Geochemistry and Mineralogy of Rare Earth Elements (REE) in Bauxitic Ores of the Catalan Coastal Range, NE Spain
Abstract
1. Introduction
2. Geological Setting
3. Sampling and Methodology
4. Results
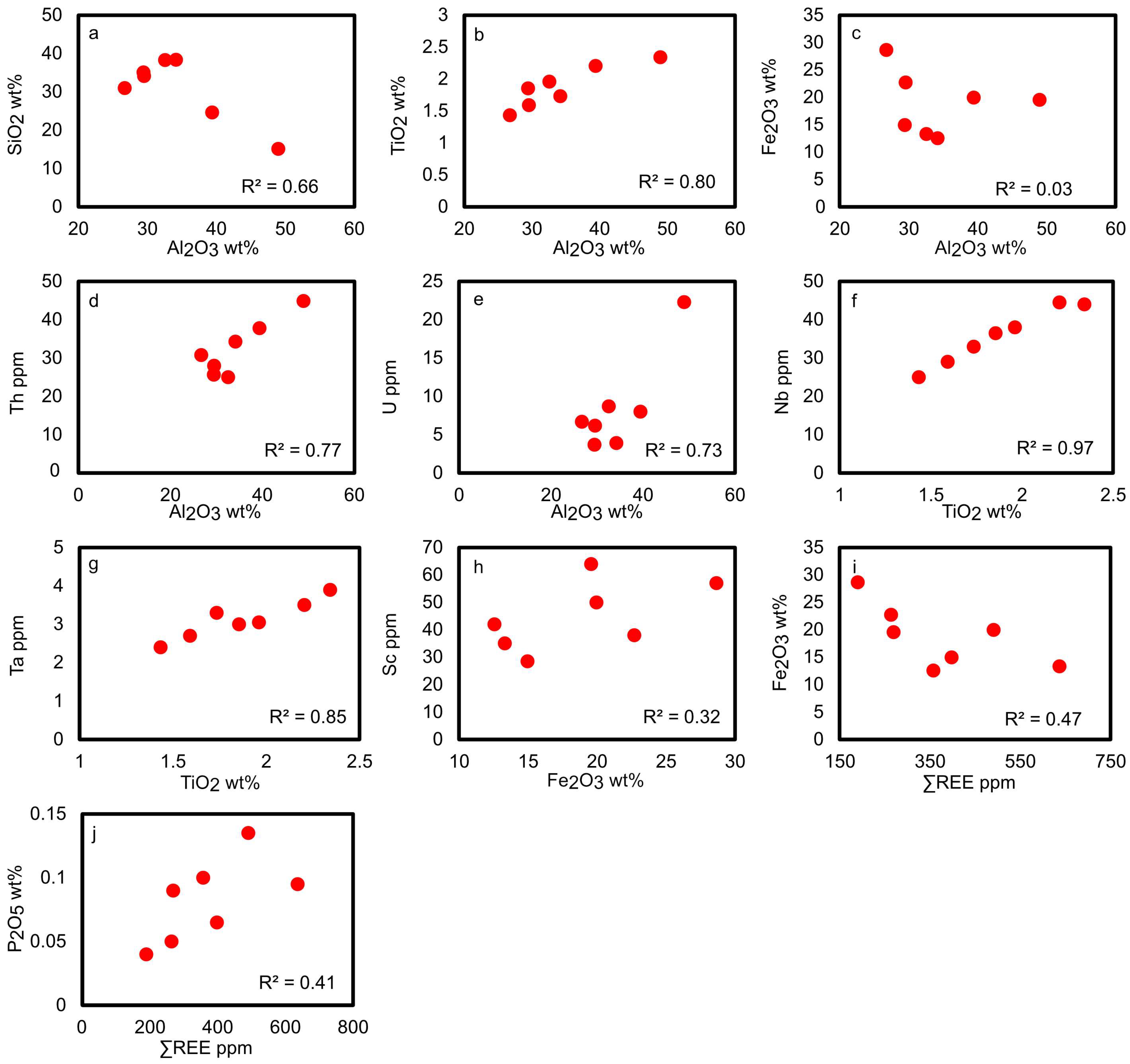
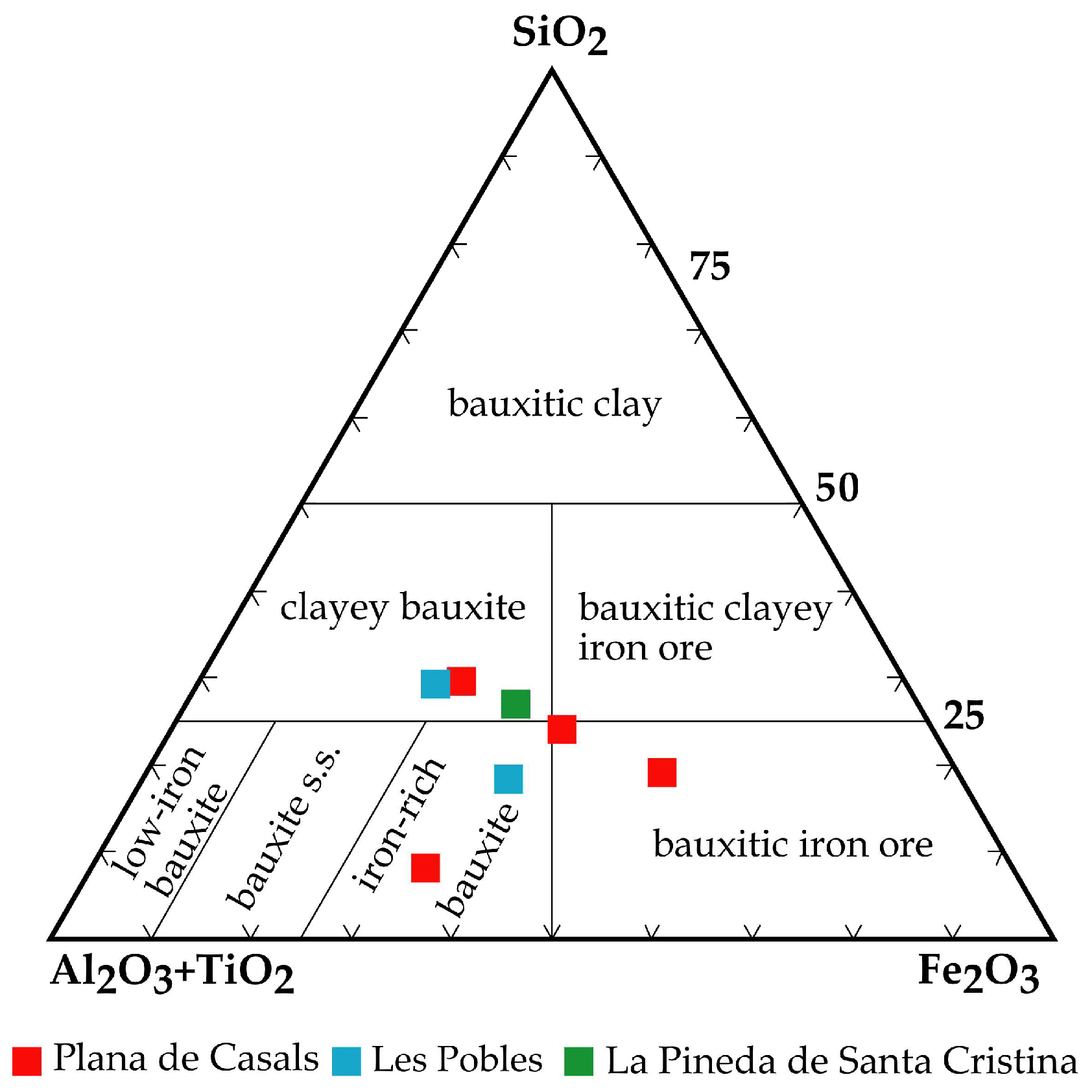
4.1. Major and Trace Element Geochemistry and Geochemical Classification
| Sample Number | Plana de Casals | El Miracle Mine | LPSC | ||||
|---|---|---|---|---|---|---|---|
| MM-1 | MM-2 | MM-3 | MM-4 1 | MI-0 1 | MI-2 | RU-1 1 | |
| SiO2 [wt %] | 15.12 | 34.17 | 31.03 | 38.31 | 24.62 | 38.39 | 35.08 |
| Al2O3 | 48.98 | 29.55 | 26.73 | 32.57 | 39.43 | 34.18 | 29.45 |
| Fe2O3(T) | 19.57 | 22.71 | 28.66 | 13.33 | 19.95 | 12.56 | 14.97 |
| MnO | 0.01 | 0.02 | 0.03 | 0.03 | 0.02 | 0.01 | 0.05 |
| MgO | 0.03 | 0.04 | 0.04 | 0.08 | 0.07 | 0.05 | 0.45 |
| CaO | 0.08 | 0.12 | 0.11 | 0.13 | 0.14 | 0.20 | 2.53 |
| Na2O | 0.02 | 0.02 | 0.02 | 0.03 | 0.03 | 0.04 | 0.06 |
| K2O | 0.02 | 0.03 | 0.02 | 0.14 | 0.13 | 0.06 | 0.49 |
| TiO2 | 2.34 | 1.59 | 1.43 | 1.96 | 2.21 | 1.74 | 1.86 |
| P2O5 | 0.09 | 0.05 | 0.04 | 0.10 | 0.14 | 0.10 | 0.07 |
| LOI | 12.77 | 11.55 | 10.67 | 12.78 | 12.36 | 12.96 | 14.77 |
| Total | 99.03 | 99.85 | 98.78 | 99.43 | 99.07 | 100.30 | 99.73 |
| Ag [ppm] | 1.50 | 1.10 | 0.90 | 1.50 | 2.00 | 1.30 | 1.40 |
| As | 29.00 | 32.00 | 45.00 | 28.00 | 38.50 | 18.00 | 13.00 |
| Ba | 32.00 | 19.00 | 12.00 | 41.50 | 39.00 | 23.00 | 78.50 |
| Be | 2.00 | 4.00 | 7.00 | 10.00 | 4.00 | 8.00 | 4.00 |
| Bi | 1.00 | 0.70 | 0.80 | 0.50 | 0.85 | 0.70 | 0.60 |
| Co | 6.00 | 6.00 | 15.00 | 16.00 | 21.50 | 18.00 | 13.00 |
| Cr | 400.00 | 230.00 | 270.00 | 155.00 | 280.00 | 220.00 | 145.00 |
| Cs | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | 8.35 |
| Cu | 20.00 | 20.00 | 30.00 | 20.00 | 30.00 | 50.00 | 40.00 |
| Ga | 63.00 | 30.00 | 39.00 | 32.00 | 52.00 | 39.00 | 33.00 |
| Ge | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 1.00 | 2.00 |
| Hf | 12.60 | 8.20 | 8.10 | 10.80 | 14.00 | 11.80 | 9.90 |
| In | 0.30 | 0.20 | 0.20 | < 0.2 | 0.25 | 0.20 | < 0.2 |
| Mo | 19.00 | 5.00 | 6.00 | 7.00 | 17.00 | 8.00 | 3.00 |
| Nb | 44.00 | 29.00 | 25.00 | 38.00 | 44.50 | 33.00 | 36.50 |
| Ni | 70.00 | 70.00 | 90.00 | 90.00 | 125.00 | 130.00 | 135.00 |
| Pb | 29.00 | 43.00 | 41.00 | 58.50 | 55.00 | 41.00 | 35.50 |
| Rb | <2 | <2 | <2 | 5.00 | 5.00 | 3.00 | 41.50 |
| Sb | 2.90 | 2.80 | 3.20 | 1.80 | 2.60 | 1.90 | 2.20 |
| Sn | 8.00 | 6.00 | 6.00 | 6.00 | 7.50 | 6.00 | 6.00 |
| Sr | 402.00 | 72.00 | 35.00 | 175.50 | 318.00 | 315.00 | 93.50 |
| Ta | 3.90 | 2.70 | 2.40 | 3.05 | 3.50 | 3.30 | 3.00 |
| Th | 44.90 | 28.00 | 30.80 | 25.00 | 37.80 | 34.30 | 25.70 |
| Tl | 0.10 | 0.10 | 0.30 | 0.10 | <0.1 | <0.1 | 1.25 |
| U | 22.30 | 6.20 | 6.70 | 8.70 | 8.00 | 3.90 | 3.70 |
| V | 531.00 | 360.00 | 471.00 | 244.50 | 393.50 | 303.00 | 238.00 |
| W | 4.00 | 3.00 | 3.00 | 4.00 | 4.50 | 3.00 | 5.50 |
| Zn | <30 | <30 | <30 | <30 | 65.00 | 40.00 | 170.00 |
| Zr | 508.00 | 332.00 | 317.00 | 434.50 | 556.00 | 466.00 | 397.00 |
| Sc | 64.00 | 38.00 | 57.00 | 35.00 | 50.00 | 42.00 | 28.50 |
| Y | 35.00 | 37.00 | 39.00 | 148.00 | 77.50 | 47.00 | 66.00 |
| La | 62.90 | 74.40 | 35.80 | 129.00 | 118.00 | 79.50 | 92.15 |
| Ce | 128.00 | 83.60 | 64.30 | 215.50 | 196.00 | 151.00 | 167.00 |
| Pr | 10.70 | 14.80 | 8.19 | 32.70 | 22.35 | 16.80 | 17.45 |
| Nd | 36.40 | 50.40 | 34.80 | 127.00 | 85.80 | 63.50 | 64.75 |
| Sm | 6.40 | 9.50 | 10.10 | 29.40 | 17.10 | 11.20 | 12.35 |
| Eu | 1.32 | 1.99 | 2.38 | 7.00 | 3.67 | 2.42 | 2.63 |
| Gd | 5.30 | 7.20 | 8.90 | 28.30 | 14.05 | 9.20 | 10.75 |
| Tb | 0.90 | 1.20 | 1.60 | 4.35 | 2.10 | 1.40 | 1.80 |
| Dy | 6.10 | 7.90 | 9.20 | 25.90 | 12.50 | 8.70 | 11.35 |
| Ho | 1.30 | 1.60 | 1.70 | 5.05 | 2.50 | 1.70 | 2.30 |
| Er | 4.00 | 4.70 | 5.30 | 14.70 | 7.45 | 5.20 | 6.80 |
| Tm | 0.61 | 0.72 | 0.82 | 2.11 | 1.10 | 0.77 | 1.01 |
| Yb | 4.40 | 5.00 | 5.80 | 13.90 | 7.45 | 5.30 | 6.60 |
| Lu | 0.67 | 0.73 | 0.84 | 2.10 | 1.15 | 0.84 | 0.99 |
| ∑REE 2 | 269.00 | 263.74 | 189.73 | 637.01 | 491.22 | 357.53 | 397.92 |
| ∑LREE 3 | 245.72 | 234.69 | 155.57 | 540.60 | 442.92 | 324.42 | 356.33 |
| ∑HREE 4 | 23.28 | 29.05 | 34.16 | 96.41 | 48.30 | 33.11 | 41.59 |
| ∑REE+[Sc+Y] | 368.00 | 338.74 | 285.73 | 820.01 | 618.72 | 446.53 | 492.42 |
| LREE/HREE | 10.55 | 8.08 | 4.55 | 5.61 | 9.17 | 9.80 | 8.57 |
| LaN/YbN 5 | 9.71 | 10.11 | 4.19 | 6.30 | 10.76 | 10.19 | 9.48 |
| LaN/SmN 6 | 6.14 | 4.89 | 2.21 | 2.74 | 4.31 | 4.43 | 4.66 |
| SmN/YbN 7 | 1.58 | 2.07 | 1.89 | 2.30 | 2.50 | 2.30 | 2.04 |
| Ce/CeN* 8 | 1.07 | 0.56 | 0.85 | 0.76 | 0.84 | 0.93 | 0.92 |
| Eu/EuN* 9 | 0.67 | 0.70 | 0.75 | 0.73 | 0.70 | 0.71 | 0.68 |
4.2. Mineralogy
4.2.1. Mineralogical and Textural Characteristics and Classification of CCR Bauxites
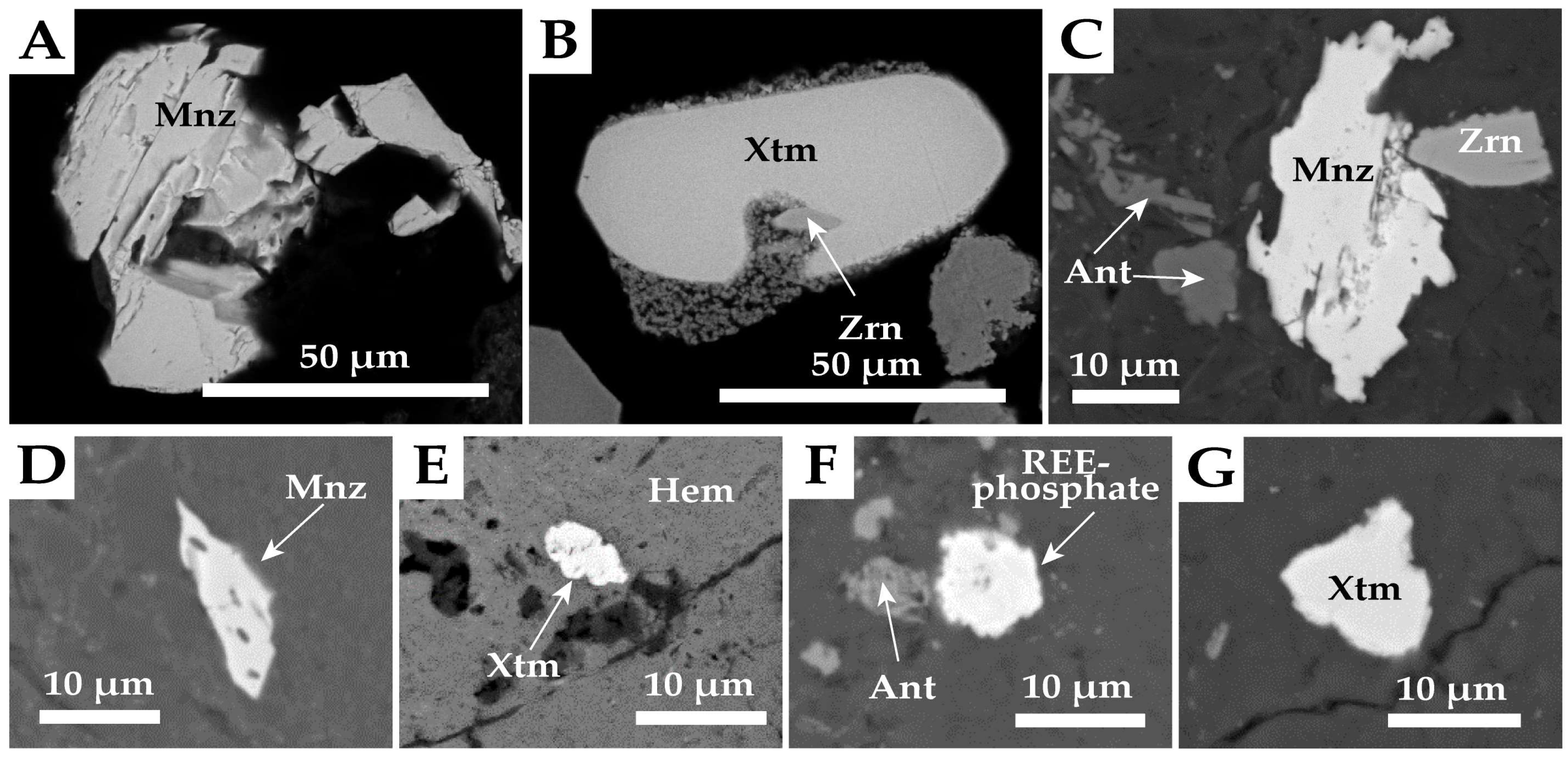
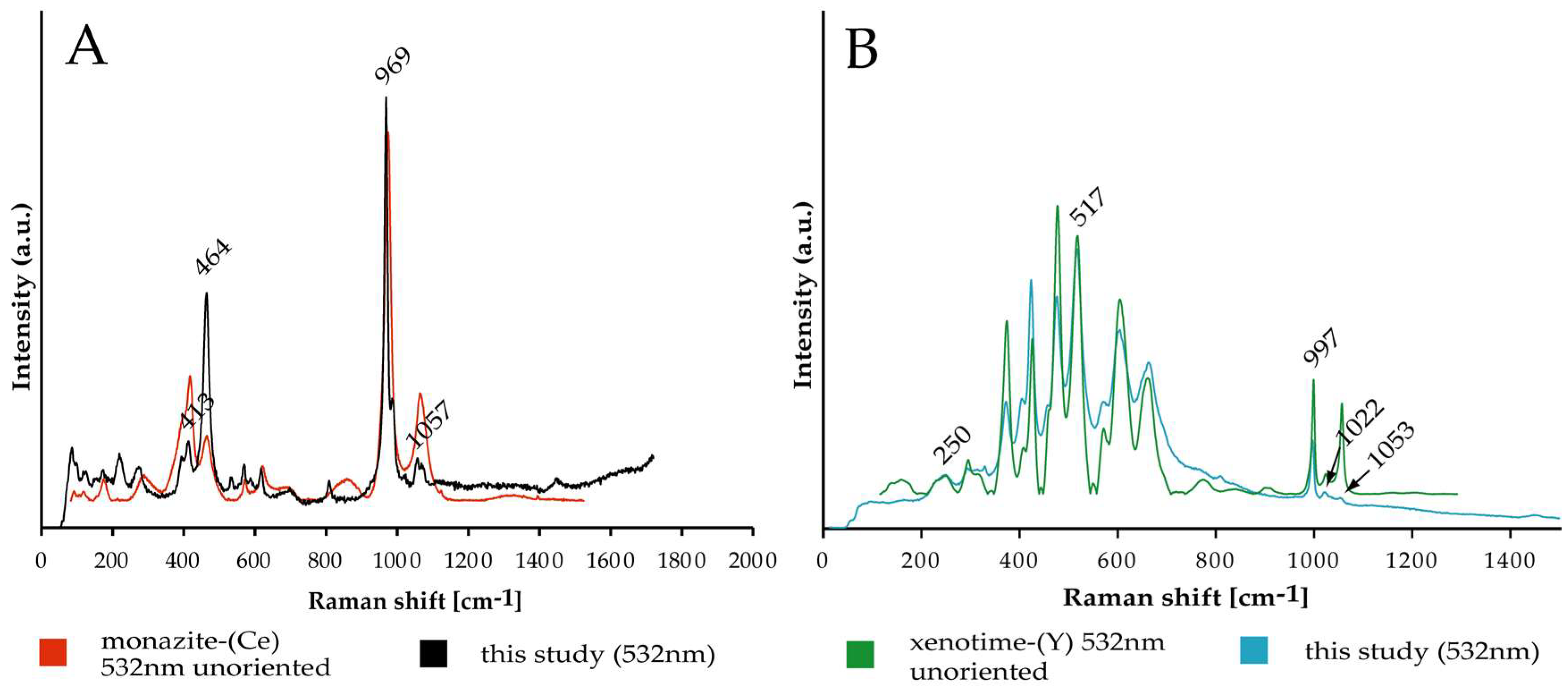
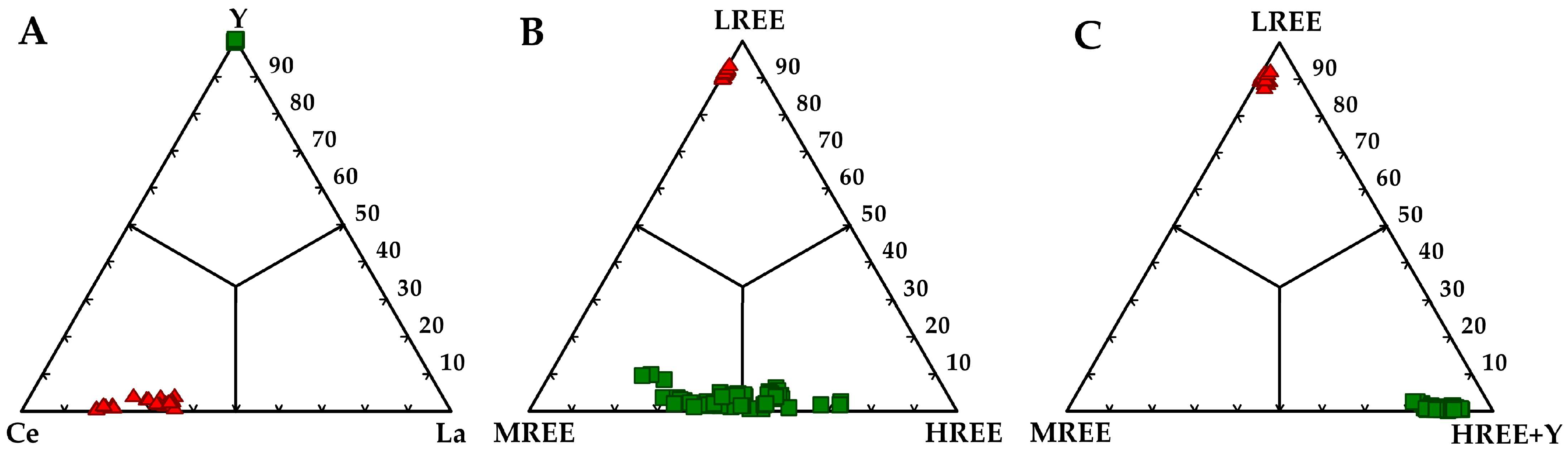
4.2.2. REE Mineralogy
4.2.3. Other Mineral Phases in Heavy Mineral Concentrates
5. Discussion
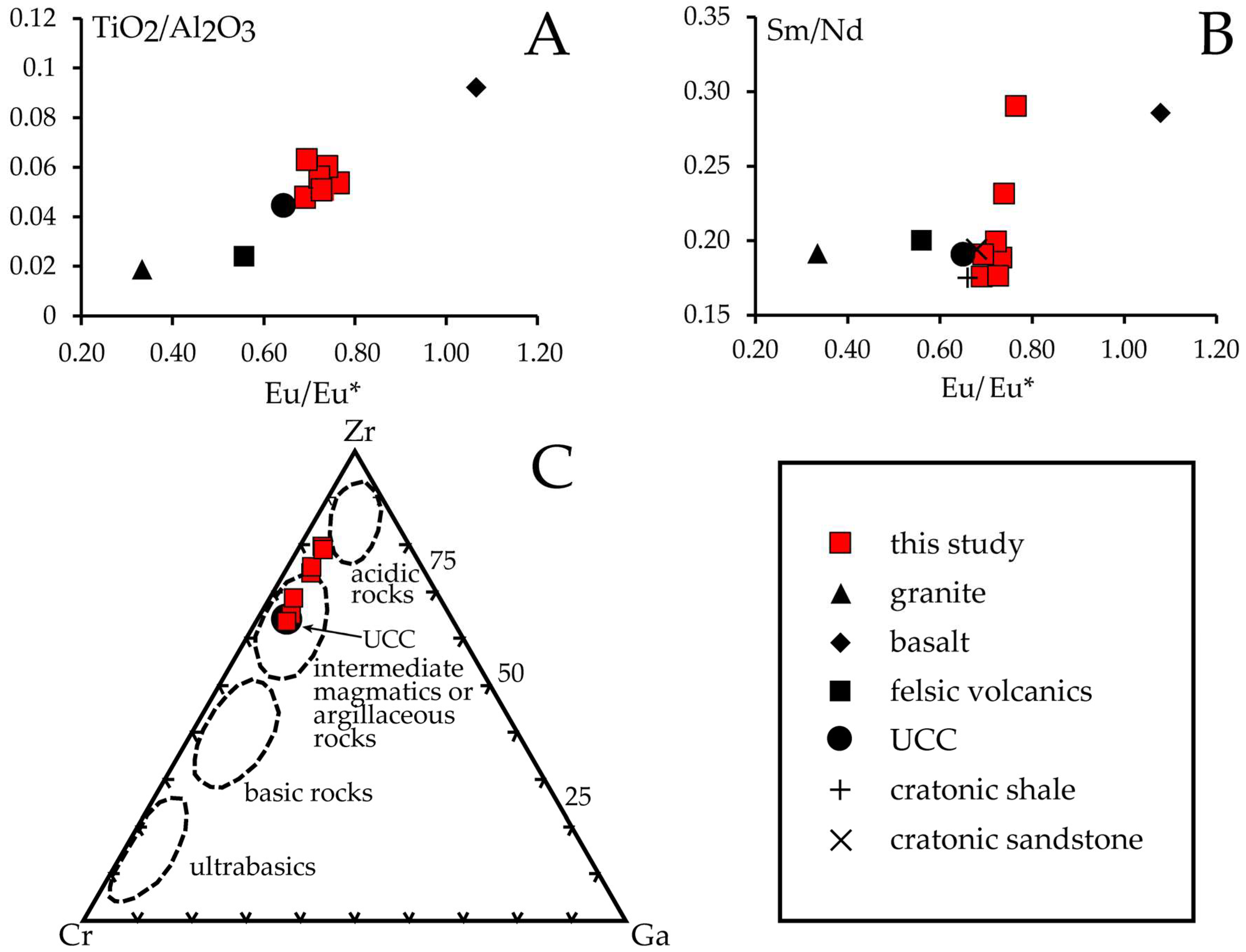
5.1. Bauxite Ore Formation
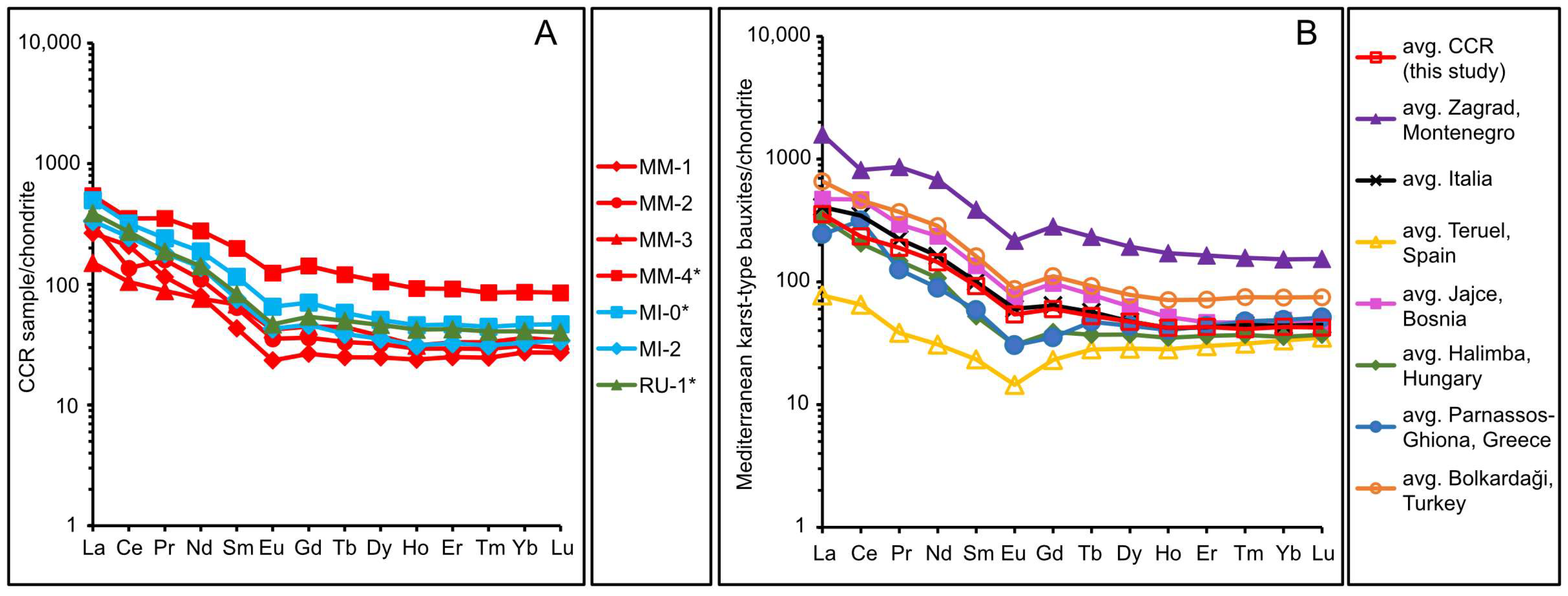
5.2. Mobilization and Fractionation of REE
5.3. Minerals Controlling REE Distribution
6. Conclusions
- The most likely precursor material of the bauxitic rocks were argillaceous sediments, possibly derived from igneous rocks of intermediate composition during the erosion of the Ebro massif paleo-high.
- The main REE-minerals are monazite-(Ce) and xenotime-(Y) of detrital origin. Unidentified REE-phosphate grains show textural evidence that may point to an authigenic origin.
- Leaching of the REE probably took place under acidic conditions, whereas the crystallization of rare earth minerals required near neutral to alkaline (pH 6~10) and overall oxidizing conditions during an incomplete bauxitization process.
- Adsorption on Fe- and Mn-oxyhydroxides and clay minerals can be ruled out as a REE scavenging mechanism in the bauxitic ores of the CCR.
- The absence of REE-bearing carbonates and the presence of phosphates as the only REE-bearing mineral phases indicates distinctly different formation conditions than in other Mediterranean-type karst bauxites and suggests relatively high initial phosphorus contents.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wall, F. Rare earth elements. In Critical Metals Handbook; Gunn, G., Ed.; John Wiley & Sons: Oxford, UK, 2013; pp. 312–339. ISBN 978-1-118-75534-1. [Google Scholar]
- Chakhmouradian, A.R.; Wall, F. Rare Earth Elements: Minerals, Mines, Magnets (and More). Elements 2012, 8, 333–340. [Google Scholar] [CrossRef]
- Goldschmidt, V.M.; Barth, T.; Lunde, G. Geochemische Verteilungsgesetze der Elemente. 5, Isomorphie und Polymorphie der Sesquioxyde. Die Lanthaniden-Kontraktion und ihre Konsequenzen; Jacob Dybwad: Oslo, Norway, 1925. (In German) [Google Scholar]
- Goodenough, K.M.; Wall, F.; Merriman, D. The Rare Earth Elements: Demand, Global Resources, and Challenges for Resourcing Future Generations. Nat. Resour. Res. 2018, 27, 201–216. [Google Scholar] [CrossRef]
- Dutta, T.; Kim, K.-H.; Uchimiya, M.; Kwon, E.E.; Jeon, B.-H.; Deep, A.; Yun, S.-T. Global demand for rare earth resources and strategies for green mining. Environ. Res. 2016, 150, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Binnemans, K.; Jones, P.T.; Müller, T.; Yurramendi, L. Rare Earths and the Balance Problem: How to Deal with Changing Markets? J. Sustain. Metall. 2018, 4, 126–146. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. Rare Earths and the Balance Problem. J. Sustain. Metall. 2015, 1, 29–38. [Google Scholar] [CrossRef]
- Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs, European Commission; Deloitte Sustainability; British Geological Survey. Toegepast Natuurwetenschappelijk Onderzoek Study on the Review of the List of Critical Raw Materials Final Report; European Commission: Brussels, Belgium, 2017; ISBN 978-92-79-47937-3. [Google Scholar]
- Goodenough, K.M.; Schilling, J.; Jonsson, E.; Kalvig, P.; Charles, N.; Tuduri, J.; Deady, E.A.; Sadeghi, M.; Schiellerup, H.; Müller, A.; et al. Europe’s rare earth element resource potential: An overview of REE metallogenetic provinces and their geodynamic setting. Ore Geol. Rev. 2016, 72, 838–856. [Google Scholar] [CrossRef]
- Mordberg, L.E. Patterns of distribution and behaviour of trace elements in bauxites. Chem. Geol. 1993, 107, 241–244. [Google Scholar] [CrossRef]
- Klyucharev, D.S.; Volkova, N.M.; Comyn, M.F. The problems associated with using non-conventional rare-earth minerals. J. Geochem. Explor. 2013, 133, 138–148. [Google Scholar] [CrossRef]
- Deady, É.; Mouchos, E.; Goodenough, K.; Wall, F. Rare Earth Elements in Karst-Bauxites: A Novel Untapped European Resource? In Proceedings of the European Rare Earth Resources Conference, Milos, Greece, 4–7 September 2014; p. 12. [Google Scholar]
- Torró, L.; Proenza, J.A.; Aiglsperger, T.; Bover-Arnal, T.; Villanova-de-Benavent, C.; Rodríguez-García, D.; Ramírez, A.; Rodríguez, J.; Mosquea, L.A.; Salas, R. Geological, geochemical and mineralogical characteristics of REE-bearing Las Mercedes bauxite deposit, Dominican Republic. Ore Geol. Rev. 2017, 89, 114–131. [Google Scholar] [CrossRef]
- Hind, A.R.; Bhargava, S.K.; Grocott, S.C. The surface chemistry of Bayer process solids: A review. Colloids Surf. Physicochem. Eng. Asp. 1999, 146, 359–374. [Google Scholar] [CrossRef]
- Deady, É.A.; Mouchos, E.; Goodenough, K.; Williamson, B.J.; Wall, F. A review of the potential for rare-earth element resources from European red muds: Examples from Seydişehir, Turkey and Parnassus-Giona, Greece. Mineral. Mag. 2016, 80, 43–61. [Google Scholar] [CrossRef]
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef]
- Borra, C.R.; Mermans, J.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Selective recovery of rare earths from bauxite residue by combination of sulfation, roasting and leaching. Miner. Eng. 2016, 92, 151–159. [Google Scholar] [CrossRef]
- Vind, J.; Malfliet, A.; Blanpain, B.; Tsakiridis, P.; Tkaczyk, A.; Vassiliadou, V.; Panias, D. Rare Earth Element Phases in Bauxite Residue. Minerals 2018, 8, 77. [Google Scholar] [CrossRef]
- Aiglsperger, T.; Proenza, J.A.; Lewis, J.F.; Labrador, M.; Svojtka, M.; Rojas-Purón, A.; Longo, F.; Ďurišová, J. Critical metals (REE, Sc, PGE) in Ni laterites from Cuba and the Dominican Republic. Ore Geol. Rev. 2016, 73, 127–147. [Google Scholar] [CrossRef]
- Radusinović, S.; Jelenković, R.; Pačevski, A.; Simić, V.; Božović, D.; Holclajtner-Antunović, I.; Životić, D. Content and mode of occurrences of rare earth elements in the Zagrad karstic bauxite deposit (Nikšić area, Montenegro). Ore Geol. Rev. 2017, 80, 406–428. [Google Scholar] [CrossRef]
- Gamaletsos, P.N.; Godelitsas, A.; Filippidis, A.; Pontikes, Y. The Rare Earth Elements Potential of Greek Bauxite Active Mines in the Light of a Sustainable REE Demand. J. Sustain. Metall. 2018. [Google Scholar] [CrossRef]
- Bárdossy, G. Karst Bauxites: Bauxite Deposits on Carbonate Rocks; Developments in Economic Geology; Distribution for the U.S.A. and Canada, Elsevier Science Pub. Co: Amsterdam, The Netherland; New York, NY, USA, 1982; ISBN 978-0-444-99727-2. [Google Scholar]
- Bárdossy, G.; Aleva, G.J.J. Lateritic Bauxites; Developments in Economic Geology; Distribution for the U.S.A. and Canada, Elsevier Science Pub. Co: Amsterdam, The Netherland; New York, NY, USA, 1990; ISBN 978-0-444-98811-9. [Google Scholar]
- Mordberg, L.E.; Stanley, C.J.; Germann, K. Mineralogy and geochemistry of trace elements in bauxites: The Devonian Schugorsk deposit, Russia. Mineral. Mag. 2001, 65, 81–101. [Google Scholar] [CrossRef]
- Wang, X.; Jiao, Y.; Du, Y.; Ling, W.; Wu, L.; Cui, T.; Zhou, Q.; Jin, Z.; Lei, Z.; Weng, S. REE mobility and Ce anomaly in bauxite deposit of WZD area, Northern Guizhou, China. J. Geochem. Explor. 2013, 133, 103–117. [Google Scholar] [CrossRef]
- Maksimović, Z.; Pantó, G. Contribution to the geochemistry of the rare earth elements in the karst-bauxite deposits of Yugoslavia and Greece. Geoderma 1991, 51, 93–109. [Google Scholar] [CrossRef]
- Maksimović, Z.; Pantó, G. Authigenic rare earth minerals in karst-bauxites and karstic nickel deposits. In Rare Earth Minerals: Chemistry, Origin, and Ore Deposits; Jones, A.P., Ed.; Mineralogical Society Series; Chapman & Hall: London, UK, 1996; pp. 257–279. ISBN 978-0-412-61030-1. [Google Scholar]
- Ochsenkühn-Petropulu, M.; Ochsenkühn, K.M. Rare earth minerals found in Greek bauxites by scanning electron microscopy and electron probe micro-analysis. Microsc. Anal. 1995, 37, 33–34. [Google Scholar]
- Mongelli, G. Ce-anomalies in the textural components of Upper Cretaceous karst bauxites from the Apulian carbonate platform (southern Italy). Chem. Geol. 1997, 140, 69–79. [Google Scholar] [CrossRef]
- Mameli, P.; Mongelli, G.; Oggiano, G.; Dinelli, E. Geological, geochemical and mineralogical features of some bauxite deposits from Nurra (Western Sardinia, Italy): Insights on conditions of formation and parental affinity. Int. J. Earth Sci. 2007, 96, 887–902. [Google Scholar] [CrossRef]
- Boni, M.; Rollinson, G.; Mondillo, N.; Balassone, G.; Santoro, L. Quantitative Mineralogical Characterization of Karst Bauxite Deposits in the Southern Apennines, Italy. Econ. Geol. 2013, 108, 813–833. [Google Scholar] [CrossRef]
- Mongelli, G.; Boni, M.; Buccione, R.; Sinisi, R. Geochemistry of the Apulian karst bauxites (southern Italy): Chemical fractionation and parental affinities. Ore Geol. Rev. 2014, 63, 9–21. [Google Scholar] [CrossRef]
- Putzolu, F.; Piccolo Papa, A.; Mondillo, N.; Boni, M.; Balassone, G.; Mormone, A. Geochemical Characterization of Bauxite Deposits from the Abruzzi Mining District (Italy). Minerals 2018, 8, 298. [Google Scholar] [CrossRef]
- Hanilçi, N. Geological and geochemical evolution of the Bolkardaği bauxite deposits, Karaman, Turkey: Transformation from shale to bauxite. J. Geochem. Explor. 2013, 133, 118–137. [Google Scholar] [CrossRef]
- Maksimović, Z.; Pantó, G. Hydroxyl-bastnaesite-(Nd), a new mineral from Montenegro, Yugoslavia. Mineral. Mag. 1985, 49, 717–720. [Google Scholar] [CrossRef]
- Molina, J.M. A review of karst bauxites and related paleokarsts in Spain. Acta Geol. Hung. 1991, 34, 179–194. [Google Scholar]
- Salas, R.; Vaquer, R.; Travé, A. Bauxitas kársticas y arcillas lateríticas berremienses de la Cadena Ibérica oriental y la Cadena Costera Catalana: Relaciones genéticas y áreas de procedencia. Geo-Temas 2004, 6, 4. (In Spanish) [Google Scholar]
- Ordóñez, S.; Fort, R.; Bustillo, M. Estudio de las tierras raras en las bauxitas karsticas del noreste de la peninsula iberica. Estud. Geol. 1990, 46, 373–384. (In Spanish) [Google Scholar] [CrossRef]
- Yuste, A.; Bauluz, B.; Mayayo, M.J. Genesis and mineral transformations in Lower Cretaceous karst bauxites (NE Spain): Climatic influence and superimposed processes: Lower Cretaceous Karst Bauxites. Geol. J. 2015, 50, 839–857. [Google Scholar] [CrossRef]
- Yuste, A.; Bauluz, B.; Mayayo, M.J. Origin and geochemical evolution from ferrallitized clays to karst bauxite: An example from the Lower Cretaceous of NE Spain. Ore Geol. Rev. 2017, 84, 67–79. [Google Scholar] [CrossRef]
- Guimerá, J. Palaeogene evolution of deformation in the northeastern Iberian Peninsula. Geol. Mag. 1984, 121, 413. [Google Scholar] [CrossRef]
- Guimerà, J.; De Vicente, G.; Rodríguez Pascua, M.A.; Muñoz Martín, A.; Vegas, R.; Simón, J.L. Cadenas con cobertura: Las cadenas Ibérica y Costera Catalana. In Geología de España; Vera, J.A., Ed.; Sociedad Geológica de España: Salamanca, Spain; Ministerio de Educación y Ciencia, Instituto Geológico y Minero de España: Madrid, Spain, 2004; pp. 602–617. ISBN 978-84-7840-546-6. (In Spanish) [Google Scholar]
- Catalunya, I.C. i G. de ICGC—Vissir3. Available online: http://www.icc.cat/vissir3/ (accessed on 18 September 2017).
- Salas, R.; Querol, X.; Fernandez Turiel, J.L.; Lopez Soler, A. The Bauxite and Lateritic Clay Deposits of NE Spain; University of Barcelona: Barcelona, Spain, 1990. [Google Scholar]
- Anadón, P.; Colombo, F.; Esteban, M.; Marzo, M.; Robles, S.; Santanach, P.; Solé Sugrañés, Y.L. Evolución tectonoestratigráfica de los Catalánides. Acta Geol. Hispànica 1979, 242–270. [Google Scholar]
- Salas, R.; Guimerá, J.; Mas, R.; Martín-Closas, C.; Meléndez, A.; Alonso, A. Evolution of the Mesozoic central Iberian rift system and its Cainozoic inversion (Iberian Chain). In Peri-Tethyan Rift/Wrench Basins and Passive Margins; Ziegler, P.A., Ed.; Peri-Tethys Memoir; Publications Scientifiques du Muséum: Paris, France, 2001; pp. 145–185. ISBN 978-2-85653-528-8. [Google Scholar]
- Combes, P.J. Recherches sur la genèse des bauxites dans le Nord-Est de l’Espagne, le Languedoc et l’Ariège (France); Foundation C.E.R.G.A.: Montpellier, France, 1969. (In French) [Google Scholar]
- Anadón, P.; Cabrera, L.; Guimerà, J.; Santanach, P. Paleogene Strike-Slip Deformation and Sedimentation Along the Southeastern Margin of the Ebro Basin. In Strike-Slip Deformation, Basin Formation, and Sedimentation; Biddle, K.T., Christie-Blick, N., Eds.; SEPM Society for Sedimentary Geology: Broken Arrow, OK, USA, 1985; pp. 303–318. [Google Scholar]
- Universitat de Barcelona Hydroseparation Laboratory Barcelona. Available online: http://hslab-barcelona.com/ (accessed on 26 September 2018).
- Cabri, L.J.; Rudashevsky, N.S.; Rudashevsky, V.N.; Oberthür, T. Electric-Pulse Disaggregation (Epd), Hydroseparation (Hs) and Their Use in Combination for Mineral Processing and Advanced Characterization of Ores. In Proceedings of the 40th Annual Canadian Mineral Processors Conference, Ottawa, ON, Canada, 22–24 January 2008; pp. 211–235. [Google Scholar]
- Aiglsperger, T.; Proenza, J.A.; Zaccarini, F.; Lewis, J.F.; Garuti, G.; Labrador, M.; Longo, F. Platinum group minerals (PGM) in the Falcondo Ni-laterite deposit, Loma Caribe peridotite (Dominican Republic). Miner. Depos. 2015, 50, 105–123. [Google Scholar] [CrossRef]
- Mongelli, G.; Boni, M.; Oggiano, G.; Mameli, P.; Sinisi, R.; Buccione, R.; Mondillo, N. Critical metals distribution in Tethyan karst bauxite: The cretaceous Italian ores. Ore Geol. Rev. 2017, 86, 526–536. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Distribution of yttrium and rare-earth elements in the Penge and Kuruman iron-formations, Transvaal Supergroup, South Africa. Precambrian Res. 1996, 79, 37–55. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.-S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; DE GRUYTER: Berlin, Germany; München, Germany; Boston, MA, USA, 2015; pp. 1–30. ISBN 978-3-11-041710-4. [Google Scholar]
- Lenz, C.; Nasdala, L.; Talla, D.; Hauzenberger, C.; Seitz, R.; Kolitsch, U. Laser-induced REE3+ photoluminescence of selected accessory minerals—An “advantageous artefact” in Raman spectroscopy. Chem. Geol. 2015, 415, 1–16. [Google Scholar] [CrossRef]
- MacLean, W.H.; Bonavia, F.F.; Sanna, G. Argillite debris converted to bauxite during karst weathering: Evidence from immobile element geochemistry at the Olmedo Deposit, Sardinia. Miner. Depos. 1997, 32, 607–616. [Google Scholar] [CrossRef]
- Lyew-Ayee, P.A. A case for the volcanic origin of Jamaican bauxites. In Proceedings of the Bauxite Symposium VI, Kingston, Jamaica, March 1986; p. 30. [Google Scholar]
- Pye, K. Bauxites gathering dust. Nature 1988, 333, 800–801. [Google Scholar] [CrossRef]
- Nesbitt, H.W. Mobility and fractionation of rare earth elements during weathering of a granodiorite. Nature 1979, 279, 206–210. [Google Scholar] [CrossRef]
- Gamaletsos, P.N.; Godelitsas, A.; Kasama, T.; Church, N.S.; Douvalis, A.P.; Göttlicher, J.; Steininger, R.; Boubnov, A.; Pontikes, Y.; Tzamos, E.; et al. Nano-mineralogy and -geochemistry of high-grade diasporic karst-type bauxite from Parnassos-Ghiona mines, Greece. Ore Geol. Rev. 2017, 84, 228–244. [Google Scholar] [CrossRef]
- Gamaletsos, P.; Godelitsas, A.; Mertzimekis, T.J.; Göttlicher, J.; Steininger, R.; Xanthos, S.; Berndt, J.; Klemme, S.; Kuzmin, A.; Bárdossy, G. Thorium partitioning in Greek industrial bauxite investigated by synchrotron radiation and laser-ablation techniques. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2011, 269, 3067–3073. [Google Scholar] [CrossRef]
- Ahmadnejad, F.; Zamanian, H.; Taghipour, B.; Zarasvandi, A.; Buccione, R.; Salamab Ellahi, S. Mineralogical and geochemical evolution of the Bidgol bauxite deposit, Zagros Mountain Belt, Iran: Implications for ore genesis, rare earth elements fractionation and parental affinity. Ore Geol. Rev. 2017, 86, 755–783. [Google Scholar] [CrossRef]
- Mongelli, G. REE and other trace elements in a granitic weathering profile from “Serre”, southern Italy. Chem. Geol. 1993, 103, 17–25. [Google Scholar] [CrossRef]
- Özlü, N. Trace-element content of “Karst Bauxites” and their parent rocks in the mediterranean belt. Miner. Depos. 1983, 18, 469–476. [Google Scholar] [CrossRef]
- MacLean, W.H.; Kranidiotis, P. Immobile elements as monitors of mass transfer in hydrothermal alteration; Phelps Dodge massive sulfide deposit, Matagami, Quebec. Econ. Geol. 1987, 82, 951–962. [Google Scholar] [CrossRef]
- Mindszenty, A. The lithology of some Hungarian bauxites—A contribution to the palaeogeographic reconstruction. Acta Geol. Acad. Sci. Hung. 1984, 27, 441–455. [Google Scholar]
- Misch, P.H. Der Bau der mittleren Südpyrenäen: Mit 6 Taf. u. 51 Abb. im Text; Weidmann: Berlin, Germany, 1934. (In German) [Google Scholar]
- Ashauer, H.; Teichmüller, R. Die Variscische und Alpidische Gebirgsbildung Kataloniens; mit 7 Tafeln und 48 Textabbildungen; Weidmann: Berlin, Germany, 1935. (In German) [Google Scholar]
- Wildeman, T.E.; Condie, K.C. Rare earths in Archean graywackes from Wyoming and from the Fig Tree Group, South Africa. Geochim. Cosmochim. Acta 1973, 37, 439–453. [Google Scholar] [CrossRef]
- Nance, W.B.; Taylor, S.R. Rare earth element patterns and crustal evolution—II. Archean sedimentary rocks from Kalgoorlie, Australia. Geochim. Cosmochim. Acta 1977, 41, 225–231. [Google Scholar] [CrossRef]
- Muzaffer Karadağ, M.; Küpeli, Ş.; Arýk, F.; Ayhan, A.; Zedef, V.; Döyen, A. Rare earth element (REE) geochemistry and genetic implications of the Mortaş bauxite deposit (Seydişehir/Konya–Southern Turkey). Chem. Erde Geochem. 2009, 69, 143–159. [Google Scholar] [CrossRef]
- Duddy, L.R. Redistribution and fractionation of rare-earth and other elements in a weathering profile. Chem. Geol. 1980, 30, 363–381. [Google Scholar] [CrossRef]
- Aubert, D.; Stille, P.; Probst, A. REE fractionation during granite weathering and removal by waters and suspended loads: Sr and Nd isotopic evidence. Geochim. Cosmochim. Acta 2001, 65, 387–406. [Google Scholar] [CrossRef]
- Fritz, B.; Tardy, Y. Etude thermodynamique du système gibbsite, quartz, kaolinite, gaz carbonique. Application à la genèse des podzols et des bauxites. Sci. Géol. Bull. 1973, 26, 339–367. [Google Scholar] [CrossRef]
- Drever, J.I. The Geochemistry of Natural Waters; Prentice Hall: Englewood Cliffs, UK, 1988; ISBN 978-0-13-351396-7. [Google Scholar]
- Johannesson, K.H.; Lyons, W.B.; Stetzenbach, K.J.; Byrne, R.H. The solubility control of rare earth elements in natural terrestrial waters and the significance of PO43− and CO32− in limiting dissolved rare earth concentrations: A review of recent information. Aquat. Geochem. 1995, 1, 157–173. [Google Scholar] [CrossRef]
- Johannesson, K.H.; Stetzenbach, K.J.; Hodge, V.F.; Berry Lyons, W. Rare earth element complexation behavior in circumneutral pH groundwaters: Assessing the role of carbonate and phosphate ions. Earth Planet. Sci. Lett. 1996, 139, 305–319. [Google Scholar] [CrossRef]
- Mao, J.; Lehmann, B.; Du, A.; Zhang, G.; Ma, D.; Wang, Y.; Zeng, M.; Kerrich, R. Re-Os Dating of Polymetallic Ni-Mo-PGE-Au Mineralization in Lower Cambrian Black Shales of South China and Its Geologic Significance. Econ. Geol. 2002, 97, 1051–1061. [Google Scholar] [CrossRef]
- Sholkovitz, E.R. The aquatic chemistry of rare earth elements in rivers and estuaries. Aquat. Geochem. 1995, 1, 1–34. [Google Scholar] [CrossRef]
- Pourret, O.; Gruau, G.; Dia, A.; Davranche, M.; Molénat, J. Colloidal Control on the Distribution of Rare Earth Elements in Shallow Groundwaters. Aquat. Geochem. 2010, 16, 31–59. [Google Scholar] [CrossRef]
- Morán, D.A. Caracterización y Estabilidad de Carbonatos de Elementos de Tierras Raras en Bauxitas Kársticas. Master’s Thesis, University of Barcelona, Barcelona, Spain, 2017. (In Spanish). [Google Scholar]
- Laveuf, C.; Cornu, S. A review on the potentiality of Rare Earth Elements to trace pedogenetic processes. Geoderma 2009, 154, 1–12. [Google Scholar] [CrossRef]
- Pourmand, A.; Dauphas, N.; Ireland, T.J. A novel extraction chromatography and MC-ICP-MS technique for rapid analysis of REE, Sc and Y: Revising CI-chondrite and Post-Archean Australian Shale (PAAS) abundances. Chem. Geol. 2012, 291, 38–54. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Zhang, Q.; Zhang, Y.; Li, Y. Genesis of REE minerals in the karstic bauxite in western Guangxi, China, and its constraints on the deposit formation conditions. Ore Geol. Rev. 2016, 75, 100–115. [Google Scholar] [CrossRef]
- Berger, A.; Janots, E.; Gnos, E.; Frei, R.; Bernier, F. Rare earth element mineralogy and geochemistry in a laterite profile from Madagascar. Appl. Geochem. 2014, 41, 218–228. [Google Scholar] [CrossRef]
- Proenza, J.A.; Aiglsperger, T.; Villanova-de-Benavent, C.; Torró, L.; Rodríguez, D.; Ramírez, A.; Rodríguez, J. Discovery of REE minerals hosted in karst bauxite ores from the Sierra de Bahoruco, Pedernales, Dominican Republic. In Proceedings of the 14th SGA Biennial Meeting, Québec City, QC, Canada, 20–23 August 2017; Volume 4, p. 5. [Google Scholar]




| Sample | Easting (m) | Northing (m) | Location | Thin Section |
|---|---|---|---|---|
| MM-1 | 378,151 | 4,595,059 | Plana de Casals | MM-1 |
| MM-2 | 378,342 | 4,595,064 | MM-2 | |
| MM-3 | 378,342 | 4,595,064 | MM-3 | |
| MM-4 | 378,291 | 4,595,024 | MM-4 and MM4B | |
| MI-0 | 369,239 | 4,579,639 | Les Pobles (El Miracle Mine) | MI-0 and MI-0B |
| MI-1 (A, B) | 369,239 | 4,579,639 | MI-1A, MI-1Arep, MI-1B | |
| MI-2 | 369,691 | 4,570,698 | MI-2 | |
| RU-1 | 369,691 | 4,570,698 | LPSC | - |
| Sample No. | Kln | Nac | Hem | Bhm | Dsp | Rt | Ant | Cal |
|---|---|---|---|---|---|---|---|---|
| MM-1 | 38 | - | 16 | 33 | 6 | tr | tr | - |
| MM-2 | 73 | - | 19 | - | - | tr | tr | - |
| MM-3 | 74 | - | 18 | - | - | 5 | tr | - |
| MM-4 | 76 | - | 16 | - | - | tr | tr | - |
| MI-0 | 45 | - | 17 | 23 | 8 | tr | tr | - |
| MI-1A 1 | 96 | - | tr | - | - | - | - | - |
| MI-1B 2 | 45 | - | 27 | - | - | - | tr | 26 |
| MI-2 | 75 | - | 14 | - | - | 5 | tr | tr |
| RU-1 | 37 | 32 | 13 | - | - | tr | tr | 10 |
| No. | MM-4 p1 | MM-4 p6 | 0.4 A mag. FC* p10 | 0.4 A mag. FC* p13 | MM-4 p13 | MM-2 p22 | 0.4 A mag. FC p1 | 0.4 A mag. FC p6 |
|---|---|---|---|---|---|---|---|---|
| P2O5 (wt %) | 28.19 | 28.42 | 28.44 | 27.12 | 35.41 | 34.41 | 34.94 | 35.12 |
| SiO2 | d.l. | 1.64 | 0.04 | d.l. | d.l. | 1.25 | 0.04 | d.l. |
| TiO2 | 0.20 | 0.25 | d.l. | d.l. | 0.02 | 0.39 | 0.04 | d.l. |
| Al2O3 | 0.48 | 2.73 | d.l. | 0.01 | 0.16 | 0.36 | d.l. | d.l. |
| FeO | 0.14 | 0.17 | 0.30 | 0.20 | 0.61 | 0.84 | 0.84 | 0.87 |
| MnO | d.l. | d.l. | d.l. | d.l. | 0.00 | 0.00 | 0.00 | d.l. |
| CoO | 0.03 | 0.02 | 0.02 | 0.01 | 0.45 | 0.28 | 0.40 | 0.38 |
| BaO | d.l. | d.l. | d.l. | d.l. | d.l. | 0.00 | 0.04 | 0.02 |
| SrO | 0.02 | d.l. | 0.03 | 0.03 | d.l. | 0.11 | d.l. | d.l. |
| CaO | 1.16 | 0.24 | 0.25 | 0.27 | 0.07 | 0.19 | 0.12 | 0.08 |
| Ga2O3 | 0.31 | 0.06 | 0.18 | 0.24 | 0.24 | 0.04 | 0.25 | 0.11 |
| Y2O3 | 1.03 | 0.36 | 0.42 | 0.41 | 41.83 | 41.03 | 39.02 | 39.77 |
| ThO2 | 4.55 | 0.80 | 1.89 | 1.82 | d.l. | 1.00 | 0.03 | 0.00 |
| UO2 | 0.19 | 0.05 | 0.26 | 0.18 | 0.29 | 0.02 | 0.43 | 0.69 |
| La2O3 | 12.70 | 6.76 | 15.79 | 16.74 | 0.04 | 0.00 | 0.09 | 0.11 |
| Ce2O3 | 27.55 | 29.49 | 31.76 | 31.29 | 0.12 | 0.00 | 0.07 | 0.13 |
| Pr2O3 | 2.96 | 3.89 | 2.83 | 3.11 | 0.05 | 0.02 | 0.02 | d.l. |
| Nd2O3 | 13.17 | 18.74 | 12.40 | 12.56 | 0.63 | 0.15 | 0.80 | 0.64 |
| Sm2O3 | 1.76 | 1.96 | 2.02 | 2.09 | 0.56 | 0.54 | 1.06 | 0.94 |
| Eu2O3 | 0.99 | 1.25 | 0.98 | 0.96 | 0.12 | 0.37 | d.l. | 0.05 |
| Gd2O3 | 2.86 | 2.46 | 3.27 | 3.15 | 2.09 | 3.51 | 3.26 | 2.98 |
| Tb2O3 | d.l. | d.l. | d.l. | d.l. | 0.44 | 0.78 | 0.93 | 0.74 |
| Dy2O3 | 0.43 | 0.32 | 0.27 | 0.44 | 4.95 | 5.98 | 6.57 | 6.33 |
| Ho2O3 | 0.04 | d.l. | d.l. | d.l. | 1.11 | 1.09 | 0.90 | 0.91 |
| Er2O3 | 0.02 | d.l. | d.l. | d.l. | 4.42 | 3.38 | 3.87 | 4.06 |
| Tm2O3 | 0.26 | 0.18 | 0.16 | 0.24 | 0.73 | 0.34 | 0.66 | 0.72 |
| Yb2O3 | d.l. | d.l. | d.l. | d.l. | 4.41 | 2.17 | 3.89 | 4.27 |
| Lu2O3 | 0.02 | d.l. | d.l. | 0.00 | 0.78 | 0.44 | 0.69 | 0.73 |
| F | 1.01 | 0.87 | 0.91 | 1.05 | 1.85 | 1.82 | 1.86 | 1.89 |
| SUM | 100.07 | 100.66 | 102.22 | 101.92 | 101.38 | 100.51 | 100.82 | 101.54 |
| P (a.p.f.u.) 1 | 0.93 | 0.89 | 0.94 | 0.91 | 0.96 | 0.93 | 0.96 | 0.96 |
| Si | - | 0.06 | 0.00 | - | - | 0.04 | 0.00 | - |
| SUM A | 0.93 | 0.95 | 0.94 | 0.91 | 0.96 | 0.97 | 0.96 | 0.96 |
| Ti | 0.01 | 0.01 | - | - | 0.00 | 0.01 | 0.00 | - |
| Al | 0.02 | 0.12 | - | 0.00 | 0.01 | 0.01 | - | - |
| Fe | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 |
| Mn | - | - | - | - | 0.00 | - | 0.00 | - |
| Co | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 |
| Ba | - | - | - | - | - | - | 0.00 | 0.00 |
| Sr | 0.00 | - | 0.00 | 0.00 | - | 0.00 | - | - |
| Ca | 0.05 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 |
| Ga | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 |
| Th | 0.04 | 0.01 | 0.02 | 0.02 | - | 0.01 | 0.00 | 0.00 |
| U | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 |
| Y | 0.02 | 0.01 | 0.01 | 0.01 | 0.71 | 0.69 | 0.67 | 0.68 |
| La | 0.18 | 0.09 | 0.23 | 0.24 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ce | 0.39 | 0.40 | 0.45 | 0.45 | 0.00 | - | 0.00 | 0.00 |
| Pr | 0.04 | 0.05 | 0.04 | 0.05 | 0.00 | 0.00 | 0.00 | - |
| Nd | 0.18 | 0.25 | 0.17 | 0.18 | 0.01 | 0.00 | 0.01 | 0.01 |
| Sm | 0.02 | 0.03 | 0.03 | 0.03 | 0.01 | 0.01 | 0.01 | 0.01 |
| Eu | 0.01 | 0.02 | 0.01 | 0.01 | 0.00 | 0.00 | - | 0.00 |
| Gd | 0.04 | 0.03 | 0.04 | 0.04 | 0.02 | 0.04 | 0.04 | 0.03 |
| Tb | - | - | - | - | 0.01 | 0.01 | 0.01 | 0.01 |
| Dy | 0.01 | 0.00 | 0.00 | 0.01 | 0.05 | 0.06 | 0.07 | 0.07 |
| Ho | 0.00 | - | - | - | 0.01 | 0.01 | 0.01 | 0.01 |
| Er | 0.00 | - | - | - | 0.04 | 0.03 | 0.04 | 0.04 |
| Tm | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.01 |
| Yb | - | - | - | - | 0.04 | 0.02 | 0.04 | 0.04 |
| Lu | 0.00 | - | - | 0.00 | 0.01 | 0.00 | 0.01 | 0.01 |
| F | 0.12 | 0.10 | 0.11 | 0.13 | 0.19 | 0.18 | 0.19 | 0.19 |
| SUM B | 1.16 | 1.13 | 1.14 | 1.20 | 1.15 | 1.14 | 1.15 | 1.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinhardt, N.; Proenza, J.A.; Villanova-de-Benavent, C.; Aiglsperger, T.; Bover-Arnal, T.; Torró, L.; Salas, R.; Dziggel, A. Geochemistry and Mineralogy of Rare Earth Elements (REE) in Bauxitic Ores of the Catalan Coastal Range, NE Spain. Minerals 2018, 8, 562. https://doi.org/10.3390/min8120562
Reinhardt N, Proenza JA, Villanova-de-Benavent C, Aiglsperger T, Bover-Arnal T, Torró L, Salas R, Dziggel A. Geochemistry and Mineralogy of Rare Earth Elements (REE) in Bauxitic Ores of the Catalan Coastal Range, NE Spain. Minerals. 2018; 8(12):562. https://doi.org/10.3390/min8120562
Chicago/Turabian StyleReinhardt, Nils, Joaquín A. Proenza, Cristina Villanova-de-Benavent, Thomas Aiglsperger, Telm Bover-Arnal, Lisard Torró, Ramon Salas, and Annika Dziggel. 2018. "Geochemistry and Mineralogy of Rare Earth Elements (REE) in Bauxitic Ores of the Catalan Coastal Range, NE Spain" Minerals 8, no. 12: 562. https://doi.org/10.3390/min8120562
APA StyleReinhardt, N., Proenza, J. A., Villanova-de-Benavent, C., Aiglsperger, T., Bover-Arnal, T., Torró, L., Salas, R., & Dziggel, A. (2018). Geochemistry and Mineralogy of Rare Earth Elements (REE) in Bauxitic Ores of the Catalan Coastal Range, NE Spain. Minerals, 8(12), 562. https://doi.org/10.3390/min8120562





