Dependence of Bubble Size on Magnesite Flotation Recovery Using Sodium Oleate (NaOL) with Different Frothers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Zeta Potential Measurements
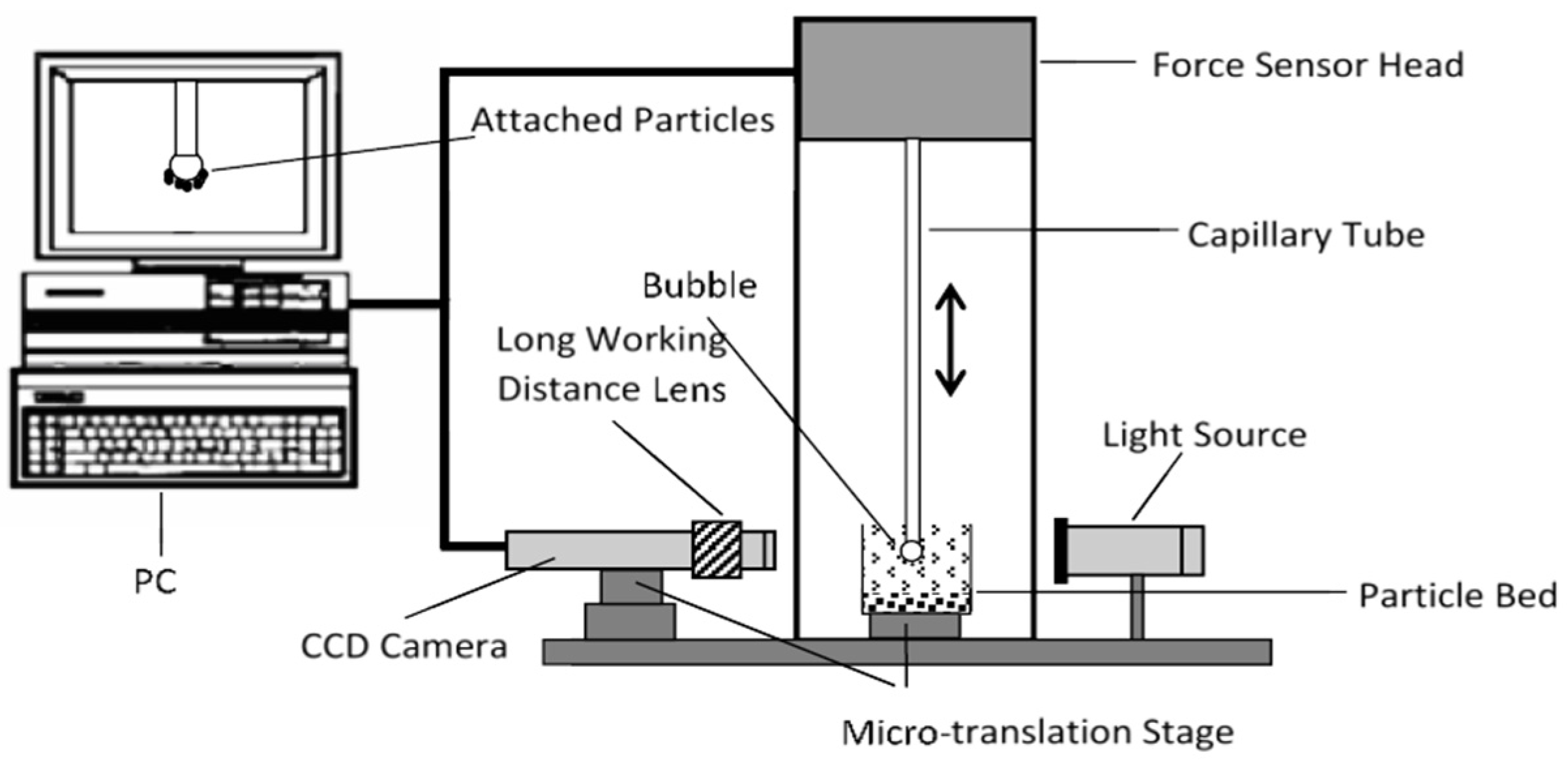
2.2.3. Bubble–Particle Attachment Measurements
2.2.4. Micro-Flotation Experiments
3. Results and Discussion
3.1. Zeta Potential Measurements
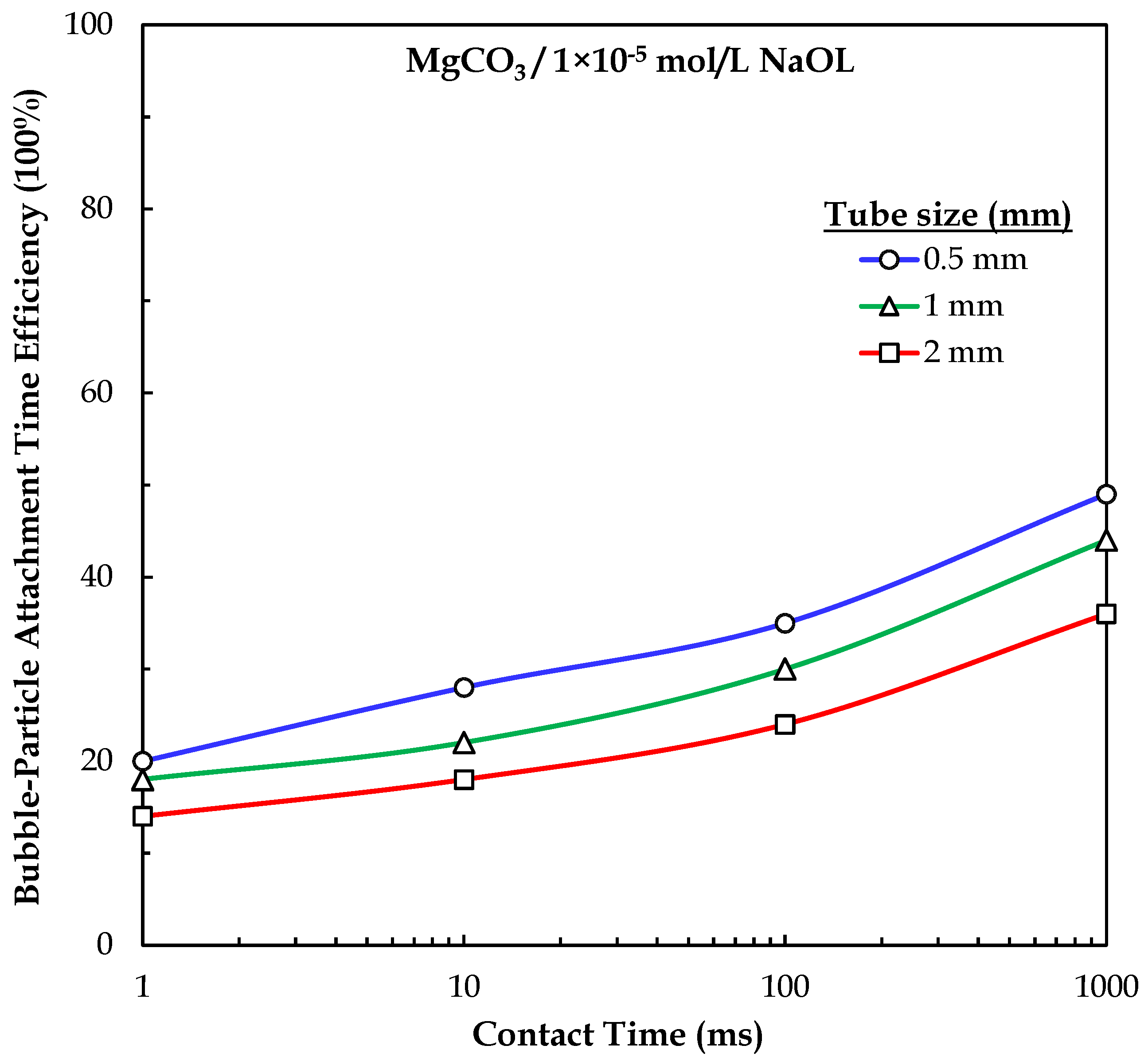
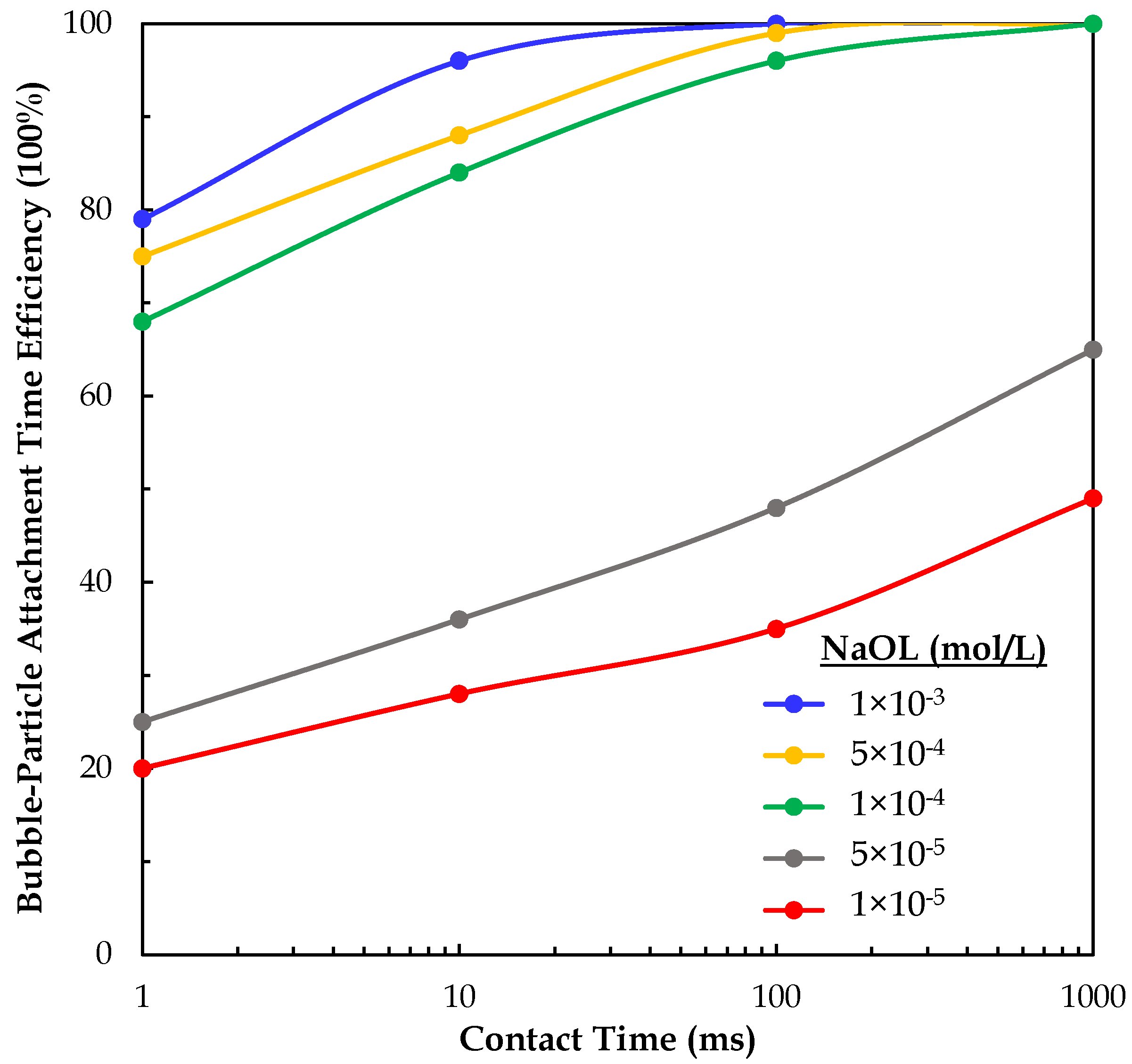
3.2. Bubble–Particle Attachment Interactions
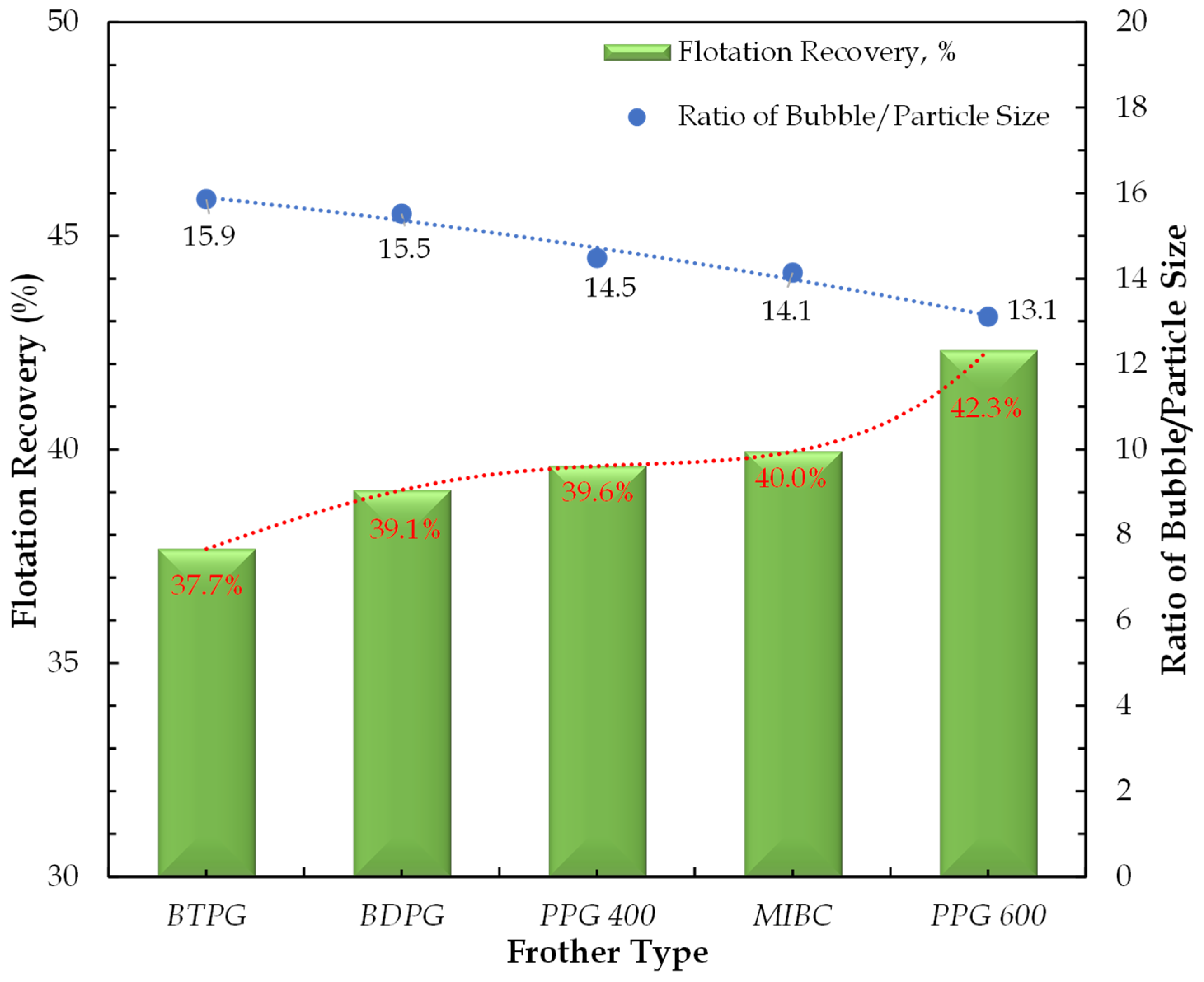
3.3. Micro-Flotation Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abkarian, M.; Subramaniam, A.B.; Kim, S.H.; Larsen, R.J.; Yang, S.M.; Stone, H.A. Dissolution arrest and stability of particle-covered bubbles. Phys. Rev. Lett. 2007, 99, 188301. [Google Scholar] [CrossRef]
- George, P.; Nguyen, A.V.; Jameson, G.J. Assessment of true flotation and entrainment in the flotation of submicron particles by fine bubbles. Miner. Eng. 2004, 17, 847–853. [Google Scholar] [CrossRef]
- Neethling, S.J.; Brito-Parada, P.R. Predicting flotation behaviour: The interaction between froth stability and performance. Miner. Eng. 2018, 120, 60–65. [Google Scholar] [CrossRef]
- Leja, J. Surface Chemistry of Froth Flotation; Plenum Press: New York, NY, USA, 1982. [Google Scholar]
- Fosu, S.; Skinner, W.; Zanin, M. Detachment of coarse composite sphalerite particles from bubbles in flotation: Influence of xanthate collector type and concentration. Miner. Eng. 2015, 71, 73–84. [Google Scholar] [CrossRef]
- Ulusoy, U.; Yekeler, M.; Hiçyilmaz, C. Determination of the shape, morphological and wettability properties of quartz and their correlations. Miner. Eng. 2003, 16, 951–964. [Google Scholar] [CrossRef]
- Koh, P.T.L.; Hao, F.P.; Smith, L.K.; Chau, T.T.; Bruckard, W.J. The effect of particle shape and hydrophobicity in flotation. Int. J. Miner. Process. 2009, 93, 128–134. [Google Scholar] [CrossRef]
- Guven, O.; Çelik, M.S. Interplay of particle shape and surface roughness to reach maximum flotation efficiencies depending on collector concentration. Miner. Process. Extr. Metall. Rev. 2016, 37, 412–417. [Google Scholar] [CrossRef]
- Rezai, B.; Rahim, M.; Aslani, M.R.; Eslamian, A.; Dehghani, F. Relationship between surface roughness of minerals and their flotation kinetics. In Proceedings of the XI International Mineral Processing and Technology Congress, Brisbane, Australia, 6–10 September 2010; pp. 232–238. [Google Scholar]
- Little, L.; Mainza, A.N.; Becker, M.; Wiese, J.G. Using mineralogical and particle shape analysis to investigate enhanced mineral liberation through phase boundary fracture. Powder Technol. 2016, 301, 794–804. [Google Scholar] [CrossRef]
- Hewitt, D.; Fornasiero, D.; Ralston, J. Bubble–particle attachment. J. Chem. Soc. Faraday Trans. 1995, 91, 1997–2001. [Google Scholar] [CrossRef]
- Güngören, C.; Baktarhan, Y.; Şahbaz, O.; Ünver, İ.K.; Özkan, Ş.G.; Özdemir, O. Effect of chloride salt ions onto coal flotation based on contact angle and bubble-particle attachment time. Dokuz Eylül Üniversitesi Mühendislik Fakültesi Fen Ve Mühendislik Derg. 2022, 24, 553–562. [Google Scholar] [CrossRef]
- Albijanic, B.; Amini, E.; Wightman, E.; Ozdemir, O.; Nguyen, A.V.; Bradshaw, D.J. A relationship between the bubble-particle attachment time and the mineralogy of a copper-sulphide ore. Miner. Eng. 2011, 24, 1335–1339. [Google Scholar] [CrossRef]
- Albijanic, B.; Ozdemir, O.; Nguyen, A.V.; Bradshaw, D. A Review of induction and attachment times of wetting thin films between air bubbles and particles and its relevance in flotation separation of particles. Adv. Colloid Interface Sci. 2010, 159, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Batjargal, K.; Güven, O.; Ozdemir, O.; Boylu, F.; Pural, Y.E.; Çelik, M.S. Correlation of flotation recoveries and bubble–particle attachment time for dodecyl ammonium hydrochloride/frother/quartz flotation system. Minerals 2023, 13, 1305. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Pradip. Zeta potentials in the flotation of oxide and silicate minerals. Adv. Colloid Interface Sci. 2005, 114–115, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Ignatkina, V.A.; Bocharov, V.A.; Aksenova, D.D.; Kayumov, A.A. Zeta potential of the surface of ultrafine sulfides and floatability of minerals. Russ. J. Non-Ferr. Met. 2017, 58, 95–100. [Google Scholar] [CrossRef]
- Muhammadi-Jam, S.; Waters, E.K.; Greenwood, R.W. A review of zeta potential measurements using electroacoustics. Adv. Colloid Interface Sci. 2022, 309, 102778. [Google Scholar] [CrossRef]
- Elmahdy, A.M.; Mirnezami, M.; Finch, J.A. Zeta potential of air bubbles in presence of frothers. Int. J. Miner. Process. 2008, 89, 40–43. [Google Scholar] [CrossRef]
- Sze, A.; Erickson, D.; Ren, L.; Li, D. Zeta-potential measurements using the Smoluchowski equation and the slope of the current-time relationship in electrostatic flow. J. Colloid Interface Sci. 2003, 261, 402–410. [Google Scholar] [CrossRef]
- Yoon, R.-H. The role of hydrodynamic and surface forces in bubble–particle interaction. Int. J. Miner. Process. 2000, 58, 129–143. [Google Scholar] [CrossRef]
- Batjargal, K.; Guven, O.; Ozdemir, O.; Karakashev, S.I.; Grozev, N.A.; Boylu, F.; Çelik, M.S. Frothing Performance of Frother-Collector Mixtures as Determined by Dynamic Foam Analyzer and Its Implications in Flotation. Minerals 2023, 13, 242. [Google Scholar] [CrossRef]
- Gaudin, A.M.; Groh, J.O.; Henderson, H.B. Effect of particle size on flotation. AIME Tech. Publ. 1931, 414, 3–23. [Google Scholar]
- Yalcin, T.; Byers, A. Dissolved gas flotation in mineral processing. Miner. Process. Extr. Metall. Rev. 2006, 27, 87–97. [Google Scholar] [CrossRef]
- Tao, Y.J.; Liu, J.T.; Yu, S.; Tao, D. Picobubble enhanced fine coal flotation. Sep. Sci. Technol. 2006, 41, 3597–3607. [Google Scholar] [CrossRef]
- Miettinen, T.; Ralston, J.; Fornasiero, D. The limits of fine particle flotation. Miner. Eng. 2010, 23, 420–437. [Google Scholar] [CrossRef]
- Cho, Y.S.; Laskowski, J.S. Effect of flotation frothers on bubble size and foam stability. Int. J. Miner. Process. 2002, 64, 69–80. [Google Scholar] [CrossRef]
- Nesset, J.E.; Finch, J.A.; Gomez, C.O. Operating variables affecting the bubble size in forced-air mechanical flotation machines. In Proceedings of the 9th Mill Operators’ Conference 2007, Fremantle, WA, Australia, 19–21 March 2007; pp. 55–65. [Google Scholar]
- Zhang, W.; Nesset, J.E.; Finch, J.A. Water recovery and bubble surface area flux in flotation. Can. Metall. Q. 2010, 49, 353–362. [Google Scholar] [CrossRef]
- Welsby, S.D.D. Pilot-scale froth testing at highland valley Copper. In Proceedings of the 46th Annual Meeting of the Canadian Mineral Processors Conference, Ottawa, ON, Canada, 21–23 January 2014; pp. 301–314. [Google Scholar]
- Zangooi, A.; Gomez, C.O.; Finch, J.A. Mapping frother distribution in industrial flotation circuits. Miner. Eng. 2017, 113, 36–40. [Google Scholar] [CrossRef]
- Walser, G. Economic Impact of World Mining; World Bank Group Mining Department: Washington, DC, USA, 2017; pp. 263–264. [Google Scholar]
- Guven, O.; Batjargal, K.; Ozdemir, O.; Karakashev, S.I.; Grozev, N.A.; Boylu, F.; Çelik, M.S. Experimental procedure for the determination of the critical coalescence concentration (CCC) of simple frothers. Minerals 2020, 10, 617. [Google Scholar] [CrossRef]
- Karakashev, S.I.; Grozev, N.A.; Batjargal, K.; Guven, O.; Özdemir, O.; Boylu, F.; Çelik, M.S. Correlations for easy calculation of the critical coalescence concentration (CCC) of simple frothers. Coatings 2020, 10, 612. [Google Scholar] [CrossRef]
- Batjargal, K.; Guven, O.; Ozdemir, O.; Karakashev, I.S.; Grozev, A.N.; Boylu, F.; Celik, S.M. Adsorption Kinetics of Various Frothers on Rising Bubbles of Different Sizes under Flotation Conditions. Minerals 2021, 11, 304. [Google Scholar] [CrossRef]
- Karakashev, S.I.; Grozev, N.A.; Ozdemir, O.; Batjargal, K.; Guven, O.; Ata, S.; Bournival, G.; Boylu, F.; Çelik, M.S. On the frother’s strength and its performance. Miner. Eng. 2021, 171, 107093. [Google Scholar] [CrossRef]
- Batjargal, K.; Guven, O.; Ozdemir, O.; Karakashev, S.; Grozev, N.A.; Boylu, F.; Çelik, M.S. Bubbling properties of frothers and collectors mix system. Physicochem. Probl. Miner. Process. 2022, 58, 152890. [Google Scholar] [CrossRef]
- Chen, G.; Tao, D. Effect of solution chemistry on floatability of magnesite and dolomite. Int. J. Miner. Process. 2004, 74, 343–357. [Google Scholar] [CrossRef]
- Ye, Y.; Miller, J.D. Bubble/particle contact time in the analysis of coal flotation. Coal Prep. 1988, 5, 147–166. [Google Scholar] [CrossRef]
- Ye, Y.; Khandrika, S.M.; Miller, J.D. Induction-time measurements at a particle bed. Int. J. Miner. Process. 1989, 25, 221–240. [Google Scholar] [CrossRef]
- Yoon, R.H.; Yordan, J.L. Induction time measurements for the quartz-amine flotation system. J. Colloid Interface Sci. 1991, 141, 374–383. [Google Scholar] [CrossRef]
- Su, L.; Xu, Z.; Masliyah, J. Role of oily bubbles in enhancing bitumen flotation. Miner. Eng. 2006, 19, 641–650. [Google Scholar] [CrossRef]
- Glembockij, V.A. The time of attachment of air bubbles to mineral particles in flotation and its measurement. Izv. Akad. Nauk SSSR (OTN) 1953, 11, 1524–1531. [Google Scholar]
- Peng, F.F. Surface energy and induction time of fine coals treated with various levels of dispersed collector and their correlation to flotation responses. Energy Fuel 1996, 10, 1202–1207. [Google Scholar] [CrossRef]
- Gu, G.; Xu, Z.; Nandakumar, K.; Masliyah, J. Effects of physical environment on induction time of air–bitumen attachment. Int. J. Miner. Process 2003, 69, 235–250. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Z.; Nandakumar, K.; Masliyah, J.H.; Xu, Z. An induction time model for the attachment of an air bubble to a hydrophobic sphere in aqueous solutions. Int. J. Miner. Process. 2005, 75, 69–82. [Google Scholar] [CrossRef]
- Ozdemir, O.; Karaguzel, C.; Nguyen, A.V.; Celik, M.S.; Miller, J.D. Contact angle and bubble attachment studies in the flotation of trona and other soluble carbonate salts. Miner. Eng. 2009, 22, 168–175. [Google Scholar] [CrossRef]
- Ozdemir, O.; Taran, E.; Hampton, M.A.; Karakashev, S.I.; Nguyen, A.V. Surface chemistry aspects of coal flotation in bore water. Int. J. Miner. Process. 2009, 92, 177–183. [Google Scholar] [CrossRef]
- Albijanic, B.; Nguyen, A.V.; Ozdemir, O.; García-Figueroa, A.A. Induction time of wetting films between air bubbles and hydrophobic particles in the presence of dodecyl amine hydrochloride: First principles model analysis and experimental validation. Molecules 2025, 30, 695. [Google Scholar] [CrossRef]
- Tang, M.; Niu, X.; Wen, S. Influences of magnesium ions in water on gelatinization characteristics of starch and its flocculation behaviors on particles. Physicochem. Probl. Miner. Process. 2024, 60, 178149. [Google Scholar] [CrossRef]
- Hwang, E.T.; Chae, M. Magnesium carbonate for physical control of poultry red mites. Surf. Interfaces 2025, 64, 106335. [Google Scholar] [CrossRef]
- Wonyen, D.G.; Kromah, V.; Gibson, B.; Nah, S.; Chelgani, S.C. A review of flotation separation of Mg carbonates (dolomite and magnesite). Minerals 2018, 8, 354. [Google Scholar] [CrossRef]
- Çelik, M.S.; Guven, O.; Karaağaçlıoğlu, İ.E.; Özdemir, O. Does hemimicelle concentration (HMC) coincide with critical aggregation concentration (CAC) in flotation? Miner. Eng. 2024, 215, 108812. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Filippova, I.; Filippov, L.; Picarra, A.; Rulyov, N.; Fornasiero, D. Flotation of fine particles in the presence of combined microbubbles and conventional bubbles. Miner. Eng. 2020, 155, 106439. [Google Scholar] [CrossRef]
- Yoon, R.N.; Lutrell, G. The Effect of bubble size on fine particle flotation. Miner. Process. Extr. Metall. Rev. 1989, 5, 101–122. [Google Scholar] [CrossRef]
- Ahmed, N.; Jameson, G. The effect of bubble size on the rate of flotation of fine particles. Int. J. Miner. Process. 1985, 14, 195–215. [Google Scholar] [CrossRef]
- Reis, A.; Reis Filho, A.M.; Demuner, L.R.; Barrozo, M.A.S. Effect of bubble size on the performance flotation of fine particles of a low-grade Brazilian apatite ore. Powder Technol. 2019, 356, 884–891. [Google Scholar] [CrossRef]
- Sivamohan, R. The problem of recovering very fine particles in mineral processing. A Review. Int. J. Miner. Process. 1990, 28, 247–288. [Google Scholar] [CrossRef]
- Rulyov, N.N. Turbulent microflotation: Theory and experiment. Colloids Surf. A Physicochem. Eng. Asp. 2001, 192, 73–91. [Google Scholar] [CrossRef]
- Rubio, J.; Capponi, F.; Matiolo, E.; Nunes, G.N. Advances in flotation of mineral fines. In Proceedings of the XXII International Mineral Processing Congress, Cape Town, South Africa, 29 September–3 October 2003; pp. 1014–1022. [Google Scholar]
- Dai, Z.; Fornasiero, D.; Ralston, J. Particle-bubble collision models—A review. Adv. Colloid Interface Sci. 2000, 85, 231–256. [Google Scholar] [CrossRef]






| Chemical | Purity (%) | Formula | Molecular Weight (g/mol) | CMC (mol/L) | Molecular Structure |
|---|---|---|---|---|---|
| Sodium oleate (NaOL) | ≥97 | C18H33NaO2 | 304.4 | 3 × 10−3 |  |
| Frothers | Formula | Molecular Weight (g/mol) | CCC (ppm) | Molecular Structure |
|---|---|---|---|---|
| PPG-400 n = 6.5 | 0H(C3H6O)nH | 360~440 | 4 |  |
| PPG-600 n = 10 | 0H(C3H6O)nH | 540~660 | 3 |  |
| BTPG n = 3 | C13H28O4 | 248.36 | 5 |  |
| BDPG n = 2 | C10H22O3 | ~250 | 17 |  |
| MIBC | C6H14O | 102.17 | 10 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batjargal, K.; Güven, O.; Ozdemir, O.; Boylu, F.; Çelik, M.S. Dependence of Bubble Size on Magnesite Flotation Recovery Using Sodium Oleate (NaOL) with Different Frothers. Minerals 2025, 15, 849. https://doi.org/10.3390/min15080849
Batjargal K, Güven O, Ozdemir O, Boylu F, Çelik MS. Dependence of Bubble Size on Magnesite Flotation Recovery Using Sodium Oleate (NaOL) with Different Frothers. Minerals. 2025; 15(8):849. https://doi.org/10.3390/min15080849
Chicago/Turabian StyleBatjargal, Khandjamts, Onur Güven, Orhan Ozdemir, Feridun Boylu, and Mehmet Sabri Çelik. 2025. "Dependence of Bubble Size on Magnesite Flotation Recovery Using Sodium Oleate (NaOL) with Different Frothers" Minerals 15, no. 8: 849. https://doi.org/10.3390/min15080849
APA StyleBatjargal, K., Güven, O., Ozdemir, O., Boylu, F., & Çelik, M. S. (2025). Dependence of Bubble Size on Magnesite Flotation Recovery Using Sodium Oleate (NaOL) with Different Frothers. Minerals, 15(8), 849. https://doi.org/10.3390/min15080849











