Dissolution of Diamond in Water–Chloride Fluids at Mantle P-T Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Starting Compositions
2.2. High-Pressure Apparatus and Analytical Techniques
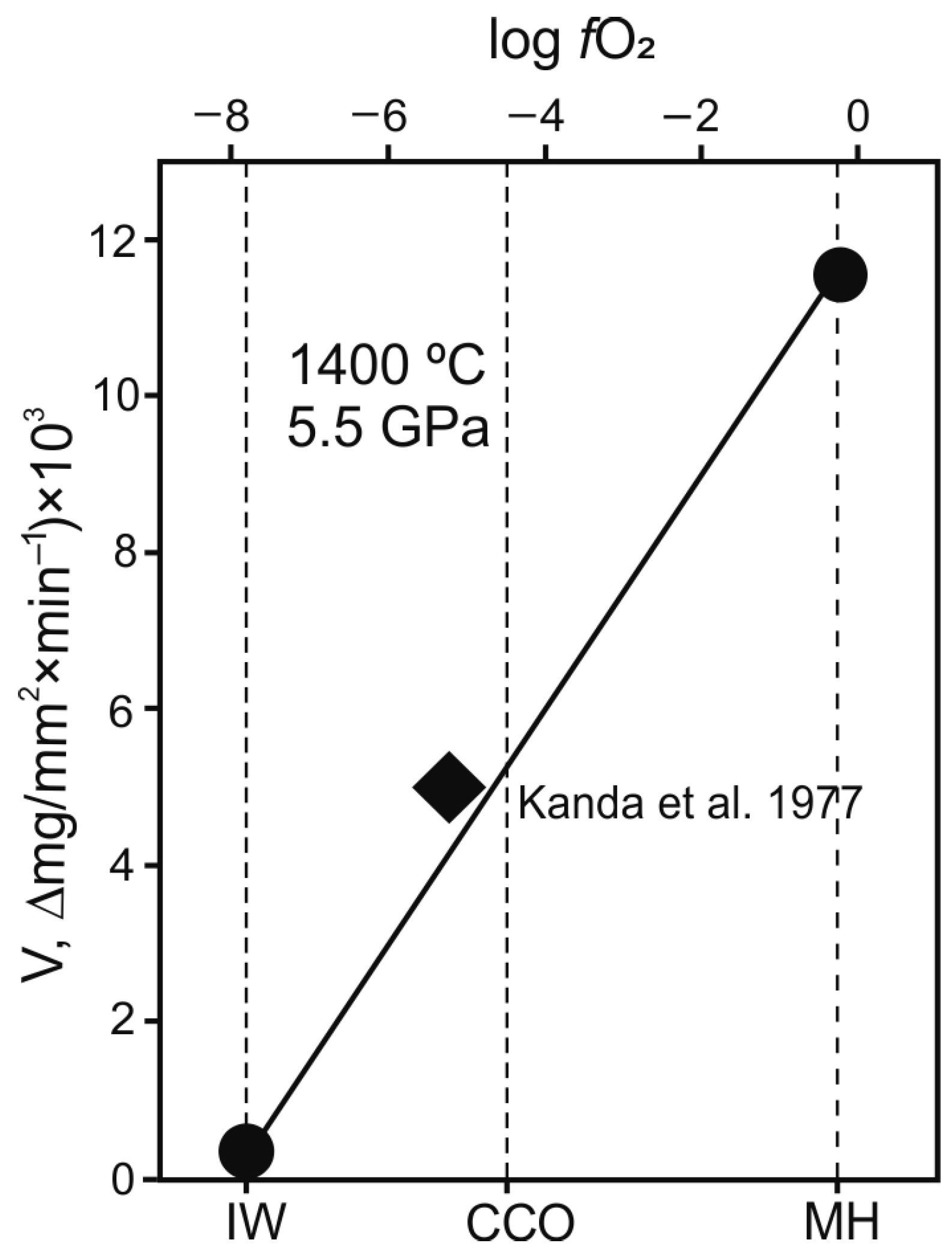
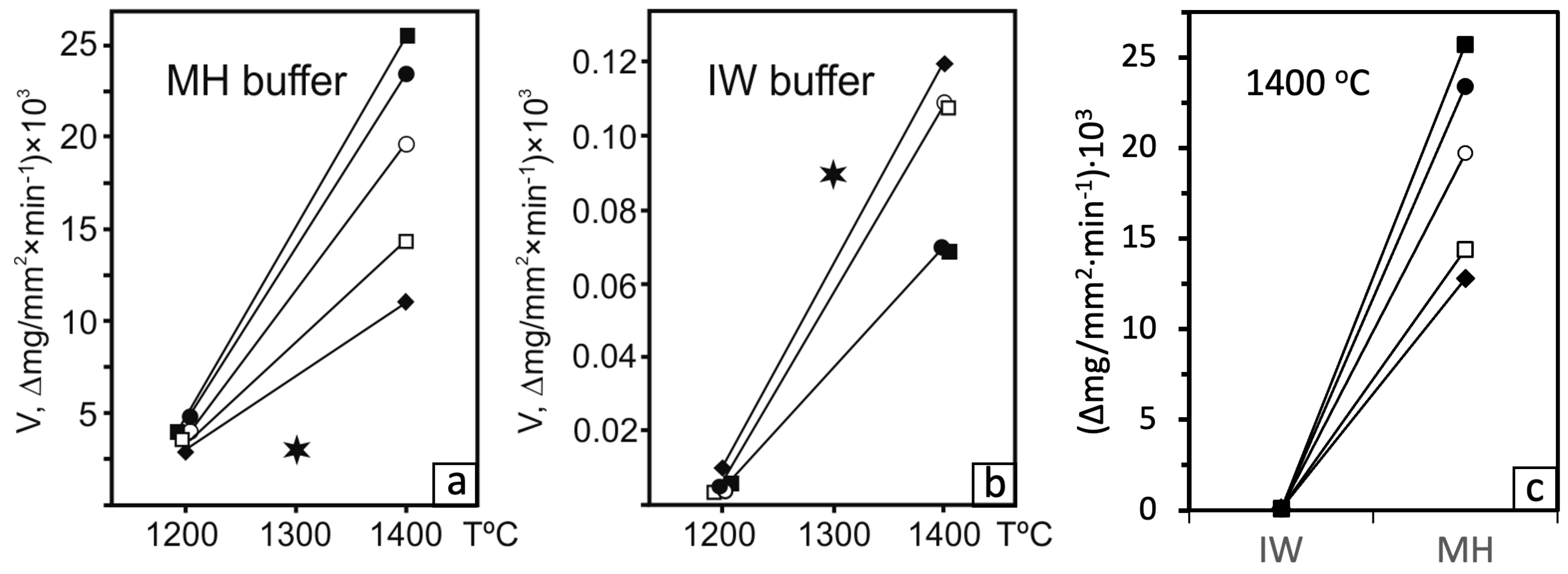
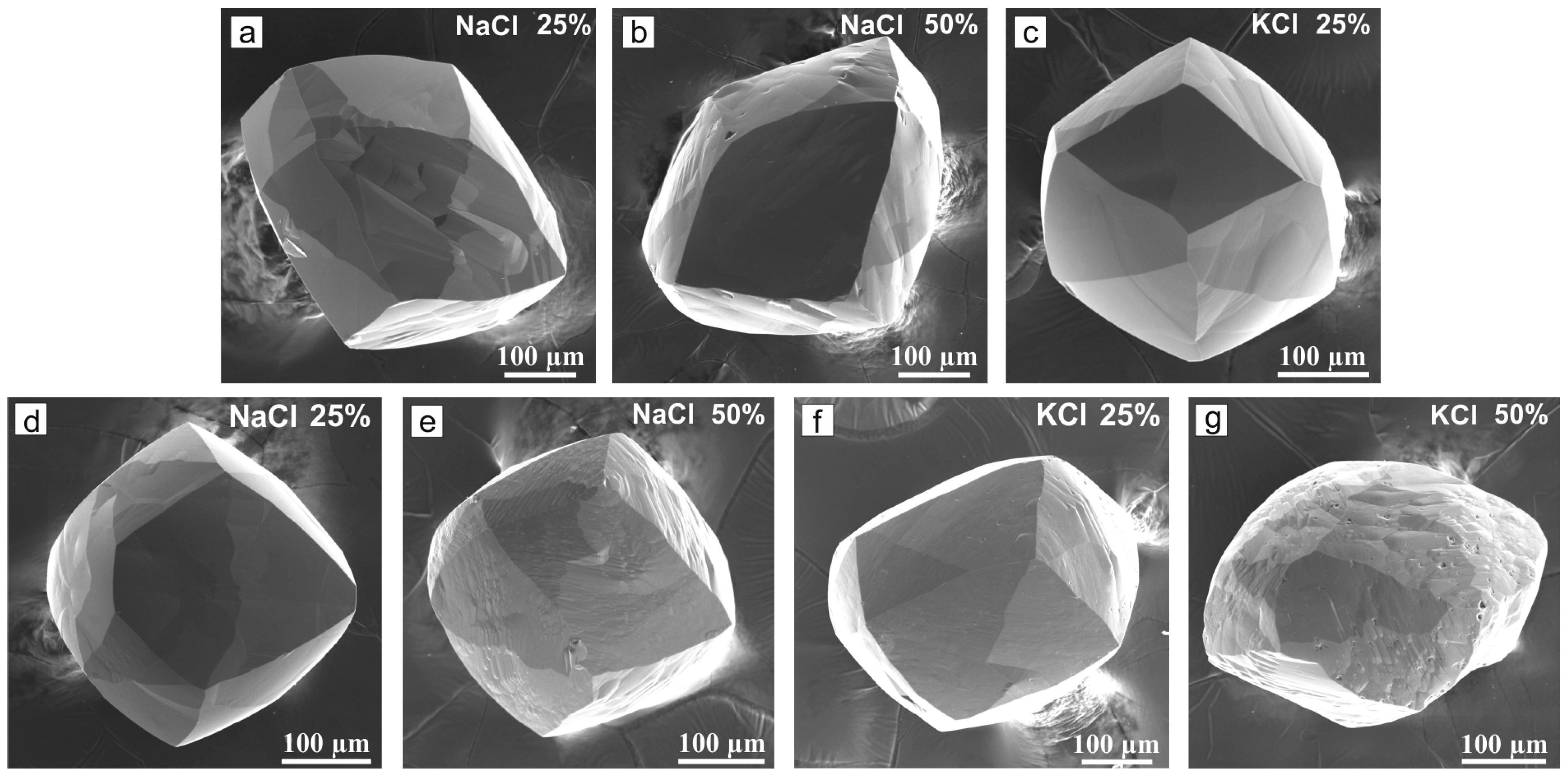
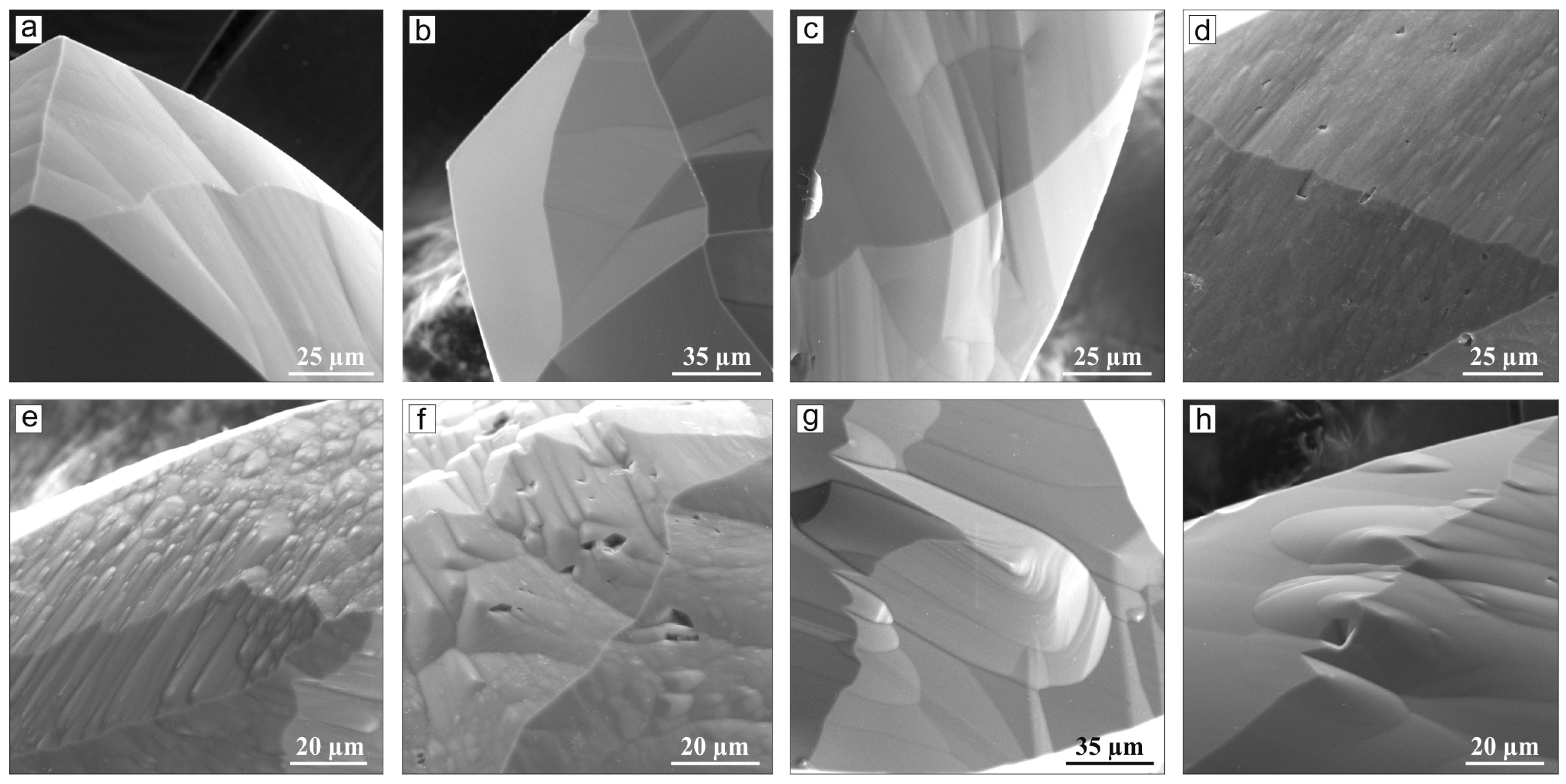
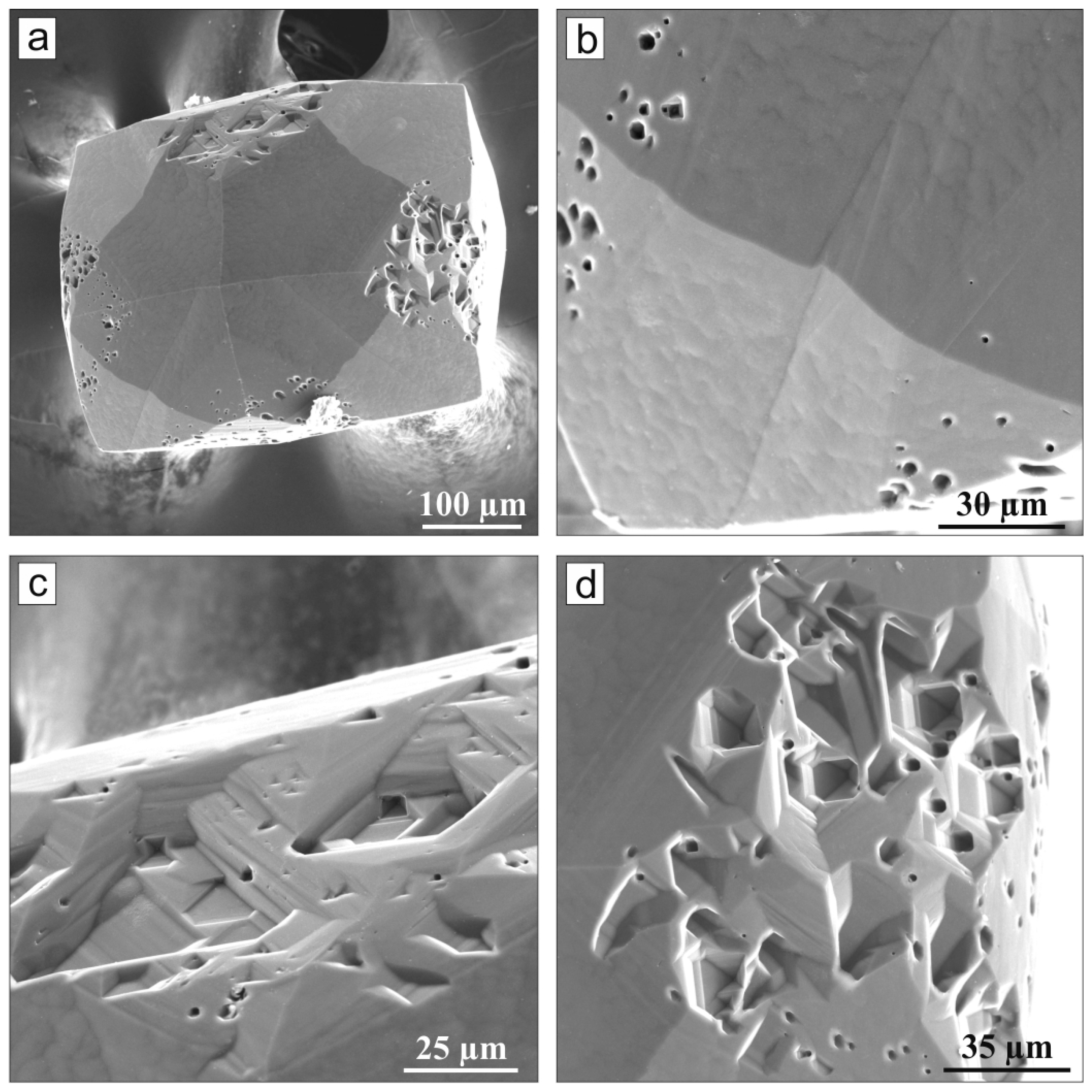
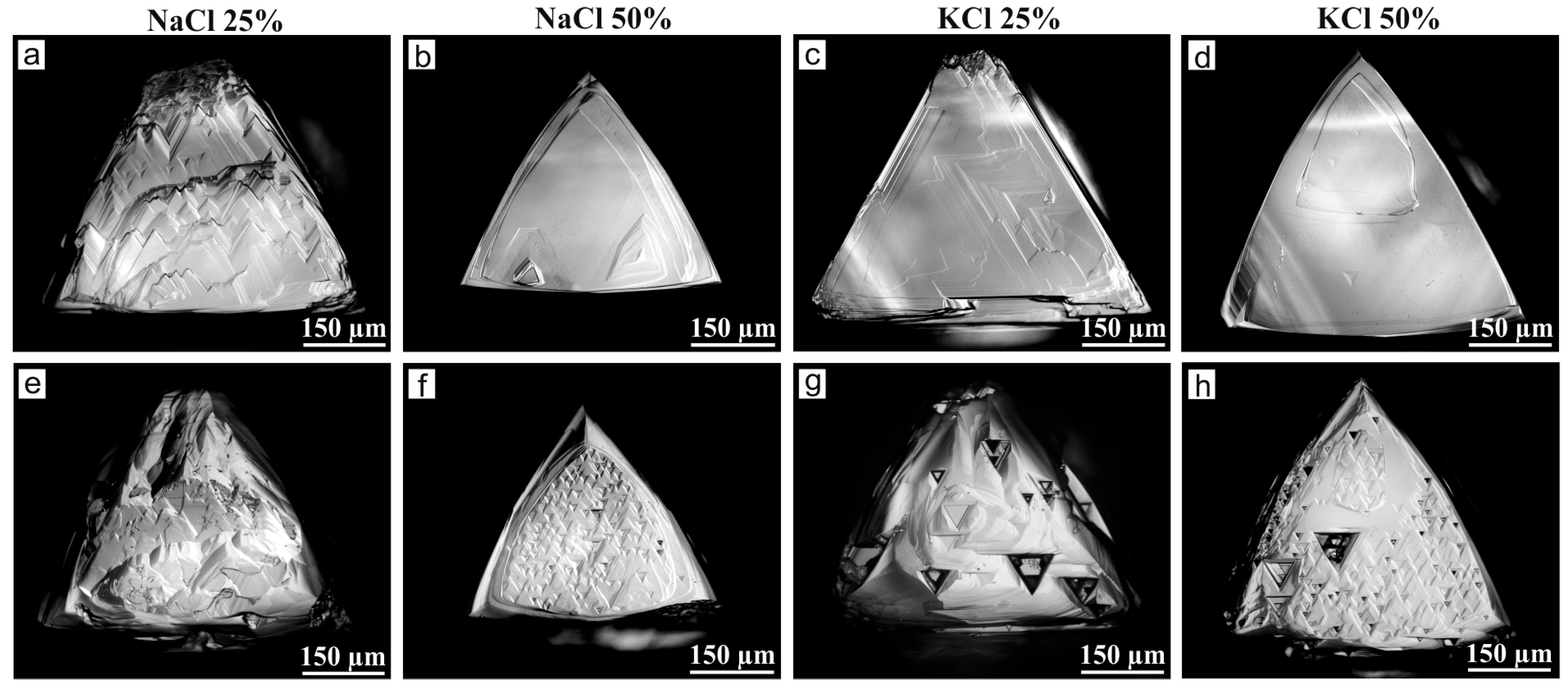
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HDF | High-density fluid |
| DIC | Differential-interference contrast |
| SEM | Scanning electron microscope |
References
- Jablon, B.M.; Navon, O. Most diamonds were created equal. Earth Planet. Sci. Let. 2016, 443, 41–47. [Google Scholar] [CrossRef]
- Navon, O. Diamond formation in the Earth’s mantle. Int. Kimberl. Conf. Ext. Abstr. 1998, 7, 618–621. [Google Scholar]
- Schrauder, M.; Navon, O.; Szafranek, D.; Kaminsky, F.; Galimov, E. Fluids in Yakutian and Indian diamonds. Mineral. Mag. 1994, 58, 813–814. [Google Scholar] [CrossRef]
- Izraeli, E.S.; Harris, J.W.; Navon, O. Brine inclusions in diamonds: A new upper mantle fluid. Earth Planet. Sci. Lett. 2001, 187, 323–332. [Google Scholar] [CrossRef]
- Izraeli, E.S.; Harris, J.W.; Navon, O. Fluid and mineral inclusions in cloudy diamonds from Koffiefontein, South Africa. Geochim. Cosmochim. Acta 2004, 68, 2561–2575. [Google Scholar] [CrossRef]
- Tomlinson, E.L.; Jones, A.P.; Harris, J.W. Co-existing fluid and silicate inclusions in mantle diamond. Earth Plan. Sci. Lett. 2006, 250, 581–595. [Google Scholar] [CrossRef]
- Weiss, Y.; Navon, O.; Goldstein, S.L.; Harris, J.W. Inclusions in diamonds constrain thermo-chemical conditions during Mesozoic metasomatism of the Kaapvaal cratonic mantle. Earth Planet. Sci. Let. 2018, 491, 134–147. [Google Scholar] [CrossRef]
- Klein-BenDavid, O.; Izraeli, E.S.; Hauri, E.; Navon, O. Fluid inclusions in diamonds from the Diavik mine, Canada and the evolution of diamond-forming fluids. Geochim. Cosmochim. Acta 2007, 71, 723–744. [Google Scholar] [CrossRef]
- Weiss, Y.; McNeill, J.; Pearson, D.G.; Nowell, G.M.; Ottley, C.J. Highly saline fluids from a subducting slab as the source for fluid-rich diamonds. Nature 2015, 524, 339–342. [Google Scholar] [CrossRef]
- Wang, Y.; Kanda, H. Growth of HPHT diamonds in alkali halides: Possible effects of oxygen contamination. Diam. Relat. Mater. 1998, 7, 57–63. [Google Scholar] [CrossRef]
- Litvin, Y.A. Alkaline-chloride components in processes of diamond growth in the mantle and high-pressure experimental conditions. Dokl. Earth Sci. 2003, 389, 388–391. [Google Scholar]
- Tomlinson, E.; Jones, A.; Milledge, J. High-pressure experimental growth of diamond using C–K2CO3–KCl as an analogue for Cl-bearing carbonate fluid. Lithos 2004, 77, 287–294. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Shatsky, V.S.; Sobolev, N.V.; Sokol, A.G. The role of mantle ultrapotassic fluids in diamond formation. Proc. Natl. Acad. Sci. USA 2007, 104, 9122–9127. [Google Scholar] [CrossRef]
- Luth, R.W. Natural versus experimental control of oxidation state; effects on the composition and speciation of COH fluids. Am. Min. 1989, 74, 50–57. [Google Scholar]
- Boettcher, A.L.; Mysen, B.O.; Allen, J.C. Techniques for the control of water fugacity and oxygen fugacity for experimentation in solid-media high-pressure apparatus. J. Geophys. Res. 1973, 78, 5898–5901. [Google Scholar] [CrossRef]
- Khokhryakov, A.F.; Palyanov, Y.N. Influence of the fluid composition on diamond dissolution forms in carbonate melts. Am. Mineral. 2010, 95, 1508–1514. [Google Scholar] [CrossRef]
- Khokhryakov, A.F.; Kruk, A.N.; Sokol, A.G. The effect of oxygen fugacity on diamond resorption in ascending kimberlite melt. Lithos 2021, 394–395, 106166. [Google Scholar] [CrossRef]
- Khokhryakov, A.F.; Kruk, A.N.; Sokol, A.G.; Nechaev, D.V. Experimental modeling of diamond resorption during mantle metasomatism. Minerals 2022, 12, 414. [Google Scholar] [CrossRef]
- Kanda, H.; Yamaoka, S.; Setaka, N.; Komatsu, H. Etching of diamond octahedrons by pressure water. J. Cryst. Growth 1977, 38, 1–7. [Google Scholar] [CrossRef]
- Stachel, N.; Luth, R.W. Diamond—Where, when and how? Lithos 2015, 200–223, 200–220. [Google Scholar] [CrossRef]
- Khokhryakov, A.F.; Pal’yanov, Y.N.; Sobolev, N.V. Crystal morphology as an indicator of redox conditions of natural diamond dissolution at the mantle PT parameters. In Doklady Earth Sciences; Pleiades Publishing, Ltd.: Moscow, Russia, 2002; Volume 385, pp. 534–537, (Translated from Doklady Akademii Nauk 2002, 384, 670–673). [Google Scholar]
- Kozai, Y.; Arima, M. Experimental study on diamond dissolution in kimberlitic and lamproitic melts at 1300–1420 °C and 1 GPa with controlled oxygen partial pressure. Am. Mineral. 2005, 90, 1759–1766. [Google Scholar] [CrossRef]
- Ballhaus, C.; Berry, R.F.; Green, D.H. High pressure experimental calibration of the olivine-orthopyroxene-spinel oxygen geobarometer: Implications for the oxidation state of the upper mantle. Contrib. Mineral. Petrol. 1991, 107, 27–40. [Google Scholar] [CrossRef]
- Kadik, A.A.; Lukanin, O.A. Degassing of the Upper Mantle During Melting; Nauka: Moscow, Russia, 1986; 120p. (In Russian) [Google Scholar]
- Frost, D.J.; Wood, B.J. Experimental measurements of the graphite C−O equilibrium and CO2 fugacities at high temperature and pressure. Contrib. Mineral. Petrol. 1995, 121, 303–308. [Google Scholar] [CrossRef]
- Rudenko, A.P.; Kulakova, I.I.; Shturman, V.L. Oxidation of natural diamond. In Novye Dannye o Mineralogii SSSR; Nauka: Moscow, Russia, 1979; Volume 28, pp. 105–125. (In Russian) [Google Scholar]
- Skvortsova, V.L.; Shiryaev, A.A.; Fedortchouk, Y. Influence of ions on diamond resorption. Diam. Relat. Mater. 2020, 104, 107764. [Google Scholar] [CrossRef]
- Khokhryakov, A.F.; Palyanov, Y.N. The evolution of diamond morphology in the process of dissolution: Experimental data. Am. Mineral. 2007, 92, 909–917. [Google Scholar] [CrossRef]
- Fedortchouk, Y.; Canil, D.; Semenets, E. Mechanisms of diamond oxidation and their bearing on the fluid composition in kimberlite magmas. Am. Mineral. 2007, 92, 1200–1212. [Google Scholar] [CrossRef]
- Fedortchouk, Y. A new approach to understanding diamond surface features based on a review of experimental and natural diamond studies. Earth-Sci. Rev. 2019, 193, 45–65. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Khokhryakov, A.F.; Kupriyanov, I.N. Crystallomorphological and crystallochemical indicators of diamond formation conditions. Crystallogr. Rep. 2021, 66, 142–155. [Google Scholar] [CrossRef]
- Luth, R.W.; Palyanov, Y.N.; Bureau, H. Experimental petrology applied to natural diamond growth. Rev. Mineral. Geochem. 2021, 88, 755–808. [Google Scholar] [CrossRef]
- Khokhryakov, A.F.; Palyanov, Y.N. Effect of crystal defects on diamond morphology during dissolution in the mantle. Am. Mineral. 2015, 100, 1528–1532. [Google Scholar] [CrossRef]
- Golovin, A.V.; Kamenetsky, V.S. Compositions of kimberlite melts: A review of melt inclusions in kimberlite minerals. Petrology 2023, 31, 143–178. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Kamenetsky, M.B.; Golovin, A.V.; Sharygin, V.V.; Maas, R. Ultrafresh salty kimberlite of the Udachnaya–East pipe (Yakutia, Russia): A petrological oddity or fortuitous discovery? Lithos 2012, 152, 173–186. [Google Scholar] [CrossRef]



| Run No. | Buffer | T (°C) | Time (h) | Composition (mg) | Diamond | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H2O | KCl | NaCl | Initial Weight (mg) | Final Weight mg) | Weight Loss (mg) | Weight Loss (%) | V 1 | ||||
| 95/4 | MH | 1200 | 1 | 5.0 | - | - | 0.34 | 0.13 | 0.21 | 62 | 2.9 |
| 3.7 | - | 1.3 | 0.32 | 0.05 | 0.27 | 84 | 3.9 | ||||
| 2.5 | - | 2.5 | 0.41 | 0.02 | 0.39 | 95 | 4.7 | ||||
| 3.7 | 1.3 | - | 0.38 | 0.11 | 0.27 | 71 | 3.5 | ||||
| 2.5 | 2.5 | - | 0.35 | 0 | 0.35 | 100 | ≥5.0 | ||||
| 37/8 | MH | 1400 | 0.17 | 5.0 | - | - | 0.40 | 0.22 | 0.18 | 45 | 12.8 |
| 3.7 | - | 1.3 | 0.43 | 0.15 | 0.28 | 65 | 19.7 | ||||
| 2.5 | - | 2.5 | 0.38 | 0.08 | 0.30 | 78 | 23.4 | ||||
| 3.7 | 1.3 | - | 0.45 | 0.24 | 0.21 | 53 | 14.4 | ||||
| 2.5 | 2.5 | - | 0.36 | 0.04 | 0.32 | 89 | 25.7 | ||||
| 39/8 | IW | 1200 | 10 | 5.0 | - | - | 0.40 | 0.40 | ≤0.40 | ≥0 | ≥0 |
| 2.5 | - | 2.5 | 0.38 | 0.38 | 0 | 0 | 0 | ||||
| 3.7 | - | 1.3 | 0.26 | 0.26 | 0 | 0 | 0 | ||||
| 3.7 | 1.3 | - | 0.32 | 0.32 | 0 | 0 | 0 | ||||
| 2.5 | 2.5 | - | 0.35 | 0.35 | 0 | 0 | 0 | ||||
| 67/3 | IW | 1200 | 40 | 5.0 | - | - | 0.35 | 0.32 | 0.03 | 9 | 0.01 |
| 3.7 | - | 1.3 | 0.32 | 0.31 | 0.01 | 3 | ≤0.01 | ||||
| 3.7 | - | 2.5 | 0.26 | ≤0.26 | ≥0 | ≥0 | ≤0.01 | ||||
| 2.5 | 1.3 | - | 0.35 | ≤0.35 | ≥0 | ≥0 | ≤0.01 | ||||
| 2.5 | 2.5 | - | 0.30 | ≤0.30 | ≥0 | ≥0 | ≤0.01 | ||||
| 40/8 | IW | 1400 | 10 | 5.0 | - | - | 0.29 | 0.21 | 0.08 | 28 | 0.12 |
| 3.7 | - | 1.3 | 0.35 | 0.27 | 0.08 | 23 | 0.11 | ||||
| 2.5 | - | 2.5 | 0.30 | 0.25 | 0.05 | 17 | 0.07 | ||||
| 3.7 | 1.3 | - | 0.29 | 0.22 | 0.07 | 24 | 0.11 | ||||
| 2.5 | 2.5 | - | 0.32 | 0.27 | 0.05 | 16 | 0.07 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khokhryakov, A.; Kruk, A.; Sokol, A.; Nechaev, D. Dissolution of Diamond in Water–Chloride Fluids at Mantle P-T Conditions. Minerals 2025, 15, 897. https://doi.org/10.3390/min15090897
Khokhryakov A, Kruk A, Sokol A, Nechaev D. Dissolution of Diamond in Water–Chloride Fluids at Mantle P-T Conditions. Minerals. 2025; 15(9):897. https://doi.org/10.3390/min15090897
Chicago/Turabian StyleKhokhryakov, Alexander, Alexey Kruk, Alexander Sokol, and Denis Nechaev. 2025. "Dissolution of Diamond in Water–Chloride Fluids at Mantle P-T Conditions" Minerals 15, no. 9: 897. https://doi.org/10.3390/min15090897
APA StyleKhokhryakov, A., Kruk, A., Sokol, A., & Nechaev, D. (2025). Dissolution of Diamond in Water–Chloride Fluids at Mantle P-T Conditions. Minerals, 15(9), 897. https://doi.org/10.3390/min15090897







