Chromium in Slag from SOEL Interconnects Remelting: Characterization and Recycling Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Slag Production
2.2. Characterization Methods
2.2.1. X-Ray Computer Tomography
2.2.2. Chemical Composition by WD-XRF
2.2.3. Mineralogical Analysis by XRD
2.2.4. Elemental Distribution and Mineral Composition by SEM-Based MLA
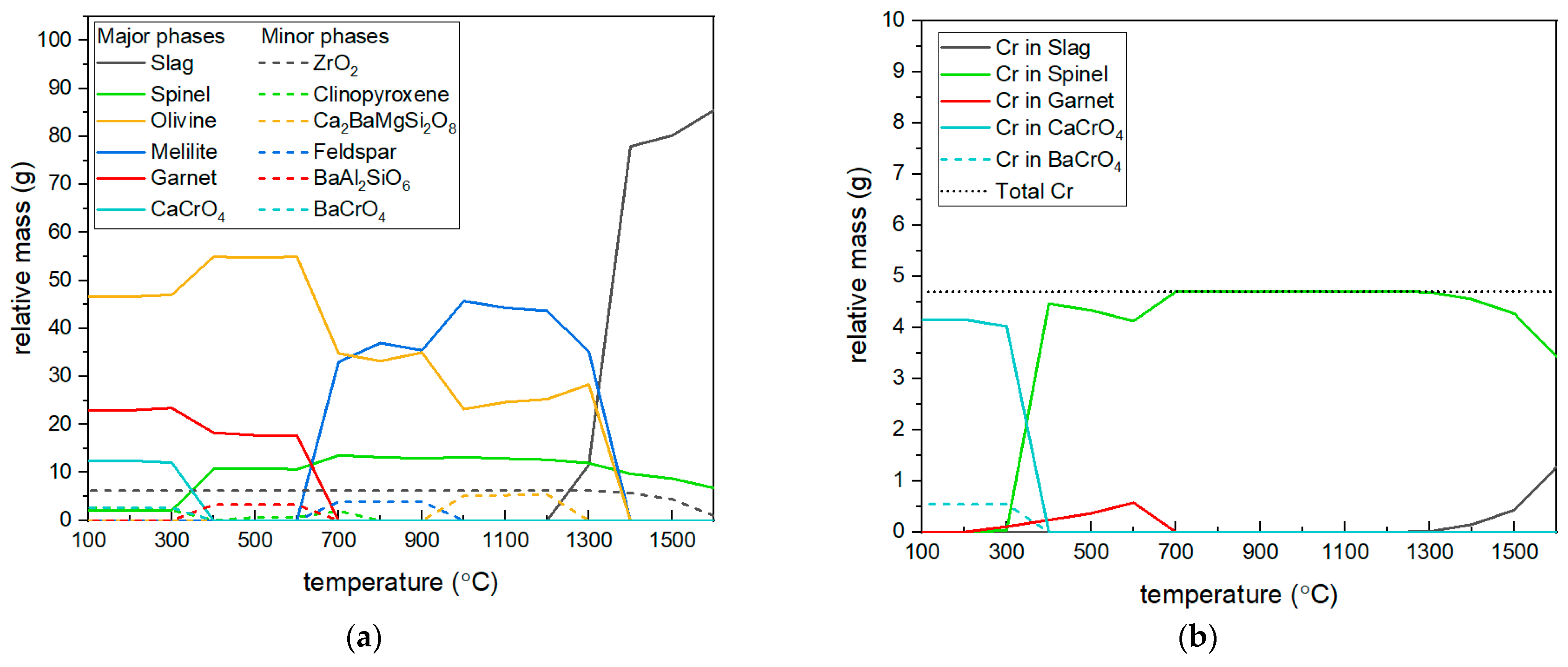
2.2.5. Thermodynamic Equilibrium Calculations
3. Results & Discussion
3.1. Slag Body Characterization by XCT
3.2. Chemical Composition
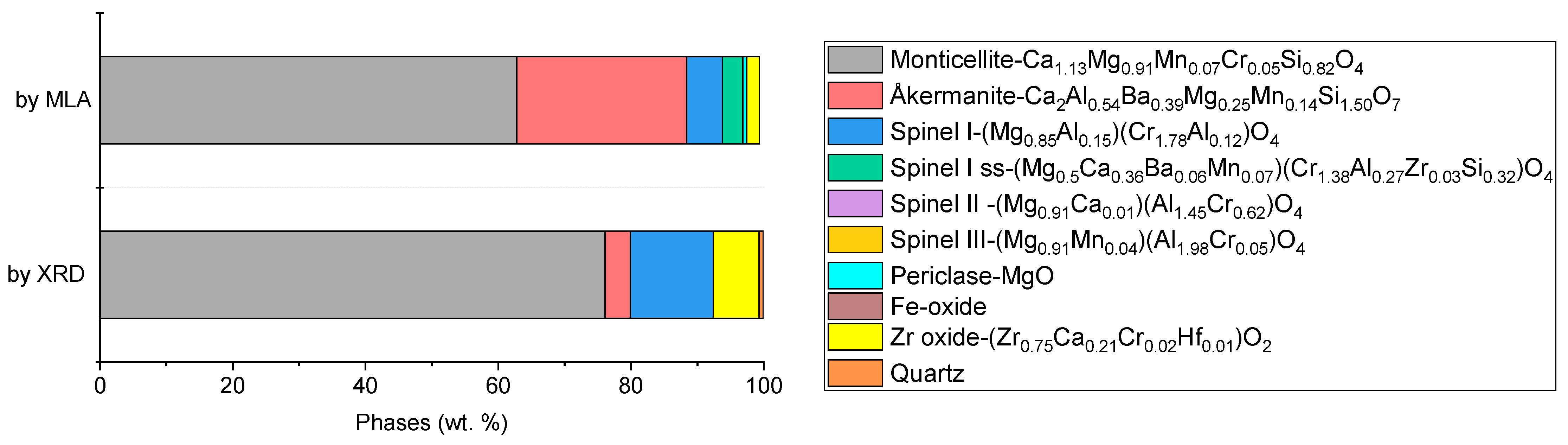
3.3. Mineralogy
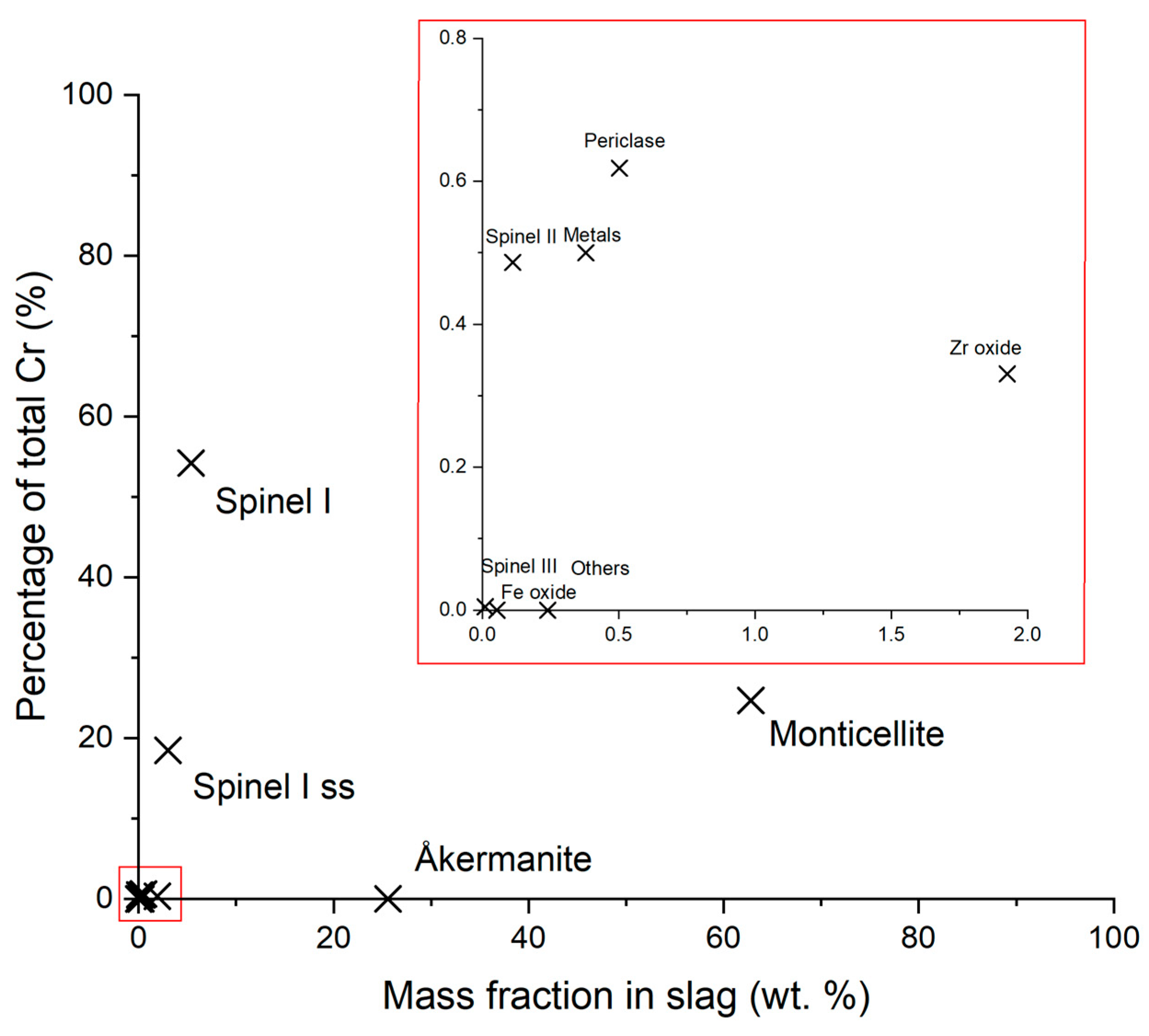
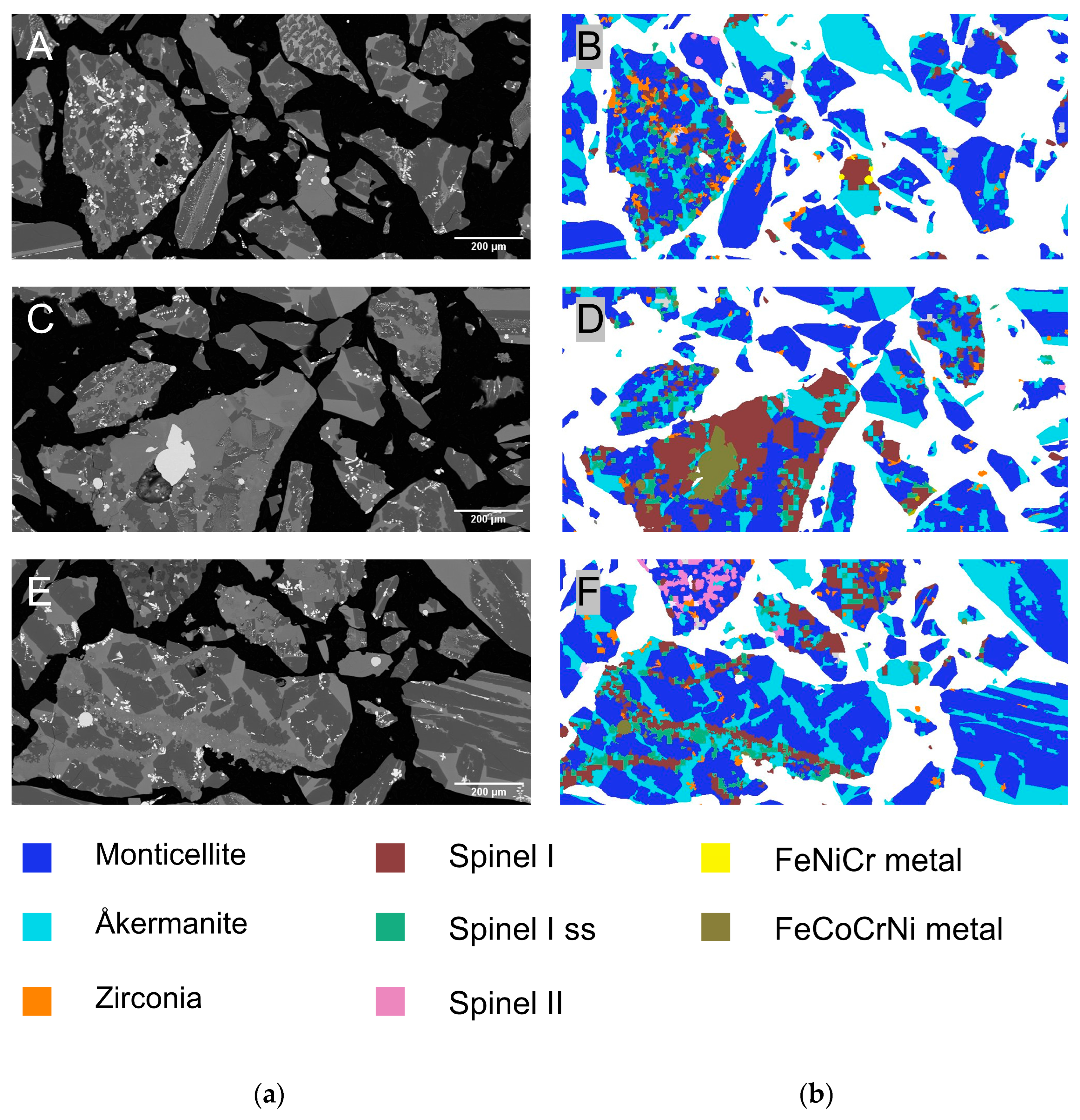
3.4. Cr Distribution & Cr-Bearing Phase Morphology
4. Conclusions and Outlook
- Thermodynamic calculation results showed good agreement with experimental data regarding Cr partitioning across mineral phases. Cr is only present in the spinel, garnet, and slag phase from the calculation.
- XCT revealed strong macroscopic heterogeneity in the slag structure. Metallic inclusions and pores were unevenly distributed, with higher densities and larger inclusions settling near the metal–slag interface.
- Chromium was mainly concentrated in magnesiochromite, which has high Cr content (~54 wt.%) representing only ~5 wt.% of the total slag. Monticellite and åkermanite, although abundant, incorporate Cr in diluted forms and are not viable for selective recovery.
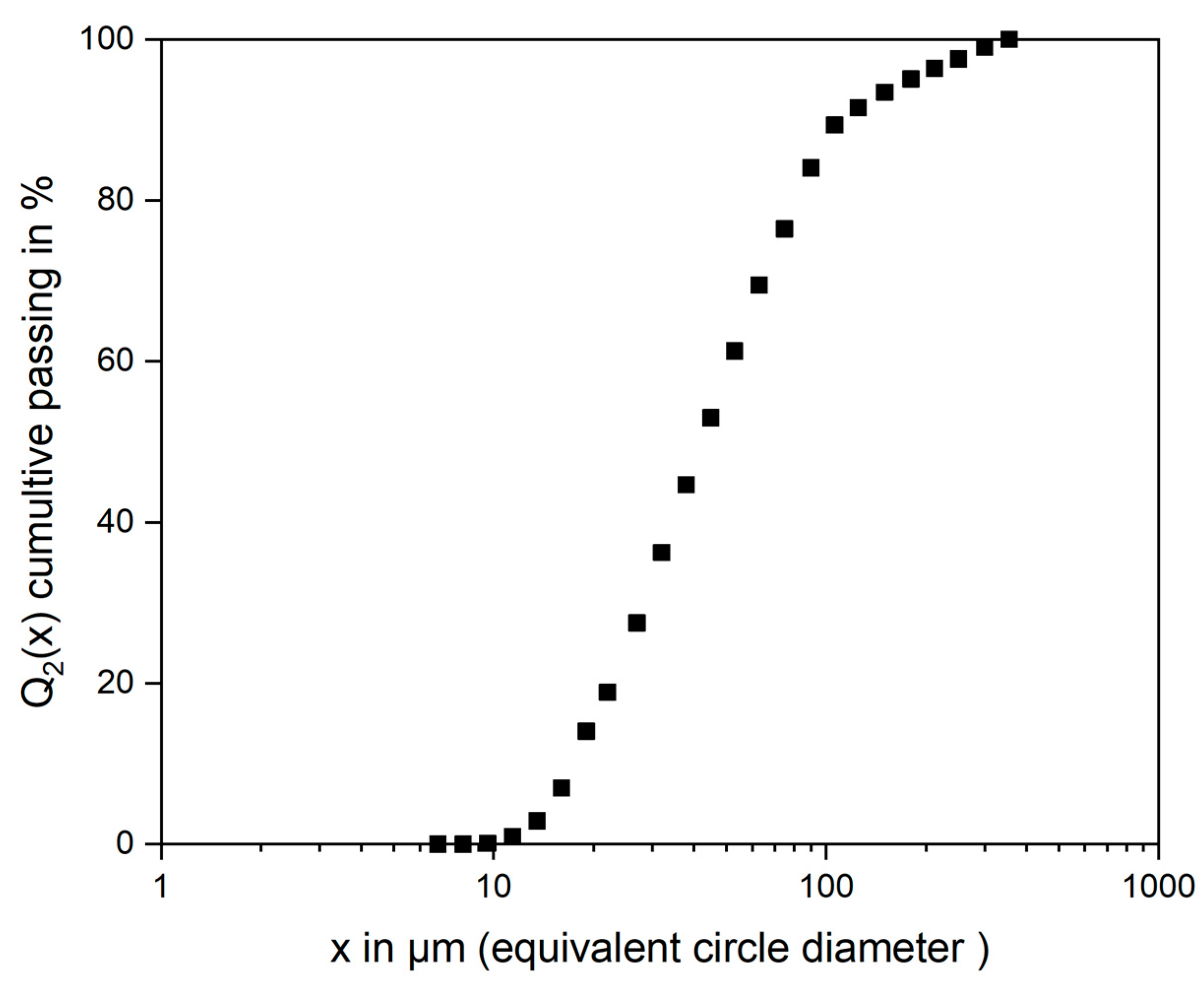
- These spinels were fine-grained (x50,2 = 55 µm) and irregular in shape. The resulting spinel size is much greater than the spinel size in industrial slags.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Identified Crystalline Phase | in wt.% |
|---|---|
| Monticellite (CaMgSiO4) | 76.1 ± 0.8 |
| Spinel (AB2O4) | 12.5 ± 0.5 |
| Gehlenite (Ca2Al(AlSiO7)) | 3.8 ± 0.7 |
| Zirconia (ZrO2) | 6.9 ± 0.2 |
| Quartz (SiO2) | 0.6 ± 0.2 |
| Sum * | 100.0 |
| Droplet Name | Fe | Cr | Ni | Co | Cu | Mn |
|---|---|---|---|---|---|---|
| in wt.% | ||||||
| FeCr | 81.6 | 18.4 | ||||
| FeNiCr | 60.1 | 17.0 | 22.9 | |||
| FeCoCrNi | 46.7 | 16.0 | 4.6 | 32.6 | ||
| FeNiCuCo | 32.7 | 0.0 | 34.7 | 7.5 | 25.1 | |
| FeCoCr | 10.9 | 11.5 | 77.6 | |||
| FeCo | 10.0 | 90.0 | ||||
| FeNiCrMn | 2.0 | 11.8 | 84.8 | 1.4 | ||
References
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Sarner, S.; Schreiber, A.; Menzler, N.H.; Guillon, O. Recycling Strategies for Solid Oxide Cells. Adv. Energy Mater. 2022, 12, 2201805. [Google Scholar] [CrossRef]
- He, S.; Zou, Y.; Chen, K.; Jiang, S.P. A critical review of key materials and issues in solid oxide cells. Interdiscip. Mater. 2023, 2, 111–136. [Google Scholar] [CrossRef]
- Nechache, A.; Hody, S. Alternative and innovative solid oxide electrolysis cell materials: A short review. Renew. Sustain. Energy Rev. 2021, 149, 111322. [Google Scholar] [CrossRef]
- Jo, K.H.; Kim, J.H.; Kim, K.M.; Lee, I.-S.; Kim, S.-J. Development of a new cost effective Fe–Cr ferritic stainless steel for SOFC interconnect. Int. J. Hydrogen Energy 2015, 40, 9523–9529. [Google Scholar] [CrossRef]
- Harboe, S.; Schreiber, A.; Margaritis, N.; Blum, L.; Guillon, O.; Menzler, N.H. Manufacturing cost model for planar 5 kWel SOFC stacks at Forschungszentrum Jülich. Int. J. Hydrogen Energy 2020, 45, 8015–8030. [Google Scholar] [CrossRef]
- Yang, Z.G.; Paxton, D.M.; Weil, K.S.; Stevenson, J.W.; Singh, P. Materials Properties Database for Selection of High-Temperature Alloys and Concepts of Alloy Design for SOFC Applications; Pacific Northwest National Laboratory: Richland, WA, USA, 2002.
- Kaiser, C.; Ahn, S.; Brünner, M.; Goes, D.; Lastam, J.; Mongoljiibuu, S.-O.; Sarner, S.; Specht, A.; Fleischer, J.; Menzler, N.H.; et al. Recycling of solid oxide electrolyzer stacks. Sustain. Mater. Technol. 2025, 45, e01435. [Google Scholar] [CrossRef]
- Lastam, J.; Sergeev, D.; Grüner, D.; Müller, M.; Schwaiger, R. Unlocking the Value of End-of-Life JÜLICH Solid Oxide Cell Stack Interconnect Assembly: A Combined Experimental and Thermodynamic Study on Metallic Resource Recyclability. Metals 2024, 14, 406. [Google Scholar] [CrossRef]
- Lastam, J.; Mongoljiibuu, S.O.; Specht, A.; Grüner, D.; Sergeev, D.; Peuker, U.A.; Müller, M.; Schwaiger, R. Recycling End-of-Life Solid Oxide Cell Interconnect Assemblies into Commercial-Grade AISI 304 Stainless Steels. Steel Res. Int. 2025, 2500384. [Google Scholar] [CrossRef]
- Engh, T.A.; Sigworth, G.K.; Kvithyld, A. Principles of Metal Refining and Recycling; Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Spooren, J.; Kim, E.; Horckmans, L.; Broos, K.; Nielsen, P.; Quaghebeur, M. In-situ chromium and vanadium recovery of landfilled ferrochromium and stainless steel slags. Chem. Eng. J. 2016, 303, 359–368. [Google Scholar] [CrossRef]
- Mombelli, D.; Mapelli, C.; Barella, S.; Di Cecca, C.; Le Saout, G.; Garcia-Diaz, E. The effect of microstructure on the leaching behaviour of electric arc furnace (EAF) carbon steel slag. Process Saf. Environ. Prot. 2016, 102, 810–821. [Google Scholar] [CrossRef]
- Engström, F. Mineralogical Influence on Leaching Behaviour of Steelmaking Slags: A Laboratory Investigation; Luleå University of Technology: Luleå, Sweden, 2010. [Google Scholar]
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250–251, 272–291. [Google Scholar] [CrossRef] [PubMed]
- Kimbrough, D.E.; Cohen, Y.; Winer, A.M.; Creelman, L.; Mabuni, C. A Critical Assessment of Chromium in the Environment. Crit. Rev. Environ. Sci. Technol. 1999, 29, 1–46. [Google Scholar] [CrossRef]
- 2003/33/EC; Council Decision of 19 December 2002 Establishing Criteria and Procedures for the Acceptance of Waste at Landfills Pursuant to Article 16 of and Annex II to Directive 1999/31/EC. E.U. Council: Brussels, Belgium, 2003.
- Bundesministerium des Innern, f.B.u.H. Muster-Verwaltungsvorschrift Technische Baubestimmungen (MVV TB), Annex 10: Anforderungen an Bauliche Anlagen bzgl. der Auswirkungen auf Boden und Gewässer (ABuG). 2024. Available online: https://izw.baw.de/publikationen/tr-w/4/Anhang_10_MVV_TB.pdf (accessed on 6 February 2025).
- Herbelin, M.; Bascou, J.; Lavastre, V.; Guillaume, D.; Benbakkar, M.; Peuble, S.; Baron, J.-P. Steel Slag Characterisation—Benefit of Coupling Chemical, Mineralogical and Magnetic Techniques. Minerals 2020, 10, 705. [Google Scholar] [CrossRef]
- Neuhold, S.; van Zomeren, A.; Dijkstra, J.J.; van der Sloot, H.A.; Drissen, P.; Algermissen, D.; Mudersbach, D.; Schüler, S.; Griessacher, T.; Raith, J.G.; et al. Investigation of Possible Leaching Control Mechanisms for Chromium and Vanadium in Electric Arc Furnace (EAF) Slags Using Combined Experimental and Modeling Approaches. Minerals 2019, 9, 525. [Google Scholar] [CrossRef]
- Horckmans, L.; Möckel, R.; Nielsen, P.; Kukurugya, F.; Vanhoof, C.; Morillon, A.; Algermissen, D. Multi-Analytical Characterization of Slags to Determine the Chromium Concentration for a Possible Re-Extraction. Minerals 2019, 9, 646. [Google Scholar] [CrossRef]
- Menad, N.-E.; Kana, N.; Seron, A.; Kanari, N. New EAF Slag Characterization Methodology for Strategic Metal Recovery. Materials 2021, 14, 1513. [Google Scholar] [CrossRef]
- Tossavainen, M.; Engstrom, F.; Yang, Q.; Menad, N.; Lidstrom Larsson, M.; Bjorkman, B. Characteristics of steel slag under different cooling conditions. Waste Manag. 2007, 27, 1335–1344. [Google Scholar] [CrossRef]
- Pillay, K.; von Blottnitz, H.; Petersen, J. Ageing of chromium(III)-bearing slag and its relation to the atmospheric oxidation of solid chromium(III)-oxide in the presence of calcium oxide. Chemosphere 2003, 52, 1771–1779. [Google Scholar] [CrossRef]
- Samada, Y.; Miki, T.; Hino, M. Prevention of Chromium Elution from Stainless Steel Slag into Seawater. ISIJ Int. 2011, 51, 728–732. [Google Scholar] [CrossRef]
- Li, Y.; Gao, J.; Lan, X.; Feng, G.; Zhang, Y.; Guo, Z. Solidification and recovery of Cr from hazardous Cr-bearing steel slag: Selective solidification, super-gravity separation and crystal characterization. Sep. Purif. Technol. 2023, 306, 122616. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Tao, M.-J.; Li, J.-G.; Zeng, Y.-N.; Qin, S.; Liu, S.-H. Carbonation of EAF Stainless Steel Slag and Its Effect on Chromium Leaching Characteristics. Crystals 2021, 11, 1498. [Google Scholar] [CrossRef]
- Kim, E.; Spooren, J.; Broos, K.; Nielsen, P.; Horckmans, L.; Vrancken, K.C.; Quaghebeur, M. New method for selective Cr recovery from stainless steel slag by NaOCl assisted alkaline leaching and consecutive BaCrO 4 precipitation. Chem. Eng. J. 2016, 295, 542–551. [Google Scholar] [CrossRef]
- Durinck, D.; Jones, P.T.; Guo, M.; Verhaeghe, F.; Heylen, G.; Hendrickx, R.; Baeten, R.; Blanpain, B.; Wollants, P. EAF Stainless Steel Refining—Part II: Microstructural Slag Evolution and its Implications for Slag Foaming and Chromium Recovery. Steel Res. Int. 2007, 78, 125–135. [Google Scholar] [CrossRef]
- Aldrian, A.; Raith, J.G.; Höllen, D.; Pomberger, R. Influence of Chromium Containing Spinels in an Electric Arc Furnace Slag on the Leaching Behaviour. J. Solid Waste Technol. Manag. 2015, 41, 357–365. [Google Scholar] [CrossRef]
- Cheremisina, E.; Schenk, J. Chromium Stability in Steel Slags. Steel Res. Int. 2017, 88, 1700206. [Google Scholar] [CrossRef]
- Rachmawati, C.; Weiss, J.; Lucas, H.I.; Löwer, E.; Leißner, T.; Ebert, D.; Möckel, R.; Friedrich, B.; Peuker, U.A. Characterisation of the Grain Morphology of Artificial Minerals (EnAMs) in Lithium Slags by Correlating Multi-Dimensional 2D and 3D Methods. Minerals 2024, 14, 130. [Google Scholar] [CrossRef]
- Qiu, H.; Li, H.; Fischlschweiger, M.; Ranneberg, M.; Graupner, T.; Lucas, H.; Stallmeister, C.; Friedrich, B.; Yagmurlu, B.; Goldmann, D. Valorization of lithium containing slags from pyrometallurgical recycling route of spent lithium-ion batteries: The enrichment of γ-LiAlO2 phase from thermodynamic controlled and modified slags. Miner. Eng. 2024, 217, 108918. [Google Scholar] [CrossRef]
- Weiss, J.; Munchen, D.; Richter, S.; Friedrich, B. Crystallization Study of a Synthetic Fayalitic Slag System with Ta Based on Thermochemical Modeling. In Proceedings of the 63rd Conference of Metallurgists, COM 2024, Toronto, ON, Canada, 21–24 August 2024; Springer Nature: Cham, Switzerland, 2025. [Google Scholar]
- Xiao, Y.; Holappa, L. Thermodynamic Properties of Chromium Bearing Slags and Minerals; Helsinki University of Technology: Espoo, Finland, 1996; pp. 39–42. [Google Scholar]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.; Eriksson, G.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J.; Pelton, A. FactSage thermochemical software and databases—2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Gondrom, S.; Zhou, J.; Maisl, M.; Reiter, H.; Kröning, M.; Arnold, W. X-ray computed laminography: An approach of computed tomography for applications with limited access. Nucl. Eng. Des. 1999, 190, 141–147. [Google Scholar] [CrossRef]
- Withers, P.J.; Bouman, C.; Carmignato, S.; Cnudde, V.; Grimaldi, D.; Hagen, C.K.; Maire, E.; Manley, M.; Du Plessis, A.; Stock, S.R. X-ray computed tomography. Nat. Rev. Methods Primers 2021, 1, 18. [Google Scholar] [CrossRef]
- Berg, S.; Kutra, D.; Kroeger, T.; Straehle, C.N.; Kausler, B.X.; Haubold, C.; Schiegg, M.; Ales, J.; Beier, T.; Rudy, M.; et al. Ilastik: Interactive machine learning for (bio)image analysis. Nat. Methods 2019, 16, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, T.; Schach, E.; Peuker, U.A. A framework for the description of multidimensional particle separation processes. Powder Technol. 2024, 433, 119165. [Google Scholar] [CrossRef]
- He, M.; Chen, M.; Wang, N.; Li, C. Sedimentation Behavior of Liquid Iron Droplets during Smelting Reduction of Converter Slag by Considering the Coalescence of Droplets. ISIJ Int. 2019, 59, 973–980. [Google Scholar] [CrossRef]
- Bellemans, I.; Cnockaert, V.; De Wilde, E.; Moelans, N.; Verbeken, K. Metal Droplet Entrainment by Solid Particles in Slags: An Experimental Approach. J. Sustain. Metall. 2017, 4, 15–32. [Google Scholar] [CrossRef]
- Iwamasa, P.K.; Fruehan, R.J. Separation of Metal Droplets from Slag. ISIJ Int. 1996, 36, 1319–1327. [Google Scholar] [CrossRef]
- Hemberger, Y.; Berthold, C.; Nickel, K.G. Wetting and corrosion of yttria stabilized zirconia by molten slags. J. Eur. Ceram. Soc. 2012, 32, 2859–2866. [Google Scholar] [CrossRef]
- Wang, W.; Xue, L.; Zhang, T.; Zhou, L.; Chen, J.; Pan, Z. Thermodynamic corrosion behavior of Al2O3, ZrO2 and MgO refractories in contact with high basicity refining slag. Ceram. Int. 2019, 45, 20664–20673. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Jiang, C.; Ding, J.; Guo, J.; Kong, L.; Bai, J.; Li, W. Influence of the Slag–Crucible Interaction on Coal Ash Fusion Behavior at High Temperatures. Energy Fuels 2020, 34, 3087–3099. [Google Scholar] [CrossRef]
- Teng, L. Chapter 3.2—Refractory Corrosion During Steelmaking Operations. In Treatise on Process Metallurgy; Seetharaman, S., Ed.; Elsevier: Boston, MA, USA, 2014; pp. 283–303. [Google Scholar]
- Renno, A.D.; Möckel, R.; Frenzel, M.; Ebert, D.; Bachmann, K.; Krause, J.; Gutzmer, J. Metal Deportment in Complex Secondary Raw Materials: The Case of Vanadium in Basic Oxygen Furnace Slags. Min. Metall. Explor. 2023, 40, 2139–2152. [Google Scholar] [CrossRef]
- Schulz, B.; Sandmann, D.; Gilbricht, S. SEM-Based Automated Mineralogy and Its Application in Geo- and Material Sciences. Minerals 2020, 10, 1004. [Google Scholar] [CrossRef]
- Võ, T.T.; Leißner, T.; Peuker, U.A. Utilizing X-ray Computed Tomography for Lithium Slag: A Guide to Analyzing Microstructure and Its Potential Influence on Liberation. Minerals 2024, 14, 42. [Google Scholar] [CrossRef]
- Bahnmüller, S.; Hirschberger, P.; Võ, T.T.; Rachmawati, C.; Kwade, A.; Peuker, U.; Kruggel-Emden, H.; Schilde, C. Particle Size-and Structure-Dependent Breakage Behaviors of EnAM-Containing Slags. Minerals 2025, 15, 195. [Google Scholar] [CrossRef]
- Murthy, Y.R.; Tripathy, S.K.; Kumar, C.R. Chrome ore beneficiation challenges & opportunities—A review. Miner. Eng. 2011, 24, 375–380. [Google Scholar]
- Schirmer, T.; Qiu, H.; Li, H.; Goldmann, D.; Fischlschweiger, M. Li-Distribution in Compounds of the Li2O-MgO-Al2O3-SiO2-CaO System—A First Survey. Metals 2020, 10, 1633. [Google Scholar] [CrossRef]
- Leißner, T.; Mütze, T.; Bachmann, K.; Rode, S.; Gutzmer, J.; Peuker, U.A. Evaluation of mineral processing by assessment of liberation and upgrading. Miner. Eng. 2013, 53, 171–173. [Google Scholar] [CrossRef]
- Reyes, F.; Lin, Q.; Cilliers, J.J.; Neethling, S.J. Quantifying mineral liberation by particle grade and surface exposure using X-ray microCT. Miner. Eng. 2018, 125, 75–82. [Google Scholar] [CrossRef]
- Ferrara, G.; Preti, U.; Meloy, T.P. Inclusion shape, mineral texture and liberation. Int. J. Miner. Process. 1989, 27, 295–308. [Google Scholar] [CrossRef]
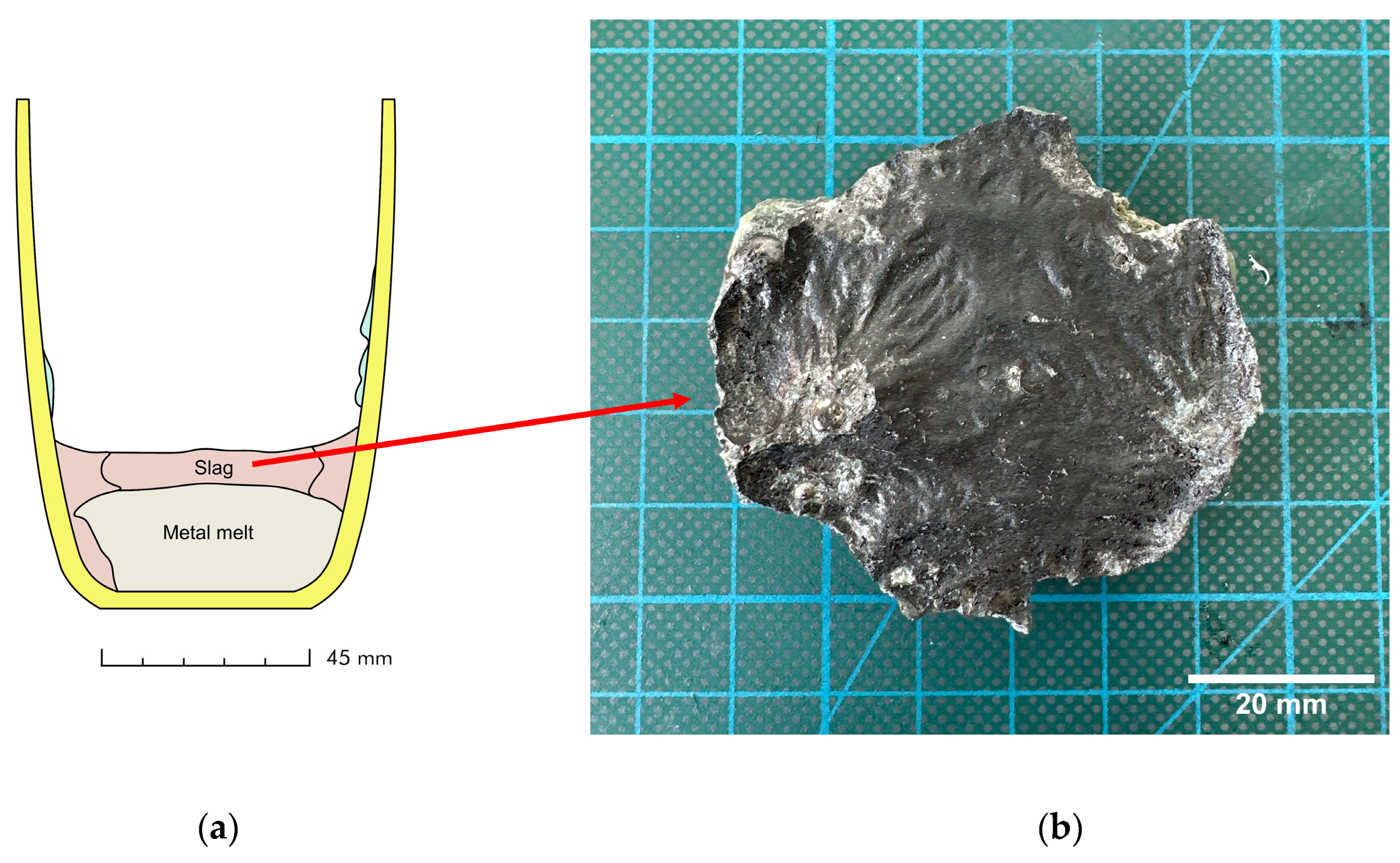
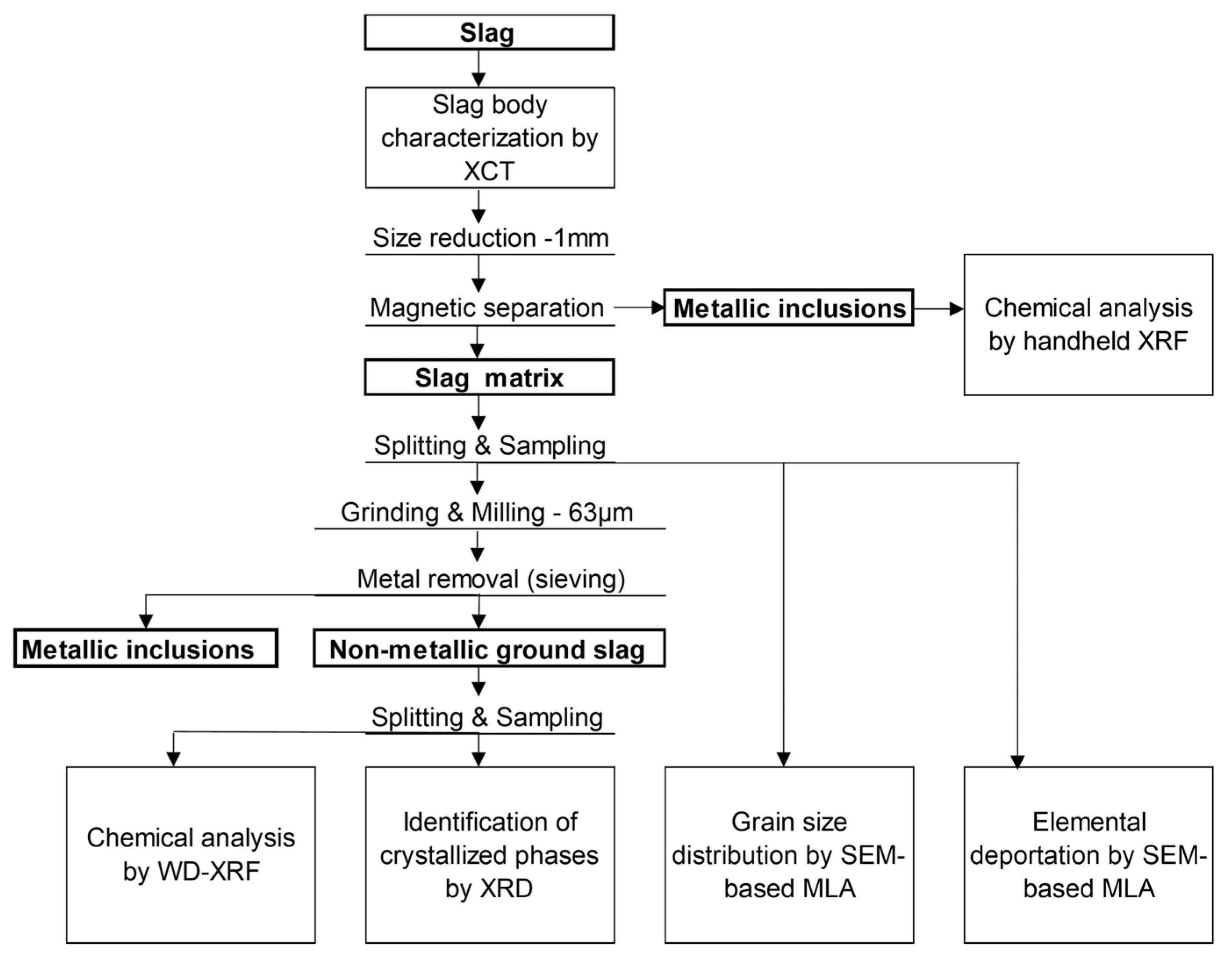

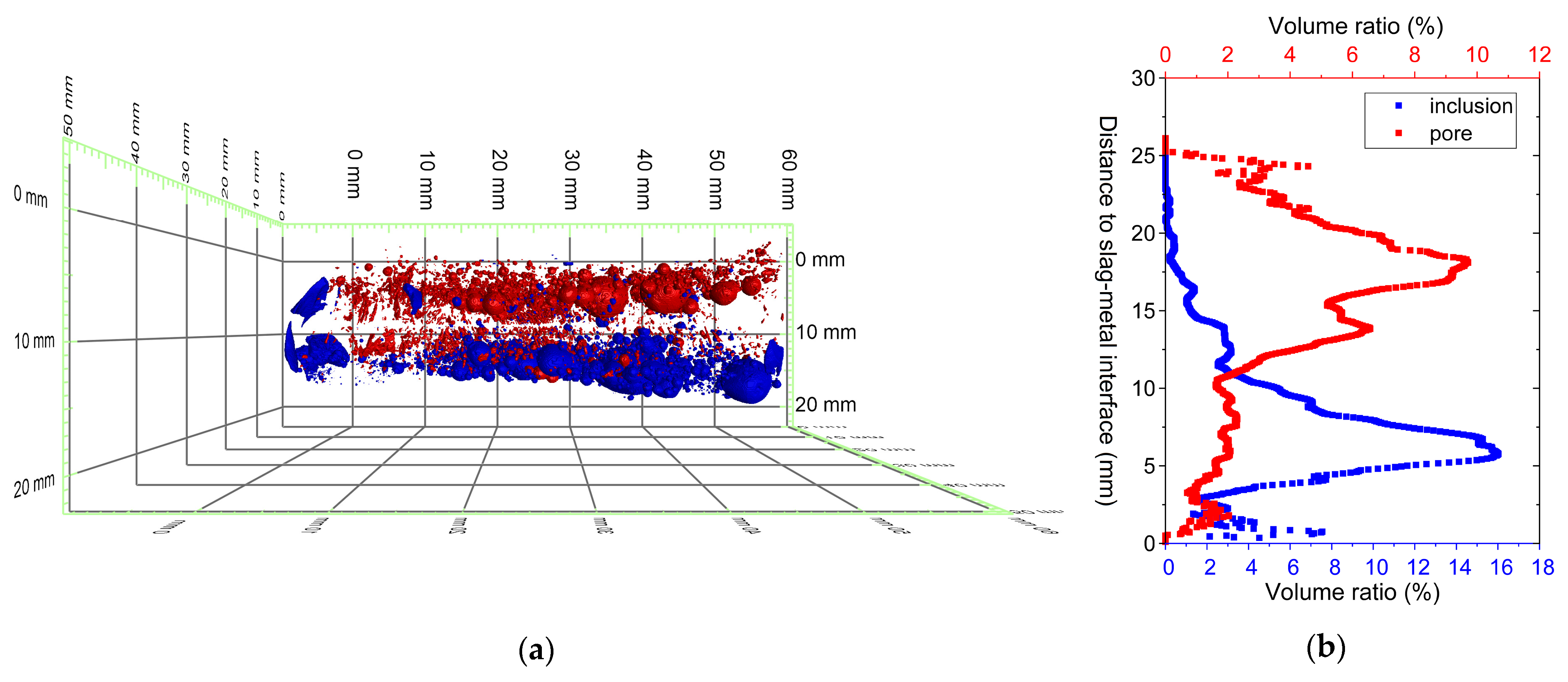
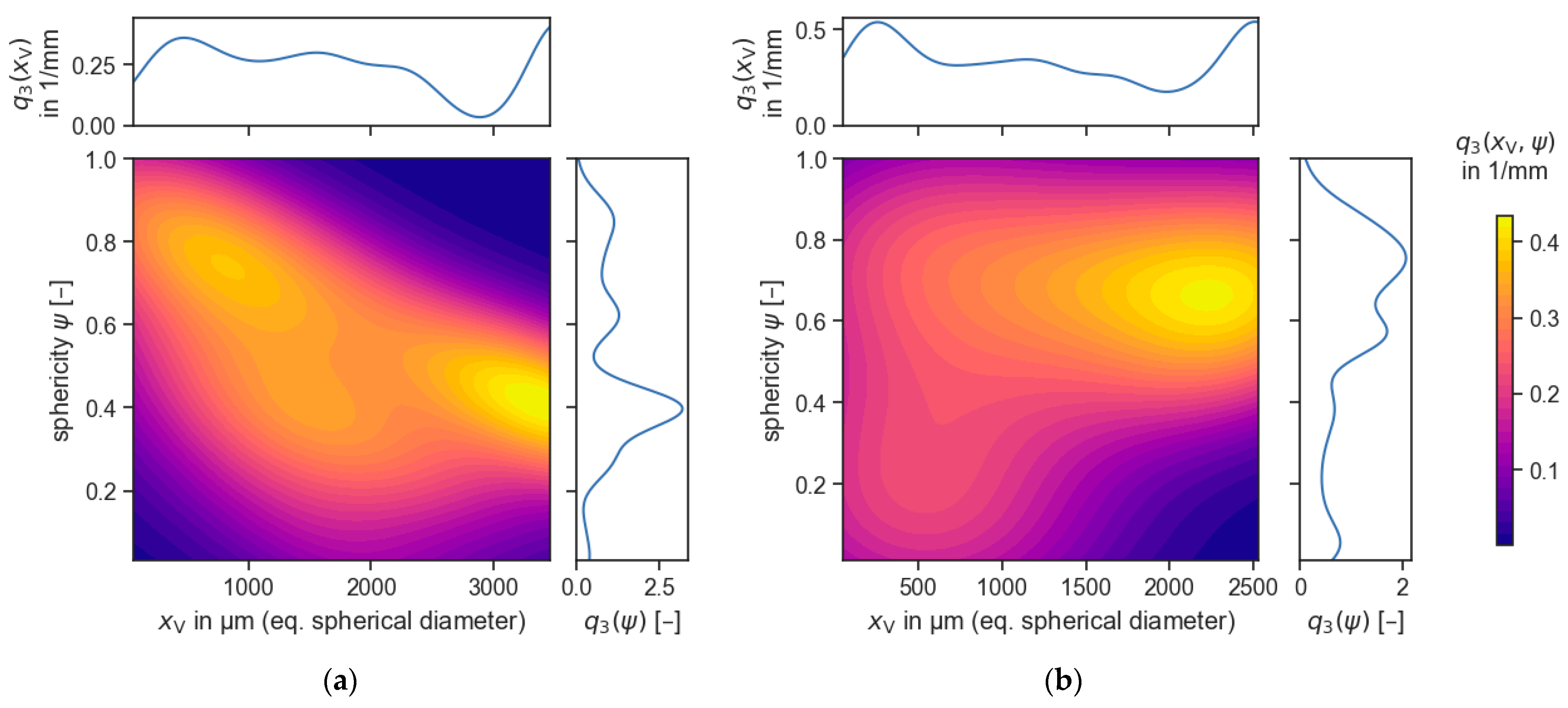
| Composition (wt.%) | Tliq (°C) | B = (CaO + MgO)/SiO2 (w/w) | |||
|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | MgO | ||
| 35.0 | 37.6 | 6.2 | 21.2 | 1453 | 1.5 |
| Feed (g) | Product (g) | Metal Yield = Metal/Sample (g/g) | |||
|---|---|---|---|---|---|
| Sample | Flux | Metal | Slag | Unrecovered | |
| 406.00 | 105.89 | 386.80 | 56.10 | 68.99 | 0.953 |
| Parameters | ||
|---|---|---|
| Scanning | Source–sample distance in mm | 140 |
| Sample–detector distance in mm | 50 | |
| Acceleration voltage in keV | 160 | |
| Electrical power in W | 10 | |
| Filter in Zeiss Standard | HE4 | |
| Optical magnification | 0.4× | |
| Exposure time in s | 4.5 | |
| Camera binning | 2 | |
| Voxel size in µm | 50.1 | |
| Number of projections | 1601 | |
| Scan angle in degree | 360 | |
| Reconstruction | Reconstruction algorithm | FBP |
| Gauss smoothing | 0.1 | |
| Beam hardening correction | 0.05 | |
| Sample Name | LOI | CaO | SiO2 | MgO | Al2O3 | Fe2O3 | ZrO2 | BaO | Cr2O3 | MnO | NiO | CuO | TiO2 | SrO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| in wt.% | ||||||||||||||
| IC | 0.59 | 26.2 | 29.1 | 16.7 | 5.2 | 1.7 | 6.2 | 1.6 | 6.9 | 1.3 | 0.58 | 0.06 | 0.3 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mongoljiibuu, S.-O.; Lastam, J.; Ditscherlein, R.; Ebert, D.; Müller, M.; Peuker, U.A. Chromium in Slag from SOEL Interconnects Remelting: Characterization and Recycling Potential. Minerals 2025, 15, 904. https://doi.org/10.3390/min15090904
Mongoljiibuu S-O, Lastam J, Ditscherlein R, Ebert D, Müller M, Peuker UA. Chromium in Slag from SOEL Interconnects Remelting: Characterization and Recycling Potential. Minerals. 2025; 15(9):904. https://doi.org/10.3390/min15090904
Chicago/Turabian StyleMongoljiibuu, Shine-Od, Jeraldine Lastam, Ralf Ditscherlein, Doreen Ebert, Michael Müller, and Urs A. Peuker. 2025. "Chromium in Slag from SOEL Interconnects Remelting: Characterization and Recycling Potential" Minerals 15, no. 9: 904. https://doi.org/10.3390/min15090904
APA StyleMongoljiibuu, S.-O., Lastam, J., Ditscherlein, R., Ebert, D., Müller, M., & Peuker, U. A. (2025). Chromium in Slag from SOEL Interconnects Remelting: Characterization and Recycling Potential. Minerals, 15(9), 904. https://doi.org/10.3390/min15090904







