Abstract
Cold-water corals (CWCs) are frequently found at cold seep areas. However, the relationship between fluid seepage and CWC development is not clear. Here, for the first time, we report the occurrences, species identification, mineralogy, carbon and oxygen isotopes, as well as elemental compositions of fossil CWC skeletons from gas-hydrate-bearing sediment in drilling cores from the South China Sea (SCS). Three sites (GMGS-08, GMGS-09B, and GMGS-16) were investigated but CWCs were only found at one site (GMGS-09B). Interestingly, the CWCs were found in three horizons and they were all embedded with authigenic carbonates. Three genera of fossil CWCs (Crispatotrochus sp., Solenosmilia sp. and Enallopsammia sp.) were identified. The CWC fragments are predominantly aragonite. The CWCs exhibit δ13C values between −8.4‰ and −0.6‰ that are significantly higher than δ13C values of the associated seep carbonates (δ13C values with an average of −55.6‰, n = 19), which indicates a carbon source other than methane for the CWCs. It appears that authigenic carbonates provide a substratum for coral colonization. Bathymetric high points, appropriate water temperature and stronger bottom-water currents at site GMGS-09B might be crucial to keep conditions favorable for the growth of CWCs in the studied area. In addition, high trace-element concentrations of Cr, Ni, Pb, U, Ba, Th, and Sr suggest that the CWCs are influenced by strong fluid seepage that can reach the water-sediment interface, and associated microbial activity. Hence, it also becomes evident that CWCs in hydrocarbon-rich seepage areas not only provide a critical constraint on the impact of fluid emission on the bottom water chemistry, but also are likely to be very precise recorders of the end time of cold seep activity.
1. Introduction
Cold-water corals (CWCs), also termed deep-water corals, belong to the phylum Cnidaria. They include black corals (Antipatharia), stony corals (Scleractinia), hydrocorals (Stylasteridae), and soft corals (Octocorallia) [1,2]. These corals commonly occur in marine settings on continental margins, slopes, seamounts, and deep-sea basins in water depths of 30–1000 m, e.g., the moderately deep parts of the Southern Ocean and North Atlantic [3,4,5]. Rather than relying on symbiotic algae, the shallow-water coral’s food supply, CWCs feed on plankton, organic matter, and tiny organisms in the oceans [1,6]. As such, CWCs are able to live in environments without sunlight and can provide paleoceanographic records in regions where other fossils are usually sparse [2].
Previous studies of CWCs have focused mainly on the geochemical record of deep-ocean changes archived in their skeletons [2,7]. Accurate dating by uranium-series disequilibrium and 14C systems are facilitated by the high contents of U in their aragonitic skeletons [8,9,10,11,12,13].Various geochemical proxies, including isotopes of carbon, oxygen, and neodymium, rare-earth elements (REEs), trace elements, and Ba/Ca, Mg/Ca and Sr/Ca ratios of CWC skeletons are commonly used to reveal oceanic processes such as carbon cycling [14,15], past water-mass changes [16,17,18], hydrothermal activity [7], changes in water temperature [19], and ocean acidification [20].
It has been proposed that the development of CWCs is controlled by various oceanographic conditions, including salinity, temperature, substrate, tractive current, and dissolved oxygen [21], as well as hydrocarbon seepage [22]. CWCs have been reported in association with hydrocarbon seeps in Norwegian fjords [6], offshore Ireland [23], the Kristen hydrocarbon field [24], the Norwegian Margin [25], the Hikurangi Margin (New Zealand) [26,27], eastern central Atlantic [28], the Gulf of Mexico [29], and the Gulf of Cádiz [30]. However, the influence of fluid seepage on CWC growth is not yet fully understood. One theory, termed the “hydraulic theory”, suggests that the energy and nutrient supply of CWCs may be directly fueled by cold seepage [22,31,32]. However, the absence of DNA from methane- and sulfide-oxidizing microbes in CWCs from an active hydrocarbon-rich seepage area of the Gulf of Cádiz indicates that CWCs do not harbor the chemosynthetic symbionts required to consume the reduced compounds in hydrocarbon-rich fluids [33]. It is possible that CWCs use authigenic carbonates, formed through anaerobic oxidation of methane (AOM) mediated by a syntrophic consortium of anaerobic methanotrophic archaea and sulfate-reducing bacteria [34,35,36,37,38,39], as a hard substrate for coral larval settlement [26,33,40,41,42]. In addition, CWCs potentially benefit from fluid-seepage-related structures such as mud volcanoes and carbonate mounds, as these structures provide morphological barriers that direct bottom currents to deliver nutrients to the corals [33,43,44].
CWCs in the South China Sea (SCS) were first discovered by Bassett-Smith [45] near the Zhongsha Islands and the Nansha Islands. Subsequently, many species of CWCs have been observed near the Xisha Islands and along the northern margin of the SCS [46]. Previous studies have focused mostly on species identification and ecological analysis with only limited reports of geochemical data. No evidence of CWCs being present in cold-seep areas of the SCS was reported until 2013, when abundant authigenic carbonates and cold-seep biota including CWCs were discovered in gas-hydrate-bearing sediments from cores GMGS2-08, GMGS2-09B, and GMGS2-16 obtained during China’s second gas-hydrate drilling expedition (GMGS-2) (Figure 1). Despite the near-identical oceanographic and sedimentological conditions of the three drill cores, CWCs were found only at core site GMGS2-09B (Figure 2). However, the exact environmental conditions that regulate the occurrence of CWCs in this region remain unresolved. These gas-hydrate drill cores represent excellent materials to study the environmental factors that affect CWC development in methane-rich settings.
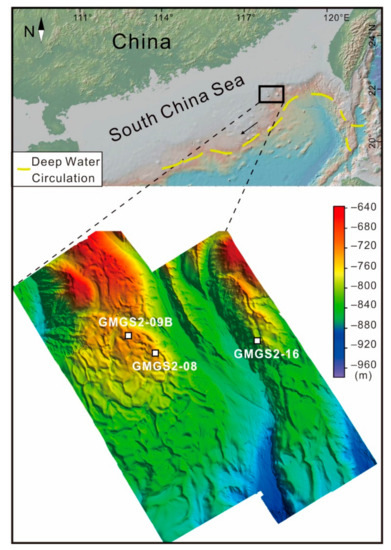
Figure 1.
Location of sites GMGS2-08, GMGS2-09B, and GMGS2-16 on the northern continental slope of the South China Sea. Deep-water currents are after Liu et al. [47].
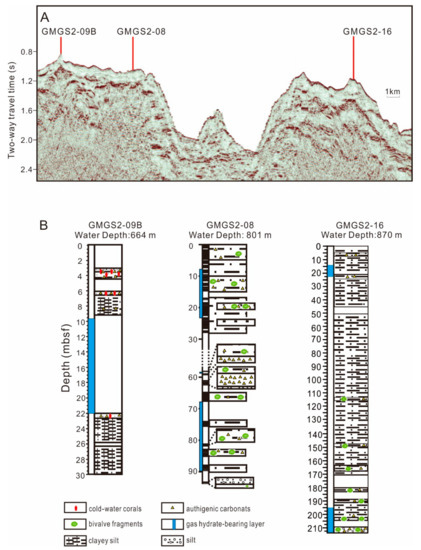
Figure 2.
(A) Seismic images of sites GMGS2-08, GMGS2-09B, and GMGS2-16. The vertical lines indicate position of cores. (B) Distribution of cold-water corals and authigenic carbonates at sites GMG S2-08, GMGS2-09B, and GMGS2-16.
The present study reports, for the first time, species identification, mineralogy, and geochemistry (carbon and oxygen isotopes and major and trace elements) of three CWC species (Crispatotrochus sp., Solenosmilia sp., and Enallopsammia sp.) retrieved from core GMGS-2 from a cold-seep area in the SCS (Figure 2). Our results provide insights into the relationship between fluid seepage and CWC development. Furthermore, the investigation helps to improve understanding of the factors that control coral growth and of interpretations of bottom-water chemistry recorded by CWC skeletons in fluid-seepage settings.
2. Geological Background
The northern SCS is an Atlantic-type passive continental margin, with extensive troughs, seamounts, and basins, including the Xisha Trough, Pearl River Mouth Basin, Beibuwan Basin, Qiongdongnan Basin, and Taixinan Basin [48]. The investigated area is located in the Dongsha area, which is situated in the eastern part of the Pearl River Mouth Basin of the SCS, at a water depth of ~664 m (Figure 1). Faults and mud diapirs, which are considered to be effective pathways for hydrocarbon migration, are common in the area [49]. The depths of bottom simulating reflectors are in the range of 160–220 m below the seafloor (mbsf) with an average depth of 180 mbsf [48]. Active cold-seep authigenic carbonates and benthic seep biota have been observed in the Dongsha area [49,50,51]. Moreover, gas hydrates have been recovered at this site during GMGS-2 in 2013 [48,52].
Gas hydrates were recovered during the GMGS-2 cruise conducted by the Chinese Geological Survey in coordination with Fugro and Geotek in 2013, with drill cores taken at sites GMGS2-08, GMGS2-09B, and GMGS2-16 (Figure 1). Authigenic carbonate nodules and chemosynthetic bivalve shells are common in the three cores. Three fossil CWC skeleton layers were identified in GMGS2-09B core samples, at depths of 3.00–4.47, 6.00–6.42, and 22.00–22.60 mbsf (Figure 2).
3. Samples and Methods
3.1. Samples
Drill core samples were collected at site GMGS2-09B. Gas hydrates containing >99% methane were recovered at depths ranging from 9 to 21 mbsf (Figure 2). There is an extraordinarily high sedimentation rate of ~73.3 cm/k.y. since 0.12 Ma at GMGS2-09B [48]. These samples exhibit both nodular and massive morphologies. The drilling depth reached ~105 mbsf in a water depth of 664 m. The dominant sediments were clayey silt and silt. Samples of fossil CWCs and authigenic carbonate nodules were obtained. The AMS14C (14C accelerator mass spectrometry) ages cluster of the CWCs obtained at 3.35–3.55 mbsf between 15.1 and 15.5 ka BP, and CWCs at 6.20–6.30 mbsf have an age of 17.1 ka BP (unpublished data). All samples were washed immediately after collection and placed in storage at 4 °C.
3.2. Analytical Methods
Well-preserved (without apparently crushed) fossil CWCs and authigenic carbonate samples were selected and further cleaned with deionized water using an ultrasonic cleaner in the laboratory. The shell fragments were separated from adhered sediments under a microscope to remove possible contaminants. CWCs were investigated using Stereo microscope (Discovery V20) and 3D X-ray microscope using an Xradia520 Versa instrument (Zeiss, Oberkochen, Germany) at the Guangzhou Marine Geological Survey and at Carl Zeiss, Shanghai Co., Ltd. (Shanghai, China), respectively. Subsamples from aragonite skeletons of CWCs were collected for geochemical analyses, and all samples were crushed and pulverized to pass through a 200 mesh.
X-ray Powder Diffraction analysis (XRD) was used for semi-quantitative characterizations of CWC mineralogy using a Rigaku D/MAX 2500PC Diffractometer (Tokyo, Japan) equipped with a diffracted beam graphite monochromator and using Cu Kα radiation at the Guangzhou Marine Geological Survey. Scans were conducted from 2.5° to 65° (2θ) at 0.01°/s, using a 300 mA current and 40 kV acceleration voltage. The relative proportions of carbonate minerals were quantified by whole pattern fitting and Rietveld refinement of the MDI Jade 6 program (V6.1, MDI, CA, United States).
Carbon and oxygen isotope values of CWCs and authigenic carbonates were analyzed at the Guangzhou Marine Geological Survey laboratory, following the procedure described by Chen et al. [53]. Samples were processed with 100% phosphoric acid at 80 °C to release CO2 for measurement using a Kiel IV online carbonate preparation line connected to a MAT 253 mass spectrometer (Thermo Scientific, Waltham, MA, United States). The isotope values are expressed using δ notation relative to the Vienna Pee Dee Belemnite (VPDB) standard. Precisions based on repeated measurements of carbonate standards are in the order of 0.1‰ (2σ) for both δ13C and δ18O.
Major- and trace-element analysis was performed with HF–HNO3 solutions following the procedure established by Hu et al. [54]. Major elements were analyzed via inductively coupled plasma–optical emission spectrometry (ICP–OES) (Optima 8300, PerkinElmer, Waltham, MA, United States) and trace elements were measured via ICP–mass spectrometry (MS) (X Series2, Thermo Fisher Scientific, Waltham, MA, United States) at the Guangzhou Marine Geological Survey. The analytical precision of element contents is better than 5%.
4. Results
4.1. Distribution and Species of CWCs
The abundance of CWCs decreases with depth, with distinct layers of higher abundance at 3.00–4.47, 6.00–6.42, and 22.00–22.60 mbsf (Figure 3). Most of the CWCs are fragments 0.063–5 cm in length. The coral fragments’ preservation, based on integrity of skeletal structure, ranges from poor to moderate throughout the core. Three genera and two unknown genera were identified, including the solitary corals Crispatotrochus sp., Solenosmilia sp., and Enallopsammia sp. (Figure 4). The most commonly observed CWC fragments were classified as Solenosmilia sp. Authigenic carbonates, mainly occurring as gray to gray-white nodules, are found in association with these CWC fragments. These carbonates display a brecciform structure and have lengths of ~0.2–1 cm.
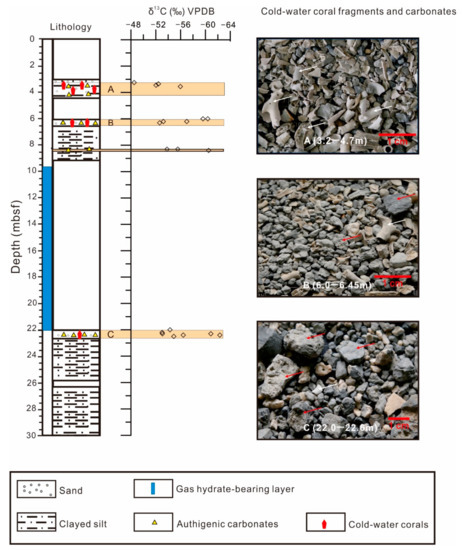
Figure 3.
Distribution of cold-water corals and stable isotopes of authigenic carbonates from site GMGS2-09B. The inset images (A–C) are coarse fragments of bulk sediments (>0.063 mm). The white arrows indicate cold-water corals and red arrow indicates the authigenic carbonates.
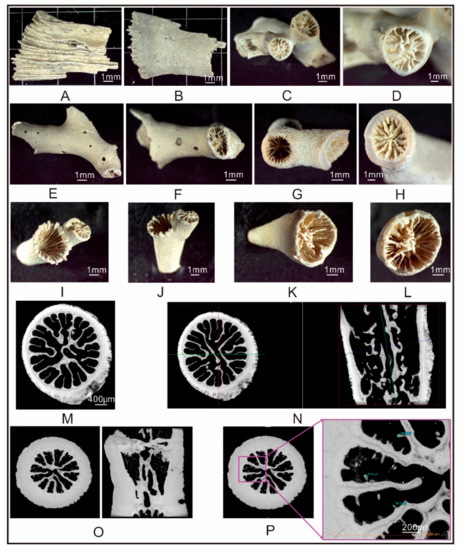
Figure 4.
Microscope and micro-CT photos of cold water corals (CWCs) from site GMGS2-09B ((A,B): Crispatotrochus sp. Fragment, 345–355 cm; (C–F): Solenosmilia sp. 345–355 cm; (G,H): Enallopsammia sp., 630–645 cm; (I,J): unknown species A 345–355 cm; (K,L): unknown species B, 600–615 cm; (M,N): Solenosmilia sp. cross-sectional view and longitudinal sectional view; (O,P): Enallopsammia sp. cross-sectional view and longitudinal sectional view).
On the basis of microstructures revealed by stereo microscope and micro-CT, three coral species were identified: including Crispatotrochus sp., Solenosmilia sp., and Enallopsammia sp. (Figure 4).
Crispatotrochus Tenison-Woods, 1878
This genus is a sessile ceratoid solitary coral, with prominent costae, no paliform lobe, and bunchy columella, and does not have a symbiotic relationship with zooxanthellae. The species distribution is Pleistocene to present. Samples: GMGS2-09B at 3.45–3.55 mbsf, incomplete (Figure 4A,B).
Solenosmilia Duncan, 1873
This species exhibits dendritic or bush-shaped complexes, thin walls, and several septa with three complete rounds, or four or five incomplete rounds. The septa of the first and second rounds are long and twisted, with a central connection. Columella are spongy or unapparent. This species does not have a symbiotic relationship with zooxanthellae. Samples: GMGS2-09B at 3.45–3.55 mbsf (Figure 4C,F).
Enallopsammia Michelotti in Sismonda, 1871
This species exhibits dendritic complexes and has thin walls covered by costae. The septa are regularly arranged. The columella is mastoid. This species does not have a symbiotic relationship with zooxanthellae. The species distribution is Pleistocene to present. Samples: GMGS2-09B at 6.30–6.45 mbsf (Figure 4G,H), 6.00–6.15 mbsf (Figure 4I,L), and 3.45–3.55 mbsf (Figure 4K,M).
4.2. CWC Mineralogy
XRD results show that the CWC samples are 95–99% aragonite, and 1–5% calcite (Table A1), and authigenic carbonate samples are dominated by aragonite, high-Mg calcite, low-Mg calcite and dolomite (unpublished data).
4.3. Carbon and Oxygen Isotopes of CWCs and Carbonates
Stable isotope values for CWCs and carbonates are listed in Table A2 and presented in Figure 5. CWC δ13C values range from −8.4‰ to −0.6‰ (mean = −5.3‰, n = 48). The mean δ13C values of Crispatotrochus sp. and Solenosmilia sp. are −6.1‰ and −5.4‰ respectively, but Enallopsammia sp. displays a slight 13C enrichment compare to the above corals, with a mean value of −3.8‰. δ18O values for CWCs vary from 0.6‰ to 4.5‰ (mean = 1.9‰, n = 48). Crispatotrochus sp. and Solenosmilia sp. exhibit similar δ18O values that range from 0.6‰ to 3.3‰ (mean = 1.7‰, n = 38). Enallopsammia sp. yields higher positive δ18O values (mean = 2.5‰, n = 7).
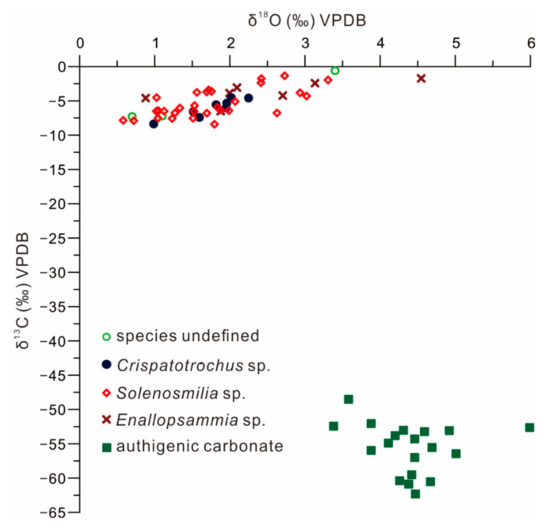
Figure 5.
Carbon and oxygen isotopic compositions of CWCs and the associated authigenic carbonates from the South China Sea.
Authigenic carbonates from site GMGS2-09B exhibit extremely negative δ13C values ranging from −62.3‰ to −48.5‰ (mean = −55.6‰, n = 19). δ18O values range from 3.4‰ to 6.0‰ (mean= 4.4‰, n = 19).
4.4. CWC Element Contents
Contents of major and trace elements in the CWCs are presented in Table A3. Mean calcium contents are 37.89% in Crispatotrochus sp., 36.72% in Solenosmilia sp., and 38.82% in Enallopsammia sp. All samples exhibit similarities in major-element abundances with Ca > Na > K > Al. Contents of Mg, Mn, and Fe are very low, and less than 0.03%.
All samples exhibit similar trace-element contents in the order Ba > Ni > Zn > U > Cr > Cu > Pb >Co > Cd (Table A3). Only Ba, Zn, and Ni show moderate to strong content in the CWC samples, with mean values of 22.36 μg/g for Ba, 9.06 μg/g for Zn, and 10.51 μg/g for Ni. Contents of Cr (2.51–5.25 μg/g), Cu (1.30–3.84 μg/g), Pb (0.79–3.26 μg/g), Co (0.73–1.40 μg/g), and Cd (0.082–0.093 μg/g) show little variation.
5. Discussion
5.1. CWCs Distribution and Factors Affect Their Growth
The factors determining CWC formation at cold seep areas are largely unknown. Hovland and Thomsen [22] established the “hydraulic theory” on the basis of the presence of cold seeps. These authors posited that hydrocarbon seeps directly fertilize CWCs, a premise supported by the fact that all the studied CWCs are embedded in authigenic carbonates. However, despite the almost identical oceanographic and sedimentological environments of the hydrate drilling area, CWCs were only observed at site GMGS-09B (Figure 2). Even at site GMGS-09B, not all the authigenic carbonate layers contain CWCs. Unlike bivalves and other seep-dwelling animals that directly use the chemical energy produced by cold-seep processes, CWCs may benefit indirectly from cold seeps through the formation of associated authigenic carbonates [33]. Authigenic carbonates potentially create a hard substrate for CWC larvae settlement [41]. Although cold-seep activity was widely observed in the research area, observations made by a remotely operated vehicle revealed that authigenic carbonates occurred in seabed only at site GMGS2-09B.
Oceanographic processes have also been regarded as an important factor in CWC development [33,55,56]. Bathymetric highs form areas where eddy currents and internal waves result in increased concentrations of nutrients and a higher food flux for CWCs [56,57]. The shallowest bathymetry recorded during the GMGS-2 survey occurred at site GMGS2-09B (water depth of ~664 m; Figure 2), and the raised seafloor might have resulted in an increased nutrient supply to drive CWC growth [55].
Additional environmental factors favoring CWC growth at site GMGS2-09B are oceanic temperatures and the prevailing hydrodynamic regime [56]. The bottom seawater temperature at site GMGS2-09B was measured as 5.35 °C, which is within the preferred temperature range of 4–13 °C reported for CWCs along continental margins worldwide [1]. In addition, the research area is located in an area where deep Pacific water flows through the Luzon Strait to enter the SCS [47]. This presence of a strong and deep seawater flow should deliver increased nutrients that drive CWC growth. Furthermore, the strong bottom current and the steep and hard seafloor structure prevent deposition of large amounts of debris that could bury CWCs. These favorable oceanographic conditions are likely a significant factor favoring the growth of CWCs in the study area.
5.2. Bottom-Water Chemistry and Fluid Seepage Archived in CWCs
5.2.1. Comparison of C and O Isotope Signatures
As CWCs use HCO3− from both ambient seawater and internally produced carbon dioxide for skeleton biomineralization, δ13C signatures of coral skeletons can be used to trace external carbon sources [33,58,59]. Food sources will also affect CWC stable isotope values [60]. CWCs may capture live zooplankton or detrital particulate organic matter from the upper water column [1]. Alternatively, it is also possible that they feed on bacterial mats or microorganisms associated with methane fluids [6]. Cold seeps are characterized by widespread chemosynthesis-based ecosystems and are commonly associated with high biomass, fueled by microbial chemosynthesis [61].
However, the data from 48 CWC samples in this study show a linear trend, with obviously high δ13C values (−8.4‰ to −0.6‰; Figure 5) compared to those of authigenic carbonates. These values are significantly different from those of the co-occurring authigenic carbonates. The highly 13C-depleted authigenic carbonates (mean = −55.6‰, n = 19) at the four intervals (3.25–3.65, 6–6.45, 8.30–8.45, and 22–22.6 mbsf) in core GMGS2-09B indicate a methane-derived isotopic signature, indicating the presence of past methane seepages at the times of deposition of these intervals (Figure 5). Biogenic methane typically exhibits δ13C values in the range of −110‰ to −50‰ [62] and thermogenic methane in the range of −50‰ to −30‰ [63]. The extremely negative δ13C values of the carbonates (−62.3‰ to −48.5‰) from core GMGS2-09B indicate that the dominant source of carbon fueling the system is biogenic methane.
A summary of δ13C and δ18O values of CWCs, coral reefs, and carbonates in the SCS and other locations are shown in Figure 6. The values exhibited by the CWCs and carbonates of the present study differ markedly from those from shallow coral reefs at the Xisha Islands [64] and Sanya Bay [65] in the SCS. Carbonates from hydrothermal sites from Mariana Forearc [66] and Lost City [67] show higher δ13C values relative to the authigenic carbonates and CWCs from site GMGS2-09B, indicating that the research area is not influenced by low-temperature hydrothermal processes. Our data are comparable with the ranges of δ13C and δ18O values in CWCs from the North Atlantic [68] and the Japan Sea [69], particularly those from the Dongsha area in the SCS [70,71], which does not have any cold-seep activity. As such, our results do not support the idea that CWCs take up methane-derived carbon at fluid-seepage areas. We therefore postulate that the primary food sources for CWCs are phytoplankton, zooplankton, and dissolved organic matter, as proposed by other studies [26,72]. However, more work on living coral skeletons is needed to verify this conclusion.
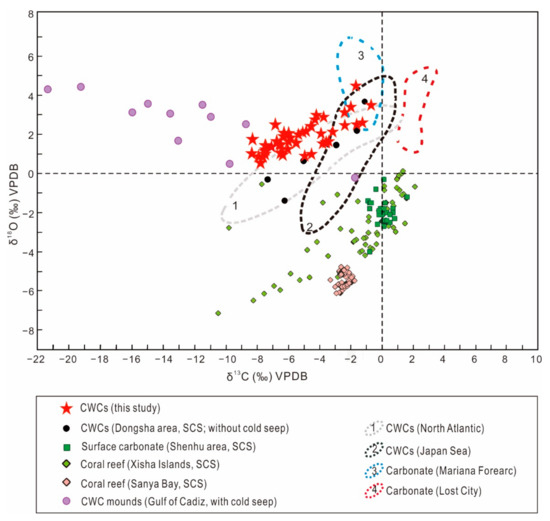
Figure 6.
A comparison of carbon and oxygen isotopic compositions of CWCs from the South China Sea and other regions. Data from Pirlet et al. [30], Zhang and He. [64], Ke et al. [65], Tran et al. [66], Früh-Green et al. [67], Blamart et al. [68], Shirai et al. [69], and Chen et al. [70].
The strong linear trend of δ18O and δ13C from CWCs may be exploited for reconstructing temperature of past climates [1]. However, δ18O appear to have a relatively weak covariance with respect to δ13C in this study (Figure 6). The most reasonable explanation is that this observation is a result of gas hydrate dissociation. Decomposition of gas hydrate could liberate 18O-rich water with methane [35,50,53]. During an intense methane seepage, this fluid is transported from the sedimentary subsurface to the seabed and into the water column, resulting in alteration of δ18O in porewater and bottom seawater [51,54]. This environment would favor heavier δ18O values of CWCs during skeleton precipitation. The variance of oxygen isotopes in CWCs caused by gas hydrate dynamics is also supported by occurrence of gas hydrate resources at depth of 9–21 mbsf in GMGS2-09B (Figure 2).
5.2.2. CWC Trace-Element Contents
Trace-element contents in skeletal corals have been used to reconstruct oceanographic conditions [73], but there is very limited information on baseline levels of trace elements in CWCs [7], particularly in cold-seep areas. This study provides the first reported trace-element contents in the fossil CWCs Crispatotrochus sp., Enallopsammia sp., and Solenosmilia sp., collected at SCS cold seeps.
Metal contents within CWC skeletons are controlled by two main factors: the external environment and vital effects [74]. It is considered that the uptake and accumulation of elements in corals during metabolic and physiological processes are influenced by the ambient environmental conditions, including organic matter availability, salinity, production cycle, and seawater temperature [75]. Trace element contents in CWCs that live in non-seep settings tend to be fairly low under the conditions of their typical habitat without an additional source of metal elements [7,76].
In general, contents of Cr, Co, Ni, Cu, Zn, Cd, Pb, U, Ba, Th, and Sr in the samples from core GMGS2-09B are similar to or higher than those observed in modern CWCs from non-seep marine environments (Figure 7). Crocket et al. [77] suggested that such high trace-element contents could be derived from contaminant phases. However, the absence of a systematic relationship between Fe, Mn, Al, and other trace elements (Figure S1) indicates that ambient contamination is not likely the primary influence on CWC trace-element contents. High metal contents are presumably related to the existence of an additional source of trace elements. The contents of Ni and Pb are in good agreement with those found at a hydrothermal site (Azores Islands) (Figure 7), supporting this interpretation. The fluids found in cold seeps contain high concentrations of dissolved trace elements (e.g., Ba), resulting in elemental enrichments in bottom seawater and surface sediments [78,79]. However, the presence of free H2S produced by intense AOM tends to scavenge trace elements from ambient porewater or seawater, causing subsequent enrichment of redox-sensitive elements (e.g., U and Mo) at cold seeps [53,80]. This is further supported by the obvious enrichment of trace elements (e.g., Mo, U, Pb, Zn and Cu) in seep-impacted aragonites that forming close to the sediment-water interface with free H2S in sediment pore water [81]. Our results suggest that CWCs might record the influence of biogeochemical processes of fluid seepage on bottom water chemistry.
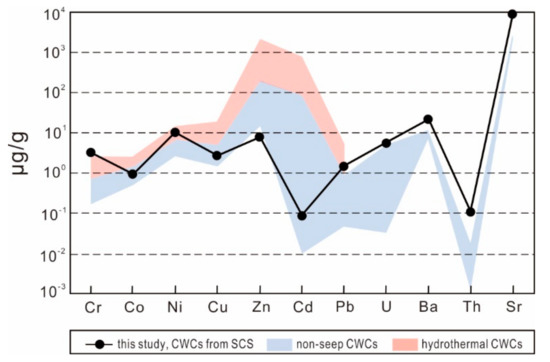
Figure 7.
Trace-element contents of CWCs from site GMGS2-09B. Published data from modern CWCs [2,7,76,77] and CWCs in a hydrothermal site [7] are included for comparison.
The geochemical and isotopic signatures and mineralogy of authigenic carbonates are widely used to trace the composition of ascending fluids, seepage intensities, and associated environmental parameters [78,82,83,84,85]. The CWCs in seep environments can record rapid changes in ocean chemistry, providing an important constraint on bottom water environmental conditions [26]. As such, the combined study of authigenic carbonates and associated CWCs in seep environments is potentially useful to calibrate the geochemical signatures within authigenic carbonates to aid in reconstruction of past conditions (Figure 8). In addition, U–Th dating of authigenic carbonates is a powerful tool that can also contribute to our understanding of the geological factors and processes controlling seabed methane release [86,87,88,89,90,91]. However, accurate dating of authigenic carbonates is difficult owing to contamination by detrital terrestrial material [84]. Furthermore, CWC geochronology can potentially provide information about the cessation of cold seep activity, as CWCs use authigenic carbonates as a substrate for colonization.
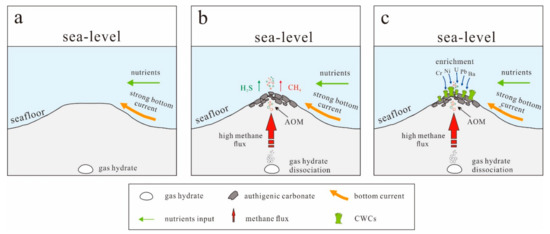
Figure 8.
A schematic growth model of formation of CWCs in cold seep area. (a) The absence of cold seep activities in the marine environment. (b) Extensive authigenic carbonates are precipitated close to the sediment-water interface during anaerobic oxidation of methane (AOM) due to enhanced fluid flow associated with dissociation of gas hydrate, providing hard substrates on the seafloor. (c) CWCs settle on the hard carbonate substrates and then are sustained by bottom current and nutrient input. Trace elements may be enriched in CWCs skeleton based on the presence of free H2S produced by intense AOM.
6. Conclusions
A combined study of three gas-hydrate-bearing sediment cores in the SCS reveal that CWCs were only found at one core and embedded with authigenic carbonates. The close relationship between CWCs and authigenic carbonates is due to the CWCs using authigenic carbonates as a substratum for colonization. Stable isotope studies suggest the source of carbon for CWCs is not directly from fluid seepage. It is suggested that the development of CWCs also require a high seabed topography, appropriate water temperature and stronger bottom-water current. However, in the case of strong fluid seepage, a large amount of nutrient elements will release into the bottom water. This information will be recorded by the coral skeletons. Therefore, CWCs from cold seep area could record the period of the latest cold seep activity, and the detailed geochemical study of CWCs will reveal environmental information during their growth period.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-163X/9/12/742/s1, Figure S1: The relationship between Fe, Al, U, Cr and Cd of cold-water coral samples from the South China Sea.
Author Contributions
Methodology, Y.D. and M.J.; data curation, J.C., H.C., Y.Z., C.W., C.Z., Y.Z. and S.C.; writing—original draft preparation, Y.D.; writing—review and editing, F.C. and N.L.; supervision, F.C.; project administration, F.C.; funding acquisition, F.C. and N.L.
Funding
This research was financial supported by the National Natural Science Foundation of China (Grants: 41776066, 41706053, 41803026 and 41976061), Key Special Project for Introduced Talents Team of Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou) (GML2019ZD0506), Yunnan University’s Research Innovation Fund for Graduate Students (2019z046), the China Ocean Mineral Resources R&D Association (Program: DY135-C1-1-04), and Key Laboratory of Marine Mineral Resources, Ministry of Natural Resources (No. KLMMR-2015-A-03).
Acknowledgments
Thanks to the crew and scientists of the GMGS-2 gas hydrate drilling expedition. We deeply appreciate Meixia Zhao (SCSIO, CAS) for identification and Longan Jiao (Carl Zeiss, Shanghai Co., Ltd.) for CT scans of the CWCs.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Mineral compositions of cold-water coral fragments from the South China Sea.
Table A1.
Mineral compositions of cold-water coral fragments from the South China Sea.
| Depth (cmbsf) | Sample | Ara | Cal |
|---|---|---|---|
| 335–345 | Cri. | 99 | 1 |
| 345–355 | Cri. | 97 | 3 |
| 345–355 | Sol. | 99 | 1 |
| 345–355 | Sol. | 95 | 5 |
| 345–355 | Sol. | 98 | 2 |
| 355–365 | Sol. | 99 | 1 |
| 355–365 | Ena. | 97 | 3 |
| 630–645 | Ena. | 99 | 1 |
Note: Cri.—Crispatotrochus species; Sol.—Solenosmilia species; Ena.—Enallopsammia species; Ara—aragonite; Cal—calcite.; cmbsf—cm below seafloor.

Table A2.
Stable carbon and oxygen isotopes of cold-water corals and associated authigenic carbonates from the gas hydrate-bearing sediments of the South China Sea.
Table A2.
Stable carbon and oxygen isotopes of cold-water corals and associated authigenic carbonates from the gas hydrate-bearing sediments of the South China Sea.
| Core Number | Depth (cmbsf) | Sample Type | δ13C (‰) | δ18O (‰) |
|---|---|---|---|---|
| 09B-2M-1a | 325–335 | Grey nodular carbonate | −48.5 | 3.6 |
| 09B-2M-1a | 335–345 | Grey nodular carbonate | −52.4 | 3.4 |
| 09B-2M-1a | 345–355 | Grey nodular carbonate | −52.0 | 3.9 |
| 09B-2M-1a | 355–365 | Grey nodular carbonate | −56.0 | 3.9 |
| 09B-2M-1a | 600–615 | Grey nodular carbonate | −59.5 | 4.4 |
| 09B-2M-1a | 620–630 | Grey nodular carbonate | −57.0 | 4.5 |
| 09B-2M-1a | 620–630 | Opalescent nodular carbonate | −53.2 | 4.6 |
| 09B-2M-1a | 630–645 | Grey nodular carbonate | −52.6 | 6.0 |
| 09B-2M-1a | 630–645 | Opalescent nodular carbonate | −60.4 | 4.3 |
| 09B-2M-1a | 830–840 | Opalescent nodular carbonate | −53.8 | 4.2 |
| 09B-2M-1a | 830–840 | Grey nodular carbonate | −55.5 | 4.7 |
| 09B-2M-1a | 840–845 | Grey nodular carbonate | −60.5 | 4.7 |
| 09B-5H-1 | 2200–2215 | Grey nodular carbonate | −54.3 | 4.5 |
| 09B-5H-1 | 2220–2230 | Grey nodular carbonate | −53.0 | 4.3 |
| 09B-5H-1 | 2230–2240 | Opalescent nodular carbonate | −60.9 | 4.4 |
| 09B-5H-1 | 2230–2240 | Grey nodular carbonate | −53.1 | 4.9 |
| 09B-5H-1 | 2240–2250 | Opalescent nodular carbonate | −62.3 | 4.5 |
| 09B-5H-1 | 2240–2250 | Grey nodular carbonate | −56.4 | 5.0 |
| 09B-5H-1 | 2250–2260 | Grey nodular carbonate | −54.9 | 4.1 |
| 09B-2M-1a | 325–335 | Crispatotrochus species | −5.6 | 1.8 |
| 09B-2M-1a | 325–335 | Crispatotrochus species | −7.4 | 1.6 |
| 09B-2M-1a | 325–335 | Crispatotrochus species | −5.3 | 2.0 |
| 09B-2M-1a | 335–345 | Crispatotrochus species | −6.6 | 1.5 |
| 09B-2M-1a | 335–345 | Crispatotrochus species | −8.4 | 1.0 |
| 09B-3M-1 | 630–645 | Crispatotrochus species | −4.6 | 2.2 |
| 09B-5H-1 | 2230–2240 | Crispatotrochus species | −6.0 | 2.0 |
| 09B-2M-1a | 345–355 | Crispatotrochus species | −4.5 | 2.0 |
| 09B-5H-1 | 2200–2215 | Enallopsammia species | −3.1 | 2.1 |
| 09B-5H-1 | 2200–2215 | Enallopsammia species | −6.5 | 1.9 |
| 09B-5H-1 | 2230–2240 | Enallopsammia species | −3.9 | 2.0 |
| 09B-5H-1 | 2220–2230 | Enallopsammia species | −4.2 | 2.7 |
| 09B-3M-1 | 630–645 | Enallopsammia species | −2.4 | 3.1 |
| 09B-3M-1 | 630–645 | Enallopsammia species | −1.7 | 4.5 |
| 09B-2M-1a | 2220–2230 | Enallopsammia species | −4.6 | 0.9 |
| 09B-2M-1a | 335–345 | Solenosmilia species | −1.3 | 2.7 |
| 09B-2M-1a | 335–345 | Solenosmilia species | −4.5 | 1.0 |
| 09B-2M-1a | 335–345 | Solenosmilia species | −6.5 | 1.1 |
| 09B-2M-1a | 335–345 | Solenosmilia species | −7.6 | 1.2 |
| 09B-2M-1a | 335–345 | Solenosmilia species | −7.5 | 1.5 |
| 09B-2M-1a | 345–355 | Solenosmilia species | −5.9 | 1.8 |
| 09B-2M-1a | 345–355 | Solenosmilia species | −6.0 | 1.3 |
| 09B-2M-1a | 345–355 | Solenosmilia species | −6.8 | 2.6 |
| 09B-2M-1a | 335–345 | Solenosmilia species | −6.4 | 2.0 |
| 09B-2M-1a | 345–355 | Solenosmilia species | −1.7 | 2.4 |
| 09B-2M-1a | 345–355 | Solenosmilia species | −3.8 | 1.6 |
| 09B-2M-1a | 345–355 | Solenosmilia species | −6.3 | 1.9 |
| 09B-2M-1a | 345–355 | Solenosmilia species | −3.7 | 1.7 |
| 09B-2M-1a | 345–355 | Solenosmilia species | −6.5 | 1.5 |
| 09B-2M-1a | 335–345 | Solenosmilia species | −5.1 | 2.1 |
| 09B-2M-1a | 345–355 | Solenosmilia species | −6.5 | 1.0 |
| 09B-2M-1a | 345–355 | Solenosmilia species | −8.4 | 1.8 |
| 09B-2M-1a | 345–355 | Solenosmilia species | -−7.9 | 0.7 |
| 09B-2M-1a | 355–365 | Solenosmilia species | -−2.0 | 3.3 |
| 09B-2M-1a | 355–365 | Solenosmilia species | −7.8 | 0.6 |
| 09B-3M-1 | 600–615 | Solenosmilia species | −3.8 | 2.9 |
| 09B-3M-1 | 600–615 | Solenosmilia species | −3.4 | 1.7 |
| 09B-3M-1 | 630–645 | Solenosmilia species | −2.4 | 2.4 |
| 09B-5H-1 | 2200–2215 | Solenosmilia species | −3.6 | 1.8 |
| 09B-5H-1 | 2230–2240 | Solenosmilia species | −4.3 | 3.0 |
| 09B-5H-1 | 2230–2240 | Solenosmilia species | −6.4 | 1.0 |
| 09B-5H-1 | 2220–2230 | Solenosmilia species | −7.5 | 1.0 |
| 09B-5H-1 | 2220–2230 | Solenosmilia species | −6.8 | 1.7 |
| 09B-2M-2a | 345–355 | Solenosmilia species | −6.8 | 1.3 |
| 09B-2M-1a | 610–615 | Solenosmilia species | −5.7 | 1.5 |
| 09B-2M-1a | 335–345 | coral (species undefined) | −7.2 | 1.1 |
| 09B-2M-1a | 345–355 | coral (species undefined) | −7.3 | 0.7 |
| 09B-2M-1a | 620–630 | coral (species undefined) | −0.6 | 3.4 |

Table A3.
Major and trace elements contents of cold-water corals from the South China Sea.
Table A3.
Major and trace elements contents of cold-water corals from the South China Sea.
| Depth | (cmbsf) | 345–355 | 345–355 | 610–615 | 2220–2230 |
|---|---|---|---|---|---|
| Al | (%) | 0.021 | 0.028 | 0.076 | 0.036 |
| Fe | (%) | 0.003 | 0.011 | 0.026 | 0.002 |
| Mn | (%) | 0.0003 | 0.001 | 0.002 | 0.006 |
| P | (%) | 0.009 | 0.023 | 0.012 | 0.006 |
| Ti | (%) | 0.0001 | 0.0002 | 0.0003 | 0.0005 |
| Ca | (%) | 37.89 | 37.09 | 36.34 | 38.82 |
| Mg | (%) | bl | bl | 0.046 | bl |
| Na | (%) | 1.52 | 1.47 | 1.22 | 2.06 |
| K | (%) | 0.021 | 0.045 | 0.048 | 0.1 |
| Cr | (μg/g) | 2.5 | 2.6 | 2.9 | 5.3 |
| Co | (μg/g) | 0.7 | 0.8 | 0.9 | 1.4 |
| Ni | (μg/g) | 9.3 | 10.6 | 10.2 | 12.0 |
| Cu | (μg/g) | 1.3 | 3.1 | 2.7 | 3.8 |
| Zn | (μg/g) | 5.0 | 8.0 | 7.0 | 12.3 |
| Cd | (μg/g) | 0.1 | 0.1 | 0.1 | 0.1 |
| Pb | (μg/g) | 0.8 | 1.0 | 1.1 | 3.3 |
| U | (μg/g) | 4.6 | 4.4 | 6.9 | 7.0 |
| Ba | (μg/g) | 16.2 | 23.4 | 20.4 | 29.5 |
| Th | (μg/g) | 0.1 | 0.1 | 0.2 | 0.1 |
| Sr | (μg/g) | 9218.0 | 9248.0 | 9706.0 | 9853.0 |
Note: bl-Below detection limit.
References
- Roberts, J.M.; Wheeler, A.J.; Freiwald, A. Reefs of the deep: The biology and geology of cold-water coral ecosystems. Science 2006, 312, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Van de Flierdt, T.; Robinson, L.F.; Adkins, J.F. Deep-sea coral aragonite as a recorder for the neodymium isotopic composition of seawater. Geochim. Cosmochim. Acta 2010, 74, 6014–6032. [Google Scholar] [CrossRef]
- Frank, N.; Paterne, M.; Ayliffe, L.; van Weering, T.; Henriet, J.P.; Blamart, D. Eastern North Atlantic deep-seacorals: Tracing up per intermediate water Delta C-14 during the Holocene. Earth Planet. Sci. Lett. 2004, 219, 297–309. [Google Scholar] [CrossRef]
- Davies, A.J.; Guinotte, J.M. Global habitat suitability for framework-forming cold-water corals. PLoS ONE 2011, 6, e18483. [Google Scholar] [CrossRef] [PubMed]
- Margolin, A.R.; Robinson, L.F.; Burke, A.; Waller, R.G.; Scanlon, K.M.; Roberts, M.L.; Auro, M.E.; Van de Flierdt, T. Temporal and spatial distributions of cold-water corals in the Drake Passage: Insights from the last 35,000 years. Deep-Sea Res. Part II 2014, 99, 237–248. [Google Scholar] [CrossRef]
- Hovland, M.; Risk, M. Do Norwegian deep-water coral reefs rely on seeping fluids? Mar. Geol. 2003, 198, 83–96. [Google Scholar] [CrossRef]
- Raimundo, J.; Vale, C.; Caetano, M.; Anes, B.; Carreiro-Silva, M.; Martins, I.; de Matos, V.; Porteiro, F.M. Element concentrations in cold-water gorgonians and black coral from Azores region. Deep-Sea Res. Part II 2013, 98, 129–136. [Google Scholar] [CrossRef]
- Edwards, R.; Chen, J.H.; Wasserburg, G.J. 238U 234U 230Th 232Th systematics and the precise measurement of time over the past 500,000 years. Earth Planet. Sci. Lett. 1987, 81, 175–192. [Google Scholar] [CrossRef]
- Mortlock, R.A.; Fairbanks, R.G.; Chiu, T.C.; Rubenstone, J. Th-230/U-234/U-238 and Pa-231/U-235 ages from a single fossil coral fragment by multi-collector magnetic-sector inductively coupled plasma mass spectrometry. Geochim. Cosmochim. Acta 2005, 69, 649–657. [Google Scholar] [CrossRef]
- Robinson, L.F.; Adkins, J.F.; Fernandez, D.P.; Burnett, D.S.; Wang, S.L.; Gagnon, A.C.; Krakauer, N. Primary U distribution in scleractinian corals and its implications for U series dating. Geochem. Geophy. Geosy. 2006, 7, Q05022. [Google Scholar] [CrossRef]
- Chen, T.Y.; Robinson, L.F.; Burke, A.; Southon, J.; Spooner, P.; Morris, P.J.; Ng, H.C. Synchronous centennial abrupt events in the ocean and atmosphere during the last deglaciation. Science 2015, 349, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.Y.; Robinson, L.F.; Beasley, M.P.; Claxton, L.M.; Andersen, M.B.; Gregoire, L.J.; Wadham, J.; Fornari, D.J.; Harpp, K.S. Ocean mixing and ice-sheet control of seawater U-234/U-238 during the last deglaciation. Science 2016, 354, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Eltgroth, S.F.; Boyle, E.A.; Adkins, J.E. The transfer of bomb radiocarbon and anthropogenic lead to the deep North Atlantic Ocean observed from a deep sea coral. Earth Planet. Sci. Lett. 2017, 458, 223–232. [Google Scholar] [CrossRef]
- Burke, A.; Robinson, L.F. The Southern Ocean’s Role in Carbon Exchange during the Last Deglaciation. Science 2012, 335, 557–561. [Google Scholar] [CrossRef]
- Hines, S.K.V.; Southon, J.R.; Adkins, J.F. A high-resolution record of Southern Ocean intermediate water radiocarbon over the past 30,000 years. Earth Planet. Sci. Lett. 2015, 432, 46–58. [Google Scholar] [CrossRef]
- Robinson, L.F.; van de Flierdt, T. Southern Ocean evidence for reduced export of North Atlantic Deep Water during Heinrich event 1. Geology 2009, 37, 195–198. [Google Scholar] [CrossRef]
- Copard, K.; Colin, C.; Henderson, G.M.; Scholten, J.; Douville, E.; Sicre, M.A.; Frank, N. Late Holocene intermediate water variability in the northeastern Atlantic as recorded by deep-sea corals. Earth Planet. Sci. Lett. 2012, 313, 34–44. [Google Scholar] [CrossRef]
- Montero-Serrano, J.C.; Frank, N.; Tisnerat-Laborde, N.; Colin, C.; Wu, C.C.; Lin, K.; Shen, C.C.; Copard, K.; Orejas, C.; Gori, A.; et al. Decadal changes in the mid-depth water mass dynamic of the Northeastern Atlantic margin (Bay of Biscay). Earth Planet. Sci. Lett. 2013, 364, 134–144. [Google Scholar] [CrossRef]
- Mitsuguchi, T.; Matsumoto, E.; Abe, O.; Uchida, T.; Isdale, P.J. Mg/Ca thermometry in coral-skeletons. Science 1996, 274, 961–963. [Google Scholar] [CrossRef]
- Turley, C.M.; Roberts, J.M.; Guinotte, J.M. Corals in deep-water: Will the unseen hand of ocean acidification destroy cold-water ecosystems? Coral Reefs 2007, 26, 445–448. [Google Scholar] [CrossRef]
- Tittensor, D.P.; Baco, A.R.; Brewin, P.E.; Clark, M.R.; Consalvey, M.; Hall-Spencer, J.; Rowden, A.A.; Schlacher, T.; Stocks, K.I.; Rogers, A.D. Predicting global habitat suitability for stony corals on seamounts. J. Biogeogr. 2009, 36, 1111–1128. [Google Scholar] [CrossRef]
- Hovland, M.; Thomsen, E. Cold-water corals—Are they hydrocarbon seep related? Mar. Geol. 1997, 137, 159–164. [Google Scholar] [CrossRef]
- Naeth, J.; di Primio, R.; Horsfield, B.; Schaefer, R.G.; Shannon, P.M.; Bailey, W.R.; Henriet, J.P. Hydrocarbon seepage and carbonate mound formation: A basin modelling study from the porcupine basin (offshore Ireland). J. Pet. Geol. 2005, 28, 147–165. [Google Scholar] [CrossRef]
- Hovland, M. Deep-Water Coral Reefs: Unique Biodiversity Hot–Spots; Praxis Publishing: Chichester, UK, 2008; p. 278. [Google Scholar]
- Lindberg, B.; Berndt, C.; Mienert, J. The Fugløy Reef at 701 N; acoustic signature, geologic, geomorphologic and oceanographic setting. Int. J. Earth Sci. 2007, 96, 201–213. [Google Scholar] [CrossRef]
- Liebetrau, V.; Eisenhauer, A.; Linke, P. Cold seep carbonates and associated cold-water corals at the Hikurangi Margin, New Zealand: New insights into fluid pathways, growth structures and geochronology. Mar. Geol. 2010, 272, 307–318. [Google Scholar] [CrossRef]
- Jones, A.T.; Greinert, J.; Bowden, D.A.; Klaucke, I.; Petersen, C.J.; Netzeband, G.L.; Weinrebe, W. Acoustic and visual characterisation of methane-rich seabed seeps at Omakere Ridge on the Hikurangi Margin, New Zealand. Mar. Geol. 2010, 272, 154–169. [Google Scholar] [CrossRef]
- León, R.; Somoza, L.; Medialdea, T.; González, F.J.; Diaz-del-Río, V.; Fernández-Puga, M.C.; Maestro, A.; Mata, M.P. Sea-floor features related to hydrocarbon seeps in deep water carbonate-mud mounds of the Gulf of Cádiz: From mud flows to carbonate precipitates. Geo.-Mar. Lett. 2007, 27, 237–247. [Google Scholar] [CrossRef]
- Schroeder, W.W. Observations of Lophelia pertusa and the surficial geology at a deep-water site in the northeastern Gulf of Mexico. Hydrobiologia 2002, 471, 29–33. [Google Scholar] [CrossRef]
- Pirlet, H.; Wehrmann, L.M.; Foubert, A.; Brunner, B.; Blamart, D.; De Mol, L.; Van Rooij, D.; Dewanckele, J.; Cnudde, V.; Swennen, R.; et al. Unique authigenic mineral assemblages reveal different diagenetic histories in two neighbouring cold-water coral mounds on Pen Duick Escarpment, Gulf of Cadiz. Sedimentology 2012, 59, 578–604. [Google Scholar] [CrossRef]
- Hovland, M.; Mortensen, P.B.; Brattegard, T.; Strass, P.; Rokoengen, K. Ahermatypic coral banks off Mid-Norway: Evidence for a link with seepage of light hydrocarbons. Palaios 1998, 13, 189–200. [Google Scholar] [CrossRef]
- Hovland, M.; Jensen, S.; Indreiten, T. Unit pockmarks associated with Lophelia coral reefs off mid-Norway: More evidence of control by ‘fertilizing’ bottom currents. Geo.-Mar. Lett. 2012, 32, 545–554. [Google Scholar] [CrossRef]
- Rincon-Tomas, B.; Duda, J.P.; Somoza, L.; Gonzalez, F.J.; Schneider, D.; Medialdea, T.; Santofimia, E.; Lopez-Pamo, E.; Madureira, P.; Hoppert, M.; et al. Cold-water corals and hydrocarbon-rich seepage in Pompeia Province (Gulf of Cadiz)-living on the edge. Biogeosciences 2019, 16, 1607–1627. [Google Scholar] [CrossRef]
- Thiel, V.; Peckmann, J.; Seifert, R.; Wehrung, P.; Reitner, J.; Michaelis, W. Highly isotopically depleted isoprenoids: Molecular markers for ancient methane venting. Geochim. Cosmochim. Acta 1999, 63, 3959–3966. [Google Scholar] [CrossRef]
- Boetius, A.; Ravenschlag, K.; Schubert, C.J.; Rickert, D.; Widdel, F.; Gieseke, A.; Amann, R.; Jorgensen, B.B.; Witte, U.; Pfannkuche, O. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 2000, 407, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Peckmann, J.; Gischler, E.; Oschmann, W.; Reitner, J. An early carboniferous seep community and hydrocarbon-derived carbonates from the Harz mountains, Germany. Geology 2001, 29, 471. [Google Scholar] [CrossRef]
- Peckmann, J.; Thiel, V. Carbon cycling at ancient methane-seeps. Chem. Geol. 2004, 205, 443–467. [Google Scholar] [CrossRef]
- Reeburgh, W.S. Oceanic methane biogeochemistry. Chem. Rev. 2007, 107, 486–513. [Google Scholar] [CrossRef]
- Feng, D.; Lin, Z.J.; Bian, Y.Y.; Chen, D.F.; Peckmann, J.; Bohrmann, G.; Roberts, H.H. Rare earth elements of seep carbonates: Indication for redox variations and microbiological processes at modern seep sites. J. Asian Earth Sci. 2013, 65, 27–33. [Google Scholar] [CrossRef]
- Van Rooij, D.; Blamart, D.; De Mol, L.; Mienis, F.; Pirlet, H.; Wehrmann, L.M.; Barbieri, R.; Maignien, L.; Templer, S.P.; de Haas, H.; et al. Cold-water coral mounds on the Pen Duick Escarpment, Gulf of Cadiz: The MICROSYSTEMS project approach. Mar. Geol. 2011, 282, 102–117. [Google Scholar] [CrossRef]
- Magalhaes, V.H.; Pinheiro, L.M.; Ivanov, M.K.; Kozlova, E.; Blinova, V.; Kolganova, J.; Vasconcelos, C.; McKenzie, J.A.; Bernasconi, S.M.; Kopf, A.J.; et al. Formation processes of methane-derived authigenic carbonates from the Gulf of Cadiz. Sediment. Geol. 2012, 243, 155–168. [Google Scholar] [CrossRef]
- Le Bris, N.; Arnaud-Haond, S.; Beaulieu, S.; Cordes, E.E.; Hilario, A.; Rogers, A.; van de Gaever, S.; Watanabe, H. Hydrothermal Vents and Cold Seeps. In The First Global Integrated Marine Assessment, United Nations; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Vandorpe, T.; Martins, I.; Vitorino, J.; Hebbeln, D.; Garcia, M.; Van Rooij, D. Bottom currents and their influence on the sedimentation pattern in the El Arraiche mud volcano province, southern Gulf of Cadiz. Mar. Geol. 2016, 378, 114–126. [Google Scholar] [CrossRef]
- Roberts, H.H.; Kohl, B. Temperature control of cold-water coral (Lophelia) mound growth by climate-cycle forcing, Northeast Gulf of Mexico. Deep-Sea Res. Part II 2018, 140, 142–158. [Google Scholar] [CrossRef]
- Bassett-Smith, P.W. XLIII.—Report on the corals from the Tizard and Macclesfield Banks, China Sea. J. Nat. Hist. Ser. 1890, 6, 353–374. [Google Scholar] [CrossRef]
- Zou, R.L. Studies on the deep water scleractinia from South China Sea—II. Record and narration of species as well as time-spatial distributional characteristics. Trop. Oceanol. 1988, 7, 74–83, (In Chinese with English Abstract). [Google Scholar]
- Liu, Z.F.; Zhao, Y.L.; Colin, C.; Stattegger, K.; Wiesner, M.G.; Huh, C.A.; Zhang, Y.W.; Li, X.J.; Sompongchaiyakul, P.; You, C.F.; et al. Source-to-sink transport processes of fluvial sediments in the South China Sea. Earth-Sci. Rev. 2016, 153, 238–273. [Google Scholar] [CrossRef]
- Zhang, G.X.; Liang, J.Q.; Lu, J.A.; Yang, S.X.; Zhang, M.; Holland, M.; Schultheiss, P.; Su, X.; Sha, Z.B.; Xu, H.N.; et al. Geological features, controlling factors and potential prospects of the gas hydrate occurrence in the east part of the Pearl River Mouth Basin, South China Sea. Mar. Pet. Geol. 2015, 67, 356–367. [Google Scholar] [CrossRef]
- Suess, E. RV SONNE cruise report SO 177, Sino-German cooperative project, South China Sea Continental Margin: Geological methane budget and environmental effects of methane emissions and gas hydrates. IFM-GEOMAR Reports 2005. Available online: http://store.pangaea.de/documentation/Reports/SO177.pdf (accessed on 29 November 2019).
- Han, X.Q.; Suess, E.; Huang, Y.Y.; Wu, N.Y.; Bohrrnann, G.; Su, X.; Eisenhauer, A.; Rehder, G.; Fang, Y.X. Jiulong methane reef: Microbial mediation of seep carbonates in the South China Sea. Mar. Geol. 2008, 249, 243–256. [Google Scholar] [CrossRef]
- Feng, D.; Cheng, M.; Kiel, S.; Qiu, J.W.; Yang, Q.H.; Zhou, H.Y.; Peng, Y.B.; Chen, D.F. Using Bathymodiolus tissue stable carbon, nitrogen and sulfur isotopes to infer biogeochemical process at a cold seep in the South China Sea. Deep-Sea Res. Part II 2015, 104, 52–59. [Google Scholar] [CrossRef]
- Sha, Z.B.; Liang, J.Q.; Zhang, G.X.; Yang, S.X.; Lu, J.G.; Zhang, Z.J.; McConnell, D.R.; Humphrey, G. A seepage gas hydrate system in northern South China Sea: Seismic and well log interpretations. Mar. Geol. 2015, 366, 69–78. [Google Scholar] [CrossRef]
- Chen, F.; Hu, Y.; Feng, D.; Zhang, X.; Cheng, S.H.; Cao, J.; Lu, H.F.; Chen, D.F. Evidence of intense methane seepages from molybdenum enrichments in gas hydrate-bearing sediments of the northern South China Sea. Chem. Geol. 2016, 443, 173–181. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, D.; Peckmann, J.; Roberts, H.H.; Chen, D.F. New insights into cerium anomalies and mechanisms of trace metal enrichment in authigenic carbonate from hydrocarbon seeps. Chem. Geol. 2014, 381, 55–66. [Google Scholar] [CrossRef]
- Foubert, A.; Depreiter, D.; Beck, T.; Maignien, L.; Pannemans, B.; Frank, N.; Blamart, D.; Henriet, J.P. Carbonate mounds in a mud volcano province off north-west Morocco: Key to processes and controls. Mar. Geol. 2008, 248, 74–96. [Google Scholar] [CrossRef]
- Somoza, L.; Ercilla, G.; Urgorri, V.; Leon, R.; Medialdea, T.; Paredes, M.; Gonzalez, F.J.; Nombela, M.A. Detection and mapping of cold-water coral mounds and living Lophelia reefs in the Galicia Bank, Atlantic NW Iberia margin. Mar. Geol. 2014, 349, 73–90. [Google Scholar] [CrossRef]
- De Mol, B.; Van Rensbergen, P.; Pillen, S.; Van Herreweghe, K.; Van Rooij, D.; McDonnell, A.; Huvenne, V.; Ivanov, M.; Swennen, R.; Henriet, J.P. Large deep-water coral banks in the Porcupine Basin, southwest of Ireland. Mar. Geol. 2002, 188, 193–231. [Google Scholar] [CrossRef]
- Zoccola, D.; Ganot, P.; Bertucci, A.; Caminiti-Segonds, N.; Techer, N.; Voolstra, C.R.; Aranda, M.; Tambutte, E.; Allemand, D.; Casey, J.R.; et al. Bicarbonate transporters in corals point towards a key step in the evolution of cnidarian calcification. Sci. Rep. 2015, 5, 9983. [Google Scholar] [CrossRef]
- Nakamura, T.; Nadaoka, K.; Watanabe, A.; Yamamoto, T.; Miyajima, T.; Blanco, A.C. Reef-scale modeling of coral calcification responses to ocean acidification and sea-level rise. Coral Reefs 2018, 37, 37–53. [Google Scholar] [CrossRef]
- Sherwood, O.A.; Jamieson, R.E.; Edinger, E.N.; Wareham, V.E. Stable C and N isotopic composition of cold-water corals from the Newfoundland and Labrador continental slope: Examination of trophic, depth and spatial effects. Deep-Sea Res. Part II 2008, 55, 1392–1402. [Google Scholar] [CrossRef]
- Levin, L.A. Ecology of cold seep sediments: Interactions of fauna with flow, chemistry and microbes. Oceanogr. Mar. Biol. 2005, 43, 1–46. [Google Scholar]
- Whiticar, M.J. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol. 1999, 161, 291–314. [Google Scholar] [CrossRef]
- Sackett, W.M. Carbon and hydrogen isotope effects during the thermocatalytic production of hydrocarbons in laboratory simulation experiments. Geochim. Cosmochim. Acta 1978, 42, 571–580. [Google Scholar] [CrossRef]
- Zhang, M.S.; He, Q.X. A study on quaternary stratigraphy of reef deposits on Xisha Islands. Quat. Sci. 1989, 9, 143–154, (In Chinese with English Abstract). [Google Scholar]
- Ke, T.; Wei, G.J.; Liu, Y.; Xie, L.H.; Deng, W.F.; Wang, G.Q.; Xu, J.F. High resolution boron isotopic compositions of a coral from the northern South China Sea and their implications for reconstruction of seawater pH. Geochimica 2015, 44, 1–8, (In Chinese with English Abstract). [Google Scholar]
- Tran, T.H.; Kato, K.; Wada, H.; Fujioka, K.; Matsuzaki, H. Processes involved in calcite and aragonite precipitation during carbonate chimney formation on Conical Seamount, Mariana Forearc: Evidence from geochemistry and carbon, oxygen, and strontium isotopes. J. Geochem. Explor. 2014, 137, 55–64. [Google Scholar] [CrossRef]
- Fruh-Green, G.L.; Kelley, D.S.; Bernasconi, S.M.; Karson, J.A.; Ludwig, K.A.; Butterfield, D.A.; Boschi, C.; Proskurowski, G. 30,000 years of hydrothermal activity at the Lost City vent field. Science 2003, 301, 495–498. [Google Scholar] [CrossRef]
- Blamart, D.; Rollion-Bard, C.; Cuif, J.P.; Juillet-Leclerc, A.; Lutringer, A.; van Weering, T.C.E.; Henriet, J.P. C and O Isotopes in A Deep-Sea Coral (Lophelia Pertusa) Related to Skeletal Microstructure. In Cold-Water Corals and Ecosystems; Freiwald, A., Roberts, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1005–1020. [Google Scholar]
- Shirai, K.; Kusakabe, M.; Nakai, S.; Ishii, T.; Watanabe, T.; Hiyagon, H.; Sano, Y. Deep-sea coral geochemistry: Implication for the vital effect. Chem. Geol. 2005, 224, 212–222. [Google Scholar] [CrossRef]
- Chen, Z.; Mo, A.; Zhao, M.X.; Zhong, Y.; Yan, W. The discovery of deep-water corals from cold seep area in the northern South China Sea and their characteristics. J. Trop. Oceanogr. 2018, 37, 64–71, (In Chinese with English Abstract). [Google Scholar]
- Xu, A.; Chen, Z.; Qu, Y.; Tian, Y.; Shu, C.; Zheng, X.; Li, G.; Yan, W.; Zhao, M. Cold-water corals in a cold seep area on the northern continental slopes of the South China sea and their isotopic characteristics. Deep-Sea Res. I 2019, 153, 103043. [Google Scholar] [CrossRef]
- Becker, E.L.; Cordes, E.E.; Macko, S.A.; Fisher, C.R. Importance of seep primary production to Lophelia pertusa and associated fauna in the Gulf of Mexico. Deep-Sea Res. Part I 2009, 56, 786–800. [Google Scholar] [CrossRef]
- Fallon, S.J.; White, J.C.; McCulloch, M.T. Porites corals as recorders of mining and environmental impacts: Misima Island, Papua New Guinea. Geochim. Cosmochim. Acta 2002, 66, 45–62. [Google Scholar] [CrossRef]
- Robinson, L.F.; Adkins, J.F.; Frank, N.; Gagnon, A.C.; Prouty, N.G.; Roark, E.B.; van de Flierdt, T. The geochemistry of deep-sea coral skeletons: A review of vital effects and applications for palaeoceanography. Deep-Sea Res. Part II 2014, 105, 118. [Google Scholar] [CrossRef]
- Ramos, A.A.; Inoue, Y.; Ohde, S. Metal contents in Porites corals: Anthro- pogenic input of river run-off into a coral reef from an urbanized area, Okinawa. Mar. Pollut. Bull. 2004, 48, 281–294. [Google Scholar] [CrossRef]
- Sinclair, D.J.; Williams, B.; Allard, G.; Ghaleb, B.; Fallon, S.; Ross, S.W.; Risk, M. Reproducibility of trace element profiles in a specimen of the deep-water bamboo coral Keratoisis sp. Geochim. Cosmochim. Acta 2011, 75, 5101–5121. [Google Scholar] [CrossRef]
- Crocket, K.C.; Lambelet, M.; de Flierdt, T.V.; Rehkamper, M.; Robinson, L.F. Measurement of fossil deep-sea coral Nd isotopic compositions and concentrations by TIMS as NdO+, with evaluation of cleaning protocols. Chem. Geol. 2014, 374, 128–140. [Google Scholar] [CrossRef]
- Torres, M.E.; McManus, J.; Huh, C.A. Fluid seepage along the San Clemente Fault scarp: Basin-wide impact on barium cycling. Earth Planet. Sci. Lett. 2002, 203, 181–194. [Google Scholar] [CrossRef]
- Feng, D.; Roberts, H.H. Geochemical characteristics of the barite deposits at cold seeps from the northern Gulf of Mexico continental slope. Earth Planet. Sci. Lett. 2011, 309, 89–99. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, D.; Liang, Q.Y.; Xia, Z.; Chen, L.Y.; Chen, D.F. Impact of anaerobic oxidation of methane on the geochemical cycle of redox-sensitive elements at cold-seep sites of the northern South China Sea. Deep-Sea Res. Part II 2015, 122, 84–94. [Google Scholar] [CrossRef]
- Liang, Q.Y.; Hu, Y.; Feng, D.; Peckmann, J.; Chen, L.Y.; Yang, S.X.; Liang, J.Q.; Tao, J.; Chen, D.F. Authigenic carbonates from newly discovered active cold seeps on the northwestern slope of the South China Sea: Constraints on fluid sources, formation environments, and seepage dynamics. Deep-Sea Res. Part II 2017, 124, 31–41. [Google Scholar] [CrossRef]
- Peckmann, J.; Birgel, D.; Kiel, S. Molecular fossils reveal fluid composition and flow intensity at a Cretaceous seep. Geology 2009, 37, 847–850. [Google Scholar] [CrossRef]
- Roberts, H.H.; Feng, D.; Joye, S.B. Cold-seep carbonates of the middle and lower continental slope, northern Gulf of Mexico. Deep-Sea Res. Part II 2010, 57, 2040–2054. [Google Scholar] [CrossRef]
- Bayon, G.; Dupre, S.; Ponzevera, E.; Etoubleau, J.; Cheron, S.; Pierre, C.; Mascle, J.; Boetius, A.; de Lange, G.J. Formation of carbonate chimneys in the Mediterranean Sea linked to deep-water oxygen depletion. Nat. Geosci. 2013, 6, 755–760. [Google Scholar] [CrossRef]
- Feng, D.; Qiu, J.W.; Hu, Y.; Peckmann, J.; Guan, H.X.; Tong, H.P.; Chen, C.; Chen, J.X.; Gong, S.G.; Li, N.; et al. Cold seep systems in the South China Sea: An overview. J. Asian Earth Sci. 2018, 168, 3–16. [Google Scholar] [CrossRef]
- Teichert, B.M.A.; Eisenhauer, A.; Bohrmann, G.; Haase-Schramm, A.; Bock, B.; Linke, P. U/Th systematics and ages of authigenic carbonates from Hydrate Ridge, Cascadia Margin: Recorders of fluid flow variations. Geochim. Cosmochim. Acta 2003, 67, 3845–3857. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nakai, S.; Hiruta, A.; Matsumoto, R.; Yoshida, K. U-Th dating of carbonate nodules from methane seeps off Joetsu, Eastern Margin of Japan Sea. Earth Planet. Sci. Lett. 2008, 272, 89–96. [Google Scholar] [CrossRef]
- Cremiere, A.; Lepland, A.; Chand, S.; Sahy, D.; Condon, D.J.; Noble, S.R.; Martma, T.; Thorsnes, T.; Sauer, S.; Brunstad, H. Timescales of methane seepage on the Norwegian margin following collapse of the Scandinavian Ice Sheet. Nat. Commun. 2016, 7, 11509. [Google Scholar] [CrossRef]
- Wu, D.D.; Sun, T.T.; Xie, R.; Pan, M.D.; Chen, X.G.; Ye, Y.; Liu, L.H.; Wu, N.Y. Characteristics of Authigenic Minerals around the Sulfate-Methane Transition Zone in the Methane-Rich Sediments of the Northern South China Sea: Inorganic Geochemical Evidence. Int. J. Env. Res. Public Health 2019, 16, 2299. [Google Scholar] [CrossRef]
- Xie, R.; Wu, D.D.; Liu, J.; Sun, T.T.; Liu, L.H.; Wu, N.Y. Geochemical Evidence of Metal-Driven Anaerobic Oxidation of Methane in the Shenhu Area, the South China Sea. Int. J. Env. Res. Public Health 2019, 16, 3559. [Google Scholar] [CrossRef]
- Chen, D.F.; Huang, Y.Y.; Yuan, X.L.; Cathles, L.M. Seep carbonates and preserved methane oxidizing archaea and sulfate reducing bacteria fossils suggest recent gas venting on the seafloor in the northeastern South China Sea. Mar. Pet. Geol. 2005, 22, 613–621. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).