1. Introduction
The main minerals found in the Lower Silesian copper deposits in Poland contain base metals such as copper, zinc, and lead, which are widely dispersed in soils and surface sediments. The literature on copper exploitation areas provides extensive geochemical data on the distribution of these metals in soils surrounding deposits, copper smelters, and old mining and metallurgical plants [
1,
2,
3,
4]. Systematic geochemical studies of soils (including both topsoil and subsoil horizons) have been conducted throughout the entire Lower Silesia region. The earliest research focused on areas near copper smelters [
5] and tailings storage facilities [
6]. Later studies expanded to numerous sites across Lower Silesia [
1,
2,
3,
4,
5,
6]. These investigations consistently show elevated copper concentrations in areas containing Cu-Ag deposits. In the early 2000s [
7], the average metal content in ore from Cu-Ag deposits was approximately 1.8 wt.% Cu, 0.2% Pb, and 0.1% Zn; however, more recent data indicate a decrease in average copper content to around 1.5 wt.% [
8]. Metal concentrations in areas adjacent to the deposits vary spatially and temporally [
9]. The issue of metal concentration is significant not only due to permissible limits for metals in soils and plants [
10,
11] but also to establish the geochemical background of the studied areas. This background largely depends on soil substrate characteristics, grain size, and mineral composition [
9,
12]. High metal contents are often found in fine soil and sediment fractions [
1]. Conversely, in abandoned areas and old tailings storage facilities (TSF), different trends are observed in the chemical fractions [
13], with higher metal concentrations associated primarily with clay minerals [
14,
15,
16]. Understanding which components hold the highest concentrations of base metals is crucial, both for potential metal recovery methods from abandoned mines and heaps [
17] and for addressing environmental issues related to sustainable development and environmental management.
This paper presents an analysis of metal concentrations in soils from the copper-bearing area of Lower Silesia, with a particular focus on copper, lead, and zinc. The main objectives of the study were to assess metal levels in various soil horizons within the copper districts of Lower Silesia and to examine differences in metal content between whole soils and different grain size fractions. Additionally, heavy minerals containing copper, zinc, lead—found in sulfides and carbonate ores—were identified and analyzed, with special attention to components associated with high clay content. The study aimed to track changes in metal concentrations in technosols within tailings storage facilities across the copper-bearing districts, from the start of mining operations to the present day. Furthermore, it sought to determine how copper, lead, and zinc concentrations vary in soils relative to their levels in TSFs in both the old and new copper districts, and how these concentrations change over increasing distances from the TFSs in agricultural and forest soils. Lastly, the study examined the variability of metal content across the selected grain fractions and soil profiles at different depths in agricultural soils, forest soils, and technosols.
2. Geological Background
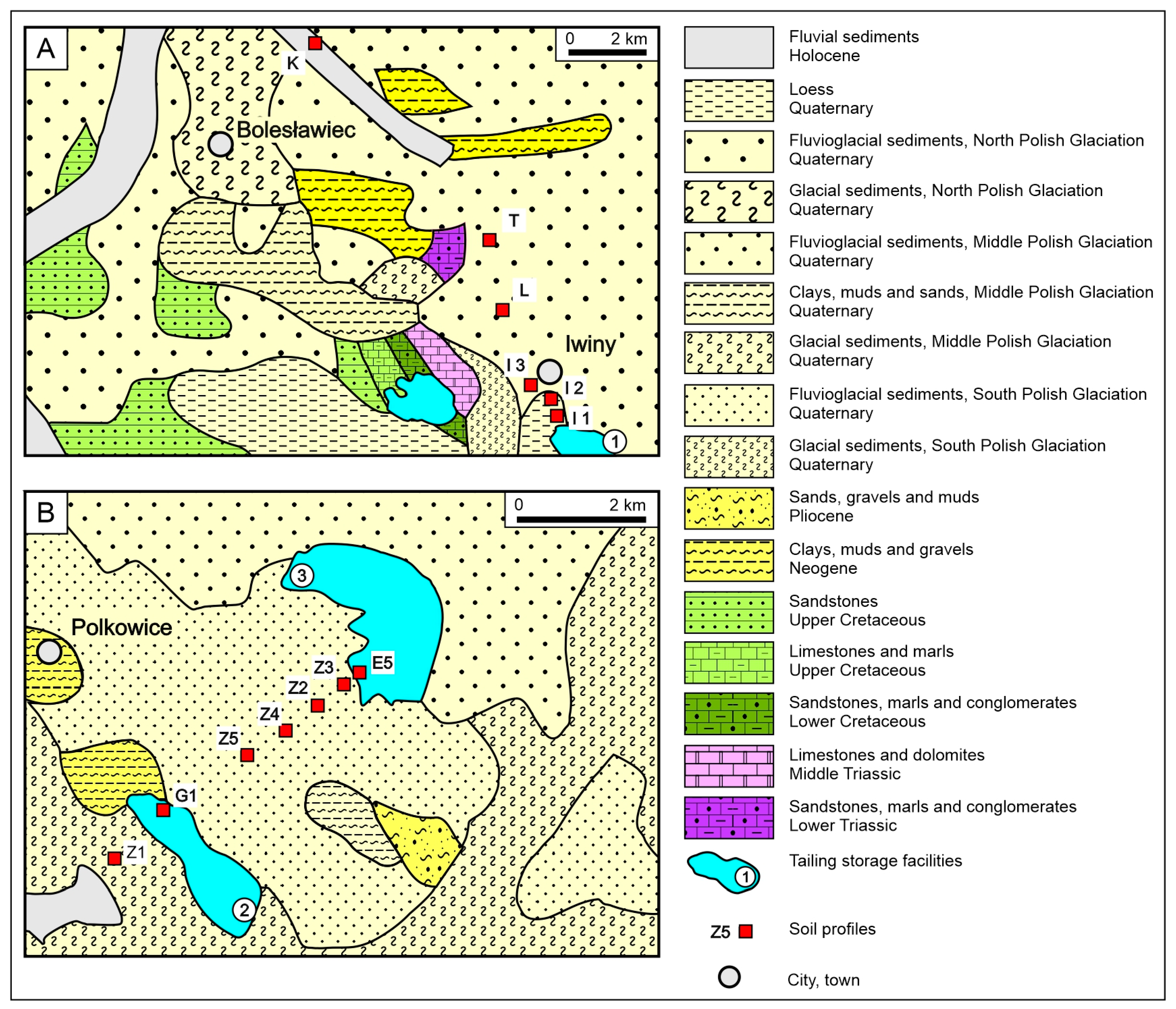
In Lower Silesia, the copper-bearing areas are commonly referred to as the old and new copper districts [
18]. Mining in the old copper district began in the 19th century at the Konrad, Lena, and Nowy Kościół mines [
19]. In 1957, new copper deposits were discovered within the Fore-Sudetic Monocline near the towns of Lubin and Głogów. By 1968, processing plants were established, and mining operations have continued in the new copper district since then [
20], covering various mining areas (
Figure 1).
Copper mineralization of the old copper district was discovered within the Zechstein deposits of the North Sudeten Basin. The deposit occurs in sandstone and Zechstein carbonate-marlstone sedimentary rocks, with a thickness ranging from 1.5 to 2 m [
11,
17,
18,
19,
20]. In this region, the Zechstein sequence includes the following lithostratigraphic units: boundary conglomerate, basal limestone, spotted marls, copper-bearing marls, and lead-bearing marls. Copper ore typically consists of various limestones and marls with loamy-organic admixtures. The deposits in the old copper district are now considered historical. The Konrad deposit, which operated the longest, ceased production in 1989 [
18,
19]. Following its closure, two tailings storage facilities remained in the old district (
Figure 2A). Mineralization occurs in the form of finely dispersed copper sulfides (such as chalcocite, digenite, and covelline), copper–iron sulfides (chalcopyrite and bornite), and lead and zinc sulfides (galena and sphalerite), along with minerals containing nickel, cobalt, and arsenic with silver admixtures [
21,
22]. The distribution of these metals is uneven: copper, often accompanied by silver, dominates in the Zechstein shales, while lead and zinc are more abundant in the carbonate rocks [
23,
24].
Figure 1.
Localization of tailings storage facility (TSF) 1 in the old copper district and 2 and 3 in the new copper district, Lower Silesia (according to [
9]), with the location of the studied agricultural soil and forest soil profiles (according to [
23]). The investigated areas marked (
A) and (
B) are shown in
Figure 2.
Figure 1.
Localization of tailings storage facility (TSF) 1 in the old copper district and 2 and 3 in the new copper district, Lower Silesia (according to [
9]), with the location of the studied agricultural soil and forest soil profiles (according to [
23]). The investigated areas marked (
A) and (
B) are shown in
Figure 2.
The deposit in the new copper district dips monoclinally from south to north, ranging in depth from approximately 300 m in the southern part to about 1500 m in the northern part of the deposit. The mineralized rocks include Permian Rotliegend sandstones, black bituminous shales, and Zechstein dolomites and dolostones. The ores of the new copper basin consist primarily of sulfide and sulfosalt minerals, which are concentrated within three main lithological rock types: sandstones, shales, and limestones or dolomites. Among these, the copper-bearing shale is the most economically important due to its high metal content [
20,
21,
22]. Within the new copper district (
Figure 2B), there is a reclaimed tailings storage facility 2, which was active from 1968 to 1980, and currently operating tailings storage 3, which is undergoing reconstruction and expansion [
7,
25]. Tailings storage 3 is the only active tailings storage facility currently operated by the KGHM Group in Poland. The reconstructed TFS surface area has been enlarged; therefore, the forest soils presented in this study are currently not available for research. It is located in the natural valley of the Rudna River, near the cities of Rudna and Polkowice. The facility’s key parameters are continuously evolving due to the ongoing deposition of waste and capacity expansions. As of now, its volume has reached approximately 800 m
3 [
26]. In the studied deposits, soils have developed on glaciofluvial, fluvial, and postglacial till deposits (
Figure 2A,B).
3. Materials and Methods
Three soil profiles—Lubków, Tomaszów, and Kraśnik, designated as L, T, and K, respectively—were located in agricultural areas near the old copper district (
Figure 2A). Additionally, three profiles (I1–I3) were collected near tailings storage No. 1 in the old copper district. Soil samples from these profiles were collected down to a depth of 1.60 m. Two more profiles were sampled near the reclaimed tailings storage No. 2, and one profile (E5) was taken from sediments deposited in the area of the abandoned tailings storage No. 3 (
Figure 2B). The E5 and G1 profiles were sampled at depths of 50 cm and 100 cm, respectively, with the consent of KGHM SA Polska Miedź. Agricultural and forest soil samples were taken at 20 cm intervals [
8,
23], whereas technogenic soils from the tailings storage facility were taken in 50 intervals. PH was measured following the method described by Mocek [
27]. Carbonate content was determined using the Scheibler method [
28], and organic carbon was quantified by the Tiurin method. Soil organic carbon (SOC) was quantified by carbon oxidation with K
2Cr
2O
7 with H
2SO
4 on a digestion block at 150 °C for 30 min, followed by titration of the excess sulfochromic with FeSO
4 [
29]. Finally, the samples were prepared for chemical analysis. The soil samples were extracted with hydrochloric acid (Merck, Darmstadt, Germany) at a 1:4 ratio at 95 °C ± 5 °C in a Mars 5 Xpress microwave digestion system (CEM, Matthews, NC, USA). Concentrations of the extracted elements were determined using atomic absorption spectrophotometry (AAS) at the Faculty of Geographical and Geological Sciences, Adam Mickiewicz University.
The concentrations of Cu, Pb, and Zn in the soil samples were determined using acetylene/air flame atomization (F-AAS) employing a fast sequential SpectrAA 280FS spectrometer manufactured by Varian (Mulgrave, Victoria, Australia). The optimized instrumental parameters, detection limits, and measurement precision are listed in
Table 1. All the reagents used were of analytical grade. Ultrapure water with a resistivity of 18.2 MΩ·cm (at 25 °C) was produced using a Direct-Q 3 Ultrapure Water Systems apparatus (Millipore Molsheim, France). Merck calibration solutions (Merck, Darmstadt, Germany) were used to calibrate the apparatus. The analytical acetone by Linde was used as the combustible gas. To verify the accuracy of the measurements, certified reference material SRM 2709 (National Institute of Standards and Technology, 2002) was used [
30]. The results showed a high level of agreement with the reference values.
The research was carried out on agricultural soils from the old copper district, forest soils from the new copper district, and technogenic soils from old (TSF 1) and from new copper districts (TSF 2 and 3).
Soil samples at two depth intervals: 20–40 cm and 100–120 cm, corresponding to the A horizon (soil substrate), were analyzed in the selected grain size fractions. Chemical analyses were also performed on bulk soil samples. In total, 111 analyses were conducted (
Table 2), and the results are presented in
Table 3 and
Tables S1–S4 (Supplementary Materials). Statistical parameters (e.g., maximum and minimum values, median, and standard deviation) relating to the soil chemistry in the new and old copper districts are included in
Table 4.
A granulometric analysis was performed using the dry sieving method with a column of sieves of the following mesh sizes: 2.0, 1.0, 0.5, 0.25, 0.16, 0.10, 0.071, and 0.063 mm. The heavy mineral fraction was separated from the 1.0–0.5 mm and 0.25–0.16 mm grain size classes of the selected samples using sodium polytungstate. The components of the heavy fraction were identified using an optical microscope, Axioplan 2 (Zaiss, Jena, Germany, at the Institute of Geology, Adam Mickiewicz University in Poznań), under transmitted and reflected light. Fractions φ < 2.0 μm and φ < 0.2 μm were obtained using a Polygen-Sigma 4–16S centrifuge. The isolated samples were dried and then subjected to mineralogical qualitative analysis using an Thermo Scientific ARL X’TRA diffractometer at the Institute of Geology, Adam Mickiewicz University in Poznań. Data analysis was performed using the Win XRD v.5 software.
4. Results
4.1. Textural Characteristics of Soils
In the agricultural soils, the finest grain fraction predominated. In contrast, forest soils near the tailings storage 3 were dominated by coarser fractions, particularly the 0.5–0.25 mm class, with fine fractions present only in a few samples. Grain size in the soils of the old basin was dominated by the fraction <0.063, but at a depth of 100–120 cm, the proportion of coarser grains increased.
Technosols from tailings storages 1 and 2 were predominantly composed of silt and silty-clay material. The most abundant grain fractions fell within the 0.071–0.063 mm range. Surface layers were characterized by coarser material, while grain size generally decreased with depth. As a result of ongoing vegetation development, a humus-rich layer had already formed at the surface.
Technosols from tailings storage 3 were primarily composed of fine sands with an admixture of medium sands. Sandy fractions accounted for 15% of the material. The dominant grain sizes were 0.16–0.1 mm (up to 50% by weight) and 0.16–0.25 mm (up to approx. 38%). Some sediments within this dam exhibited a high proportion of particles larger than 0.063 mm (silt fraction). Sand layers occurred irregularly and alternated with dust, but enrichment with the dust fraction was observed at a depth of 100–150 cm. The grain size distribution of the analyzed soils is presented in
Table S4 in Supplementary Materials.
4.2. Physicochemical Characteristics
In all forest soils, carbonates were detected only in the selected samples. The pH values ranged from 5 to 6, and the content of Corg was lower than the total organic matter content. Agricultural soils and technosols contained carbonates in amounts exceeding 5%. Their pH ranged from 7 to 9, and the Corg content did not exceed 1% by weight.
4.3. Geochemical Features
The content of metals in the soils, as presented in
Table 3 and
Table 4 and
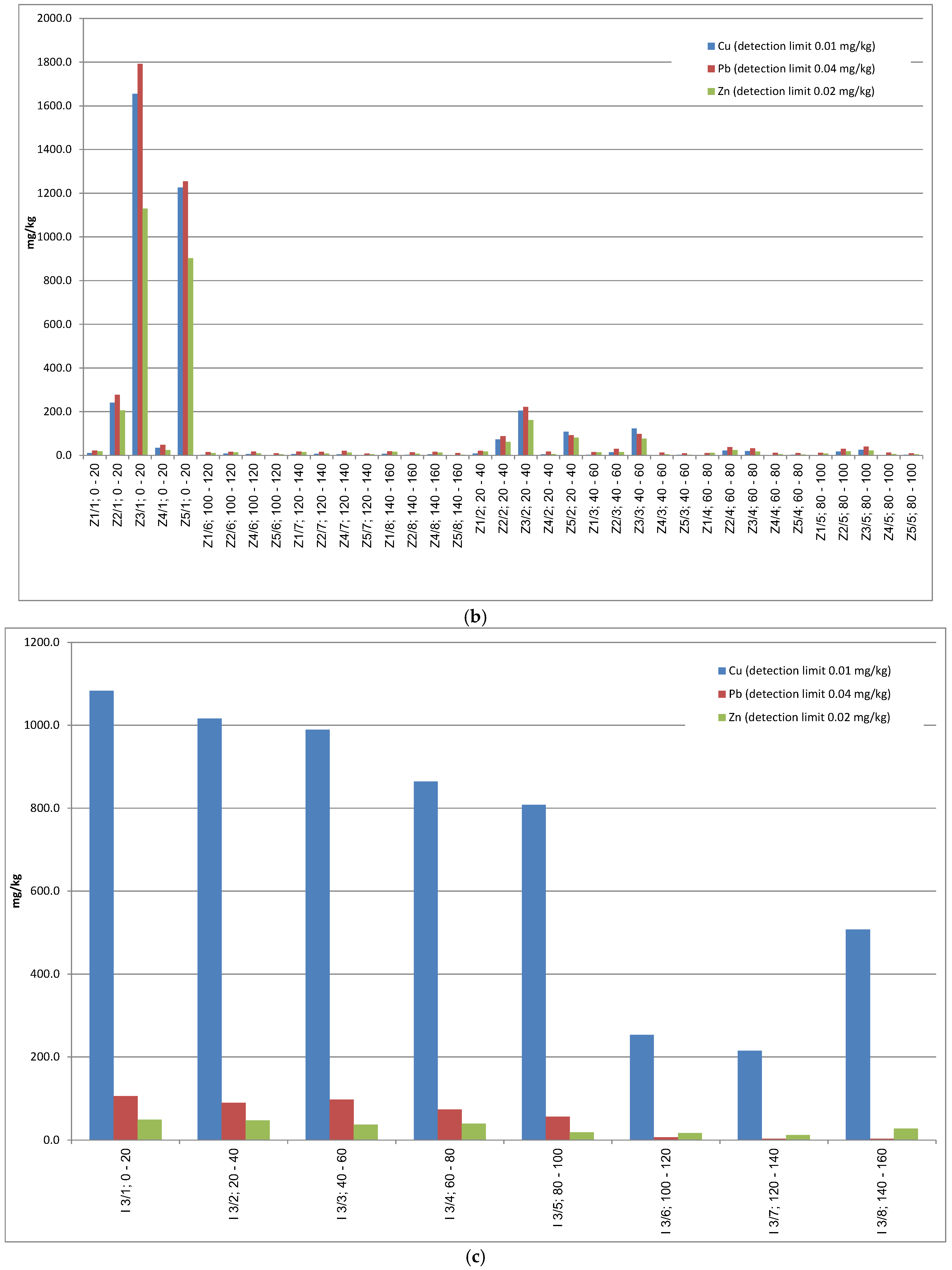
Tables S1–S3 in Supplementary Materials, is highly variable and spans a wide range. In agricultural soils surrounding tailings storage 1, Cu concentrations reached up to 1200 mg/kg and zinc up to 250 mg/kg. Within this tailings storage, copper content was higher, amounting to approximately 2000 mg/kg, while lead and zinc concentrations reached up to 100 and 20 mg/kg, respectively.
In forest soils adjacent to tailings storages 2 and 3, the maximum recorded content concentrations were approximately 1600 mg/kg for Cu, 1200 mg/kg for Pb, and 90 mg/kg for Zn. In technogenic soils from tailings storages 2 and 3, copper concentrations increased up to approx. 11,000 mg/kg, with lead exceeding 289 mg/kg and zinc exceeding 108 mg/kg (
Table 3). An inverse relationship between metal concentration and grain size was observed: higher concentrations of Cu, Pb, and Zn were found in coarser fractions, particularly those greater than 0.16 mm in diameter (
Table S2, Supplementary Materials). Across both study areas, a clear decrease in metal concentrations with soil depth was evident, as well as a consistent decline in metal content in finer grain fractions within surface samples. At depths of 20–40 cm, corresponding to the subsoil zone, metal concentrations are typically lower than those found in the topsoil (
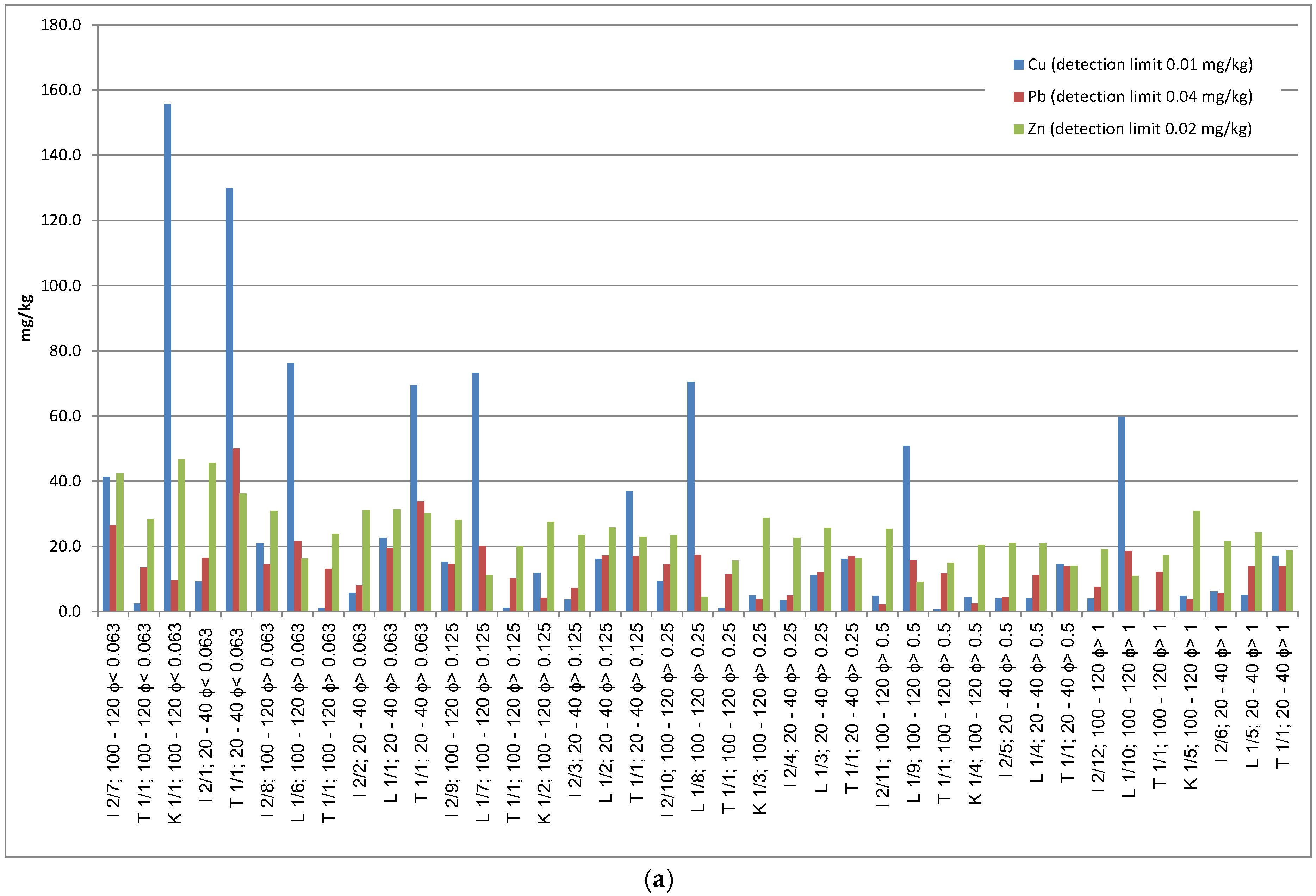
Figure 3a–c).
In soil horizons sampled at a depth of 100–120 cm, the concentration of all the tested metals generally did not exceed several dozen mg/kg. The exception is sample I3, where concentrations reached up to 2000 mg/kg for Cu, 1300 mg/kg for Pb, and 68.5 mg/kg for Zn (
Table S1, Supplementary Materials).
In sample I1, metal concentrations at 100–120 cm were significantly higher than those at 20–40 cm. At a depth of 100–120 cm in the finest grain fraction of <0.063 mm, copper content reached 2856.3 mg/kg, Pb 83.9 mg/kg, and Zn even 68.5 mg/kg—the latter being the highest Zn concentration recorded in this region. In the same grain fraction at 20–40 cm depth, copper content was 371.8 mg/kg, Pb 45.0 mg/kg, and Zn 63.6 mg/kg.
4.4. Results of Mineralogical Studies
The sediments contained minerals such as quartz, feldspar, copper oxides, copper carbonates, fragments of carbonate ore, and ore minerals—primarily chalcopyrite and covellite. Gypsum was also detected in some samples. The coarse fractions contained a higher proportion of metal ores (up to 20%) compared to fine fractions (up to 2%). Besides lithoclasts, isolated chalcopyrites and pyrites were identified within the sandy fraction. The clay fractions were predominantly composed of illite and kaolinite. In tailings storage 1, the light fractions were dominated by quartz and calcite (
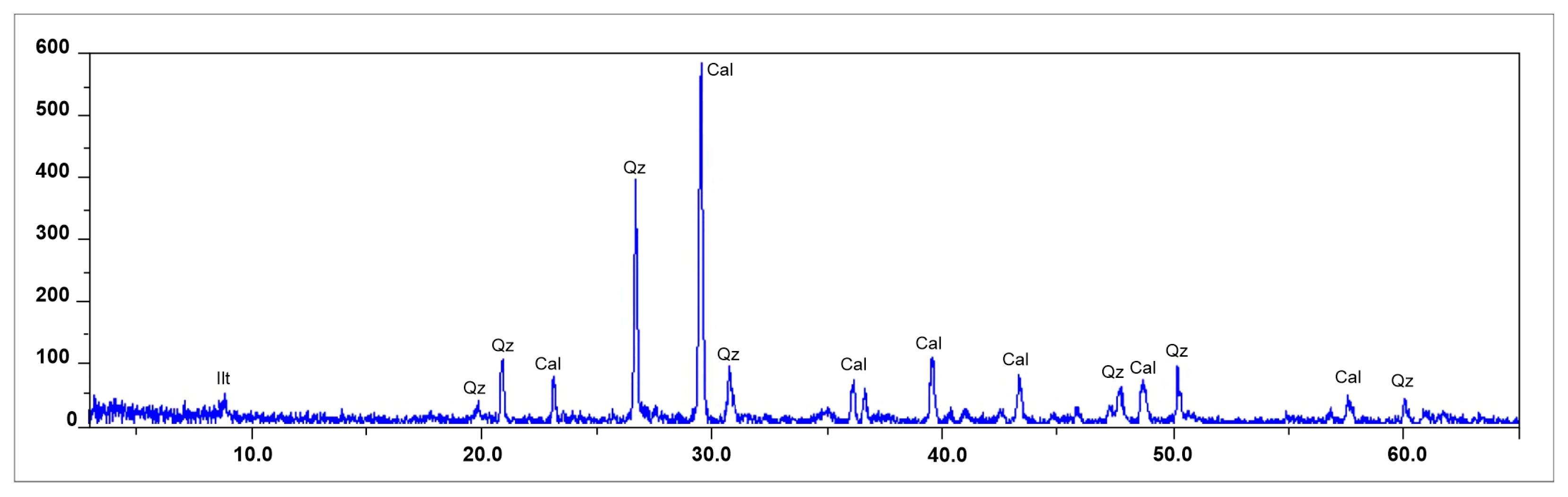
Figure 4) or quartz and dolomite.
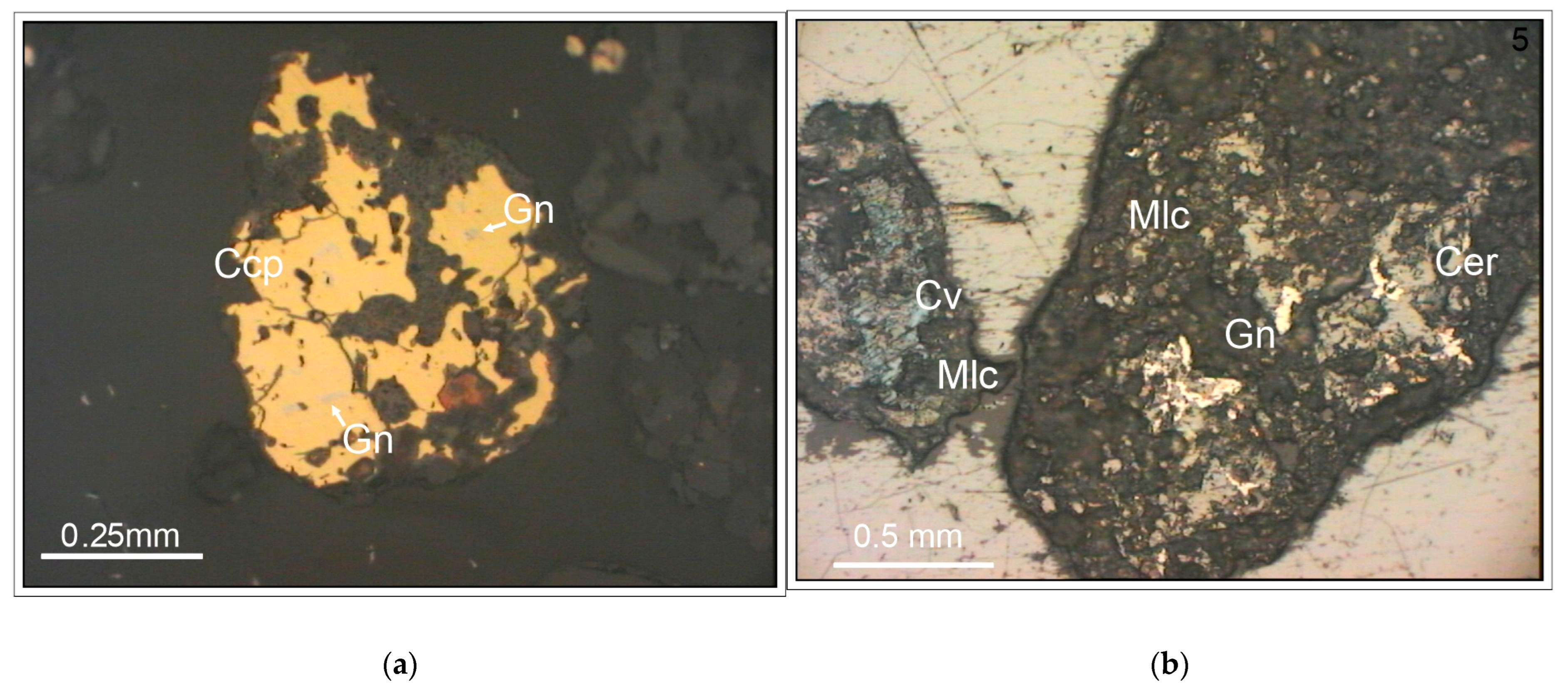
The heavy fraction primarily consisted of lithoclastic carbonate and siltstone rocks. The lithoclasts were generally poorly mineralized with sulfide minerals. Ore grains made up a small portion—up to 5%—mostly consisting of pyrite or marcasite. Additionally, there were individual grains of covelline, chalcocite, digenite, bornite, and polymineral intergrowths of other phases, usually dominated by sulfides from the Cu-Fe-S group. Among the copper minerals, chalcopyrite and malachite (
Figure 5b) were present. Minerals originally associated with copper deposits, such as galena (
Figure 5a,b) and sphalerite, were also found in trace amounts.
In agricultural soils surrounding the old copper district, dolomite—a component of the coarser grain fractions—dominated among the main rock-forming minerals. In contrast, forest soils from the vicinity of tailings storages TFS No. 2 and 3 were primarily composed of quartz and feldspar. In the heavy fraction from both areas, iron sulfides occurred mainly as pyrite (commonly framboidal), and, in some locations, copper and iron sulfides (chalcopyrite) were present but poorly transformed. Copper minerals were primarily represented by malachite. Soils from the new copper basin also contained admixtures of other carbonates, including dolomite and cerussite. The mineral composition of the clay fractions included illite, smectite, and illite–smectite mixture phases. In several surface samples, admixtures of chlorite or mixed-layer phases of chlorite–smectite were also identified.
In the new copper district, dolomites and shales constituted approximately 42 to 58% of the heavy fraction and contained microinclusions of sulfides and Cu, Pb, Zn, and Fe sulfosalts. Among opaque minerals, pyrite was the most abundant, forming the primary component of many grains. It occasionally occurred as individual clusters, accounting for up to 20% of the entire heavy fraction, and was sometimes accompanied by marcasite. The dominant copper sulfides were covellite, digenite, and chalcocite, comprising up to 11% of the heavy fraction. Grains dominated by bornite represented 4–9% by weight, while those dominated by chalcopyrite accounted for 2 to 3% and were typically present as isolated grains. The most common lead mineral—galena—was frequently found in association with cerussite and/or sphalerite, comprising up to 1–2 wt.%. Around galena grains, PbCO3 and PbO were commonly observed, along with malachite, which formed linings around sulfides. The light fraction was predominantly composed of clay minerals, mainly illite and kaolinite.
5. Discussion
Since the onset of copper ore mining in Lower Silesia, significant changes in the concentration of base metals have been observed in surface soil layers. Research has primarily focused on soils in the vicinity of copper smelters, i.e., sources of copper, lead, and zinc emissions as a result of ore processing. In the early years of smelter operation, Roszyk and Roszyk [
10] reported concentrations of 30–100 mg/g Cu and 15–40 mg/kg Pb. In 1995, Cu concentrations reached 2240 ppm [
1], and by 2015, exceeded 3700 mg/kg [
9]. In soils around TFS 3, Cu concentrations ranged 4.5–67 mg/kg [
1]. In the soils successively tested by the monitoring system, the highest copper content—up to 142 mg/kg—was found east of the storage [
2,
31], while in the latest research, Cu ranged 36–337 mg/kg [
32]. Based on the analyses conducted in this study, it was determined that in the surface soil layer (0–20 cm) near the dam, maximum concentrations reached over 1655 mg/kg for Cu, 1792 mg/kg for Pb, and 1130 mg/kg for Zn. Below this layer, metal concentrations decreased several times (
Tables S1 and S3, Supplementary Materials), and in the lower part of the profiles, values decreased even further, reaching as low as 1 mg/kg (
Table S2, Supplementary Materials).
5.1. Forest Soils
In some soil profiles, there was no clear correlation between metal concentration and depth, with concentrations ranging from 1 to 10 mg/kg (see profile G1 in
Table 3 and I3 in
Table S3, Supplementary Materials). However, with increasing distance from the tailings storage, metal concentrations in the uppermost forest soil layers tended to decrease, reaching approx. 35 mg/kg for Cu, 48 mg/kg for Pb, and up to 24 mg/kg for Zn. In the bottom layers of these profiles, the concentrations were significantly lower, typically not exceeding 3 mg/kg, which is primarily attributed to the increasing proportion of fine fractions <0.063 mm. A notable increase in lead (up to 1800 mg/kg) and zinc (over 1100 mg/kg) concentrations was observed in the surface layer of forest soils and at a depth of 20–40 cm (over 220 mg/kg Pb and over 160 mg/kg Zn) near TSF 3 (profile Z3); Pb is positively correlated with the copper content. It is typical of soils where the highest metal concentration is usually associated with the soil surface and the subsoil horizon [
28]. In the technosols, the lead content exceeded that of copper. However, in the E5 profile, copper consistently dominated over lead (see
Figure 2,
Table 4). This regularity trend has been previously documented in other copper mining regions of Lower Silesia [
1,
2]. This profile exhibited the highest pH value (over 7.8) and relatively elevated contents of organic carbon and organic matter. Furthermore, the Z3 profile was distinguished by a higher share of coarser fractions of 0.25 mm, which is the highest among all the profiles analyzed in this region.
The dominance of lead over copper is likely attributable to its stronger accumulation in surface organic soil layers, particularly when associated with wind-blown material originating from the tailing pond. Alternatively, it may indicate the impact of other emission sources, such as proximity to a paved, frequented road. In profile Z5, where elevated lead concentrations were also observed, the highest proportion of colloidal fractions (<0.05 mm) was recorded, along with equally high concentrations of other metals. Although the grain size distribution in profiles Z1–Z2 and Z3 and Z5 was similar, in all cases, the 0.25–0.5 mm fraction represented the greatest share (
Table S2, Supplementary Materials). The largest share of fine fractions was found in profiles Z2 and Z4 at depths of 20–60 cm, where the concentrations of metals were not high (
Table S4, Supplementary Materials). This indicates that the abundance of fine fractions alone does not directly affect the concentration of metals. Rather, the key factors affecting both mineralogical and chemical composition are the high proportions of fractions coarser than 0.25 mm. Additionally, the distance from the TFS and the relative position of the profile in relation to the forest also influence metal concentrations in the sediments.
In profile Z5, located in agricultural soils at the greatest distance from TFS 3, the metal concentrations were, respectively: from 1 to 10.7 mg/kg for Cu, from 11.2 to 29 for Pb, and 10.0 to 18.7 mg/kg for Zn, i.e., significantly less than the average for soils and below average in sedimentary rocks (
Table S2, Supplementary Materials). As such, these soils are not considered contaminated with copper or lead. The slightly elevated zinc levels appear to correlate with an increased presence of lead and copper. In most of the analyzed profiles, zinc concentrations do not exceed 60 mg/kg and are frequently below a few mg/kg, indicating levels lower than the average for soils. Slightly acidic or neutral pH in forest soils surrounding TFS No. 2 and 3 [
23,
28], on which the humus layer had developed, was related to the remobilization of lead in fragments of organic matter and increased concentration of lead, which also promoted the mobility of zinc.
5.2. Agricultural Soils
In the vicinity of TSF 1, significant variability in metal concentrations was observed, influenced by distance from the storage, soil profile depth, and the proportion of fine fractions. The highest copper concentration, exceeding 2800 mg/kg, was recorded in the I1 profile, located about 100 m from the tailings storage, at depths of 100–120 cm. In contrast, the copper concentration at a depth of 20–40 cm in the same profile was lower. This profile reflects a site affected by a past spill of material from TFS 1 into the adjacent areas [
28], with the upper portion of the soil likely having undergone reclamation. In other profiles located at varying distances from storage 1 (
Table S1, Supplementary Materials), metal concentrations decreased substantially, with copper levels falling to several mg/kg and lead and zinc to a dozen or so mg/kg (T profile). The higher proportion of zinc in this profile may be attributed to the alkaline pH and the presence of carbonates, which favor the natural remobilization of zinc—and likely lead—through the formation of carbonates.
The profiles I1 and I3 contained up to 50% of carbonates by weight. In profiles T, L, and K, an increase in carbonate was observed at the top of profiles T, L, and K. This translates into a slight increase in the pH of the environment (up to 8.2), which correlates with an increase in metal concentration in the sediments ranging from 500 to 1000 mg/kg for Cu, 3.5–106 mg/kg for Pb and 27–49 mg/kg for Zn.
The high copper concentrations were accompanied by significantly lower levels of lead and zinc compared to the forest soils surrounding tailings storage facility 3, where zinc frequently predominates over lead (see
Table S2, Supplementary Materials). At a depth of 100–120 cm, the lead content was consistently several times lower than that of zinc. In profile K, located furthest from TFS 1, the copper content in the <0.063 mm grain fraction at a depth of 100–120 cm exceeded 150 mg/kg, while the zinc and lead contents were 45 mg/kg and 9 mg/kg, respectively.
Given the considerable distance from TFS 1 and the depth of the profile, the concentration of metals in this profile is likely more closely related to the original metal content of the soil or the influence of the city of Bolesław than to pollution from emissions of the copper industry. The profiles from this area exhibit a high proportion of fine-grained particles, reaching up to 90% by weight, while the organic matter content remains relatively low, ranging from 1 to 6%. In addition to carbonates, copper and lead may also be associated with clay minerals (illite and smectite–illite). Notably, the copper content in technogenic soils within the tailings storages (
Figure 3c) is significantly higher than in the surrounding agricultural soils (
Figure 3a,b).
In light of existing legal regulations in Poland, permissible concentrations in soils depend on water permeability [
33]. The requirements for copper, lead, and zinc content in soils are closely related to the requirements for topsoils, and are established separately for agricultural and forest soils [
33]. Limits for Cu were set at 150 and 300 mg/kg, Pb—250 and 500 mg/kg, and for Zn—500 and 1000 mg/kg, for agricultural and forest soils, respectively. The agricultural topsoils in the old copper basin covered by this study meet applicable standards, while forest soils in the new copper basin in the immediate vicinity of the TSF 3 significantly exceed these standards. Guideline values for metals in soils in Europe have also been determined for topsoils as well [
34] and were established for Cu in a range of 150 to 200 mg/kg at level of environmental risk, Zn 250–400, and for Pb 250–750 mg/kg for health and environmental risk. Thresholds were established at 100 mg/kg for Cu, 60 for Pb, and 200 for Zn [
34]. In the studied agricultural and forest soil profiles, these thresholds were exceeded only in topsoils in areas adjacent to TSF 1 and 3.
5.3. Technogenic Soils
Copper, zinc, and lead in surface soils can also occur in the form of sulfide minerals. Among these, chalcopyrite has been identified as the most persistent under soil conditions. It is commonly found in tailings storages 2 and 3 as very small inclusions and growths in rock-forming minerals (
Figure 5 and
Figure 6). In the profiles located in the immediate vicinity of the storages, the heavy fraction of surface sediments also contains traces of Cu and Fe sulfides (chalcopyrite), and more frequently copper carbonates such as malachite, which may serve as sources of copper. Even a single sediment grain containing copper sulfide or carbonate can reveal a very high concentration of copper in the sediment [
6]. Medyńska–Juraszek et al. and Kabała [
2,
31] noted that in forest soils, which primarily function as closed systems, all elements undergo internal cycling with very low metal losses. Additionally, the presence of an organic layer in forest soils slows the leaching of metals. Kabała and Singh [
6] observed that the content of metals near copper smelters, as determined in chemical fractions, depends largely on the total content in the samples. The processes of metal accumulation in the forest soils investigated in this study may be comparable. The variation in copper, lead, and zinc concentrations in other regions of Poland and worldwide is influenced by the geochemical background and the physicochemical conditions of the soils and sediments [
35,
36,
37,
38,
39].
In the tested profiles, pH levels and carbonate content appear to play a key role in influencing metal concentrations. A decrease in pH facilitates the separation of lead and zinc and the migration of zinc. Conversely, an increase in pH, coupled with increased carbonate content, encourages the accumulation of zinc. The most significant increase in metal concentrations in soils is associated with the presence of grain fractions coarser than 0.25 mm. This trend has been observed in technogenic soils in tailings storage facilities and is supported by previous studies of such deposits [
40,
41]. Notably, this pattern also holds true for sediments located in close proximity to tailings storages [
28,
42]. Similar findings were reported by Ciszewski et al. [
43], who observed metal distribution patterns in sediments from the Przemsza River near Kraków, where authigenic sphalerite and pyrite were identified.
The spread of metals is generally confined to zones immediately adjacent to emission sources, as predicted by Kijewski [
1]. The several-kilometre distance of the studied profiles from the TFS limits the influence on the mineralogical and chemical composition of the soils. Nevertheless, compared to the initial period of deposit exploitation, copper and lead concentrations in these areas have increased by over thirtyfold [
1,
2,
10,
28]. The distribution of the studied metals in soils from both the old and new copper districts is limited, and an increase in copper, lead, and zinc concentrations occurs only at depths of 0–20 cm and 20–40 cm. At depths of 100–120 cm, increased metal concentrations are only apparent in profiles near the area where sludge overflowed tailings from TFS 1, particularly within the fine-grained fractions (<0.063 mm). Other profiles at depths of 100–120 cm show low metal concentrations consistent with average levels found in post-glacial sediments, fluvial soils, and glacigenic sediments [
8,
33]. High metal concentrations in soils in copper-mining areas may pose an environmental risk [
44,
45,
46], although these risks can be reduced in forest and agricultural soils by controlling their grain size and mineralogical composition. The main mechanism driving metal dispersion around a tailings storage facility is likely the deflation of dust material from above the facility’s surface. Additionally, elevated metal concentrations in some locations may also be influenced by traffic-related emissions.
6. Conclusions
The concentrations of copper, lead, and zinc in the studied soils are closely correlated. In the old cooper district adjacent to tailings storage 1, concentration ranges from 50 up to 2800 mg/kg for Cu, 1 to 150 mg/kg for Pb, and 3.5 to 65 mg/kg for Zn. In contrast, in the new cooper district adjacent to tailings storages 2 and 3, the ranges are from 3 to 1700 for Cu, 5 to 1800 for Pb, and 1,5 to 700 mg/kg for Zn. This significant variation in metal content is attributed to remobilization processes and differences in the proportion of the finest grain sizes within the soil profiles.
Slight fluctuations in metal concentrations are associated with changes in pH and the presence of carbonates, which lead to an increase in zinc concentration and a decrease in lead content. With increasing distance from the tailings storage and the depths down to 40 cm, the concentrations of copper, zinc, and lead decrease to values below the average for soils, reflecting the geochemical characteristics of the parent rock material.
No clear correlation exists between metal content and grain fraction in soils from the new copper district. However, grain fractions coarser than 0.025 mm contribute to elevated concentrations of copper, lead, and zinc in the studied profiles. Copper, zinc, and lead sulfides were identified within these fractions.
Copper concentration in the sediments shows slight variations depending on the location relative to the emission source and the sampling depth. The highest concentrations—comparable to those found in technogenic soils in the tailings storages 1 and 3—are observed in profiles nearest to the emission source.
Compared to the original metal content in copper ore, concentrations near the tailings storages have increased by several dozen times. However, the impact of emission sources is largely confined to areas immediately adjacent to the landfill dams.
Supplementary Materials
The following supporting information can be downloaded at:
https://www.mdpi.com/article/10.3390/min15090992/s1, Table S1: Concentrations of Cu, Pb, and Zn in agricultural soils profiles in old copper district; measured by the F- AAS method [mg/kg]. The detection limits are shown in
Table 1. Data according to [
23]; Table S2: Concentrations of Cu, Pb, and Zn in forest soils profiles (Z1–Z5) situated near tailing storage facility 3 in new copper district; measured by the F- AAS method [mg/kg]. The detection limits are shown in
Table 1. Data according to [
23]; Table S3: Concentrations of Cu, Pb, and Zn technogenic soils from tailing storage facility 1 in old copper district in selected grain fraction 20–40 and 100–120 cm (in profile I1), without sieving in depths up to 100 cm (samples I3 and IA), measured by F-AAS method [mg/kg]. The detection limits are shown in
Table 1. Data according to [
23]. Table S4: Grain size variation in samples collected from forest soils (Z1–Z5) in the new copper district. The sample numbers according to [
23].
Author Contributions
Conceptualization, A.D.-C. and N.H.; methodology, A.D.-C. and M.S. (Maciej Swęd); software, N.H. and M.S. (Marcin Siepak); validation, M.S. (Marcin Siepak) and A.D.-C.; formal analysis, A.D.-C.; investigation, N.H.; resources, N.H. and M.S. (Maciej Swęd); data curation, N.H. and M.S. (Marcin Siepak); writing—original draft preparation, A.D.-C.; writing—review and editing, A.D.-C. and N.H.; visualization, M.S. (Maciej Swęd); supervision, A.D.-C.; project administration, A.D.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. This study was supported by the Institute of Geology and Faculty of Geography and Geological Sciences, Adam Mickiewicz University in Poznan, Poland.
Data Availability Statement
Data are available through contact with the authors.
Acknowledgments
We acknowledge the valuable support in sampling and data curation to Marcin Zimny and Daniel Zimny, who prepared the materials for experiments as well as interpretation of their data. Michał Kubiak is acknowledged for the support with fine fraction analysis on XRD. Special thanks to Jacek Czernikiewicz for preparing the drawings. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
Natalia Hoska is an employee of Bureau Veritas Porto Portugal. The paper reflects the views of the scientists and not the company.
Abbreviations
The following abbreviations are used in this manuscript:
| TSF | Tailing Storage Facility |
| Cal | Calcite |
| Qz | Quartz |
| Ccp | Chalcopyrite |
| Cv | Covellite |
| Gn | Galena |
| Ilt | Illite |
| Mlc | Malachite |
| Cer | Cerussite |
References
- Kijewski, P. Occurrence of heavy metals in area of central Odra embankment in the zone of the impact of copper industry. Phys. Probl. Hydrometall. 1995, 29, 47–54. [Google Scholar]
- Medyńska-Juraszek, A.; Kabała, C. Heavy metal pollution of forest soils affected by the copper industry. J. Elem. 2012, 17, 441–451. [Google Scholar] [CrossRef]
- Medyńska, A.; Kabała, C.; Chodak, T.; Jezierski, P. Concentration of copper, zinc, lead and cadmium in plants cultivated in the surroundings of Żelazny Most copper ore tailings impoundment. J. Elem. 2009, 14, 729–736. [Google Scholar]
- Lis, J.; Pasieczna, A. Antropogenic soils pollution within the Legnica-Głogów copper district. In: Proceedings of the conference: Valorization of the environment in the areas exposed to long term industrial and mining activities Poland. Pol. Geol. Inst. Bull. Sp. Pap. 2005, 17, 42–48. [Google Scholar]
- Konstantynowicz, E. 1973 Genesis of Permian copper deposits in Poland. Int. Geol. Rev. 1973, 15, 1054–1066. [Google Scholar] [CrossRef]
- Kabała, C.; Singh, B.R. Fractionation mobility of copper lead zinc in soil profiles in the vicinity of a copper smelter. J. Environ. Qual. 2001, 30, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Potysz, A.; Kierczak, J.; Pietranik, A.; Kądziołka, K. Mineralogical, geochemical, and leaching study of historical Cu-slags issued from processing of the Zechstein formation (Old Copper Basin, southwestern Poland). Appl. Geochem. 2018, 98, 22–35. [Google Scholar] [CrossRef]
- Duczmal-Czernikiewicz, A.; Suchan, J. The accumulation of metals in post-flotation waste facilities in Lower Silesia. Pol. Geol. Inst. Bull. 2015, 465, 67–76. [Google Scholar]
- Duczmal-Czernikiewicz, A. Mineralogy and Geochemistry of Post-Floatation Sediments in Old and New Cooper District; Serie: Studies in Geography and Geology; Bogucki Sci. Publisher: Princeton, NJ, USA, 2013; Volume 31, 203p. [Google Scholar]
- Roszyk, E.; Roszyk, S. Effect of copper metallurgy on some soil properties and chemical composition of plants Part I. The second emission year. Roczn. Glebozn. 1975, 26, 277–291. (In Polish) [Google Scholar]
- Kucha, H. Geology, mineralogy and geochemistry of the Kupferschiefer, Poland. In Eurtope’s Major Base Metal Deposits; Kelly, J.G., Andrew, C.J., Ashton, J.H., Boland, M.B., Earls, G., Fuscardi, L., Stanley, G., Eds.; Irish Association for Economic Geology: Dublin, Ireland, 2003; pp. 215–238. [Google Scholar]
- Salminen, R.; Batista, M.J.; Bidovec, M.; Demetriades, A.; De Vivo, B.; De Vos, W.; Duris, M.; Gilucis, A.; Gregorauskiene, V.; Halamic, J.; et al. FOREGS Geochemical Atlas of Europe, Part 1: Methodology and Maps; Salminen, R., Ed.; Geological Survey of Finland: Espoo, Finland, 2005; p. 526p. Available online: http://www.gtk.fi/publ/foregsatlas (accessed on 15 August 2025).
- Niesiobędzka, K. Transfer of Copper, Lead and Zinc in Soil–Grass Ecosystem in Aspect of Soils Properties, in Poland. Bull. Environ. Contam. Toxicol. 2012, 88, 627–633. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001; 425p. [Google Scholar]
- Pasieczna, A. Atlas of Urban Soils Contamination in Poland; Polish Geological Institute: Warszawa, Poland, 2003; p. 83. [Google Scholar]
- Lis, J.; Pasieczna, A. Geochemical anomalies in the area of mining and copper metallurgy (LGOM). Geol. Rev. 1998, 46, 940–944. (In Polish) [Google Scholar]
- Lis, J.; Pasieczna, A.; Bojakowska, I.; Gliwicz, F.; Frankowski, Z.; Pasławski, P.; Popiołek, E.; Sokołowska, G.; Strzelecki, R.; Wołkowicz, S. Geochemical Atlas of Legnica—Głogów Copper District. 1:250 000; Polish Geological Institute Publisher: Warszawa, Poland, 1999; p. 31. [Google Scholar]
- Paździora, J. Old Copper District. In KGHM Polska Miedź SA Monograph; Piestrzyński, A., Ed.; Cuprum Publisher: Wrocław, Poland, 2008; pp. 15–25. (In Polish) [Google Scholar]
- Banaszak, A.; Leszczynski, R. History, resources and documentation of the Polish Copper Deposits on the Fore-Sudetic Monocline. Pol. Geol. Inst. Bullet. 2007, 423, 43–48. [Google Scholar]
- Mikulski, S.Z.; Oszczepalski, S.; Sadłowska, K.; Chmielewski, A.; Małek, R. Trace Element Distributions in the Zn-Pb (Mississippi Valley-Type) and Cu-Ag (Kupferschiefer) Sediment-Hosted Deposits in Poland. Minerals 2020, 10, 75. [Google Scholar] [CrossRef]
- Oszczepalski, S.; Speczik, S.; Zieliński, K.; Chmielewski, A. The Kupferschiefer Deposits and Prospects in SW Poland: Past, Present and Future. Minerals 2019, 9, 592. [Google Scholar] [CrossRef]
- Oszczepalski, S. Origin of the Kupferschiefer polymetallic mineralization in Poland. Miner. Depos. 1999, 34, 599–613. [Google Scholar] [CrossRef]
- Duczmal-Czernikiewicz, A.; Hoska, N.; Zimny, M.; Zimny, D. Copper, nickel, lead and zinc in the zones affected by exploitation of copper deposits in the Lower Silesia. Prz. Geol. 2019, 67, 154–155. [Google Scholar] [CrossRef]
- Salamon, W. Precious metals in black shale on the Fore-sudetic Monocline (in Polish: Metale szlachetne w czarnych łupkach Monokliny Przedsudeckiej). Rudy Met. Nieżelazne 1976, 21, 472–477. [Google Scholar]
- Jamiolkowski, M.; Masella, A. Geotechnical Characterization of Copper Tailings at Zelazny Most Site. 2015. Available online: https://share.google/VHAjQEUu1hoVMlU1S (accessed on 20 July 2025).
- Truty, A. Dynamic analysis of Zelazny Most tailings dams. A Case Study. Available online: https://www.zsoil.com/publications/Dynamic_analysis_of_Zelazny_Most_tailings_dams.pdf (accessed on 20 July 2025).
- Mocek, A.; Drzymała, S.; Maszner, P. Origin, Analysis and Soils Classification; Poznan University of Technology: Poznan, Poland, 2010; 120p. [Google Scholar]
- Duczmal-Czernikiewicz, A.; Diatta, J.; Rachwał, L. Mineralogical composition of post-flotation copper ore wastes and the possibility of their agricultural use. Pol. Geol. Inst. Bull. 2012, 448, 371–380. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis Part 3: Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; pp. 996–998. [Google Scholar]
- National Institute of Standards and Technology, 2002. Available online: https://www.nist.gov/srm (accessed on 20 July 2025).
- Kabała, C. (Ed.) Soils of Lower Silesia: Origins, Diversity and Protection, Monograph; Polish Society of Soil Science, Wrocław Branch, Ultima-Druk: Wrocław, Poland, 2015; 255p. [Google Scholar]
- Hołtra, A.; Zamorska-Wojdyła, D. The pollution indices of trace elements in soils and plants close to the copper and zinc smelting works in Poland’s Lower Silesia. Environ. Sci. Pollut. Res. 2020, 27, 16086–16099. [Google Scholar] [CrossRef]
- Journal of Laws, Regulation of the Minister of the Environment. 2016. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20160001395 (accessed on 20 July 2025).
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Lin, Z.; Herbert, R.B., Jr. Heavy metals retention in secondary precipitates from mine rock dump and underlying soil, Dalarna, Sweden. Environ. Geol. 1997, 33, 1–12. [Google Scholar] [CrossRef]
- Jordan, G.; D’Alessandro, M. (Eds.) Mining, Mining Waste and Related Environmental Issues: Problems and Solutions in Central and Eastern European Candidate Countries; EUR 20868 EN; Joint Research Centre of the European Commission: Ispra, Italy, 2004; 208p, ISBN 92-894-4935-7. [Google Scholar]
- Sracek, O.; Mihaljevic, M.; Kribek, B.; Majer, V.; Veselovsky, F. Geochemistry and mineralogy of Cu and Co in mine tailings at the Copperbelt, Zambia. J. Afric. Earth Sci. 2010, 57, 14–30. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M.; Dołęgowska, S.; Michalik, A.; Duczmal-Czernikiewicz, A. Geochemical background of potentially toxic trace elements in soils of the historic copper mining area: A case study from Miedzianka Mt., Holy Cross Mountains, south-central Poland. Environ. Earth Sci. 2015, 74, 4586–4605. [Google Scholar] [CrossRef]
- Swęd, M.; Niedzielski, P. Geochemistry and mineralogy of technogenic soils developed on old mine heaps of abandoned iron ore mines in the Ławęczna area (Holy Cross Mountains, south-central Poland). Soil Sci. Annu. 2018, 69, 28–38. [Google Scholar] [CrossRef]
- Karczewska, A.; Kaszubkiewicz, J.; Jezierski, P.; Kabala, C.; Król, K. Copper, lead and cadmium concentration in soils of Legnica smelter protective zone, in years 1982–2005. Soil Sci. Annu. 2010, 61, 45–51. [Google Scholar]
- Lewińska, K.; Duczmal-Czernikiewicz, A.; Karczewska, A.; Dadrach, A. Arsenic Forms in Soils of Various Settings in the Historical Ore Mining and Processing Site of Radzimowice, Western Sudetes. Minerals 2021, 11, 491. [Google Scholar] [CrossRef]
- Duczmal-Czernikiewicz, A.; Baibatsha, A.; Bekbotaeva, A.; Omarova, G.; Baisalova, A. Ore Minerals and Metal Distribution in Tailings of Sediment-Hosted Stratiform Copper Deposits from Poland and Kazakhstan. Minerals 2021, 11, 752. [Google Scholar] [CrossRef]
- Ciszewski, D.; Kucha, H.; Skwarczek, M. Authigenic minerals and sediments in the hyporheic zone of Biala Przemsza River polluted by metal ore mining. Prz. Geol. 2017, 65, 650–660. [Google Scholar]
- Kashyap, R.; Sharma, R.; Uniyal, S.K. Distribution of heavy metals in habitation land-use soils with high ecological risk in urban and peri-urban areas. Intern. J. Environ. Sci. Technol. 2019, 16, 8093–8106. [Google Scholar] [CrossRef]
- Jönsson, A.; The Report Approved. Ni, Cu, Zn, Cd and Pb in Sediments in the City-Centre of Stockholm, Sweden Origins, Deposition Rates and Bio-Availability, Report; Swedish Environment Research Institute: Stockholm, Sweden, 2013; 56p. [Google Scholar]
- Hudson-Edwards, K.A.; Kemp, D.; Torres-Cruz, L.A.; Macklin, M.G.; Brewer, P.A.; Owen, J.R.; Franks, D.M.; Marquis, E. Tailings storage facilities, failures and disaster risk. Nat. Rev. Earth Environ. 2024, 5, 612–630. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).