1. Introduction
The exploration of polymetallic deep-sea nodules as a multi-metallic resource is an ongoing research object since the first extensive exploration phase in the 1960s and 1970s. Due to an increase in metal prices and a continuously high demand for various metals, another extensive phase of exploration started at the beginning of this century. Several governmental and semi-governmental institutions signed contracts with the International Seabed Authority to investigate polymetallic deep-sea nodules and since 2010 private enterprises have also done so. The most promising area for exploitation of this marine resource is the Clarion-Clipperton Fracture Zone (CZZ) in the Pacific Ocean [
1]. Around 15 kg of manganese nodules per square meter on average cover the seafloor in an area the size of five million square kilometers [
2].
Metals of interest in deep-sea nodules are especially copper, cobalt and nickel. Due to the high abundance of nodules on the deep-sea floor, the reserves for those metals are significant [
3,
4]. Furthermore, the recovery of manganese, molybdenum and vanadium is of interest or at least the enrichment of a marketable by-product can add value to an extraction process [
3].
Table 1 shows a comparison of estimated reserves for the mentioned valuable metals in manganese nodules and in land-based ore bodies, which highlights why manganese nodules are an interesting potential resource [
4].
The expected resources of cobalt, manganese and nickel in manganese nodules solely in the CCZ surpass the reserves of those metals on land [
4]. Due to the abundance of high-grade manganese ore on land, utilizing the nodules as a source of copper and nickel only is of interest [
5]. A process that additionally recovers cobalt is desirable due to the criticality of cobalt and its importance in the area of clean energy technology [
6]. More than one half of global cobalt mine production currently comes from the Democratic Republic of the Congo and since the predicted consumption increases faster than the world cobalt refinery capacity, a limited supply is expected. Most cobalt is already mined as a by-product of nickel and copper production [
7], therefore the recovery of cobalt while extracting nickel and copper from manganese nodules can be considered a viable option to close the forecasted supply gap. A rising demand is also predicted for manganese alloys, however, metallurgical grade ores on land are distributed more evenly than cobalt [
8], which minimizes a supply risk. To optimize the profitability of a metallurgical process and to decrease the mass of produced by-products with low or no economic value, the production of marketable manganese alloys seems to be an apparent necessity to utilize manganese-rich streams.
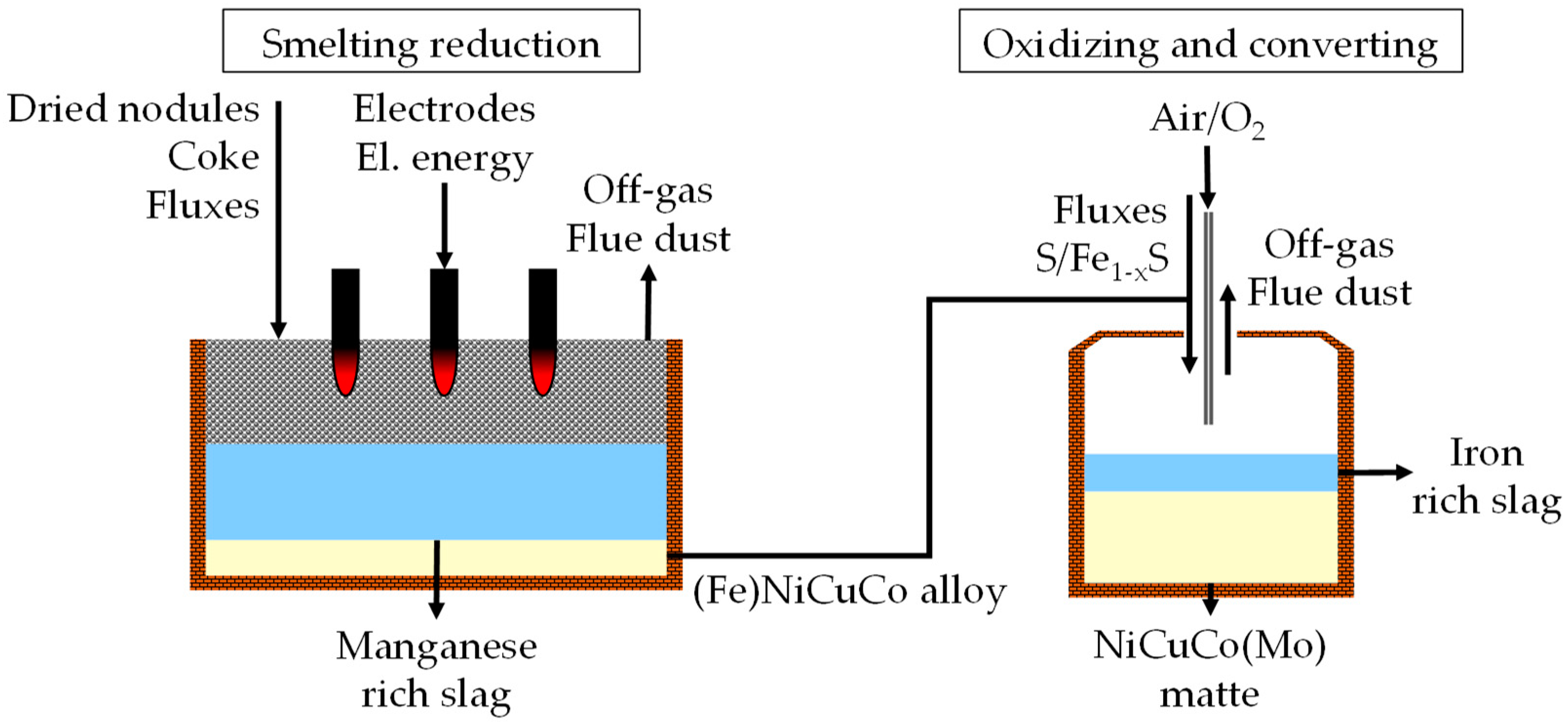
Several metallurgical processes underwent experimental trials. In general, different extraction processes for utilizing manganese nodules could be categorized into hydrometallurgical processes and pyrometallurgical enrichment processes with subsequent hydrometallurgical downstream processing to separate valuable metals. Already in the 1970s, the International Nickel Corporation (Inco) proposed a process, the so-called “Inco-process” [
5], to utilize polymetallic deep-sea nodules as a resource for nickel, copper and cobalt. The process is based on well-known metallurgical unit operations like drying, selective reduction, smelting, oxidizing, sulfiding and converting. Valuable metals like nickel, copper and cobalt are concentrated in an early stage of the process by reduction into a metallic phase and manganese is mainly discarded in the manganese-silicate slag. Excess iron and manganese in the metal is converted to reduce the weight of the valuable metal stream. A matte is produced by sulfiding the reduced metal. Compared to the weight of the nodules, the matte weighs only 5 wt % and is processed further by hydrometallurgical unit operations to separate nickel, copper and cobalt. Due to the abundance of high-grade manganese deposits on land, the recovery of manganese from the manganese-silicate slag was not investigated in detail by Inco [
5]. A simplified flowchart of the pyrometallurgical enrichment process by Inco is displayed in
Figure 1.
In comparison to the pyrometallurgical treatment of polymetallic nodules, there are also several hydrometallurgical processes under investigation; however there are a few major disadvantages with these processes, especially, the high consumption of chemicals like acids or bases and considerable amounts of leach residues and precipitation by-products that seem to be a high burden for the environment [
9]. A sole hydrometallurgical process can only be economically feasible if reagents are cheap or can be recycled easily. The major disadvantage of a pyrometallurgical process is the energy consumption of drying and smelting of nodules [
10], however, less extensive downstream processing to recover valuable metals and the possibility of manganese recovery from slag can possibly outweigh these disadvantages [
9,
11].
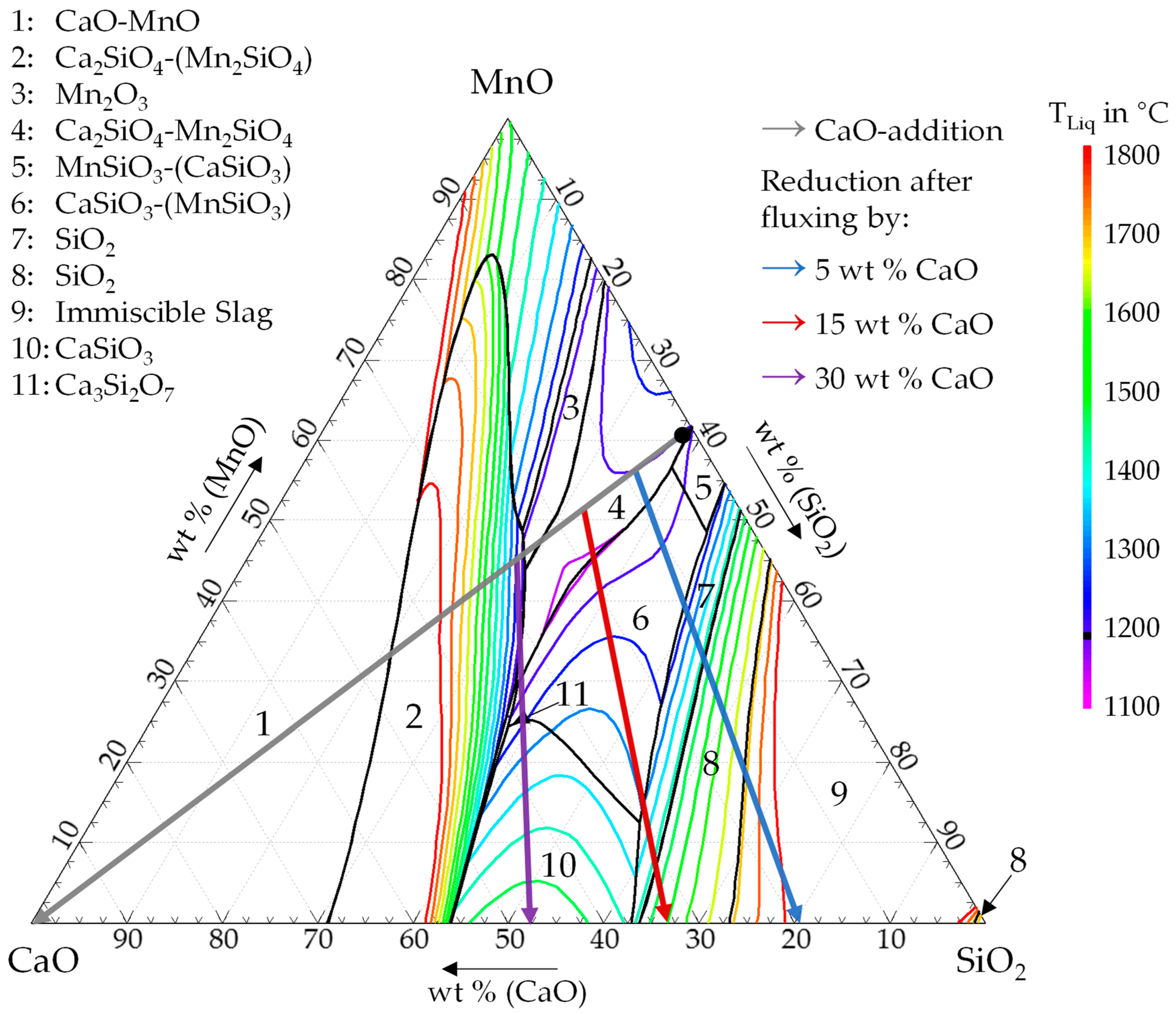
The aim of this study was to investigate the smelting reduction operation from the Inco-process. Inco proposed a smelting reduction operation without the addition of fluxes [
5]. However, to decrease the liquidus temperature and to improve the recovery of metals, the addition of fluxes can be beneficial [
3]. Previous research explored the option of adding silica [
3,
12] or silica and lime [
13]. To alter the smelting behavior and to increase the degree of reduction for valuable metals, this paper explored the usage of different additives and variations in the added amount of fluxes to the nodules. To support the design of the experimental trials FactSage
TM 7.0 was used. FactSage
TM is a thermochemical modeling program [
14]. In this article liquidus temperatures of smelting operations, activities of oxides, degree of reduction and the composition of phases like slag or alloys were predicted by FactSage
TM.
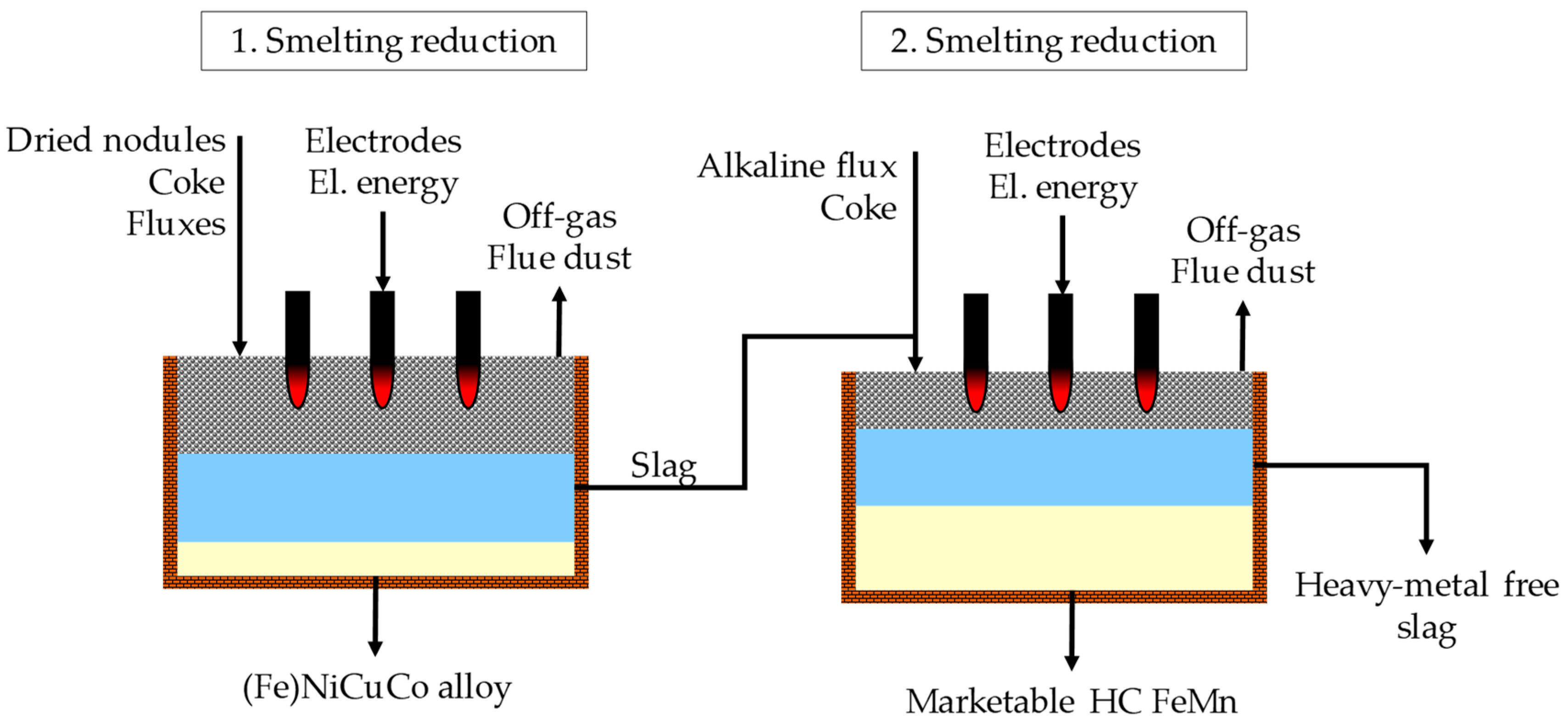
Furthermore, the possibility of manganese recovery utilizing the resulting manganese-rich slag as a resource to produce high carbon ferromanganese (HC FeMn) in a second smelting reduction process was proposed. The effects of adding lime and magnesia as a flux in the second smelting reduction step on the composition of the final alloy and slag were examined and modeled by FactSage
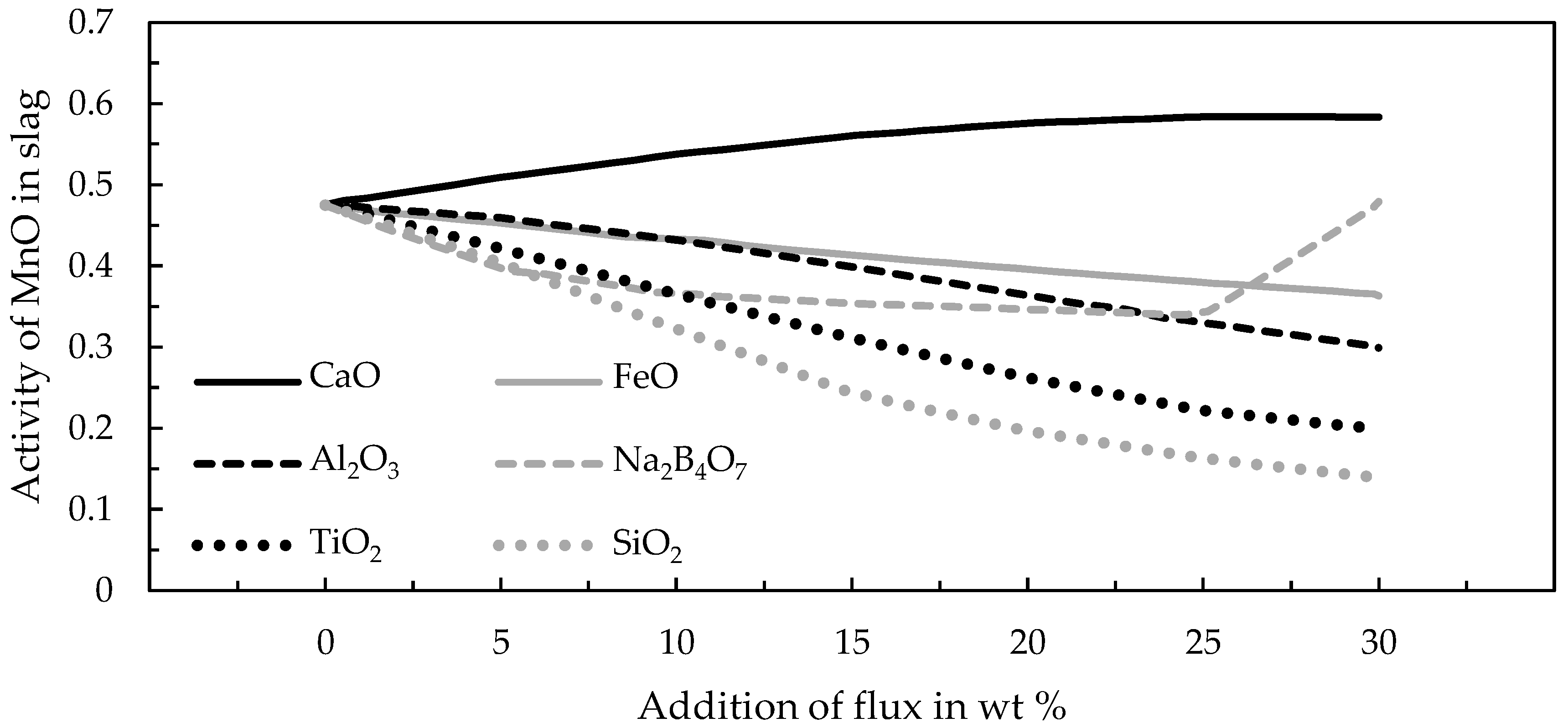
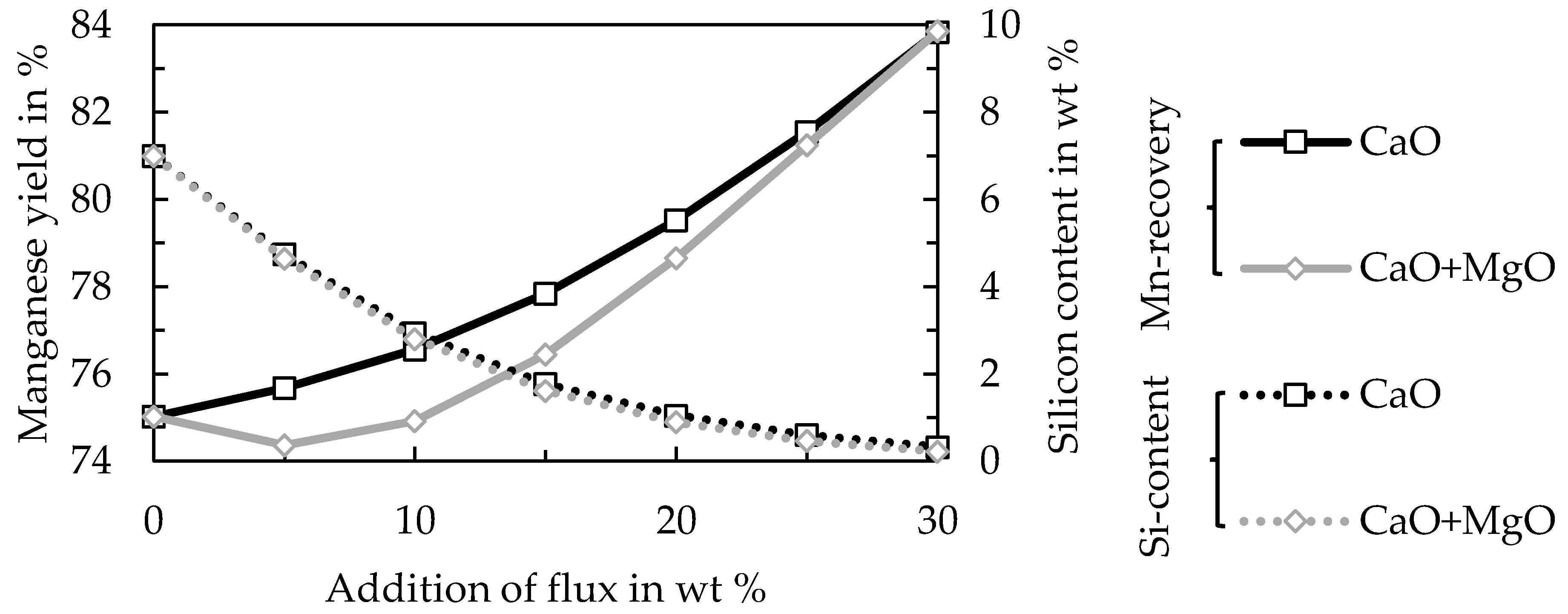
TM. Those fluxes are commonly used in the production of ferromanganese in a submerged arc furnace from land-based ore bodies. The main objective of the addition of basic fluxes was to increase the manganese yield and to decrease the silicon content in the produced alloy [
15].
Figure 2 shows the flowchart of the process investigated by this paper. For both reduction operations, submerged arc furnaces were recommended. The advantages of such a furnace for processing manganese nodules were already highlighted in previous research [
3,
5]. For producing ferromanganese, the submerged arc furnace is already the dominant furnace in the industry [
15].
By combining two smelting reduction processes, the following advantages were expected:
Valuable metals are concentrated in an alloy in the first reduction step, to minimize hydrometallurgical downstream processing.
By production of marketable ferromanganese, value can be added to the entire production process.
After two reduction processes, it is envisaged to generate a heavy-metal free slag, which can be used as a construction material to avoid landfilling of slag and therefore reduce the burden on the environment.
5. Conclusions
This paper investigated the pyrometallurgical enrichment process of valuable metals from manganese nodules and explored the utilization of the manganese-bearing slag.
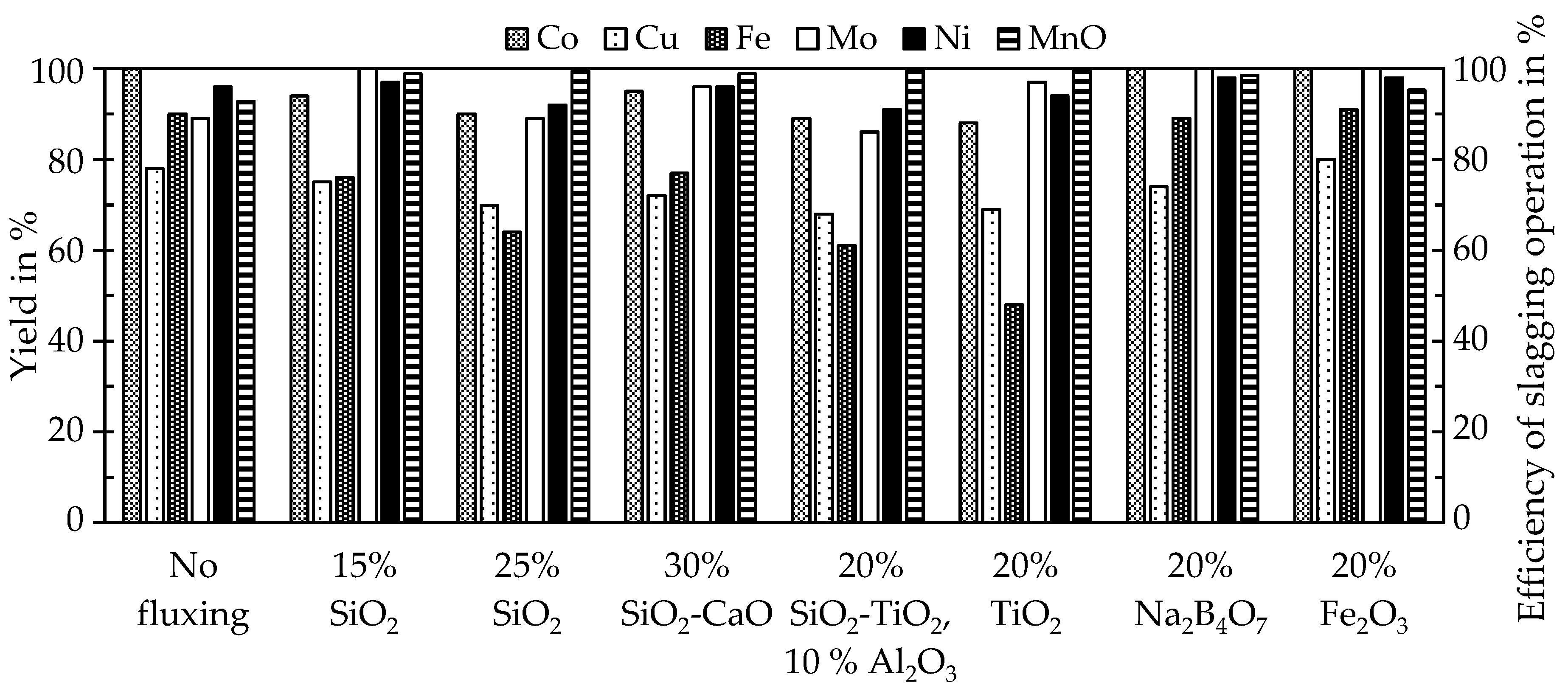
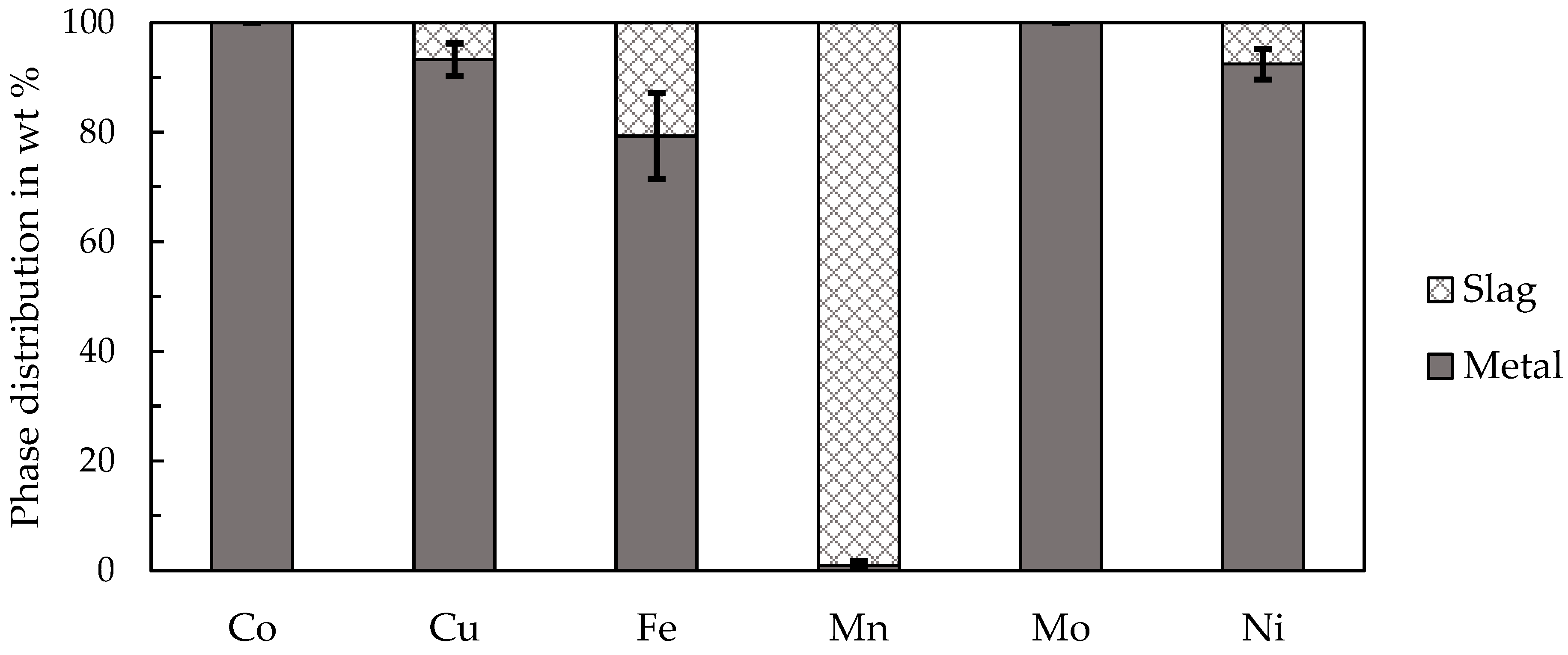
Pre-trials and further scale-up experiments highlighted the beneficial effects of fluxing by SiO2 in the smelting reduction process of manganese nodules. Optimal parameters were a smelting temperature of 1400 °C and the addition of 9.4 wt % SiO2 to recover over 90% and up to 100% of cobalt, copper, molybdenum, nickel and manganese was successfully discarded into the slag to a significant extent (>97%). The enrichment of valuable metals in an alloy while discarding gangue and manganese with the slag is an advantage of the process, since it significantly reduces the amount of material, which needs to be processed further by hydrometallurgical methods.
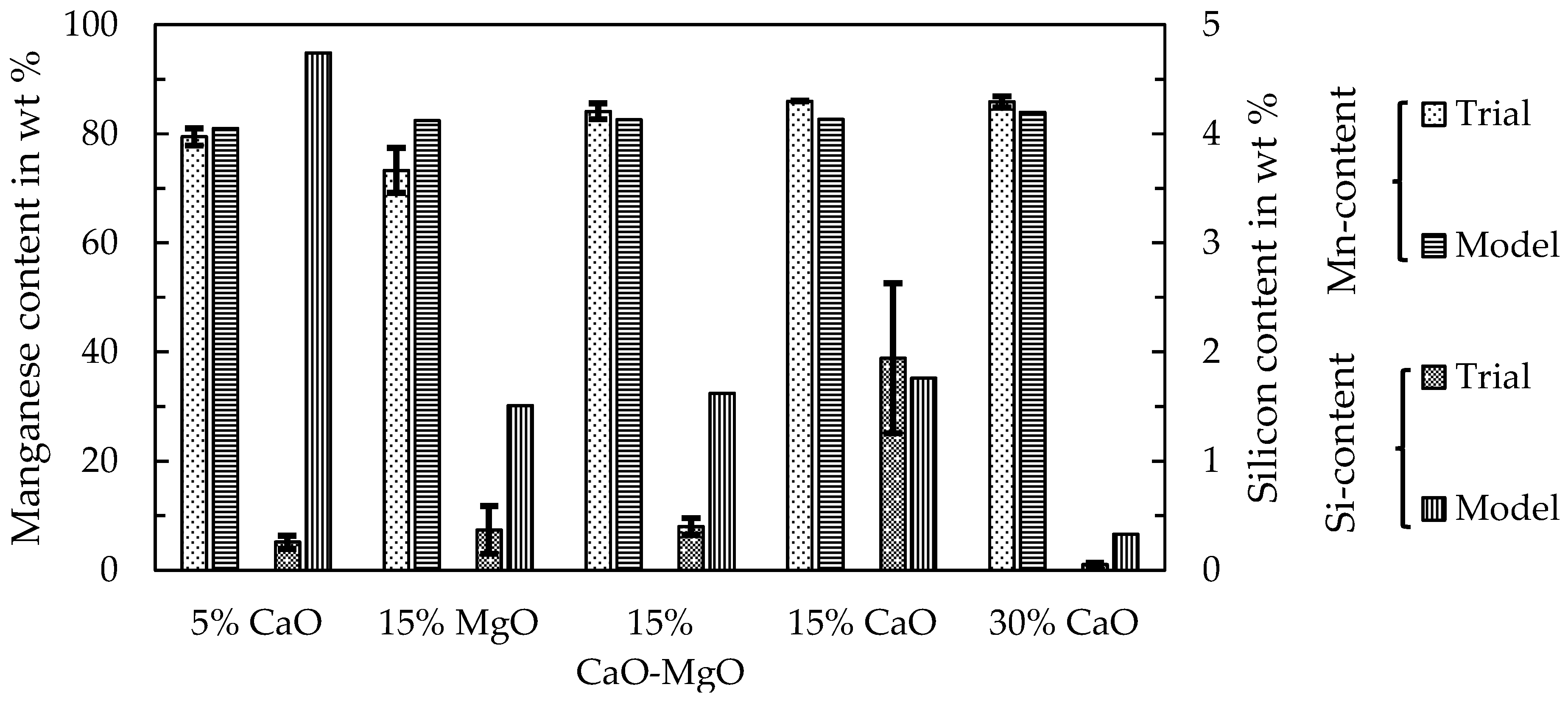
The trials to produce ferromanganese showed that the manganese-bearing slag could be used as a resource to produce an alloy that conformed with the ASTM standard. Recovering manganese as a marketable alloy has two significant advantages, (i) the generation of revenue and (ii) by further reduction the total amount of slag is decreased and heavy-metals are reduced and collected in the metal, which is relevant for utilizing the slag as a mineral product, for example in the construction industry. Different fluxes and the amount of added fluxes were investigated. The results showed that the addition of 5–15 wt % of lime provided the best results. However, further studies and a scale-up are necessary especially for the second reduction step. Copper impurities are another problem in the alloy, which make further investigation of the copper behavior during the reduction and phase separation necessary. Furthermore, the utilization of the resulting slag as a mineral product has to be proven in
supplementary studies, however, the chemical analysis does not show elevated contents for heavy-metals and is therefore promising.