Kinetics of Sulfide Dissolution Controlled by Sulfur Radical Diffusion: Implications for Sulfur Transport and Triggering of Volcanic Eruptions
Abstract
1. Introduction
2. Materials and Methods
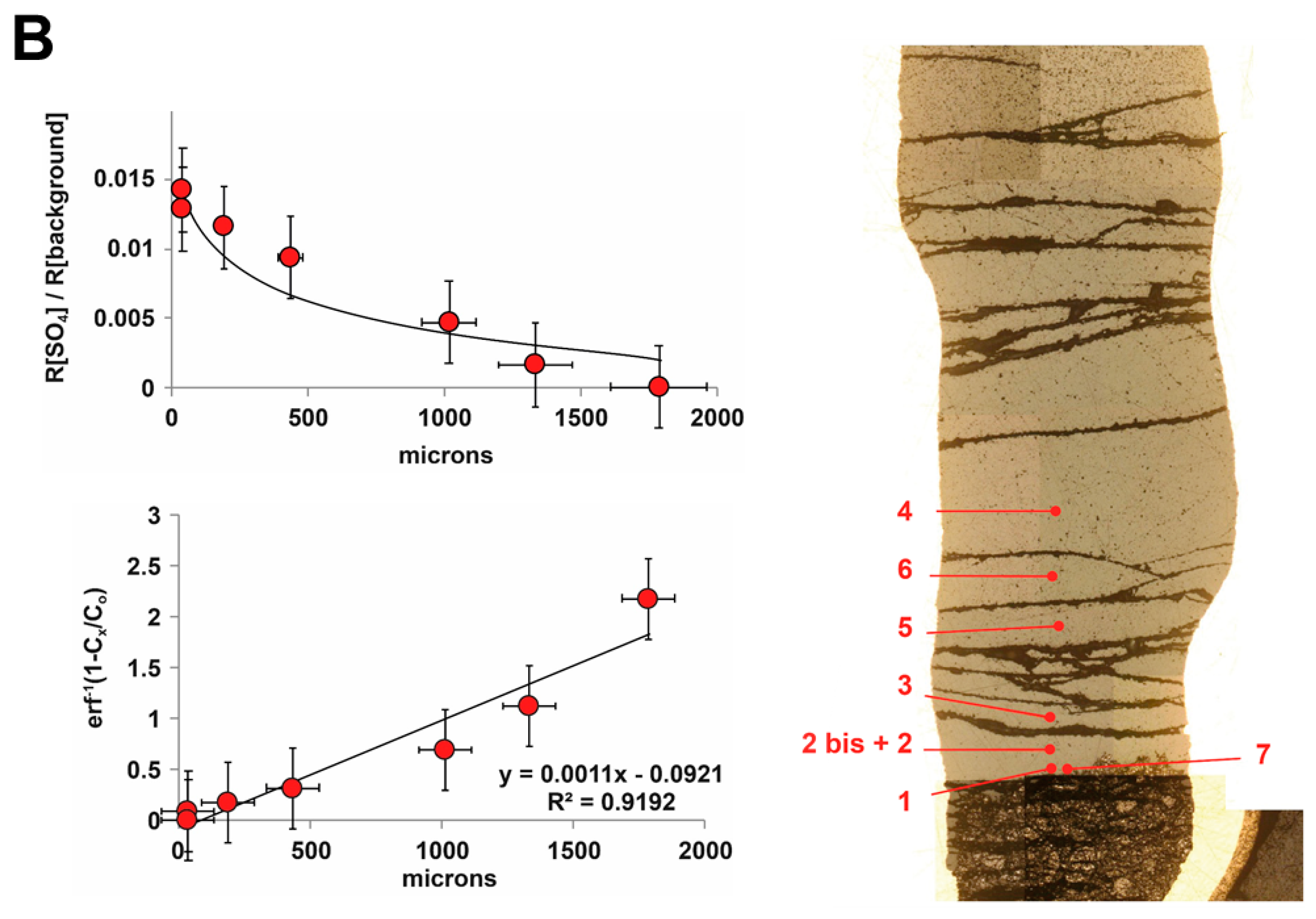
2.1. Experimental Method
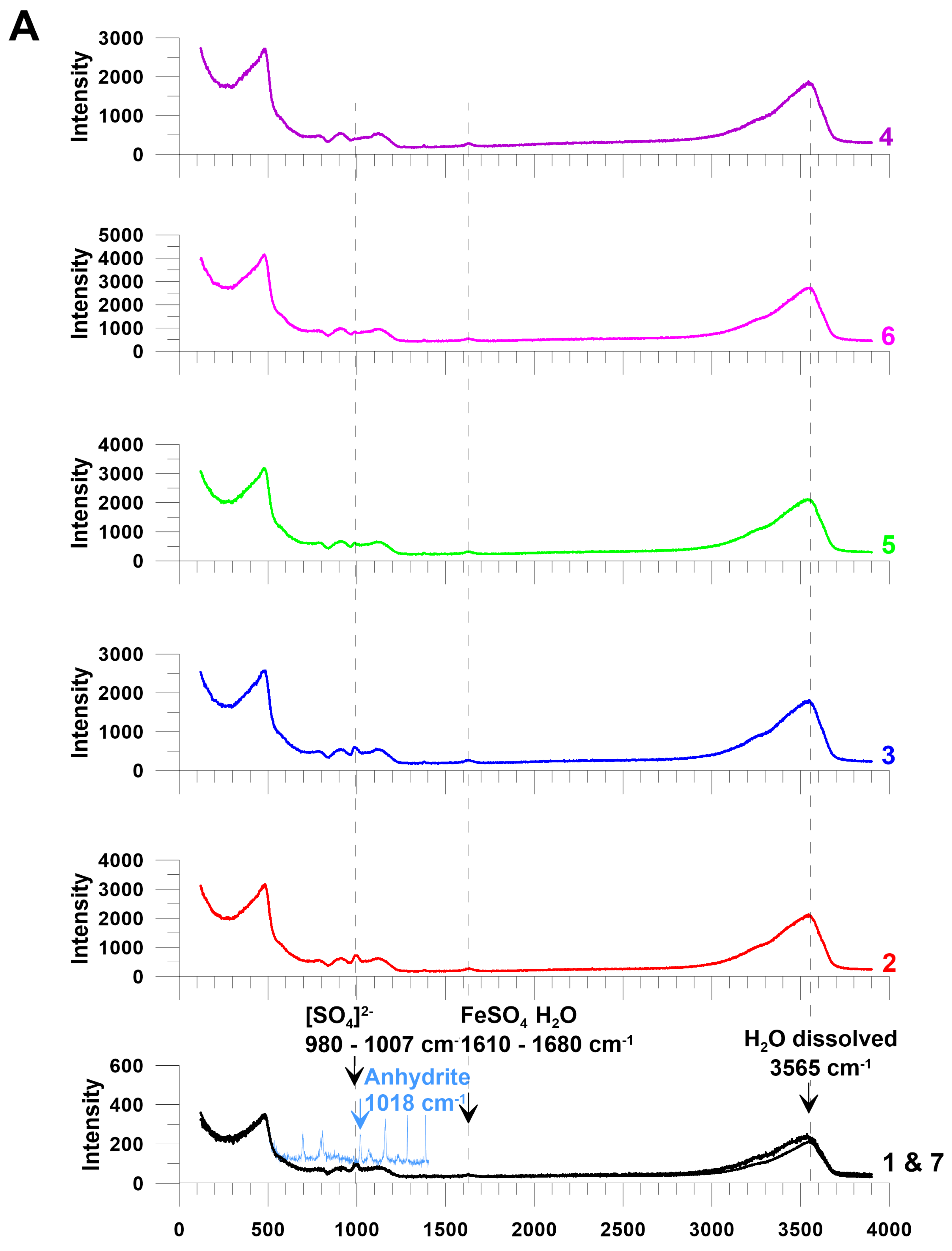
2.2. Micro-Raman and ATR-FTIR Spectroscopy
2.3. Electron Probe Microanalysis
2.4. Thermodynamic Modeling
3. Results
Sulfide Dissolution Reactions and the Reaction Products
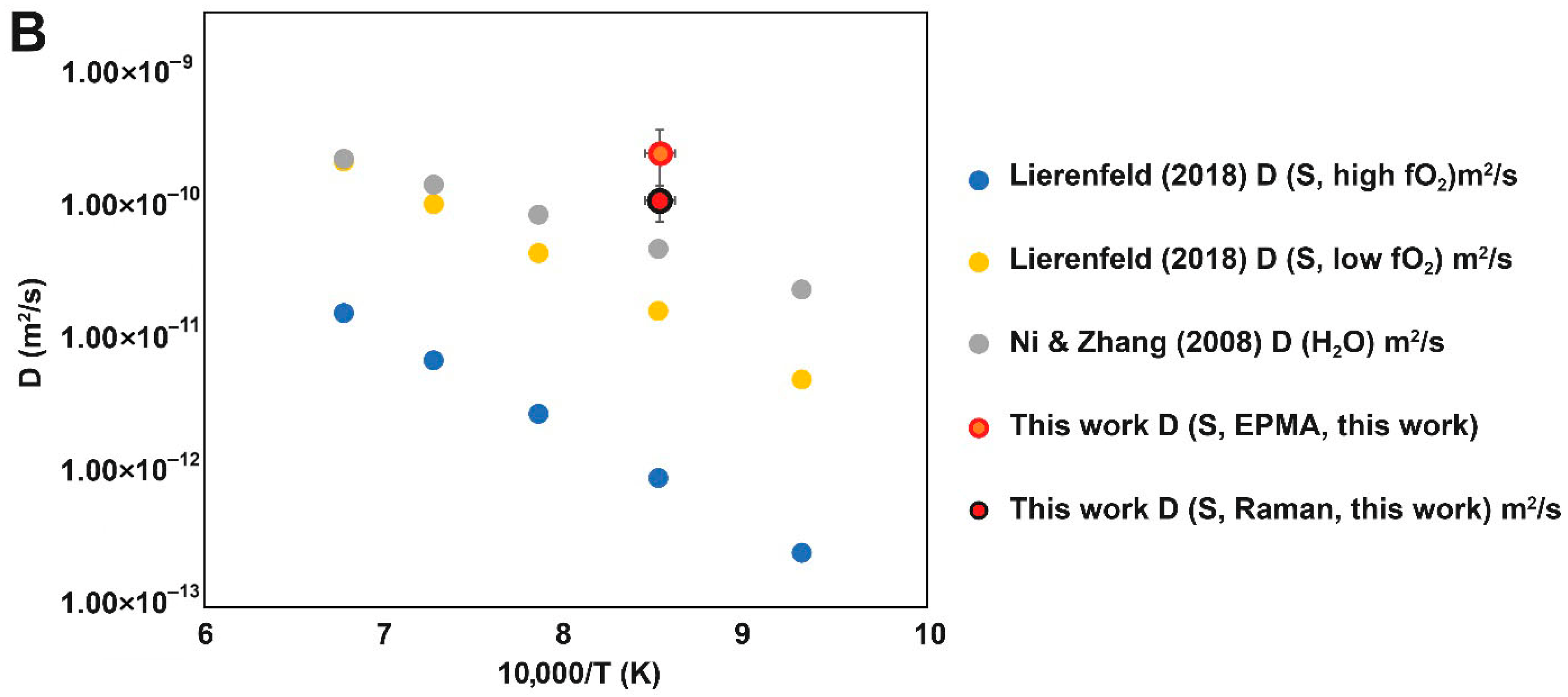
4. Discussion
Sulfur Diffusion and Speciation in Hybrid System
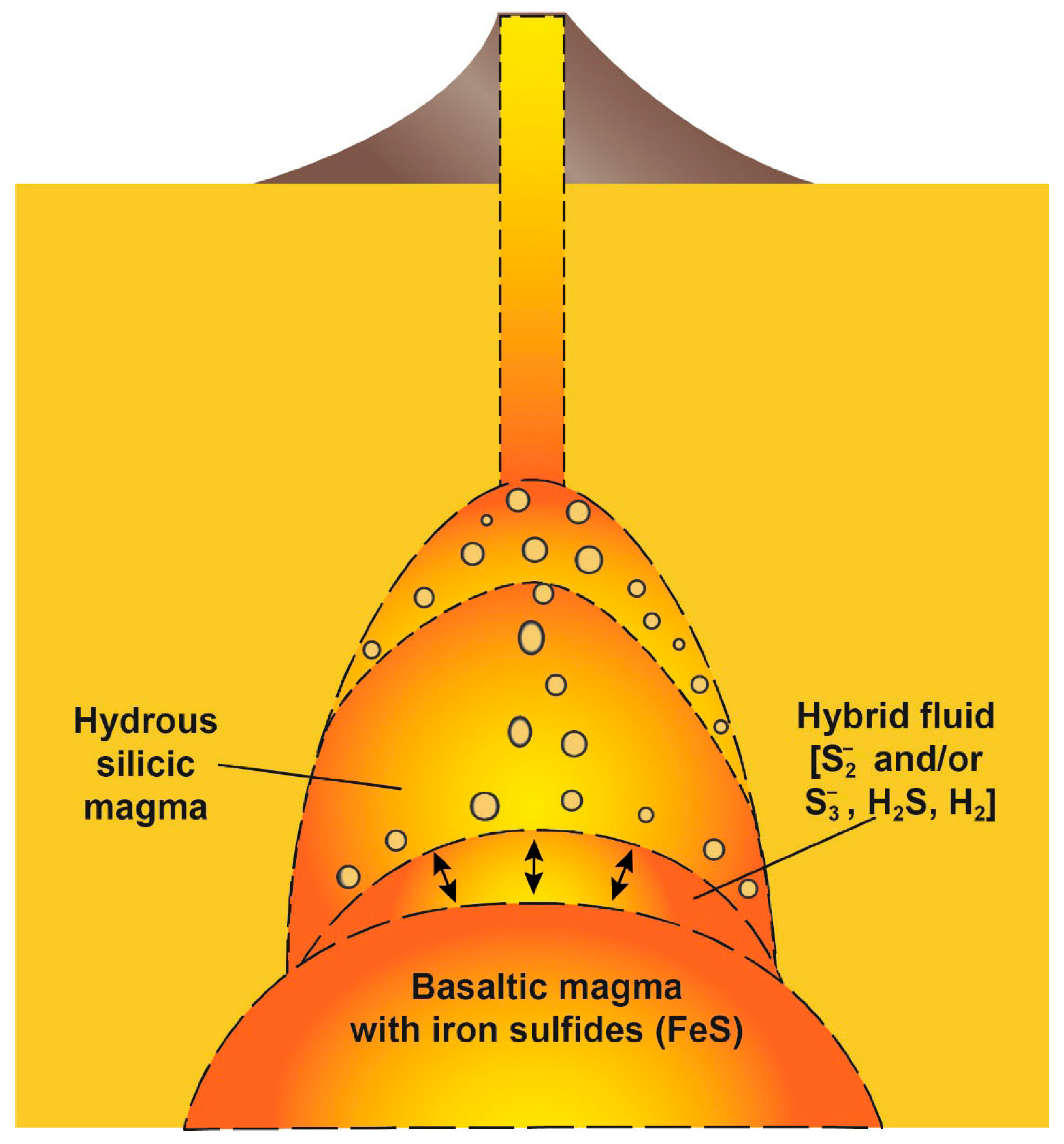
5. Implications
5.1. Hybrid S-Rich Magma and Its Role in Explosive Eruptions
5.2. Sulfur-Loving (Chalcophile) Metal-Rich Magma Formation
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPMA | Electron Probe Microanalysis |
| FTIR | Fourier Transform Infrared Spectroscopy |
| XANES | X-ray absorption near edge structure |
References
- Sparks, S.R.J.; Sigurdsson, H.; Wilson, L. Magma mixing: A mechanism for triggering acid explosive eruptions. Nature 1977, 267, 315–318. [Google Scholar] [CrossRef]
- Matthews, S.J.; Jones, A.P.; Bristow, C.S. A simple magma-mixing model for sulphur behaviour in calc-alkaline volcanic rocks: Mineralogical evidence from Mount Pinatubo 1991 eruption. J. Geol. Soc. 1992, 149, 863–866. [Google Scholar]
- Hattori, K. High-sulfur magma, a product of fluid discharge from underlying mafic magma: Evidence from Mount Pinatubo, Philippines. Geology 1993, 21, 1083–1086. [Google Scholar] [CrossRef]
- Kress, V. Magma mixing as a source for Pinatubo sulfur. Nature 1997, 389, 591–593. [Google Scholar] [CrossRef]
- Hattori, K.; Sato, H. Magma evolution recorded in plagioclase zoning in 1991 Pinatubo eruption products. Am. Mineral. 1996, 81, 982–994. [Google Scholar] [CrossRef]
- Bluth, G.J.S.; Doiron, S.D.; Schnetzler, C.C.; Krueger, A.J.; Walter, L.S. Global tracking of the SO2 clouds from the June 1991 Mount Pinatubo eruption. Geophys. Res. Lett. 1992, 19, 151–154. [Google Scholar] [CrossRef]
- Pallister, J.S.; Hoblitt, R.P.; Reyes, A.G. A basalt trigger for the 1991 eruptions of Pinatubo volcano? Nature 1992, 356, 426–428. [Google Scholar] [CrossRef]
- Borisova, A.Y.; Toutain, J.-P.; Dubessy, J.; Pallister, J.; Zwick, A.; Salvi, S. H2O–CO2–S fluid triggering the 1991 Mount Pinatubo climactic eruption (Philippines). Bull. Volcanol. 2014, 76, 800. [Google Scholar] [CrossRef]
- Johnson, M.C.; Anderson, A.T., Jr.; Rutherford, M.J. Pre-eruptive volatile contents of magmas. In Reviews in Mineralogy, Volatiles in Magmas; Carroll, M.R., Holloway, J.R., Eds.; De Gruyte: Berlin, Germany, 1994; pp. 281–323. [Google Scholar]
- Van Hoose, A.E.; Streck, M.J.; Pallister, J.S.; Wälle, M. Sulfur evolution of the 1991 Pinatubo magmas based on apatite. J. Volcanol. Geotherm. Res. 2013, 257, 72–89. [Google Scholar] [CrossRef]
- Borisova, A.Y.; Zagrtdenov, N.R.; Toplis, M.J.; Ceuleneer, G.; Safonov, O.G.; Pokrovski, G.S.; Bychkov, A.Y. Hydrated peridotite–basaltic melt interaction Part II: Fast assimilation of serpentinized mantle by basaltic magma. Front. Earth Sci. 2020, 8, 84. [Google Scholar] [CrossRef]
- Borisova, A.Y.; Zagrtdenov, N.R.; Toplis, M.J.; Bohrson, W.A.; Nédélec, A.; Safonov, O.G.; Pokrovski, G.S.; Ceuleneer, G.; Bindeman, I.N.; Melnik, O.E.; et al. Hydrated Peridotite–Basaltic Melt Interaction Part I: Planetary Felsic Crust Formation at Shallow Depth. Front. Earth Sci. 2021, 9, 640464. [Google Scholar] [CrossRef]
- Vikentyev, I.; Vikent’eva, O.; Tyukova, E.; Nikolsky, M.; Ivanova, J.; Sidorova, N.; Palyanova, G. Noble metal speciations in hydrothermal sulphides. Minerals 2021, 11, 488. [Google Scholar] [CrossRef]
- Borisova, A.Y.; Pokrovski, G.S.; Pichavant, M.; Freydier, R.; Candaudap, F. Amorphous Materials: Properties, Structure, and Durability. Arsenic enrichment in hydrous peraluminous melts: Insights from femtosecond laser ablation-inductively coupled plasma-quadrupole mass spectrometry, and in situ X-ray absorption fine structure spectroscopy. Am. Mineral. 2010, 95, 1095–1104. [Google Scholar]
- Bromiley, G.; Hilaret, N.; McCammon, C. Solubility of hydrogen and ferric iron in rutile and TiO2 (II): Implications for phase assemblages during ultrahigh-pressure metamorphism and for the stability of silica polymorphs in the lower mantle. Geophys. Res. Lett. 2004, 31, 310. [Google Scholar] [CrossRef]
- Matjuschkin, V.; Woodland, A.B.; Yaxley, G.M. Methane-bearing fluids in the upper mantle: An experimental approach. Contrib. Mineral Petrol. 2019, 174, 1. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Dubrovinsky, L.S. The S3–ion is stable in geological fluids at elevated temperatures and pressures. Science 2011, 331, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Colin, A.; Schmidt, C.; Pokrovski, G.S.; Wilke, M.; Borisova, A.Y.; Toplis, M.J. In situ determination of sulfur speciation and partitioning in aqueous fluid-silicate melt systems. Geochem. Persp. Let. 2020, 14, 31–35. [Google Scholar] [CrossRef]
- King, P.L.; Ramsey, M.S.; McMillan, P.F.; Swayze, G. Laboratory Fourier transform infrared spectroscopy methods for geologic samples. Infrared Spectrosc. Geochem. Explor. Remote Sens. 2004, 33, 57–91. [Google Scholar]
- Jarosewich, E.; Nelen, J.A.; Norberg, J.A. Reference samples for electron microprobe analysis. Geostand. Newsl. 1980, 4, 43–47. [Google Scholar] [CrossRef]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Enzweiler, J. Determination of reference values for NIST SRM 610–617 glasses following ISO guidelines. Geostand. Geoanalytical Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Shvarov, Y.V. A suite of programs, OptimA, OptimB, OptimC, and OptimS compatible with the Unitherm database, for deriving the thermodynamic properties of aqueous species from solubility, potentiometry and spectroscopy measurements. Appl. Geochem. 2015, 55, 17–27. [Google Scholar]
- Helgeson, H.C.; Kirkham, D.H.; Flowers, G.C. Theoretical prediction of thermodynamic behavior of aqueous electrolytes at high temperatures and pressures. IV. Calculation of activity coefficients, osmotic coefficients, and apparent molal and standard and relative partial molal properties to 5 kb and 600 °C. Am. J. Sci. 1981, 281, 1249–1516. [Google Scholar]
- Holland, T.J.B.; Powell, R. An improved and extended internally consistent thermodynamic dataset for phases of petrological interest, involving a new equation of state for solids. J. Metamorph. Geol. 2011, 29, 333–383. [Google Scholar] [CrossRef]
- Chio, C.H.; Sharma, S.K.; Muenow, D.W. The hydrates and deuterates of ferrous sulfate (FeSO4): A Raman spectroscopic study. J. Raman Spectrosc. 2007, 38, 87–99. [Google Scholar]
- Harrison, T.M.; Watson, E.B. Kinetics of zircon dissolution and zirconium diffusion in granitic melts of variable water content. Contrib. Mineral. Petrol. 1983, 84, 66–72. [Google Scholar]
- Borisova, A.Y.; Bindeman, I.N.; Toplis, M.J.; Zagrtdenov, N.R.; Guignard, J.; Safonov, O.G.; Bychkov, A.Y.; Shcheka, S.; Melnik, O.E.; Marchelli, M.; et al. Zircon survival in shallow asthenosphere and deep lithosphere. Am. Mineral. 2020, 105, 1662–1671. [Google Scholar]
- Lierenfeld, M.B.; Zajacz, Z.; Bachmann, O.; Ulmer, P. Sulfur diffusion in dacitic melt at various oxidation states: Implications for volcanic degassing. Geochim. Cosmochim. Acta 2018, 226, 50–68. [Google Scholar]
- Ni, H.; Zhang, Y. H2O diffusion models in rhyolitic melt with new high pressure data. Chem. Geol. 2008, 250, 68–78. [Google Scholar] [CrossRef]
- Zajacz, Z. The effect of melt composition on the partitioning of oxidized sulfur between silicate melts and magmatic volatiles. Geochim. Cosmochim. Acta 2015, 158, 223–244. [Google Scholar] [CrossRef]
- Xu, T.; Liu, X.; Xiong, X.; Wang, J. Sulfur dissolution capacity of highly hydrated and fluid-saturated dacitic magmas at the lower crust and implications for porphyry deposit formation. Geochim. Cosmochim. Acta 2022, 333, 107–123. [Google Scholar] [CrossRef]
- De Hoog, J.C.M.; Hattori, K.H.; Hoblitt, R.P. Oxidized sulfur-rich mafic magma at Mount Pinatubo, Philippines. Contrib. Mineral. Petrol. 2004, 146, 750–761. [Google Scholar] [CrossRef]
- Pallister, J.S.; Hoblitt, R.P.; Meeker, G.P.; Knight, R.J.; Siems, D.F. Magma mixing at Mount Pinatubo: Petrographic and chemical evidence from the 1991 deposits. In FIRE and MUD Eruptions and Lahars of Mount Pinatubo, Philippines; U.S.Geological Survey: Reston, VA, USA, 1996; pp. 687–731. Available online: https://pubs.usgs.gov/pinatubo/index.html (accessed on 12 September 2025).
- Di Muro, A.; Pallister, J.; Villemant, B.; Newhall, C.; Semet, M.; Martinez, M.; Mariet, C. Pre-1991 sulfur transfer between mafic injections and dacite magma in the Mt. Pinatubo reservoir. J. Volcanol. Geotherm. Res. 2008, 175, 517–540. [Google Scholar] [CrossRef]
- Jégo, S.; Dasgupta, R. The fate of sulfur during fluid-present melting of subducting basaltic crust at variable oxygen fugacity. J. Petrol. 2014, 55, 1019–1050. [Google Scholar]
- Hervo, M.; Quennehen, B.; Kristiansen, N.I.; Boulon, J.; Stohl, A.; Fréville, P.; Pichon, J.-M.; Picard, D.; Labazuy, P.; Gouhier, M.; et al. Physical and optical properties of 2010 Eyjafjallajökull volcanic eruption aerosol: Ground-based, Lidar and airborne measurements in France. Atmos. Chem. Phys. 2012, 12, 1721–1736. [Google Scholar] [CrossRef]
- Gerlach, T.M.; Westrich, H.R.; Symonds, R.B. Fire and mud: Eruptions and lahars of Mount Pinatubo. Philippines 1996, 415, 33. [Google Scholar]
- Li, L.; Liu, X.; Xu, T.; Xiong, X.; Wang, J.; Li, L. Sulfur partitioning between aqueous fluids and felsic melts at high pressures: Implications for sulfur migration in subduction zones. Sci. Rep. 2025, 15, 4273. [Google Scholar] [CrossRef]
- Prouteau, G.; Scaillet, B.; Pichavant, M.; Maury, R. Evidence for mantle metasomatism by hydrous silicic melts derived from subducted oceanic crust. Nature 2001, 410, 197–200. [Google Scholar] [CrossRef]
- Jenner, F.E. Cumulate causes for the low contents of sulfide-loving elements in the continental crust. Nat. Geosci. 2017, 10, 524–529. [Google Scholar] [CrossRef]
- Liu, S.A.; Rudnick, R.L.; Liu, W.R.; Teng, F.Z.; Wu, T.H.; Wang, Z.Z. Copper isotope evidence for sulfide fractionation and lower crustal foundering in making continental crust. Sci. Adv. 2023, 9, eadg6995. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Kokh, M.A.; Guillaume, D.; Borisova, A.Y.; Gisquet, P.; Hazemann, J.L.; Dubessy, J. Sulfur radical species form gold deposits on Earth. Proc. Natl. Acad. Sci. USA 2015, 112, 13484–13489. [Google Scholar] [CrossRef] [PubMed]
- Pokrovski, G.S.; Kokh, M.A.; Desmaele, E.; Laskar, C.; Bazarkina, E.F.; Borisova, A.Y.; Saitta, A.M. The trisulfur radical ion S3−controls platinum transport by hydrothermal fluids. Proc. Natl. Acad. Sci. USA 2021, 118, e2109768118. [Google Scholar] [CrossRef]
- Chiaradia, M.; Caricchi, L. Supergiant porphyry copper deposits are failed large eruptions. Commun. Earth Environ. 2022, 3, 107. [Google Scholar] [CrossRef]
- Borisova, A.Y.; Pichavant, M.; Polvé, M.; Wiedenbeck, M.; Freydier, R.; Candaudap, F. Trace element geochemistry of the 1991 Mt. Pinatubo silicic melts, Philippines: Implications for ore-forming potential of adakitic magmatism. Geochim. Cosmochim. Acta 2006, 70, 3702–3716. [Google Scholar] [CrossRef]
- Borisova, A.Y.; Martel, C.; Gouy, S.; Pratomo, I.; Sumarti, S.; Toutain, J.P.; Bindeman, I.N.; de Parseval, P.; Métaxian, J.P. Highly explosive 2010 Merapi eruption: Evidence for shallow-level crustal assimilation and hybrid fluid. J. Volcanol. Geotherm. Res. 2013, 261, 193–208. [Google Scholar] [CrossRef]
- He, D.Y.; Qiu, K.F.; Simon, A.C.; Pokrovski, G.S.; Yu, H.C.; Connolly, J.A.; Deng, J. Mantle oxidation by sulfur drives the formation of giant gold deposits in subduction zones. Proc. Natl. Acad. Sci. USA 2024, 121, e2404731121. [Google Scholar] [CrossRef] [PubMed]
- Pieterek, B.; Tribuzio, R.; Matusiak-Małek, M.; Ciążela, J.; Horn, I.; Weyer, S.; Strauss, H.; Kuhn, T.; Muszyński, A. Underplated melts control sulfide segregation at the continental crust-mantle transition. Commun. Earth Environ. 2024, 5, 45. [Google Scholar] [CrossRef]

| Parameters | Dissolution Experiment and Thermodynamic Modeling Data | Parameters for the Explosive Eruption | 1991 Mount Pinatubo Eruption | ||
|---|---|---|---|---|---|
| S in FeS | 37.7 wt% | 377,000 ppm S | Mass of ash (literature) **** | 13,000 | Mt |
| SO3 melt at the interface | 0.5 wt% | 2000 ppm S | Mass of aqueous fluid (literature) **** | 95 | Mt |
| Fluid Sthermodynamics | 7.0– 20 wt% | 70,000–300,000 ppm S | |||
| Fluid SO3 * | 25 wt% | - | Mass of erupted S | 10–13 | Mt |
| Fluid S *** experiment | 10 wt% | 100,126 ppm S | |||
| KD (fluid/sulfide) Estimate ** | 0.2–0.5 | Mass of liberated SO2 (literature) **** | 19–27 | Mt | |
| KD (fluid/melt) *** | 50–150 | ||||
| Model calculations $ | |||||
| 1991 Mount Pinatubo eruption | |||||
| Mass of FeS in basalt | 25–32 | Mt | |||
| FeS in the hybrid magma | 0.2–0.6 | wt% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borisova, A. Kinetics of Sulfide Dissolution Controlled by Sulfur Radical Diffusion: Implications for Sulfur Transport and Triggering of Volcanic Eruptions. Minerals 2025, 15, 989. https://doi.org/10.3390/min15090989
Borisova A. Kinetics of Sulfide Dissolution Controlled by Sulfur Radical Diffusion: Implications for Sulfur Transport and Triggering of Volcanic Eruptions. Minerals. 2025; 15(9):989. https://doi.org/10.3390/min15090989
Chicago/Turabian StyleBorisova, Anastassia. 2025. "Kinetics of Sulfide Dissolution Controlled by Sulfur Radical Diffusion: Implications for Sulfur Transport and Triggering of Volcanic Eruptions" Minerals 15, no. 9: 989. https://doi.org/10.3390/min15090989
APA StyleBorisova, A. (2025). Kinetics of Sulfide Dissolution Controlled by Sulfur Radical Diffusion: Implications for Sulfur Transport and Triggering of Volcanic Eruptions. Minerals, 15(9), 989. https://doi.org/10.3390/min15090989









