1. Introduction
Currently, due to the global shortage of high-grade ores, the demand to process refractory, low-grade raw materials has become increasingly urgent. To improve the recovery of valuable components from such ores, various activation methods are employed. These include mechanical, thermal, biological, ultrasonic, and chemical treatments [
1,
2,
3].
Mechanical activation involves grinding solid material into fine powder. The deformation and partial destruction of the crystal structure reduce the material’s strength, accelerating its interaction with the leaching agent [
4,
5]. There are two approaches: preliminary grinding followed by leaching, and a combined approach—simultaneous grinding and chemical reaction with the leaching solution (mechanochemical process). Mechanochemical leaching enables reactions that are thermodynamically unlikely under standard conditions [
6].
Thermal activation enhances the solubility of solids by exposing them to high temperatures. The process is based on the weakening of chemical bonds, disruption of the crystal lattice, development of thermal stresses, and formation of simpler, more soluble compounds. These changes destabilize the structure and accelerate dissolution [
7]. An example is the thermal decomposition of sulfides in metallurgy. Metal sulfides such as pyrite, arsenopyrite, and chalcopyrite are poorly soluble in non-oxidizing acids, but after thermal decomposition, they convert into simpler sulfide forms that are easily leached in acids [
8].
Biological activation (bacterial leaching) utilizes microorganisms to extract metals from ores. They act on sulfide minerals or accelerate the oxidation of ferrous iron (Fe
2+) to ferric iron (Fe
3+). Thiobacillus ferrooxidans is a microorganism widely used in hydrometallurgy, operating in acidic media (optimal pH 1.7–2.5) and losing activity above pH 6. Th. ferrooxidans secretes enzymes that catalyze chemical reactions by binding to substrates and forming intermediate complexes, which decompose into final products. The formation of such complexes lowers the activation energy, enhancing leaching rates. Th. ferrooxidans catalyzes the oxidation of Fe
2+ to Fe
3+, which then facilitates the leaching of non-ferrous metal sulfides, such as copper. The bacteria re-oxidizes the resulting FeSO
4 to Fe
2(SO
4)
3, creating a closed cycle [
9].
Ultrasonic treatment (frequency above 20 kHz) accelerates leaching by generating alternating pressure zones in liquid media. Inhomogeneities, such as on solid particle surfaces, cause the liquid to break into cavitation bubbles. When these bubbles collapse, shock waves are generated. The high pressure inside cavitation bubbles (up to several thousand atmospheres) and its rapid release damage the solid phase by crushing particles, breaking crystal structures, and removing surface films. Additionally, ultrasound generates vortex flows that reduce diffusion resistance at phase boundaries, further enhancing leaching rates [
10].
The present study tested the application of thermochemical activation using a NaHCO3 solution for various types of mineral raw materials, including slag from the reductive smelting of red mud prior to acid leaching, ash before chemical beneficiation, gibbsite–kaolinite bauxite prior to gravity separation, and nephelines, for which the traditional sintering process was replaced with chemical beneficiation.
The technical outcome of the thermochemical activation technology is the transformation of the mineral composition of raw materials. This is achieved by treating the raw material with a solution containing 120–150 g/dm3 sodium bicarbonate at a temperature of 120–160 °C. Under these conditions, transformations occur in the mineral structure. The required sodium bicarbonate concentration of 120–150 g/dm3 is chosen based on its solubility limit. The necessary treatment temperature of 120–200 °C was determined experimentally and requires the use of an autoclave. At temperatures below 120 °C, the rate of mineral structure transformation is too low.
At temperatures of 60–200 °C, sodium bicarbonate decomposes according to the following reaction [
11]:
This reaction is reversible. When carried out in an autoclave, all components of sodium bicarbonate decomposition and formation are present in the solution in an active, freshly formed state. The components of the mineral raw material react with the solution to form water-soluble bicarbonate salts and are removed from the mineral structure. Subsequently, due to the elevated temperature, these salts decompose to form less soluble carbonates, which serve as the basis for the formation of a new mineral structure.
Slag from the reductive smelting of red mud can be utilized for the extraction of rare earth elements (REEs) and other valuable components. The primary mineral phase of this slag consists of acid-resistant calcium silicate minerals, which is why it is currently not employed for REE or other element recovery. The proposed technology for the preliminary transformation of the slag’s phase composition enables its effective processing via acid leaching.
Thermochemical activation of the slag leads to significant changes in its phase structure. After activation, leaching in nitric acid solution yielded the following extraction rates: 96% REEs, 92% TiO2, 98% CaO, 98% Al2O3, 50% Fe2O3. Without activation, the extraction rates were significantly lower: 20% REEs, 40% CaO, 40% Al2O3, 35% Fe2O3.
Fly ash and slags, rich in silica and aluminosilicate phases, represent another category of high-tonnage, high-silica mineral raw materials.
To process low-grade, high-silica aluminosilicate raw materials, preliminary beneficiation with silica extraction into solution is required. The efficiency of this process depends on the potential transformation of the refractory initial mineral composition. Major sources of high-silica aluminosilicate material are ash and slag., A known method for their processing involves preliminary sintering with limestone and soda at 1150–1300 °C before leaching [
11]. With this method, limestone is added to bind silica, while soda converts alumina into sodium aluminate. However, this method does not enable any prior transformation of the initial phase composition, which results in high energy consumption and significant limestone consumption during sintering.
In recent decades, the global accumulation of ash-related waste has led to increased demand for efficient processing technologies. For fly ash beneficiation, methods such as magnetic separation and alkaline treatment are commonly used. For REE extraction from ash, acid leaching is applied [
12]. A promising option for ash processing is magnetic separation, which enables partial iron removal [
13]. However, iron is inert during alumina extraction from alkaline solutions.
The most problematic impurity in ash is silica, and without preliminary removal of the maximum possible amount of silica, alumina extraction becomes difficult.
Globally, alumina production is primarily based on high-grade bauxite processed using the Bayer process [
14,
15,
16,
17]. This method is applicable only to bauxites with a silica modulus (μSi) greater than 7, where
The classic Bayer process is only suitable for high-grade bauxites. Bauxites with μSi > 7 are more appropriately processed via sintering or using combined methods. Kazakh low-grade, high-silica gibbsite–kaolinite bauxites, with silica modulus values as low as 4, cannot be directly processed using the Bayer method. However, preliminary beneficiation to remove the high-silica kaolinite fraction can increase the silica modulus.
Thermochemical pre-activation of bauxite has proven effective in enhancing gravity separation performance, raising the silica modulus to the required value of μSi > 7 [
18,
19,
20].
For processing high-silica nepheline syenites, the raw material is pretreated via chemical beneficiation prior to alumina extraction [
11]. According to this method, nepheline rock is treated with an alkaline solution containing 200 g/dm
3 Na
2O at 200 °C. As a result, up to 45% of the silica can be extracted into solution. The disadvantage of this method is the low degree of silica extraction, which is determined by the refractory initial mineral composition of the syenites. Without transforming this composition, it is not possible to increase the recovery rate.
This study aims to investigate the impact of preliminary thermochemical activation involving the mineral phase transformation of various types of raw materials on the outcomes of subsequent processing.
2. Materials and Methods
To investigate the feasibility of preliminary thermochemical activation of raw materials, various ores and materials from the Republic of Kazakhstan were studied:
- -
Slag from the reductive smelting of red mud derived by processing high-iron bauxites of the Koktal deposit;
- -
Ash from the combustion of coals of the Ekibastuz deposit;
- -
Kaolinite–gibbsite bauxites of the Krasnogorsk deposit;
- -
Nepheline syenites of the Kubasadyr deposit.
For the thermochemical activation, the raw materials were ground to a particle size of −0.1 mm. The thermochemical activation was carried out in a sodium bicarbonate solution with a concentration of 120–150 g/dm3 at a temperature of 120–160 °C in an autoclave.
The charge for the reduction smelting process was prepared from red mud, calcium oxide, and a reducing agent, the amounts of which were calculated to obtain a hydraulic modulus in the range of 0.55–0.88, defined as the ratio CaO/(Al2O3 + SiO2). Maintaining the specified hydraulic modulus is necessary to obtain a fluid slag. After briquetting, the charge was subjected to reduction smelting in a graphite crucible in a muffle furnace at 1300 °C for 2 h.
After reductive smelting and magnetic separation, the pig iron slag was leached in a solution of 8 M HNO3 at 80 °C for 2 h.
After thermochemical activation, the ash was enriched via leaching in an alkaline solution containing 100 g/dm3 Na2O at a temperature of 100 °C for 60 min in a reactor.
The low-grade gibbsite–kaolinite bauxite of the Krasnogorsk deposit was enriched using gravity concentration by separating the light-clay fraction from the heavy-sand fraction.
The nepheline was enriched through treatment in an alkaline solution containing 240 g/dm
3 Na
2O at a temperature of 280 °C for 40 min. At 150–280 °C, the saturated vapor pressure of water is approximately 2.0–50.0 bar [
21].
To investigate the physicochemical composition of the raw materials and processed products, a range of analytical techniques were employed. X-ray fluorescence (XRF) analysis was conducted using a Venus 200 spectrometer with wavelength-dispersive capabilities (Malvern PANalytical B.V., Almelo, The Netherlands). Chemical composition was determined using an inductively coupled plasma optical emission spectrometer (ICP-OES), Optima 2000 DV (PerkinElmer, Waltham, MA, USA). X-ray diffraction (XRD) data were collected on a Bruker D8 ADVANCE diffractometer (Bruker Corporation, Billerica, MA, USA) using copper Kα radiation at an accelerating voltage of 36 kV and a current of 25 mA. The sample, which was ground to a particle size of 0.056 mm, was placed in a cuvette pretreated with alcohol. The sample was evenly distributed throughout the cuvette, compressed, and placed in the apparatus. The International Centre for Diffraction Data ICDD PDF-2 2023 database was used to confirm the accuracy of phase identification.
3. Results and Discussion
3.1. Thermochemical Activation of Slag from Reductive Smelting of Red Mud from the Processing of High-Iron Bauxites of the Koktal Deposit
During the processing of low-grade, high-silica bauxites, the technologies employed involved the additional utilization of red mud—residues from the Bayer leaching of bauxites—in order to recover additional alumina. Red mud represents a large-scale anthropogenic waste stream; however, its comprehensive processing enables the production of valuable commercial products such as aluminum, titanium, and rare earth elements (REEs).
Processing red mud via reductive smelting results in the separation of iron as pig iron and the production of slag containing titanium and rare earth elements (REEs) [
22,
23,
24,
25,
26,
27]. The main challenge in the hydrometallurgical treatment of pig iron slag for REE and titanium recovery lies in its composition, which includes chemically stable and refractory silicate minerals. For this reason, slag is generally not considered a suitable raw material for comprehensive processing. To enable effective recovery of valuable components, it is necessary to activate the slag through the preliminary transformation of its mineral composition.
The slag used in this study was obtained through the reductive smelting of red mud, which is itself a by-product of bauxite processing through the Bayer process. The charge mixture for reductive smelting consisted of red mud, calcium oxide, and coke. Calcium oxide was added to achieve a free-flowing slag with a hydraulic modulus in the range of 0.55–0.80 [
28].
The chemical composition of the slag (wt%) was as follows: 7.6% Al2O3, 23.0% SiO2, 20.9% CaO, 5.3% Fe, 10.0% TiO2, and 0.32% Na2O.
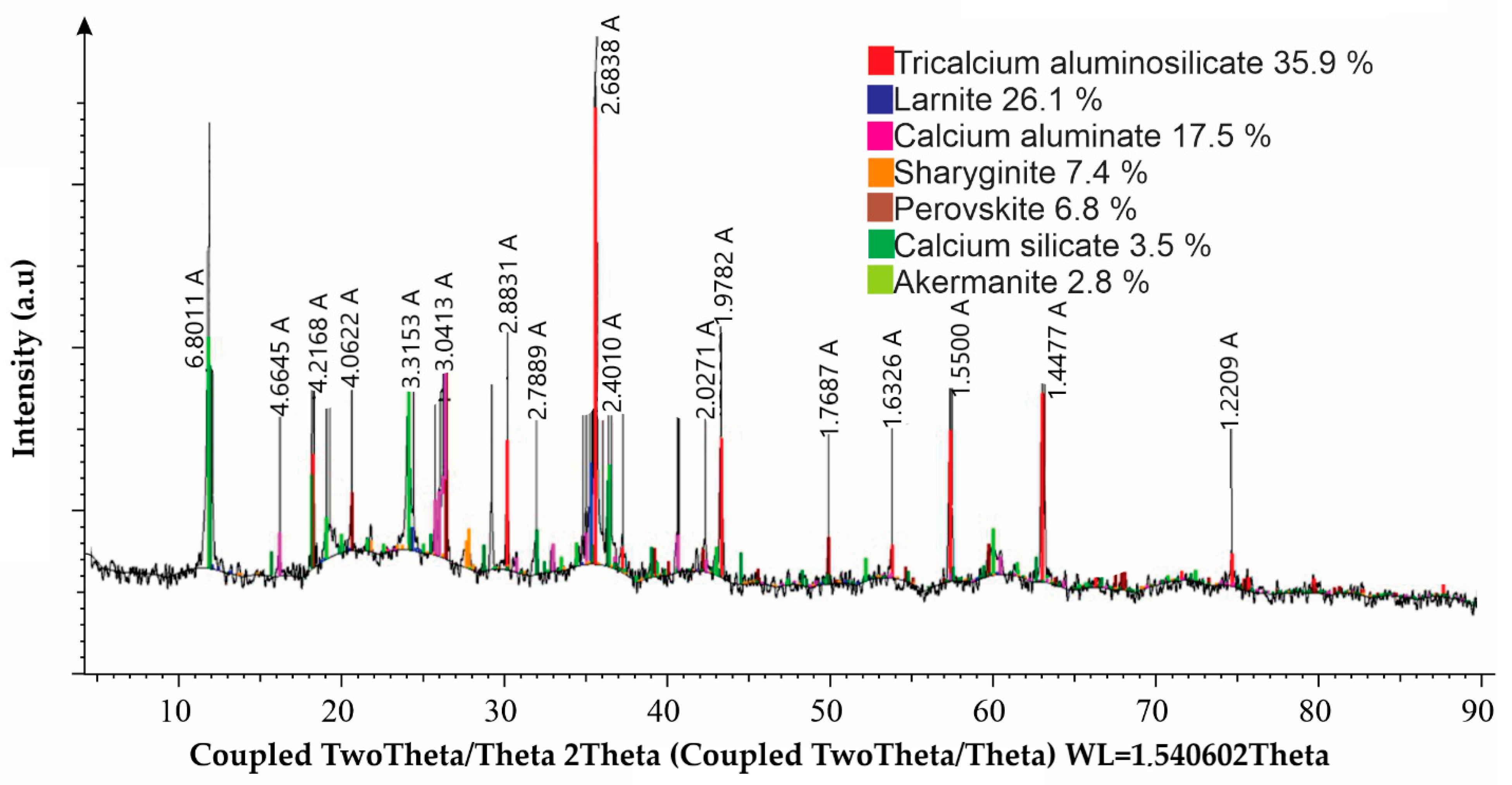
The phase composition of the slag from the reductive smelting of red mud is shown in
Figure 1.
The most intense peaks are attributed to tricalcium aluminosilicate, suggesting it is the dominant crystalline phase. The presence of perovskite confirms that titanium is incorporated into the slag in a crystalline form. Peaks corresponding to larnite and calcium aluminate are also significant, indicating a high content of calcium-bearing silicates and aluminates.
The results of this study show that the acid treatment of the slag without prior activation, using 8N HNO3 at 80 °C, resulted in a recovery rate of only 32.0% for REEs and titanium. Further research was conducted to evaluate the effect of preliminary thermochemical activation of slag in a NaHCO3 solution on the efficiency of valuable component extraction during subsequent processing.
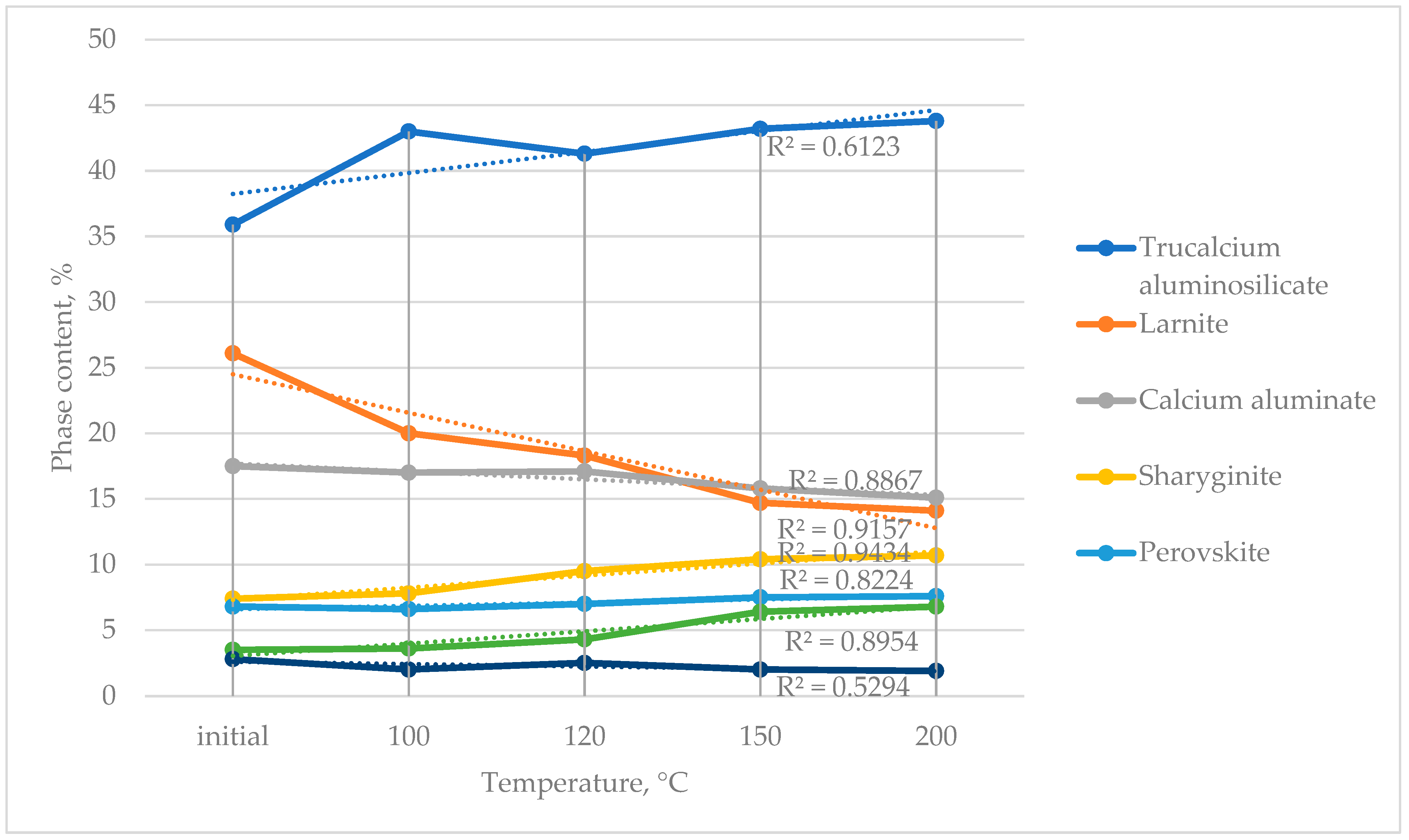
Studies were carried out to determine the optimal temperature of thermochemical activation that ensures the maximum alteration in the phase composition of the slag (
Figure 2).
As a result of the activation, the content of the dicalcium silicate (larnite) phase in the slag decreased, while the proportion of other phases increased correspondingly. XRD analysis revealed that calcium oxide, released from the decomposition of dicalcium silicate, transformed into other calcium-containing phases. The silica liberated from the dicalcium silicate contributed to the formation of an amorphous component in the slag.
According to the obtained results (
Figure 2), the optimal activation temperature is 150 °C, at which the maximum transformation of the slag’s phase composition is achieved.
Increasing the activation temperature beyond 150 °C is not justified, as it does not yield further structural changes and may lead to additional energy consumption.
As a result of activation, a transformation of calcium-containing phases occurs within the slag: the content of dicalcium silicate (larnite) decreases, while the amounts of tricalcium hydroaluminate, calcium silicate, and sharyginite increase proportionally.
The following phase transformation reactions are assumed to occur during this process, which are thermodynamically favorable, as confirmed by their negative Gibbs free energy values (ΔG < 0):
According to Reaction (3), larnite decomposes to form calcium silicate and calcium carbonate. Calcium carbonate was not detected via XRD, which may indicate its amorphous nature. During activation, calcium silicate decomposes (Reaction (4)), yielding free silica and calcium carbonate. The absence of silica peaks in the XRD pattern can be explained through its involvement in the formation of tricalcium aluminosilicate via Reaction (5), where larnite also takes part and is consumed.
Calcium-bearing phases represent the dominant fraction of the slag both in terms of quantity and function, particularly in ensuring the fluidity of the slag, which is crucial for successful smelting and iron separation. In addition to these, the slag contains iron- and titanium-bearing phases—notably perovskite and sharyginite—whose effect on slag properties is considered negligible.
Note that the goal of this study was not the synthesis of specific mineral phases, but the overall transformation of the slag to enable the effective hydrometallurgical recovery of valuable components through acid leaching.
The chemical composition of the slag after activation at 150 °C (wt%) was as follows: 21.1% Al2O3, 20.3% SiO2, 45.8% CaO, 0.22% Fe, 9.8% TiO2, 2.3% Na2O, 0.055% ΣREE, and loss on ignition (LOI) 0.425%. The overall chemical composition of the slag remained nearly unchanged.
The activated slag was subsequently leached in a nitric acid solution under specified conditions. As a result, the extraction rates into the solution reached approximately 96% for REEs, 92% for TiO2, ~98% for calcium oxides, ~98% for aluminum oxides, and ~50% for iron.
In contrast, leaching the slag without prior thermochemical activation resulted in markedly lower extraction rates: approximately 20% for REEs, ~40% for calcium and aluminum, and ~35% for iron.
The leach residue was further processed to produce REE and titanium concentrates.
Thus, preliminary thermochemical activation of the slag from reductive smelting proved effective for enhancing the recovery of REEs and TiO2 into solution, thereby achieving the goal of comprehensive red mud utilization.
3.2. Thermochemical Activation of Ash from Combustion of Ekibastuz Coal
The ash consists of 95–98% mineral compounds, including oxides of silicon, aluminum, iron, calcium, magnesium, alkali oxides, sulfates, sulfides, and rare metals. The mineral products of ash can be converted into valuable commodities [
29,
30,
31,
32].
The main challenge in processing ash is its high silicon content and the associated complex structure of the mineral phase, which is mainly composed of aluminosilicates. Traditional methods for processing ash and slag wastes typically employ acid and alkaline treatments, as well as sulfating roasting of ash followed by acid leaching. However, these methods do not sufficiently break down the initial material, and the resulting products require additional complex technological operations.
Ash is a high-silica material, and for its comprehensive hydrometallurgical processing, it is necessary to preliminarily remove excess silica, which preferentially dissolves in the alkaline solution during leaching. As a result of the preliminary alkaline treatment, with the removal of excess silica, the ash becomes enriched in other components. This operation is commonly referred to as ash beneficiation. The chemical beneficiation of ash yields a silicate solution and an ash concentrate.
This study aimed to investigate the effect of the preliminary thermochemical activation of ash on enhancing the degree of excess silica removal during the chemical beneficiation of ash.
The chemical composition of ash (wt%) is as follows: 44.2% SiO2, 20.7% Al2O3, 9.1% Fe2O3, 2.5 CaO, 0.804 MgO, 0.94% Na2O, 0.34% K2O, 0.004% Ga2O3, 0.02% V2O5, 0.98% TiO2, 0.2% MnO2, 0.164% SO3.
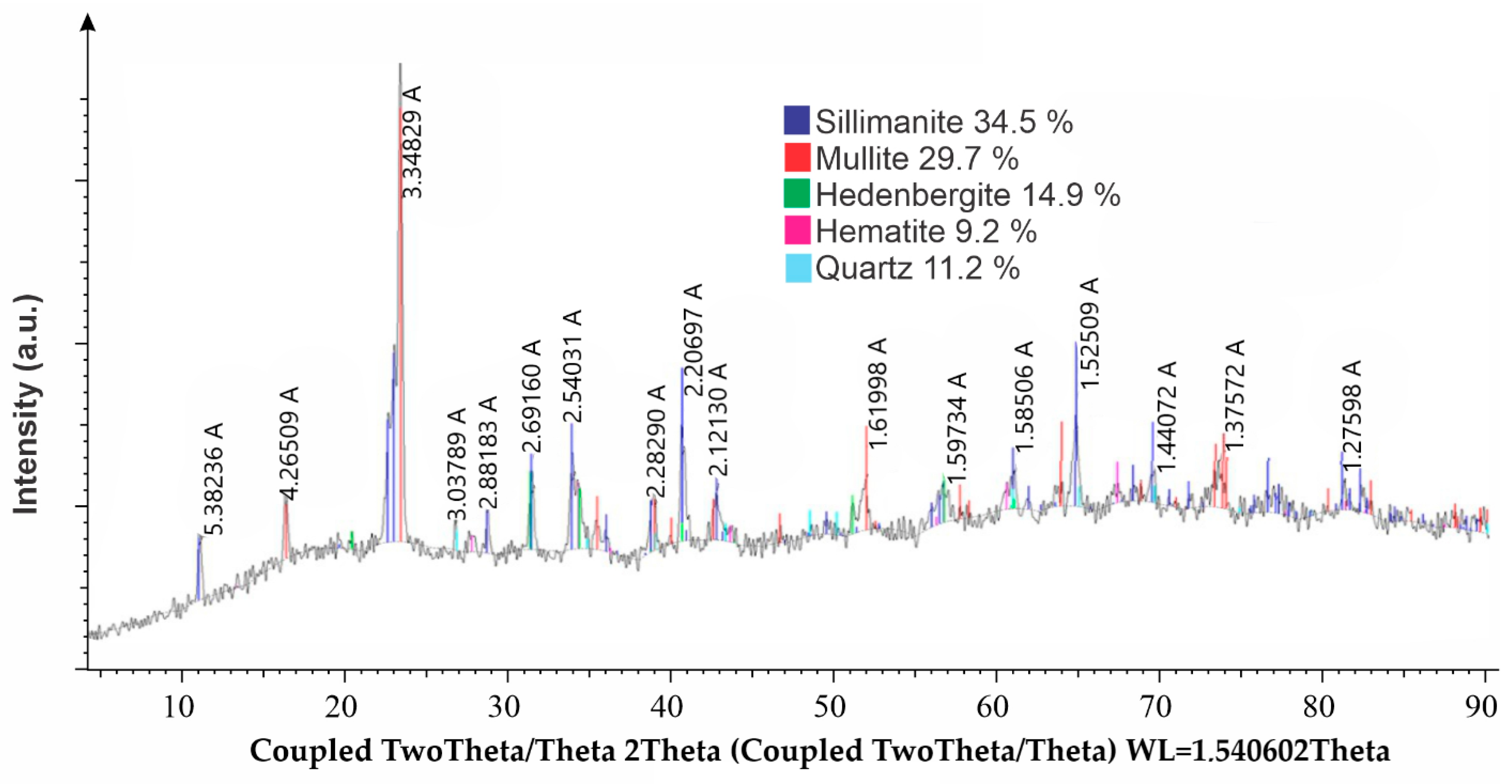
The results of the X-ray phase analysis of the ash sample are shown in
Figure 3.
This XRD pattern (
Figure 3) reveals a multi-phase mineral composition dominated by sillimanite (34.5%) and mullite (29.7%), indicating the presence of high-temperature aluminosilicate phases. Other identified phases include hedenbergite (14.9%), quartz (11.2%), and hematite (9.2%), indicating a complex crystalline structure.
Various phase transformations were observed as a result of the thermochemical activation of ash at temperatures ranging from 120 to 200 °C (
Figure 4).
The following possible reactions can represent changes in the mineral composition of ash during thermochemical activation in a sodium bicarbonate solution at elevated temperature and pressure in an autoclave, with the formation of phases identified via XRD analysis:
Thus, the following changes occur in the mineral structure of the ash as a result of activation:
- -
The phases of aluminum silicate—sillimanite (Al2SiO5) and hedenbergite (CaFe(SiO3)2)—disappear (Reactions (6) and (7));
- -
The content of mullite (2Al2O3·SiO2) increases (Reaction (6));
- -
The amount of free silica (SiO2) increases from 11.2 to 39.0% (Reactions (6) and (7));
- -
The calcite phase (CaCO3) increases.
The overall chemical composition of the ash remains unchanged during activation.
Preliminary chemical activation of ash was found to improve the efficiency of subsequent beneficiation, as reflected by a progressive increase in the extent of silica extraction into solution with rising activation temperatures: 55.0% at 120 °C, 61.2% at 140 °C, and 68.0% at 160 °C.
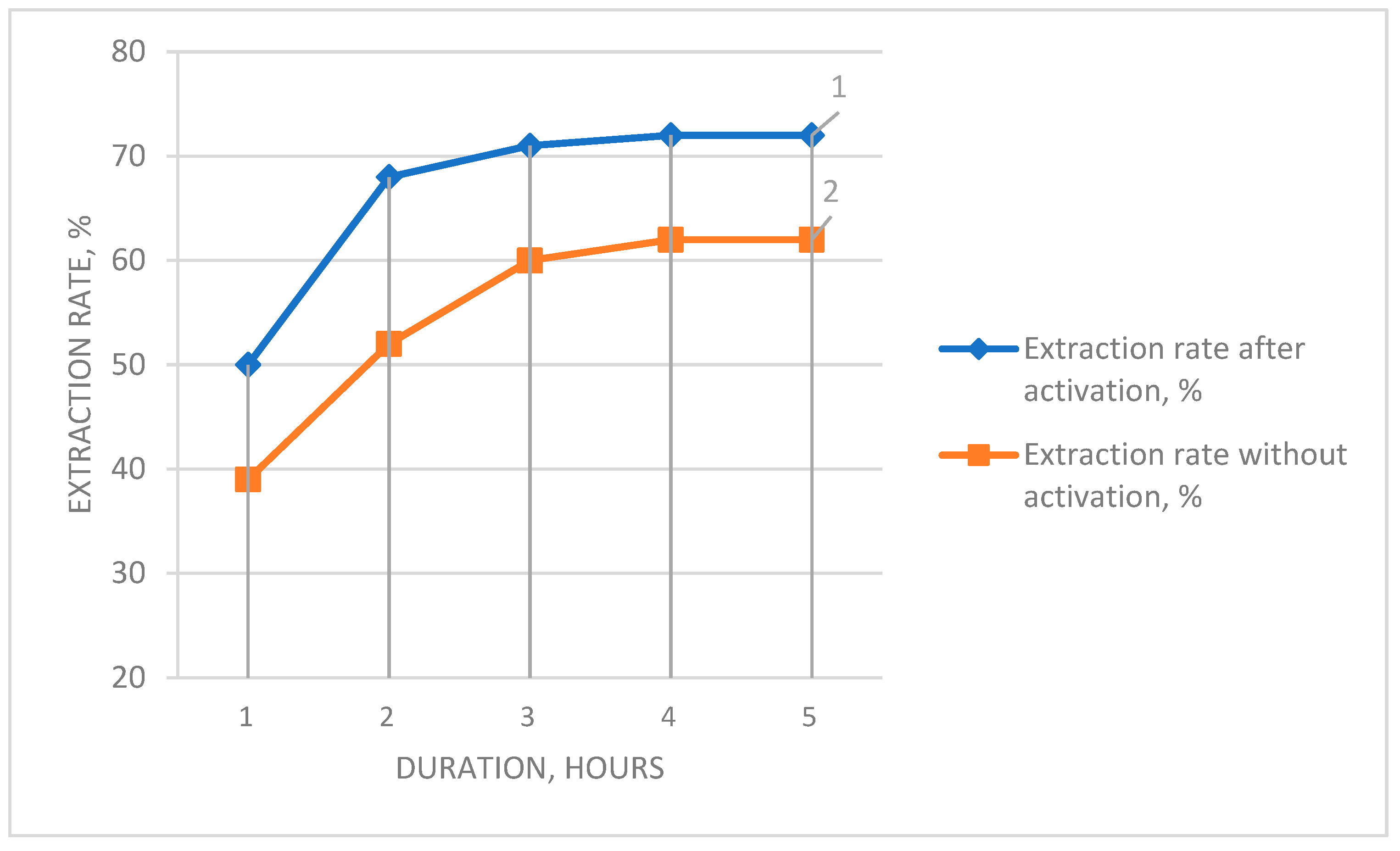
To determine the influence of preliminary activation on the chemical beneficiation process, leaching experiments were conducted on both untreated and pre-activated ash as a function of leaching duration (
Figure 5).
It was found that preliminary activation intensifies the beneficiation process, and after 120 min, the degree of silica extraction into solution reached 68.3%, which is 15% higher compared to the process without activation. This trend is characteristic across the entire duration range. The increase in beneficiation efficiency is attributed to the transformation of the original ash phase composition, leading to the formation of free silica (quartz).
3.3. Thermochemical Activation of Gibbsite–Kaolinite Bauxites from the Krasnogorsk Deposit
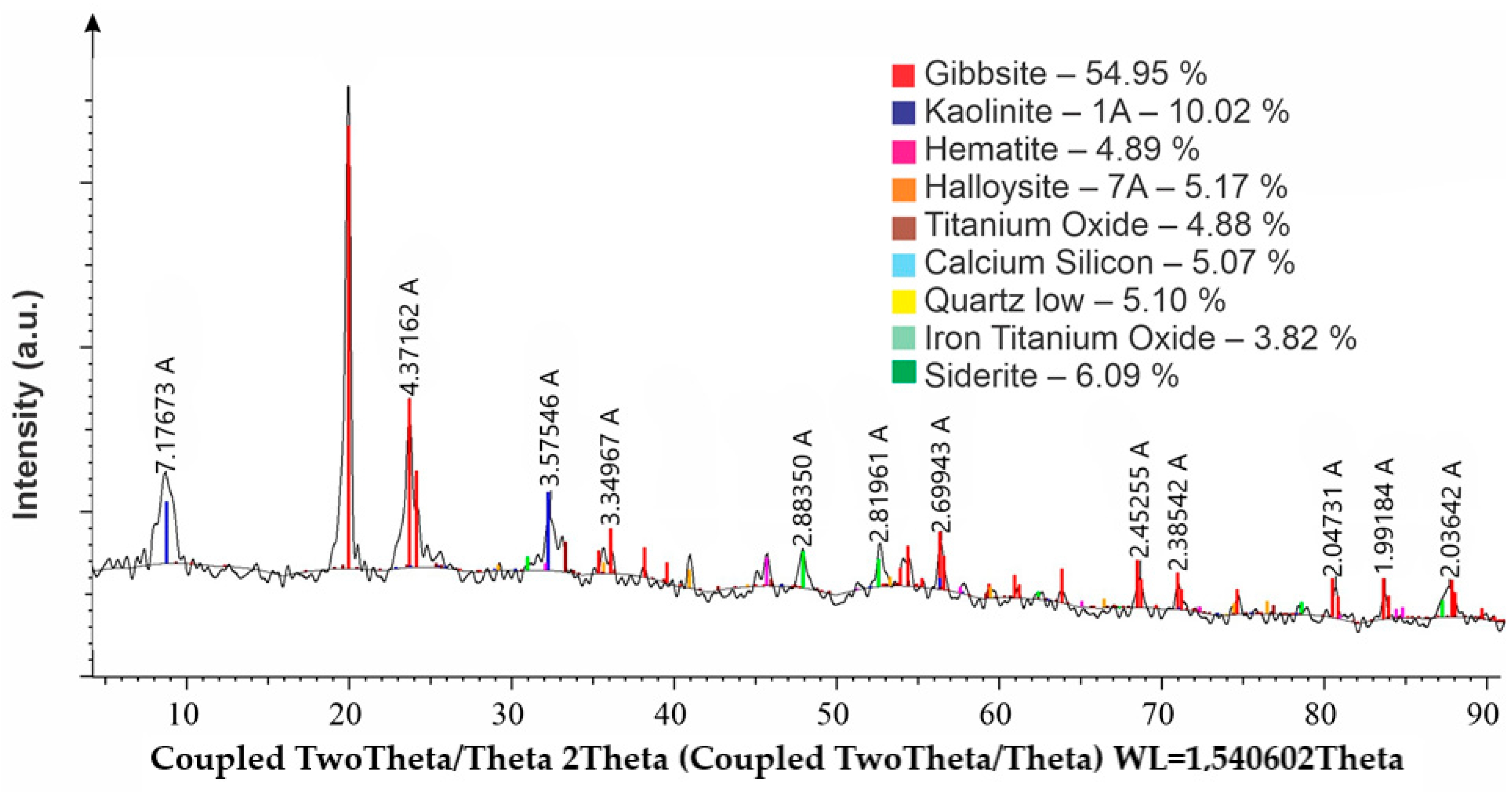
In the experiments, a sample of gibbsite–kaolinite bauxite was used, with the following chemical composition (wt%): 43.1% Al2O3, 19.11% SiO2, 19.8% Fe2O3, 1.06% CaO, 0.23% Na2O, 0.16% MgO, 0.02% K2O, 2.04% TiO2, 0.27% SO3, 0.02% Cl, μSi 3.7.
According to the results of the X-ray phase analysis (
Figure 6), the gibbsite–kaolinite bauxite sample consists predominantly of aluminum hydroxide (gibbsite), accounting for nearly 55%. In addition, clay minerals—kaolinite and halloysite—were identified (approximately 15% combined), along with iron and titanium oxides (hematite, FeTiO
3, TiO
2) amounting to about 13% in total. Minor amounts of iron carbonate (siderite), quartz, and calcium silicate were also detected.
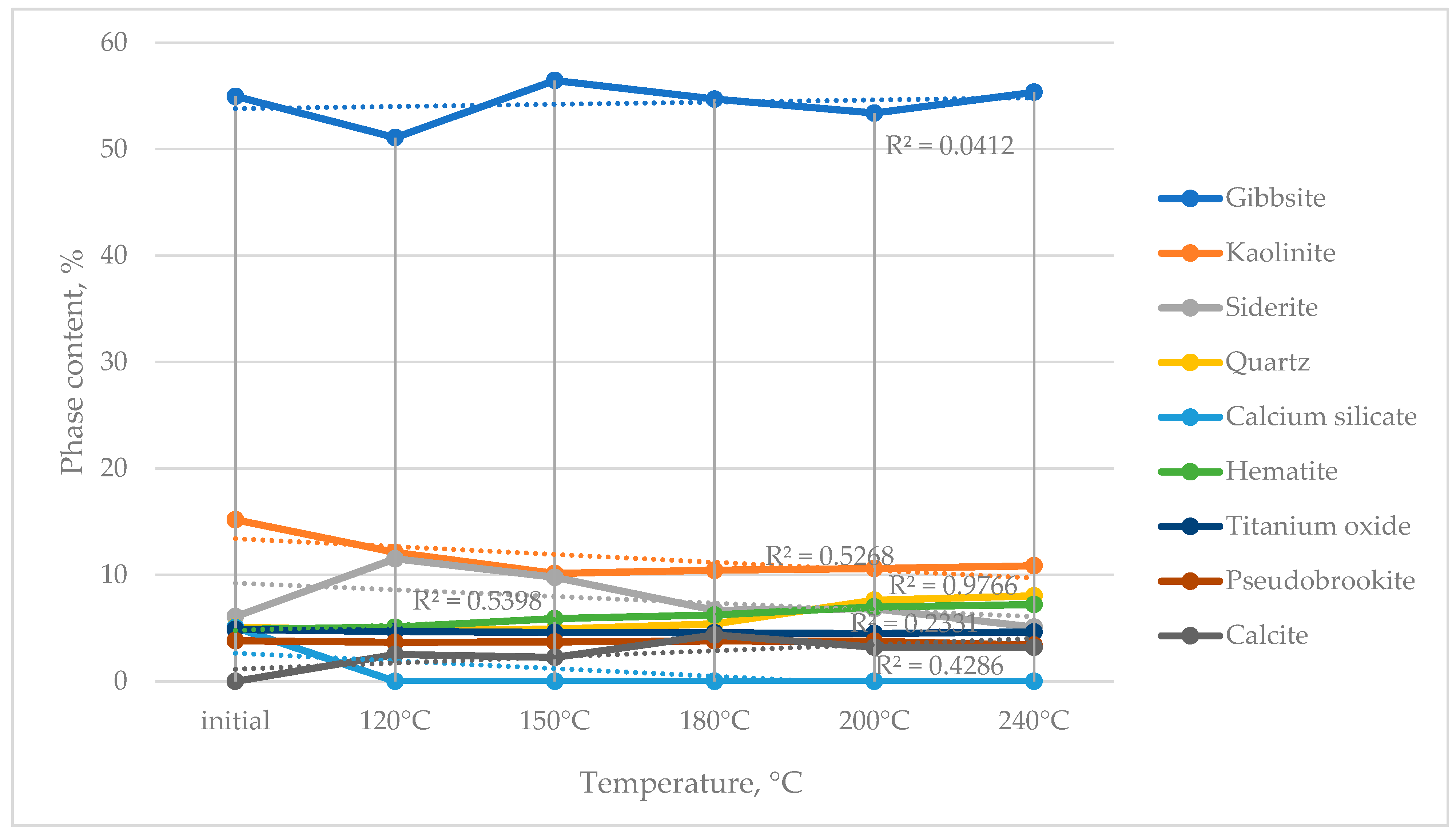
The effect of the thermochemical activation of bauxite in a sodium bicarbonate solution on phase composition was investigated as a function of temperature (120–240 °C) and duration (20–240 min) (
Figure 7,
Figure 8 and
Figure 9).
X-ray phase analysis of the samples after activation revealed notable changes in the bauxite phase composition:
- -
At 90 °C, the calcium silicate phase disappeared, accompanied by the formation of calcite (Reaction (8));
- -
With increasing activation temperature, the contents of kaolinite and siderite decreased, while the amounts of quartz and hematite increased (Reaction (9)).
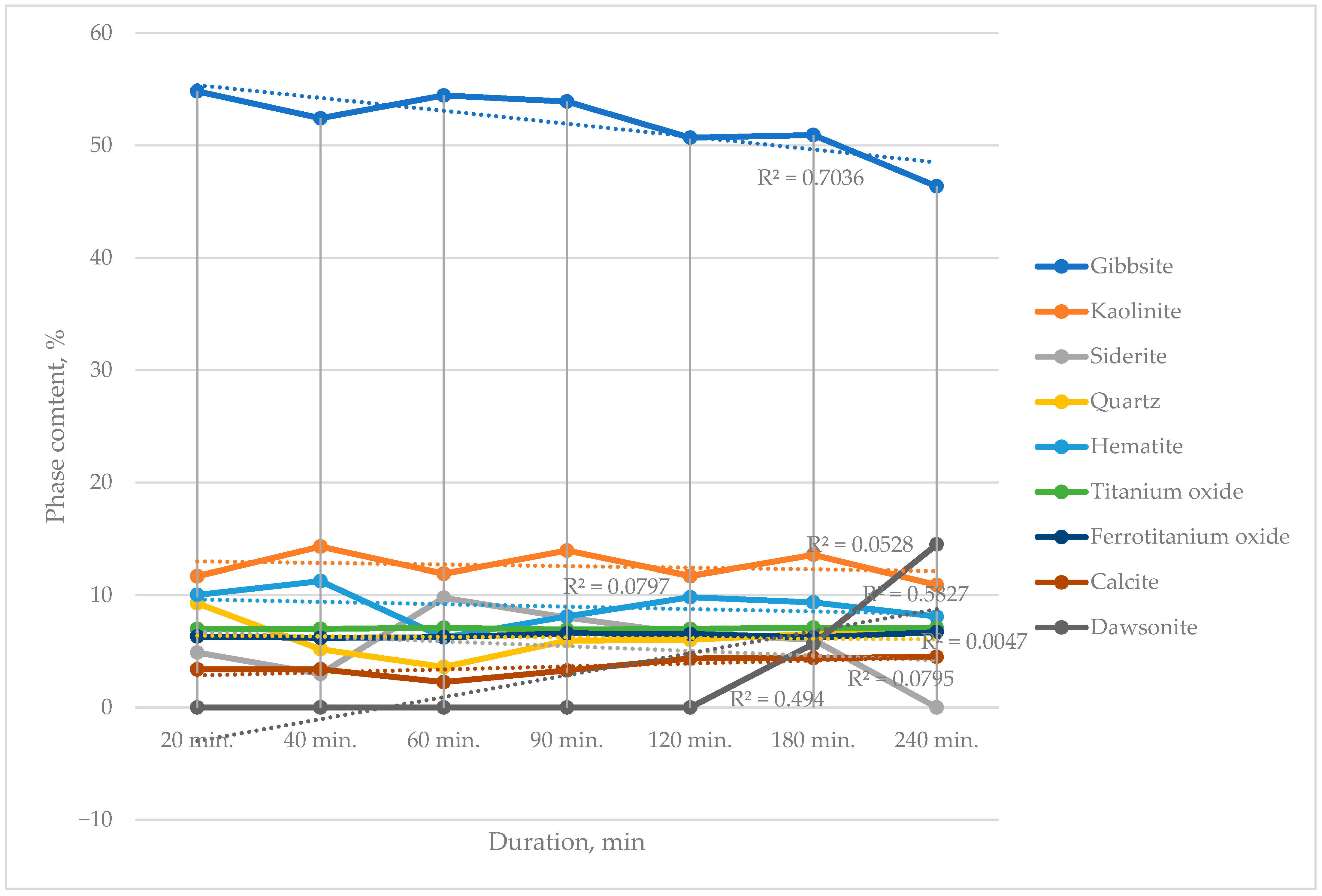
The X-ray phase analysis results for the bauxite samples as a function of the duration of chemical activation at 120 °C indicate the following:
- -
The content of the gibbsite phase remains approximately constant. The observed decrease in the relative proportion of gibbsite at activation durations beyond 120 min is attributed to an increase in the final sample weight.
- -
The contents of kaolinite, siderite, quartz, and hematite exhibit a wave-like (non-monotonic) behavior, suggesting their participation in ongoing chemical reactions with the solution containing CO32− and HCO3− ions, as well as interaction with the gas phase in the autoclave.
- -
The calcium silicate phase disappears within the first few minutes of activation, forming calcite, in accordance with Reaction (8).
In bauxite samples activated for 180 and 240 min, a new phase—sodium dawsonite (NaAlCO
3(OH)
2)—was identified, formed according to Reaction (10):
Experimental data indicate that elevated pressure at 120 °C and a duration exceeding 180 min makes Reaction (9) possible. The formation of the dawsonite phase is undesirable as it hinders subsequent gravity beneficiation.
The X-ray phase analysis results for the bauxite samples as a function of the duration of chemical activation at 200 °C indicate the following:
- -
The contents of kaolinite, quartz, titanium dioxide, and ferrotitanium oxide phases remain approximately constant. The observed decrease in the relative proportions of these phases at activation durations beyond 60 min is attributed to an increase in the final weight of the sample.
- -
The calcium silicate phase disappears within the first few minutes of activation, forming calcite (Reaction (8)).
- -
In bauxite samples activated for 60 and 90 min, new phases were identified: sodium dawsonite (NaAlCO3(OH)2) and sodium bicarbonate (NaHCO3) (Reaction (8)).
During the activation of bauxite at 200 °C for 90 min, a solid phase was obtained that absorbed the entire sodium bicarbonate solution. The mass of the moist solid phase increased more than tenfold compared to the initial bauxite sample. After drying, the solid phase underwent dehydration, and a residue was obtained with a mass equal to the sum of the initial bauxite and sodium carbonate.
To assess the effect of preliminary thermochemical activation, a comparative study of gravity beneficiation was conducted on both untreated and pre-activated bauxite.
After preliminary thermochemical activation, gravity beneficiation produced a coarse crystalline sand fraction with μSi 5.4, which is 35.9% higher than the silica modulus of the sand fraction obtained without activation. μSi of the fine fraction was 1.97.
The sieve analysis of the coarse sand fraction of the bauxite concentrate is shown in
Table 1.
The silica modulus of the coarse sand fraction ranged from 4.2 to 5.4 (
Table 2). The fine fraction consisted of 80% particles smaller than 0.1 mm, with a silica modulus ranging from 1.87 to 1.97.
Gravity beneficiation of the untreated bauxite yielded a coarse crystalline sand fraction with a yield of 36% and μSi 3.8, and a fine fraction with μSi2.32.
To achieve the required μSi ≥ 7, the bauxite obtained after gravity beneficiation with μSi 5.4 was further treated in an alkaline solution containing 100 g/dm3 Na2O at 100 °C for 2 h. As a result, bauxite with μSi 9.5 was obtained, suitable for processing using the economical Bayer method.
Thus, the goal of beneficiating high-silica gibbsite–kaolinite bauxite was achieved through the application of preliminary thermochemical activation.
3.4. Thermochemical Activation of Nepheline from the Kubasadyr Deposit
The growing demand for alumina and the shortage of Bayer-grade bauxites prompted an investigation into the use of alternative raw materials, specifically nepheline syenites from the Kubasadyr deposit. Nepheline is an alkaline, high-alumina mineral [
33,
34].
Conventional technology for the integrated processing of nephelines involves sintering the ore with limestone. However, the high energy consumption, high capital cost, and significant atmospheric emissions are the main disadvantages of the sintering process. As an alternative, the feasibility of applying a hydrometallurgical, alkaline–sulfate processing technology for nepheline syenites was studied [
35]. To implement this approach, the effect of preliminary thermochemical activation of the raw material prior to leaching was investigated.
The chemical composition of nephelines from the Kubasadyry deposit (wt%) was as follows: 17.77% Al2O3, 48.37% SiO2, 3.18% Fe2O3, 2.89% CaO, 4.24% Na2O, 7.7% K2O, 0.34% MgO, 0.3% Cl, 0.21% S.
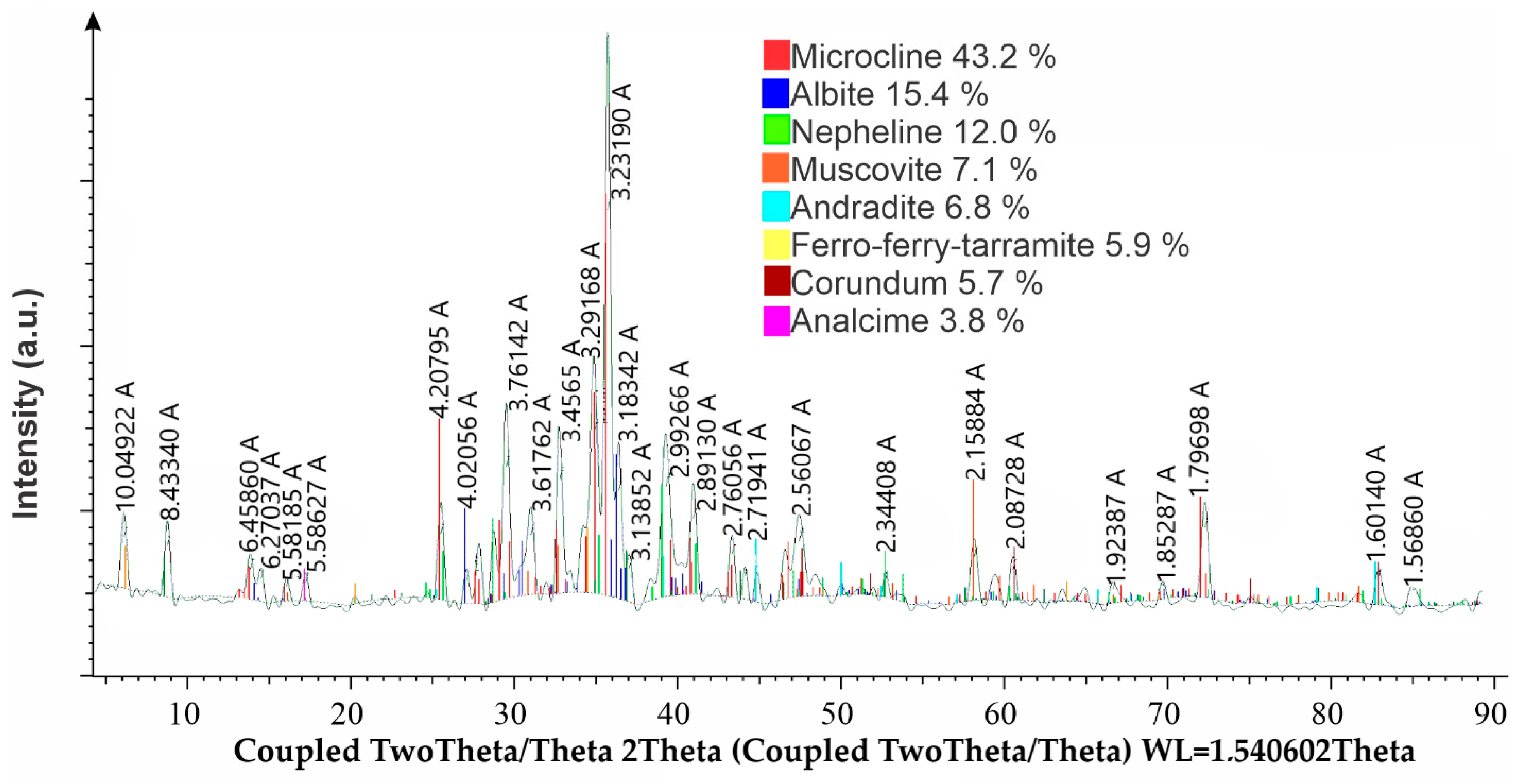
The phase composition of nephelines is shown in
Figure 10.
According to
Figure 10, the phase composition of the nepheline sample consists predominantly of microcline (intermediate) KAlSi
3O
8, accounting for approximately 43.2% of the sample. Other significant phases include albite (Na
0.98Ca
0.02)(Al
1.02Si
2.98O
8) at 15.4%, nepheline (K
1.6Na
6/Al
7.49Si
8.51O
32) at 12.0%, and muscovite-2M1 (KAl
2(Si
3Al)O
10(OH)
2) at 7.1%. In addition, minor phases were identified, including andradite (aluminoan) Ca
3(Fe
1.42Al
0.56)(SiO
4)
3 at 6.8%, ferro-ferri-taramite (Na
2CaFe
5Al
2Si
6O
22(OH)
2) at 5.9%, corundum (synthetic) Al
2O
3 at 3.7%, and analcime-1Q NaAl(Si
2O
6) at 3.8%.
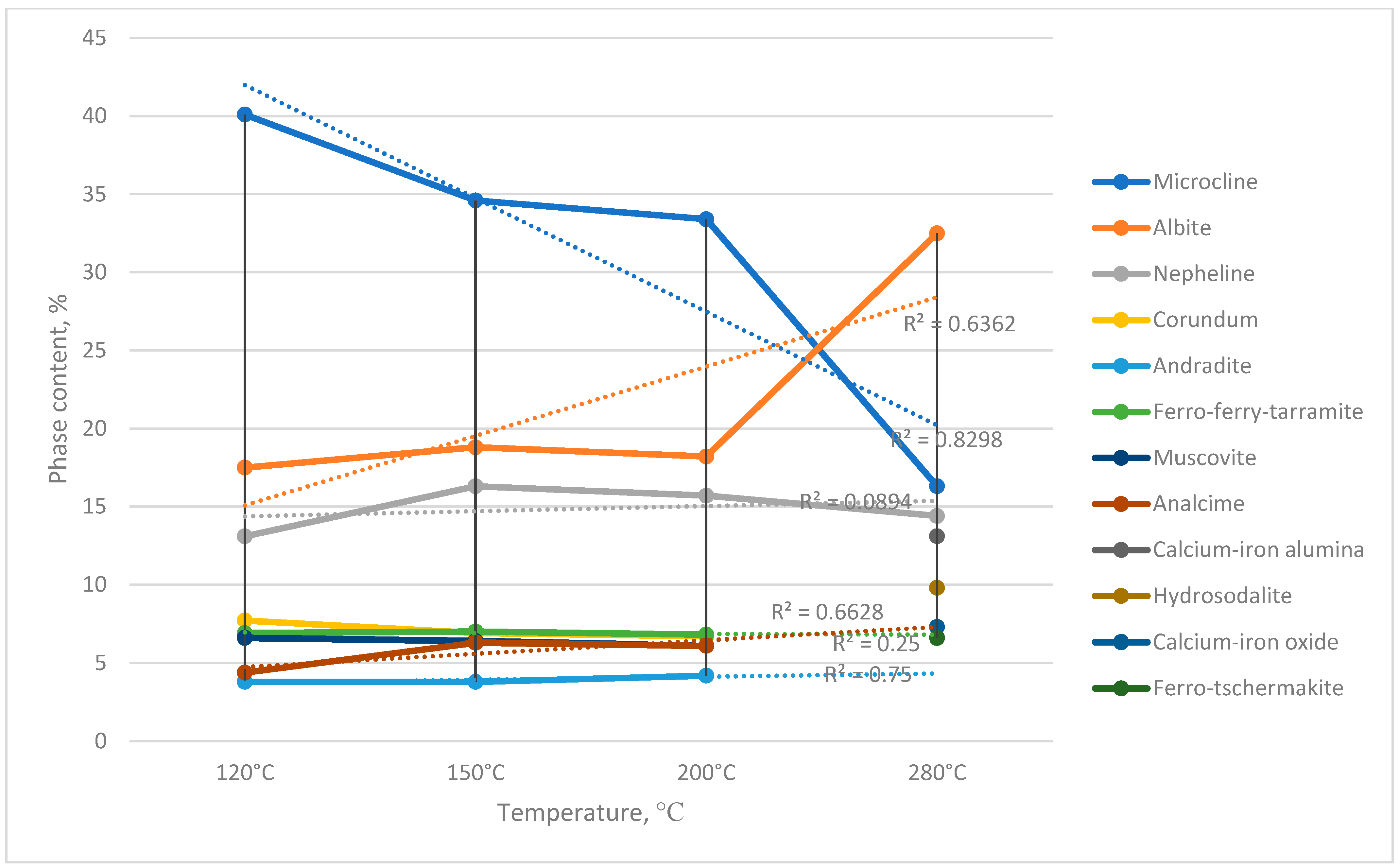
During chemical activation, temperature-dependent changes in the phase composition of nepheline syenites were observed (
Figure 11):
- -
The content of microcline (KAlSi3O8) decreased from 43.0% to 15.3% (at 280 °C), accompanied by the formation of albite (NaAlSi3O8) (Reaction (11)). This suggests a substitution of potassium ions with sodium ions, which have a smaller ionic radius and can more easily fit into the dense microcline structure, rendering it less stable.
- -
Hydrosodalite phases were formed (Reaction (12)), representing hydrated nepheline, (NaAlSiO4)6·(H2O)8. This indicates that hydration increased the size of the nepheline molecule and transformed its dense mineral structure into a more porous form.
- -
The disappearance of corundum (Al2O3) can be attributed to its interaction with andradite (Ca3(Fe1.42Al0.56)(SiO4)3), resulting in the formation of a calcium–iron aluminosilicate (Ca2Fe2O5.12).
The proposed reaction of microcline with sodium bicarbonate, according to Gibbs free energy calculations, proceeds only at a temperature of 360 °C, i.e., above the activation temperature of 280 °C. In practice, however, the obtained results indicate the possibility of this reaction occurring with the formation of albite and analcime.
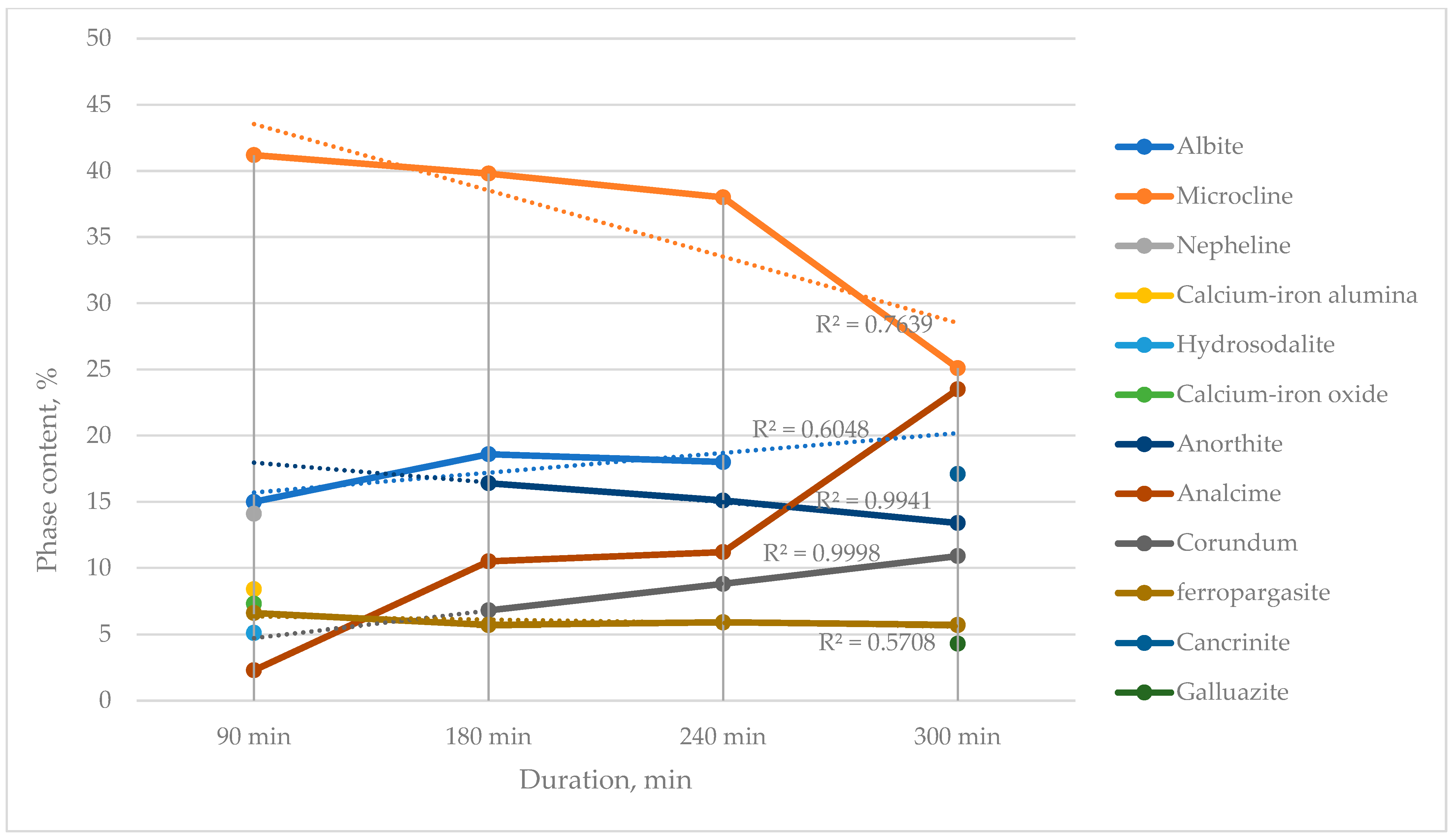
The phase compositions of the nepheline concentrate samples as a function of activation duration are presented in
Figure 12.
It is well known that albite is a phase that is readily leached during nepheline processing. The formation of this phase during preliminary preparation prior to leaching can replace the energy-intensive calcination step, which represents a novel aspect determining the efficiency of the developed technology.
In the beneficiation of the initial (untreated) nepheline, the degree of silica extraction into solution was 33.58%. After preliminary activation of nepheline ore under optimal conditions [
35], the degree of silica extraction into solution increased to 59.6%.
Thus, preliminary chemical activation enables more efficient desilication of nepheline, facilitating its subsequent processing, reducing reagent consumption, and minimizing material flows.
4. Conclusions
The proposed method for the thermochemical activation of raw materials in a NaHCO3 solution under elevated pressure and temperature in an autoclave enables the transformation of the initial chemically stable and refractory phase composition, thereby significantly enhancing the extraction of valuable components during subsequent processing.
The method has been tested on various types of mineral raw materials, including slag from the reductive smelting of red mud from alumina production prior to acid leaching, ash before chemical beneficiation, gibbsite–kaolinite bauxite prior to gravity separation, and nephelines, for which the sintering process was replaced with chemical beneficiation.
For the slag from the reductive smelting of red mud, activation caused a decrease in the larnite content and an increase in the tricalcium silicate phase. Leaching the activated slag in nitric acid solution achieved extraction rates of approximately 96% for REEs, 92% for TiO2, ~98% for CaO and Al2O3, and 50% for Fe2O3. In contrast, leaching unactivated slag yielded only ~20% REE, ~40% CaO and Al2O3, and 35% Fe2O3 recovery.
As a result of the thermochemical activation of ash, the aluminosilicate phases sillimanite and hedenbergite disappeared, the amount of mullite and free silica increased, and a calcite phase was formed. The application of preliminary thermochemical activation during ash beneficiation improved the silica recovery rate by 15–20% compared to non-activated samples.
For gibbsitic–kaolinitic bauxite, activation led to changes in the content of kaolinite, siderite, quartz, and hematite. The calcium silicate phase decomposed within the first minutes of activation with the formation of calcite. After activation, gravity beneficiation produced a coarse crystalline sand fraction with a silica modulus (μSi) of 5.4, which is 35.9% higher than that of the sand fraction obtained without activation. At the second stage, chemical beneficiation yielded bauxite with a silica modulus (μSi) of 9.5, suitable for processing using the Bayer method.
Thermochemical activation of nepheline ores prior to beneficiation reduced the content of microcline and promoted the formation of albite, hydrosodalite, and calcium–iron aluminosilicate phases, while corundum and andradite disappeared. During the beneficiation of nepheline ore after preliminary activation under optimal conditions, the degree of silica extraction into solution reached 59.6%, which is 25% higher compared to the process without activation. Without activation, the degree of silica extraction into solution was 33.58%.
Thus, the proposed thermochemical activation method enables the transformation of the original phase composition of mineral raw materials and significantly increases the efficiency of subsequent processing. It does not require additional reagent consumption, since the sodium bicarbonate solution used can be easily regenerated using a gas containing CO2.