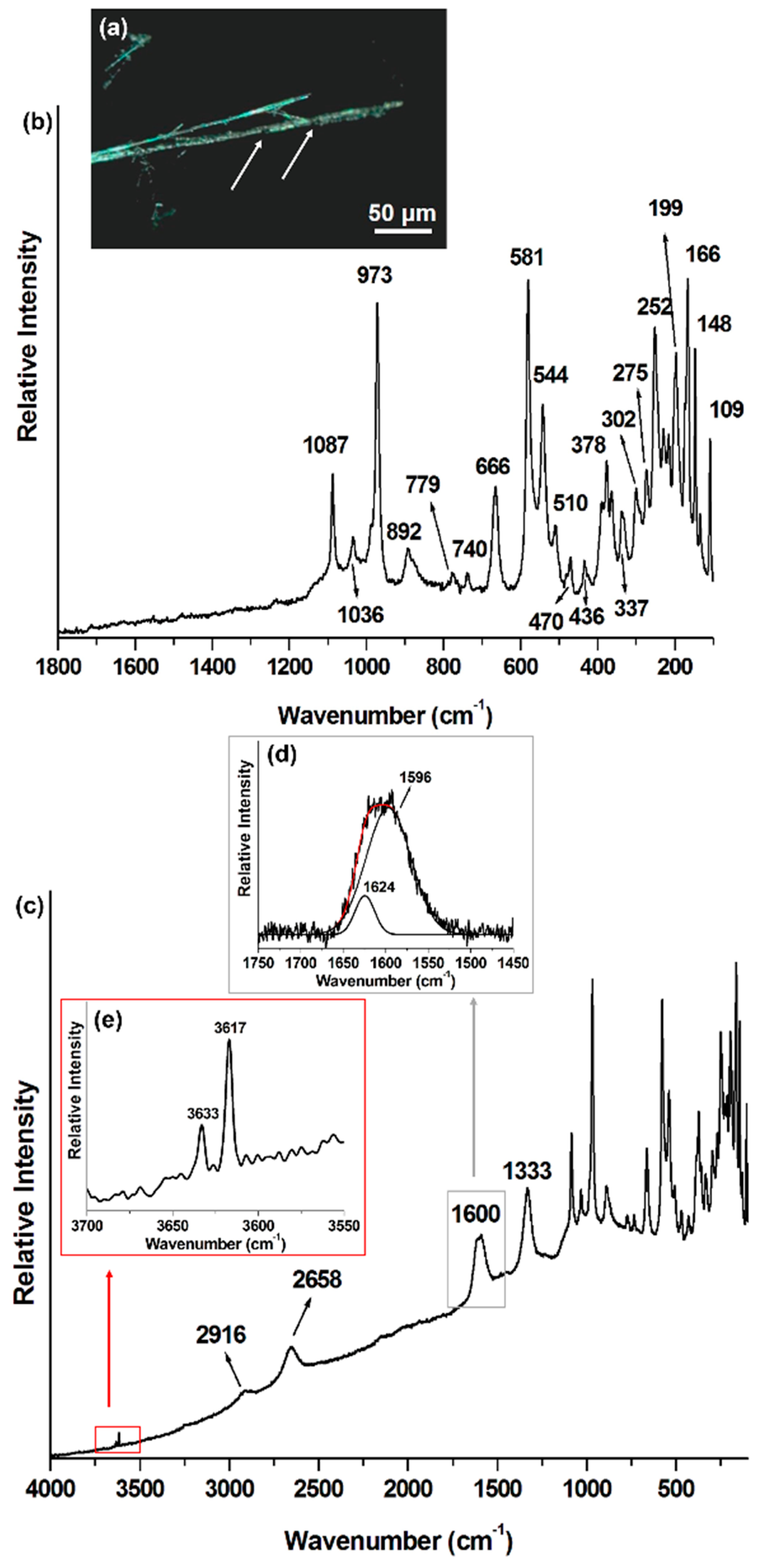
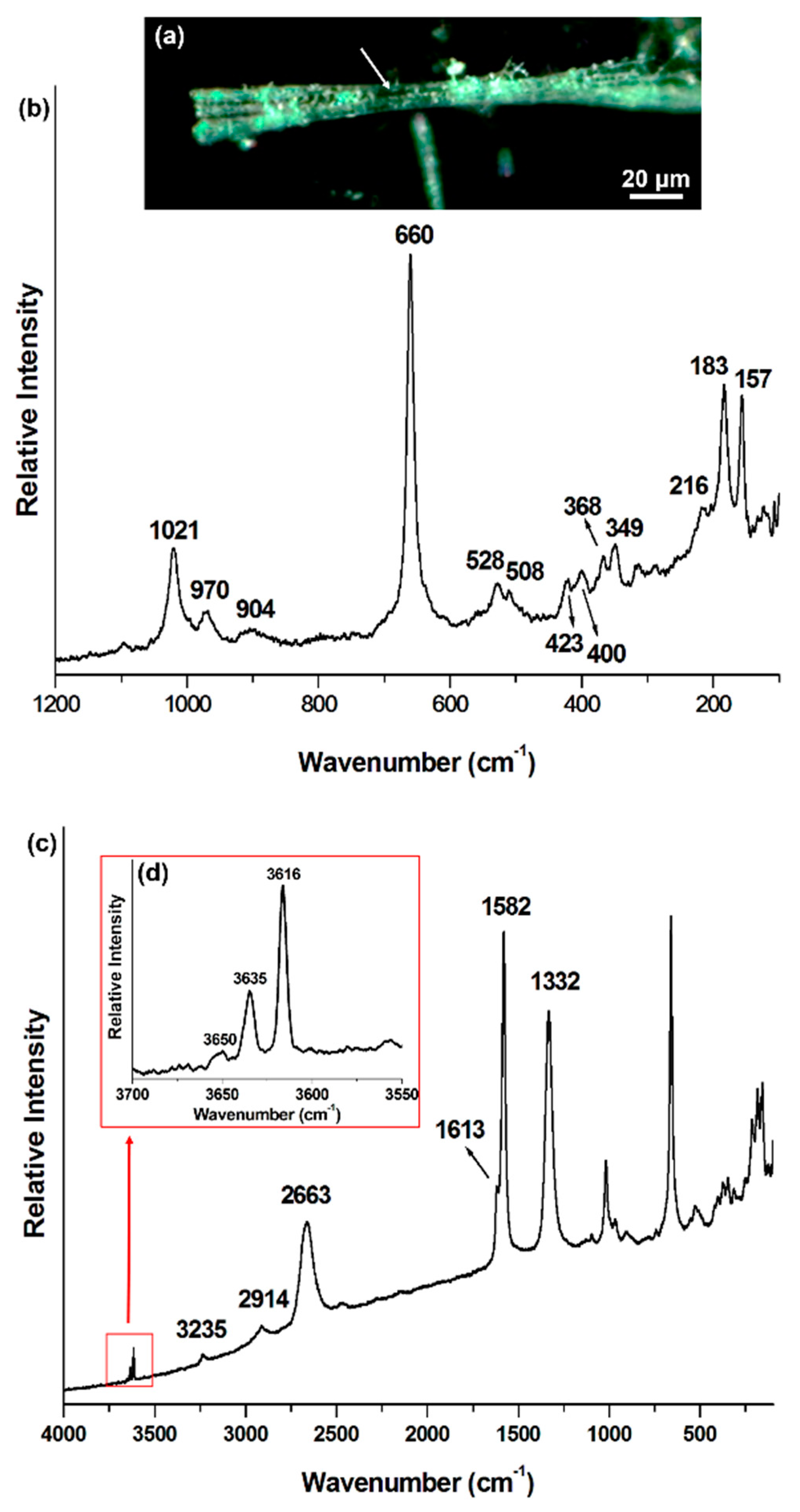
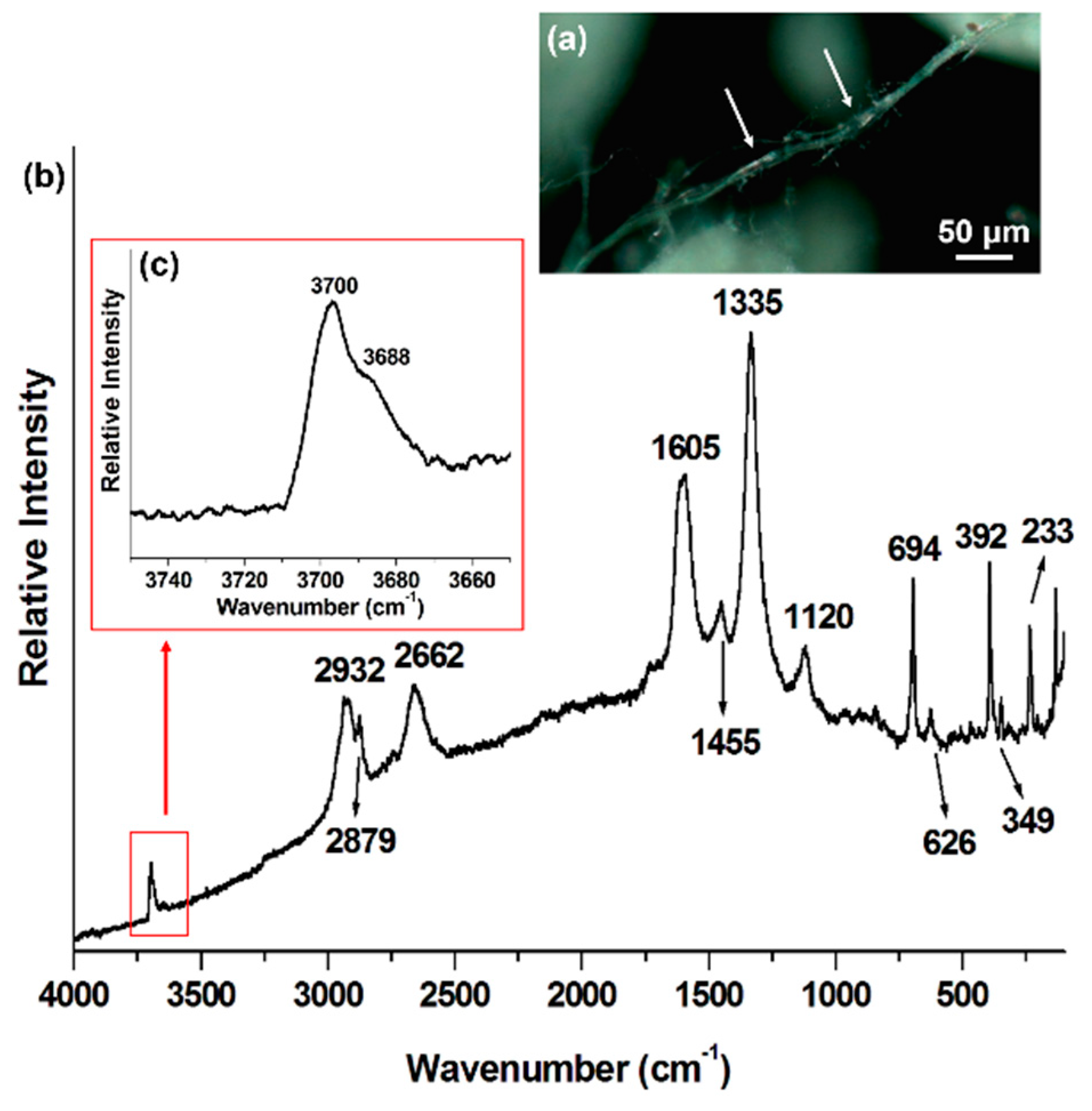
| Mineral Phase | Crocidolite This Work | Crocidolite Rinaudo et al., 2004 [5] | Amosite This Work | Amosite Rinaudo et al., 2004 [5] | Chrysotile This Work | Chrysotile Rinaudo et al., 2003 [6] |
|---|---|---|---|---|---|---|
| Mineral Raman Bands (cm−1) | 109 | 157 | 155 | 233 | 231 | |
| 148 | 183 | 182 | 349 | 345 | ||
| 166 | 162 | 216 | 216 | 392 | 389 | |
| 199 | 195 | 252 | 626 | 620 | ||
| 252 | 246 | 289 | 694 | 692 | ||
| 275 | 272 | 307 | 1105 | |||
| 302 | 300 | 349 | 348 | |||
| 337 | 331 | 368 | 368 | |||
| 360 | 400 | 400 | ||||
| 378 | 374 | 423 | 423 | |||
| 436 | 428 | 508 | 507 | |||
| 470 | 470 | 528 | 528 | |||
| 510 | 506 | 660 | 659 | |||
| 544 | 537 | 904 | 904 | |||
| 581 | 577 | 970 | 968 | |||
| 666 | 664 | 1021 | 1020 | |||
| 740 | 733 | 1093 | ||||
| 779 | 771 | |||||
| 892 | 889 | |||||
| 973 | 967 | |||||
| 1036 | 1030 | |||||
| 1087 | 1082 | |||||
| Carbonaceous phase Raman bands (cm−1) | n. a. * | n. a. | 1120 (C–O–C) | n. a. | ||
| 1333 (D) | 1332 (D) | 1335 (D) | ||||
| 1455 (C–H) | ||||||
| 1596 (G) | 1582 (G) | |||||
| 1624 (D′) | 1613 (D′) | 1605 (G) | ||||
| 2658 (G′) | 2663 (G′) | 2662 (G′) | ||||
| 2879 (C–H) | ||||||
| 2916 (C–H) | 2914 (C–H) | 2932 (C–H) | ||||
| 3235 (2D′) | ||||||
| OH− mineral Raman bands (cm−1) | 3617 | n. a. | 3616 | n. a. | 3668 | n. a. |
| 3633 | 3635 | 3670 | ||||
| 3650 | ||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croce, A.; Arrais, A.; Rinaudo, C. Raman Micro-Spectroscopy Identifies Carbonaceous Particles Lying on the Surface of Crocidolite, Amosite, and Chrysotile Fibers. Minerals 2018, 8, 249. https://doi.org/10.3390/min8060249
Croce A, Arrais A, Rinaudo C. Raman Micro-Spectroscopy Identifies Carbonaceous Particles Lying on the Surface of Crocidolite, Amosite, and Chrysotile Fibers. Minerals. 2018; 8(6):249. https://doi.org/10.3390/min8060249
Chicago/Turabian StyleCroce, Alessandro, Aldo Arrais, and Caterina Rinaudo. 2018. "Raman Micro-Spectroscopy Identifies Carbonaceous Particles Lying on the Surface of Crocidolite, Amosite, and Chrysotile Fibers" Minerals 8, no. 6: 249. https://doi.org/10.3390/min8060249
APA StyleCroce, A., Arrais, A., & Rinaudo, C. (2018). Raman Micro-Spectroscopy Identifies Carbonaceous Particles Lying on the Surface of Crocidolite, Amosite, and Chrysotile Fibers. Minerals, 8(6), 249. https://doi.org/10.3390/min8060249






