Abstract
Multiple ironstone beds formed during the Callovian-Oxfordian times as a consequence of intense continental weathering, upwelling, and hydrothermal activity. This study examines the compositional differences between core and rim, and the origin of iron ooids along the northwestern margin of the Neo-Tethys Ocean to highlight sea-level fluctuations, redox conditions, and elemental influx. An integrated sedimentological study, including petrography, mineralogy, micro-texture, and mineral chemistry, was carried out to explain the origin and implications of ironstones. The ~14 m thick Callovian-Oxfordian, marginal marine deposits in the Kutch Basin, in western India, exhibit iron ooids, predominantly formed in oolitic shoals during transgression, associated with lagoonal siliciclastics. Callovian shoals interbedded with lagoonal facies record minor sea-level fluctuations, whereas the Oxfordian deposit records a major transgression and condensation, resulting in extensive ironstone deposits. The ooid cortices and nuclei exhibit distinctive mineralogy and micro-textures: glauconitic smectite exhibits poorly-developed rosettes, chamosite displays flower-like, and goethite shows rod-like features. Three types of ooids are formed: (i) monomineralic ooids are entirely of chamosite or goethite, (ii) quartz-nucleated ooids, and (iii) composite ooids with either chamosite core and goethite rim, or chamosite core and glauconitic smectite rim. The assemblages within iron ooids reflect variation in depositional redox conditions: glauconitic smectite develops under suboxic lagoonal flank, chamosite forms in anoxic central lagoon, and goethite precipitates on oxic shoals.
Keywords:
Neo-Tethys; iron ooids; shallow marine; chamosite; glauconitic smectite; goethite; Callovian; Oxfordian 1. Introduction
Ironstones formed abundantly during the Jurassic period [1,2,3,4,5,6]. The ironstones are significant not only for economic potential but also for reconstructing paleoclimate, sea-level fluctuations, and paleotectonic events [7,8,9]. The ironstones formed abundantly during the Jurassic, with rich deposits forming along the boundary of the Indian plate and in the Neo-Tethyan domain [2,10,11,12,13]. Ironstones are widespread throughout the Neo-Tethys Ocean, which began opening during the Permian at the eastern margin of Gondwana [14]. The mineralogy of ironstones varies depending on the depositional conditions. Iron ooid is a common constituent in most ironstones. Iron ooids are made up of an admixture of iron silicates and iron oxide, including glauconite, Fe-illite, Fe-smectite, odinite, chamosite, berthierine, chlorite, goethite, hematite, and siderite [15,16]. Iron-rich minerals can form either by altering substrates such as fecal pellets, quartz, and feldspar or by direct precipitation at the sediment-ocean water interface [17,18,19,20,21,22,23,24,25,26,27]. Iron ooids form in diverse depositional settings, including nearshore, shelf, slope, and, less commonly, in lacustrine, lagoonal, deltaic, or deeper marine settings [1,28,29,30,31,32,33]. Iron ooids forming in different depositional conditions show wide variations in mineralogy [2,34,35,36,37,38]. However, in most ironstone deposits, the mineralogy of the iron ooids is consistent from the core to rim [34,35,38,39,40]. Iron ooids may show diverse micro-textures, which may be inherited from the subtle fluctuations in depositional conditions. However, the exact reasons behind the variety of micro-textures in iron ooids remain poorly understood. Further, the origin of the complex iron ooids, with mineralogical contrast between the core and rim, needs proper explanation.
Iron ooids are abundant in the Neo-Tethyan basins along the north-western margin of India, circumscribing Kutch, Jaisalmer, Indus, and Himalayan basins [11,41]. The Callovian-Oxfordian succession of the Kutch basin hosts a series of iron-rich beds, varying in thickness from 0.4 to 1 m [42,43,44,45]. While previous studies mention the presence of ferruginous ooids within Callovian Golden Oolite in the Kutch Basin [43,44,45,46,47], detailed chemico-mineralogical analysis is lacking for the Oxfordian iron ooids. A comprehensive investigation of mineralogy, micro-texture, and mineral chemistry is necessary in order to understand the origin of the Callovian-Oxfordian iron ooids. The objectives of the paper are to (a) understand the origin of iron ooids, (b) interpret the compositional variation within iron ooids, and (c) relate iron ooid formation to the paleoenvironmental context in the Neo-Tethyan domain. To achieve the objectives, this study combines petrographical, mineralogical, micro-textural, and chemical analyses of the iron ooids.
2. Geological Background
Kutch Basin is a pericratonic rift basin at the western margin of India [48,49,50] (Figure 1a). The rifting began during the Late Triassic with the E-W trending axis, with a southwestward tilting, following the breakup of the Indian plate from Gondwana [49]. The central Neo-Tethys Ocean at the northern margin of India, including Rajasthan, Kutch, and the Himalayan basins (Figure 1b), eventually closed after the collision of the Indian and Eurasian plates [11,51]. The Kutch basin is situated in the northwestern part of Neo-Tethys (Figure 1b). The Jurassic sedimentation in the Kutch Basin took place in Kutch Mainland, Wagad Uplift, and Island Belts like Patcham, Khadir, Bela, and Chorad. The Jurassic succession of the Kutch Mainland comprises the Jhurio, Jumara, and Jhuran formations in ascending stratigraphic order [42,48,52] (Figure 1c). The Aalenian-Bathonian Jhurio formation is composed of thick limestone with intercalations of silty shale and fine sandstone [48] (Figure 1c). The Callovian-Oxfordian Jumara Formation is made up of iron-rich limestone (ironstone), silty shale, and sandstone, while the Kimmeridgian-Tithonian Jhuran formation is mainly dominated by siliciclastic components, comprised mainly of sandstone, siltstone, and shale [48] (Figure 1c).
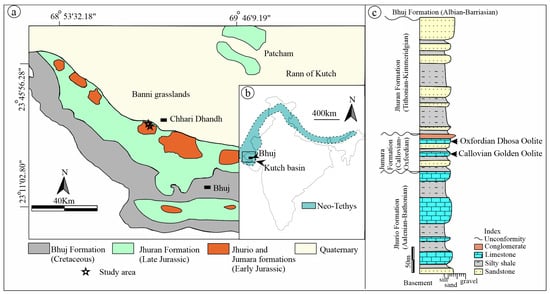
Figure 1.
Geological map showing outcrop of Mesozoic sediments of the Kutch Basin, map modified after [48] (a). Map of India within the inset showing the extent of the Neo-Tethyan ocean [11] (b). Composite log of the study area with relevant stratigraphic elaborations (c) [48,49,50]. Litholog showing the ironstone intervals in the Jumara Formation with relevant stratigraphic details (arrows marking Oxfordian Dhosa Oolite and Callovian Golden Oolite) (c).
This study focuses on the ironstone deposition at two intervals of the Callovian-Oxfordian Jumara Formation of the Kutch Mainland (Figure 1c). Two distinct ironstone deposits occur in the formation as Callovian ironstone and Oxfordian ironstone (Figure 1c). The Callovian ironstone bed is bounded by shale at the bottom and the top, whereas the Oxfordian ironstone is bounded by sandstone at the base and conglomerate at the top (Figure 1c). The Callovian ironstone bed is locally referred to as Golden Oolite because of its color (Figure 1b) [48], while the Oxfordian ironstone bed is known as Dhosa Oolite (Figure 1c) [43,44,48,49,52]. The 1–2 m thick Dhosa oolite, which is widespread across the entire Kutch mainland, represents a condensed horizon [43,44]. Foraminifera and ammonite biostratigraphy indicate the Callovian age for the basal part of the Jumara Formation [52,53,54], and ammonite biostratigraphy indicates Oxfordian age for the Dhosa Oolite [42,52].
3. Samples and Methods
Sample collections: Fieldwork was conducted in the Kutch mainland at Keera dome, exposing the Jumara Formation (23°34′45.39″ N, 69°14′29.66″ E to 23°35′5.36″ N, 69°14′43.46″ E). Lithologs were prepared, sample positions were marked, and samples were collected from the measured sections (Figure 2).
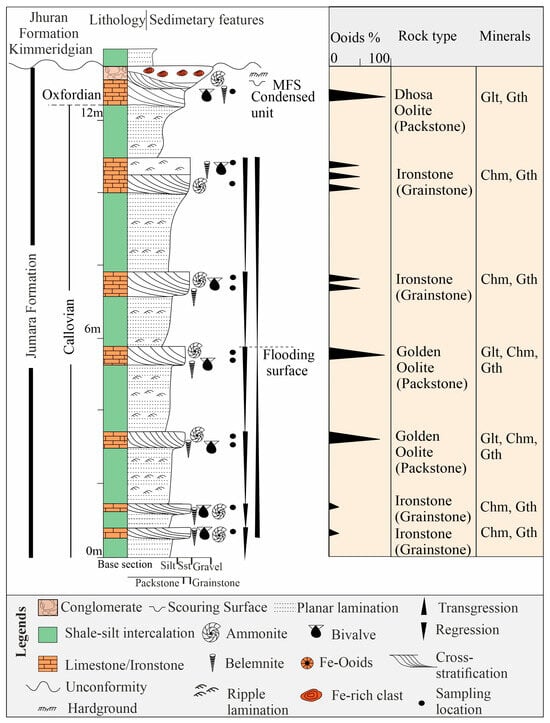
Figure 2.
Litholog of the Callovian-Oxfordian deposits of the Keera section in the Kutch Basin, India, showing sedimentary structures, invertebrate fossils, and ironstone distribution with mineralogy and oolite content. Oolites show maximum concentration near the major flooding surfaces in Golden Oolite and Dhosa Oolite beds (MFS). Note the m-scale shoaling up cycles of lagoon-bar deposits (indicated by small inverted triangles) and transgressive-regressive cycles. Mineral abbreviations: Glt-Glauconitic smectite, Chm-Chamosite, Gth-Goethite.
Analytical procedure: Thin sections were prepared and examined using a Leica DM 4500P polarizing microscope from Leica (Wetzlar, Germany), at the Department of Earth Sciences, Indian Institute of Technology (IIT) Bombay. X-Ray Diffraction (XRD) analysis was performed on seven oolitic ironstone samples and clay-bearing limestone samples. The samples were powdered and sieved using a 230 ASTM (63 µm) sieve for bulk XRD analysis. For carbonate removal, 5 g of the bulk sample was treated with 0.6 N diluted acetic acid in a 1:10 ratio and left for 30 min. Once the carbonate had dissolved, the samples were separated by centrifugation. Clay fractions (<2 µm grain size) were separated by centrifugation after suspension in 15–18 min and sedimentation of coarse, non-clay fraction in a Milli-Q (18 MΩ) water column [55,56]. Clay samples were analyzed as oriented smear-mounted preparations under three conditions: air-dried, glycolated (ethylene glycol solvent for 1 h), and heated at 550 °C [57,58]. Scanning was performed from 4° to 70° with a step size of 0.026° 2θ and a scan speed of 96 s/step using nickel-filtered copper radiation on an Empyrean X-Ray Diffractometer with a Pixel 3D detector from Malvern Panalytical (Almelo, The Netherlands), at IIT Bombay. Clay minerals were identified based on basal (hkl) planes [55] using Panalytical© HighScore™ 5.1a software. Elemental mapping of iron silicates in oolitic ironstone was performed using a Cameca SX Five Electron Probe Micro Analyzer (EPMA) from CAMECA (Gennevilliers, France), with an accelerating voltage of 15 kV, specimen current of 40 nA, and a beam diameter of 1 μm (peak: 10–20 s and background counting: 5–10 s) at the Department of Earth Sciences, IIT Bombay. Natural minerals, including albite (for Na Kα), orthoclase (for K Kα), diopside (for Ca Kα, Mg Kα), apatite (for P Kα), and rhodonite (for Mn Kα), as well as synthetic mineral phases including CaSiO3 (for Si Kα), Fe2O3 (for Fe Kα), and Al2O3 (for Al Kα), were used as standards for the calibration of the major oxide analysis with an analytical error of <1%. For micro-textural analysis, handpicked individual ooids were broken using an agate mortar. Micro-textural analysis and major oxide analysis of the concentric laminae in ooids were carried out using a JSM-7600F Field Emission Gun-Scanning Electron Microscope (FEG-SEM) from JEOL (Tokyo, Japan), under an accelerating voltage of 10 kV at SAIF and the JEOL JSM-IT800 Field Emission Gun Scanning Electron Microscope (FEG-SEM) and Energy Dispersive X-ray Fluorescence (EDXRF) Analysis System from JEOL (Tokyo, Japan), at the Department of Earth Sciences, IIT Bombay, with an analytical error <1%.
4. Results
4.1. Sedimentary Facies and Architecture
The measured sections of the ironstone-bearing Jumara Formation consist of clastic and carbonate deposits, comprising three facies: shale, limestone, and conglomerate (Figure 2). Greenish shale occurs at the basal part of the studied section, and its thickness varies from 0.5 m to 4.6 m. The shale incorporates a few siltstone bands of 2–3 cm thickness (Figure 3a). The thickness of the limestone facies varies from ~0.5 m to ~1.5 m, which occurs on the top of the greenish shale (Figure 3b). The bases of the limestone beds are sharper than their tops (Figure 3b). The limestone beds show cross-stratification internally and are topped by ripple laminae (Figure 3c). Bioclasts, such as ammonite, belemnite, and bivalves, are common constituents of the limestone beds (Figure 2 and Figure 3d). The alternation of shale and limestone forms up to seven cycles in the studied section, varying in thickness from 1 to 4 m (Figure 2). The Oxfordian Dhosa Oolite bed exhibits a sharp erosional contact with the underlying shale facies. The conglomerate facies comprises 20–30 cm thick, randomly oriented, subrounded to angular lime clasts larger than 2 mm, along with bioclasts (Figure 2). A 10–12 cm-thick ferruginous crust marks the top of the conglomerate bed. The conglomerate bed occurs across the onshore Kutch Basin, and is considered a useful marker horizon, known as the Dhosa Conglomerate bed [44]. Ammonites, belemnites, bivalves, and gastropods are the main skeletal components of this conglomerate (Figure 2).

Figure 3.
Field photographs showing shale facies containing thin siltstone beds (marked with arrow) in between (a), facies transition from limestone to shale ((b), hammer length 12 inches), cross-bedded oolitic limestone (c), external mold of an ammonite in Dhosa Oolite ((d); lens length = 21 mm).
The Callovian succession contains six ironstone beds, including two Golden Oolite beds. These beds may be either oolitic limestone or bioclastic limestone. In contrast, the Oxfordian contains a single oolitic ironstone bed known as Dhosa Oolite (Figure 2). The ironstones are mainly dominated by iron ooids. The Callovian limestone beds include packstone and grainstone, whereas the Oxfordian limestone bed is a packstone (Figure 2). The limestone contains abundant iron silicates and iron oxides besides the carbonate allochems.
Packstone: It is grain-supported, consisting of ooids (up to 80% of total allochems), along with peloids, intraclasts, bioclastic fragments, besides micrite and microspar. The ooids may be whole or broken. The entire ooids occur as rounded to elliptical or composite grains. Bioclastic fragments of bivalves, belemnites, diatoms, and echinoids form the nucleus of these ooids. The diameter of spherical ooids varies from 283 to 390 µm, while the long dimension of elliptical ooids ranges from 200 to 560 µm.
Grainstone: The beds consist of oolite, peloid, bioclast, and rarely intraclasts, exhibiting a grain-supported texture, and are devoid of micrite. The ooids are mainly elliptical, with long diameters varying from 100 µm to 500 µm. Besides whole ooids, some broken ooids are also present. The nuclei of the ooids are primarily composed of bioclastic fragments, quartz, and feldspar, and are surrounded by concentric lamellae. Grainstone beds may be dominated either by ooids or by bioclasts.
4.2. Petrography of Ooids
The limestone facies, which include grainstone and packstone, comprise ooids, peloids, and bioclasts, including bivalves, diatoms, ammonites, belemnites, gastropods, micrites, and microspar, along with minor clastic grains such as subangular to subrounded quartz and feldspar. The grainstones are either bioclastic or oolitic, whereas packstones are mainly oolitic. Morphologically, the ooids occur as: (i) composite ooids, showing a larger ooid grain circumscribing 2–3 smaller ooids, (Figure 4a), (ii) superficial ooids, showing a thin concentric rim on bioclast/quartz nuclei (Figure 4b,c), (iii) elliptical to spherical ooids, in which the nuclei are surrounded by several concentric laminae (simple type; Figure 4d). The ooids are predominantly composed of iron silicate and iron oxide. The iron silicates appear as light brown to dark brown under plane-polarized light, and show higher-order interference colour under crossed-polars (Figure 4c,d). Whereas the iron oxide phases are dark red under both plane-polarized light and crossed-polars (Figure 4e). Ooids show variable core and rim mineralogy, with three broad types: (i) quartz-nucleated (Figure 4c). (ii) monomineralic (Figure 4d), and (iii) composite core-rim type. (Figure 4b,e,f). Bioclast nuclei are more commonly observed in monomineralic and composite ooids (Figure 4b,d,e), with partial bioclast relics in the nucleus (Figure 4f). The cortices of most ooids are generally homogeneous.
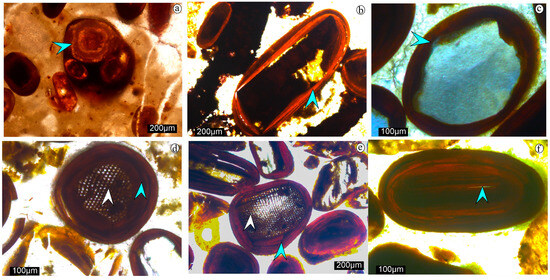
Figure 4.
Photomicrographs showing a composite ooid (marked with blue arrow) (a), superficial ooid (marked with blue arrow) (b), Quartz-nucleated ooid, surrounded by iron silicate (marked with blue arrow) (c), Monomineralic ooid with bioclastnuclei containing iron silicate in the intraparticle pores (marked with white arrow), surrounded by iron silicate (marked with blue arrow) (d), Composite ooids with bioclast nuclei containing iron silicates in the intra-particle pores (marked with white arrow), surrounded by iron oxides (marked with blue arrow) (e), Composite ooids completely altered by iron silicate/oxide, with only relics of carbonate remaining in the nucleus (marked with blue arrow) (f).
4.3. Mineralogy and Micro-Texture
The randomly oriented X-ray diffraction (XRD) of the whole rock sample of the oolitic ironstone exhibits distinct peaks at 4.9 Å, 4.1 Å, 3.8 Å, 3.3 Å, 2.6 Å, and 2.5 Å (Figure 5a). The XRD analysis of decalcified clay separated samples displays well-defined, sharp peaks at ~10 Å, ~7 Å, ~14 Å, and ~13 Å under air-dried conditions. The ~10 Å peak remains unchanged even after glycolation, but the peak intensity increases after heating to 550 °C (Figure 5b). The ~7 Å peak remains unchanged after glycolation but collapses after heating at 550 °C (Figure 5b). The ~14 Å peak shifts to ~15 Å after glycolation (Figure 5c), and after heating, the same peak collapses to ~10 Å, and the ~13 Å peak emerges (Figure 5c). The ~13 Å, ~9 Å, ~7 Å, and ~5 Å peaks remain prominent even after glycolation and heating (Figure 5d). The different mineral assemblages, with their characteristic peaks, and air-drying, glycolation, and heating at 550 °C of the rock samples from different ironstone beds are described in Table 1.
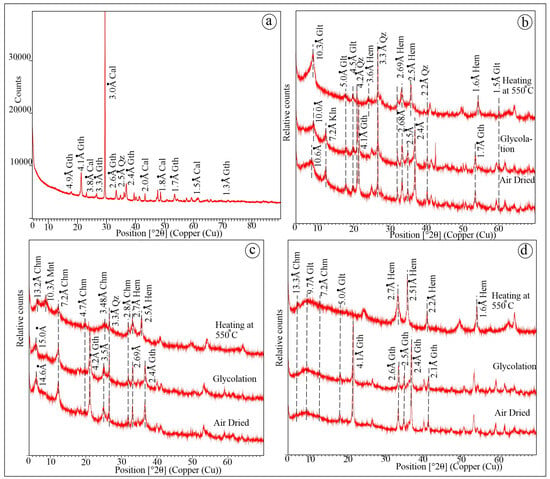
Figure 5.
X-Ray Diffractogram (XRD) of randomly oriented powdered ironstone (a). Decalcified and clay separated XRD of Oxfordian Dhosa Oolite (packstone) (b), Callovian ironstone (grainstone) (c), Callovian Golden Oolite (packstone) (d). Note: Cal-Calcite, Qz-Quartz, Glt-Glauconitic smectite, Chm-Chamosite, Gth-Goethite, Hem-Hematite, Kln-Kaolinite, Mnt-Montmorillonite.

Table 1.
Identified peak positions (in Å) through X-ray diffractogram in bulk samples and decalcified-clay separated samples, air-dried, glycolated, and following heating at 550 °C.
The randomly oriented X-ray diffraction analysis shows strong reflections 3.8 Å (012), 3.0 Å (104), and 2.0 Å (202) with maximum intensity at (104) planes, confirming calcite (Figure 5a) [55] that masked the other mineral peaks. The basal peaks of 10 Å (001), 5.0 Å (002), 4.5 Å (020), and the (001) to (002) reflection intensity ratio are characteristic of glauconite [17,55,59,60,61] (Figure 5b,d). The distance between the (001) and (020) reflections indicates the slightly evolved nature of glauconite [62]. The change in intensity of the (001) basal reflection after heating suggests smectite interstratification in the glauconite [23,60,63]. The prominent 7.2 Å peak, observed in the air-dried and glycolated samples, corresponds to the (001) reflection of kaolinite (Figure 5b), which collapses after heating at 550 °C. The peak shift from 14.6 Å (air-dried) to 15.0 Å after glycolation represents a swelling-type of clay mineral (Figure 5c). However, the appearance of two different diffraction patterns at 13.2 Å and 10.3 Å after heating indicates the presence of two distinct minerals, with 13.2 Å peaks partially masked by the broad peak of the swelling clay (Figure 5c). The presence of the 13.2 Å (001) peak (Figure 5c), along with the other prominent peaks at 7.2 Å (002), 4.7 Å (003), and 3.4 Å (004), throughout air-dried, glycolated, and heated samples, confirms chamosite, with the (002) reflection showing the maximum intensity (Figure 5c,d). The swelling-type of clay with the 14.6 Å peak (air-dried) expands up to 15 Å and then collapses at 10.3 Å after heating, which is identified as montmorillonite [55] (Figure 5c). The (001) reflection of chamosite and montmorillonite overlap at 14.6 Å and 15.0 Å in the air-dried and glycolated samples, and are separated after heating, indicating the presence of two different minerals (Figure 5c). The strong diffraction peaks in the air-dried samples at 4.1 Å (101), 2.68 Å (301), and 2.5 Å (210) indicate the presence of goethite, which remains unchanged after glycolation. However, the shifts in peaks at 3.6 Å (012), 2.69 Å (104), 2.5 Å (110), and 1.6 Å (116) after heating at 550 °C (Figure 5b,d) correspond to hematite, indicating that goethite transforms to hematite upon heating.
The micro-textural analysis using FEG-SEM reveals the concentric cortex encircling the rounded nucleus (Figure 6a). The nucleus of the ooid displays an ill-developed rosette texture (Figure 6b), while the cortex and nucleus of some ooids exhibit a flower-like texture (Figure 6c). Rod-like micro-crystals characterize the cortex and rarely occur in the nucleus (Figure 6d). The poorly-developed rosette features with ~22 wt% Fe2O3T and ~1.5 wt% K2O indicate glauconitic smectite (Figure 6b). The flower-like features showing ~35 wt% Fe2O3T and 0.34 wt% K2O confirm the presence of chamosite (Figure 6c). The rod-like micro-crystals with Fe2O3T content ~70 wt% indicate goethite (Figure 6d).

Figure 6.
SEM-EDS of oolite morphology and cortex showing concentric lamellae (marked with an arrow) (a) rosette-like texture of glauconitic smectite (marked with arrows) (b), flower-like texture of chamosite (marked with arrows) (c), rod-like micro-crystals of goethite (marked with arrows) (d).
4.4. Mineral Chemistry of Iron Ooids
The mineral chemistry of iron ooids indicates the presence of two types of iron silicates along with an iron oxide within their micro-structures (Supplementary Data). The K2O and Fe2O3T (FeO total) contents of the first variety of iron silicate range from 1.0 to 1.72 wt% and from 23.5 to 42.05 wt%, respectively. The K2O content of the second variety of iron silicate is always less than 1 wt%, and Fe2O3T content ranges from 27.79 to 45.64 wt% (av. 37.71 wt%). The iron oxide exhibits a high Fe2O3T content, exceeding 46 wt%. The cross-plot between Fe2O3T and K2O shows three broad clusters: (i) Fe- and K-bearing iron silicate, identified as glauconitic smectite (cf. [21,64]), (ii) high Fe and K-depleted iron silicate, identified as chamosite (cf. [46,65,66]), and (iii) very high Fe2O3T (>46 wt%), identified as goethite (Figure 7; cf. [46]). Petrography and elemental mapping reveal the precipitation of iron silicates within the composite iron ooids (Figure 8). The nucleus of the constituent ooids is calcitic (Figure 8a,b), which shows distinct alteration to Fe-rich, K-depleted iron silicate in places. The rim is made up of the same Fe-rich, K-depleted iron silicate (Figure 8c,f). The interparticle pore spaces of composite ooids contain a Fe- and K-bearing iron silicate (Figure 8). The K-poor, Fe-enriched iron silicate replacing the calcite nucleus is interpreted as chamosite, while the Fe- and K-bearing iron silicate is identified as glauconitic smectite (Figure 8b–f).
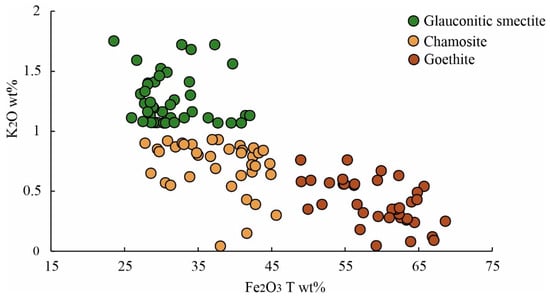
Figure 7.
The cross-plot between Fe2O3T vs. K2O shows three broad clusters corresponding to glauconitic smectite, chamosite, and goethite.
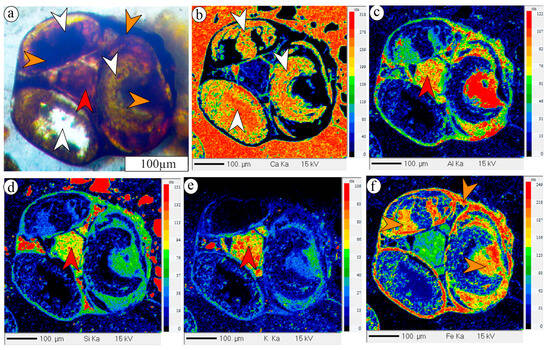
Figure 8.
Photomicrograph of composite ooids containing calcitic nucleus (white arrows) infilled with iron silicates (a). Elemental mapping of the same grain showing compositional variation of Ca (b), Al (c), Si (d), K (e), and Fe (f). Note: Ca-enriched portion indicates calcite. K-depleted, Fe-enriched iron silicate marked with orange arrows marks chamosite. K-enriched variety of iron silicate marked with a red arrow within a composite ooid identified as glauconitic smectite.
SEM-EDS analysis shows distinct core-to-rim mineralogic variation in ooids. Glauconitic smectite is characterized by ~1.5 wt% K2O and ~27 wt % TFe2O3, chamosite by ~0.6 wt % K2O and ~44 wt% TFe2O3, and goethite by high TFe2O3 (~64 wt%). Based on these compositions, three types of ooids are identified: (i) quartz nucleus with chamosite rim (Figure 9a); (ii) monomineralic ooids, including (a) chamosite within the nucleus mantled by chamosite rim (Figure 9b) (b) goethite within the nucleus surrounded by goethite rim (Figure 9c); and (iii) Composite ooids showing core-rim mineralogical contrasts, such as (a) chamosite nucleus, surrounded by goethite rim (Figure 9c), (b) chamosite nucleus surrounded by glauconitic smectite rim (Figure 9d).
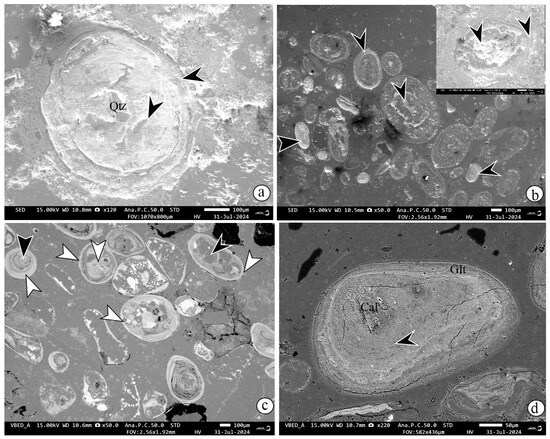
Figure 9.
SEM-EDS images showing three types of ooids. (i) Quartz nucleus surrounded by chamosite rim (mark with black arrow) (a). Monomineralic ooids, where chamosite precipitated in the intraparticle pores of bioclastic nuclei, surrounded by chamositic rim and chamositic peloids (marked with black arrows) (b). Monomineralic goethitic ooids (marked with white arrow) (c). Composite ooids, chamosite in the core (marked with black arrow), and goethite in the rim (marked with white arrow) (c). Composite ooids showing (a) chamosite nucleus (marked with black arrow) with relics of calcite surrounded by glauconitic smectite (d). (Mineral abbreviations: -Qz—quartz, Glt—glauconitic smectite, Cal—calcite).
5. Discussion
5.1. Depositional Environment
The iron-rich Callovian limestones are mainly dominated by oolitic packstone, oolitic grainstone, and bioclastic grainstone. The ooids in the limestone are mostly autochthonous, although broken ooids are also present in minor amounts. The cross-bedded limestone with Fe-ooids, peloids, and bioclasts like bivalves, diatoms, ammonites, belemnites, and gastropods indicates that the limestone beds are primarily deposited as carbonate shoals, above the fair-weather wave base [67] (Figure 10a). Although shoals may be oolitic and bioclastic, the former dominates in the study area. The shale facies alternating with the limestone represent low-energy lagoonal deposits. The studied sections, dominated by shale and limestone, represent a mixed siliciclastic-carbonate succession deposited in a shoal-lagoon complex, which constitutes an integral part of the Neo-Tethys ocean [38,41]. The iron ooids form in carbonate shoals during transgression (Figure 2). The repeated lagoon-to-shoals transition, expressed as periodic coarsening-up hemi-cycles, suggests short-term sea-level oscillation. The relatively higher order facies transition from grainstone to packstone shows a fining upward transgressive phase followed by a coarsening-up regressive phase up to grainstone (indicated by coarsening-up cycles in Figure 2). The abundance of iron ooids increases during transgression, reaching a peak near the flooding surface. The maximum occurrence of iron ooids, along with chamosite and glauconitic smectite, marks the flooding surface at the top of the packstone (Figure 2).
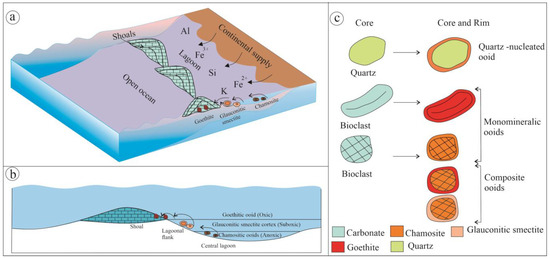
Figure 10.
Depositional settings and formation of iron ooids in the shoal-lagoon system (a). The schematic diagram below shows the cross-section across the shoal-lagoon complex, showing ooid distribution: goethite ooids within the shoals, glauconitic smectite rims forming on the suboxic lagoon flank, and chamosite ooids originating within the lagoon center (b). Schematic diagram showing the distinct mineralogical variation in the core and rim of ooids. Different ooid types are as follows: (i) quartz nucleus with chamosite rim, (ii) bioclasts altering to monomineralic ooid of either chamosite or goethite mineralogy, and chamosite nucleus with rims of either glauconitic smectite or goethite, (ii) composite ooids involving all previously formed ooids (c).
The Oxfordian ironstone bed with a less than 2 m thick deposit has been interpreted as a condensed horizon by the previous workers [43,44,52], forming an oolitic limestone bed that is widespread in the Kutch mainland, indicating a pronounced stratigraphic condensation. The Oxfordian oolitic limestone represents a major transgression in Neo-Tethys that led to the formation of iron ooids, hardgrounds, intraformational conglomerate, and ferruginous crust at the top of the conglomerate bed (Figure 2) [44]. The abundance of iron ooids in the oolitic limestone (Dhosa oolite), along with the presence of glauconitic smectite, reflects deposits corresponding to the Oxfordian maximum flooding surface (MFS). The lag conglomerate at the top of the Dhosa Oolite bed indicates unconformity lying on the top of the Oxfordian deposits.
5.2. Genesis of Iron Ooids and Factors Controlling the Internal Variations
Petrographical and mineralogical observations reveal that the iron ooids are composed of calcite, quartz, feldspar, and iron silicates such as glauconitic smectite and chamosite, and iron oxide such as goethite (Figure 10b). Quartz and feldspar impurities in the iron ooids reflect the influx of siliciclastics from land. Generally, iron ooids are reported from various depositional environments, including deltaic, lagoonal, and shoreface to offshore transition [28,32,68,69]. However, it is less common in fluvial, estuarine, and lacustrine environments [69]. A few studies indicated the mineralogical variation in ooids, with goethite, hematite, berthierine, chamosite, and siderite as the constituent phases [35,38,70]. Goethite, siderite, berthierine/chamosite-bearing iron ooids predominantly form in shoal-lagoon settings [41], whereas hematitic ooids are mainly associated with shoal, and chamosite tends to form in restricted lagoon [38,71]. But in the Kutch basin, goethite, chamosite, and glauconitic-smectite bearing iron ooids are present in the shoal-lagoon complex (Figure 10b,c). Additionally, iron ooids showing mineralogical variation from the core to the rim are rare.
Iron ooids are broadly of three types: (i) monomineralic ooids composed entirely of chamosite or goethite, (ii) quartz-nucleated with a chamositic rim, and (iii) composite ooids with distinct core-rim show mineralogy, such as (a) chamosite core and goethite rim, and (b) chamosite core and glauconitic smectite rim (Figure 10c). Glauconite and glauconitic smectite typically form in suboxic conditions [72,73,74,75,76,77], chamosite in anoxic conditions [72,78], and goethite in oxic conditions [72,79]. The sharp mineralogical contrast between the core and rim of the composite ooid indicates formation in different depositional settings within the shoal-lagoon complex. Petrographical observation further suggests an autochthonous to para-autochthonous origin. The compositional variation of the composite ooids indicates fluctuation in depositional redox conditions [80]. Authigenesis of glauconitic smectite and chamosite occurs at a very early stage of diagenesis, close to the seafloor, but under contrasting environments [19,74,75,81,82]. Glauconitic smectite precipitates at the margin of a lagoon in suboxic conditions. Chamosite primarily forms by replacing calcitic bioclasts, and also as precipitates in the cortex (Figure 10b,c) in anoxic lagoon. Goethite may have formed through two possible pathways: either as an alteration product of iron silicate on the sea floor or as a newly precipitated mineral under oxic conditions. In either case, its formation requires oxic depositional conditions. Goethite can form directly as a product of continental weathering in the marginal marine setting under oxic conditions [83].
5.3. Callovian-Oxfordian Iron Ooids Across the Paleo-Tethys
The maximum number of ironstones corresponds to five significant time intervals in the Jurassic, such as Sinemurian, Pliensbachian, Aalenian, Callovian, and Oxfordian [28], with the Callovian-Oxfordian being the most dominant. Globally, transgression begins in the Early Callovian [84,85]. However, the major transgression took place at the end of the Callovian and the end of the Oxfordian [6]. Globally, the Oxfordian oolitic ironstone, only a few meters thick, represents a condensed section [86]. Within the condensed section, iron ooids are generally enriched in goethite and often reworked [32]. In the Kutch basin, however, iron ooids mainly form during the Callovian-Oxfordian, with the basin experiencing its peak transgression during the Oxfordian. The Oxfordian oolitic ironstone bed is referred to as condensed deposits [43,44]. Callovian-Oxfordian iron ooids mainly form in offshore transition, shoreface, shoal-lagoon systems, where the sedimentation rate remains low [28,32,38,85]. Callovian iron ooids reported from eastern Neo-Tethys from the Zanskar range, Nepal, and Tibet are marked by chamosite formation in reducing conditions on the mid-shelf to outer shelf settings [87,88]. In contrast, the presence of glauconitic smectite, chamosite, and goethite in the northwestern part of the Neo-Tethys reflects fluctuations in sea-level, transitioning through anoxic, suboxic, to oxic conditions in the marginal marine setting. The enhanced chemical weathering in continental areas contributed to an elemental influx into the shallow marine settings, promoting iron silicate authigenesis, under warm and humid climates in the northwestern part of the Neo-Tethys during these periods [89,90]. Additionally, extensive upwelling, hydrothermal activity, and intense continental weathering supply adequate iron for the formation of multiple ironstone beds in Neo-Tethys [46,88].
6. Conclusions
The research work deciphers the formation of iron ooids, depositional conditions, and the development of authigenic iron silicates. The main conclusions of the study are as follows.
- The limestone facies are primarily deposited as bioclastic and oolitic shoals associated with clastic lagoons. The repeated transition from shoal to lagoon reflects sea-level oscillations during the Callovian. The Oxfordian interval records a major transgression and condensation in Neo-Tethys, during which iron ooids and glauconitic smectite become abundant, near the maximum flooding surface, forming a condensed oolitic ironstone horizon. Iron ooids mainly form during transgression at a slow sedimentation rate.
- Iron ooids are composed of a mixture of iron silicates and iron oxide. Iron silicates like glauconitic smectite exhibit poorly-developed rosette micro-texture, while chamosite shows a flower-like micro-texture. The iron oxide goethite reflects a rod-like micro-texture. Based on the internal mineralogy and micro-textural distribution, ooids can be classified into three types (i) monomineralic, composed of either chamosite or goethite, (ii) quartz-nucleated, and (iii) composite ooids characterized by a chamosite core with either goethite or glauconitic smectite rim.
- The distinct compositional contrast between core and rim indicates their formation in different redox conditions. Glauconitic smectite develops in the suboxic settings, chamosite forms in the anoxic conditions, and goethite ooids precipitate under oxic conditions, within the different sub-environments of the bar-lagoon complex.
- The Jurassic ironstones formed in a marginal marine setting, which belonged to the Neo-Tethys Ocean. Intense continental weathering supplies elemental input in the shallow sea, facilitating ironstone formation in a warm, humid climate, which was further promoted by hydrothermal activity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/min15090990/s1. Supplementary Data: Mineral chemistry of glauconitic smectite, chamosite, and goethite from Jumara Formation, Kutch basin, India, presented in wt%, was generated using SEM-EDS.
Author Contributions
A.C.: Writing—review and editing, Writing—original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation. S.B.: Writing—review and editing, Writing—original draft, Visualization, Validation, Supervision, Software, Resources, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. S.A.B.: Primary data collection. S.M.: Primary data collection. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the Indian Institute of Technology (IIT) Bombay and the Birbal Sahni Institute of Palaeosciences for providing technical infrastructure support. Arpita Chakraborty (PMRF ID: 1302641) expresses gratitude to the Ministry of Education, New Delhi, Government of India, for granting the Prime Minister’s Research Fellow (PMRF) scheme. Fieldwork of AC was financially supported by the contingency under the PMRF scheme. SM is thankful to the Director, Birbal Sahni Institute of Palaeosciences, for permitting his field visits for this work. The authors thank the Department of Science and Technology, Government of India, for the FEG-SEM facility at the Department of Earth Sciences, IIT Bombay, through the FIST grant no. SR/FST/ES-II/2019/63. The authors extend their gratitude to S.C. Patel and Javed M. Shaikh for allowing and assisting with mineral analyses through the EPMA, a national facility in the Department of Earth Sciences, IIT Bombay.
Data Availability Statement
The data generation and analysis during the study are presented in the article and the Supplementary file.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| XRD | X-Ray Diffraction |
| EPMA | Electron Probe Micro-Analyzer |
| SEM-EDS | Scanning Electron Microscopy-Energy Dispersive Spectroscopy |
References
- Van Houten, F.B.; Bhattacharyya, D.P. Phanerozoic Oolitic Ironstones- Geological Record and Facies Model. Annu. Rev. Earth Planet. Sci. 1982, 10, 441. [Google Scholar] [CrossRef]
- Young, T.P.; Taylor, W.E.G. Phanerozoic Ironstones; The Geological Society London: London, UK, 1989; ISBN 0903317435. [Google Scholar]
- Aurell, M.; Fernandez-Lopez, S.; Melendez, G. The Middle-Upper Jurassic oolitic Ironstone level in the Tererian Range (Spain) Eustatic Implications. Geobios 1994, 27, 549–561. [Google Scholar] [CrossRef]
- Taylor, K.G.; Macquaker, J.H.S. Spatial and Temporal Distribution of Authigenic Minerals in Continental Shelf Sediments: Implications for Sequence Stratigraphic Analysis. In Marine Authigenesis: From Global to Microbial; SEPM: Broken Arrow, OK, USA, 2000; Volume 66, pp. 309–323. [Google Scholar] [CrossRef]
- Mücke, A.; Farshad, F. Whole-Rock and Mineralogical Composition of Phanerozoic Ooidal Ironstones: Comparison and Differentiation of Types and Subtypes. Ore Geol. Rev. 2005, 26, 227–262. [Google Scholar] [CrossRef]
- Ramajo, J.; Aurell, M. Long-Term Callovian-Oxfordian Sea-Level Changes and Sedimentation in the Iberian Carbonate Platform (Jurassic, Spain): Possible Eustatic Implications. Basin Res. 2008, 20, 163–184. [Google Scholar] [CrossRef]
- Van Houten, F.B. Oolitic Ironstones and Contrasting Ordovician and Jurassic Paleogeography. Geology 1986, 13, 722–724. [Google Scholar] [CrossRef]
- McGregor, F.; Ramanaidou, E.; Wells, M. Phanerozoic Ooidal Ironstone Deposits—Generation of Potential Exploration Targets. Trans. Inst. Min. Metall. Sect. B Appl. Earth Sci. 2010, 119, 60–64. [Google Scholar] [CrossRef]
- Bekker, A.; Planavsky, N.J.; Krapež, B.; Rasmussen, B.; Hofmann, A.; Slack, J.F.; Rouxel, O.J.; Konhauser, K.O. Iron Formations: Their Origins and Implications for Ancient Seawater Chemistry. Treatise Geochem. Second Ed. 2014, 9, 561–628. [Google Scholar] [CrossRef]
- Young, T.P. Ooidal Ironstones from Ordovician Gondwana: A Review. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1992, 99, 321–347. [Google Scholar] [CrossRef]
- Valdiya, K.S. The Making of India Geodynamic Evolution, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 9783319250274. [Google Scholar]
- Van Houten, F.B.; Hou, H.F. Stratigraphic and Palaeogeographic Distribution of Palaeozoic Oolitic Ironstones. Geol. Soc. Mem. 1990, 12, 87–93. [Google Scholar] [CrossRef]
- Todd, S.E.; Pufahl, P.K.; Murphy, J.B.; Taylor, K.G. Sedimentology and Oceanography of Early Ordovician Ironstone, Bell Island, Newfoundland: Ferruginous Seawater and Upwelling in the Rheic Ocean. Sediment. Geol. 2019, 379, 1–15. [Google Scholar] [CrossRef]
- Muttoni, G.; Gaetani, M.; Kent, D.V.; Sciunnach, D.; Angiolini, L.; Berra, F.; Garzanti, E.; Mattei, M.; Zanchi, A. Opening of the Neo-Tethys Ocean and the Pangea B to Pangea A Transformation during the Permian. GeoArabia 2009, 14, 17–48. [Google Scholar] [CrossRef]
- Huggett, J.M. Minerals: Glauconites and Green Clays; Elsevier Inc.: Amsterdam, The Netherlands, 2013; ISBN 9780124095489. [Google Scholar]
- Megwara, J.U.; Aba’a, S.I.; Funtua, I.I. Mineralogical and Geochemical Studies of Ironstones around Koton Karfi, Part of Southern Bida Basin, North Central Nigeria. J. Emerg. Technol. Innov. Res. 2019, 6, 112–135. [Google Scholar]
- Odin, G.S.; Matter, A. De Glauconiarum Origine. Sedimentology 1981, 28, 121–151. [Google Scholar] [CrossRef]
- Van Houten, F.B.; Purucker, M.E. Glauconitic Peloids and Chamositic Ooids—Favorable Factors, Constraints, and Problems. Earth Sci. Rev. 1984, 20, 211–243. [Google Scholar] [CrossRef]
- Tang, D.; Shi, X.; Ma, J.; Jiang, G.; Zhou, X.; Shi, Q. Formation of Shallow-Water Glaucony in Weakly Oxygenated Precambrian Ocean: An Example from the Mesoproterozoic Tieling Formation in North China. Precambrian Res. 2017, 294, 214–229. [Google Scholar] [CrossRef]
- Tounekti, A.; Boukhalfa, K.; Choudhury, T.R.; Soussi, M.; Banerjee, S. Global and Local Factors behind the Authigenesis of Fe-Silicates (Glauconite/Chamosite) in Miocene Strata of Northern Tunisia. J. Afr. Earth Sci. 2021, 184, 104342. [Google Scholar] [CrossRef]
- Banerjee, S.; Bansal, U.; Vilas Thorat, A. A Review on Palaeogeographic Implications and Temporal Variation in Glaucony Composition. J. Palaeogeogr. 2016, 5, 43–71. [Google Scholar] [CrossRef]
- Baldermann, A.; Grathoff, G.H.; Nickel, C. Micromilieu-Controlled Glauconitization in Fecal Pellets at Oker (Central Germany). Clay Miner. 2012, 47, 513–538. [Google Scholar] [CrossRef]
- Baldermann, A.; Warr, L.N.; Grathoff, G.H.; Dietzel, M. The Rate and Mechanism of Deep-Sea Glauconite Formation at the Ivory Coast-Ghana Marginal Ridge. Clays Clay Miner. 2013, 61, 258–276. [Google Scholar] [CrossRef]
- Baldermann, A.; Warr, L.N.; Letofsky-Papst, I.; Mavromatis, V. Substantial Iron Sequestration during Green-Clay Authigenesis in Modern Deep-Sea Sediments. Nat. Geosci. 2015, 8, 885–889. [Google Scholar] [CrossRef]
- Baldermann, A.; Banerjee, S.; Czuppon, G.; Dietzel, M.; Farkaš, J.; Löhr, S.; Moser, U.; Scheiblhofer, E.; Wright, N.M.; Zack, T. Impact of Green Clay Authigenesis on Element Sequestration in Marine Settings. Nat. Commun. 2022, 13, 1527. [Google Scholar] [CrossRef] [PubMed]
- El Albani, A.; Meunier, A.; Fürsich, F. Unusual Occurrence of Glauconite in a Shallow Lagoonal Environment (Lower Cretaceous, Northern Aquitaine Basin, SW France). Terra Nov. 2005, 17, 537–544. [Google Scholar] [CrossRef]
- Huggett, J.; Adetunji, J.; Longstaffe, F.; Wray, D. Mineralogical and Geochemical Characterisation of Warm-Water, Shallow-Marine Glaucony from the Tertiary of the London Basin. Clay Miner. 2017, 52, 25–50. [Google Scholar] [CrossRef]
- Bayer, U. Stratigraphic and Environmental Patterns of Ironstone Deposits. Phaneroz. Ironstones Geol. Soc. Spec. Publ. 1989, 46, 105–117. [Google Scholar] [CrossRef]
- Chan, M.A. Oolitic Ironstone of The Cretaceous Western Interior Seaway, East-Cental Utah. J. Sediment. Res. 1992, 64, 693–705. [Google Scholar]
- Burkhalter, R.M. Ooidal Ironstones and Ferruginous Microbialites: Origin and Relation to Sequence Stratigraphy (Aalenian and Bajocian, Swiss Jura Mountains). Sedimentology 1995, 42, 57–74. [Google Scholar] [CrossRef]
- Macquaker, J.H.S.; Taylor, K.G.; Young, T.P.; Curtis, C.D. Sedimentological and Geochemical Controls on Ooidal Ironstone and “bone-Bed” Formation and Some Comments on Their Sequence-Stratigraphical Significance. Geol. Soc. Spec. Publ. 1996, 103, 97–107. [Google Scholar] [CrossRef]
- Collin, P.Y.; Loreau, J.P.; Courville, P. Depositional Environments and Iron Ooid Formation in Condensed Sections (Callovian-Oxfordian, South-Eastern Paris Basin, France). Sedimentology 2005, 52, 969–985. [Google Scholar] [CrossRef]
- Novoselov, K.A.; Belogub, E.V.; Kotlyarov, V.A.; Filippova, K.A.; Sadykov, S.A. Mineralogical and Geochemical Features of Oolitic Ironstones from the Sinara–Techa Deposit, Kurgan District, Russia. Geol. Ore Depos. 2018, 60, 265–276. [Google Scholar] [CrossRef]
- Rudmin, M.; Mazurov, A.; Banerjee, S. Origin of Ooidal Ironstones in Relation to Warming Events: Cretaceous-Eocene Bakchar Deposit, South-East Western Siberia. Mar. Pet. Geol. 2019, 100, 309–325. [Google Scholar] [CrossRef]
- Rudmin, M.; Banerjee, S.; Maximov, P.; Novoselov, A.; Trubin, Y.; Smirnov, P.; Abersteiner, A.; Tang, D.; Mazurov, A. Origin of Ooids, Peloids and Micro-Oncoids of Marine Ironstone Deposits in Western Siberia (Russia). J. Asian Earth Sci. 2022, 237, 105361. [Google Scholar] [CrossRef]
- Clement, A.M.; Tackett, L.S.; Ritterbush, K.A.; Ibarra, Y. Formation and Stratigraphic Facies Distribution of Early Jurassic Iron Oolite Deposits from West Central Nevada, USA. Sediment. Geol. 2020, 395, 105537. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, M.; Tian, J.; Ren, K.; Wang, B.; Zhao, Z.; Chen, X.; Ettensohn, F.R.; Adatte, T. Microbe-Mediated, Marine Authigenic Formation of Ooidal Chamosite: Insights from Upper Ordovician Carbonates of the South-Western Yangtze Platform (China). Sedimentology 2023, 70, 1655–1678. [Google Scholar] [CrossRef]
- Luan, X.; Sproat, C.D.; Jin, J.; Zhan, R. Depositional Environments, Hematite–Chamosite Differentiation and Origins of Middle Ordovician Iron Ooids in the Upper Yangtze Region, South China. Sedimentology 2024, 71, 2210–2247. [Google Scholar] [CrossRef]
- Rudmin, M.; Banerjee, S.; Abdullayev, E.; Ruban, A.; Filimonenko, E.; Lyapina, E.; Kashapov, R.; Mazurov, A. Ooidal Ironstones in the Meso-Cenozoic Sequences in Western Siberia: Assessment of Formation Processes and Relationship with Regional and Global Earth Processes. J. Palaeogeogr. 2020, 9, 1. [Google Scholar] [CrossRef]
- Belasy, A.E.; Grammer, G.M.; Wanas, H.A.; Anan, T. Facies Architecture and sequence stratigraphy of the Coniacian-Santonian Mixed Siliciclastic Carbonate Matulla Formation, West Central Sinal, Egypt. Geol. Soc. Am. Abstr. Programs 2015, 47, 54. [Google Scholar]
- Saboor, A.; Wang, C.; Li, Y.; Reolid, M.; Jafarian, A.; Xi, C.; Wang, L.; Wang, M. Sedimentology, Geochemistry and Palaeoenvironments of Jurassic Oolitic Ironstones from the Eastern Tethys (Indus Basin, Northwestern Himalayas, Pakistan). Facies 2025, 71, 5. [Google Scholar] [CrossRef]
- Fürsich, F.T.; Oschmann, W.; Jaitly, A.K.; Singh, I.B. Faunal Response to Transgressive-Regressive Cycles: Example from the Jurassic of Western India. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1991, 85, 149–159. [Google Scholar] [CrossRef]
- Fürsich, F.T.; Oschmann, W.; Singh, I.B.; Jaitly, A.K. Hardgrounds, Reworked Concretion Levels and Condensed Horizons in the Jurassic of Western India: Their Significance for Basin Analysis. J.-Geol. Soc. 1992, 149, 313–331. [Google Scholar] [CrossRef]
- Alberti, M.; Fürsich, F.T.; Pandey, D.K. Deciphering Condensed Sequences: A Case Study from the Oxfordian (Upper Jurassic) Dhosa Oolite Member of the Kachchh Basin, Western India. Sedimentology 2013, 60, 574–598. [Google Scholar] [CrossRef]
- Alberti, M.; Fürsich, F.T.; Pandey, D.K.; Ramkumar, M. Stable Isotope Analyses of Belemnites from the Kachchh Basin, Western India: Paleoclimatic Implications for the Middle to Late Jurassic Transition. Facies 2012, 58, 261–278. [Google Scholar] [CrossRef]
- Bansal, U.; Banerjee, S.; Chauhan, G.; Rudmin, M.; Borgohain, D.; Upadhyay, A. Geochemistry of Callovian Ironstone in Kutch and Its Stratigraphic Implications. In Mesozoic Stratigraphy of India; Springer International Publishing: Cham, Switzerland, 2021; pp. 215–239. ISBN 9783030713706. [Google Scholar]
- Fürsich, F.T.; Callomon, J.H.; Pandey, D.K.; Jaitly, A.K. Environments and Faunal Patterns in the Kachchh Rift Basin, Western India, during the Jurassic. Riv. Ital. Paleontol. Stratigr. 2004, 110, 181–190. [Google Scholar]
- Biswas, S.K.; Mahender, K.; Chauhan, G.D. Field Guide Book of Geology of Kutch (Kachchh) Basin, Gujarat, India; Springer: Berlin/Heidelberg, Germany, 2021; ISBN 9783030874698. [Google Scholar]
- Biswas, S.K. Regional Tectonic Framework, Structure and Evolution of the Western Marginal Basins of India. Tectonophysics 1987, 135, 307–327. [Google Scholar] [CrossRef]
- Krishna, J. An Overview of the Mesozoic Stratigraphy of Kachchh and Jaisalmer Basins. J. Palaeontol. Soc. India 1987, 32, 136–149. [Google Scholar] [CrossRef]
- Nerlich, R.; Colli, L.; Ghelichkhan, S.; Schuberth, B.; Bunge, H.P. Constraining Central Neo-Tethys Ocean Reconstructions with Mantle Convection Models. Geophys. Res. Lett. 2016, 43, 9595–9603. [Google Scholar] [CrossRef]
- Krishna, J. The Indian Mesozoic Chronicle; Springer: Singapore, 2017; Volume 10, ISBN 9789811024764. [Google Scholar]
- Talib, A.; Gaur, K.N.; Bhalla, S.N. Callovian-Oxfordian Boundary in Kutch Mainland, India—A Foraminiferal Approach. Rev. Paleobiol. 2007, 26, 625–630. [Google Scholar]
- Talib, A.; Jain, S.; Irshad, R. Integrated Benthic Foraminiferal and Ammonite Biostratigraphy of Middle to Late Jurassic Sediments of Keera Dome, Kachchh, Western India; Scientific Publishers: Jodhpur, India, 2017; pp. 71–81. [Google Scholar]
- Moore, D.M.; Reynolds, R.C., Jr. X-Ray Diffraction and the Identification and Analysis of Clay Minerals; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Singh, P.; Banerjee, S.; Wagh, D.; Pande, K.; Bhattacharya, S. Origin of K-Rich Green Clays within Late Cretaceous Deccan Basalts: A Local K-Depository. Appl. Clay Sci. 2024, 250, 107270. [Google Scholar] [CrossRef]
- Roy Choudhury, T.; Banerjee, S.; Khanolkar, S.; Saraswati, P.K.; Meena, S.S. Glauconite Authigenesis during the Onset of the Paleocene-Eocene Thermal Maximum: A Case Study from the Khuiala Formation in Jaisalmer Basin, India. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 571, 110388. [Google Scholar] [CrossRef]
- Mandal, S.; Roy Choudhury, T.; Das, A.; Sarkar, S.; Banerjee, S. Shallow Marine Glauconitization during the Proterozoic in Response to Intrabasinal Tectonics: A Study from the Proterozoic Lower Bhander Sandstone, Central India. Precambrian Res. 2022, 372, 106596. [Google Scholar] [CrossRef]
- Banerjee, S.; Mondal, S.; Chakraborty, P.P.; Meena, S.S. Distinctive Compositional Characteristics and Evolutionary Trend of Precambrian Glaucony: Example from Bhalukona Formation, Chhattisgarh Basin, India. Precambrian Res. 2015, 271, 33–48. [Google Scholar] [CrossRef]
- Banerjee, S.; Farouk, S.; Nagm, E.; Choudhury, T.R.; Meena, S.S. High Mg-Glauconite in the Campanian Duwi Formation of Abu Tartur Plateau, Egypt and Its Implications. J. Afr. Earth Sci. 2019, 156, 12–25. [Google Scholar] [CrossRef]
- Singh, P.; Banerjee, S.; Choudhury, T.R.; Bhattacharya, S.; Pande, K. Distinguishing Celadonite from Glauconite for Environmental Interpretations: A Review. J. Palaeogeogr. 2023, 12, 179–194. [Google Scholar] [CrossRef]
- Amorosi, A.; Sammartino, I.; Tateo, F. Evolution Patterns of Glaucony Maturity: A Mineralogical and Geochemical Approach. Deep. Res. Part II Top. Stud. Oceanogr. 2007, 54, 1364–1374. [Google Scholar] [CrossRef]
- Thompson, G.R.; Hower, J. The Mineralogy of Glauconite. Clays Clay Miner. 1975, 23, 289–300. [Google Scholar] [CrossRef]
- López-Quirós, A.; Sánchez-Navas, A.; Nieto, F.; Escutia, C. New insights into the nature of glauconite. Am. Mineral. 2020, 105, 674–686. [Google Scholar] [CrossRef]
- Sanità, E.; Di Rosa, M.; Lardeaux, J.M.; Marroni, M.; Tamponi, M.; Lezzerini, M.; Pandolfi, L. Deciphering the Pressure—temperature Path in Low-Grade Metamorphic Rocks by Combining Crystal Chemistry, Thermobarometry and Thermodynamic Modelling: An Example in the Marguareis Massif, Western Ligurian Alps, Italy. Mineral. Mag. 2024, 89, 203–224. [Google Scholar] [CrossRef]
- Sanità, E.; Di Rosa, M.; Lardeaux, J.M.; Marroni, M.; Pandolfi, L. The Moglio-Testico Unit (Ligurian Alps, Italy) as Subducted Metamorphic Oceanic Fragment: Stratigraphic, Structural and Metamorphic Constraints. Minerals 2022, 12, 1343. [Google Scholar] [CrossRef]
- Fürsich, F.T.; Oschma; Pandey, D.K.; Jaitly, A.K.; Singh, I.B.; Liu, C. Paleoecology of the Middle to Lower Upper Jurassic Macofaunas of the Kachchh Basin Western India. J. Palaeontol. Soc. India 2004, 49, 1–26. [Google Scholar] [CrossRef]
- Kimberley, M.M. Origin of the oolitic Iron Formation. J. Sediment. Res. 1979, 49, 111–131. [Google Scholar]
- Van Houten, F.B. Review of Cenozoic Ooidal Ironstones. Sediment. Geol. 1992, 78, 101–110. [Google Scholar] [CrossRef]
- Hallam, A.; Maynard, J.B. The Iron Ores and Associated Sediments of the Chichali Formation (Oxfordian to Valanginian) of the Trans-Indus Salt Range, Pakistan. J. Geol. Soc. Lond. 1987, 144, 107–114. [Google Scholar] [CrossRef]
- Talbot, M.R. Ironstones in the Upper Oxfordian of Southern England. Sedimentology 1974, 21, 433–450. [Google Scholar] [CrossRef]
- Porrenga, D.H. Glauconite and Chamosite as Depth Indicators in the Marine Environment. Mar. Geol. 1967, 5, 495–501. [Google Scholar] [CrossRef]
- Porrenga, D.H. Non-Marine Glauconitic Illite in the Lower Oligocene of Aardebrug, Belgium. Clay Miner. 1968, 7, 421–430. [Google Scholar] [CrossRef]
- Bansal, U.; Pande, K.; Banerjee, S.; Nagendra, R.; Jagadeesan, K.C. The Timing of Oceanic Anoxic Events in the Cretaceous Succession of Cauvery Basin: Constraints from 40 Ar/39 Ar Ages of Glauconite in the Karai Shale Formation. Geol. J. 2019, 54, 308–315. [Google Scholar] [CrossRef]
- López-Quirós, A.; Escutia, C.; Sánchez-Navas, A.; Nieto, F.; Garcia-Casco, A.; Martín-Algarra, A.; Evangelinos, D.; Salabarnada, A. Glaucony Authigenesis, Maturity and Alteration in the Weddell Sea: An Indicator of Paleoenvironmental Conditions before the Onset of Antarctic Glaciation. Sci. Rep. 2019, 9, 13580. [Google Scholar] [CrossRef] [PubMed]
- Tribovillard, N.; Bout-Roumazeilles, V.; Delattre, M.; Ventalon, S.; Abraham, R.; Nzié, O. Syndepositional Glauconite as a Paleoenvironmental Proxy—The Lower Cenomanian Chalk of Cap Blanc Nez (N-France). Chem. Geol. 2021, 584, 120508. [Google Scholar] [CrossRef]
- Tribovillard, N.; Bout-Roumazeilles, V.; Abraham, R.; Ventalon, S.; Delattre, M.; Baudin, F. The Contrasting Origins of Glauconite in the Shallow Marine Environment Highlight This Mineral as a Marker of Paleoenvironmental Conditions. Comptes Rendus-Geosci. 2023, 355, 213–228. [Google Scholar] [CrossRef]
- Tang, D.; Shi, X.; Jiang, G.; Zhou, X.; Shi, Q. Ferruginous Seawater Facilitates the Transformation of Glauconite to Chamosite: An Example from the Mesoproterozoic Xiamaling Formation of North China. Am. Mineral. 2017, 102, 2317–2332. [Google Scholar] [CrossRef]
- Berner, R.A. A New Geochemical Classification Of Sedimentary Environments. J. Sediment. Res. 1981, 51, 359–365. [Google Scholar] [CrossRef]
- Kalinina, N.A.; Rudmin, M.A.; Sherstyukov, M.; Maximov, P.; Kerimov, A.G. Origin of Iron-Rich Minerals, Ooids and Pisoids in the Jurassic Ooidal Ironstones of the Labino-Malkin Region (Caucasus). J. Palaeogeogr. 2024, 13, 475–494. [Google Scholar] [CrossRef]
- Banerjee, S.; Chattoraj, S.L.; Saraswati, P.K.; Dasgupta, S.; Sarkar, U. Substrate Control on Formation and Maturation of Glauconites in the Middle Eocene Harudi Formation, Western Kutch, India. Mar. Pet. Geol. 2012, 30, 144–160. [Google Scholar] [CrossRef]
- Banerjee, S.; Bansal, U.; Pande, K.; Meena, S.S. Compositional Variability of Glauconites within the Upper Cretaceous Karai Shale Formation, Cauvery Basin, India: Implications for Evaluation of Stratigraphic Condensation. Sediment. Geol. 2016, 331, 12–29. [Google Scholar] [CrossRef]
- Baldermann, A.; Dietzel, M.; Mavromatis, V.; Mittermayr, F.; Warr, L.N.; Wemmer, K. The Role of Fe on the Formation and Diagenesis of Interstratified Glauconite-Smectite and Illite-Smectite: A Case Study of Lower Cretaceous Shallow-Water Carbonates. Chem. Geol. 2017, 453, 21–34. [Google Scholar] [CrossRef]
- Wright, J.; Schrader, H.; Holser, W.T. Paleoredox Variations in Ancient Oceans Recorded by Rare Earth Elements in Fossil Apatite. Geochim. Cosmochim. Acta 1987, 51, 631–644. [Google Scholar] [CrossRef]
- Powell, J.H.; Riding, J.B. Stratigraphy, Sedimentology and Structure of the Jurassic (Callovian to Lower Oxfordian) Succession at Castle Hill, Scarborough, North Yorkshire, UK. Proc. Yorksh. Geol. Soc. 2016, 61, 109–133. [Google Scholar] [CrossRef]
- Meléndez, G.; Ramajo, J.; Bello, J.; Page, K.N. Callovian and the Callovian—Oxfordian Transition Sedimentary Record in NE Iberian Chain: Taphonomic Analysis and Palaeogeography. J. Iber. Geol. 2007, 33, 261–282. [Google Scholar]
- Jadoul, F.; Berra, F.; Garzanti, E. The Tethys Himalayan Passive Margin from Late Triassic to Early Cretaceous (South Tibet). J. Asian Earth Sci. 1998, 16, 173–194. [Google Scholar] [CrossRef]
- Han, K.; Han, Z.; Garzanti, E.; Zhu, S.; Yao, H.; Guo, H.; Liu, X.; Wang, C. Middle Jurassic Ooidal Ironstones (Southern Tibet): Formation Processes and Implications for the Paleoceanography of Eastern Neo-Tethys. Front. Earth Sci. 2023, 10, 1055957. [Google Scholar] [CrossRef]
- Dera, G.; Pellenard, P.; Neige, P.; Deconinck, J.F.; Pucéat, E.; Dommergues, J.L. Distribution of Clay Minerals in Early Jurassic Peritethyan Seas: Palaeoclimatic Significance Inferred from Multiproxy Comparisons. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 271, 39–51. [Google Scholar] [CrossRef]
- Dera, G.; Brigaud, B.; Monna, F.; Laffont, R.; Pucéat, E.; Deconinck, J.F.; Pellenard, P.; Joachimski, M.M.; Durlet, C. Climatic Ups and Downs in a Disturbed Jurassic World. Geology 2011, 39, 215–218. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).