Decoding of Mantle Processes in the Mersin Ophiolite, Turkey, of End-Member Arc Type: Location of the Boninite Magma Generation
Abstract
1. Introduction
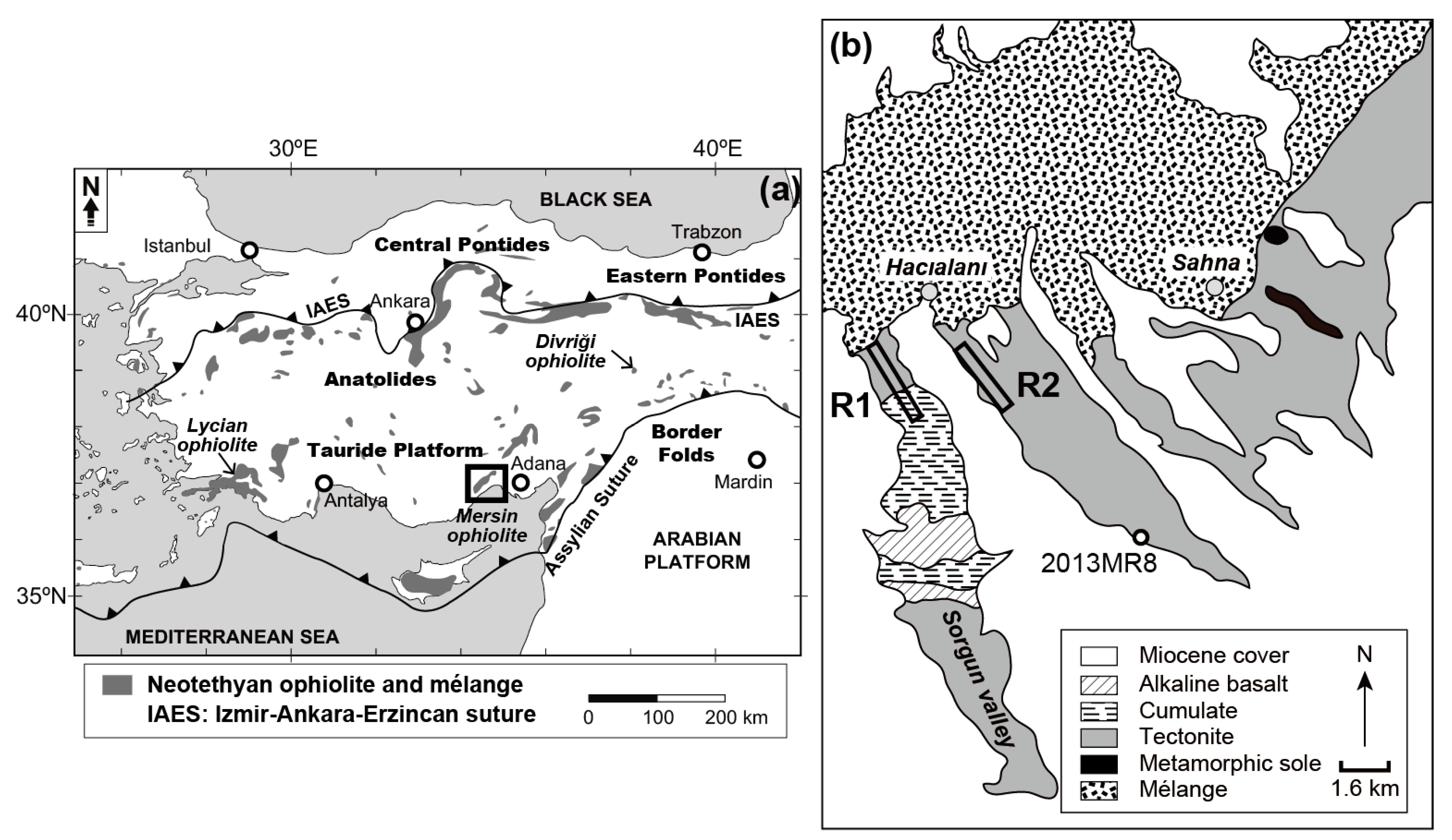
2. Geological Setting
2.1. Regional Geology
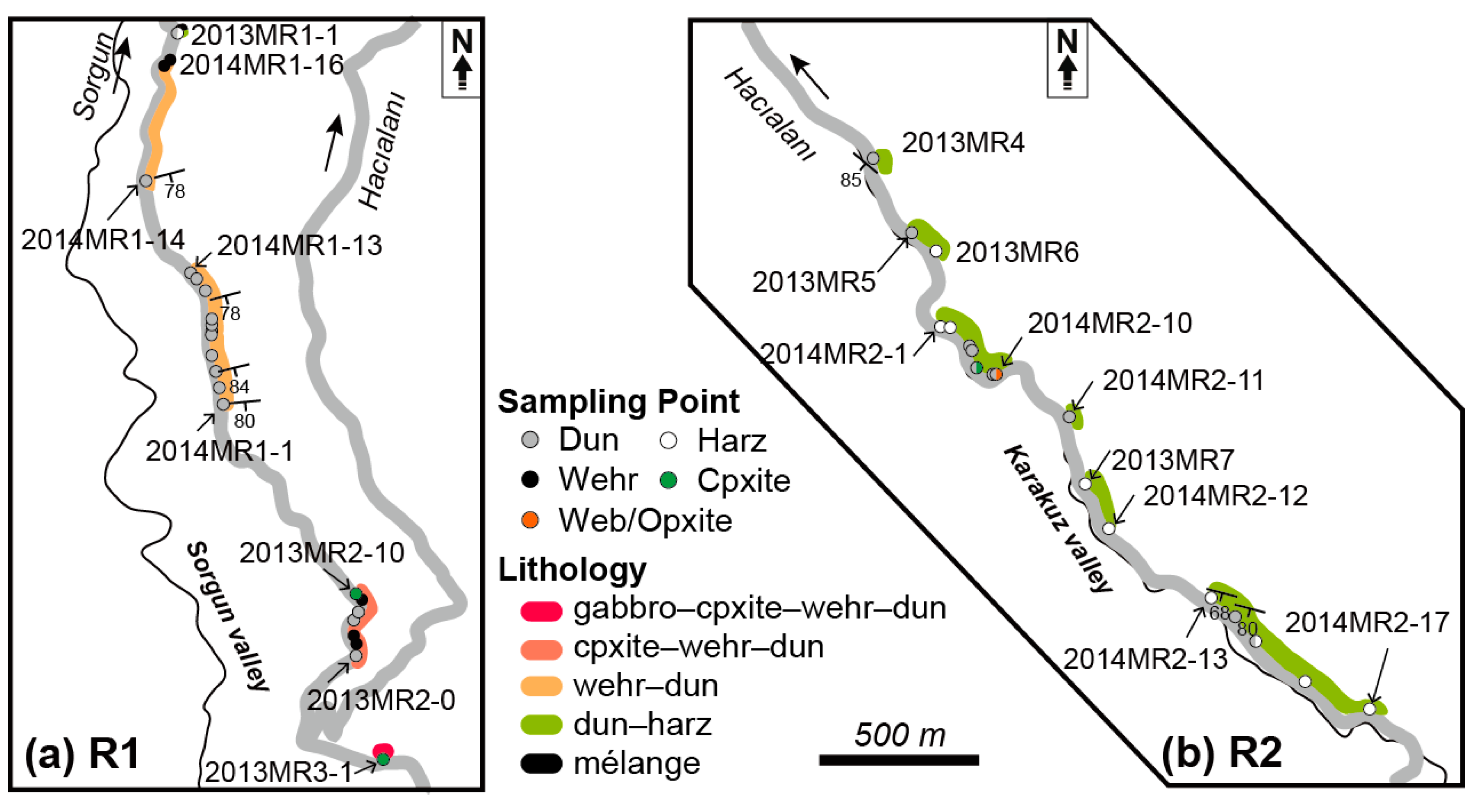
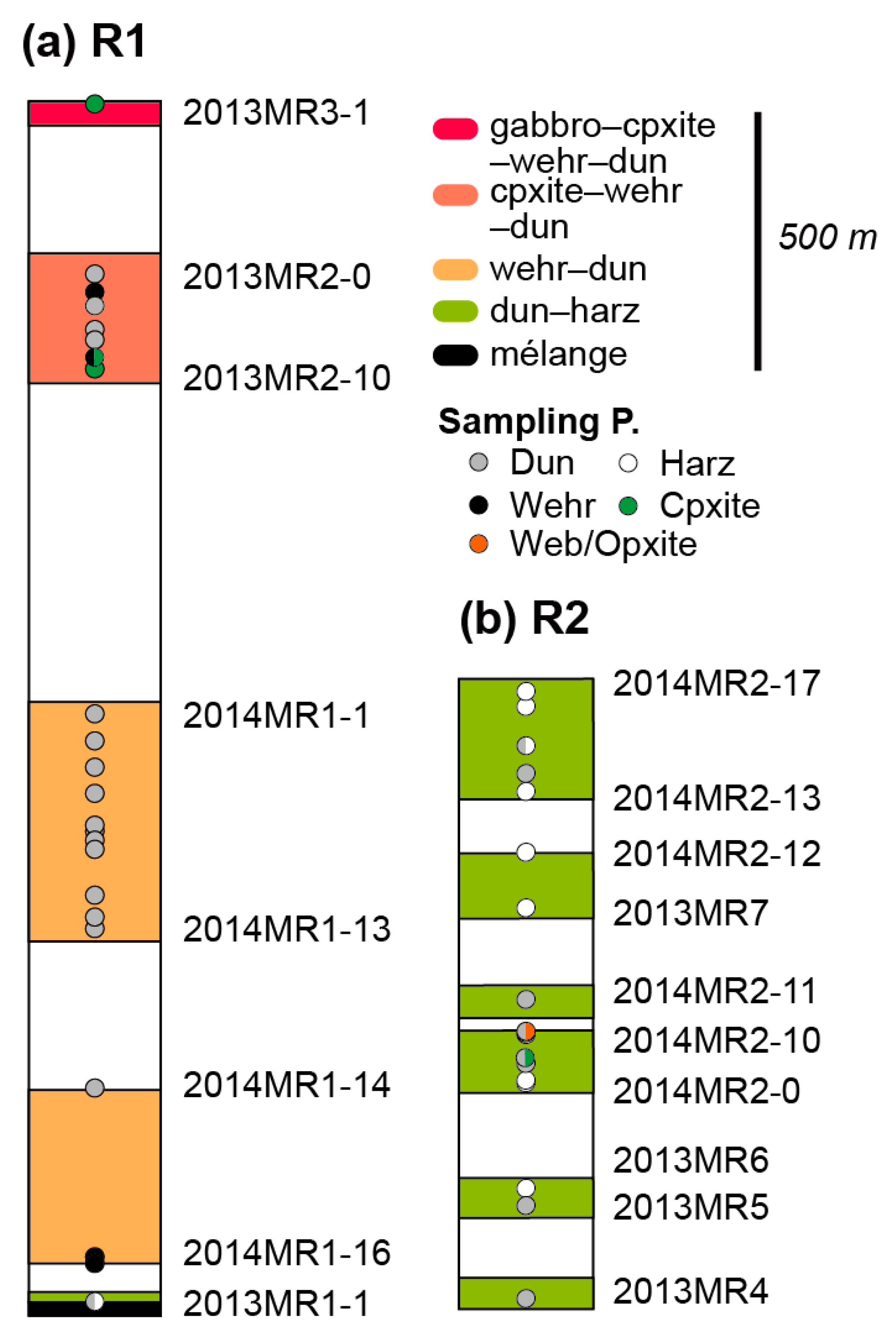
2.2. Sampling
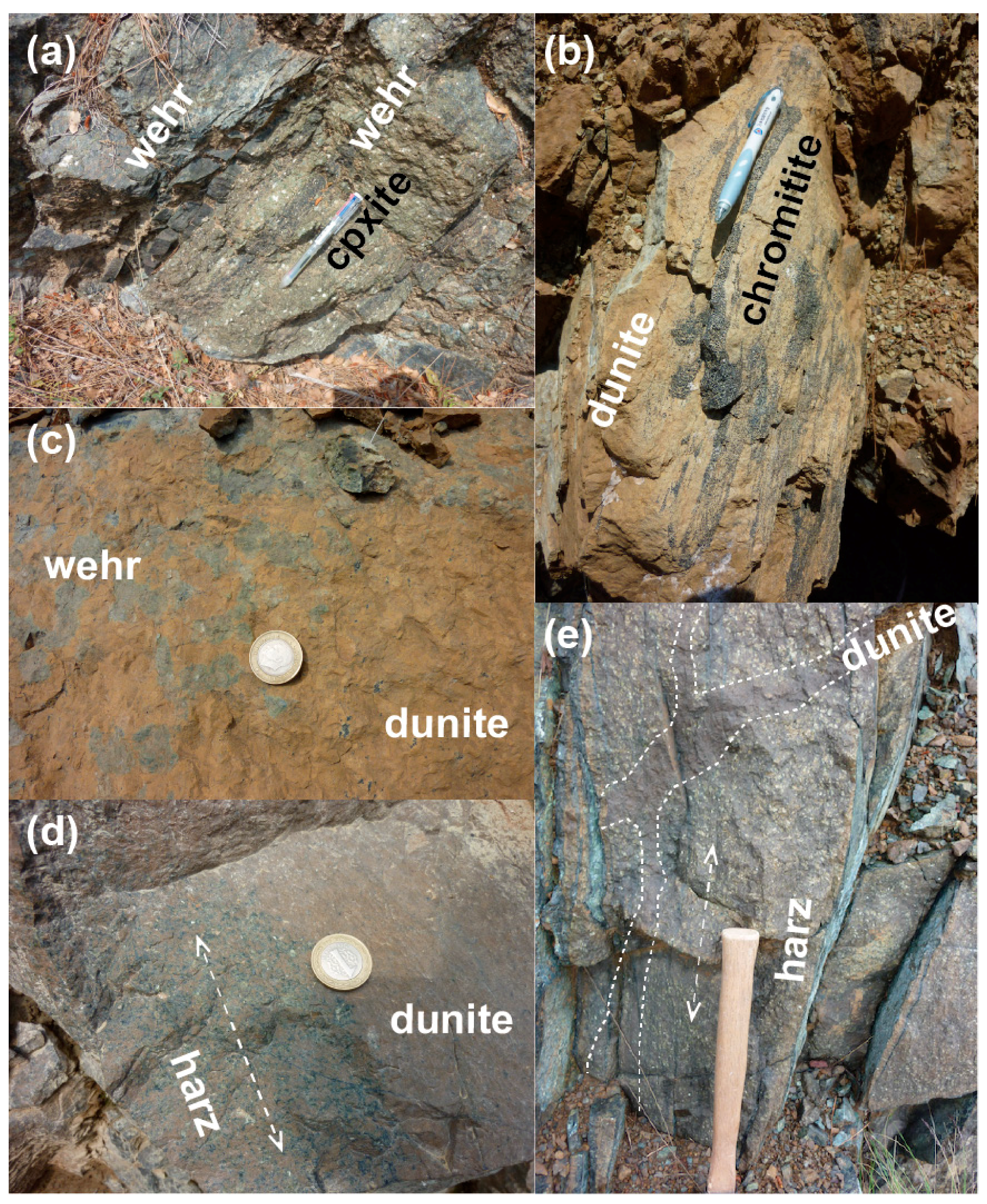
3. Petrographic Features of Ultramafic Rocks
3.1. LU Rocks (Dunite–Wehrlite–Clinopyroxenite of R1)
3.2. MTZ Peridotites (Dunite–Wehrlite–Harzburgite of R1)
3.3. Mantle Peridotites (Ultramafic Rocks of R2)
4. Geochemical Analyses and the Results
4.1. Analytical Methods
4.2. Major-Element Compositions
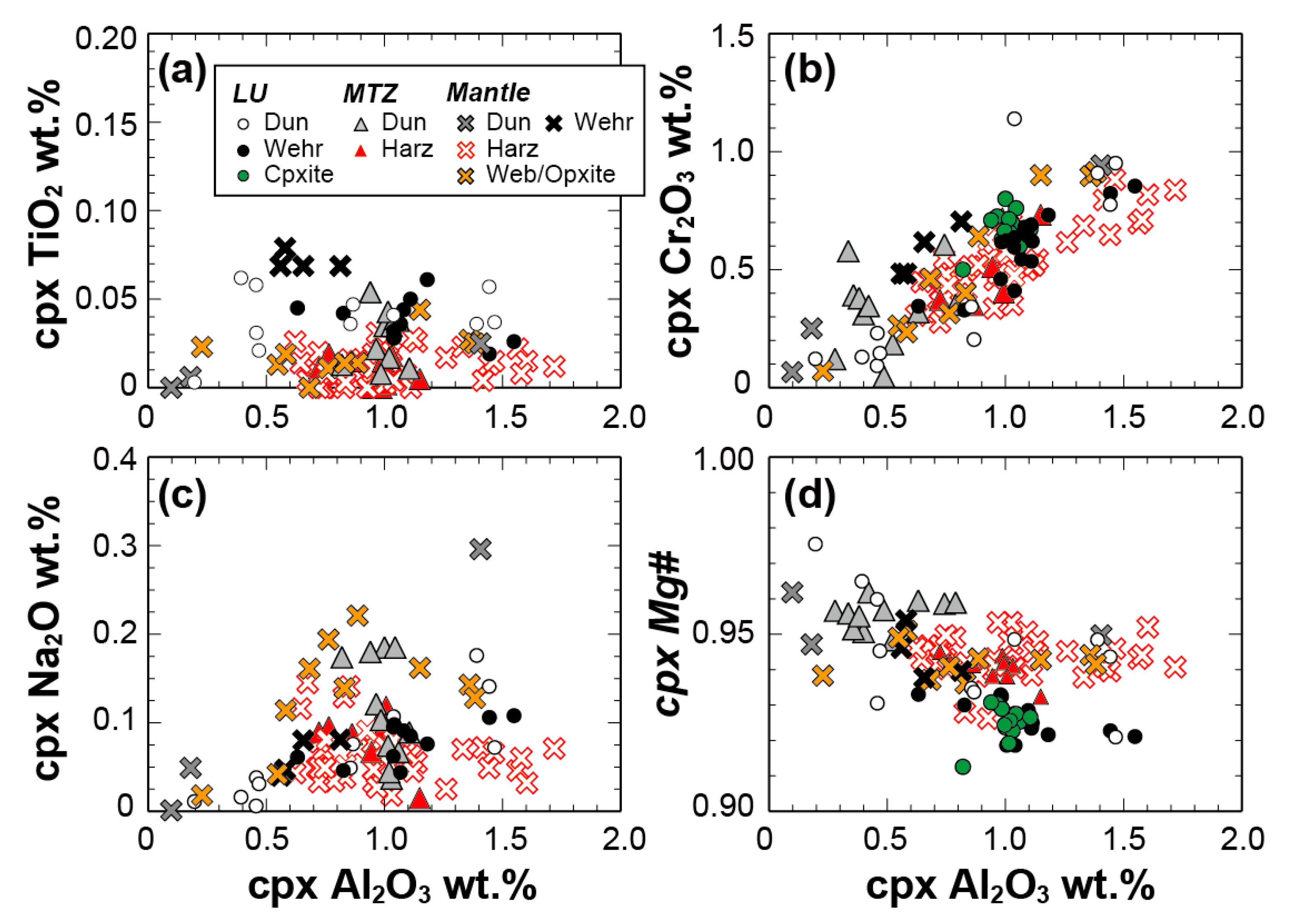
4.2.1. LU Rocks (Dunite–Wehrlite–Clinopyroxenite of R1)
4.2.2. MTZ Peridotites (Dunite–Wehrlite–Harzburgite of R1)
4.2.3. Mantle Peridotites (Ultramafic Rocks of R2)
4.3. Trace-Element Compositions
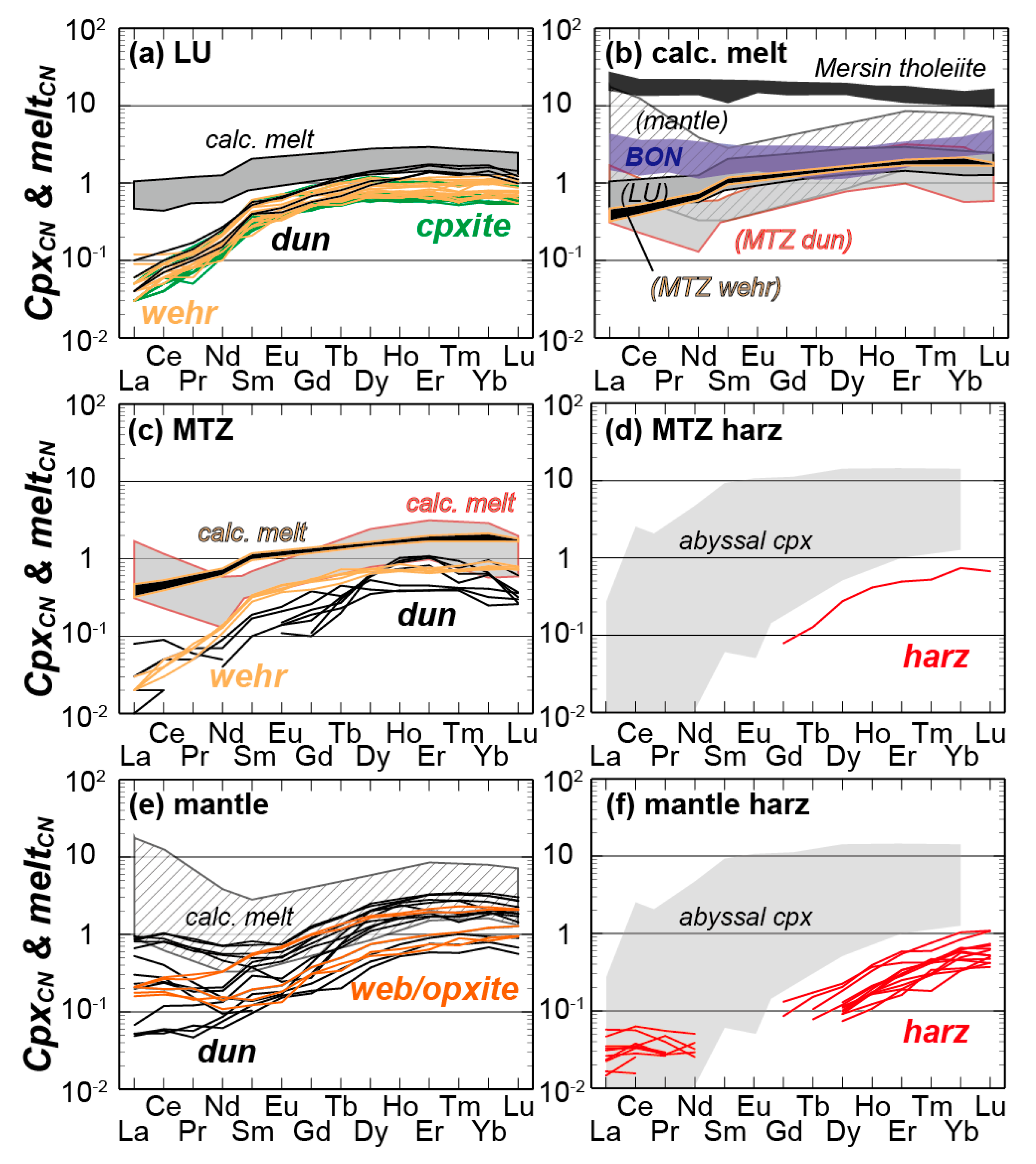
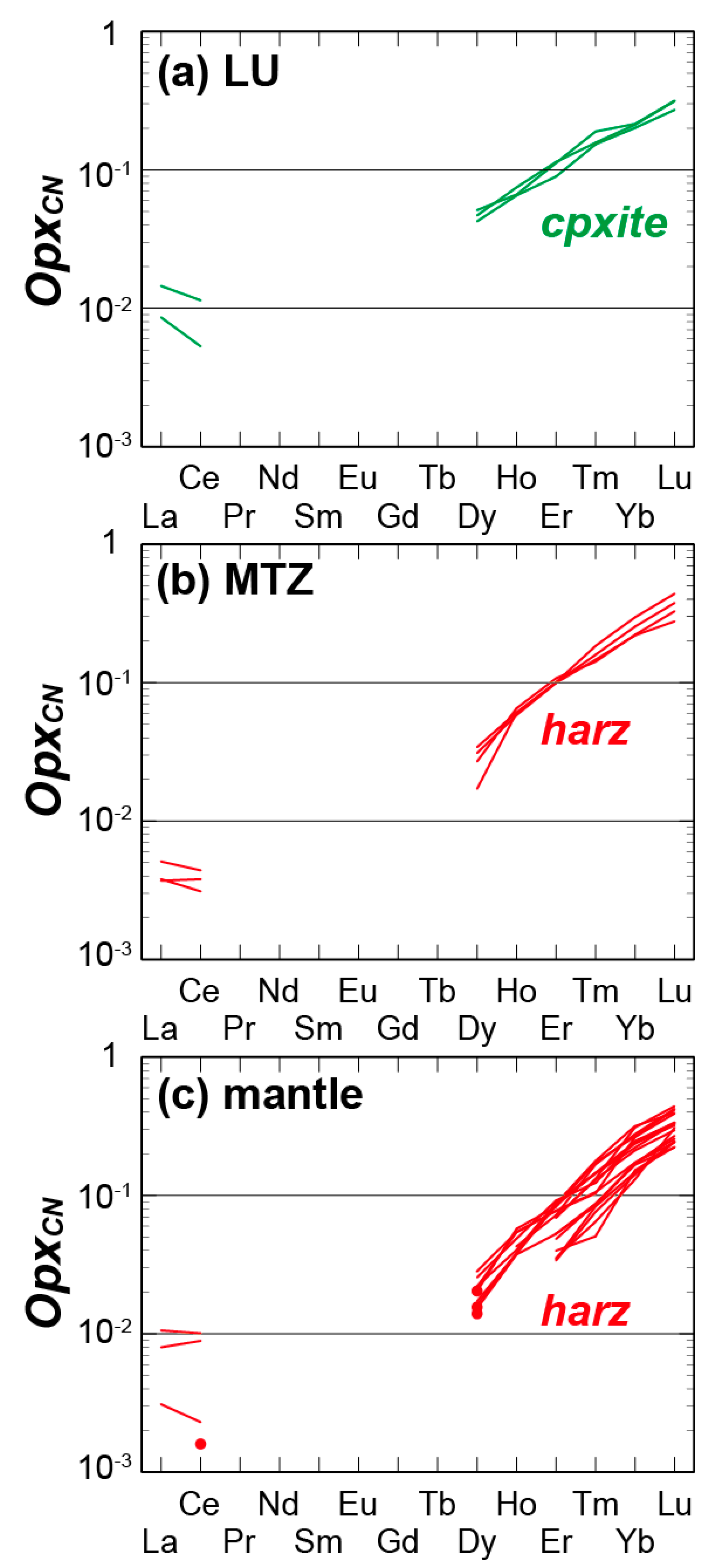
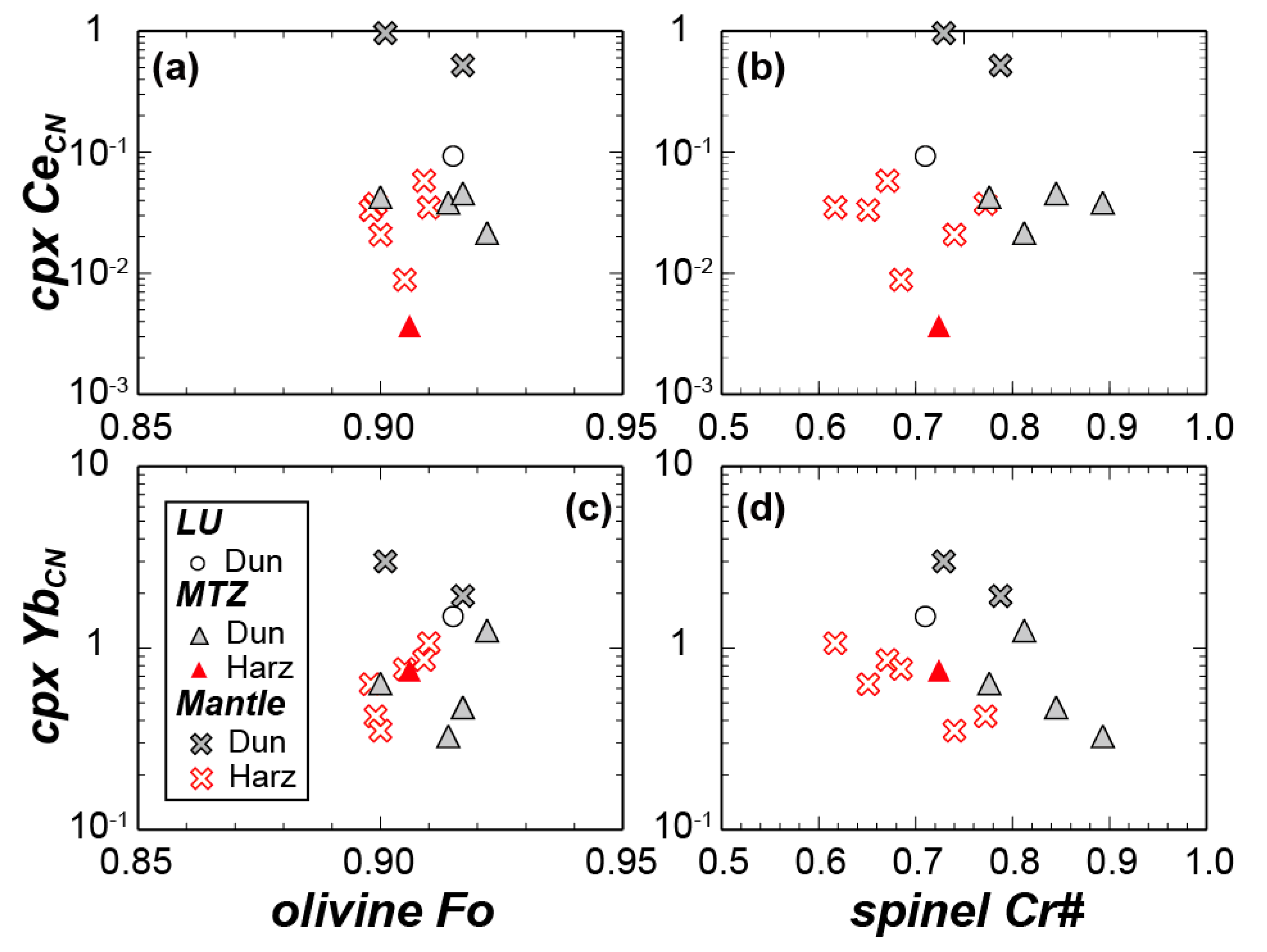
4.3.1. LU Rocks (Dunite–Wehrlite–Clinopyroxenite of R1)
4.3.2. MTZ Peridotites (Dunite–Wehrlite–Harzburgite of R1)
4.3.3. Mantle Peridotites (Ultramafic Rocks of R2)
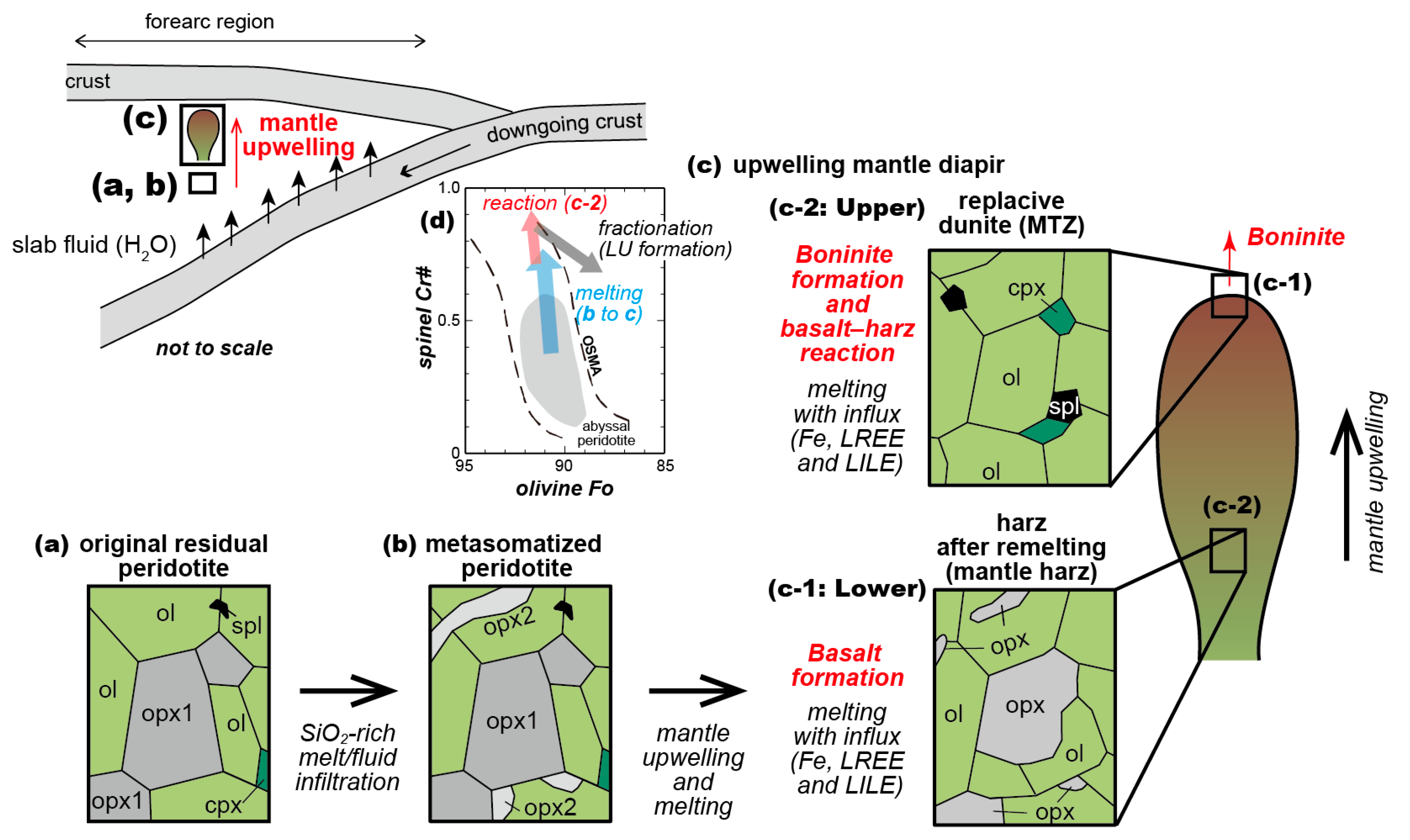
5. Discussion and Summary
5.1. Partial Melting Process Recorded in Mantle Harzburgite
5.2. Metasomatism by SiO2-Rich Fluid/Melt Suggested by High-Ni Olivine of Harzburgite
5.3. Nature and Formation Process of Dunites, Wehrlite and Pyroxenites
5.4. Implications for Tectonic Setting and History of the Mersin Ophiolite
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arai, S.; Ishimaru, S. Insights into petrological characteristics of the lithosphere of mantle wedge beneath arcs through peridotite xenoliths: A review. J. Petrol. 2008, 49, 665–695. [Google Scholar] [CrossRef]
- Lippard, S.J.; Shelton, A.W.; Gass, I.G. The Ophiolite of Northern Oman. Geol. Soc. Lond. Mem. 1986, 11, 178. [Google Scholar]
- Dilek, Y.; Furnes, H. Ophiolite genesis and global tectonics: Geochemical and tectonic fingerprinting of ancient oceanic lithosphere. Geol. Soc. Am. Bull. 2011, 123, 387–411. [Google Scholar] [CrossRef]
- Boudier, F.; Nicolas, A.; Bouchez, J.L. Kinematics of oceanic thrusting and subduction from basal sections of ophiolites. Nature 1982, 296, 825–828. [Google Scholar] [CrossRef]
- Arai, S.; Kadoshima, K.; Morishita, T. Widespread arc-related melting in the mantle section of the northern Oman ophiolite as inferred from detrital chromian spinels. J. Geol. Soc. Lond. 2006, 163, 869–879. [Google Scholar] [CrossRef]
- Uysal, I.; Ersoy, E.Y.; Karslı, O.; Dilek, Y.; Sadıklar, M.B.; Ottley, C.J.; Tiepolo, M.; Meisel, T. Coexistence of abyssal and ultra-depleted SSZ type mantle peridotites in a Neo-Tethyan Ophiolite in SW Turkey: Constraints from mineral composition, whole-rock geochemistry (major–trace–REE–PGE), and Re–Os isotope systematics. Lithos 2012, 132–133, 50–69. [Google Scholar] [CrossRef]
- Kay, S.M.; Kay, R.W. Role of crystal cumulates and the oceanic crust in the formation of the lower crust of the Aleutian arc. Geology 1985, 13, 461–464. [Google Scholar] [CrossRef]
- Parlak, O. The Tauride ophiolites of Anatolia (Turkey): A review. J. Earth Sci. 2016, 27, 901–934. [Google Scholar] [CrossRef]
- Parlak, O.; Delaloye, M.; Bíngöl, F. Geochemistry of the volcanic rocks in the Mersin ophiolite (Southern Turkey) and their tectonic significance in the Eastern Mediterranean geology. In Proceedings of the International Earth Sciences Colloquium on the Aegean Region (IESCA-95), Izmir-Güllük, Turkey, 9–14 October 1995; Piskin, Ö., Ergün, M., Savaşçın, M.Y., Tarcan, G., Eds.; Dokuz Eylul University: İzmir, Turkey, 1997; pp. 441–463. [Google Scholar]
- Parlak, O.; Delaloye, M. Geochemistry and timing of post-metamorphic dyke emplacement in the Mersin Ophiolite (southern Turkey): New age constraints from 40Ar/39Ar geochronology. Terra Nova 1996, 8, 585–592. [Google Scholar] [CrossRef]
- Ketin, I. Tectonic units of Anatolia. MTA Bull. 1966, 66, 23–24. [Google Scholar]
- Şengör, A.M.C.; Yılmaz, Y. Tethyan Evolution of Turkey: A Plate Tectonic Approach. Tectonophysics 1981, 75, 181–241. [Google Scholar] [CrossRef]
- Robertson, A.H.F.; Dixon, J.A. Introduction: Aspects of the Geological Evolution of the Eastern Mediterranean. In The Geological Evolution of the Eastern Mediterranean; Dixon, J.A., Robertson, A.H.F., Eds.; Geological Society of London: London, UK, 1984; Volume 17, pp. 1–74. [Google Scholar]
- Görür, N.; Oktay, F.Y.; Seymen, İ.; Şengör, A.M.C. Palaeotectonic Evolution of the Tuzgölü Basin Complex, Central Turkey: Sedimentary Record of a Neotethyan Closure. In The Geological Evolution of the Eastern Mediterranean; Dixon, J.A., Robertson, A.H.F., Eds.; Geological Society of London: London, UK, 1984; Volume 17, pp. 467–482. [Google Scholar]
- Okay, A.İ. High-Pressure/Low-Temperature Metamorphic Rocks of Turkey. In Blueschists and Eclogites; Evans, B.W., Brown, E.H., Eds.; Geological Society of America: Boulder, CO, USA, 1986; Volume 164, pp. 333–348. [Google Scholar]
- Yılmaz, Y. New evidence and model on the evolution of the southeast Anatolian Orogen. Geol. Soc. Am. Bull. 1993, 105, 251–271. [Google Scholar] [CrossRef]
- Yılmaz, Y.; Yiğitbaş, E.; Genç, Ş.C. Ophiolitic and metamorphic assemblages of southeast Anatolia and their significance in the geological evolution of the orogenic belt. Tectonics 1993, 12, 1280–1297. [Google Scholar] [CrossRef]
- Okay, A.İ.; Tüysüz, O. Tethyan Sutures of Northern Turkey. In The Mediterranean Basins: Tertiary Extension within the Alpine Orogeny; Mascle, A., Jolivet, L., Eds.; Geological Society of London: London, UK, 1999; Volume 156, pp. 475–516. [Google Scholar]
- Robertson, A.H.F. Overview of the genesis and emplacement of Mesozoic ophiolites in the Eastern Meditterranean Tethyan region. Lithos 2002, 65, 1–67. [Google Scholar] [CrossRef]
- Stampfli, G.M.; Borel, G.D. A plate tectonic model for the Palaeozoic and Mesozoic constrained by dynamic plate boundaries and restored synthetic oceanic isochrones. Earth Planet. Sci. Lett. 2002, 169, 17–33. [Google Scholar] [CrossRef]
- Robertson, A.H.F.; Ustaomer, T. Tectonic evolution of the Intra-Pontide suture in the Armautlu Peninsula, NW Turkey. Tectonophysics 2004, 381, 175–209. [Google Scholar] [CrossRef]
- Oberhansli, R.; Candan, O.; Bousquet, R.; Rimmele, G.; Okay, A.; Goff, J.B. Alpine HP evolution of the eastern Bitlis complex, SE Turkey. In Sedimentary Basin Tectonics from the Black Sea and Caucasus to the Arabian Sea; Sosson, M., Kaymakci, N., Stephenson, R., Bergerat, F., Starostenko, V., Eds.; Geological Society of London: London, UK, 2010; Volume 340, pp. 461–483. [Google Scholar]
- Pourteau, A.; Candan, O.; Oberhänsli, R. High-Pressure metasediments in Central Turkey: Constraints on the Neotethyan Closure History. Tectonics 2010, 29, TC5004. [Google Scholar] [CrossRef]
- Robertson, A.H.F.; Parlak, O.; Ustaömer, T. Overview of the Palaeozoic-Neogene Evolution of Neotethys in the Eastern Mediterranean Region (Southern Turkey, Cyprus, Syria). Petrol. Geosci. 2012, 18, 381–404. [Google Scholar] [CrossRef]
- Robertson, A.H.F.; Parlak, O.; Ustaömer, T. Late-Palaeozoic–Early Cenozoic tectonic development of Southern Turkey and the easternmost Mediterranean region: Evidence from the inter-relations of continental and oceanic units. In Geological Development of Anatolia and the Easternmost Mediterranean Region; Robertson, A.H.F., Parlak, O., Ünlügenç, U.C., Eds.; Geological Society of London: London, UK, 2013; Volume 372, pp. 9–48. [Google Scholar]
- Andrew, T.; Robertson, A.H.F. The Beyşehir-Hoyran-Hadim Nappes: Genesis and Emplacement of Mesozoic Marginal and Oceanic Units of the Northern Neotethys in Southern Turkey. J. Geol. Soc. 2002, 159, 529–543. [Google Scholar] [CrossRef]
- Parlak, O.; Robertson, A.H.F. The ophiolite-related Mersin mélange, southern Turkey: Its role in the tectonic—Sedimentary setting of Tethys in the Eastern Mediterranean region. Geol. Mag. 2004, 141, 257–286. [Google Scholar] [CrossRef]
- Robertson, A.H.F. Development of Concepts Concerning the Genesis and Emplacement of Tethyan Ophiolites in the Eastern Mediterranean and Oman Regions. Earth Sci. Rev. 2004, 66, 331–387. [Google Scholar] [CrossRef]
- Parlak, O.; Delaloye, M.; Bíngöl, F. Mineral chemistry of ultramafic and mafic cumulates as an indicator of the arc-related origin of the Mersín ophiolite (southern Turkey). Geol. Rund. 1996, 85, 647–661. [Google Scholar] [CrossRef]
- Parlak, O.; Delaloye, M.; Bíngöl, F. Phase and cryptic variation through the ultramafic–mafic cumulates in the Mersin ophiolite (southern Turkey). Ofioliti 1996, 21, 81–92. [Google Scholar]
- Boudier, F.; Nicolas, A. Nature of the Moho transition xone in the Oman ophiolite. J. Petrol. 1995, 36, 777–796. [Google Scholar] [CrossRef]
- Parlak, O.; Delaloye, M.; Bíngöl, F. Origin of sub-ophiolitic metamorphic rocks beneath the Mersin ophiolite, southern Turkey. Ofioliti 1995, 20, 97–110. [Google Scholar]
- Çelik, Ö.F. Detailed Geochemistry and K-Ar Geochronology of the Metamorphic Sole Rocks and Their Mafic Dykes from the Mersin Ophiolite, Southern Turkey. Turk. J. Earth Sci. 2008, 17, 685–708. [Google Scholar]
- Dilek, Y.; Thy, P.; Hacker, B.; Grundvig, S. Structure and petrology of Tauride ophiolites and mafic dike intrusions (Turkey): Implications for the Neotethyan ocean. Geol. Soc. Am. Bull. 1999, 111, 1192–1216. [Google Scholar] [CrossRef]
- Arai, S. Igneous mineral equilibria in some Alpine-type peridotites in Japan. In Materials Science in Earth’s Interior; Sunagawa, I., Ed.; Terra: Tokyo, Japan, 1984; pp. 445–460. [Google Scholar]
- Morishita, T.; Ishida, Y.; Arai, S.; Shirasaka, M. Determinaion of meltiple trace element compositions in thin (<30 µm) lasers of NIST SRM 614 and 616 using laser albration-inductively coupled plasma-mass spectrometry (LA-ICP-MS). Geostand. Geoanal. Res. 2005, 29, 107–122. [Google Scholar] [CrossRef]
- Leake, B.E.; Woolley, A.R.; Arps, C.E.S.; Birch, W.D.; Gilbert, M.C.; Grice, J.D.; Hawthorne, F.C.; Kato, A.; Kisch, H.J.; Krivovichev, V.G.; et al. Nomenclature of amphiboles report of the subcommittee on amphiboles of the international mineralogical association commission on new minerals and mineral names. Eur. J. Miner. 1997, 9, 623–651. [Google Scholar] [CrossRef]
- Takahashi, E. Origin of basaltic magmas: Implications from peridotite melting experiments and an olivine fractionation model. Bull. Vol. Soc. Jpn. 1986, 30, S17–S40, (In Japanese with English Abstract). [Google Scholar]
- Herzburg, C.; Asimow, P.D.; Ionov, D.A.; Vidito, C.; Jackson, M.G.; Geist, D. Nickel and helium evidence for melt above the core-mantle boundary. Nature 2013, 494, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Arai, S. Origin of ophiolitic peridotites. J. Geogr. 1989, 3, 45–54, (In Japanese with English abstract). [Google Scholar] [CrossRef]
- Tamura, A.; Arai, S. Harzburgite–dunite–orthopyroxenite suite as a record of supra-subduction zone setting for the Oman ophiolite mantle. Lithos 2006, 90, 43–56. [Google Scholar] [CrossRef]
- Arai, S.; Okamura, H.; Kadoshima, K.; Tanaka, C.; Suzuki, K.; Ishimaru, S. Chemical characteristics of chromian spinel in plutonic rocks: Implications for deep magma processes and discrimination of tectonic setting. Island Arc 2011, 20, 125–137. [Google Scholar] [CrossRef]
- Arai, S. Dunite–harzburgite–chromitite complexes as refractory residue in the Sangun–Yamaguchi zone, western Japan. J. Petrol. 1980, 21, 141–165. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.-S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Johnson, K.T.M.; Dick, H.J.B. Open system melting and temporal and spatial variation of peridotite and basal at the Atlantis II fracture zone. J. Geophys. Res. 1992, 97, 9219–9241. [Google Scholar] [CrossRef]
- Workman, R.K.; Hart, S.R. Major and trace element composition of the depleted MORB mantle (DMM). Earth Planet. Sci. Lett. 2005, 231, 53–72. [Google Scholar]
- Warren, J.M.; Shimizu, N. Cryptic variations in abyssal peridotite compositions: Evidence for shallow-level melt infiltration in the oceanic lithosphere. J. Petrol. 2010, 51, 395–423. [Google Scholar] [CrossRef]
- Kanayama, K.; Umino, S.; Ishizuka, O. Eocene volcanism during the incipient stage of Izu–Ogasawara Arc: Geology and petrology of the Mukojima Island Group, the Ogasawara Islands. Island Arc 2012, 21, 288–316. [Google Scholar] [CrossRef]
- Dick, H.J.B.; Bullen, T. Chromian spinel as a petrogenetic indicator in abyssal and alpine-type peridotites as spatially associated lavas. Contrib. Miner. Petrol. 1984, 86, 54–76. [Google Scholar] [CrossRef]
- Arai, S. Characterization of spinel peridotites by olivine-spinel compositional relationships: Review and interpretation. Chem. Geol. 1994, 113, 191–204. [Google Scholar] [CrossRef]
- Arai, S. Compositional variation of olivine-chrominal spinel in Mg-rich magmas as a guide to their residual spinel peridotites. J. Volcanol. Geoth. Res. 1994, 59, 279–293. [Google Scholar] [CrossRef]
- Rampone, R.; Piccardo, G.B.; Vannucci, R.; Bottazzi, P.; Ottolini. Subsolidus reactions monitored by traceelement partitioning: The spinel- to plagioclase-facies transition in mantle peridotites. Contrib. Miner. Petrol. 1993, 115, 1–17. [Google Scholar] [CrossRef]
- Hellebrand, E.; Snow, J.E.; Dick, H.J.; Hofmann, A.W. Coupled major and trace elements as indicators of the extent of melting in mid-ocean-ridge peridotites. Nature 2001, 410, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Matsukage, K.N.; Kubo, K. Chromian spinel during melting experiments of dry peridotite (KLB-1) at 1.0–2.5 GPa. Am. Miner. 2003, 88, 1271–1278. [Google Scholar] [CrossRef]
- Johnson, K.T.M.; Dick, H.J.B.; Shimizu, N. Melting in the oceanic upper mantle: An ion microprobe study of diopsides in abyssal peridotites. J. Geophys. Res. 1990, 95, 2661–2678. [Google Scholar] [CrossRef]
- Witt-Eickschen, G.; O’Neill, H.S. The effect of temperature on the equilibrium distribution of trace elements between clinopyroxene, orthopyroxene, olivine and spinel in upper mantle peridotite. Chem. Geol. 2005, 221, 65–101. [Google Scholar] [CrossRef]
- Sun, C.; Liang, Y. An assessment of subsolidus re-equilibration on REE distribution among mantle minerals olivine, orthopyroxene, clinopyroxene and garnet in peridotite. Chem. Geol. 2014, 372, 80–91. [Google Scholar] [CrossRef]
- Salters, V.J.M.; Stracke, A. Composition of the depleted mantle. Geochem. Geophys. Geosyst. 2004, 5, Q05004. [Google Scholar] [CrossRef]
- Scott, J.M.; Liu, J.; Pearson, D.G.; Waight, T.E. Mantle depletion and metasomatism recorded in orthopyroxene in highly depleted peridotites. Chem. Geol. 2016, 441, 280–291. [Google Scholar] [CrossRef]
- Hirose, K.; Kawamoto, T. Hydrous partial melting of lherzolite at 1 GPa: The effect of H2O on the genesis of basaltic magmas. Earth Planet. Sci. Lett. 1995, 133, 463–473. [Google Scholar] [CrossRef]
- Kelemen, P.B.; Hart, S.R.; Bernstein, S. Silica enrichment in the continental upper mantle via melt/rock reaction. Earth Planet. Sci. Lett. 1998, 164, 387–406. [Google Scholar] [CrossRef]
- Straub, S.M.; LaGatta, A.B.; Martin-Del Pozzo, A.L.; Langmuir, C.H. Evidence from high-Ni olivines for a hybridized peridotite/pyroxenite source for orogenic andesites from the central Mexican Volcanic Belt. Geochem. Geophys. Geosyst. 2008, 9, Q03007. [Google Scholar] [CrossRef]
- Mysen, B.; Kushiro, I. Compositional variations of coexisting phases with degree of melting of peridotite in the upper mantle. Am. Miner. 1977, 62, 843–865. [Google Scholar]
- Prinzhofer, A.; Allégre, C.J. Residual peridotites and the mechanisms of partial melting. Earth Planet. Sci. Lett. 1985, 74, 251–265. [Google Scholar] [CrossRef]
- Presnall, D.C. The geometrical analysis of partial fusion. Am. J. Sci. 1969, 267, 1178–1194. [Google Scholar] [CrossRef]
- Nicolas, A.; Prinzhofer, A. Cumulative or residual origin for the transition zone in ophiolites: Structural evidence. J. Petrol. 1983, 24, 188–206. [Google Scholar] [CrossRef]
- Arai, S.; Yurimoto, H. Podiform chromitites of the Tari-Misaka ultramafic complex, southwestern Japan, as mantle-melt interaction products. Econ. Geol. 1994, 89, 1279–1288. [Google Scholar] [CrossRef]
- Kessel, R.; Shmidt, M.W.; Ulmer, P.; Pettke, T. Trace element signature of subduction-zone fluids, melts and supercritical liquids at 120-180 km depth. Nature 2005, 437, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Elthon, D.; Casey, J.F.; Komor, S. Mineral chemistry of ultramafic cumulates from the North Arm Mountain massif of the Bay of Islands ophiolite: Evidence for high-pressure crystal fractionation of oceanic basalt. J. Geophys. Res. 1982, 87, 8717–8734. [Google Scholar] [CrossRef]
- Barnouin-Jha, K.; Parmentier, E.M.; Sparks, D.W. Buoyant mantle upwelling and crustal production at oceanic spreading centers: On-axis segmentation and off-axis melting. J. Geophys. Res. 1997, 102, 11979–11989. [Google Scholar] [CrossRef]
- Hernlund, J.W.; Stevenson, D.J.; Tackley, P.J. Buoyant melting instabilities beneath extending lithosphere: 1. Numerical models. J. Geophys. Res. 2008, 113, B04405. [Google Scholar] [CrossRef]
- Hernlund, J.W.; Stevenson, D.J.; Tackley, P.J. Buoyant melting instabilities beneath extending lithosphere: 2. Linear analysis. J. Geophys. Res. 2008, 113, B04406. [Google Scholar] [CrossRef]
- Fisk, M.R. Basalt magma interaction with harzburgite and the formation of high-magnesian andesites. Geophys. Res. Lett. 1986, 13, 467–470. [Google Scholar] [CrossRef]
- Kelemen, P.B. Reaction between ultramafic rocks and fractionating basaltic magma. I. Phase relations, the origin of calc-alkaline magma series, and the formation of discordant dunite. J. Petrol. 1990, 31, 51–98. [Google Scholar] [CrossRef]
- Nicolas, A.; Boudier, F.; Ildefonse, B. Variable crustal thickness in the Oman ophiolite: Implication for oceanic crust. J. Geophys. Res. 1996, 191, 17941–17950. [Google Scholar] [CrossRef]
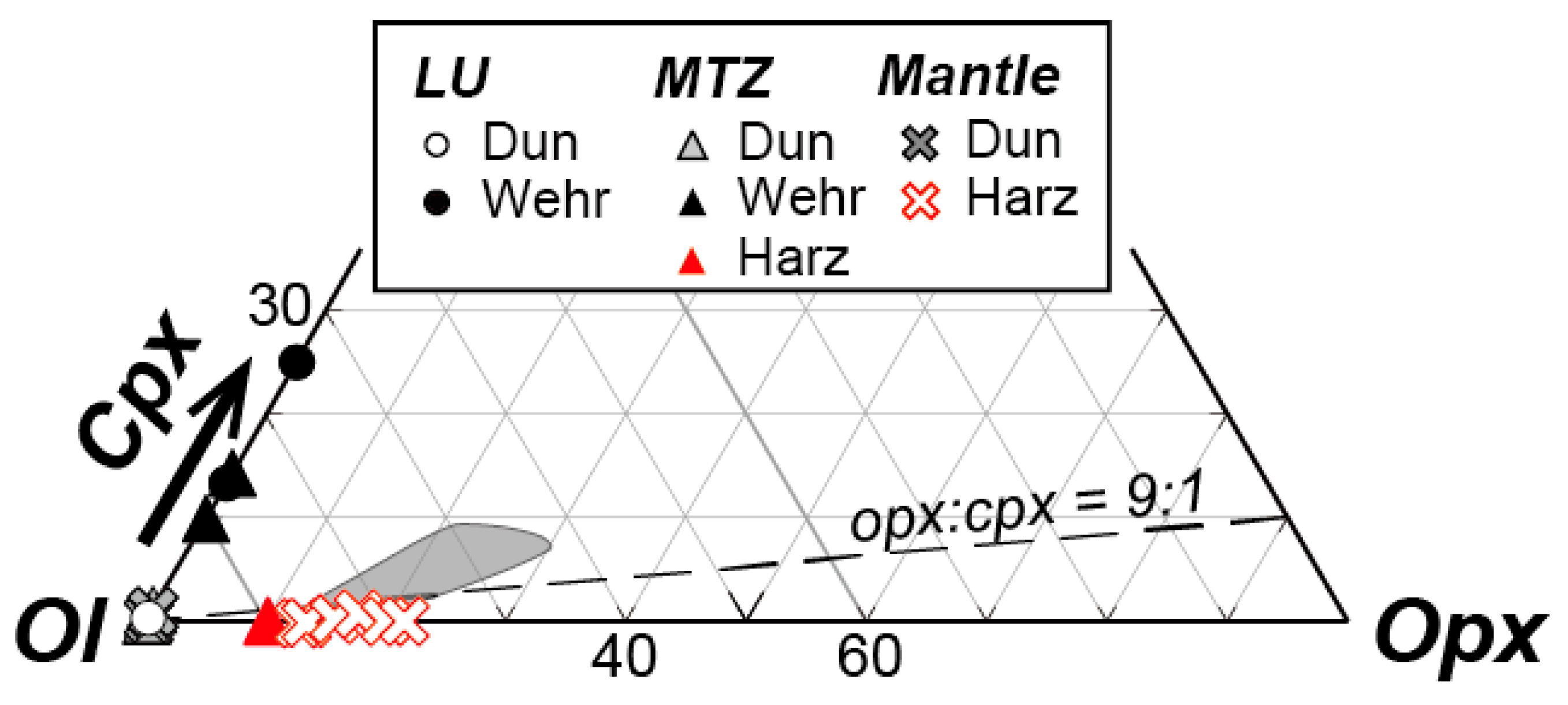

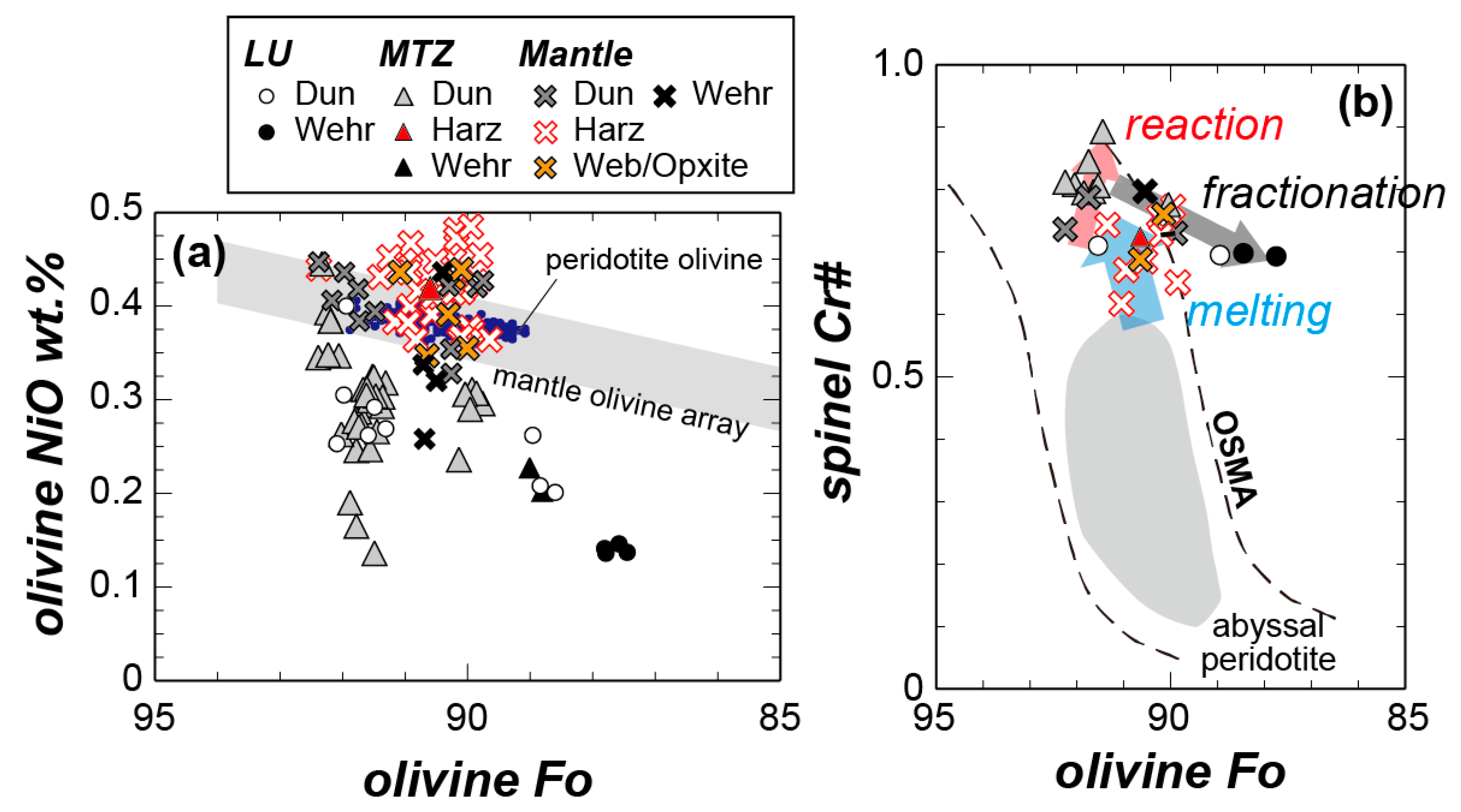
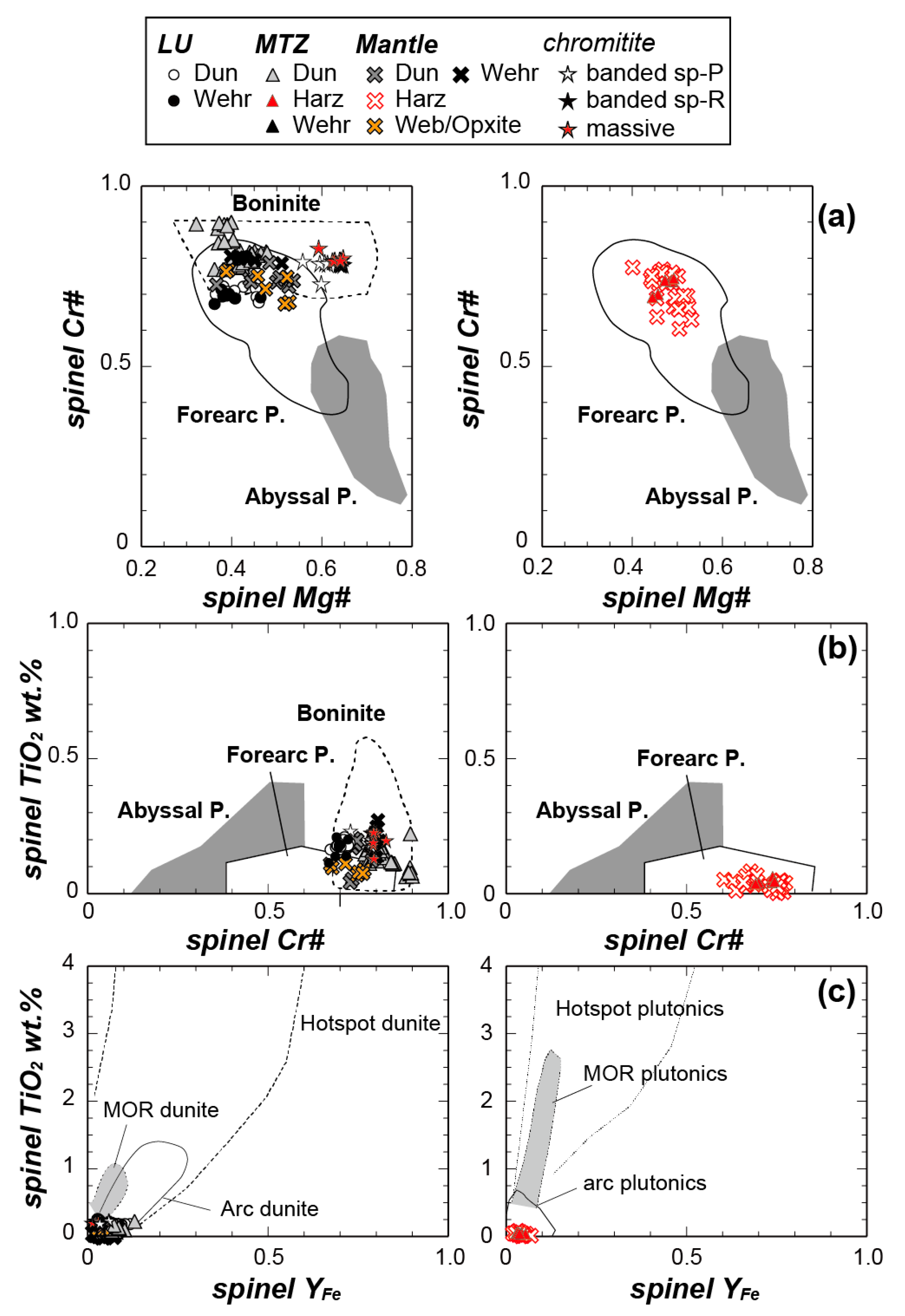
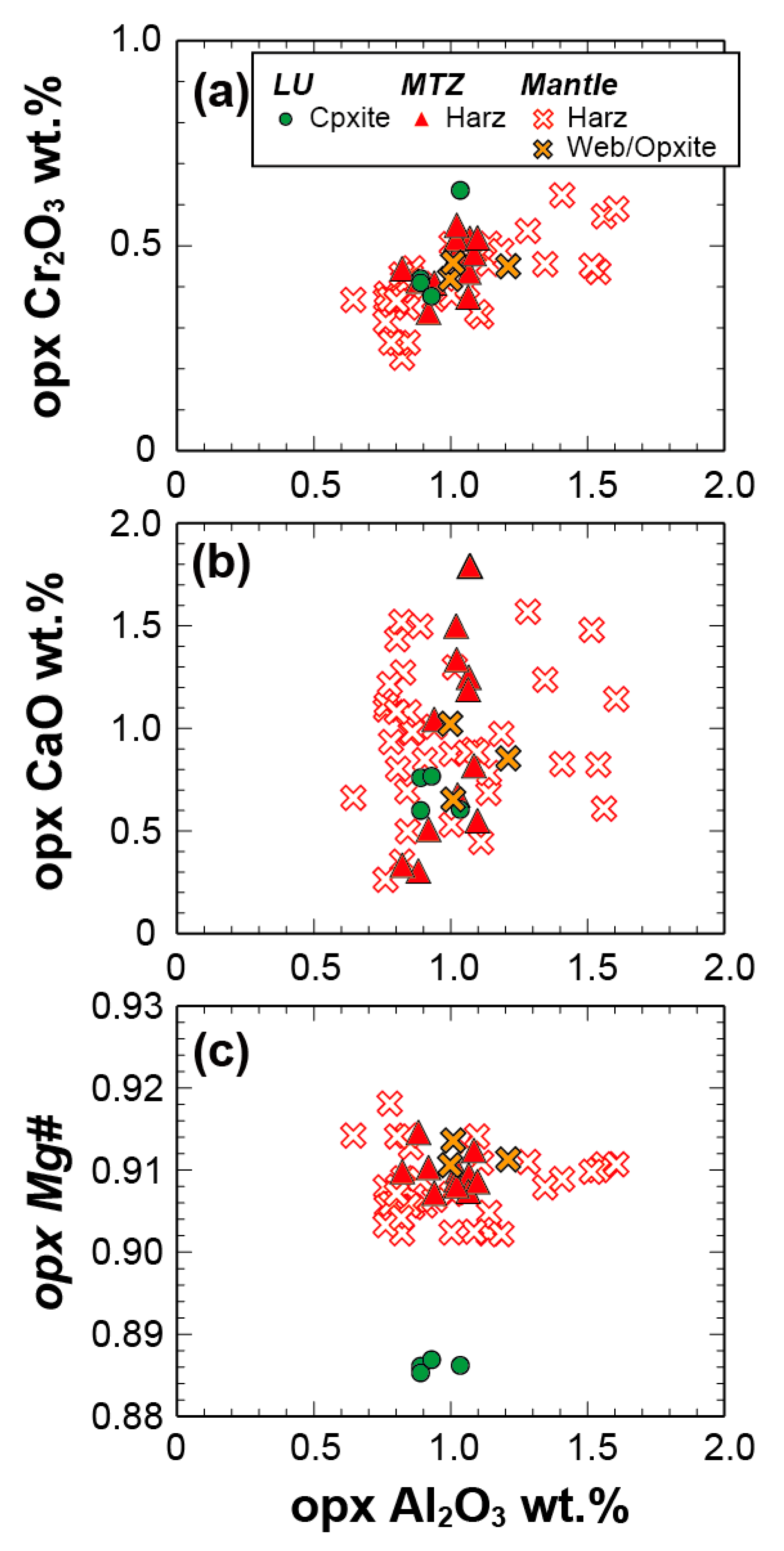
| Route | Sample | Rock Type | Latitude | Longitude | Foliation | Notes |
|---|---|---|---|---|---|---|
| R1 | Layered Ultramafic (LU) rocks | |||||
| 2013MR3-01 | Cpxite | N36°48.551′ | E34°10.350′ | Gabbro-cpxite | ||
| 2013MR3-02 | Wehrlite | Cpxite in wehrlite | ||||
| 2013MR2-0 | Dunite | |||||
| 2013MR2-02 | Wehrlite | |||||
| 2013MR2-06 | Wehrlite | |||||
| 2013MR2-07 | Dunite | |||||
| 2013MR2-08 | Cpxite | |||||
| 2013MR2-09 | Wehrlite | |||||
| 2013MR2-10 | Cpxite | |||||
| Moho transition zone (MTZ) rocks | ||||||
| 2014MR1-01 | Dunite | N36°49.214′ | E34°09.938′ | EW80°S | ||
| 2014MR1-03 | Dunite | N36°49.250′ | E34°09.954′ | N82°E84°S | ||
| 14T-MR01 | Chromitite | Massive chromitite | ||||
| 14T-MR03 | Dunite | Host dunite of 14T-MR01 | ||||
| 2014MR1-04 | Dunite | N36°49.289′ | E34°09.933′ | |||
| 2014MR1-05 | Dunite | N36°49.315′ | E34°09.925′ | |||
| 2014MR1-06 | Dunite | N36°49.358′ | E34°09.918′ | |||
| 2014MR1-09 | Dunite | N36°49′433′ | E34°09′910′ | N80°E78°SE | ||
| 2014MR1-13 | Dunite | |||||
| 2014MR1-14 | Dunite | N36°49.714′ | E34°09.724′ | N80°E78°SE | ||
| 2014MR1-16 | Wehrlite | |||||
| 2013MR1-02 | Harz | N36°50.081′ | E34º09.787′ | |||
| R2 | Mantle rocks | |||||
| 2014MR2-16 | Harz | N36°48.535′ | E34°13.229′ | |||
| 2014MR2-15 | Harz | N36°48.608′ | E34°13.114′ | |||
| 2014MR2-14 | Dunite | N36°48.653′ | E34°13.067′ | N70°W80°SW | ||
| 2014MR2-13 | Harz | N36°48.688′ | E34°13.010′ | N70°W68°SW | ||
| 2014MR2-12 | Harz | N36°48.809′ | E34°12.751′ | |||
| 2013MR7-01 | Harz | N36°48.901′ | E34°12.702′ | |||
| 2014MR2-11 | Dunite | N36°49.026′ | E34°12.674′ | |||
| 2014MR2-10 | Websterite | N36°49.117′ | E34°12.490′ | Websterite dike in dunite | ||
| 2014MR2-09 | Dunite/Harz | N36°49.113′ | E34°12.480′ | Dunite-Harz boundary | ||
| 2014MR2-07 | Dunite | N36°49.126′ | E34º12.442′ | Dunite with websterite dike | ||
| 2014MR2-06 | Harz | |||||
| 2014MR2-05 | Dunite | N36°49.159′ | E34°12.430′ | Host dunite of 14T-MR12 | ||
| 14T-MR12 | Chromitite | Banded chromitite | ||||
| 2014MR2-03 | Harz | N36°49.199′ | E34°12.374′ | N30°W78°W | ||
| 14T-MR04 | Wehrlite | N36°49.202′ | E34°12.381′ | |||
| 2014MR2-01 | Harz | N36°49.205′ | E34°12.346′ | |||
| 2013MR5-01 | Dunite | N36°49.394′ | E34°12.267′ | |||
| 2013MR5-02 | Websterite | Websterite dike in dunite | ||||
| 2013MR4-02 | Opxite | N36°49.557 | E34°12.158′ | N45°W85°SW | Harz with opxite dike | |
| 2013MR4-03 | Dunite | |||||
| 2013MR4-04 | Harz | |||||
| 2013MR4-05 | Dunite | Dunite with websterite dike | ||||
| 2013MR8-01 | Harz | N36°45.789′ | E34°16.019′ | N70°W, 64°S | ||
| Rock Suite | Layered Ultramafic (LU) Rocks | Moho Transition Zone (MTZ) Rocks | Mantle Rocks | |||||||||||||
| Rock Type | Dunite | Wehrlite | Cpxite | Dunite | Harz | Harz | ||||||||||
| Sample No. | 2013MR2-0 | 2013MR2-07 | 2013MR2-02 | 2013MR2-09 | 2013MR2-08 | 2013MR2-10 | 2014MR1-01 | 2014MR1-03 | 14T-MR03 | 2014MR1-04B | 2014MR1-05 | 2014MR1-06 | 2014MR1-09 | 2014MR1-14 | 2013MR1-02 | 2014MR2-16 |
| No. of Analysis | (N = 3) | (N = 3) | (N = 2) | (N = 4) | (N = 4) | (N = 5) | (N = 6) | (N = 6) | (N = 3) | (N = 5) | (N = 4) | (N = 6) | (N = 5) | (N = 3) | (N = 1) | (N = 2) |
| SiO2 | 41.57 | 41.24 | 41.82 | 41.01 | 39.60 | 40.38 | 40.81 | 41.29 | 40.66 | 41.17 | 41.36 | 40.94 | 40.96 | 41.28 | 41.00 | 41.92 |
| TiO2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| Al2O3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.03 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | ||||
| Cr2O3 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.02 | 0.03 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | ||
| FeO* | 8.42 | 10.82 | 10.76 | 11.97 | 11.77 | 11.87 | 9.71 | 7.56 | 8.03 | 8.22 | 8.02 | 8.34 | 8.05 | 8.41 | 9.22 | 9.84 |
| MnO | 0.12 | 0.12 | 0.14 | 0.16 | 0.13 | 0.11 | 0.16 | 0.12 | 0.11 | 0.12 | 0.11 | 0.11 | 0.09 | 0.13 | ||
| MgO | 50.63 | 48.63 | 48.35 | 47.70 | 46.76 | 47.72 | 48.77 | 50.46 | 51.84 | 50.58 | 50.31 | 50.06 | 49.93 | 50.78 | 49.88 | 49.41 |
| CaO | 0.05 | 0.02 | 0.02 | 0.02 | 0.06 | 0.06 | 0.09 | 0.06 | 0.08 | 0.08 | 0.06 | 0.06 | 0.00 | 0.02 | ||
| Na2O | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | ||||
| K2O | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| NiO | 0.27 | 0.24 | 0.21 | 0.14 | 0.29 | 0.38 | 0.32 | 0.28 | 0.19 | 0.30 | 0.29 | 0.31 | 0.42 | 0.44 | ||
| Total | 101.08 | 101.08 | 101.33 | 101.01 | 98.13 | 99.96 | 99.79 | 99.89 | 101.23 | 100.45 | 100.09 | 99.86 | 99.41 | 100.96 | 100.61 | 101.76 |
| Mg# | 0.915 | 0.889 | 0.889 | 0.877 | 0.876 | 0.878 | 0.900 | 0.922 | 0.920 | 0.916 | 0.918 | 0.914 | 0.917 | 0.915 | 0.906 | 0.899 |
| Rock Suite | Mantle Rocks | |||||||||||||||
| Rock Type | Harz | (Harz Part) | Harz | Dunite | (Dunite Part) | G Chromitite | Wehrlite | Websterite | Opxite | |||||||
| Sample No. | 2014MR2-15 | 2014MR2-13 | 2014MR2-12 | 2013MR7-01 | 2014MR2-09 | 2014MR2-06 | 2014MR2-03 | 2014MR2-01 | 2013MR8-01 | 2014MR2-14 | 2014MR2-11 | 2014MR2-09 | 14T-MR12(p) | 14T-MR04 | 2013MR5-02 | 2013MR4-02 |
| No. of Analysis | (N = 4) | (N = 5) | (N = 4) | (N = 3) | (N = 3) | (N = 5) | (N = 2) | (N = 2) | (N = 4) | (N = 3) | (N = 3) | (N = 1) | (N = 2) | (N = 4) | (N = 3) | (N = 4) |
| SiO2 | 42.10 | 41.24 | 41.80 | 41.09 | 40.61 | 41.08 | 40.89 | 41.01 | 41.86 | 41.19 | 41.62 | 41.33 | 40.62 | 40.41 | 41.23 | 41.42 |
| TiO2 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.02 | 0.00 | 0.00 |
| Al2O3 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.03 | 0.00 | 0.00 |
| Cr2O3 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.02 | 0.01 | 0.02 | 0.00 | 0.02 | 0.01 | 0.00 | 0.43 | 0.04 | 0.00 | 0.00 |
| FeO* | 9.80 | 9.78 | 8.81 | 9.81 | 9.55 | 8.56 | 9.86 | 8.97 | 9.40 | 7.78 | 8.21 | 9.87 | 3.99 | 9.41 | 9.10 | 9.62 |
| MnO | 0.14 | 0.11 | 0.10 | 0.09 | 0.10 | 0.10 | 0.11 | 0.10 | 0.11 | 0.10 | 0.08 | 0.13 | 0.09 | 0.12 | 0.13 | |
| MgO | 49.97 | 50.27 | 50.25 | 48.71 | 49.22 | 50.43 | 49.97 | 50.22 | 50.02 | 51.49 | 50.58 | 49.05 | 54.01 | 50.58 | 49.30 | 49.15 |
| CaO | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.09 | 0.02 | 0.01 | 0.07 | 0.04 | 0.01 | 0.04 |
| Na2O | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.02 | 0.02 | 0.00 | 0.00 |
| K2O | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.02 | 0.02 | 0.00 | 0.00 |
| NiO | 0.39 | 0.45 | 0.44 | 0.41 | 0.39 | 0.42 | 0.37 | 0.37 | 0.42 | 0.43 | 0.40 | 0.42 | 0.58 | 0.20 | 0.41 | 0.39 |
| Total | 102.42 | 101.88 | 101.42 | 100.15 | 99.89 | 100.64 | 101.22 | 100.70 | 101.82 | 101.10 | 100.93 | 100.84 | 99.83 | 100.78 | 100.17 | 100.76 |
| Mg# | 0.901 | 0.902 | 0.910 | 0.898 | 0.902 | 0.913 | 0.900 | 0.909 | 0.905 | 0.922 | 0.917 | 0.899 | 0.960 | 0.905 | 0.906 | 0.901 |
| Rock Suite | LU Rocks | MTZ Rocks | Mantle Rocks | |||||||||||||
| Rock Type | Dunite | Wehrlite | Dunite | M Chromitite | Harz | Harz | ||||||||||
| Sample No. | 2013MR2-0 | 2013MR2-07 | 2013MR2-02 | 2013MR2-09 | 2014MR1-01 | 2014MR1-03 | 14T-MR03 | 2014MR1-04B | 2014MR1-05 | 2014MR1-06 | 2014MR1-09 | 2014MR1-14 | 14T-MR01 | 2013MR1-02 | 2014MR2-16 | 2014MR2-13 |
| No. of Analysis | (N = 7) | (N = 6) | (N = 6) | (N = 2) | (N = 8) | (N = 7) | (N = 3) | (N = 6) | (N = 3) | (N = 6) | (N = 5) | (N = 6) | (N = 7) | (N = 6) | (N = 2) | (N = 3) |
| SiO2 | 0.04 | 0.04 | 0.03 | 0.05 | 0.04 | 0.02 | 0.08 | 0.05 | 0.03 | 0.16 | 0.01 | 0.02 | 0.07 | 0.05 | 0.06 | 0.02 |
| TiO2 | 0.18 | 0.18 | 0.18 | 0.13 | 0.14 | 0.14 | 0.17 | 0.17 | 0.17 | 0.08 | 0.11 | 0.16 | 0.20 | 0.05 | 0.02 | 0.02 |
| Al2O3 | 13.45 | 14.65 | 14.33 | 14.37 | 10.35 | 8.78 | 9.35 | 9.58 | 9.60 | 4.84 | 7.03 | 9.20 | 10.21 | 13.67 | 10.94 | 12.11 |
| Cr2O3 | 49.04 | 49.66 | 49.43 | 48.39 | 53.38 | 56.73 | 57.10 | 56.08 | 55.68 | 60.08 | 57.22 | 56.82 | 60.22 | 53.53 | 55.06 | 56.14 |
| Fe2O3 | 7.16 | 5.36 | 5.61 | 6.67 | 6.08 | 4.75 | 4.42 | 5.21 | 4.92 | 5.99 | 5.92 | 4.84 | 2.21 | 3.17 | 4.09 | 3.20 |
| FeO* | 21.17 | 21.38 | 22.12 | 22.80 | 21.12 | 19.84 | 19.97 | 20.18 | 20.35 | 21.31 | 21.14 | 19.92 | 13.66 | 19.18 | 20.12 | 19.61 |
| MnO | 0.37 | 0.38 | 0.39 | 0.41 | 0.41 | 0.38 | 0.30 | 0.41 | 0.37 | 0.47 | 0.41 | 0.39 | 0.16 | 0.31 | 0.36 | 0.31 |
| MgO | 8.37 | 8.45 | 7.91 | 7.50 | 8.03 | 8.69 | 8.88 | 8.78 | 8.51 | 7.45 | 7.67 | 8.87 | 13.06 | 9.68 | 8.68 | 9.50 |
| CaO | 0.00 | 0.07 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.03 | 0.01 | 0.02 | 0.00 |
| Na2O | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.00 | 0.00 | 0.00 |
| K2O | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 |
| NiO | 0.07 | 0.06 | 0.04 | 0.03 | 0.05 | 0.09 | 0.08 | 0.03 | 0.02 | 0.03 | 0.03 | 0.07 | 0.11 | 0.06 | 0.08 | 0.03 |
| Total | 99.84 | 100.22 | 100.04 | 100.34 | 99.61 | 99.46 | 100.45 | 100.49 | 99.66 | 100.42 | 99.55 | 100.29 | 100.01 | 99.71 | 99.42 | 100.95 |
| Mg# | 0.414 | 0.413 | 0.389 | 0.370 | 0.404 | 0.439 | 0.442 | 0.437 | 0.427 | 0.384 | 0.393 | 0.442 | 0.630 | 0.473 | 0.435 | 0.463 |
| Cr# | 0.710 | 0.695 | 0.698 | 0.693 | 0.776 | 0.812 | 0.804 | 0.797 | 0.795 | 0.893 | 0.845 | 0.806 | 0.798 | 0.724 | 0.772 | 0.757 |
| YCr | 0.646 | 0.648 | 0.649 | 0.635 | 0.716 | 0.763 | 0.759 | 0.745 | 0.746 | 0.823 | 0.780 | 0.756 | 0.776 | 0.696 | 0.732 | 0.727 |
| YAl | 0.264 | 0.285 | 0.281 | 0.281 | 0.207 | 0.176 | 0.185 | 0.190 | 0.192 | 0.099 | 0.143 | 0.183 | 0.196 | 0.265 | 0.217 | 0.234 |
| YFe | 0.090 | 0.067 | 0.070 | 0.083 | 0.078 | 0.061 | 0.056 | 0.066 | 0.063 | 0.078 | 0.077 | 0.061 | 0.027 | 0.039 | 0.052 | 0.039 |
| Rock Suite | Mantle rocks | |||||||||||||||
| Rock Type | Harz | (Harz part) | Harz | Dunite | (Dunite part) | G Chromitite | Wehrlite | Websterite | Opxite | |||||||
| Sample No. | 2014MR2-12 | 2013MR7-01 | 2014MR2-09 | 2014MR2-06 | 2014MR2-03 | 2014MR2-01 | 2013MR8-01 | 2014MR2-14 | 2014MR2-11 | 2014MR2-09 | 14T-MR12 (p) | 14T-MR12 (r) | 14T-MR04 | 2013MR5-02 | 2013MR4-02 | |
| No. of Analysis | (N = 2) | (N = 2) | (N = 2) | (N = 5) | (N = 4) | (N = 2) | (N = 4) | (N = 5) | (N = 1) | (N = 2) | (N = 4) | (N = 4) | (N = 7) | (N = 2) | (N = 3) | |
| SiO2 | 0.01 | 0.04 | 0.04 | 0.02 | 0.03 | 0.04 | 0.02 | 0.02 | 0.52 | 0.03 | 0.08 | 0.06 | 0.10 | 0.02 | 0.09 | |
| TiO2 | 0.05 | 0.02 | 0.04 | 0.04 | 0.03 | 0.07 | 0.05 | 0.19 | 0.12 | 0.05 | 0.20 | 0.21 | 0.20 | 0.11 | 0.08 | |
| Al2O3 | 20.19 | 17.25 | 13.47 | 12.79 | 12.86 | 16.67 | 16.15 | 13.05 | 10.21 | 12.99 | 10.83 | 10.92 | 9.76 | 15.72 | 11.71 | |
| Cr2O3 | 48.39 | 48.05 | 53.83 | 55.01 | 54.45 | 50.71 | 52.27 | 54.24 | 56.40 | 52.28 | 58.16 | 58.67 | 57.78 | 51.69 | 55.33 | |
| Fe2O3 | 2.02 | 4.84 | 3.23 | 2.87 | 3.36 | 2.98 | 2.38 | 3.57 | 2.98 | 4.28 | 2.96 | 2.89 | 2.54 | 2.68 | 3.22 | |
| FeO* | 18.36 | 19.53 | 19.28 | 18.92 | 19.95 | 17.99 | 18.29 | 17.70 | 18.25 | 21.33 | 14.14 | 13.25 | 20.01 | 18.17 | 21.12 | |
| MnO | 0.28 | 0.31 | 0.28 | 0.34 | 0.32 | 0.31 | 0.32 | 0.32 | 0.39 | 0.37 | 0.17 | 0.16 | 0.28 | 0.32 | 0.40 | |
| MgO | 11.07 | 9.84 | 9.63 | 9.79 | 9.21 | 10.76 | 10.63 | 10.66 | 9.61 | 8.15 | 12.67 | 13.33 | 8.77 | 10.49 | 8.33 | |
| CaO | 0.00 | 0.00 | 0.01 | 0.03 | 0.00 | 0.02 | 0.01 | 0.00 | 0.01 | 0.00 | 0.03 | 0.02 | 0.04 | 0.00 | 0.01 | |
| Na2O | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 | 0.04 | 0.05 | 0.00 | 0.00 | |
| K2O | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.02 | 0.03 | 0.02 | 0.00 | 0.00 | |
| NiO | 0.08 | 0.09 | 0.09 | 0.06 | 0.09 | 0.05 | 0.04 | 0.10 | 0.06 | 0.05 | 0.14 | 0.08 | 0.08 | 0.03 | 0.05 | |
| Total | 100.43 | 99.97 | 99.90 | 99.86 | 100.29 | 99.59 | 100.15 | 99.85 | 98.56 | 99.52 | 99.44 | 99.65 | 99.64 | 99.24 | 100.34 | |
| Mg# | 0.518 | 0.473 | 0.471 | 0.480 | 0.451 | 0.516 | 0.509 | 0.518 | 0.484 | 0.405 | 0.615 | 0.642 | 0.439 | 0.507 | 0.413 | |
| Cr# | 0.617 | 0.651 | 0.728 | 0.743 | 0.740 | 0.671 | 0.685 | 0.736 | 0.788 | 0.730 | 0.783 | 0.783 | 0.799 | 0.688 | 0.760 | |
| YCr | 0.602 | 0.613 | 0.699 | 0.716 | 0.709 | 0.647 | 0.665 | 0.704 | 0.758 | 0.690 | 0.754 | 0.755 | 0.773 | 0.665 | 0.730 | |
| YAl | 0.374 | 0.328 | 0.261 | 0.248 | 0.250 | 0.317 | 0.306 | 0.252 | 0.204 | 0.256 | 0.209 | 0.210 | 0.195 | 0.302 | 0.230 | |
| YFe | 0.024 | 0.059 | 0.040 | 0.036 | 0.042 | 0.036 | 0.029 | 0.044 | 0.038 | 0.054 | 0.036 | 0.035 | 0.032 | 0.033 | 0.040 | |
| Rock Suite | LU Rocks | MTZ Rocks | Mantle Rocks | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rock Type | Cpxite | Harz | Harz | (Harz Part) | Harz | Websterite | ||||||||
| Sample No. | 2013MR2-08 | 2013MR2-10 | 2013MR1-02 | 2014MR2-16 | 2014MR2-15 | 2014MR2-13 | 2014MR2-12 | 2013MR7-01 | 2014MR2-09 | 2014MR2-06 | 2014MR2-03 | 2014MR2-01 | 2013MR8-01 | 2013MR5-02 |
| No. of Analysis | (N = 4) | (N = 2) | (N = 12) | (N = 2) | (N = 2) | (N = 6) | (N = 4) | (N = 4) | (N = 4) | (N = 4) | (N = 5) | (N = 3) | (N = 3) | (N = 3) |
| SiO2 | 56.13 | 55.81 | 58.15 | 57.49 | 58.54 | 57.25 | 57.34 | 57.96 | 57.08 | 57.52 | 57.70 | 57.68 | 57.66 | 57.24 |
| TiO2 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 | 0.02 | 0.01 | 0.02 | ||
| Al2O3 | 0.89 | 0.97 | 1.00 | 0.76 | 0.86 | 0.83 | 1.55 | 1.14 | 0.99 | 0.77 | 0.83 | 1.09 | 1.34 | 1.07 |
| Cr2O3 | 0.44 | 0.48 | 0.46 | 0.38 | 0.31 | 0.37 | 0.51 | 0.48 | 0.43 | 0.34 | 0.38 | 0.36 | 0.54 | 0.44 |
| FeO* | 7.65 | 7.53 | 6.09 | 6.23 | 6.59 | 6.31 | 5.98 | 6.49 | 6.27 | 5.78 | 6.40 | 5.90 | 6.07 | 5.85 |
| MnO | 0.13 | 0.14 | 0.15 | 0.13 | 0.10 | 0.11 | 0.14 | 0.11 | 0.13 | 0.13 | 0.13 | 0.14 | ||
| MgO | 33.36 | 33.05 | 34.53 | 34.02 | 34.81 | 34.87 | 34.07 | 33.87 | 33.86 | 34.98 | 34.78 | 34.49 | 34.11 | 33.97 |
| CaO | 0.74 | 0.70 | 0.94 | 1.11 | 0.60 | 0.83 | 1.01 | 0.83 | 0.93 | 0.95 | 1.22 | 0.74 | 1.21 | 0.84 |
| Na2O | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 | ||
| K2O | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| NiO | 0.11 | 0.11 | 0.07 | 0.10 | 0.07 | 0.12 | 0.12 | 0.10 | 0.11 | 0.09 | 0.11 | 0.10 | ||
| Total | 99.21 | 98.54 | 101.41 | 100.26 | 101.94 | 100.70 | 100.66 | 101.01 | 99.85 | 100.57 | 101.56 | 100.49 | 101.19 | 99.67 |
| Mg# | 0.886 | 0.887 | 0.910 | 0.907 | 0.904 | 0.908 | 0.910 | 0.903 | 0.906 | 0.915 | 0.906 | 0.912 | 0.909 | 0.912 |
| Rock Suite | LU Rocks | MTZ Rocks | Mantle Rocks | |||||||||||
| Rock Type | Dunite | Wehrlite | Cpxite | Dunite | Harz | Harz | ||||||||
| Sample No. | 2013MR2-0 | 2013MR2-07 | 2013MR2-02 | 2013MR2-09 | 2013MR2-08 | 2013MR2-10 | 2014MR1-01 | 2014MR1-03 | 2014MR1-05 | 2014MR1-06 | 2014MR1-09 | 2014MR1-14 | 2013MR1-02 | 2014MR2-16 |
| No. of Analysis | (N = 6) | (N = 5) | (N = 5) | (N = 7) | (N = 5) | (N = 5) | (N = 3) | (N = 2) | (N = 1) | (N = 3) | (N = 1) | (N = 1) | (N = 9) | (N = 2) |
| SiO2 | 55.61 | 55.11 | 54.75 | 54.74 | 53.73 | 53.57 | 54.67 | 54.65 | 55.48 | 54.59 | 53.42 | 53.88 | 55.58 | 55.96 |
| TiO2 | 0.04 | 0.03 | 0.03 | 0.04 | 0.03 | 0.04 | 0.01 | 0.01 | 0.04 | 0.05 | 0.01 | 0.00 | ||
| Al2O3 | 0.83 | 0.82 | 1.11 | 1.03 | 1.02 | 0.98 | 0.47 | 0.69 | 0.28 | 0.36 | 0.42 | 0.79 | 0.94 | 0.74 |
| Cr2O3 | 0.54 | 0.38 | 0.68 | 0.53 | 0.72 | 0.65 | 0.18 | 0.46 | 0.12 | 0.45 | 0.35 | 0.35 | 0.55 | 0.51 |
| FeO* | 1.41 | 2.24 | 2.84 | 2.52 | 2.53 | 2.69 | 1.57 | 1.31 | 1.48 | 1.50 | 1.23 | 1.32 | 2.01 | 1.92 |
| MnO | 0.04 | 0.07 | 0.09 | 0.09 | 0.03 | 0.01 | 0.00 | 0.04 | 0.04 | 0.01 | 0.06 | 0.04 | ||
| MgO | 17.37 | 17.53 | 17.50 | 17.49 | 17.72 | 18.28 | 17.47 | 17.25 | 18.30 | 17.52 | 17.41 | 17.31 | 17.79 | 17.68 |
| CaO | 25.46 | 24.43 | 23.87 | 23.80 | 23.73 | 23.09 | 25.17 | 24.75 | 24.42 | 24.57 | 25.12 | 25.35 | 24.08 | 24.27 |
| Na2O | 0.08 | 0.05 | 0.09 | 0.07 | 0.12 | 0.11 | 0.17 | 0.08 | 0.07 | 0.18 | 0.08 | 0.06 | ||
| K2O | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| NiO | 0.04 | 0.03 | 0.03 | 0.04 | 0.04 | 0.04 | 0.08 | 0.05 | 0.04 | 0.09 | 0.07 | 0.03 | ||
| Total | 101.42 | 100.69 | 100.99 | 100.36 | 99.43 | 99.26 | 99.75 | 99.31 | 100.35 | 99.17 | 98.14 | 99.34 | 101.17 | 101.22 |
| Mg# | 0.956 | 0.933 | 0.917 | 0.925 | 0.926 | 0.924 | 0.952 | 0.959 | 0.957 | 0.954 | 0.962 | 0.959 | 0.940 | 0.943 |
| Rock Suite | Mantle Rocks | |||||||||||||
| Rock Type | Harz | (Harz Part) | Harz | Dunite | (Dunite Part) | Wehrlite | Websterite | Opxite | ||||||
| Sample No. | 2014MR2-15 | 2014MR2-13 | 2014MR2-12 | 2013MR7-01 | 2014MR2-09 | 2014MR2-06 | 2014MR2-03 | 2014MR2-01 | 2013MR8-01 | 2014MR2-11 | 2014MR2-09 | 14T-MR04 | 2013MR5-02 | 2013MR4-02 |
| No. of Analysis | (N = 1) | (N = 3) | (N = 8) | (N = 3) | (N = 6) | (N = 3) | (N = 3) | (N = 2) | (N = 10) | (N = 2) | (N = 1) | (N = 4) | (N = 6) | (N = 4) |
| SiO2 | 54.22 | 54.41 | 54.18 | 55.13 | 54.38 | 53.37 | 53.99 | 54.51 | 55.10 | 55.06 | 55.16 | 53.69 | 54.19 | 55.04 |
| TiO2 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.00 | 0.07 | 0.02 | 0.01 |
| Al2O3 | 0.83 | 0.83 | 1.37 | 0.95 | 0.80 | 0.72 | 0.78 | 0.93 | 1.10 | 0.79 | 0.10 | 0.65 | 0.91 | 0.75 |
| Cr2O3 | 0.46 | 0.46 | 0.61 | 0.45 | 0.42 | 0.47 | 0.43 | 0.41 | 0.59 | 0.60 | 0.07 | 0.59 | 0.56 | 0.43 |
| FeO* | 2.82 | 2.02 | 1.87 | 1.93 | 1.89 | 1.93 | 1.95 | 1.76 | 1.80 | 1.70 | 1.26 | 1.89 | 1.96 | 1.96 |
| MnO | 0.09 | 0.07 | 0.05 | 0.07 | 0.07 | 0.05 | 0.08 | 0.06 | 0.06 | 0.05 | 0.04 | 0.13 | 0.06 | 0.05 |
| MgO | 20.34 | 18.05 | 18.00 | 17.89 | 17.93 | 17.91 | 18.21 | 17.78 | 17.87 | 17.55 | 17.75 | 18.22 | 18.06 | 17.75 |
| CaO | 21.02 | 24.56 | 23.95 | 24.15 | 24.20 | 23.90 | 24.67 | 23.95 | 24.49 | 24.19 | 25.47 | 24.56 | 23.25 | 24.30 |
| Na2O | 0.13 | 0.05 | 0.05 | 0.05 | 0.06 | 0.13 | 0.05 | 0.07 | 0.05 | 0.17 | 0.00 | 0.07 | 0.11 | 0.16 |
| K2O | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 |
| NiO | 0.02 | 0.06 | 0.07 | 0.06 | 0.05 | 0.03 | 0.05 | 0.04 | 0.05 | 0.05 | 0.07 | 0.11 | 0.06 | 0.05 |
| Total | 99.93 | 100.51 | 100.16 | 100.70 | 99.79 | 98.53 | 100.22 | 99.54 | 101.13 | 100.18 | 99.91 | 100.00 | 99.19 | 100.50 |
| Mg# | 0.928 | 0.941 | 0.945 | 0.943 | 0.942 | 0.943 | 0.943 | 0.947 | 0.947 | 0.948 | 0.962 | 0.945 | 0.943 | 0.942 |
| Rock Suite | LU Rocks | MTZ Rocks | |||||||||||||
| Rock Type | Dunite | Wehrlite | Cpxite | Dunite | Wehrlite | Harz | |||||||||
| Sample No. | 2013MR2-0 | 2013MR3-02 | 2013MR2-02 | 2013MR2-06 | 2013MR2-09 | 2013MR3-01 | 2013MR2-08 | 2013MR2-10 | 2014MR1-01 | 2013-MR1-03 | 2014MR1-06 | 2014MR1-09 | 2014MR1-13 | 2014MR1-16 | 2013MR1-02 |
| No. of Analysis | (N = 4) | (N = 4) | (N = 5) | (N = 4) | (N = 6) | (N = 3) | (N = 6) | (N = 4) | (N = 3) | (N = 1) | (N = 3) | (N = 1) | (N = 2) | (N = 5) | (N = 1) |
| Rb | (<0.040) | (<0.040) | (<0.040) | (<0.040) | 0.072 | (<0.040) | 0.047 | (<0.040) | 0.096 | (<0.040) | (<0.040) | (<0.040) | (<0.040) | (<0.040) | (<0.040) |
| Sr | 3.751 | 3.157 | 3.872 | 2.674 | 2.53 | 3.646 | 2.413 | 2.171 | 1.049 | 0.457 | 1.662 | 0.627 | 1.988 | 2.499 | 4.307 |
| Y | 1.95 | 1.319 | 1.338 | 0.959 | 0.948 | 1.453 | 0.886 | 0.832 | 1.191 | 1.725 | 0.576 | 0.862 | 1.313 | 0.946 | 0.555 |
| Zr | 0.449 | 0.203 | 0.249 | 0.27 | 0.132 | 0.231 | 0.103 | 0.114 | 0.282 | 0.2 | 0.089 | 0.162 | 0.21 | 0.1 | 0.053 |
| Nb | 0.045 | 0.037 | 0.043 | 0.042 | 0.034 | 0.019 | 0.032 | 0.036 | 0.021 | 0.022 | 0.023 | 0.022 | 0.035 | 0.033 | 0.021 |
| Cs | (<0.023) | (<0.023) | (<0.023) | (<0.023) | (<0.023) | (<0.023) | (<0.023) | (<0.023) | (<0.023) | (<0.023) | (<0.023) | (<0.023) | (<0.023) | (<0.023) | (<0.023) |
| Ba | 0.258 | 0.05 | 0.286 | 0.246 | 0.242 | 0.059 | 0.111 | 0.096 | 0.155 | 0.086 | 0.078 | (<0.045) | 0.108 | 0.108 | 0.349 |
| La | 0.014 | 0.011 | 0.017 | 0.008 | 0.008 | 0.012 | 0.007 | 0.007 | 0.009 | 0.006 | 0.006 | 0.006 | 0.018 | 0.005 | (<0.002) |
| Ce | 0.057 | 0.051 | 0.056 | 0.036 | 0.037 | 0.055 | 0.029 | 0.031 | 0.026 | 0.013 | 0.023 | 0.028 | 0.053 | 0.026 | 0.002 |
| Pr | 0.012 | 0.011 | 0.011 | 0.007 | 0.008 | 0.013 | 0.007 | 0.006 | 0.003 | 0.002 | 0.004 | 0.005 | 0.007 | 0.006 | (<0.002) |
| Nd | 0.097 | 0.091 | 0.091 | 0.064 | 0.064 | 0.104 | 0.057 | 0.059 | 0.016 | 0.014 | 0.031 | 0.035 | 0.045 | 0.06 | (<0.011) |
| Sm | 0.07 | 0.074 | 0.063 | 0.046 | 0.048 | 0.081 | 0.045 | 0.044 | (<0.011) | 0.018 | 0.022 | 0.03 | 0.028 | 0.047 | (<0.011) |
| Eu | 0.031 | 0.033 | 0.029 | 0.021 | 0.023 | 0.04 | 0.022 | 0.021 | 0.008 | 0.01 | 0.011 | 0.012 | 0.015 | 0.023 | (<0.006) |
| Gd | 0.15 | 0.14 | 0.147 | 0.11 | 0.107 | 0.157 | 0.098 | 0.093 | 0.031 | 0.089 | 0.056 | 0.067 | 0.091 | 0.1 | 0.017 |
| Tb | 0.034 | 0.03 | 0.03 | 0.023 | 0.023 | 0.034 | 0.022 | 0.019 | 0.009 | 0.028 | 0.012 | 0.015 | 0.023 | 0.022 | 0.005 |
| Dy | 0.297 | 0.235 | 0.235 | 0.18 | 0.172 | 0.276 | 0.165 | 0.163 | 0.152 | 0.265 | 0.104 | 0.151 | 0.238 | 0.174 | 0.069 |
| Ho | 0.072 | 0.052 | 0.053 | 0.038 | 0.038 | 0.058 | 0.036 | 0.033 | 0.049 | 0.073 | 0.022 | 0.031 | 0.055 | 0.04 | 0.023 |
| Er | 0.248 | 0.155 | 0.155 | 0.113 | 0.117 | 0.17 | 0.103 | 0.1 | 0.16 | 0.195 | 0.065 | 0.106 | 0.139 | 0.115 | 0.08 |
| Tm | 0.036 | 0.023 | 0.025 | 0.016 | 0.016 | 0.023 | 0.015 | 0.016 | 0.017 | 0.033 | 0.01 | 0.012 | 0.019 | 0.018 | 0.013 |
| Yb | 0.24 | 0.156 | 0.176 | 0.11 | 0.123 | 0.175 | 0.109 | 0.095 | 0.103 | 0.201 | 0.053 | 0.076 | 0.11 | 0.128 | 0.121 |
| Lu | 0.029 | 0.024 | 0.027 | 0.016 | 0.018 | 0.025 | 0.015 | 0.014 | 0.008 | 0.021 | 0.008 | 0.009 | 0.015 | 0.018 | 0.017 |
| Hf | 0.029 | (<0.026) | (<0.026) | (<0.026) | (<0.026) | (<0.026) | (<0.026) | (<0.026) | (<0.026) | (<0.026) | (<0.026) | (<0.026) | (<0.026) | (<0.026) | (<0.026) |
| Ta | (<0.007) | (<0.007) | 0.009 | (<0.007) | (<0.007) | (<0.007) | (<0.007) | (<0.007) | (<0.007) | (<0.007) | (<0.007) | (<0.007) | (<0.007) | (<0.007) | (<0.007) |
| Pb | 0.119 | 0.061 | 0.594 | 0.181 | 0.201 | 0.051 | 0.164 | 0.189 | 0.648 | 0.126 | 0.068 | 0.113 | (<0.035) | 0.093 | 0.106 |
| Th | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) |
| U | (<0.005) | (<0.005) | 0.013 | 0.016 | 0.011 | (<0.005) | (<0.005) | (<0.005) | 0.008 | (<0.005) | (<0.005) | (<0.005) | 0.006 | (<0.005) | (<0.005) |
| Rock Suite | Mantle Rocks | ||||||||||||||
| Rock Type | Harz | Dunite | (Dunite part) | Dunite | (Dunite part) | Dunite | Websterite | (Web part) | |||||||
| Sample No. | 2014MR2-16 | 2014MR2-12 | 2013MR7-01 | 2014MR2-03 | 2014MR2-01 | 2013MR4-04 | 2013MR8-01 | 2014MR2-14 | 2014MR2-11 | 2014MR2-07 | 2013MR5-01 | 2013MR4-05 | 2013MR4-03 | 2014MR2-10 | 2014MR2-07 |
| No. of Analysis | (N = 2) | (N = 2) | (N = 4) | (N = 2) | (N = 1) | (N = 1) | (N = 3) | (N = 3) | (N = 3) | (N = 1) | (N = 1) | (N = 2) | (N = 3) | (N = 4) | (N = 2) |
| Rb | (<0.040) | (<0.040) | (<0.040) | (<0.040) | (<0.040) | (<0.040) | (<0.040) | 0.679 | (<0.040) | 0.049 | (<0.040) | (<0.040) | (<0.040) | (<0.040) | (<0.040) |
| Sr | 1.225 | 1.273 | 1.123 | 0.972 | 1.517 | 1.81 | 0.637 | 9.896 | 25.548 | 5.184 | 17.423 | 7.334 | 3.413 | 8.971 | 8.09 |
| Y | 0.156 | 0.438 | 0.282 | 0.191 | 0.549 | 0.515 | 0.466 | 2.811 | 3.84 | 1.935 | 3.57 | 2.192 | 0.86 | 1.033 | 2.523 |
| Zr | 0.083 | 0.055 | 0.045 | 0.045 | 0.043 | 0.102 | 0.053 | 2.979 | 1.722 | 0.328 | 1.486 | 0.551 | 0.084 | 0.315 | 0.522 |
| Nb | 0.03 | 0.036 | 0.035 | 0.031 | 0.03 | 0.036 | 0.027 | 0.247 | 0.062 | 0.04 | 0.056 | 0.045 | 0.015 | 0.039 | 0.058 |
| Cs | (<0.023) | (<0.023) | (<0.023) | (<0.023) | (<0.023) | (<0.023) | (<0.023) | 0.033 | (<0.023) | (<0.023) | (<0.023) | (<0.023) | (<0.023) | (<0.023) | (<0.023) |
| Ba | (<0.045) | (<0.045) | (<0.045) | (<0.045) | (<0.045) | (<0.045) | (<0.045) | 0.613 | 0.109 | 0.04 | (<0.045) | (<0.045) | (<0.045) | 0.079 | (<0.045) |
| La | 0.007 | 0.006 | 0.007 | 0.004 | 0.014 | 0.011 | (<0.002) | 0.14 | 0.189 | 0.016 | 0.192 | 0.051 | 0.012 | 0.045 | 0.05 |
| Ce | 0.023 | 0.019 | 0.019 | 0.013 | 0.036 | 0.04 | 0.006 | 0.319 | 0.589 | 0.073 | 0.494 | 0.148 | 0.035 | 0.114 | 0.167 |
| Pr | 0.004 | 0.002 | (<0.002) | (<0.002) | 0.004 | 0.005 | (<0.002) | 0.033 | 0.073 | 0.011 | 0.057 | 0.018 | 0.005 | 0.016 | 0.026 |
| Nd | 0.017 | 0.011 | (<0.011) | 0.014 | 0.012 | 0.024 | (<0.011) | 0.107 | 0.303 | 0.062 | 0.248 | 0.09 | 0.034 | 0.062 | 0.152 |
| Sm | (<0.011) | (<0.011) | (<0.011) | (<0.011) | (<0.011) | (<0.011) | (<0.011) | 0.03 | 0.105 | 0.043 | 0.073 | 0.061 | 0.019 | 0.024 | 0.082 |
| Eu | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | 0.009 | 0.039 | (<0.006) | 0.028 | 0.027 | 0.009 | 0.011 | 0.039 |
| Gd | (<0.016) | (<0.016) | (<0.016) | 0.017 | 0.018 | 0.027 | (<0.016) | 0.067 | 0.227 | 0.099 | 0.176 | 0.145 | 0.039 | 0.068 | 0.199 |
| Tb | (<0.004) | (<0.004) | (<0.004) | (<0.004) | 0.006 | 0.006 | (<0.004) | 0.03 | 0.057 | 0.025 | 0.049 | 0.037 | 0.01 | 0.016 | 0.046 |
| Dy | 0.021 | 0.042 | 0.027 | 0.026 | 0.052 | 0.057 | 0.048 | 0.401 | 0.564 | 0.274 | 0.507 | 0.342 | 0.112 | 0.161 | 0.42 |
| Ho | 0.008 | 0.016 | 0.01 | 0.008 | 0.022 | 0.021 | 0.017 | 0.123 | 0.145 | 0.075 | 0.145 | 0.089 | 0.033 | 0.04 | 0.1 |
| Er | 0.033 | 0.084 | 0.053 | 0.029 | 0.096 | 0.069 | 0.072 | 0.359 | 0.491 | 0.271 | 0.527 | 0.29 | 0.12 | 0.134 | 0.329 |
| Tm | 0.006 | 0.019 | 0.011 | 0.008 | 0.015 | 0.011 | 0.016 | 0.05 | 0.079 | 0.042 | 0.084 | 0.046 | 0.019 | 0.023 | 0.052 |
| Yb | 0.068 | 0.151 | 0.103 | 0.057 | 0.139 | 0.097 | 0.111 | 0.312 | 0.483 | 0.316 | 0.553 | 0.335 | 0.13 | 0.169 | 0.353 |
| Lu | 0.013 | 0.027 | 0.018 | 0.011 | 0.027 | 0.013 | 0.017 | 0.048 | 0.064 | 0.035 | 0.075 | 0.046 | 0.018 | 0.028 | 0.052 |
| Hf | (<0.026) | (<0.026) | (<0.026) | (<0.026) | (<0.026) | (<0.026) | (<0.026) | 0.079 | 0.073 | 0.019 | 0.057 | (<0.026) | (<0.026) | (<0.026) | (<0.026) |
| Ta | (<0.007) | (<0.007) | (<0.007) | (<0.007) | (<0.007) | (<0.007) | (<0.007) | 0.018 | (<0.007) | (<0.007) | (<0.007) | (<0.007) | (<0.007) | (<0.007) | (<0.007) |
| Pb | 0.062 | 0.066 | 0.052 | 0.121 | 0.044 | 0.089 | 0.124 | 0.383 | 0.081 | 0.037 | 0.045 | 0.06 | 0.043 | 0.523 | 0.065 |
| Th | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | 0.055 | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | 0.006 |
| U | (<0.005) | (<0.005) | (<0.005) | (<0.005) | (<0.005) | (<0.005) | (<0.005) | 0.048 | (<0.005) | (<0.005) | (<0.005) | (<0.005) | (<0.005) | (<0.005) | (<0.005) |
| Rock Suite | LU Rocks | MTZ Rocks | Mantle Rocks | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rock Type | Cpxite | Harz | Harz | |||||||
| Sample No. | 2013MR2-08 | 2013MR2-10 | 2013MR1-02 | 2014MR2-16 | 2014MR2-12 | 2013MR7-01 | 2014MR2-03 | 2014MR2-01 | 2013MR4-04 | 2013MR8-01 |
| No. of Analysis | (N = 1) | (N = 1) | (N = 4) | (N = 3) | (N = 1) | (N = 1) | (N = 3) | (N = 4) | (N = 1) | (N = 3) |
| Rb | 0.077 | 0.017 | 0.034 | 0.033 | 0.014 | 0.054 | (<0.010) | 0.041 | (<0.010) | 0.035 |
| Sr | 0.200 | 0.115 | 3.508 | 0.079 | 0.120 | 0.199 | 0.034 | 0.102 | 0.035 | 0.113 |
| Y | 0.104 | 0.111 | 0.089 | 0.025 | 0.057 | 0.040 | 0.037 | 0.065 | 0.074 | 0.062 |
| Zr | 0.029 | 0.119 | 0.030 | 0.041 | 0.032 | 0.031 | 0.042 | 0.029 | 0.049 | 0.025 |
| Nb | 0.018 | 0.028 | 0.029 | 0.028 | 0.030 | 0.043 | 0.024 | 0.029 | 0.038 | 0.025 |
| Cs | 0.015 | (<0.005) | 0.011 | (<0.005) | (<0.005) | (<0.005) | (<0.005) | (<0.005) | (<0.005) | 0.006 |
| Ba | 0.208 | 0.142 | 0.263 | 0.023 | 0.016 | (<0.010) | 0.019 | 0.014 | (<0.010) | 0.011 |
| La | (<0.001) | 0.003 | 0.001 | 0.002 | (<0.001) | (<0.001) | (<0.001) | (<0.001) | 0.001 | (<0.001) |
| Ce | (<0.001) | 0.005 | 0.002 | 0.004 | (<0.001) | (<0.001) | 0.002 | (<0.001) | 0.001 | (<0.001) |
| Pr | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) |
| Nd | (<0.004) | (<0.004) | (<0.004) | (<0.004) | (<0.004) | (<0.004) | (<0.004) | (<0.004) | (<0.003) | (<0.004) |
| Sm | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) |
| Eu | (<0.002) | (<0.002) | (<0.002) | (<0.002) | (<0.002) | (<0.002) | (<0.002) | (<0.002) | (<0.002) | (<0.002) |
| Gd | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) |
| Tb | (<0.002) | (<0.002) | (<0.002) | (<0.002) | (<0.002) | (<0.002) | (<0.002) | (<0.002) | (<0.002) | (<0.002) |
| Dy | 0.013 | 0.011 | 0.007 | (<0.003) | (<0.003) | (<0.003) | 0.003 | 0.005 | 0.007 | 0.005 |
| Ho | 0.004 | 0.004 | 0.003 | (<0.002) | (<0.001) | (<0.002) | (<0.002) | 0.003 | 0.003 | 0.002 |
| Er | 0.018 | 0.016 | 0.016 | 0.007 | 0.014 | 0.011 | 0.007 | 0.013 | 0.014 | 0.014 |
| Tm | 0.005 | 0.004 | 0.004 | 0.002 | 0.004 | 0.003 | 0.002 | 0.003 | 0.003 | 0.004 |
| Yb | 0.035 | 0.033 | 0.040 | 0.024 | 0.051 | 0.050 | 0.025 | 0.040 | 0.030 | 0.038 |
| Lu | 0.008 | 0.007 | 0.009 | 0.007 | 0.010 | 0.011 | 0.006 | 0.009 | 0.007 | 0.008 |
| Hf | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) | (<0.006) |
| Ta | (<0.002) | 0.004 | (<0.002) | (<0.002) | (<0.002) | (<0.002) | (<0.002) | (<0.002) | (<0.002) | (<0.002) |
| Pb | 0.137 | 0.944 | 0.160 | 0.268 | 0.028 | 0.060 | 0.324 | 0.093 | 0.156 | 0.043 |
| Th | (<0.003) | (<0.003) | (<0.003) | (<0.003) | (<0.003) | (<0.003) | (<0.003) | (<0.003) | (<0.003) | (<0.004) |
| U | (<0.003) | (<0.003) | (<0.003) | (<0.003) | (<0.003) | (<0.003) | (<0.003) | (<0.003) | (<0.003) | (<0.003) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishimaru, S.; Saikawa, Y.; Miura, M.; Parlak, O.; Arai, S. Decoding of Mantle Processes in the Mersin Ophiolite, Turkey, of End-Member Arc Type: Location of the Boninite Magma Generation. Minerals 2018, 8, 464. https://doi.org/10.3390/min8100464
Ishimaru S, Saikawa Y, Miura M, Parlak O, Arai S. Decoding of Mantle Processes in the Mersin Ophiolite, Turkey, of End-Member Arc Type: Location of the Boninite Magma Generation. Minerals. 2018; 8(10):464. https://doi.org/10.3390/min8100464
Chicago/Turabian StyleIshimaru, Satoko, Yuji Saikawa, Makoto Miura, Osman Parlak, and Shoji Arai. 2018. "Decoding of Mantle Processes in the Mersin Ophiolite, Turkey, of End-Member Arc Type: Location of the Boninite Magma Generation" Minerals 8, no. 10: 464. https://doi.org/10.3390/min8100464
APA StyleIshimaru, S., Saikawa, Y., Miura, M., Parlak, O., & Arai, S. (2018). Decoding of Mantle Processes in the Mersin Ophiolite, Turkey, of End-Member Arc Type: Location of the Boninite Magma Generation. Minerals, 8(10), 464. https://doi.org/10.3390/min8100464






