Abstract
Ore mining and smelting are often related to environmental pollution. This study provides information about the geochemical features of Technosols at historical mining and metallurgical sites in the Tatra Mountains, southern Poland, evaluating the contents of potentially toxic trace elements (PTTE) and their behaviours in soils, as well as the influence of soil properties on PTTE mobility. Thirteen soil profiles were studied in eight abandoned mining and smelting sites. PTTE concentrations, including rare earth elements (REE), were measured using ICP-MS and ICP-OES. Selected elements (Cu, Zn, Pb, Cd, As, Sb, Ba, Sr, Co, Ni, Mn and Cr) were fractionated using the modified European Community Bureau of Reference (BCR) four-step sequential extraction. Contamination of soils with PTTE was compared against Polish regulatory limits, which were exceeded for Cu, Zn, Pb, Mo, Hg, As, Co, Ni and Ba, with concentrations exceeding limits by 16, 18, 34 and 160 times for Cu, Hg, As and Ba, respectively, in some profiles. Based on geochemical features depending on parent material properties, the soils examined were divided into three groups. Group I Technosols (near-neutral soils developed from Fe/Mn-ore and carbonate-bearing mining waste) were particularly enriched in Co, Ni, Mn and REE. Group II Technosols (acidic soils developed from polymetallic ore-bearing aluminosilicate mining waste) contained elevated concentrations of Cu, Zn, Hg, As, Sb, Bi, Co, Ag, Ba, Sr, U and Th; they contained lower contents of REE than Group I Technosols. Group III Technosols (soils developed in smelting-affected areas and containing metallurgical waste) were rich in Cu, As, Sb, Ba, Hg, Co and Ag and contained the lowest REE contents among the studied soils. Sequential BCR extraction revealed that PTTE mobility varied strongly according to soil group, with higher mobility of Mn, Cu and Zn in acidic polymetallic ore-derived soils (Group II), while carbonate-rich soils (Group I) showed mainly immobile forms. Metallurgical slag-derived soils (Group III) exhibited complex PTTE behaviour controlled by organic matter and Fe/Mn oxides. Soil properties (pH, carbonates and TOC) seem to control PTTE mobility.
1. Introduction
The Tatra Mountains nowadays are known for their rich biodiversity and unique geological and geomorphological features [1]. The area of the Tatra Mountains is now under strict environmental protection as part of Tatra National Park (TNP), which aims to preserve the natural ecosystem. However, the area contains sites affected by historical mining and smelting operations [2,3]. Metals such as Cu, Ag, Fe and Mn were mined and processed at these sites from the 15th century until the end of the 19th century [4,5,6]. Although nowadays the Tatra Mountains are mainly associated with tourism and natural protection, the area was an important mining region where mines and smelters were developed for centuries. In the 19th century, industrial activities in the Tatra Mountains declined due to the depletion of easily accessible ore deposits, competition from cheaper mines in other regions and rising production costs [2]. Historical mining and smelting in the Tatra Mountains, particularly in Kościeliska Valley, Chochołowska Valley (for example, in the area known as Huciańskie Banie), Pyszniańska Valley, Ornak ridge (including Banisty Żleb and Żleb pod Banie) and Kuźnice, have left a legacy in the form of mining and metallurgical waste deposited on the land surface. Technogenic soils (Technosols) have developed in the superficial parts of waste disposal sites.
Technosols are soils containing artefacts, i.e., materials made, modified or exposed due to human activity that would not otherwise be present on the Earth’s surface [7,8]. Mining and metallurgical wastes are common parent materials for Spolic Technosols, which comprise a subgroup of technogenic soils particularly rich in artefacts [9,10,11,12,13,14]. Vegetation growth on anthropogenic materials can promote their weathering and stimulate the progress of pedogenic processes in post-industrial areas [15,16]. In recent years, the first information about Technosols in the Tatra Mountains was published [17,18,19]. These studies focused on the determination of soil properties as well as their mineral and chemical composition to classify the soils and identify the first effects of soil-forming processes contributing to their development.
Mining and metallurgical wastes, as well as Technosols developing from these wastes, often contain high concentrations of potentially toxic trace elements (PTTE) (e.g., Cu, Zn, Pb, Cd and As), which can be harmful to plants, animals and humans [20,21,22,23,24,25,26]. Contamination of Technosols developed from these wastes is a significant environmental problem due to the possible release of PTTE into the environment [27]. The mobility of PTTE in post-mining soils is affected by various factors, including soil pH, redox conditions, quantity and quality of soil organic matter (SOM), mineral composition of waste and the specific nature of the PTTE [28,29,30].
Studies on the determination of PTTE in soils in various areas of the Tatra Mountains have been conducted by numerous researchers. Differences in the contents of trace elements in soils around Morskie Oko and Kasprowy Wierch in the TNP with increasing altitude were studied [31]. Research on trace element pollution in Polish national parks, including the TNP, investigated the presence of trace elements in spruce (Picea abies (L.) H. Karst) stands, accumulation on the surface and inside the needles, as well as the concentration of bioavailable trace elements in the soil [32]. The influence of spruce and other tree species’ habitat types on the nature and occurrence of selected elements was also studied [33]. Moreover, the bioavailability of elements in TNP soils in Chochołowska, Kościeliska, Strążyska and Mała Łąka Valleys was examined [34]. Besides determining the activity of selected radionuclides, the contents of trace elements in the topsoil from Chochołowska, Kościeliska, Bystra, Suchej Wody and Rybiego Potoku Valleys were also investigated [35,36]. The trace element contents in podzols was the subject of further studies [37,38]. Furthermore, trace elements in TNP’s initial soils (regosols and leptosols) were analysed [39,40,41]. Trace element contents were also examined in peat-bog and fen soils [42] and in soils from grazed mountain glades in the TNP [43]. Recently, forms of elements in soil were determined by comparing the co-occurrence of special forms of metals in forest areas of the TNP [44]. Trace elements were also investigated in selected non-forest soils of the TNP [45,46]. Metal contamination in dustfall, nettle and soils in Chochołowska and Strążyska Valleys and near Morskie Oko, reflecting the impact of long-term trace element emissions, was also assessed [47]. In recent studies, concentrations of trace metals (Zn, Pb and Cd) were analysed in the uppermost layers of non-forest soils from the TNP, with soil surface horizon samples and bedrock samples collected from the bottom of excavation pits [48]. According to research conducted in Slovakia, the most serious soil contaminants in the TNP were Cd and Fe, with transport and tourism identified as the main pollution sources. Although the contents of some PTTE decreased with increasing altitude, this trend could not be unequivocally confirmed [49].
Although the knowledge about the contents of PTTE in soils of the Tatra Mountains is extensive, until now there have not been many studies focusing on PTTE in soils of historical mining and smelting areas, including sites where post-industrial waste was deposited. This study fills this gap by presenting detailed analyses of total PTTE content and their geochemical forms using sequential BCR extraction in Technosols developed from various post-industrial parent materials, thereby extending the knowledge of trace element contamination, mobility and environmental risks in the unique environment of the Tatra Mountains. A study on Technosols developed on mining dumps near abandoned iron ore mines in Jaworzynka Valley in the Tatra Mountains stated that these soils did not contain high contents of As, Cd, Co, Cr, Cu, Ni, Pb and Zn [19]. However, results about the contents and geochemical forms of selected PTTE in other Technosols representing several important abandoned mining and smelting sites in the Tatra Mountains have not yet been presented.
The purpose of the present study was (1) to recognise the total contents of selected PTTE (Cu, Zn, Pb, Cd, Mo, Hg, As, Sb, Bi, Co, Ni, Ba, Sr, Ag, Au and Sn), radioactive elements (U and Th) and rare earth elements (REE) (Sc, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb and Lu), as well as (2) to determine the geochemical forms of selected PTTE (Cu, Zn, Pb, Cd, As, Sb, Ba, Sr, Co, Ni, Mn and Cr) in soils from the Tatra Mountains using the BCR sequential extraction procedure. In addition to recognising total contents and determining the geochemical forms of selected PTTE, this study aims (3) to identify levels above limits (permissible contents) to evaluate the contamination status of Technosols by comparing measured PTTE concentrations against current Polish regulatory limits. This allowed for an assessment of the extent and potential environmental risks of trace element pollution in soils developed on historical mining and metallurgical wastes in the Tatra Mountains. The results allowed for a discussion on the degree of soil contamination, the origin of PTTE in soils and the potential mobility of PTTE in soils. Moreover, the results contribute to a more detailed recognition of the geochemical features of Technosols developed in the Tatra Mountains from different technogenic parent materials, Supplementary Data presented in previous studies [17,18,19].
2. Materials and Methods
2.1. Study Area and Object
The field research was conducted in 2020 in the Tatra Mountains, southern Poland, in historical mining and smelting areas. The study sites differed in the types of anthropogenic parent materials, including mining waste and metallurgical slags. These materials differed in the mineral composition of artefacts present in the soils, influencing soil properties [17]. Research was carried out in the following eight selected areas:
- Huciańskie Banie (Mn and Fe ore mine)—Profiles 1 and 2.
- Mouth of Kościeliska Valley (Fe ore mine)—Profile 3.
- Kościeliska Valley near the Ornak tourist shelter (Cu and Ag ore prospection adit)—Profile 4.
- Pyszniańska Valley (Cu, Ag and Fe ore mine)—Profiles 5 and 6.
- Żleb pod Banie (Pod Banie Couloir) at the Ornak ridge (Cu, Fe and Ag ore mine)—Profiles 7 and 8.
- Banisty Żleb (Banisty Couloir) at the Ornak ridge (Cu, Ag, Sb and Fe ore mine)—Profiles 9 and 10.
- Kościeliska Valley—an old steelwork (a former iron and non-ferrous metal smelting area at Stare Kościeliska)—Profiles 11 and 12.
- The Kuźnice steelworks area (metal smelter)—Profile 13.
Soil profiles were located on surfaces of small heaps at adit outlets, as well as near collapsed shafts and historic smelters. The exact location was provided elsewhere [17].
Soil samples taken from each soil horizon distinguished in the field were analysed in the laboratory. Soil samples were first cleared of roots and organic debris, followed by air-drying at room temperature. The dried material was then passed through a 2 mm mesh sieve to separate the fine earth fraction (<2 mm). Standard pedological techniques, as described in established soil analysis references [50,51,52], were used to assess the physical and chemical properties of the fine earth. All studied soils were classified as Spolic Technosols or Coarsic Spolic Technosols [17] according to the WRB soil system [8].
2.2. Laboratory Analyses
2.2.1. Total Contents of PTTE
In the laboratory, living roots were removed from soil samples, and then the samples were dried at room temperature and sieved through a 2 mm sieve to obtain the fine earth fraction (<2 mm). Fine earth was used for all subsequent laboratory analyses. To determine the contents of PTTE (Cu, Zn, Pb, Cd, Mo, Hg, As, Sb, Bi, Co, Ni, Ba, Sr, Ag, Au and Sn), radioactive elements (U and Th) and rare earth elements (REE) (Sc, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb and Lu), the fine earth soil samples were ground and mixed with LiBO2/Li2B4O7 flux. Crucibles were fused in a furnace. The cooled beads were dissolved in American Chemical Society (ACS)-grade nitric acid. Contents of elements were analysed using the inductively coupled plasma-mass spectroscopy (ICP-MS) method. Analyses were conducted at the Bureau Veritas Minerals Laboratories (BVML), Canada. The contents of Cu, Hg, Sb and Ag in Profiles 9 and 12 were higher than the maximum limit of detection of the method used. Therefore, these results were described adequately (e.g., >100) (Table S1).
2.2.2. BCR Sequential Extraction Analysis
Operationally defined chemical forms of selected PTTE (Cu, Zn, Pb, Cd, As, Sb, Ba, Sr, Co, Ni, Mn and Cr) were determined in soil samples using the BCR procedure [53,54,55]. This is a four-step sequential extraction protocol described in more detail in Table 1. In the laboratory, fine earth fractions (<2 mm) were used for BCR extraction. Samples were prepared by removing any remaining roots or organic debris and homogenised prior to fractionation.

Table 1.
Fractions and reagents used in BCR sequential extraction [54,55].
Contents of elements in soil extracts gained during fractionation studies were determined using inductively coupled plasma-optical emission spectrometry (ICP–OES). Soil extracts were analysed in duplicate. Blanks were run in duplicate with each set of fractions. Analytical-grade reagents were used in the analyses. Analyses were performed at the Department of Soil Science and Agricultural Chemistry, Facultad de Ciencias, Universidad de Granada, Spain.
2.3. Statistical Analysis
The distribution of the results was checked for normality using the Shapiro–Wilk test. Since the assumption of normality was not consistently met, the non-parametric Spearman’s rank correlation was applied to evaluate the strength and direction of the relationships between the PTTE concentrations bound in operationally defined fractions and soil properties (pH, carbonate content and total organic carbon (TOC)). The correlation coefficients (ρ) and corresponding p-values were calculated, with the statistical significance level at p < 0.05. Statistical analysis was performed in StatisticaTM 13.3 (Tibco Software Inc., Palo Alto, CA, USA).
3. Results
3.1. Soil Properties
The studied Technosols were divided into three distinct groups differing in the nature of the anthropogenic parent material and soil properties [17]. A summary of the results showing the soil characteristics is presented below and in Table S2.
Group I Technosols (Profiles 1–3) were carbonate-rich mining waste soils. They displayed neutral to alkaline pHH2O (7.2–8.1; with the exception of P3, horizons Oe—5.7, O/C—6.8) due to high carbonate content (9.6%–31.2% CaCO3 eq.) (Table S2). These soils, derived from Fe- and Mn-ore and carbonate-bearing mining waste, were strongly buffered by carbonates. The total organic carbon (TOC) content was very high in organic horizons (43% on average; with the exception of P3, horizons O/C—24%) and lower in mineral horizons (4% on average) (Table S2), following typical soil depth-dependent trends [17].
Group II Technosols (Profiles 5–10) were soils derived from aluminosilicate mining waste near abandoned polymetallic ores. They were acidic soils (pHH2O 3.6–6.0) (Table S2), reflecting their origin from non-carbonate igneous (granite) and metamorphic (gneiss) rocks. Surface layers under coniferous vegetation (e.g., Pyszniańska Valley, Banisty Couloir and Pod Banie Couloir) exhibited the lowest pH (pHH20 4.0 on average). The TOC content averaged 2% (in mineral horizons), with the highest accumulation in organic horizons (39% on average) (Table S2) [17].
Group III Technosols (Profiles 11–13) were soils containing metallurgical waste. They exhibited variable pHH2O (5.6–8.6) and low carbonate content (on average 3% CaCO3 eq.) (Table S2). Profiles 11 and 12 had lower pH levels than Profile 13. The TOC contents throughout the soil profiles were notably higher (7% on average in mineral horizons; 43% on average in organic horizons) than in other soils, peaking at 15% in Profile 12 (2C horizon) (Table S2) due to the occurrence of charcoal-rich anthropogenic materials [17].
Profile 4 exhibited intermediate properties between Group I and Group II, with pH (Table S2) influenced by mixed carbonate and aluminosilicate parent materials [17].
3.2. Total Contents of PTTE in the Studied Soils
In Group I Technosols (Profiles 1–3), derived from carbonate-bearing mining waste near abandoned Fe and Mn ore mines, the most notable feature was elevated contents of Co (up to 205 mg·kg−1) and Ni (up to 431 mg·kg−1) (Figure 1; Table S1). This feature was typical for Profiles 1 and 2. These values far exceeded those found in Groups II and III, where the Ni and Co contents reached up to 74 mg·kg−1 and 79 mg·kg−1 (Figure 1; Table S1), respectively. These soils contained relatively low contents of other PTTE (e.g., Cu, Hg, Sb, Cr, Bi and Ag), although Zn, Mo and Sr showed elevated contents compared to soils in other studied locations.
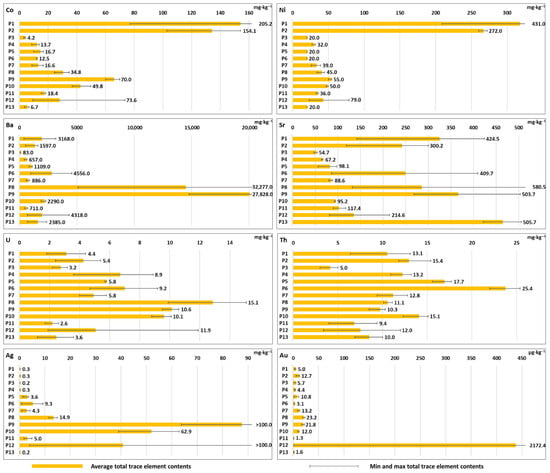
Figure 1.
Average (marked as orange bars), minimal and maximal (marked as black lines; numbers near the bar show maximum concentration of the element) total concentrations of Co, Ni, Ba, Sr, U, Th, Ag and Au in the studied soil profiles (P1–P13). Total concentrations of elements in individual soil horizons are shown in Table S1 (Supplementary Materials).
Group II Technosols (Profiles 5–10), derived from aluminosilicate mining waste near abandoned polymetallic ores, showed very high variability in PTTE contents. Profile 8 from Pod Banie Couloir contained particularly high amounts of Mo (up to 20 mg·kg−1), Ba (up to 32,277 mg·kg−1), Sr (up to 581 mg·kg−1), U (up to 15 mg·kg−1) and Au (up to 23 µg·kg−1) (Figure 1 and Figure 2; Table S1). Profile 9 from Banisty Couloir showed significant enrichment in many PTTE, including Cu (up to 1666 mg·kg−1), Zn (up to 159 mg·kg−1), Hg (up to 37 mg·kg−1), As (up to 347 mg·kg−1), Sb (up to 1263 mg·kg−1), Bi (up to 21 mg·kg−1), Co (up to 70 mg·kg−1), Ba (up to 27,828 mg·kg−1), Sr (up to 504 mg·kg−1), U (up to 11 mg·kg−1), Th (up to 10 mg·kg−1), Ag (>100 mg·kg−1) and Au (up to 22 µg·kg−1) (Figure 1 and Figure 2, Table S1). Profile 6 from Pyszniańska Valley contained the highest Th content (up to 25 mg·kg−1) in this group, while Profile 10, located at the same site as Profile 9, contained the same elements but in lower concentrations than Profile 9 (with the exception of Th—15 mg·kg−1) (Figure 1; Table S1).
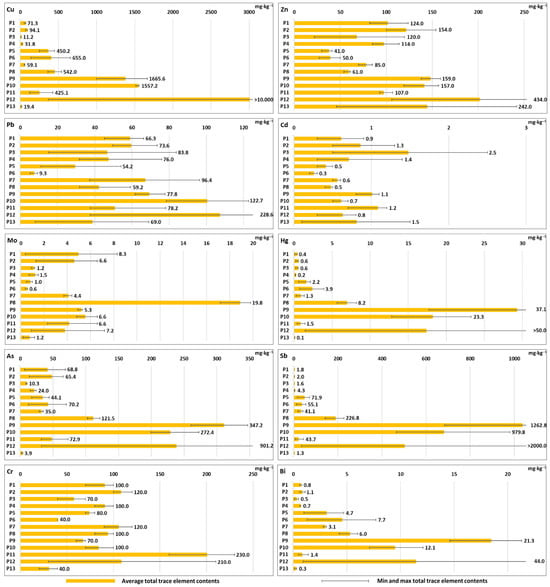
Figure 2.
Average (marked as orange bars), minimal and maximal (marked as black lines; numbers near the bar show maximum concentration of the element) total concentrations of Cu, Zn, Pb, Cd, Mo, Hg, As, Sb, Cr and Bi in the studied soil profiles (P1–P13). Total concentrations of elements in individual soil horizons are shown in Table S1 (Supplementary Materials).
The most complex contamination patterns appeared in Group III Technosols (Profiles 11–13), representing soils in old smelting areas. The concentrations of all studied elements in Profile 11 were typically low in comparison with the other soils; however, Cr reached its maximum concentration in this profile, up to 230 mg·kg−1. Profile 12 showed particularly significant PTTE variation between horizons, with high total contents of As, Cu, Sb and Ba throughout the soil profile. The 3C horizon in Profile 12 contained extreme concentrations of Cu (>10,000 mg·kg−1), Zn (434 mg·kg−1), Hg (>50 mg·kg−1), As (901 mg·kg−1), Sb (>2000 mg·kg−1), Bi (44 mg·kg−1), Ag (>100 mg·kg−1) and Au (2172 µg·kg−1) (Figure 1 and Figure 2; Table S1). However, the 3C horizon in Profile 12 constituted a layer of ore-bearing rocks (not metallurgical waste) preserved within the soil profile. Profile 13 showed lower contents of the aforementioned PTTE except for Sr (up to 506 mg·kg−1), which showed one of the highest contents of that element among the studied soils (Figure 1; Table S1).
Profile 4 showed moderate concentrations of PTTE. Zinc reached up to 114 mg·kg−1, Cr up to 100 mg·kg−1, Pb up to 76 mg·kg−1, As up to 24 mg·kg−1, Co up to about 14 mg·kg−1 and Ni up to 32 mg·kg−1. Barium (Ba) showed higher levels, up to 657 mg·kg−1, while Sr reached about 67 mg·kg−1. Uranium and Th were present up to about 9 mg·kg−1 and 13 mg·kg−1, respectively. Profile 4 contained relatively low contents of other PTTE (e.g., Cu, Cd, Mo, Hg, Sb, Bi, Ag and Au) (Figure 1 and Figure 2; Table S1).
3.3. Total Contents of Rare Earth Elements (REE) in the Studied Soils
The studied soils showed distinct REE (Sc, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb and Lu) distribution patterns across the three Technosol groups (Table 2), reflecting differences in parent material composition.

Table 2.
Concentrations of rare earth elements (REE) in the studied Technosols.
Group I Technosols (Profiles 1–3), in general, contained the highest contents of REE in comparison with the other soils (from 61–786 mg·kg−1) (Table 2). This was particularly true for Profiles 1 and 2, where the most common REE were Ce (107–293 mg·kg−1), La (50–116 mg·kg−1), Nd (45–120 mg·kg−1), Y (33–113 mg·kg−1), Sm (8.5–23 mg·kg−1), Gd (7.6–25 mg·kg−1) and Dy (6–19 mg·kg−1). Profile 3 contained the lowest total REE concentrations (61–126 mg·kg−1), as it was developed in a mining area other than the site where Profiles 1 and 2 were located.
Total REE contents (61–346 mg·kg−1) in Group II Technosols (Profiles 5–10) were lower than in Group I, with the highest values in Profile 6 (C horizon) (Table 2). Soils of that group showed relative enrichment in Ce (61–127 mg·kg−1), La (31–61 mg·kg−1), Nd (28–59 mg·kg−1) and Y (20–45 mg·kg−1).
Group III Technosols in historical smelting areas (Profiles 11–13) displayed the lowest total REE contents (59–237 mg·kg−1). Profile 13 (AC horizon: 237 mg·kg−1) contained the highest contents of REE in that group. Cerium (19–89 mg·kg−1) and La (11–36 mg·kg−1) were predominant REE, but their contents were significantly lower than in Groups I and II Technosols. Profile 12 showed irregular REE distribution, with an abrupt decrease in total contents of REE in the 2C and 3C horizons (Table 2).
The total REE contents (159–214 mg·kg−1) (Table 2) in Profile 4 were relatively low in comparison with the other soils studied.
3.4. Geochemical Forms of Selected PTTE in the Studied Soils Based on BCR Sequential Extraction
Geochemical forms of Cu, Zn, Pb, Cd, As, Sb, Ba, Sr, Co, Ni, Mn and Cr in the investigated Technosols varied between soil profiles as well as between soil horizons within each soil profile, showing high heterogeneity of soil substrates (Figure 3, Figure 4 and Figure 5).
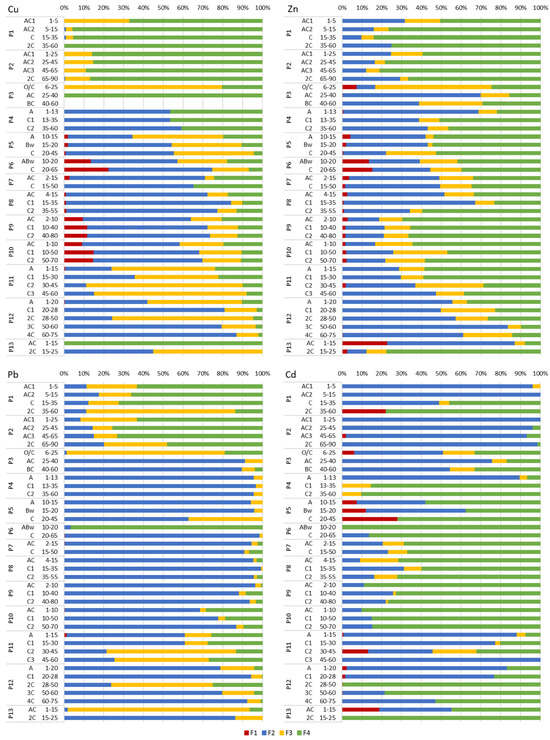
Figure 3.
Chemical forms of Cu, Zn, Pb and Cd in the studied soils based on BCR sequential extraction. Operational definitions of fractions: F1—exchangeable, soluble in acidic medium; F2—susceptible to reduction, including element forms bound to Fe and Mn oxides; F3—oxidisable, including element forms associated with organic matter and sulphides; F4—residual. Detailed descriptions of fractions (from F1 to F4) are presented in Table 1.
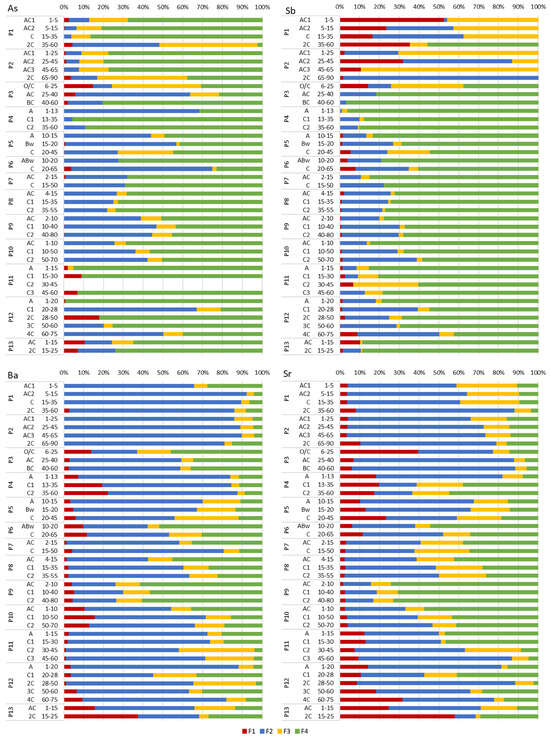
Figure 4.
Chemical forms of As, Sb, Ba and Sr in the studied soils based on BCR sequential extraction. Operational definitions of fractions: F1—exchangeable, soluble in acidic medium; F2—susceptible to reduction, including element forms bound to Fe and Mn oxides; F3–oxidisable, including element forms associated with organic matter and sulphides; F4—residual. Detailed descriptions of fractions (from F1 to F4) are presented in Table 1.
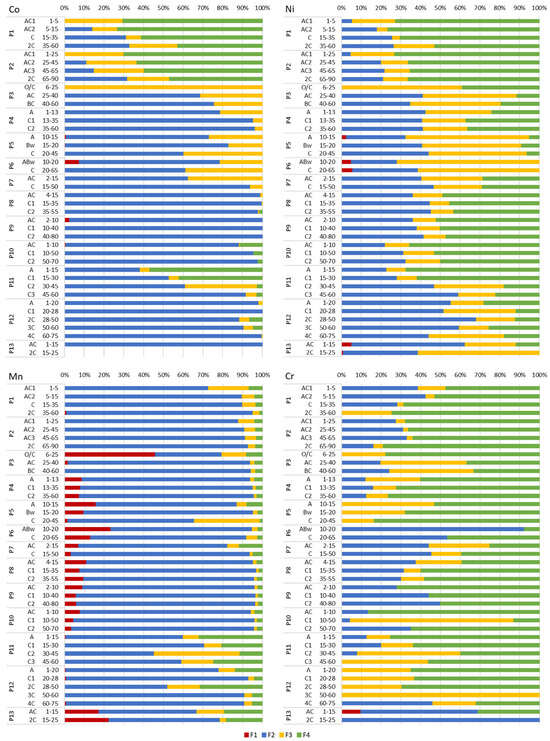
Figure 5.
Chemical forms of Co, Ni, Mn and Cr in the studied soils based on BCR sequential extraction. Operational definitions of fractions: F1—exchangeable, soluble in acidic medium; F2—susceptible to reduction, including element forms bound to Fe and Mn oxides; F3—oxidisable, including element forms associated with organic matter and sulphides; F4—residual. Detailed descriptions of fractions (from F1 to F4) are presented in Table 1.
In Group I Technosols (Profiles 1–3), Cu dominated the residual fraction (F4, 67%–100%), except for Profile 3 (P3) topsoil (F3—oxidisable forms, 80%) (Figure 3). Zinc prevailed in F4 (50%–84%) in Profile 1 (P1) and Profile 2 (P2). Profile 3 contained Zn in F3 (59%) in the topsoil and F2 (reducible forms) (up to 69%) in the subsoil. Lead dominated F4 (up to 75%), except for P3 (F3, 80%) and P1 (F3, 75%). Profile 3 subsoil had Pb in F2 (up to 91%). Cadmium occurred mainly in F2 (45%–100%), except for the deepest horizon of P1 (F4, 78%). Arsenic dominated F4 (67%–86%), with exceptions (F3) in the deepest horizons of P1–P2 and (F2) the AC horizon of P3 (Figure 4). A significant share of As in P3 topsoil was present in F1. Antimony showed high diversity of forms and occurred in F4 (up to 97% in P3), F3 (up to 89% in P2), F2 (up to 98% in P2) and F1 (exchangeable forms) (up to 53% in P1). Barium prevailed in F2, except for P3 topsoil (F4, 46%). Strontium dominated F2, with a slightly higher share in F1 in P3 topsoil (40%). Cobalt dominated F4 (43%–73%) in P1 and P2 (Figure 5). Profile 3 contained Co in F3 (100%) in topsoil and F2 (69%–76%) in subsoil. Nickel dominated F4 (53%–77%) in P1–P2. Profile 3 subsoil showed Ni in F3 (46%–61%). Cobalt and Ni exhibited similar behaviours in P1 and P2 throughout the soil profiles. Manganese dominated F2 (73%–94%); however, a high share of F1 (46%) was typical of P3 topsoil. Chromium prevailed in F4 (47%–79%), except for P3 subsoil (F3, up to 44%) (Figure 5).
In Group II Technosols (Profiles 5–10), the residual fraction (F4) was dominated by Zn (up to 69%), Cd (up to 100%), As (up to 74%) and Sb (up to 85%), though reducible forms (F2) were significant for Cu (up to 83%), Pb (up to 99%), Co (up to 100%), Mn (up to 93) and Ba (up to 76%) (Figure 3, Figure 4 and Figure 5). Nickel was present in F2 (up to 47%), F3 (up to 72%) and F4 (up to 66%). Antimony occurred in F2 (up to 57%) and in F4 (up to 74%). Chromium was bound mostly in F2 (up to 92%) and in F4 (up to 86%) (Figure 3, Figure 4 and Figure 5). Exceptions included Cu in F3 (46%) in topsoil of P5; Zn in F2 (46%–66%) in the horizons AC and C of P7 and horizons AC and C1 of P8; Pb in F4 (97%) in the ABw horizon of P6; Cd in F2 (50%) in the Bw horizon of P5; As in F2 (56%) in the Bw horizon of P5, (71%) in the C horizon of P6 and (46%) in the C1 horizon of P9; Ba in F4 (52%) in the ABw horizon of P6, (45%) in the AC horizon of P8 and (up to 61%) in P9; and Cr in F3 (83%) in the C1 horizon of P10 (Figure 3, Figure 4 and Figure 5).
Group III Technosols (Profiles 11–13) showed complex element distributions. Profile 11 showed Cu in F3 (up to 79%) (Figure 3). In P12, Cu occurred in F2 (81%–87%) in the C1, 3C and 4C horizons and in F3 in the A (48%) and 2C (71%) horizons. Zinc dominated F2 (up to 83%), except for F4 (up to 78%) in AC and C1 of P11 and in 2C of P13 (78%). Lead occurred mostly in F2 (up to 94% in P12) and F3 (up to 92% in P13). Cadmium dominated F2 (up to 100% in P11 and in the subsoil horizons of P12) and F4 (up to 100% in P13 and deeper horizons of P12). Arsenic dominated F4, except for the C1 and 4C horizons of P12 (F2, 67% and 50%) (Figure 4). Antimony dominated F4 (42%–88%). Barium occurred mostly in F2, except for the subsoil of P13 (F1, 38%). Strontium prevailed in F2, except for the subsoil of P13 (F1, 58%), as well as for the A and C1 horizons of P11 and the C1 horizon of P12 (F4). Barium and Sr showed similar trends in P11–P13. Cobalt, Mn and Ni dominated F2, except for a few soil horizons (Figure 5). Chromium in P11 and P12 occurred mostly in F4, and in P13 mostly in F2, except for the C2 horizon of P11 (F3) and the 3C (F3) and 4C (F2) horizons of P12.
Profile 4 showed varied geochemical forms of PTTE. Copper and Zn were mainly present in F2 (up to 68%) and F4 (up to 51%) (Figure 3). Lead, Co and Mn were found largely in F2 (up to 97%) (Figure 3 and Figure 5). Cadmium and As occurred significantly in F2 (up to 90%) in the topsoil and F4 (up to 95%) in the subsoil. Antimony was mainly present in F4 (88%–96%). Barium and Sr primarily occurred in F2 (up to 77%) and up to 22% in F1 (Figure 3 and Figure 4). Nickel and Cr were mostly in F2 (up to 43%) and F4 (up to 76%) (Figure 5).
4. Discussion
4.1. Contamination of Technosols Based on the Total Contents of PTTE Against Polish Regulations and by Comparison with Other Soils in the Tatra Mountains
The PTTE contents in the analysed soils were evaluated against Poland’s regulatory standards, which serve as the benchmark for assessing land surface contamination [56]. In the case of Poland, national soil regulations are influenced by European Union directives [57]. These regulations establish permissible contents of selected metal(loid)s (i.e., As, Ba, Cr, Sn, Zn, Cd, Co, Cu, Mo, Ni, Pb and Hg) in the soil for various land use categories, differentiating permissible contents for two depths (0–25 cm and below 25 cm). Polish law classifies land into four groups (I–IV): (1) land group I includes urban and developed areas inhabited by human; (2) land group II includes agricultural lands (arable fields, orchards, meadows), but also national parks and natural reserves; (3) land group III includes forested and shrub-covered regions; and (4) land group IV includes industrial zones, transportation corridors, production facilities, storage areas, technical infrastructure sites and mining districts. Additionally, land group II is further divided into three subgroups (II-1, II-2 and II-3) based on specific soil characteristics such as texture, pH and organic carbon content. For soils below 25 cm, permissible trace element levels are dependent on water permeability, with a threshold value of 1 × 10−7 m·s−1 [56].
The results obtained in this study were compared with the permissible contents of metal(loid) standards in soil for proper subgroups in land group II (Table S1) [56]. Permissible levels of elements were exceeded for the following metal(loid)s: Cu (Profiles P5, P6, P8–P12), Zn (P12—3C horizon), Pb (P10, P12), Mo (P8), Hg (P5, P6, P8–P10, P12), As (P1, P2, P4–P12), Co (P1, P2, P8–P10, P12—3C horizon), Ni (P1, P2) and Ba (P1, P2, P4–P13) (Table S1). The copper content in Technosols exceeded permissible limits by more than 16 times (in Profile P9), and Pb and Mo by 1.2 and 2 times, respectively. Permissible values for Hg were exceeded by 18 times and for As by 34 times in these soils. Cobalt and Ni reached values 6 and 4 times higher, respectively, than those specified in Polish regulations. Barium reached values 160 times higher than the threshold values. The 3C horizon of Profile 12, which is most likely a layer containing remnants of ore material containing high concentrations of all studied elements, exceeded permissible contents of Cu (66 times), Zn (1.4 times), Pb (1.7 times), Hg (16 times), As (45 times), Co (2.5 times) and Ba (7.5 times). By contrast, the concentrations of Cr, Cd and Sn in all studied soils did not exceed permissible limits.
The elevated contents of PTTE such as Cu, Zn, Pb, Mo, Hg, As, Co, Ni and Ba in the studied Technosols derived from mining waste and old smelting areas often exceeded concentrations reported for native soils in the Tatra Mountains. For instance, Cu, Zn, Pb and Ni contents in Group II Technosols (up to 1666 mg·kg−1 of Cu, 159 mg·kg−1 of Zn, 123 mg·kg−1 of Pb and 55 mg·kg−1 of Ni) were significantly higher than those detected in a study of natural mountain podzols of the Tatra Mountains [38], where authors reported much lower trace element contents (up to 36 mg·kg−1 of Cu, 89 mg·kg−1 of Zn, 50 mg·kg−1 of Pb and 27 mg·kg−1 of Ni) attributed to natural lithology and podzolisation processes. Lower average contents of Cu (up to 13 mg·kg−1), Zn (up to 92 mg·kg−1), Pb (up to 67 mg·kg−1) and Ni (up to 16 mg·kg−1), in comparison to the studied Technosols, were also recorded in soils presented in another study [47]. Similarly, lower average contents of heavy metals such as Cu (up to 15 mg·kg−1), Zn (up to 125 mg·kg−1) and Ni (up to 16 mg·kg−1) were recorded in soils near Lake Morskie Oko and Kasprowy Wierch, with pollution indices generally suggesting low to moderate contamination mostly influenced by long-range atmospheric deposition rather than local mineralisation or metallurgical activities [31]. Although higher concentrations of Zn, Pb and Cd in non-forest soils of Tatra National Park than in the analysed Technosols were reported [48], their spatial distribution was strongly controlled by natural factors like parent rock composition, with less influence from anthropogenic contamination. It can be concluded that the PTTE levels in the studied Technosols markedly exceed concentrations reported in the aforementioned studies, reflecting local anthropogenic pollution from mining and smelting activities, whereas the trace metal contents in undisturbed mountain soils of the Tatra region generally remain within natural or slightly elevated ranges associated with geological and atmospheric factors [31,48].
4.2. Origin of PTTE in the Studied Technosols
The analysed Technosols developed from three different types of mining and industrial wastes. Therefore, the origin of the high contents of PTTE in the investigated Technosols is complex and depends on the nature of anthropogenic parent material.
Group I Technosols (Profiles 1–3), in particular Profiles 1 and 2, contained high concentrations of Ni (up to 431 mg·kg−1) and Co (up to 205 mg·kg−1) (Table S1). Soils around the world contain Ni over a very wide range, but the average levels reported for different countries are in the relatively low range of 13–37 mg·kg−1 [58]. Nickel content is the lowest in sandy and organic soils and highest in heavy loamy and calcareous soils [58]. The world average Co content in soils was estimated at 10 mg·kg−1 [59]. High contents of Ni and Co in Profiles 1 and 2 from the Tatra Mountains were most likely related to high concentrations in the parent material, i.e., mining waste containing remnants of Fe and Mn ores. Nickel and Co are frequently enriched in Fe-Mn ores due to adsorption and incorporation mechanisms in Fe and Mn oxide minerals [60]. Moreover, there were also high contents of Ba (up to 3168 mg·kg−1) in Group I Technosols. Barium is typically found in low concentration in soils. High levels of Ba in soils can come from industrial activities, such as mining [58]. Barium is commonly associated with carbonate rocks [61], as it is often incorporated into carbonate minerals or is found adsorbed on carbonate phases in marine and sedimentary environments [62]. Studies have shown that Ba concentrations can vary in carbonate rocks due to their association with carbonate mineral phases such as calcite and aragonite [63]. Additionally, Ba can be present in barite (BaSO4), which can form in marine sedimentary settings, often associated with carbonate deposits [64].
Group II Technosols (Profiles 5–10), developed from aluminosilicate igneous and metamorphic rocks containing remnants of polymetallic ores, had very high Cu (up to 1666 mg·kg−1 in Profile 9) and As (up to 347 mg·kg−1 in Profile 9) contents. In the soils of European Union countries, average Cu contents range from 5 to 50 mg·kg−1 [59]. The average As content in soils generally ranges from about 1 to 15 mg·kg−1, depending on the region, with natural background levels typically below 10 mg·kg−1 [59]. High Cu and As contents are typical of polymetallic ore-related mining waste [28,65,66,67], where these elements are primarily linked to the sulphide mineralisation in rocks [68,69]. Similar mineralogical controls were documented on metal occurrence in sulphide-rich Technosols developed from mining waste [9,67]. Metal(loid)-rich minerals in the area of Ornak ridge, where historical Cu-Ag-Sb-Fe ore mining was conducted, are, for example, chalcopyrite (CuFeS2)—a primary copper-bearing sulphide, pyrite (FeS2) often associated with arsenic as an impurity—as well as arsenopyrite (FeAsS) and enargite (Cu3AsS4), which are arsenic-rich minerals [68]. High contents of Hg (up to 37 mg·kg−1) were found in Profiles P8–P10, representing Group II Technosols. The presence of Hg is linked to remnants of polymetallic ore minerals scattered in aluminosilicate igneous and metamorphic rocks. Mercury in the rocks constituting the parent materials of the studied soils was most likely accumulated due to hydrothermal mineralisation processes occurring in rocks during ore formation [68]. Typically, Hg is present primarily in sulphide minerals that form in hydrothermal veins [70]. Cinnabar (HgS) is the major mercury mineral, but Hg is also found as a trace element in other sulphide minerals, including sphalerite (ZnS), chalcopyrite and tetrahedrite, within hydrothermal mineral deposits [71]. Group II Technosols contained the highest Ba content (up to 32,277 mg·kg−1) among the studied Technosols. This was primarily due to the presence of barite (BaSO4), which is associated with polymetallic ore mineralisation (Cu, Fe and Ag) in this area [68]. In the Ornak ridge mining area, barite is found in veins with chalcopyrite, pyrite and galena (PbS). Barite frequently occurs alongside sulphide minerals in ore deposits [72]. Barite was also identified in Group II Technosols based on microscopic studies and SEM-EDS analyses of soil thin sections [18]. Contents of Pb and Zn were elevated in some profiles representing Group II Technosols (e.g., Profiles 9 and 10) (Figure 2). Lead and Zn are related to the remnants of polymetallic ores occurring in mining waste deposited in dumps [68].
Group III Technosols (Profiles 11–13) were characterised by elevated contents of Cu, Pb, As and Sb, likely originating from historical metallurgical activities. These PTTE commonly occur in metallurgical slags where they are trapped during cooling, forming metallic aggregates (e.g., native Cu, Pb and Fe) or mineral phases (e.g., sulphides and oxides) [73]. Metal(loid)s are subsequently released into surrounding soils during slag weathering, creating persistent geochemical anomalies [74,75,76]. The presence of Cu, As and Sb reflected their association with polymetallic ores processed in smelters. High contents of PTTE are typical of metallurgical waste [77]. The elevated contents of metal(loid)s in metallurgical waste reflect the original ore composition, processing chemistry and metallurgical partitioning behaviours, leading to enrichment of slags in various metal(loid)s, as metallurgical processes are not fully effective in the extraction of metal(loid)s from ore [13,78]. Extremely high contents of Cu (>10,000 mg·kg−1), As (up to 901 mg·kg−1) and Hg (>50 mg·kg−1) in the 3C horizon of P12 were most likely due to the fact that it was a layer of crushed rocks rich in polymetallic ore. That horizon contained mining waste (not metallurgical waste), which were preserved in the examined soil profile as a separate layer buried subsequently by newer anthropogenic materials.
4.3. Assessment of Mobility of PTTE in the Studied Technosols Based on BCR Sequential Extraction
BCR sequential extraction revealed distinct chemical behaviours of PTTE across the three Technosol groups, strongly influenced by their properties, which were controlled by the mineral and chemical composition of the parent materials. These findings aligned with the results of other authors [20,61], who emphasised that metal(loid)s fractionation in soils is governed by geogenic and anthropogenic factors.
In Group I Technosols, the predominance of residual fractions (F4) for PTTE such as Cu, Zn, Pb, As, Co, Ni and Cr reflected low mobility of these elements. Therefore, carbonate minerals present in these soils likely play the most important role in immobilisation of these elements by maintaining the soil pH at a high level (Table S2). The strong positive correlation between CaCO3 and pH (r = 0.81; p < 0.05; Table S3) confirmed the dominant effect of calcium carbonates on soil pH. High pH of soil limits the solubility of many PTTE, causing them to form weakly soluble mineral phases [20]. This favours the precipitation of metals as insoluble carbonates or hydroxides, effectively reducing their solubility and bioavailability [30,59,79]. Additionally, carbonate minerals like calcite and dolomite have relatively high chemical stability, particularly in soils having high pH [80]. Under such conditions, carbonates provide surfaces for adsorption, which further immobilises metals by binding metal ions and preventing their release into the soil solution [81]. Together, these processes decrease the bioavailability and leaching potential of metals, contributing to their retention primarily within the residual soil fraction [30]. In Profile 3, representing Group I Technosols, oxidisable Cu (F3, 80%) and reducible Zn and Pb (F2, up to 91%) (Figure 3) forms occurred in the topsoil, which suggested that SOM and Fe/Mn oxides, respectively, contributed to the binding of Cu, Zn and Pb [82]. Cadmium seemed to be a rather mobile element in all Group I Technosols, as it occurred mostly in F2 (metals supposed to be related to Fe/Mn oxides). Cadmium tends to be more mobile in soils than many other PTTE because it often occurs in easily soluble or exchangeable forms in soils [67,83]. The high residual Cd (F4, 78%) in Profile 1 subsoil (Figure 3) contrasted with its typical high or moderate mobility, likely due to carbonate co-precipitation.
In Group II Technosols (acidic soils developed from aluminosilicate mining waste), the predominance of the reducible fraction (F2) of Co, Pb, Cu, Ag, Mn and Ba reflected the important role of Fe and Mn oxides in controlling the mobility of these elements. The low pH range (3.6–6.0) in soils enhances the solubility of such metals as Pb, Zn, Cu, Cd and others, promoting desorption and leaching [84,85,86]. BCR analyses showed that the most mobile elements in Group II Technosols were Mn, Cu and Zn (Figure 3 and Figure 5), as a considerable share of these metals occurred in F1 in these soils. Furthermore, a rather small amount of investigated elements in F3 in Group II Technosols (except for Cu, Ni and Cr in some studied soils) indicated that SOM has a limited metal stabilising effect, which corresponded with the observations in other Technosols [66]. Moreover, Cd was a mobile element in Profile 5. On the other hand, some elements typically mobile in acidic soils (e.g., Zn, Cd and Ni) often occurred in the residual fraction (F4) in Group II Technosols. Such a feature is most likely due to the physical blocking of metal-bearing minerals inside quartz or aluminosilicate grains, which were the major minerals occurring in the studied Technosols [17]. This makes it impossible for the reagents used during the first three steps of sequential extraction to dissolve these minerals. Quartz and aluminosilicates are minerals resistant to weathering [87]; therefore, they are not dissolved during the first three steps of sequential extraction and constituted part of F4. Moreover, these metals can be present as stable sulphides and oxides, such as chalcopyrite and hematite, which further limit their mobility until they dissolve or weather [88,89].
Group III Technosols from historical smelting areas displayed the most complex chemical behaviours of PTTE. BCR analysis showed that Co, Mn, Ni, Ba, Sr, Pb, Zn and Cu were the most mobile elements in soils (Figure 1, Figure 2 and Figure 3). On the other hand, As, Sb and Cd seemed to be the least mobile in Group III Technosols. Reducible Pb (F2, up to 94%) and oxidisable Cu (F3, up to 79%) (Figure 3) indicated slag-derived metals bound to Fe/Mn oxides and SOM, respectively [11,90]. Construction materials in the 2C horizon of Profile 13 artificially elevated pH and Cr mobility [17].
Thanks to the four-step BCR analysis, it was possible to identify the contents of PTTE present in the first fraction (F1), i.e., in the most mobile forms in soil (Table 3). In Group II Technosols (Profiles 9 and 10), high contents of Cu reaching up to 174 mg·kg−1 (horizon C1 of P10) were found in F1. Relatively high contents of Cu and Sb (43 and 7 mg·kg−1, respectively) were recorded in the 3C horizon of Profile 12, which was a layer of ore-bearing rocks (not metallurgical waste) preserved within the soil profile. The highest content of Zn in F1 was measured at the old steelworks in Kuźnice (P13), amounting to 50 mg·kg−1 (horizon AC). In other locations, the contents of Zn reached a maximum at 9 mg·kg−1 (P3, horizon O/C) or around 3–4 mg·kg−1 (P6, horizons Bw and C, respectively). In F1, Pb contents of up to 0.9 mg·kg−1 (P11, horizon A), As up to 1.2 mg·kg−1 (P3, horizon O/C), Co up to 1.1 mg·kg−1 (P9, horizon AC), Ni and Cd up to 0.3 mg·kg−1, Cr up to 0.9 mg·kg−1, Sr up to 58 mg·kg−1, Ba up to 213 mg·kg−1 and Mn up to 2242 mg·kg−1 (P13, horizon AC) were recorded. These results indicated the potential mobility of several PTTE in the studied Technosols, which could pose environmental risks through possible uptake of PTTE by plants and leaching of PTTE into groundwater and surface water, including mountain streams typically found in the vicinity of sampling points near waste disposal sites, adits, shafts and historical smelting areas. Such findings were consistent with previous studies emphasising the importance of the exchangeable fraction (F1) as a key indicator of bioavailable and mobile metals in contaminated soils [29,30,44,67,69].

Table 3.
Contents of PTTE in the most mobile fraction (F1) based on BCR sequential extraction.
4.4. Relationship Between Element Fractionation and Soil Properties
Table S3 presents the correlation coefficients between soil pH, carbonate content, TOC and the concentrations of elements in different BCR fractions (F1–F4). Correlation of pH with contents of elements in fractions (F1–F4) showed negative correlations between soil pH and contents of Cu, Zn, Pb, Sb, Ba, Co and Mn in F1 (Table S3), which was considered to be the most mobile fraction. This indicated that the lower the soil pH, the higher the contents of the aforementioned elements in F1. Therefore, in acidic soils, one should expect the highest mobility of Cu, Zn, Pb, Sb, Ba, Co and Mn. On the other hand, correlations between soil pH and contents of As and Sr in F1 (Table S3) were positive, which indicated that the higher the pH (within the ranges of pH found in the studied soils), the higher the mobility of As and Sr in the studied soils.
Statistical analyses showed significant negative correlations between carbonate contents and Cu, Zn, Pb, Sb, Ba, Co and Mn concentrations in F1 (Table S3). These results showed that the occurrence of carbonates in soils reduced the mobility of the aforementioned elements. This result perfectly fit the aforementioned correlation between element concentrations and pH.
Statistical analyses also showed significant positive correlations between TOC contents and Zn, Pb and Cr concentrations in F3 (Table S3), which was considered to be a fraction related to SOM. These results showed that the aforementioned elements had a high affinity for SOM. On the other hand, As was an element not related to SOM in the studied Technosols, which was corroborated by the negative correlation between TOC contents and concentration of As in F3. These findings were in line with the results of other authors [91,92,93].
4.5. Geochemical Features of Technosol Groups Based on the Total Contents of PTTE and REE
The total PTTE and REE contents revealed distinct geochemical signatures across the three groups of Technosols, highlighting the critical role of the parent material in controlling the chemical composition of the investigated soils, which was postulated in our previous study [17].
Group I Technosols (in particular Profiles 1 and 2), developed from carbonate-rich mining waste, were characterised by t elevated concentrations of siderophile elements (Co and Ni) (Figure 1; Table S1), as well as Fe and Mn [17], which reflected the geogenic origin of these elements from rocks containing dispersed Fe- and Mn-ore bearing minerals. Group I Technosols were also characterised by relatively high total REE contents (up to 786 mg·kg−1) (in comparison with other soils), which was most likely due to the high abundance of REE-bearing minerals and the strong affinity of REE for carbonate minerals in rocks [94].
Group II Technosols (Profiles 5–10), developed from aluminosilicate mining waste containing remnants of polymetallic ores, exhibited very high variability in PTTE contents, with notable enrichments in chalcophile elements (Cu, Mo, Hg, As, Sb and Bi), but also Ba, Sr, U and Th (Figure 1; Table S1). These patterns reflected the polymetallic nature of ores scattered in the parent rocks. The lower total REE contents (up to 346 mg·kg−1) (Table 2) compared to Group I suggested most likely a lower abundance of REE-bearing minerals in the parent material.
Group III Technosols (Profiles 11–13), containing waste from smelting activities, displayed the most complex contamination patterns. Excluding the 3C horizon from Profile 12 (as it was most likely a layer of ore-bearing rocks preserved in the soil profile), it should be stated that these soils were enriched in a number of metal(loids) (Cu, As, Sb, Ba, Hg, Co and Ag) coming from the ores processed in smelters, which were mostly represented by polymetallic ores. The relatively low REE contents (<237 mg·kg−1) (Table 2) was also a feature of Group III Technosols.
5. Conclusions
- The studied Technosols in the Tatra Mountains exhibit significant contamination with several PTTE exceeding Polish regulatory limits for Cu, Zn, Pb, Mo, Hg, As, Co, Ni and Ba.
- The three Technosol groups distinguished in the study are characterised by different geochemical features. Group I Technosols (near-neutral soils developed from Fe/Mn-ore and carbonate-bearing mining waste) are particularly enriched in Co, Ni and REE and contain Cu, Zn, Pb, As, Co, Ni and Cr mostly in immobile forms.
- Group II Technosols (acidic soils developed from polymetallic ore-bearing aluminosilicate mining waste) contained elevated concentrations of Cu, Zn, Hg, As, Sb, Bi, Co, Ag, Ba, Sr, U and Th; the contents of REE were lower than in Group I Technosols. PTTE (in particular Mn, Cu and Zn) exhibited the highest mobility among the studied soils, and Fe and Mn oxides seem to control the mobility of Co, Pb, Cu, Ag, Mn and Ba.
- Group III Technosols (soils developed in smelting-affected areas and containing metallurgical waste) contained high total concentrations of Cu, As, Sb, Ba, Hg, Co and Ag, the lowest REE contents among the studied soils and were characterised by complex behaviours of PTTE, with Pb and Cu having high affinity to Fe/Mn oxides and organic matter, respectively.
- The study showed relationships between element fractionation and soil properties such as pH, presence of carbonates and contents of SOM. Statistical analyses showed increases in Cu, Zn, Pb, Sb, Ba, Co and Mn mobility along with decreases in soil pH and carbonate contents. However, the behaviours of As and Sr exhibited the opposite trend: the higher the pH, the higher the mobility of these elements. The results also showed that Zn, Pb and Cr have a high affinity for SOM. On the other hand, As is an element whose mobility is not related to SOM in the studied Technosols.
- The four-step BCR analysis allowed for the identification of PTTE in the most mobile forms (fraction F1). High contents of Cu, Zn, Sb, Pb, As, Co, Ni, Cd, Cr, Sr, Ba and Mn were found in F1 in specific horizons of several Technosols, indicating that these elements may be readily mobilised. This highlights the potential environmental risks of PTTE related to possible uptake of PTTE by plants and leaching of PTTE into groundwater and surface water.
- The findings highlight opportunities for further research to better understand the mechanisms driving PTTE mobility in contaminated soils. Future studies could explore the accumulation of PTTE in plants, as well as how pH variations and interactions with groundwater and surface water influence the mobilisation of individual elements. Such research may also support predictions of how PTTE could spread from contaminated soils, which could improve environmental risk assessment in Tatra National Park.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/min15090988/s1, Table S1: Total concentrations of trace elements in the studied soils; Table S2: Selected chemical properties of the studied soils [17]; Table S3: Statistical analysis.
Author Contributions
Conceptualization, M.T., Ł.U., W.K. and A.P.; methodology, M.T., Ł.U., W.K., A.P. and F.J.M.-P.; validation, M.T., Ł.U., W.K., A.P. and F.J.M.-P.; investigation, M.T., Ł.U., W.K., A.P. and F.J.M.-P.; data curation, M.T. and Ł.U.; writing—original draft preparation, M.T., Ł.U. and A.P.; writing—review and editing, M.T., Ł.U., W.K., A.P. and F.J.M.-P.; visualization, M.T.; supervision, Ł.U. and W.K.; project administration, M.T.; funding acquisition, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Science Centre, Poland, under research project no. 2021/41/N/ST10/03129.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, and further inquiries can be directed to the corresponding author.
Acknowledgments
The Minister of Climate and Environment of Poland, as well as the Director of Tatra National Park (TNP), are acknowledged for giving permission to conduct studies in the TNP. Maria Król and Magdalena Sitarz (TNP), as well as Arletta Kochańska-Jeziorska, are acknowledged for their help with field work and in finding the literature related to the topic of the study. María del Carmen Carrasco Sierra (Universidad de Granada, Spain) is acknowledged for her assistance with laboratory analyses.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| BCR | The modified European Community Bureau of Reference |
| F | Fraction |
| ICP-MS | Inductively coupled plasma-mass spectroscopy |
| ICP-OES | Inductively coupled plasma-optical emission spectrometry |
| P | Profile |
| PTTE | Potentially toxic trace elements |
| REE | Rare earth elements |
| SEM-EDS | Scanning electron microscope—energy dispersive X-ray spectroscopy |
| SOM | Soil organic matter |
| TNP | Tatra National Park |
| TOC | Total organic carbon |
References
- Skrzydłowski, T. Przewodnik Przyrodniczy po Tatrach Polskich; Wydawnictwo Tatrzańskiego Parku Narodowego: Zakopane, Poland, 2019; pp. 1–432. [Google Scholar]
- Jost, H. O górnictwie i Hutnictwie w Tatrach Polskich; Wydawnictwo Naukowo-Techniczne: Warsaw, Poland, 1962; pp. 1–182. (In Polish) [Google Scholar]
- Górecki, J.; Sermet, E. Hawiarskie Szlaki Tatr Polskich, Dzieje Górnictwa–Element Europejskiego Dziedzictwa Kultury; Oficyna Wydawnicza Politechniki Wrocławskiej: Wrocław, Poland, 2012. (In Polish) [Google Scholar]
- Zwoliński, S. Badania nad Historią Górnictwa i Hutnictwa w Tatrach Polskich. Etnogr. Pol. 1962, 6, 163–191. (In Polish) [Google Scholar]
- Jach, R. Ślady dawnego wydobycia rud manganu w Tatrach Zachodnich. Przegląd Geol. 2002, 50, 1159–1164. (In Polish) [Google Scholar]
- Gawęda, A. How to find a gold on the touristic path-a gold-mining in the Tatra Mts. Geoturystyka 2010, 2, 59–64. [Google Scholar] [CrossRef][Green Version]
- Kabała, C.; Greinert, A.; Charzyński, P.; Uzarowicz, Ł. Technogenic soils—Soils of the year 2020 in Poland. Concept, properties and classification of technogenic soils in Poland. Soil Sci. Annu. 2020, 71, 267–280. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Néel, C.; Bril, H.; Courtin-Nomade, A.; Dutreuil, J.P. Factors affecting natural development of soil on 35-year-old sulphide-rich mine tailings. Geoderma 2003, 111, 1–20. [Google Scholar] [CrossRef]
- Grünewald, G.; Kaiser, K.; Jahn, R. Alteration of secondary minerals along a time series in young alkaline soils derived from carbonatic wastes of soda production. Catena 2007, 71, 487–496. [Google Scholar] [CrossRef]
- Huot, H.; Simonnot, M.O.; Watteau, F.; Marion, P.; Yvon, J.; De Donato, P.; Morel, J.L. Early transformation and transfer processes in a Technosol developing on iron industry deposits. Eur. J. Soil Sci. 2014, 65, 470–484. [Google Scholar] [CrossRef]
- Uzarowicz, Ł.; Charzyński, P.; Greinert, A.; Hulisz, P.; Kabała, C.; Kusza, G.; Kwasowski, W.; Pędziwiatr, A. Studies of technogenic soils in Poland: Past, present, and future perspectives. Soil Sci. Annu. 2020, 71, 281–299. [Google Scholar] [CrossRef]
- Kierczak, J.; Pietranik, A.; Piatak, N.M. Weathering of Slags. In Metallurgical Slags: Environmental Geochemistry and Resource Potential; Piatak, N.M., Ettler, V., Eds.; The Royal Society of Chemistry: London, UK, 2021; Volume 3, pp. 125–150. [Google Scholar]
- Watteau, F.; Morel, J.L.; Liu, C.; Tang, Y.; Huot, H. Technosol micromorphology reveals the early pedogenesis of abandoned rare earth element mining sites undergoing reclamation in south China. Minerals 2025, 15, 514. [Google Scholar] [CrossRef]
- Huot, H.; Simonnot, M.O.; Morel, J.L. Pedogenetic trends in soils formed in technogenic parent materials. Soil Sci. 2015, 180, 182–192. [Google Scholar] [CrossRef]
- Uzarowicz, Ł.; Kwasowski, W.; Lasota, J.; Błońska, E.; Górka-Kostrubiec, B.; Tarnawczyk, M.; Murach, D.; Gilewska, M.; Gryczan, W.; Pawłowicz, E.; et al. Vegetation cover as an important factor affecting the properties and evolution of Spolic Technosols: A case study from a dump of the abandoned iron ore mine in central Poland. Catena 2025, 254, 108906. [Google Scholar] [CrossRef]
- Tarnawczyk, M.; Uzarowicz, Ł.; Kwasowski, W.; Górka-Kostrubiec, B.; Pędziwiatr, A. Soil-forming factors controlling Technosol formation in historical mining and metallurgical sites in the high-alpine environment of the Tatra Mountains, southern Poland. Catena 2024, 247, 108521. [Google Scholar] [CrossRef]
- Tarnawczyk, M.; Uzarowicz, Ł.; Pędziwiatr, A.; Kwasowski, W. Micromorphological, submicromorphological and chemical indicators of pedogenesis in Spolic Technosols developed at historical mining and metallurgical sites in the Tatra Mountains, southern Poland. Soil Sci. Annu. 2025, 76, 205499. [Google Scholar]
- Feluch, K.; Uzarowicz, Ł. Soil development and contents of selected trace elements in Technosols on dumps of historical iron ore mines in the Jaworzynka Valley, Tatra Mountains, southern Poland. Soil Sci. Annu. 2025, 76, 205499. [Google Scholar] [CrossRef]
- Alloway, B.; Ayres, D.C. Chemical Principles of Environmental Pollution, 2nd ed.; Blackie Academic: London, UK, 1997; pp. 3–85. [Google Scholar]
- Gomez-Ros, J.M.; Garcia, G.; Peñas, J.M. Assessment of restoration success of former metal mining areas after 30 years in a highly polluted Mediterranean mining area: Cartagena-La Unión. Ecol. Eng. 2013, 57, 393–402. [Google Scholar] [CrossRef]
- Pellegrini, S.; García, G.; Peñas-Castejon, J.M.; Vignozzi, N.; Costantini, E.A.C. Pedogenesis in mine tails affects macroporosity, hydrological properties, and pollutant flow. Catena 2016, 136, 3–16. [Google Scholar] [CrossRef]
- Dradrach, A.; Karczewska, A.; Szopka, K. Arsenic accumulation by red fescue (Festuca rubra) growing in mine-affected soils—Findings from the field and greenhouse studies. Chemosphere 2020, 248, 126045. [Google Scholar] [CrossRef]
- Dradrach, A.; Karczewska, A.; Szopka, K.; Lewińska, K. Accumulation of arsenic by plants growing in the sites strongly contaminated by historical mining in the Sudetes region of Poland. Int. J. Environ. Res. Public Health 2020, 17, 3342. [Google Scholar] [CrossRef]
- Ferronato, C.; Vianello, G.; Feudis, M.D.; Vittori Antisari, L. Technosols development in an abandoned mining area and environmental risk assessment. Appl. Sci. 2021, 11, 6982. [Google Scholar] [CrossRef]
- Castillo Corzo, M.; Peña Rodríguez, V.; Manrique Nugent, M.; Villarreyes Peña, E.; Byrne, P.; Gonzalez, J.C.; Patiño Camargo, G.; Barnes, C.H.W.; Sánchez Ortiz, J.F.; Saldaña Tovar, J.; et al. Potentially toxic elements and radionuclides contamination in soils from the vicinity of an ancient mercury mine in Huancavelica, Peru. Soil Sci. Annu. 2025, 76, 204389. [Google Scholar] [CrossRef]
- García-Lorenzo, M.L.; Crespo-Feo, E.; Esbrí, J.M.; Higueras, P.; Grau, P.; Crespo, I.; Sánchez-Donoso, R. Assessment of potentially toxic elements in technosols by tailings derived from Pb–Zn–Ag mining activities at San Quintín (Ciudad Real, Spain): Some insights into the importance of integral studies to evaluate metal contamination pollution hazards. Minerals 2019, 9, 346. [Google Scholar] [CrossRef]
- Kierczak, J.; Neel, C.; Aleksander-Kwaterczak, U.; Helios-Rybicka, E.; Bril, H.; Puziewicz, J. Solid speciation and mobility of potentially toxic elements from natural and contaminated soils: A combined approach. Chemosphere 2008, 73, 776–784. [Google Scholar] [CrossRef]
- Kodirov, O.; Kersten, M.; Shukurov, N.; Peinado, F.J.M. Trace metal (loid) mobility in waste deposits and soils around Chadak mining area, Uzbekistan. Sci. Total Environ. 2018, 622, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Kicińska, A.; Pomykała, R.; Izquierdo-Diaz, M. Changes in soil pH and mobility of heavy metals in contaminated soils. Eur. J. Soil Sci. 2022, 73, e13203. [Google Scholar] [CrossRef]
- Korzeniowska, J.; Krąż, P. Heavy Metals Content in the Soils of the Tatra National Park Near Lake Morskie Oko and Kasprowy Wierch—A Case Study (Tatra Mts, Central Europe). Minerals 2020, 10, 1120. [Google Scholar] [CrossRef]
- Staszewski, T.; Łukasik, W.; Kubiesa, P. Contamination of Polish national parks with heavy metals. Environ. Monit. Assess. 2012, 184, 4597–4608. [Google Scholar] [CrossRef]
- Kwapuliński, J.; Paprotny, L.; Paukszto, A.; Kowol, J.; Rochel, R.; Nogaj, E.; Musielińska, R.; Celinski, R. Influence of the type of tree habitat on the character of co-occurrence of Fe, Mn, Zn, Cu, Pb, Ni, Cr and Co in the soil of the Tatra Mountain National Park. Ann. Agric. Environ. Med. 2013, 20, 494–499. [Google Scholar]
- Kwapuliński, J.; Paukszto, A.; Paprotny, Ł.; Musielińska, R.; Kowol, J.; Nogaj, E.; Rochel, R. Bioavailability of Lead, Cadmium, and Nickel in Tatra Mountain National Park Soil. Pol. J. Environ. Stud. 2012, 21, 407–413. [Google Scholar]
- Kubica, B.; Kwiatek, W.M.; Stobiński, M.; Skiba, S.; Skiba, M.; Gołaś, J.; Kubica, M.; Tuleja-Krysa, M.; Wrona, A.; Misiak, R.; et al. Concentrations of 137Cs, 40K radionuclides and some heavy metals in soil samples of Chochołowska Valley from Tatra National Park. Pol. J. Environ. Stud. 2007, 16, 723–729. [Google Scholar]
- Stobiński, M.; Kubica, B. Chemometric analysis of 137Cs activity and heavy metals distribution in the Tatras’ soil. Int. J. Environ. Sci. Technol. 2017, 14, 1217–1224. [Google Scholar] [CrossRef]
- Wieczorek, J.; Zadrożny, P. Content of Cd, Pb and Zn in podzols Tatra National Park. Proc. ECOpole 2013, 7, 57. (In Polish) [Google Scholar] [CrossRef]
- Kowalska, J.B.; Gąsiorek, M.; Zadrożny, P.; Nicia, P.; Waroszewski, J. Deep subsoil storage of trace elements and pollution assessment in mountain Podzols (Tatra Mts., Poland). Forests 2021, 12, 291. [Google Scholar] [CrossRef]
- Niemyska-Łukaszuk, J. Całkowita zawartość ołowiu w profilach rankerów Tatrzańskiego Parku Narodowego. Zesz. Probl. Postępów Nauk. Rol. 1999, 467, 429–437. (In Polish) [Google Scholar]
- Niemyska-Łukaszuk, J.; Miechówka, A. Zawartość cynku, ołowiu i kadmu w poziomach powierzchniowych gleb obszarów nieleśnych Tatrzańskiego Parku Narodowego. Przemiany Sr. Przyr. Tatr 2002, 99–103. (In Polish) [Google Scholar]
- Miechówka, A.; Niemyska-Łukaszuk, J. Content diversity of Zn, Pb and Cd in Lithic Leptosols of the Tatra National Park (Poland). Oecologia Mont. 2004, 13, 1–5. [Google Scholar]
- Miechówka, A.; Niemyska-Lukaszuk, J.; Gasiorek, M. Content of Zn, Pb, Cd and Ni in peat-bog and fen soils in the Tatra National Park. Acta Agrophys. 2002, 67, 163–172. [Google Scholar]
- Miechówka, A.; Gąsiorek, M.; Zaleski, T. Contents of cadmium and nickel in soils and plants on grazed mountain glades in Tatra National Park. Zesz. Probl. Postępów Nauk. Rol. 1997, 448, 197–202. [Google Scholar]
- Paprotny, Ł.; Kwapuliński, J.; Wianowska, D.; Gnatowski, M.; Kasprzyk-Pochopień, J.; Piekoszewski, W. A Comparison of Co-Occurrence of Special Forms of Selected Metals in Soil, on the Example of Sycamore, Beech, and Spruce Forest Complexes in Urbanized and Non-Urbanized Regions of Tatra National Park. Pol. J. Environ. Stud. 2024, 33, 4273–4282. [Google Scholar] [CrossRef] [PubMed]
- Miechówka, A. Zawartość różnych form żelaza w rędzinach położonych powyżej górnej granicy lasu w Tatrach. Rocz. Glebozn. Soil Sci. Annu. 2001, 52, 135–143. (In Polish) [Google Scholar]
- Miechówka, A.; Niemyska-Łukaszuk, J.; Ciarkowska, K. Heavy metals in selected non-forest soils from the Tatra National Park. Chem. I Inżynieria Ekol. 2002, 9, 1433–1438. [Google Scholar]
- Paukszto, A.; Mirosławski, J. Using stinging nettle (Urtica dioica L.) to assess the influence of long-term emission upon pollution with metals of the Tatra National Park area (Poland). Atmos. Pollut. Res. 2019, 10, 73–79. [Google Scholar] [CrossRef]
- Ciarkowska, K.; Miechówka, A. Identification of the factors determining the concentration and spatial distribution of Zn, Pb and Cd in the soils of the non-forest Tatra Mountains (southern Poland). Environ. Geochem. Health 2022, 44, 4323–4341. [Google Scholar] [CrossRef]
- Demková, L.; Bobuľská, L.; Árvay, J.; Homolová, Z.; Michalko, M.; Bálintová, M. Potentially toxic elements in soil and air along an altitudinal gradient in Tatra National Park. J. Geochem. Explor. 2023, 252, 107268. [Google Scholar] [CrossRef]
- Van Reeuwijk, L.P. Procedures for Soil Analysis; Technical Paper 9; ISRIC: Wageningen, The Netherlands, 2002. [Google Scholar]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis. Mineralogical, Organic and Inorganic Methods; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Soil Science Division Staff. Soil Survey Manual; Ditzler, C., Scheffe, K., Monger, H.C., Eds.; USDA Handbook 18; Government Printing Office: Washington, DC, USA, 2017. [Google Scholar]
- Ure, A.M.; Quevauviller, P.; Muntau, H.; Griepink, B. Speciation of heavy metals in soils and sediments—An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. Int. J. Environ. Anal. Chem. 1993, 51, 135–151. [Google Scholar] [CrossRef]
- Mossop, K.F.; Davidson, C.M. Comparison of original and modified BCR sequential extraction procedures for the fractionation of copper, iron, lead, manganese and zinc in soils and sediments. Anal. Chim. Acta 2003, 478, 111–118. [Google Scholar] [CrossRef]
- Rauret, G.; López-Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR three-step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef]
- Chancellery of the Sejm of the Republic of Poland. Regulation of the Minister of Climate and Environment of 31 October 2024 Amending the Regulation on the Method of Conducting the Assessment of Ground Surface Pollution, 2024, No. 2024, Item 1657. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20240001657 (accessed on 13 September 2025).
- Charzyński, P.; Plak, A.; Hanaka, A. Influence of the soil sealing on the geoaccumulation index of heavy metals and various pollution factors. Environ. Sci. Pollut. Res. 2017, 24, 4801–4811. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007; pp. 283–319. [Google Scholar]
- Kabata-Pendias, A.; Szteke, B. Trace Elements in Abiotic and Biotic Environments; Taylor & Francis Group: Boca Raton, FL, USA, 2015; p. 468. [Google Scholar]
- Sujith, P.P.; Gonsalves, M.J.B.D. Ferromanganese oxide deposits: Geochemical and microbiological perspectives of interactions of cobalt and nickel. Ore Geol. Rev. 2021, 139, 104458. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2000; p. 432. [Google Scholar] [CrossRef]
- Pingitore, N.E.; Eastman, M.P. Barium partitioning in carbonates: Theory and applications. AAPG Bull. 1984, 68, 516. [Google Scholar] [CrossRef]
- Wu, K.; Ueno, Y.; Surma, J.; Nakajo, C. Separate determination of strontium and barium mass fractions in calcite and dolomite in carbonate rocks by a multi-step sequential leaching procedure. Geostand. Geoanalytical Res. 2025, 1–19. [Google Scholar] [CrossRef]
- Canet, C.; Anadón, P.; Alfonso, P.; Prol-Ledesma, R.M.; Villanueva-Estrada, R.E.; García-Vallès, M. Gas-seep related carbonate and barite authigenic mineralization in the northern Gulf of California. Mar. Pet. Geol. 2013, 43, 147–165. [Google Scholar] [CrossRef]
- Karczewska, A.; Lewińska, K.; Agata, M.; Krysiak, A. Soil pollution by arsenic within the allotment gardens in Zloty Stok. Ecol. Chem. Eng. 2010, 17, 927–933. [Google Scholar]
- Tarnawczyk, M.; Uzarowicz, Ł.; Perkowska-Pióro, K.; Pędziwiatr, A.; Kwasowski, W. Effect of land reclamation on soil properties, mineralogy and trace-element distribution and availability: The example of technosols developed on the tailing disposal site of an abandoned Zn and Pb mine. Minerals 2021, 11, 559. [Google Scholar] [CrossRef]
- Uzarowicz, Ł.; Swęd, M.; Kwasowski, W.; Pędziwiatr, A.; Kaczmarek, D.; Koprowska, D.; Górka-Kostrubiec, B.; Pawłowicz, E.; Murach, D. Initial pedogenic processes, mineral and chemical transformations and mobility of trace elements in Technosols on dumps of the former copper mines in Miedziana Góra and Miedzianka, the Świętokrzyskie Mts., south-central Poland. Catena 2024, 245, 108293. [Google Scholar] [CrossRef]
- Sitarz, M.; Gołębiowska, B.; Nejbert, K.; Dimitrova, D.; Milovský, R. Hydrothermal ore mineralization from the Polish part of the Tatra Mts., Central Western Carpathians. Geol. Geophys. Environ. 2021, 47, 159–179. [Google Scholar] [CrossRef]
- Swęd, M.; Uzarowicz, Ł.; Duczmal-Czernikiewicz, A.; Kwasowski, W.; Pędziwiatr, A.; Siepak, M.; Niedzielski, P. Forms of metal(loid)s in soils derived from historical calamine mining waste and tailings of the Olkusz Zn–Pb ore district, southern Poland: A combined pedological, geochemical and mineralogical approach. Appl. Geochem. 2022, 139, 105218. [Google Scholar] [CrossRef]
- Hazen, R.M.; Golden, J.; Downs, R.T.; Hystad, G.; Grew, E.S.; Azzolini, D.; Sverjensky, D.A. Mercury (Hg) mineral evolution: A mineralogical record of supercontinent assembly, changing ocean geochemistry, and the emerging terrestrial biosphere. Am. Mineral. 2012, 97, 1013–1042. [Google Scholar] [CrossRef]
- Jolly, J.L.; Van Heyl, A. Mercury and Other Trace Elements in Sphalerite and Wallrocks from Central Kentucky, Tennessee, and Appalachian Zinc Districts; United States Government Printing Office: Washington, DC, USA, 1968; p. 29. [Google Scholar] [CrossRef]
- Tombros, S.F.; Seymour, K.S.; Williams-Jones, A.E.; Zhai, D.; Liu, J. Origin of a barite-sulfide ore deposit in the Mykonos intrusion, Cyclades: Trace element, isotopic, fluid inclusion and Raman spectroscopy evidence. Ore Geol. Rev. 2015, 67, 139–157. [Google Scholar] [CrossRef]
- Warchulski, R. Zn-Pb slag crystallization: Evaluating temperature conditions on the basis of geothermometry. Eur. J. Mineral. 2016, 28, 375–384. [Google Scholar] [CrossRef]
- Kierczak, J.; Potysz, A.; Pietranik, A.; Tyszka, R.; Modelska, M.; Néel, C.; Ettler, V.; Mihaljevič, M. Environmental impact of the historical Cu smelting in the Rudawy Janowickie Mountains (south-western Poland). J. Geochem. Explor. 2013, 124, 183–194. [Google Scholar] [CrossRef]
- Potysz, A.; van Hullebusch, E.D.; Kierczak, J.; Grybos, M.; Lens, P.N.; Guibaud, G. Copper metallurgical slags–current knowledge and fate: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2424–2488. [Google Scholar] [CrossRef]
- Tyszka, R.; Pietranik, A.; Potysz, A.; Kierczak, J.; Schulz, B. Experimental simulations of ZnPb slag weathering and its impact on the environment: Effects of acid rain, soil solution, and microbial activity. J. Geochem. Explor. 2021, 228, 106808. [Google Scholar] [CrossRef]
- Ilutiu-Varvara, D.A. Researching the hazardous potential of metallurgical solid wastes. Pol. J. Environ. Stud. 2016, 25, 147–152. [Google Scholar] [CrossRef]
- Kupczak, K.; Warchulski, R.; Ettler, V.; Mihaljevič, M. The impact of buried historical copper slags on contemporary soil contamination. J. Geochem. Explor. 2025, 273, 107743. [Google Scholar] [CrossRef]
- Ding, Q.; Cheng, G.; Wang, Y.; Zhuang, D. Effects of natural factors on the spatial distribution of heavy metals in soils surrounding mining regions. Sci. Total Environ. 2017, 578, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Doner, H.E.; Lynn, W.C. Carbonate, Halide, Sulfate, and Sulfide Minerals. In Minerals in Soil Environments; Dixon, J.B., Weed, S.B., Eds.; American Society for Agronomy and the Soil Science Society of America: Madison, WI, USA, 1989; pp. 279–330. [Google Scholar]
- Lee, H.H. Adsorption characteristics of cadmium onto calcite and its agricultural environmental relevance. Heliyon 2024, 11, e40241. [Google Scholar] [CrossRef]
- Kubová, J.; Matúš, P.; Bujdoš, M.; Hagarová, I. Utilization of optimized BCR three-step sequential and dilute HCl single extraction procedures for soil–plant metal transfer predictions in contaminated lands. Talanta 2008, 75, 1110–1122. [Google Scholar] [CrossRef]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef]
- Chuan, M.C.; Shu, G.Y.; Liu, J.C. Solubility of heavy metals in a contaminated soil: Effects of redox potential and pH. Water Air Soil Pollut. 1996, 90, 543–556. [Google Scholar] [CrossRef]
- Rieuwerts, J.S.; Thornton, I.; Farago, M.E.; Ashmore, M.R. Factors influencing metal bioavailability in soils: Preliminary investigations for the development of a critical loads approach for metals. Chem. Speciat. Bioavailab. 1998, 10, 61–75. [Google Scholar] [CrossRef]
- Martínez, C.E.; Motto, H.L. Solubility of lead, zinc and copper added to mineral soils. Environ. Pollut. 2000, 107, 153–158. [Google Scholar] [CrossRef]
- Wilson, M.J. Weathering of the primary rock-forming minerals: Processes, products and rates. Clay Miner. 2004, 39, 233–266. [Google Scholar] [CrossRef]
- Sracek, O. Formation of secondary hematite and its role in attenuation of contaminants at mine tailings: Review and comparison of sites in Zambia and Namibia. Front. Environ. Sci. 2015, 2, 64. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, D.; Gou, J.; Ni, X.; Zeng, X.; Zhang, X.; Liu, W.; Liang, S.; Deng, C. Chalcopyrite geochemistry: Advancements and implications in ore deposit research. Ore Geol. Rev. 2025, 179, 106528. [Google Scholar] [CrossRef]
- Guillevic, F.; Rossi, M.; Develle, A.-L.; Spadini, L.; Martins, J.M.F.; Arnaud, F.; Poulenard, J. Pb dispersion pathways in mountain soils contaminated by ancient mining and smelting activities. Appl. Geochem. 2023, 150, 105556. [Google Scholar] [CrossRef]
- Romero-Freire, A.; Sierra-Aragón, M.; Ortiz-Bernad, I.; Martín-Peinado, F.J. Toxicity of arsenic in relation to soil properties: Implications to regulatory purposes. J. Soils Sediments 2014, 14, 968–979. [Google Scholar] [CrossRef]
- Hattab, N.; Motelica-Heino, M.; Faure, O.; Bouchardon, J.L. Effect of fresh and mature organic amendments on the phytoremediation of technosols contaminated with high concentrations of trace elements. J. Environ. Manag. 2015, 159, 37–47. [Google Scholar] [CrossRef]
- Paniagua-López, M.; Aguilar-Garrido, A.; Contero-Hurtado, J.; García-Romera, I.; Sierra-Aragón, M.; Romero-Freire, A. Ecotoxicological assessment of polluted soils one year after the application of different soil remediation techniques. Toxics 2023, 11, 298. [Google Scholar] [CrossRef]
- Liang, T.; Li, K.; Wang, L. State of rare earth elements in different environmental components in mining areas of China. Environ. Monit. Assess. 2014, 186, 1499–1513. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).