Ultrasonic Pulp Conditioning-Induced Nanoparticles: A Critical Driver for Sonication-Assisted Ultrafine Smithsonite Flotation
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
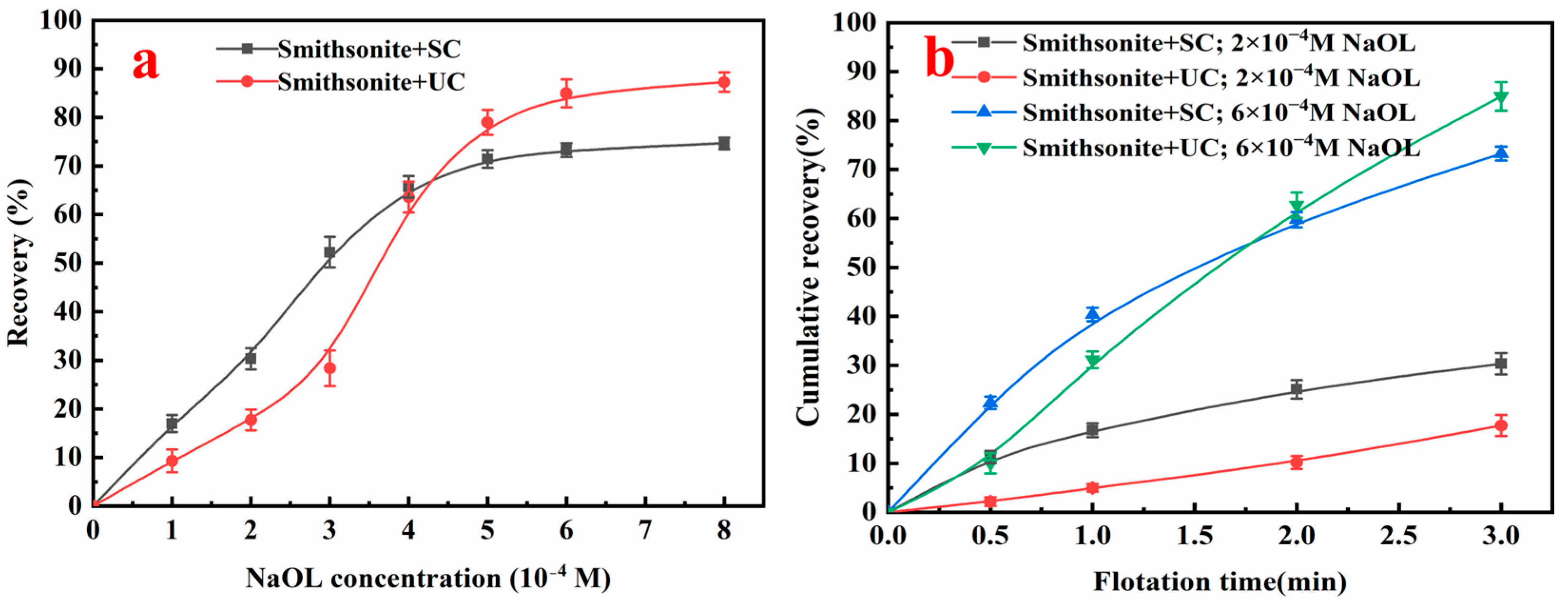
2.2. Flotation Procedure
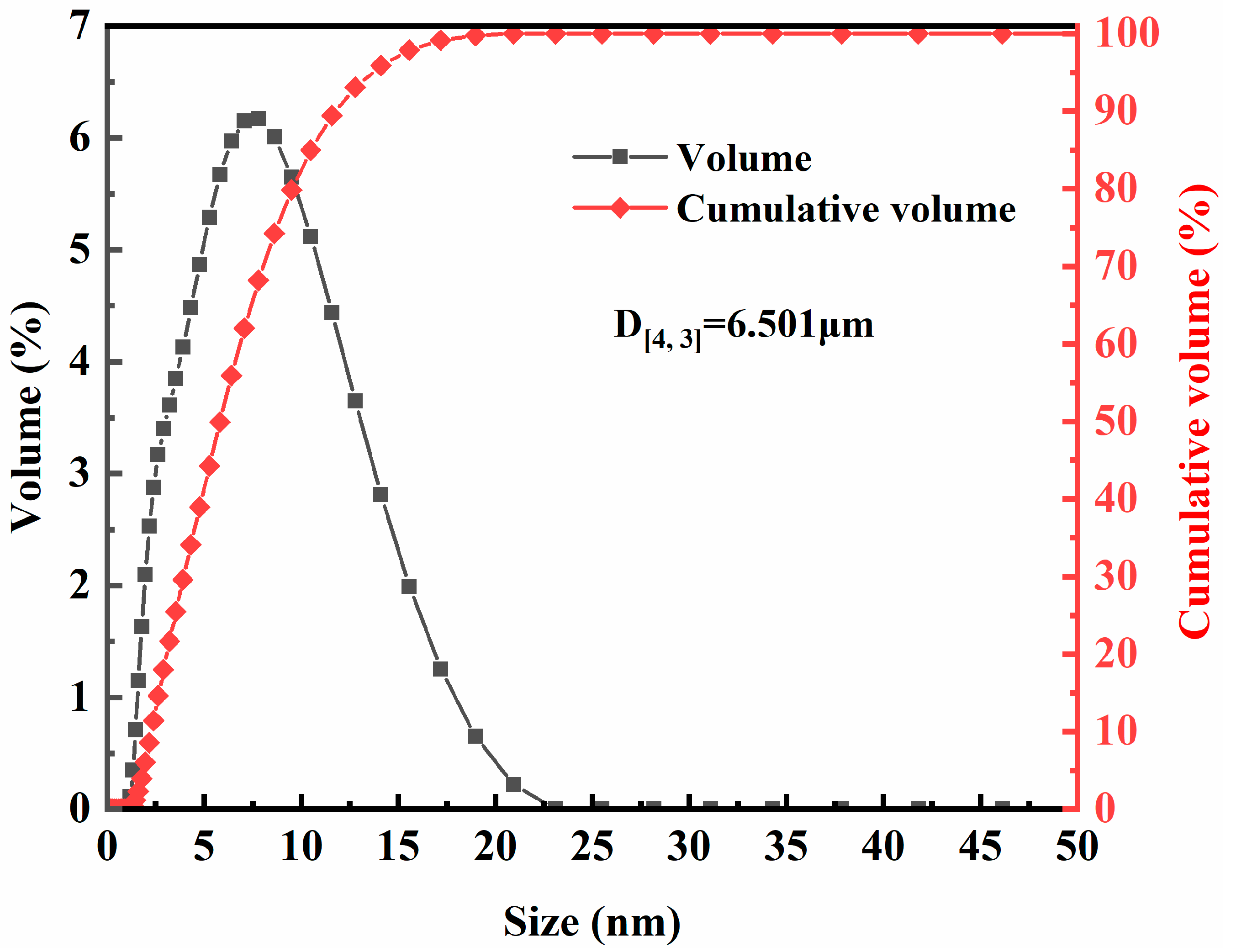
2.3. Geometrical and Morphological Characterizations of Smithsonite Particles
2.4. Nanoparticle Tracking Within the Smithsonite Slurry
2.5. Adsorption Experiments
2.6. Zeta Potential Measurements
2.7. XPS Analysis
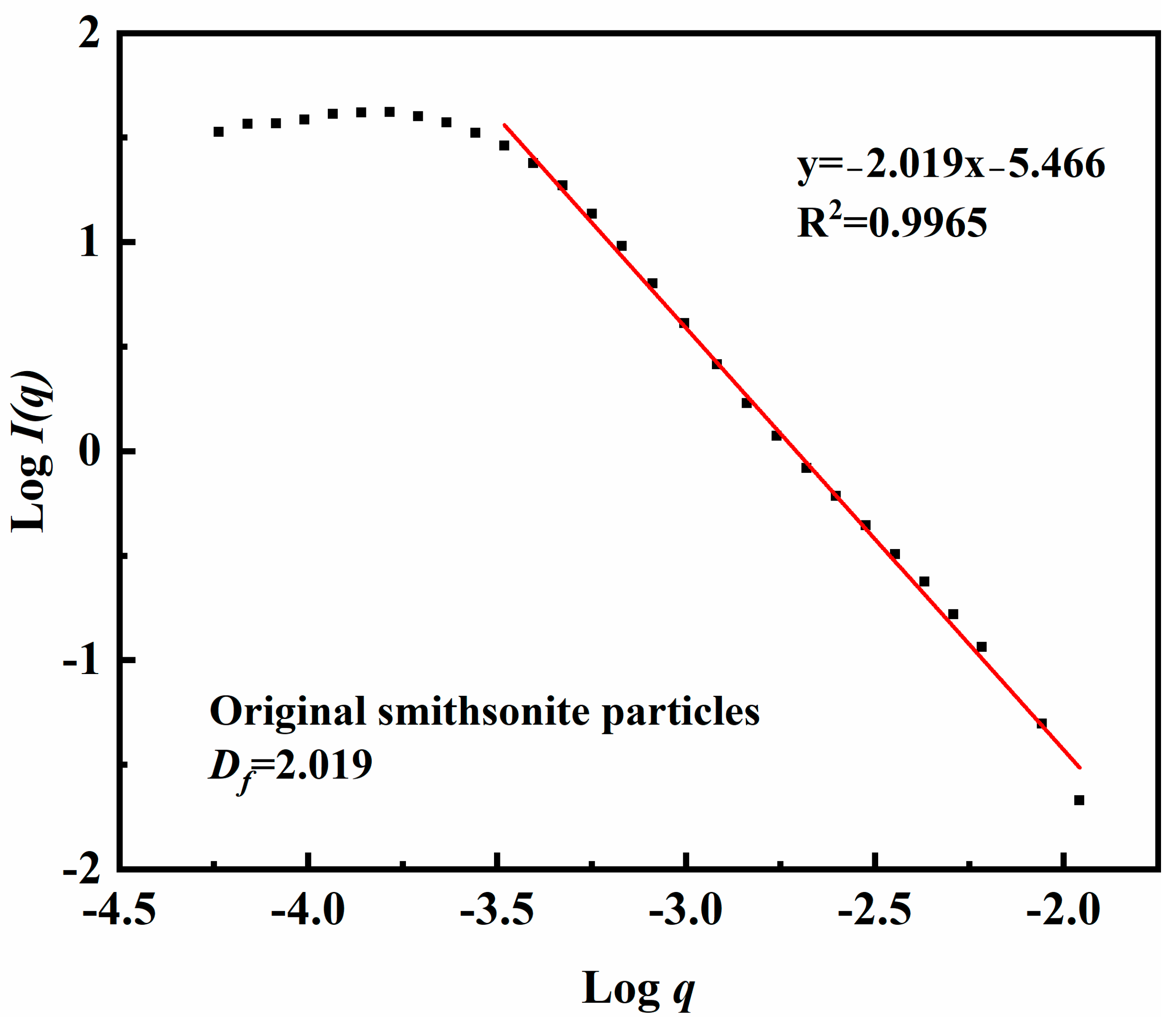
2.8. Aggregates Size and Mass Fractal Dimension Measurements
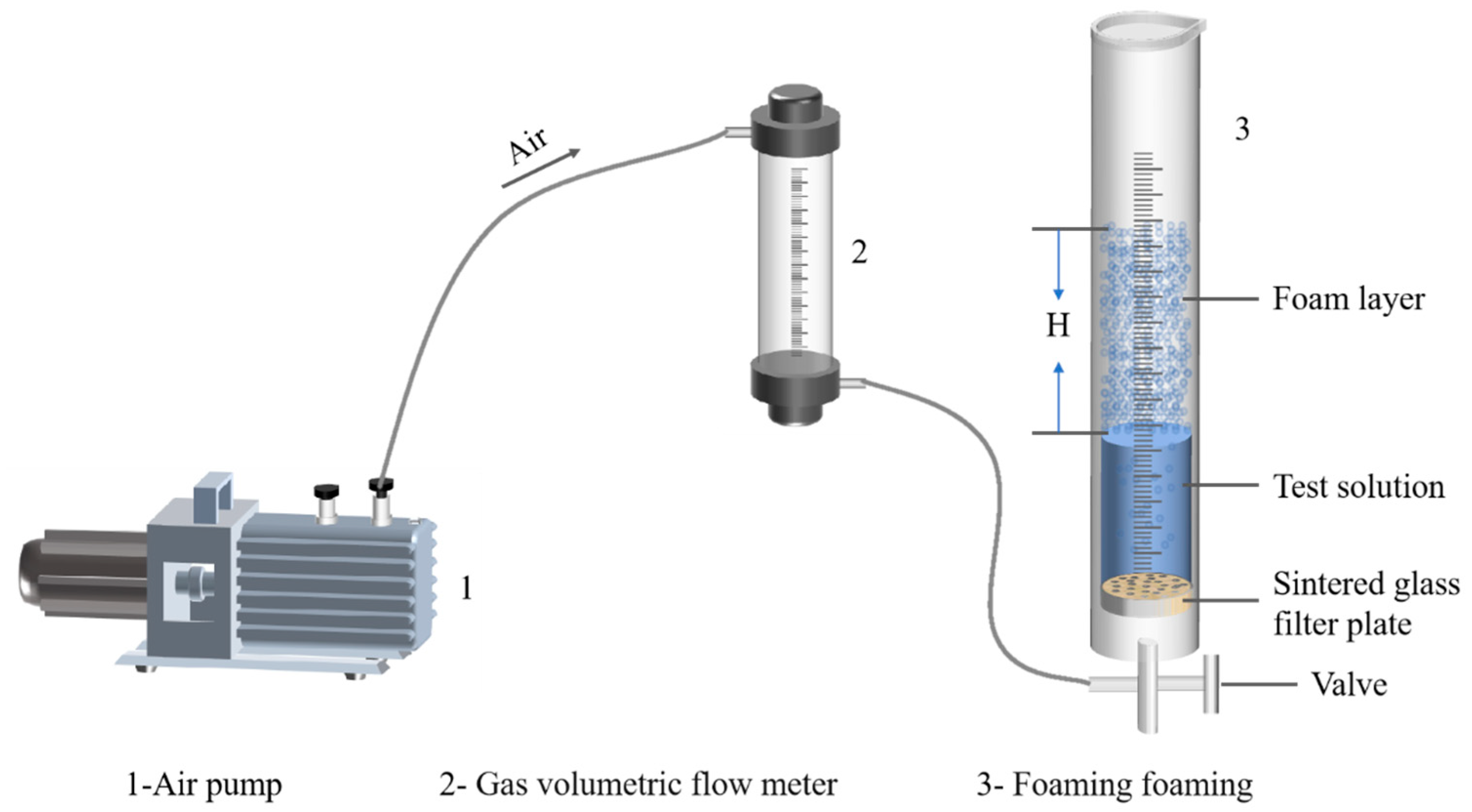
2.9. Frothing Ability of Smithsonite Pulp in the Presence of NaOL
3. Results and Discussion
3.1. Flotation of Ultrafine Smithsonite with and Without UC
3.2. Sonication-Assisted Interaction Between Ultrafine Smithsonite and NaOL
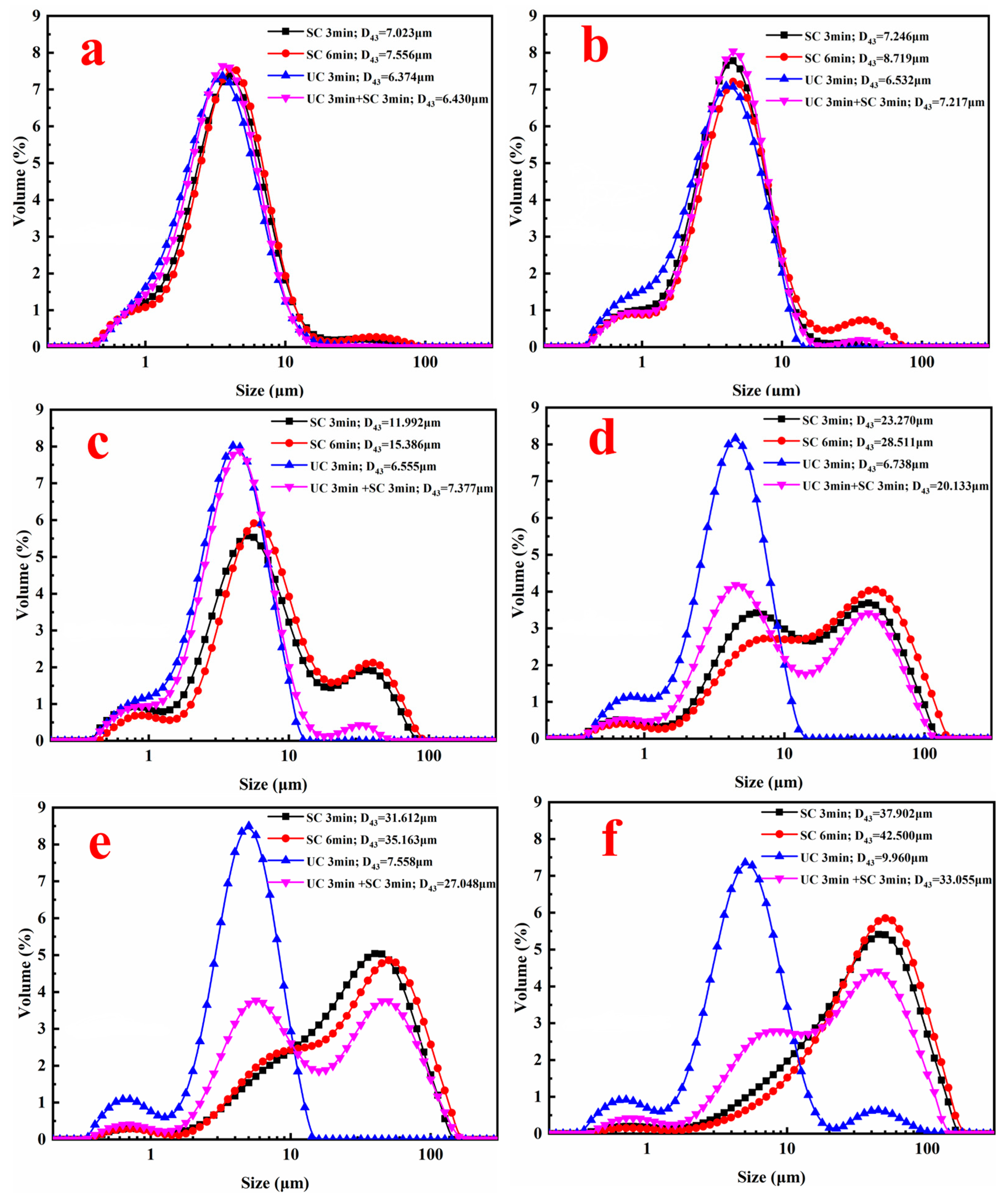
3.2.1. Geometrical and Morphological Characterizations of Ultrafine Smithsonite
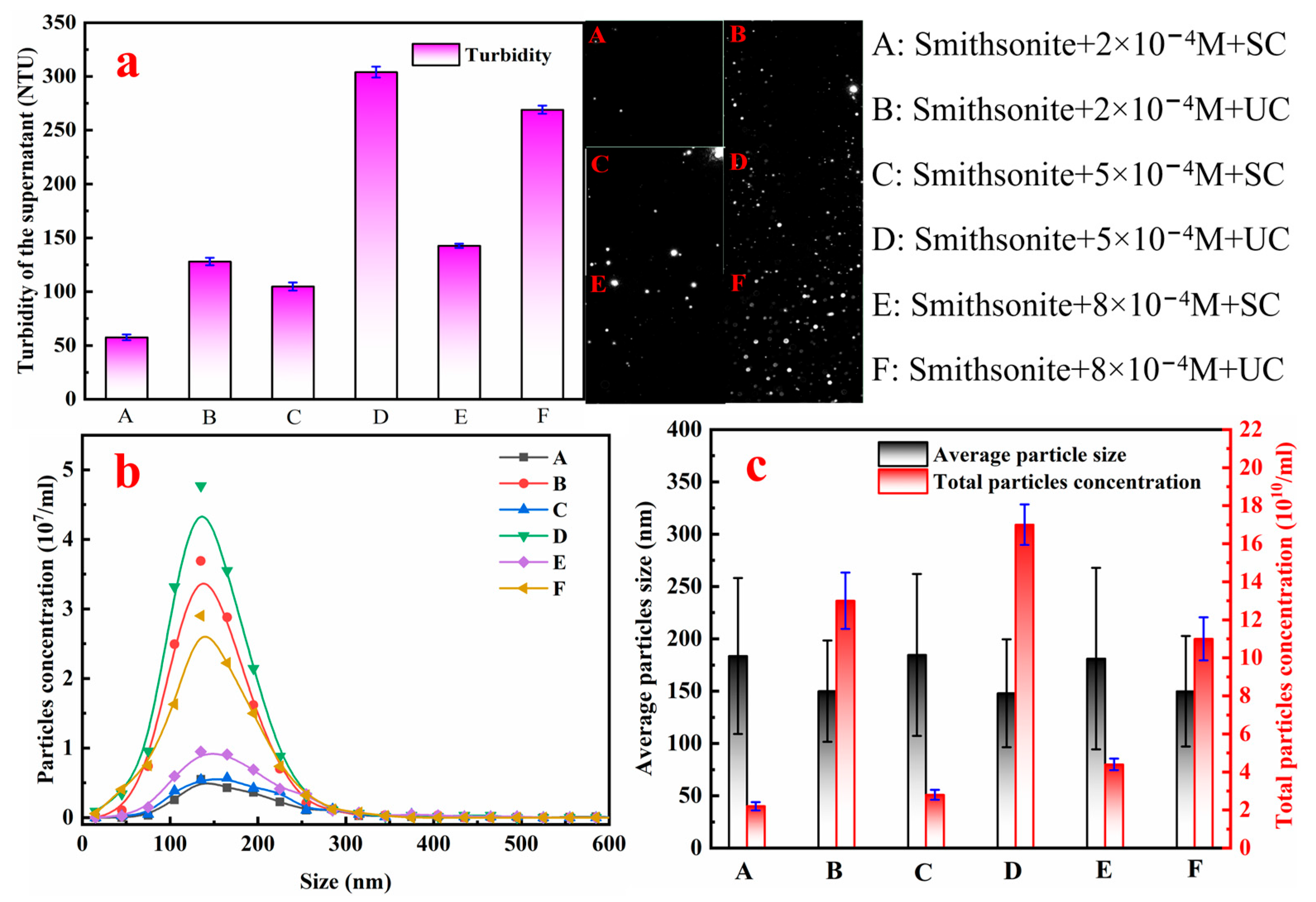
3.2.2. Nanoparticle Detection Results of Different Smithsonite Pulps
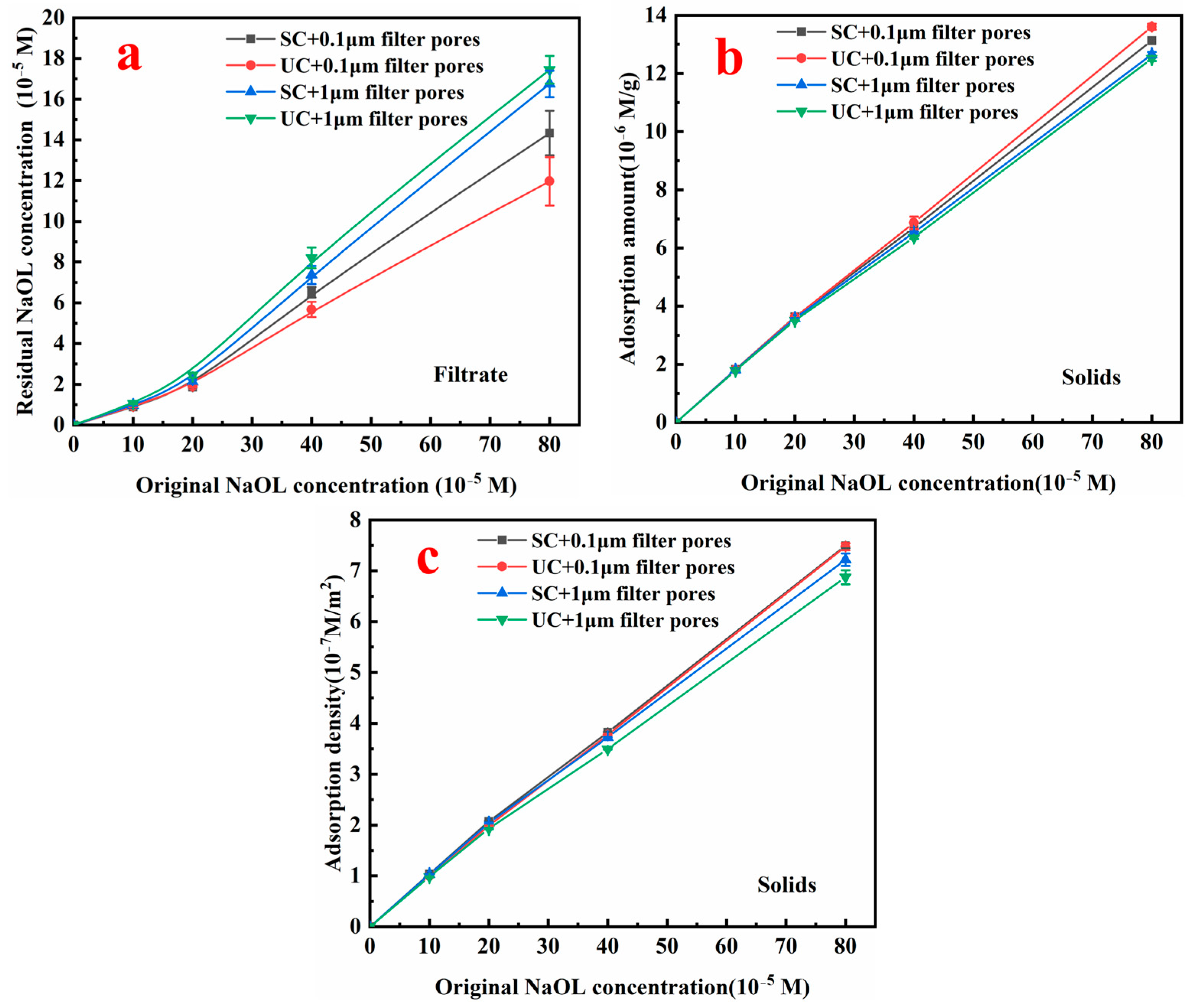
3.2.3. Quantitative Evaluation of NaOL Adsorption on Smithsonite Surfaces Under Variable Conditions
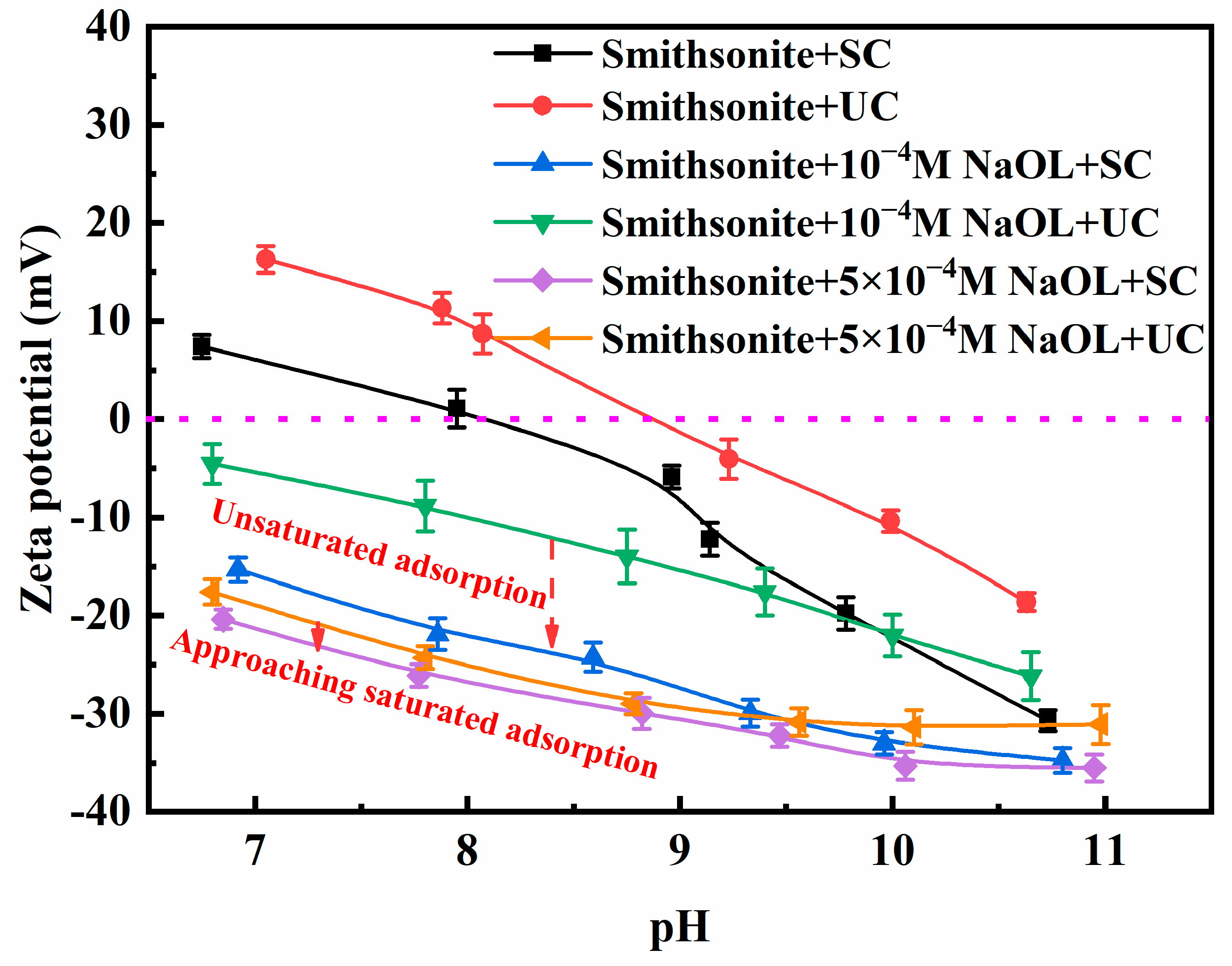
3.2.4. Electrokinetic Potential Results of Smithsonite Particles
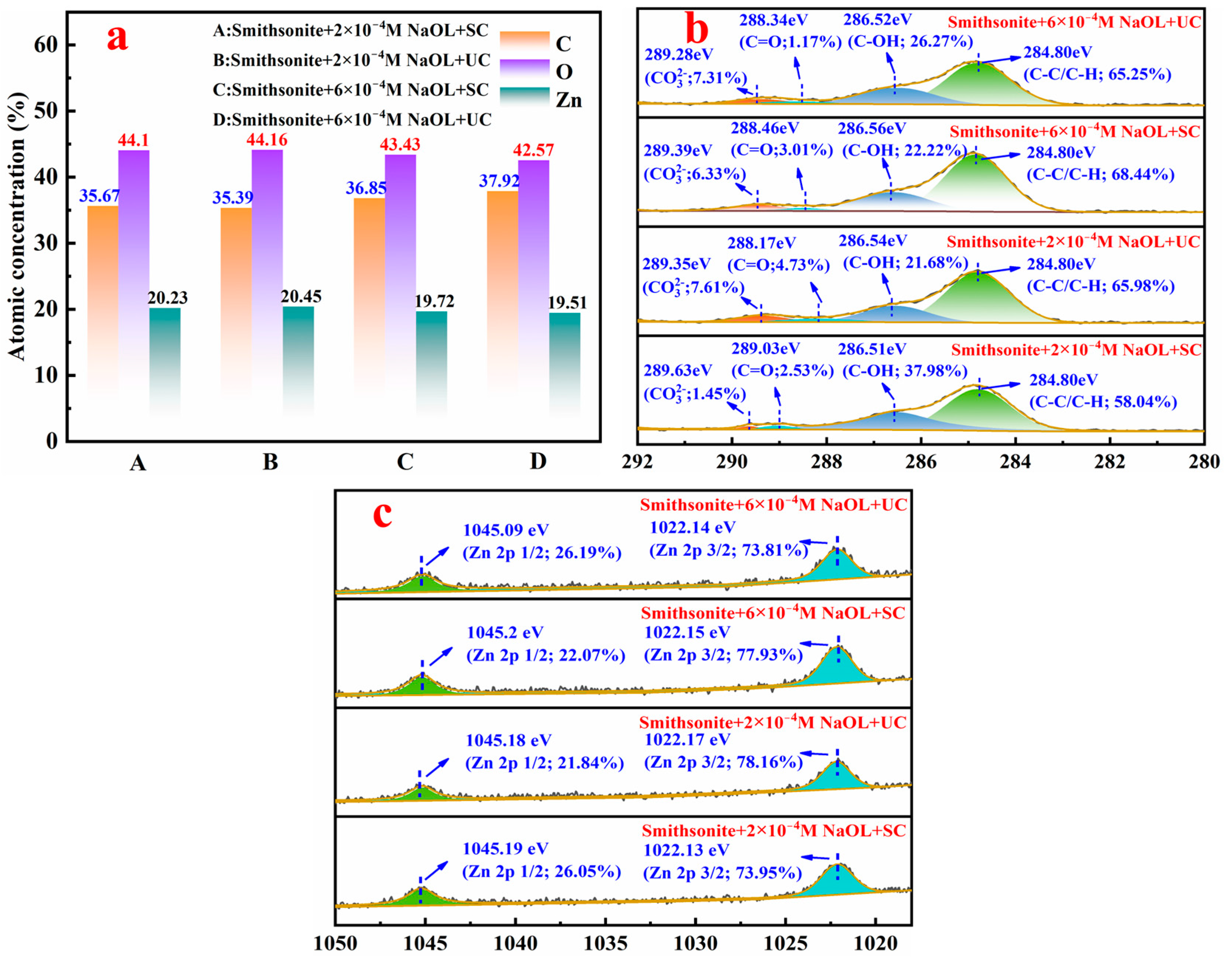
3.2.5. XPS Analysis Results
3.3. Aggregation/Dispersion of Smithsonite Particles Under Different Conditions
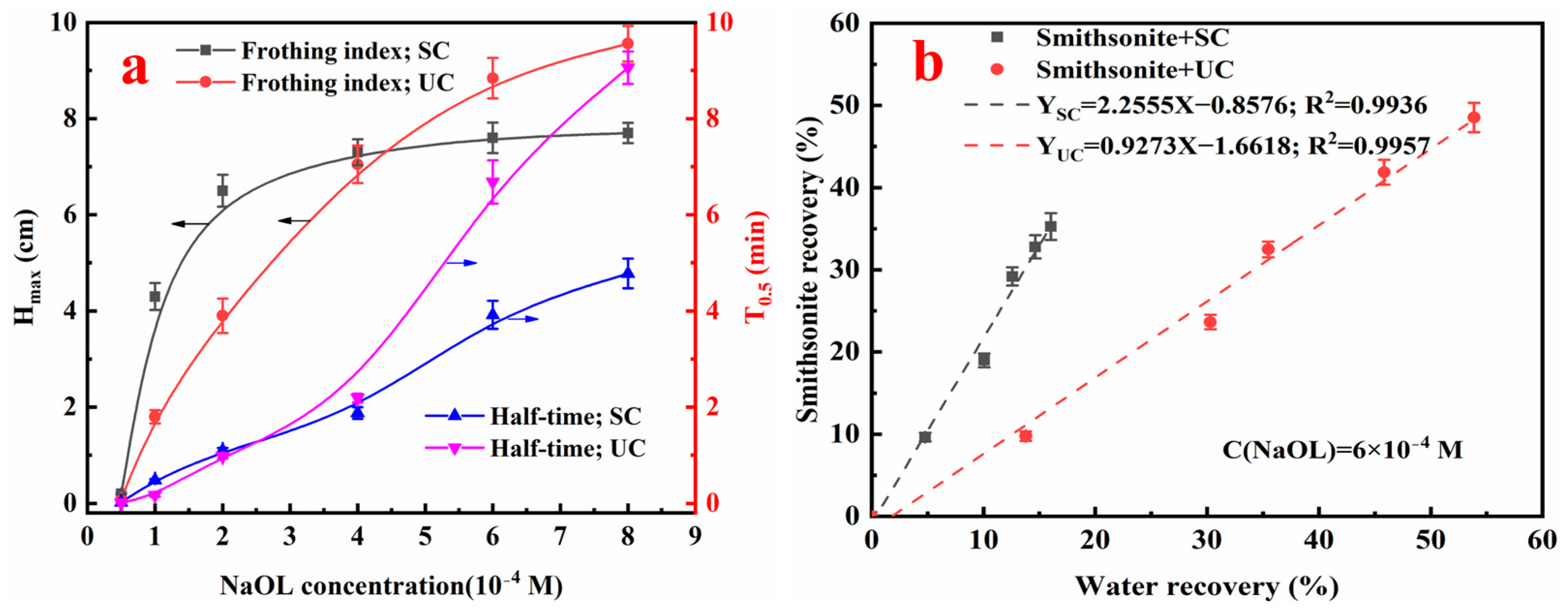
3.4. Frothing of Smithsonite Pulp Under Different NaOL Concentrations
4. Conclusions
- (1)
- UC significantly alters ultrafine smithsonite flotation, but its influence is governed by the NaOL concentration. Below 4 × 10−4 M NaOL, UC lowers both recovery and kinetics relative to SC. Once the concentration exceeds this threshold, UC delivers higher recovery than SC, although the initial flotation kinetics remain slower.
- (2)
- UC reshapes the smithsonite surface far more drastically than SC. The intense acoustic field dissolves lattice ions and nucleates zinc-rich nanoparticles, thereby creating extra adsorption sites for NaOL. At low NaOL concentrations, sonication marginally enhances NaOL chemisorption, yet the simultaneous exposure of fresh Zn-rich sites aggravates collector undersaturation. When NaOL is ample, both the mineral surface and dissolved zinc species are fully hydrophobized by oleate, forming a hydrophobic shell that likely promotes the deposition of nanosolids and the interfacial anchoring of gaseous nanoparticles.
- (3)
- During SC, gentle shear steadily enlarges smithsonite flocs as the NaOL concentration rises, whereas UC violently disperses all particles regardless of the NaOL level. Although sonication destroys flocs, re-starting SC allows the dispersed particles to re-flocculate, and the extent of re-aggregation is set by the prevailing NaOL concentration. At low NaOL levels, only partial surface hydrophobization occurs, yielding weak and slow re-aggregation that accounts for the poor recovery and sluggish kinetics observed after UC. When NaOL concentration is high, two parallel changes occur: complete hydrophobization of both solid particles and dissolved zinc species, and a sharp rise in CBs. These synergistic effects accelerate hydrophobic aggregation. Once NaOL reaches 6 × 10−4 M, nanoparticles bridging triggers the rapid formation of large, loose aggregates and flotation performance improves dramatically.
- (4)
- The frothability of smithsonite pulp is controlled by two inter-related variables: the NaOL concentration and the conditioning route. At low NaOL concentrations, UC produces froths that are markedly weaker and less stable than those generated by SC. As NaOL rises, smithsonite surfaces become more hydrophobized and the pulp accumulates both solid and gaseous nanoparticles. Together these changes enlarge and stabilize the froth layer. Once NaOL exceeds 4 × 10−4 M, UC surpasses SC in overall smithsonite recovery, albeit with greater water recovery. Nevertheless, the vigorous dispersion imparted by ultrasound still suppresses UC’s initial flotation rate below that of SC, even at elevated NaOL levels.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Ejtemaei, M.; Gharabaghi, M.; Irannajad, M. A review of zinc oxide mineral beneficiation using flotation method. Adv. Colloid Interface Sci. 2014, 206, 68–78. [Google Scholar] [CrossRef]
- Chandra, A.P.; Gerson, A.R. A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite. Adv. Colloid Interface Sci. 2009, 145, 97–110. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Irannajad, M.; Gharabaghi, M. Influence of important factors on flotation of zinc oxide mineral using cationic, anionic and mixed (cationic/anionic) collectors. Miner. Eng. 2011, 24, 1402–1408. [Google Scholar] [CrossRef]
- Pereira, C.A.; Peres, A.E.C. Reagents in calamine zinc ores flotation. Miner. Eng. 2005, 18, 275–277. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, W.; Duan, H.; Yang, T.; Li, Z.; Zhou, S. Sodium carbonate effects on the flotation separation of smithsonite from quartz using N,N′-dilauroyl ethylenediamine dipropionate as a collector. Miner. Eng. 2018, 126, 1–8. [Google Scholar] [CrossRef]
- Jia, K.; Jin, Y.; Liu, S.; Qin, W.; Zhang, C.; Li, G.; Cao, Y. The application and mechanism of degradable activator biologically small molecule organic acids in smithsonite flotation. Appl. Surf. Sci. 2024, 660, 159907. [Google Scholar] [CrossRef]
- Liao, R.; Wen, S.; Liu, J.; Bai, S.; Feng, Q. Synergetic adsorption of dodecylamine and octyl hydroxamic acid on sulfidized smithsonite: Insights from experiments and molecular dynamics simulation. Sep. Purif. Technol. 2024, 329, 125106. [Google Scholar] [CrossRef]
- Wu, D.; Wen, S.; Deng, J.; Liu, J.; Mao, Y. Study on the sulfidation behavior of smithsonite. Appl. Surf. Sci. 2015, 329, 315–320. [Google Scholar] [CrossRef]
- Rao, S.R.; Finch, J.A. Base metal oxide flotation using long chain xanthates. Int. J. Miner. Process. 2003, 69, 251–258. [Google Scholar] [CrossRef]
- Zhou, W.; Cao, W.; Wei, H.; Shi, S.; Li, C.; Dong, L. Sonication-assisted surface erosion and its impact on the flotation of ultrafine smithsonite. Metals 2025, 15, 731. [Google Scholar] [CrossRef]
- Chen, Y.; Truong, V.N.T.; Bu, X.; Xie, G. A review of effects and applications of ultrasound in mineral flotation. Ultrason. Sonochemistry 2020, 60, 104739. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Chen, L.; Lu, D.; Wang, Y.; Zheng, X.; Zhu, G. Ultrasound application in alkaline pretreatment process of spodumene to improve particle floatability. Int. J. Min. Sci. Technol. 2023, 33, 883–891. [Google Scholar] [CrossRef]
- Fang, S.; Xu, L.; Wu, H.; Xu, Y.; Wang, Z.; Shu, K.; Hu, Y. Influence of surface dissolution on sodium oleate adsorption on ilmenite and its gangue minerals by ultrasonic treatment. Appl. Surf. Sci. 2020, 500, 144038. [Google Scholar] [CrossRef]
- Mao, Y.; Xia, W.; Peng, Y.; Xie, G. Ultrasonic-assisted flotation of fine coal: A review. Fuel Process. Technol. 2019, 195, 106150. [Google Scholar] [CrossRef]
- Barma, S.D. Ultrasonic-assisted coal beneficiation: A review. Ultrason. Sonochemistry 2019, 50, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Ziarati, A.; Sobhani-Nasab, A.; Rahimi-Nasrabadi, M.; Ganjali, M.R.; Badiei, A. Sonication method synergism with rare earth based nanocatalyst: Preparation of NiFe2–xEuxO4 nanostructures and its catalytic applications for the synthesis of benzimidazoles, benzoxazoles, and benzothiazoles under ultrasonic irradiation. J. Rare Earths 2017, 35, 374–381. [Google Scholar] [CrossRef]
- Diehl, L.O.; Gatiboni, T.L.; Mello, P.A.; Muller, E.I.; Duarte, F.A.; Flores, E.M.M. Ultrasound-assisted extraction of rare-earth elements from carbonatite rocks. Ultrason. Sonochemistry 2018, 40, 24–29. [Google Scholar] [CrossRef]
- Wang, W.; Liu, D.; Tu, Y.; Jin, L.; Wang, H. Enrichment of residual carbon in entrained-flow gasification coal fine slag by ultrasonic flotation. Fuel 2020, 278, 118195. [Google Scholar] [CrossRef]
- Videla, A.R.; Morales, R.; Saint-Jean, T.; Gaete, L.; Vargas, Y.; Miller, J.D. Ultrasound treatment on tailings to enhance copper flotation recovery. Miner. Eng. 2016, 99, 89–95. [Google Scholar] [CrossRef]
- Kruszelnicki, M.; Hassanzadeh, A.; Legawiec, K.J.; Polowczyk, I.; Kowalczuk, P.B. Effect of ultrasound pre-treatment on carbonaceous copper-bearing shale flotation. Ultrason. Sonochemistry 2022, 84, 105962. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, Y.; Ma, Y.; Chen, K. Effects of ultrasonic treatment on the flotation behavior of magnesite and dolomite in a sodium oleate system. Green Smart Min. Eng. 2024, 1, 76–84. [Google Scholar] [CrossRef]
- Bu, X.; Alheshibri, M. The effect of ultrasound on bulk and surface nanobubbles: A review of the current status. Ultrason. Sonochemistry 2021, 76, 105629. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.A.; Xu, Z.; Finch, J.A.; Masliyah, J.H.; Chow, R.S. On the role of cavitation in particle collection in flotation—A critical review. II. Miner. Eng. 2009, 22, 419–433. [Google Scholar] [CrossRef]
- Dong, X.; Price, M.; Dai, Z.; Xu, M.; Pelton, R. Mineral-mineral particle collisions during flotation remove adsorbed nanoparticle flotation collectors. J. Colloid Interface Sci. 2017, 504, 178–185. [Google Scholar] [CrossRef]
- Olszok, V.; Rivas-Botero, J.; Wollmann, A.; Benker, B.; Weber, A.P. Particle-induced nanobubble generation for material-selective nanoparticle flotation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 592, 124576. [Google Scholar] [CrossRef]
- Cilek, E.C.; Karaca, S. Effect of nanoparticles on froth stability and bubble size distribution in flotation. Int. J. Miner. Process. 2015, 138, 6–14. [Google Scholar] [CrossRef]
- Sun, J.; Dai, L.; Lv, K.; Wen, Z.; Li, Y.; Yang, D.; Yan, H.; Liu, X.; Liu, C.; Li, M.-C. Recent advances in nanomaterial-stabilized pickering foam: Mechanism, classification, properties, and applications. Adv. Colloid Interface Sci. 2024, 328, 103177. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, K.; Wang, L.; Zhou, B.; Niu, J.; Ou, L. The role of bulk micro-nanobubbles in reagent desorption and potential implication in flotation separation of highly hydrophobized minerals. Ultrason. Sonochemistry 2020, 64, 104996. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, X.; Long, Y.; Xie, G.; Chen, Y. Monitoring effects of hydrodynamic cavitation pretreatment of sodium oleate on the aggregation of fine diaspore particles through small-angle laser scattering. Ultrason. Sonochemistry 2023, 100, 106574. [Google Scholar] [CrossRef]
- Tang, S.; Preece, J.M.; McFarlane, C.M.; Zhang, Z. Fractal Morphology and Breakage of DLCA and RLCA Aggregates. J. Colloid Interface Sci. 2000, 221, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Atrafi, A.; Gomez, C.O.; Finch, J.A.; Pawlik, M. Frothing behavior of aqueous solutions of oleic acid. Miner. Eng. 2012, 36–38, 138–144. [Google Scholar] [CrossRef]
- Shi, Q.; Feng, Q.; Zhang, G.; Deng, H. Electrokinetic properties of smithsonite and its floatability with anionic collector. Colloids Surf. A Physicochem. Eng. Asp. 2012, 410, 178–183. [Google Scholar] [CrossRef]
- Gurpinar, G.; Sonmez, E.; Bozkurt, V. Effect of ultrasonic treatment on flotation of calcite, barite and quartz. Miner. Process. Extr. Metall. 2004, 113, 91–95. [Google Scholar] [CrossRef]
- Awad, S.B. High-power ultrasound in surface cleaning and decontamination. In Ultrasound Technologies for Food and Bioprocessing; Springer: Berlin/Heidelberg, Germany, 2010; pp. 545–558. [Google Scholar]
- Calgaroto, S.; Wilberg, K.Q.; Rubio, J. On the nanobubbles interfacial properties and future applications in flotation. Miner. Eng. 2014, 60, 33. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Z.; Wei, Z.; Hu, J.; Guo, Y.; Deng, T. Titanium-based ion sieve with enhanced post-separation ability for high performance lithium recovery from geothermal water. Chem. Eng. J. 2021, 410, 128320. [Google Scholar] [CrossRef]
- Roberts, D.R.; Ford, R.G.; Sparks, D.L. Kinetics and mechanisms of Zn complexation on metal oxides using EXAFS spectroscopy. J. Colloid Interface Sci. 2003, 263, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Miao, Y.; Li, C.; Ye, G.; Wu, W.; Li, B.; Zhang, G. Deep insight into the effect of smithsonite particle size on surface heterogeneity and adsorption behavior: A case of fatty acid system. J. Mol. Liq. 2024, 414, 126214. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, G.; Li, C.; Li, B.; Ye, G. The surface dissolution process of smithsonite and its effect on flotation behaviour. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132118. [Google Scholar] [CrossRef]
- Trainor, T.P.; Brown, G.E.; Parks, G.A. Adsorption and Precipitation of Aqueous Zn(II) on Alumina Powders. J. Colloid Interface Sci. 2000, 231, 359–372. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, Q.; Li, X.; Wang, X.; Ke, B.; Li, X.; Shen, Z. Effect of the morphology of adsorbed oleate on the wettability of a collophane surface. Appl. Surf. Sci. 2018, 444, 87–96. [Google Scholar] [CrossRef]
- Zhang, Q.; Wen, S.; Song, Z.; Tang, Y.; Feng, Q. Advances in zinc oxide mineral flotation: Fundamentals, practices and perspectives. Miner. Eng. 2025, 233, 109621. [Google Scholar] [CrossRef]
- Sun, K.; Nguyen, N.N.; Nguyen, A.V. Soluble mineral flotation paradox: Improved recovery with no sign of collector adsorption on minerals signifies colloidal attraction between bubble-bound collector colloids and mineral particles. Miner. Eng. 2024, 218, 109018. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Z.; Jiao, F.; Qin, W. Understanding bubble growth process under decompression and its effects on the flotation phenomena. Miner. Eng. 2020, 145, 106066. [Google Scholar] [CrossRef]
- Aarab, I.; Derqaoui, M.; El Amari, K.; Yaacoubi, A.; Abidi, A.; Etahiri, A.; Baçaoui, A. Influence of surface dissolution on reagents’ adsorption on low-grade phosphate ore and its flotation selectivity. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127700. [Google Scholar] [CrossRef]
- Yin, X.; Yao, J.; Yang, B.; Yin, W.; Song, N.; Xie, Y.; Liu, J. Inhibition effect and mechanism of actinolite dissolution characteristics on quartz flotation. Miner. Eng. 2025, 232, 109491. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, G.; Feng, Q.; Deng, H. Effect of solution chemistry on the flotation system of smithsonite and calcite. Int. J. Miner. Process. 2013, 119, 34–39. [Google Scholar] [CrossRef]
- Sun, W.; Han, H.; Sun, W.; Wang, R.; Wei, Z. Novel insights into the role of colloidal calcium dioleate in the flotation of calcium minerals. Miner. Eng. 2022, 175, 107274. [Google Scholar] [CrossRef]
- Horta, D.; de Mello Monte, M.B.; de Salles Leal Filho, e.L. The effect of dissolution kinetics on flotation response of apatite with sodium oleate. Int. J. Miner. Process. 2016, 146, 97–104. [Google Scholar] [CrossRef]
- Yu, A.; Ding, Z.; Yuan, J.; Yu, P.; Chen, L.; Zhang, Y.; Wen, S.; Bai, S. Influence of carboxymethyl chitosan on selective flotation separation of smithsonite from calcite with sodium oleate. Adv. Powder Technol. 2024, 35, 104604. [Google Scholar] [CrossRef]
- Lu, Y.; Drelich, J.; Miller, J.D. Oleate adsorption at an apatite surface studied by ex-situ FTIR internal reflection spectroscopy. J. Colloid Interface Sci. 1998, 202, 462–476. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Z.; Luo, X.; Qin, W.; Jiao, F. Effect of nanobubbles on adsorption of sodium oleate on calcite surface. Miner. Eng. 2019, 133, 127–137. [Google Scholar] [CrossRef]
- Xiao, W.; Zhao, Y.; Yang, J.; Ren, Y.; Yang, W.; Huang, X.; Zhang, L. Effect of sodium oleate on the adsorption morphology and mechanism of nanobubbles on the mica surface. Langmuir 2019, 35, 9239. [Google Scholar] [CrossRef] [PubMed]
- Fuerstenau, D.W.; Pradip. Zeta potentials in the flotation of oxide and silicate minerals. Adv. Colloid Interface Sci. 2005, 114–115, 9–26. [Google Scholar] [CrossRef]
- Atrafi, A.; Pawlik, M. Foamability of fatty acid solutions and surfactant transfer between foam and solution phases. Miner. Eng. 2017, 100, 99–108. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Li, L.; Jin, S. Effect of energy input on flocculation process and flotation performance of fine scheelite using sodium oleate. Miner. Eng. 2017, 112, 27–35. [Google Scholar] [CrossRef]
- Qamar, S.; Warnecke, G.; Elsner, M.P. On the solution of population balances for nucleation, growth, aggregation and breakage processes. Chem. Eng. Sci. 2009, 64, 2088–2095. [Google Scholar] [CrossRef]
- Yin, W.; Fan, Y.; Xie, Y. Application of nano-collector in mineral flotation: A review. Powder Technol. 2025, 466, 121430. [Google Scholar] [CrossRef]
- Yang, B.; Huang, P.; Song, S.; Luo, H.; Zhang, Y. Hydrophobic agglomeration of apatite fines induced by sodium oleate in aqueous solutions. Results Phys. 2018, 9, 970–977. [Google Scholar] [CrossRef]
- Zou, S.; Wang, S.; Zhong, H.; Qin, W. Hydrophobic agglomeration of rhodochrosite fines in aqueous suspensions with sodium oleate. Powder Technol. 2021, 377, 186–193. [Google Scholar] [CrossRef]
- Liu, Q.; Wannas, D.; Peng, Y. Exploiting the dual functions of polymer depressants in fine particle flotation. Int. J. Miner. Process. 2006, 80, 244–254. [Google Scholar] [CrossRef]
- Liu, L.; Hu, S.; Wu, C.; Liu, K.; Weng, L.; Zhou, W. Aggregates characterizations of the ultra-fine coal particles induced by nanobubbles. Fuel 2021, 297, 120765. [Google Scholar] [CrossRef]
- Lei, W.; Zhang, M.; Zhang, Z.; Zhan, N.; Fan, R. Effect of bulk nanobubbles on the entrainment of kaolinite particles in flotation. Powder Technol. 2020, 362, 84–89. [Google Scholar] [CrossRef]
- Hu, N.; Li, Y.; Wu, Z.; Lu, K.; Huang, D.; Liu, W. Foams stabilization by silica nanoparticle with cationic and anionic surfactants in column flotation: Effects of particle size. J. Taiwan Inst. Chem. Eng. 2018, 88, 62–69. [Google Scholar] [CrossRef]
- AlYousef, Z.A.; Almobarky, M.A.; Schechter, D.S. The effect of nanoparticle aggregation on surfactant foam stability. J. Colloid Interface Sci. 2018, 511, 365–373. [Google Scholar] [CrossRef]




| ZnO | Fe | Al2O3 | SiO2 | CaO | L.O.I |
|---|---|---|---|---|---|
| 64.1 | 0.10 | 0.015 | 0.75 | 0.05 | 34.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Cao, W.; Li, C.; Peng, Y.; Cui, Y.; Dong, L. Ultrasonic Pulp Conditioning-Induced Nanoparticles: A Critical Driver for Sonication-Assisted Ultrafine Smithsonite Flotation. Minerals 2025, 15, 927. https://doi.org/10.3390/min15090927
Zhou W, Cao W, Li C, Peng Y, Cui Y, Dong L. Ultrasonic Pulp Conditioning-Induced Nanoparticles: A Critical Driver for Sonication-Assisted Ultrafine Smithsonite Flotation. Minerals. 2025; 15(9):927. https://doi.org/10.3390/min15090927
Chicago/Turabian StyleZhou, Weiguang, Weiwei Cao, Chenwei Li, Yaoli Peng, Yanru Cui, and Liuyang Dong. 2025. "Ultrasonic Pulp Conditioning-Induced Nanoparticles: A Critical Driver for Sonication-Assisted Ultrafine Smithsonite Flotation" Minerals 15, no. 9: 927. https://doi.org/10.3390/min15090927
APA StyleZhou, W., Cao, W., Li, C., Peng, Y., Cui, Y., & Dong, L. (2025). Ultrasonic Pulp Conditioning-Induced Nanoparticles: A Critical Driver for Sonication-Assisted Ultrafine Smithsonite Flotation. Minerals, 15(9), 927. https://doi.org/10.3390/min15090927






