The Utilization of the Copper Smelting Slag: A Critical Review
Abstract
1. Introduction
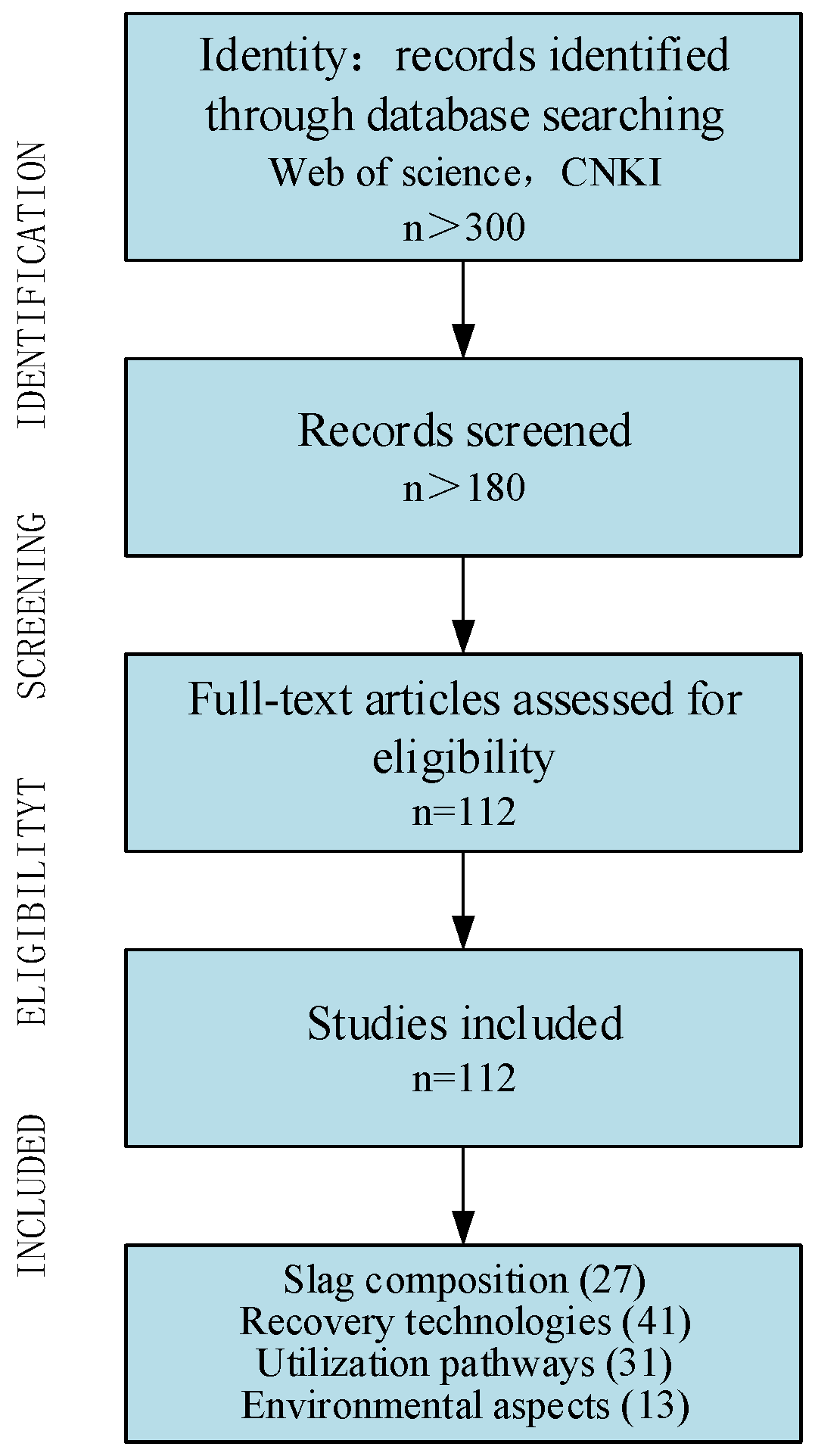
2. Methodology
3. Generation and Properties of Slag
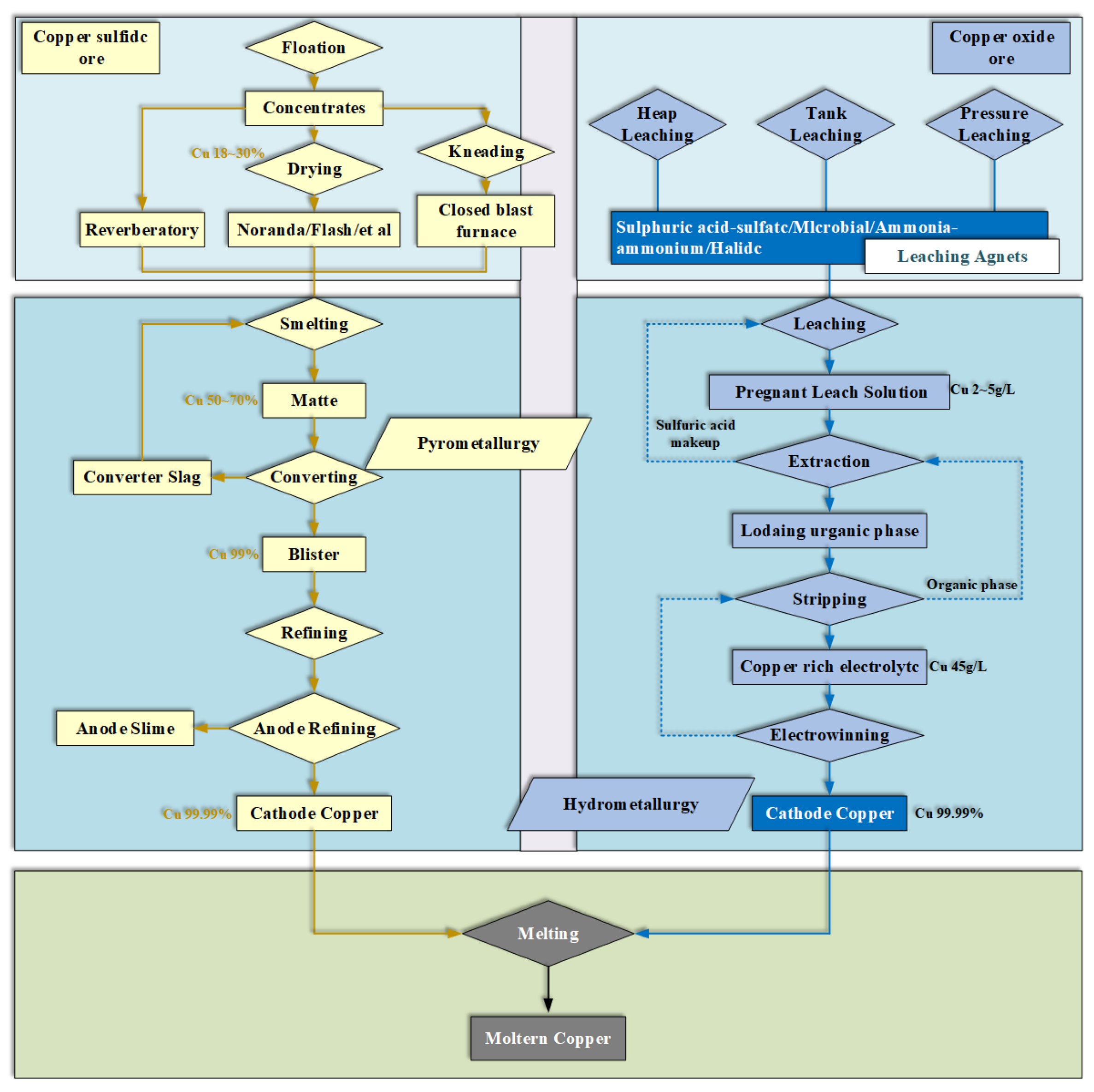
3.1. Copper Smelting Process
3.2. Present Situation of Copper Slag
3.3. Properties
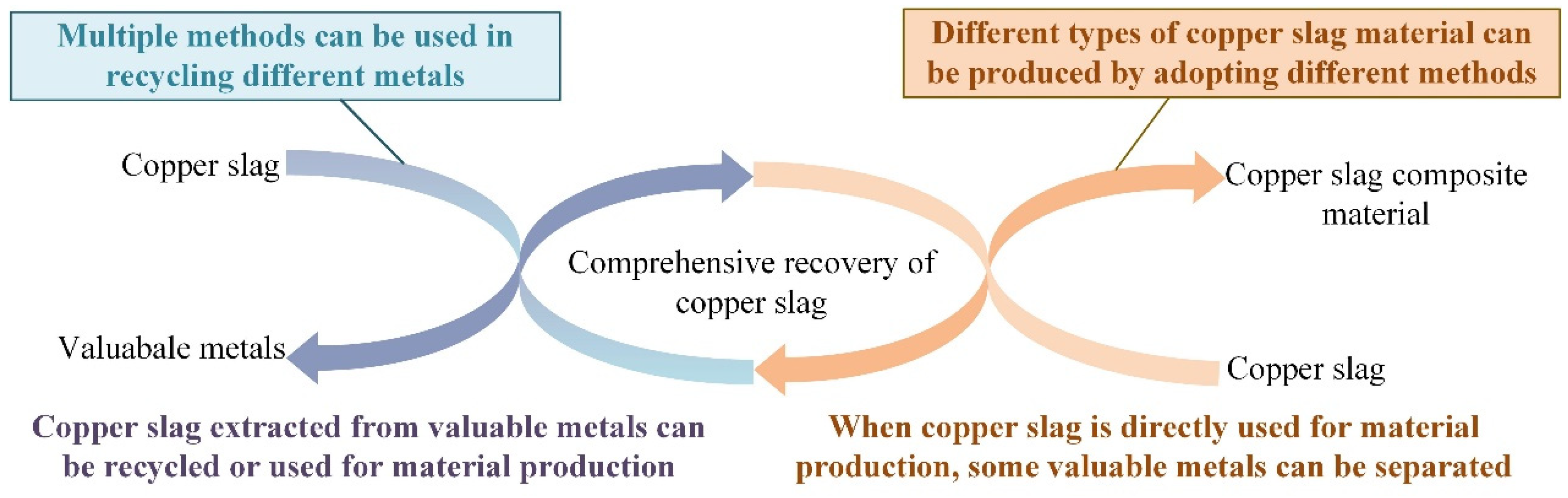
4. Valuable Metal Recycling
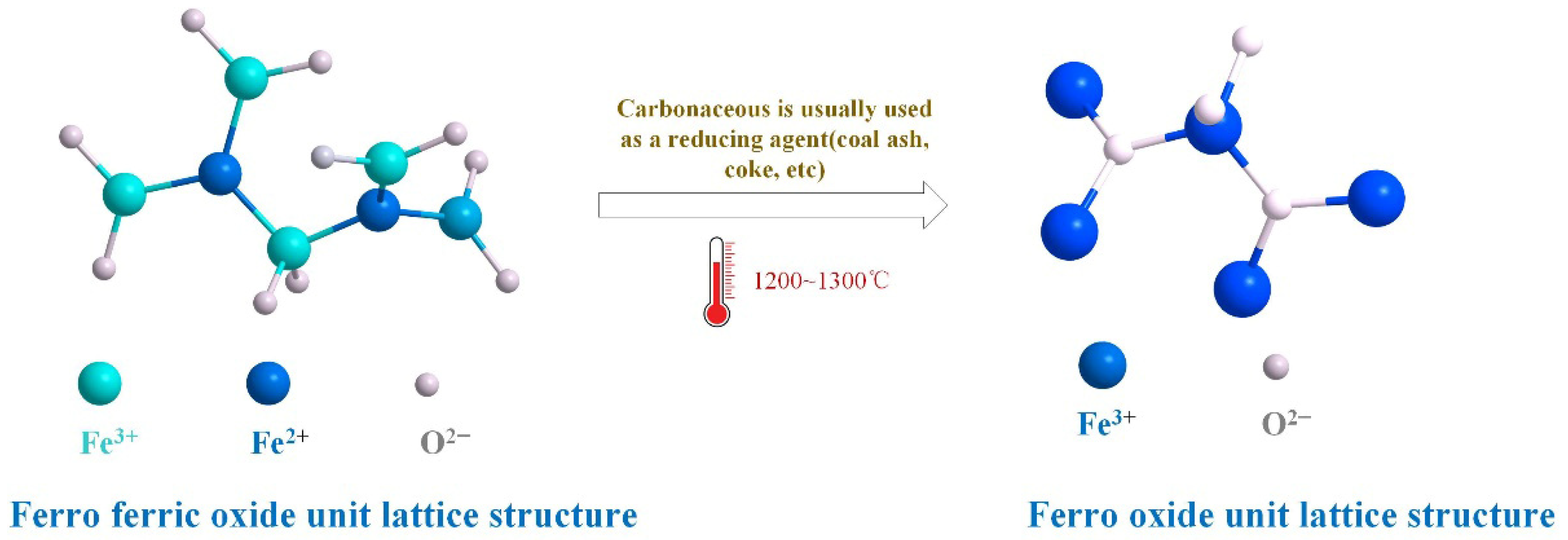
4.1. Pyrometallurgical Impoverishment
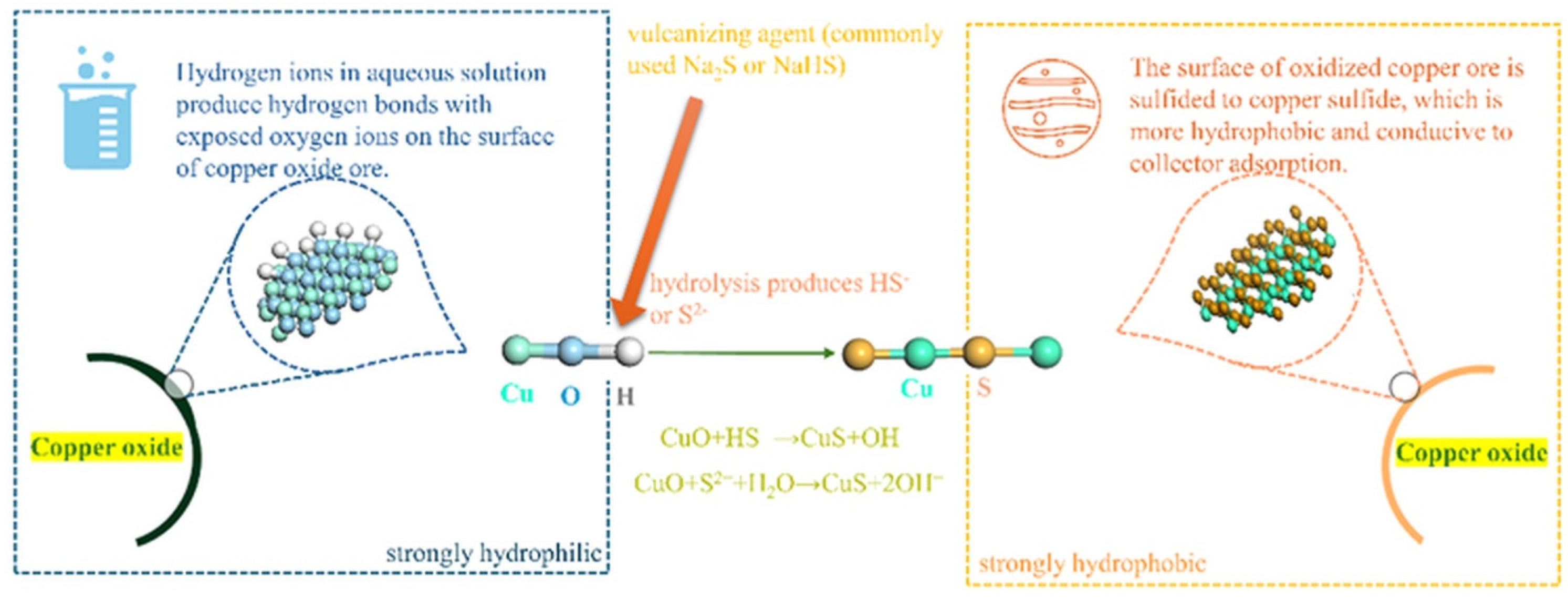
4.2. Mineral Processing Method Recovery
4.3. Hydrometallurgical Recovery
4.4. Other Recycling Methods
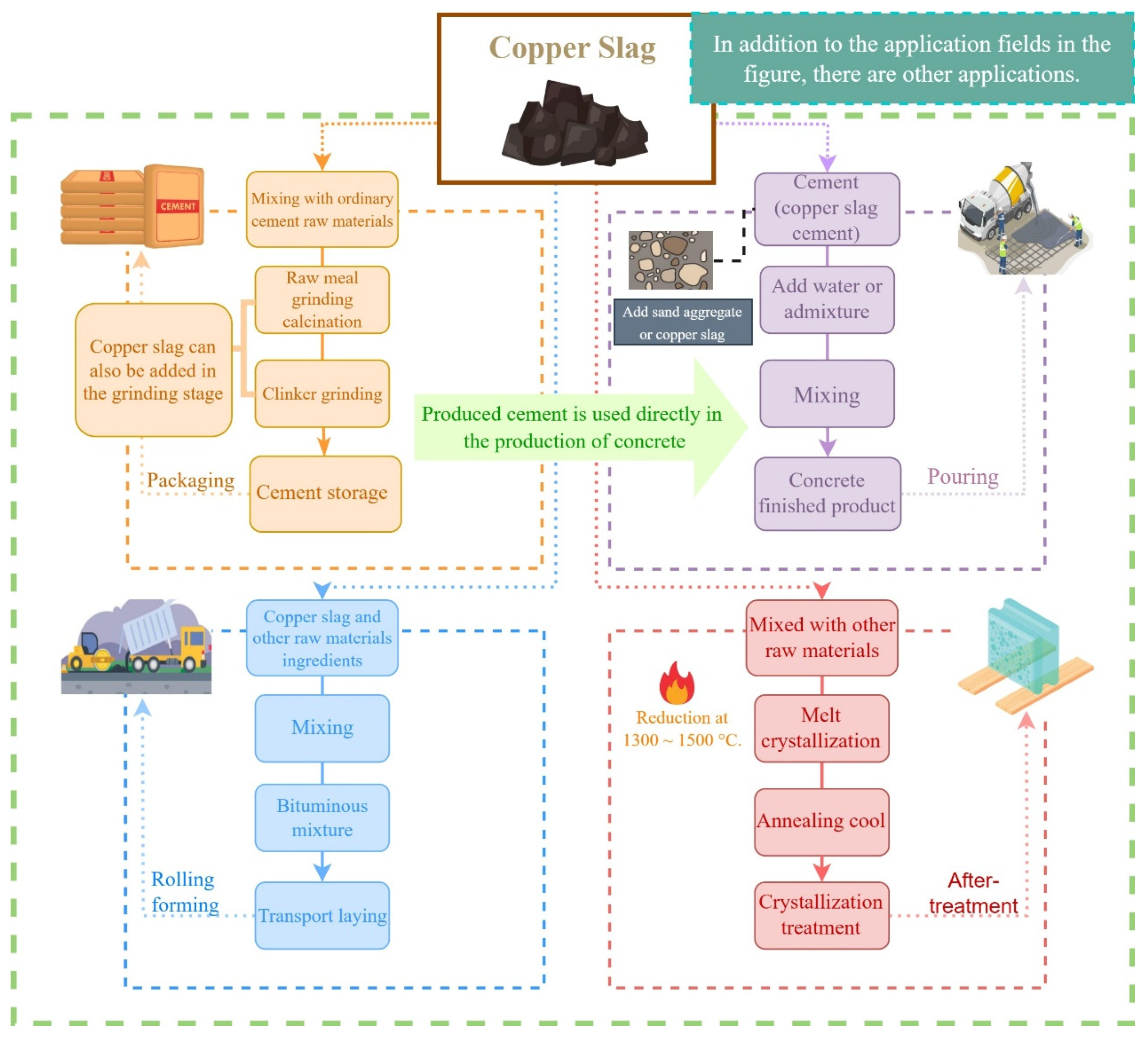
5. Material Application
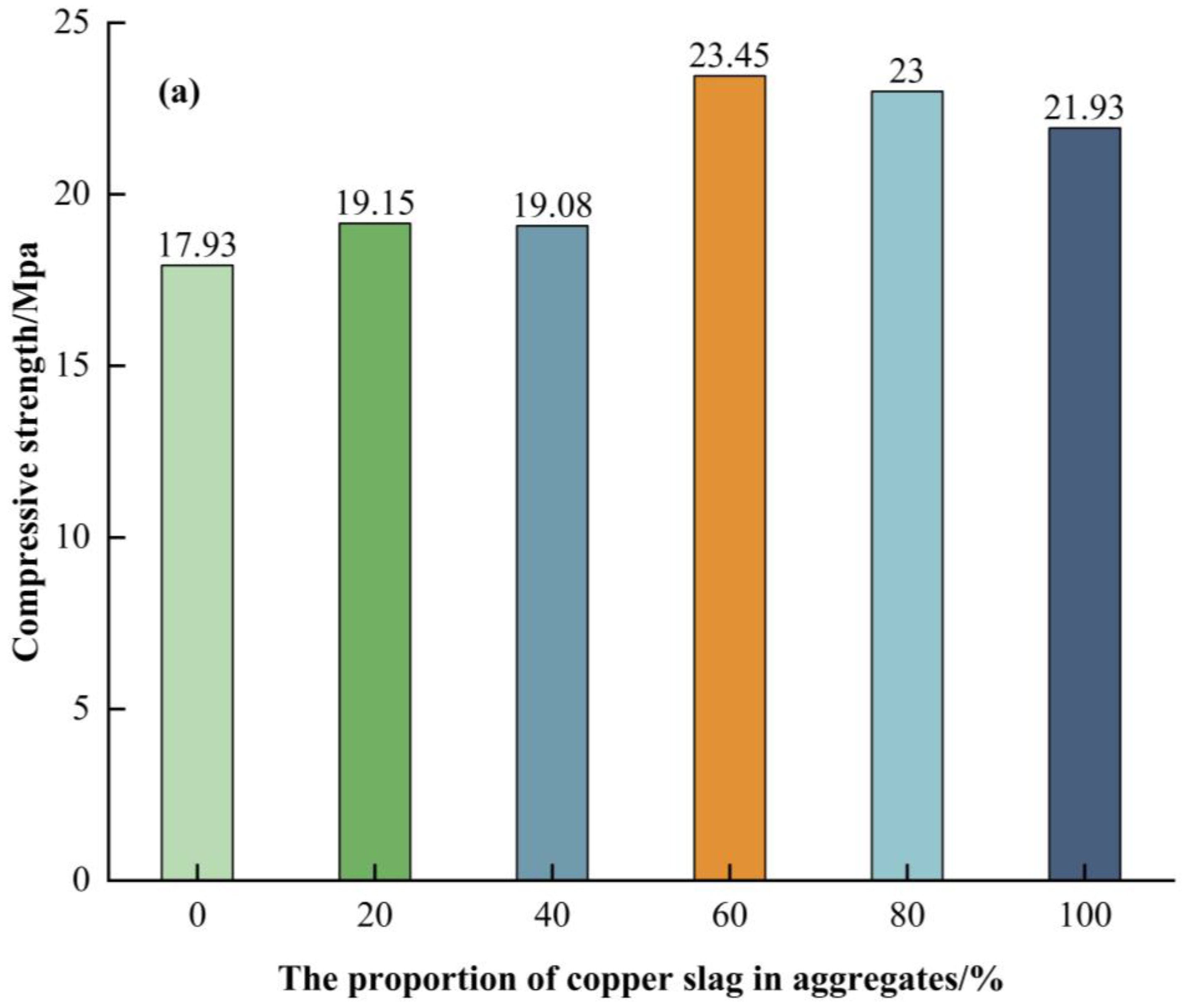
5.1. Concrete
5.2. Cement
5.3. Asphalt
5.4. Glass-Ceramics
5.5. Other Applications
- Copper slag oxidizes for 2 h at temperatures above 800 °C.
- Oxidized slag undergoes hydrothermal treatment with NaOH (140 g/L) at 190 °C for 3 h.
- Heat filtration.
- Change pH to hydrolyze liquid silicate into gel.
- Dry at 80 °C to obtain amorphous silica gel.
5.6. Large-Scale Implementation and Technical Limitations
6. Conclusions and Outlook
- The low recovery rate of valuable metals in copper slag results from their complex embedding with gangue minerals, limitations of single-method recovery, and high costs outweighing profits. Recent studies focus on efficient, cost-effective, and environmentally sustainable recovery techniques driven by economic and environmental needs as a key future research direction.
- The pyrometallurgical impoverishment of copper slag mainly targets the recovery of copper from copper slag and has almost no effect on other valuable metals, which has certain limitations. The differences in raw ore properties and copper smelting processes can lead to changes in the properties of copper slag. If flotation recovery is used, the reagents usually need to be replaced, and there are issues such as the cost of reagents and whether the reagents are toxic. The single magnetic separation method can only separate part of the iron phase from the copper slag, and the magnetic separation concentrate is usually accompanied by some gangue minerals, which affects the magnetic separation recovery rate. The magnetic flotation combined process can treat the most valuable metals in copper slag. However, at present, the demand for copper slag treatment is significant, and it cannot be widely applied in industry in the short term. In particular, integrating magnetic–flotation technology with environmentally friendly leaching agents could offer a promising direction for future research and provide a more sustainable and efficient approach to copper slag treatment.
- Copper slag has a wide range of material fields but mainly focuses on cement, concrete aggregates, and the production of glass-ceramic materials. Domestic and foreign research scholars have made abundant achievements in these two areas, but there is a lack of feasible solutions for large-scale industrial production. In the future, the direct recycling and utilization of copper slag needs to be considered from the perspective of industrial practice.
- The comprehensive recovery of copper smelting slag is a promising research topic that can improve resource utilization efficiency while reducing environmental pollution. However, further technological research and the strengthening of large-scale applications are still needed to achieve effective comprehensive recycling and sustainable development.
Funding
Conflicts of Interest
References
- Chen, J.H.; Shuai, J.; Zhao, Y.J.; Duan, H.R.; Shuai, C.M. Risk assessment and prediction of critical mineral resources supply for China: A case of copper. Resour. Sci. 2023, 45, 1778–1788. [Google Scholar] [CrossRef]
- Lin, Y.M.; Han, B.S.; Jiang, L.S.; Li, X.Y.; Xu, W.T.; Xie, H.Y. Research status and prospects of flotation methods and reagents for oxidized copper ore. Multipurp. Util. Miner. Resour. 2024, 45, 112–120. [Google Scholar] [CrossRef]
- International Copper Study Group. Copper Market Forecast 2023/2024; Press Releases International Copper Study Group (icsg.org); International Copper Study Group: Lisbon, Portugal, 2023. [Google Scholar]
- International Copper Study Group. Copper: Preliminary Data C for March 2024; Press Releases–International Copper Study Group (icsg.org); International Copper Study Group: Lisbon, Portugal, 2024. [Google Scholar]
- Wu, L.; Hao, Y.D. The investigation of utilization status of copper slag resources and efficient utilization. China Nonferrous Metall. 2015, 44, 61–64. [Google Scholar]
- Gabasiane, T.S.; Danha, G.; Mamvura, T.A.; Mashifana, T.; Dzinomwa, G. Characterization of copper slag for beneficiation of iron and copper. Heliyon 2021, 7, e06757. [Google Scholar] [CrossRef]
- Chew, S.H.; Bharati, S.K. Use of recycled copper slag in cement-treated Singapore marine clay. In Advances in Environmental Geotechnics; Springer: Berlin/Heidelberg, Germany, 2010; pp. 705–710. [Google Scholar] [CrossRef]
- Echeverry-Vargas, L.; Rojas-Reyes, N.R.; Estupiñán, E. Characterization of copper smelter slag and recovery of residual metals from these residues. Rev. Fac. Ing 2017, 44, 61–71. [Google Scholar] [CrossRef]
- Gorai, B.; Jana Premchand, R.K. Characteristics and utilization of copper slag—A review. Resour. Conserv. Recycl. 2023, 39, 299–313. [Google Scholar] [CrossRef]
- Liu, Z.W. Study on the Recovery of Metal Values from Copper Converter Slag. Master’s Dissertation, Wuhan University of Science and Technology, Wuhan, China, 2017. [Google Scholar]
- Lai, X.S.; Huang, H.J. Current status of the comprehensive utilization technology of copper slag. Met. Mine 2017, 2017, 205–208. [Google Scholar] [CrossRef]
- Gümüşsoy, A.; Başyïğït, M.; Kart, E.U. Economic potential and environmental impact of metal recovery from copper slag flotation tailings. Resour. Policy 2023, 80, 103232. [Google Scholar] [CrossRef]
- Liao, Y.L.; Ye, Z.; Wang, Y.Y.; Cao, L. Resource utilization of copper smelt slag—A state-of-the-arts review. Chem. Ind. Eng. Prog. 2017, 36, 3066–3073. [Google Scholar] [CrossRef]
- Arslanoğlu, H.; Altundoğan, H.S.; Tümen, F. Extraction of copper, Cobalt and Nickel by leaching of Iron (III) Sulfate from Copper Slags. Trans. Indian Inst. Met. 2022, 75, 1759–1766. [Google Scholar] [CrossRef]
- Yang, T.; Dan, Z.; Ni, H.F.; Xu, F. Research status of copper slag resource utilization technology. Recycl. Resour. Circ. Econ. 2021, 14, 29–35. [Google Scholar]
- Liu, H.T.; Cao, Y.J.; Fan, G.X. Progress in comprehensive recovery and utilization of copper smelting slag. Conserv. Util. Miner. Resour. 2021, 41, 34–42. [Google Scholar] [CrossRef]
- Zhou, J. Advances in copper smelting and converting process and technical upgrading in Chinese smelters. Nonferrous Met. (Extr. Metall.) 2019, 8, 1–10. [Google Scholar]
- Chen, S.P.; Wu, Z.L.; Lan, B.B.; Guo, Q.Z. Summarize on the technology of copper pyrometallurgy. Copp. Eng. 2010, 2010, 44–49. [Google Scholar]
- Xie, H.; Li, X. Technology status and application of copper hydrometallurgy in domestic and aboard. China Nonferrous Metall. 2015, 44, 15–20. [Google Scholar]
- Wang, S. The current status and development trend of pyrometallurgical copper smelting technology. Jiangxi Build. Mater. 2015, 284–285. [Google Scholar] [CrossRef]
- Bao, H.G.; Wu, X.S.; Yang, Q. Research on techniques of blister copper oxygen-enriched pyrometallurgy. Resour. Inf. Eng. 2022, 37, 98–101. [Google Scholar] [CrossRef]
- Xinhua News Agency. In 2023, China’s Production of Ten Non-Ferrous Metals Exceeded 70 Million Tons for the First Time. 2023. Available online: www.gov.cn (accessed on 15 March 2024).
- Xie, R.Q.; Huang, R.; Zhao, S.F.; Yang, J.P.; Zhang, J.Z. Research progress in resource utilization of copper slag. Conserv. Util. Miner. Resour. 2020, 40, 149–154. [Google Scholar] [CrossRef]
- Kundu, T.; Senapati, S.; Das, S.K.; Angadi, S.I.; Rath, S.S. A comprehensive review on the recovery of copper values from copper slag. Powder Technol. 2023, 426, 118693. [Google Scholar] [CrossRef]
- Jiang, P.G.; Wu, P.F.; Hu, X.J.; Zhou, G.Z. Copper slag comprehensive utilization development and new technology is put forward. China Min. Mag. 2016, 25, 76–79. [Google Scholar]
- Li, J.L.; Liao, Y.L.; Ma, H.F.; Liu, Q.F.; Wu, Y. Review on Comprehensive Recovery Valuable Metals and Utilization of Copper Slag. J. Sustain. Metall. 2023, 2, 439–458. [Google Scholar] [CrossRef]
- Zhu, Z.Z.; He, J.Q. Modern Copper Metallurgy; Science Press: Beijing, China, 2003. [Google Scholar]
- Liu, H.; Lu, H.; Chen, D.; Wang, H.; Xu, H.; Zhang, R. Preparation and properties of glass–ceramics derived from blast-furnace slag by a ceramic-sintering process. Ceram. Int. 2009, 35, 3181–3184. [Google Scholar] [CrossRef]
- Xu, M. Preliminary Study on Recovery Copper from Copper Slag. Master’s Dissertation, Northeastern University, Boston, MA, USA, 2009. [Google Scholar]
- Klaffenbach, E.; Montenegro, V.; Guo, M.X.; Blanpain, B. Sustainable and Comprehensive Utilization of Copper Slag: A Review and Critical Analysis. J. Sustain. Metall. 2023, 2, 468–496. [Google Scholar] [CrossRef]
- Phiri, T.C.; Singh, P.; Nikoloski, A.N. The potential for copper slag waste as a resource for a circular economy: A review–Part II. Miner. Eng. 2021, 172, 107150. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Hao, J.; Dou, Z.H.; LV, G.Z.; Zhang, T.A. A novel slag cleaning method to recover copper from molten copper converter slag. Trans. Nonferrous Met. Soc. China 2023, 8, 2511–2522. [Google Scholar] [CrossRef]
- Li, X.F.; Dou, Z.H.; Zhang, T.A.; Liu, Y. Progress in comprehensive utilization of copper smelting slag. Nonferrous Met. (Extr. Metall.) 2021, 2021, 108–118. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, C.A.; Xia, Z.F. Application of slag concentration technology in China. World Nonferrous Met. 2022, 2022, 40–42. [Google Scholar]
- Shen, H.T.; Forssberg, E. An overview of recovery of metals from slags. Waste Manag. 2003, 23, 933–949. [Google Scholar] [CrossRef]
- Wang, L.S.; Gao, Z.Y.; Yang, Y.; Han, H.S.; Wang, L.; Sun, W. Research progress on comprehensive recovery and utilization of copper slag. Chem. Ind. Eng. Prog. 2021, 40, 5237–5250. [Google Scholar] [CrossRef]
- Zhou, S.W.; Wei, Y.G.; Shi, Y.; Li, B.; Wang, H. Characterization and Recovery of Copper from Converter Copper Slag Via Smelting Separation. Metall. Mater. Trans. B-Process Metall. Mater. Process. Sci. 2018, 49, 2458–2468. [Google Scholar] [CrossRef]
- Chi, X.P.; Liu, H.Y.; Xia, J.; Wen, W.; Zhong, S.P. Research status and prospects of dilution of copper slag for copper recovery. Met. Mine 2024, 2024, 293–303. [Google Scholar] [CrossRef]
- Chen, H.Q.; Li, P.X.; Liu, S.G.; Zhang, Z.J. Study on the strengthening depletion of copper from copper smelter slag by pyro-process. Hunan Nonferrous Met. 2006, 2006, 16–18. [Google Scholar]
- Cao, H.Y.; Zhang, L.; Fu, N.X.; Xia, F.S.; Sui, Z.T.; Feng, N.X. Review of copper slag impoverishment. J. Mater. Metall. 2009, 8, 33–39. [Google Scholar]
- Qin, Q.W.; Huang, Z.L.; Li, M.; Liao, G.D. A study on comprehensive utilization of copper smelting slags from reverberator. Copp. Eng. 2010, 2010, 49–54. [Google Scholar]
- Guo, X.J.; Ni, X.M.; Ma, D.; Yong, H.Q.; Luo, L. Copper slag treatment and comprehensive utilization. Nonferrous Met. Eng. Res. 2017, 38, 23–26. [Google Scholar]
- Chang, H.Q.; Zhang, T.A.; Niu, L.P.; Dou, Z.H.; Du, Y.J. Research progress of copper slag dilution technology. Chinese Metal Society, Metallurgical Reaction Engineering Branch. In Proceedings of the 17th National Metallurgical Reaction Engineering Academic Conference Proceedings, Azores, Portugal, 24–26 April 2013; Volume I. [Google Scholar]
- Li, X.F. Hot Copper Slag Direct Electric Furnace Eddy Current Depletion. Master’s Dissertation, Northeastern University, Boston, MA, USA, 2021. [Google Scholar]
- Wei, G.Z.; Wuth, W.; Ye, G.R. Impoverishment of copper converter slags by a laboratory D. C electric furnace. J. Northeast. Univ. (Nat. Sci.) 1989, 1989, 388–393. [Google Scholar]
- Zhang, H.W. Study of Copper Slag Cleaning Process Based on DC Electric Field and C-H2 Mixed Reduction. Master’s Dissertation, Shanghai University, Shanghai, China, 2014. [Google Scholar]
- Tang, Y.X.; Mo, D.C. Preliminary study on vacuum depletion of furnace slag. Hunan Nonferrous Met. 1990, 1990, 62–63. [Google Scholar]
- Du, Q.Z. Physical chemistry of vacuum slag cleaning. J. Kunming Univ. Sci. Technol. (Nat. Sci.) 1995, 1995, 107–110. [Google Scholar]
- Zhai, Q.L.; Liu, R.Q.; Wang, C.T.; Sun, W.; Tang, C.J.; Min, X.B. Simultaneous recovery of arsenic and copper from copper smelting slag by flotation: Redistribution behavior and toxicity investigation. J. Clean. Prod. 2023, 425, 138811. [Google Scholar] [CrossRef]
- Guo, Y.G.; Li, D.B.; Chen XGLiang, S.B.; Wang, Y. Research status and prospect of iron, zinc and lead recovery from copper slag. Min. Metall. 2021, 30, 103–108. [Google Scholar] [CrossRef]
- Osborn, G.A.; Garner, F.A.; Veasey, T.J. Recovery of metal values from secondary copper slags. In Proceedings of the 1st International Mineral Processing Symposium, Kurashiki, Japan, 31 October–3 November 1986; pp. 46–64. [Google Scholar]
- Andreev, G.N.; Barzev, A. Raman spectroscopic study of some chalcopyrite-xanthate flotation products. J. Mol. Struct. 2003, 661–662, 325–332. [Google Scholar] [CrossRef]
- Sarrafi, A.; Rahmati, B.; Hassani, H.; Shirazi, H. Recovery of copper from reverberatory furnace slag by flotation. Miner. Eng. 2003, 17, 457–459. [Google Scholar] [CrossRef]
- Shamsi, M.; Noaparast, M.; Shafaie, S.Z.; Gharabaghi, M. Synergism effect of collectors on copper recovery in flotation of copper smelting slags. Geosystem Eng. 2016, 19, 57–68. [Google Scholar] [CrossRef]
- Sun, W.; Liu, J.Y.; He, Z.; Zhou, B.Z. Study on flotation of copper slag. Multipurp. Util. Miner. Resour. 2019, 2019, 112–114. [Google Scholar] [CrossRef]
- Jin, R.; Wang, J.S.; Long, Q.R. On the flotation technology of complicated copper smelting slag. Nonferrous Met. Sci. Eng. 2009, 23, 12–14. [Google Scholar]
- Fan, J.; Li, H.; Wei, L.; Li, C.; Sun, S. The Recovery of Copper from Smelting Slag by Flotation Process. In Applications of Process Engineering Principles in Materials Processing, Energy and Environmental Technologies; Wang, S., Free, M., Alam, S., Zhang, M., Taylor, P., Eds.; The Minerals, Metals & Materials Series; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Zuo, Z.; Feng, Y.; Luo, S.; Dong, X.; Li, X.; Ren, D.; Yu, Q.; Guo, J. Element Distribution and Migration Behavior in the Copper Slag Reduction and Separation Process. Front. Energy Res. 2021, 9, 760312. [Google Scholar] [CrossRef]
- Kim, B.S.; Jo, S.K.; Shin, D.Y.; Lee, J.C.; Jeong, S.B. A physico-chemical separation process for upgrading iron from waste copper slag. Int. J. Miner. Process. 2013, 2013, 124–127. [Google Scholar] [CrossRef]
- Luo, L.Q.; Zhang, X.X.; Wang, H.Y. Distribution of elements in copper slag during magnetic separation. J. Cent. South Univ. (Sci. Technol.) 2022, 53, 2843–2850. [Google Scholar] [CrossRef]
- Sun, S.S. Study on Desiliconization and Dezincification Process of Copper Slag. Master’s Dissertation, Dalian University of Technology, Dalian, China, 2021. [Google Scholar]
- Li, S.W.; Guo, Z.Q.; Pan, J.; Zhu, D.Q.; Dong, T.; Lu, S.H. Stepwise utilization process to recover valuable components from copper slag. Minerals 2021, 2, 211. [Google Scholar] [CrossRef]
- Roy, S.; Sarkar, S.; Datta, A.; Rehani, S. Importance of mineralogy and reaction kinetics for selecting leaching methods of copper from copper smelter slag. Sep. Sci. Technol. 2016, 1, 135–146. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, L.K.; Rao, B.; Wang, F.W.; Gao, G.Y.; Peng, K.B. Research status of resource efficient recovery of copper smelting slag from hydrometallurgy leaching. Min. Metall. 2022, 31, 88–97. [Google Scholar]
- Khalid, M.K.; Hamuyuni, J.; Agarwal, V.; Pihlasalo, J.; Haapalainen, M.; Lundström, M. Sulfuric acid leaching for capturing value from copper rich converter slag. J. Clean. Prod. 2019, 2019, 1005–1013. [Google Scholar] [CrossRef]
- Bulut, G. Recovery of copper and cobalt from ancient slag. Waste Manag. Res. 2006, 24, 118–124. [Google Scholar] [CrossRef]
- Karimov, K.A.; Naboichenko, S.S.; Kritskii, A.V.; Tret’yak, M.A.; Kovyazin, A.A. Oxidation sulfuric acid autoclave leaching of copper smelting production fine dust. Metallurgist 2019, 11–12, 1244–1249. [Google Scholar] [CrossRef]
- He, S.M.; Wang, R.X.; Han, H.J.; Zhong, X.C.; Deng, G.F. Removal of arsenic and copper from black copper slag by oxygen pressure leaching with sulfuric acid. Min. Metall. Eng. 2018, 38, 84–87. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, J.J.; Tang, M.; Dai, Z.; Yao, Z.W.; Zheng, R.H.; Kou, Q.J. Research progress of wet process of copper slag. Conserv. Util. Miner. Resour. 2019, 39, 81–87. [Google Scholar] [CrossRef]
- Nadirov, R.; Syzdykova, L.; Zhussupova, A. Copper smelter slag treatment by ammonia solution: Leaching process optimization. J. Cent. South Univ. 2018, 12, 2799–2804. [Google Scholar] [CrossRef]
- Bidari, E.; Aghazadeh, V. Investigation of copper ammonia leaching from smelter slags: Characterization, Leaching and Kinetics. Metall. Mater. Trans. B-Process Metall. Process. Sci. 2015, 5, 2305–2314. [Google Scholar] [CrossRef]
- Yu, Y.F.; Liu, Y.Y.; Qin, Q.W.; Chen, X.Q. Research on leaching kinetics of copper from electro slag by ammoniacal solution. Hydrometall. China 2012, 31, 230–233+236. [Google Scholar] [CrossRef]
- Aracena, A.; Fernández, F.; Jerez, O.; Jaques, A. Converter slag leaching in ammonia medium/column system with subsequent crystallization with NaSH. Hydrometallurgy 2019, 188, 31–37. [Google Scholar] [CrossRef]
- Dimitrijevic, M.; Urosevic, D.; Milic, S.; Sokic, M.; Markovic, R. Dissolution of copper from smelting slag by leaching in chloride media. J. Min. Metall. Sect. B-Metall. 2017, 3, 407–412. [Google Scholar] [CrossRef]
- Yu, Z.S.; Ye, Y.M.; Zeng, J.; Ma, H.H.; Wei, J. Experimental study of chlorinated leaching of matte-slag. Shandong Chem. Ind. 2023, 52, 37–40+43. [Google Scholar] [CrossRef]
- Zhu, X.L.; Ma, Y.; Feng, H. Leaching of gold, palladium, silver and lead from leaching residue of copper anode mud using chlorate. Hydrometall. China 2022, 41, 108–112. [Google Scholar] [CrossRef]
- Mikoda, B.; Potysz, A.; Kmiecik, E. Bacterial leaching of critical metal value from Polish copper metallurgical slags using Acidthiobacillus thiooxidans. J. Environ. Manag. 2019, 236, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Mishra, S.; Rao, D.S.; Pradhan, N.; Mohapatra, U.; Angadi, S.; Mishra, B.K. Extraction of copper from copper slag: Mineralogical insights, physical beneficiation and bioleaching studies. Korean J. Chem. Eng. 2015, 4, 667–676. [Google Scholar] [CrossRef]
- Behera, K.; Sandeep, P.; Mulaba-Bafubiandi, A.F. Valorization of copper smelt slag through the recovery of metals values by a synergistic bioprocess system of bio-flotation and bioleaching. Environ. Qual. Manag. 2022, 32, 233–241. [Google Scholar] [CrossRef]
- Lan, X.; Gao, J.T.; Huang, Z.L.; Guo, Z.C. Rapid separation of copper phase and iron-rich phase from copper slag at low temperature in a super-gravity field. Metall. Mater. Trans. B-Process Metall. Mater. Process. Sci. 2018, 3, 1165–1173. [Google Scholar] [CrossRef]
- Shibayama, A.; Takasaki, Y.; William, T.; Yamatodani, A.; Higuchi, Y.; Sunagawa, S.; Ono, E. Treatment of smelting residue for arsenic removal and recovery of copper using pyro-hydrometallurgical process. J. Hazard. Mater. 2010, 1–3, 1016–1023. [Google Scholar] [CrossRef]
- Kurniati, E.O.; Pederson, F.; Kim, H.J. Application of steel slags, ferronickel slags, and copper mining waste as construction materials: A review. Resour. Conserv. Recycl. 2023, 198, 107175. [Google Scholar] [CrossRef]
- Shi, G.C.; Liao, Y.L.; Zhang, Y.; Su, B.W.; Wang, W. Research progress on preparation of building materials and functional materials with copper metallurgical slag. Mater. Rep. 2020, 34, 13044–13049+13057. [Google Scholar]
- Sharma, R.; Khan, R.A. Durability assessment of self compacting concrete incorporating copper slag as fine aggregates. Constr. Build. Mater. 2017, 155, 617–629. [Google Scholar] [CrossRef]
- Shirdam, R.; Amini, M.; Bakhshi, N. Investigating the Effects of Copper Slag and Silica Fume on Durability, Strength, and Workability of Concrete. Int. J. Environ. Res. 2019, 6, 909–924. [Google Scholar] [CrossRef]
- Lori, A.R.; Hassani, A.; Sedghi, R. Investigating the mechanical and hydraulic characteristics of pervious concrete containing copper slag as coarse aggregate. Constr. Build. Mater. 2019, 197, 130–142. [Google Scholar] [CrossRef]
- Ambily, P.S.; Umarani, C.; Ravisankar, K.; Prem, P.R.; Bharatkumar, B.H.; Iyer, N.R. Studies on ultra high performance concrete incorporating copper slag as fine aggregate. Constr. Build. Mater. 2015, 77, 233–240. [Google Scholar] [CrossRef]
- He, W.; Zhou, Y.Q.; Wang, Q. Advances in copper slag as concrete admixture. Mater. Rep. 2018, 32, 4125–4134. [Google Scholar]
- Wang, K.; Liu, Y.; Hao, J.; Dou, Z. Copper recovery from molten converter slag by depletion method. Trans. Nonferrous Met. Soc. China 2023, 33, 2511–2522. [Google Scholar] [CrossRef]
- Jiang, G.; Wu, A.; Wang, Y.; Wang, H.; Lan, C. Effect of composite activator on the reactivity of copper slag and preparation of filling materials. Chin. J. Eng. Sci. 2017, 39, 1305–1312. [Google Scholar]
- Nazer, A.; Pavez, O.; Toledo, I. Effect of type cement on the mechanical strength of copper slag mortars. REM-Rev. Esc. Minas 2013, 1, 85–90. [Google Scholar] [CrossRef]
- Pavez, O.; Nazer, A.; Rivera, O.; Salinas, M.; Araya, B. Copper slag from different dumps in the Atacama Region used in mortars as partial replacement of cement. Mater.-Rio Janieiro 2019, 2, e12349. [Google Scholar] [CrossRef]
- Mao, K.; Li, L.; Xu, M. Copper and iron recovery from copper slag by molten reduction using spent cathode carbon from aluminum electrolysis as additive. J. Cent. South Univ. 2021, 28, 2010–2021. [Google Scholar] [CrossRef]
- Raposeiras, A.C.; Movilla-Quesada, D.; Muñoz-Cáceres OAndrés-Valeri, V.C.; Lagos-Varas, M. Production of asphalt mixes with copper industry wastes: Use of copper slag as raw material replacement. J. Environ. Manag. 2021, 293, 112867. [Google Scholar] [CrossRef]
- Ziari, H.; Moniri, A.; Imaninasab, R.; Nakhaei, M. Effect of copper slag on performance of warm mix asphalt. Int. J. Pavement Eng. 2020, 7, 775–781. [Google Scholar] [CrossRef]
- Modarres, A.; Bengar, P.A. Investigating the indirect tensile stiffness, toughness and fatigue life of hot mix asphalt containing copper slag powder. Int. J. Pavement Eng. 2019, 8, 977–985. [Google Scholar] [CrossRef]
- Lin, Q.; Yang, Z.H.; Xie, H.J.; Ke, Y.; Liao, G.D. Research on preparation of glass ceramics with copper slag. Bull. Chin. Ceram. Soc. 2012, 31, 1204–1207+1211. [Google Scholar] [CrossRef]
- Shang, W.X.; Peng, Z.W.; Huang, Y.W.; Gu, F.Q.; Zhang, J.; Tang, H.M.; Yang, L.; Tian, W.G.; Rao, M.J.; Li, G.H.; et al. Production of glass-ceramics from metallurgical slags. J. Clean. Prod. 2021, 317, 128220. [Google Scholar] [CrossRef]
- Yang, Z.H.; Lin, Q.; Lu, S.C.; He, Y.; Liao, G.D.; Ke, Y. Effect of CaO/SiO2 ratio on the preparation and crystallization of glass-ceramics from copper slag. Ceram. Int. 2014, 5, 7297–7305. [Google Scholar] [CrossRef]
- Mohamed, E.; Shahsavari, P.; Eftekhari-Yekta, B.; Marghussian, V.K. Preparation and Characterization of Glass Ceramic Foams Produced from Copper Slag. Trans. Indian Ceram. Soc. 2015, 1, 1–5. [Google Scholar] [CrossRef]
- Sarfo, P.; Wyss, G.; Ma, G.J.; Das, A.; Young, C. Carbothermal reduction of copper smelter slag for recycling into pig iron and glass. Miner. Eng. 2017, 107, 8–19. [Google Scholar] [CrossRef]
- Blenau, L.W.; Sander, S.A.H.; Fuhrmann, S.; Charitos, A. Holistic valorization of fayalitic slag to pig iron and glass fibers. J. Clean. Prod. 2023, 418, 137990. [Google Scholar] [CrossRef]
- Yao, C.L.; Liu, Z.N.; Teng YFan, X.X.; Zhang, J.L. Comprehensive utilization development and prospect of copper slag. Min. Metall. 2019, 28, 77–81+96. [Google Scholar]
- Erdenebold, U.; Choi, H.M.; Wang, J.P. Recovery of pig iron from copper smelting slag by reduction smelting. Arch. Metall. Mater. 2018, 4, 1793–1798. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, B.; Song, J.X.; Volski, V.; Vandenbosch, G.A.E.; Guo, M.X. An innovated application of reutilize copper smelt slag for cement-based electromagnetic interference composites. Sci. Rep. 2018, 8, 16155. [Google Scholar] [CrossRef] [PubMed]
- Gyurov, S.; Marinkov, N.; Kostova, Y.; Rabadjieva, D.; Kovacheva, D.; Tzvektkova, C.; Gentscheva, G.; Penkov, I. Technological scheme for copper slag processing. Int. J. Miner. Process. 2017, 158, 1–7. [Google Scholar] [CrossRef]
- Xu, Y.M.; Wang, L.B.; Xie, W.J.; Chen, Y.; Zhang, K.S.; Du, Y.G. A novel way to prepare battery-grade FePO4·2H2O from copper slag and Life cycle assessment. Sep. Purif. Technol. 2024, 339, 126686. [Google Scholar] [CrossRef]
- Dos Anjos, M.A.G.; Ribeiro, D.V.; Labrincha, J.A. Blasted copper slag as fine aggregate in Portland cement concrete. J. Environ. Manag. 2017, 196, 607–616. [Google Scholar] [CrossRef]
- Vukadinović, D.; Mladenović, A.; Ivanović, L. Mechanical properties and durability of concrete with water-cooled copper slag aggregate. Waste Biomass Valorization 2017, 8, 1841–1854. [Google Scholar]
- Liu, R.; Chen, Y.; Zhang, X. Utilization status and environmental risk assessment of copper slag in China. J. Clean. Prod. 2022, 344, 130987. [Google Scholar]
- Wang, Y.; Tao, G.; Wang, P. Regulation of refractive index and thermo-optic coefficient of chalcogenide glass. Infrared Laser Eng. 2020, 49, 0303003. [Google Scholar]


| Main Process Flow | Advantages | Disadvantages | |
|---|---|---|---|
| Pyrometallurgy | Smelting—Converting—Refining | High smelting efficiency, Mature technology | High cost, Poor environmental friendliness |
| Hydrometallurgy | Leaching—Extracting—Electrodeposition process | Can handle low-grade copper ore and multi-impurity metal copper, relatively environmentally friendly | Long cycle |
| Methods of Smelting | Slag Mass Fraction/% | |||||||
|---|---|---|---|---|---|---|---|---|
| Cu | FeO | Fe3O4 | SiO2 | S | Al2O3 | CaO | MgO | |
| Imperial smelt furnace | 0.4–2.8 | 33–42 | 5–9 | 32–34 | - | 3–8 | 3–10 | 1–4 |
| Outokumpu flash smelting (without impoverishment) | 1.5 | 44.4 | 11.8 | 26.6 | 1.6 | - | - | - |
| Outokumpu flash smelting (impoverishment) | 0.78 | 44.06 | - | 29.7 | 1.4 | 7.8 | 0.6 | - |
| Inco smelting | 0.9–1.7 | 44–54 | 10–12 | 32–35 | 1.1 | 4–5 | 1.5–2.5 | 1.4–2.2 |
| Noranda smelting process | 2.5–5 | 40–52 | 15–29 | 21–25 | 1.7 | 5.0 | 0.5–2 | 1.0–1.5 |
| Vanukov smelting | Cu | FeO | Fe3O4 | SiO2 | S | Al2O3 | CaO | MgO |
| Baiyin smelting | 0.45 | 35 | 3.15 | 35 | 0.7 | 3.3 | 8 | 1.4 |
| Teninte converter smelting | 1.1–4.6 | 43–65 | 12–29 | 16–28 | 0.8 | 5–10 | 1–2 | 5–10 |
| Isasmelt | 0.7–2.0 | 36.6–45 | 6–8 | 31–34 | 0.8 | 3.64 | 1.5–4.4 | 1.0–2.0 |
| Ausmelt | 0.65 | 34 | 7.5 | 31 | 2.8 | 7.5 | 5.0 | - |
| Mitsubishi process smelting | 0.6–2.4 | 38–58 | - | 30–35 | 0.6 | 2–6 | 5–8 | - |
| Collector | Frother | Depressant | Sulfurizing Agent | pH | Cu Recovery | Particle Size μm |
|---|---|---|---|---|---|---|
| SBX | MIBC | HEC | 82% | |||
| PAX | Pine oil | 8–9 | 96% | |||
| PAX + FC7245 or FC 4146 | Flotanol CO7 | Na2S | ~70%–80%, up to 90% after regrinding | d80 = 45 | ||
| PAX + FC7245 or FC 4146 | Flotanol CO7 | Na2S | ~80%–85%, >90% after regrinding | d80 = 45 | ||
| PAX + FC7245 or FC 4146 | Flotanol CO7 | Na2S | 5%–15% | d80 = 45 | ||
| BX | Pine oil (Terpineol) | 10 | 80% | <74 | ||
| PAX + AERO 3477 | Pine oil (Terpineol) | 11 | 80% | d80 = 48 | ||
| SIPX/DTP(40:160 g/t) | A65, MIBC | CMC | 9 | 85% | d80 = 75 | |
| KBX | Pine oil (Terpineol) | 73% | d90 = 43 |
| Acid Leaching | Ammonia Leaching | Chlorination Leaching | Bioleaching | |
|---|---|---|---|---|
| Advantages | Simplicity of operation, Fast reaction rate, Wide application range | Strong complexation ability, Low corrosivity, Environmentally friendly | Without elevated temperature required, Recoverable precious metals | Environmentally friendly, Low energy consumption and cost |
| Disadvantages | Strong corrosion, Environmental pollution | Long leaching time, Limited scope of application, Excessive cost | Equipment corrosion, Low safety, Impact on the environment | Difficulty in microbial culture, Harsh terms, Long period |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Xie, H.; Han, B. The Utilization of the Copper Smelting Slag: A Critical Review. Minerals 2025, 15, 926. https://doi.org/10.3390/min15090926
Liu J, Xie H, Han B. The Utilization of the Copper Smelting Slag: A Critical Review. Minerals. 2025; 15(9):926. https://doi.org/10.3390/min15090926
Chicago/Turabian StyleLiu, Jiaxing, Haoyu Xie, and Baisui Han. 2025. "The Utilization of the Copper Smelting Slag: A Critical Review" Minerals 15, no. 9: 926. https://doi.org/10.3390/min15090926
APA StyleLiu, J., Xie, H., & Han, B. (2025). The Utilization of the Copper Smelting Slag: A Critical Review. Minerals, 15(9), 926. https://doi.org/10.3390/min15090926






