1. Introduction
Rutile (TiO
2), ilmenite (FeTiO
3), and titanite (CaTiSiO
5) are known as the main titanium minerals. Among them, rutile is thought to be the most valuable source of titanium due to its relatively higher titanium grade and valuable high-end titanium pigment products [
1]. Rutile (TiO
2) typically contains 60% Ti, and has a hardness of about 6–6.5 Mohs, high resistance to abrasion, specific gravity of about 4 g/cm
3, and colors varying from yellowish red to reddish brown or black. However, commercially valuable or enriched rutile concentrates contain 95% TiO
2, with the remaining 5% comprising minerals such as SiO
2, CrO
3, and Fe
2O
3. Rutile and ilmenite are generally found together with heavy minerals such as quartz, zircon, monazite, and magnetite in placer deposits [
2]. It is possible to increase the grade of low-grade rutile ore by separating its heavy minerals using enrichment methods. Widely used methods for enriching titanium ores include froth flotation, gravity, and magnetic and electrostatic separation methods, or combinations of them. In some cases, roasting and pickling processes can be performed in addition to enhance separation efficiency [
3,
4,
5,
6]. Titanium minerals can be separated from quartz via the flotation of quartz with amines or the flotation of titanium minerals with fatty acids.
Flotation is one of the most widely used methods of fine-particle ore enrichment, utilizing froth flotation based on the fundamental principles of physico, surface, and colloidal chemistry [
7,
8]. The main operational parameters of this method are pH adjustment, particle size, pulp properties, pulp density, and pulp temperature. Moreover, flotation is particularly useful in recovering low-grade and complex ores that would otherwise be economically unviable [
9,
10]. Another method employed in mineral beneficiation is enrichment by agglomeration or flocculation, which enhances the transportability and processing characteristics of fine ores by converting them into larger, more manageable agglomerates. These procedures are also used in the recovery of minerals that are rare but have high economic value, wherein hydrophobic particles are brought together using a second liquid that adsorbs to hydrophobic particles in suspension and does not mix with water. The amount of oil used determines the shape and humidity of the agglomerate [
11].
The large amounts of gangue found in titanium ores contain varied silicate types. These gangue components can often be separated using magnetic separation and enriched at relatively low cost due to the magnetic properties of iron-rich titanium ore [
12,
13,
14]. However, because of the complexity of mineral dissemination, rutile ores need to be finely ground to liberate valuable minerals for recovery. During this process, gangue slimes are generated in excess [
15,
16,
17]. Accordingly, it has been well established that techniques like agglomeration and flocculation can improve the recovery efficiency of fine-grained rutile ores [
18]. Moreover, eclogite rutile ores usually contain a certain amount of clay, or flaky gangue minerals such as biotite or muscovite, while fine rutile particles are often associated with gangue minerals, resulting in the loss of valuable rutile ore.
Most of the natural rutile used in industry is sourced from heavy mineral placer deposits, which are expected to provide approximately 50% of global titanium for mineral production [
19]. However, these conventional sources are limited in quantity and are rapidly depleted due to increasing global demand [
20]. Titanium (Ti) is one of many strategically important metals, with a wide range of applications, especially in aerospace, machinery, marine, medicine, and chemical industries [
21]. It is estimated that the Western Anatolia region of Türkiye holds over 100 million tons of rutile ore reserves, with an average TiO
2 grade ranging between 0.5 and 1% [
22]. This grade amount can be increased with ore enrichment methods and technological developments [
23], and there is a possibility that these mines will be put into production [
24,
25,
26].
Rutile is the main source of titanium, with significant reserves found in the Menderes Massif region of Manisa, Türkiye where approximately 1.2 million tons resource within a total of 100 million tons of titanium reserves have been estimated. The aim of this study is to recover TiO2 from titanium-rich ores containing rutile and associated minerals. To achieve this, flotation was employed under different pH conditions and reagents depending on the specific mineralogical characteristics of the ore.
2. Materials and Methods
In this study, we investigated the flotation enrichment conditions of rutile ore samples, titanium-bearing minerals obtained from Alasehir/Esme district of Manisa province in the Aegean Region of western Turkey. All experimental procedures were conducted in the ore preparation and enrichment laboratory of the Mining Engineering Department of Dokuz Eylul University. Following size reduction of the ore sample through crushing and grinding, the flocculated product was processed using a combined enrichment technique incorporating conventional flotation and flocculation. This approach enabled the recovery of fine particles smaller than 63 μm, which corresponds to the calculated liberation size of the ore.
The analysis of the titanium (Ti) content across the different particle size fractions of the ore sample, specifically within the −800 + 38 µm range, reveals that the Ti content varies between approximately 4.15% and 5.92 across different particle sizes. Based on this data, it is evident that conventional gravity separation and flotation methods result in a significant loss of Ti, particularly within the fine-grained fraction (particles smaller than 63 µm), which constitutes 17.38% of the total material which is typically discarded as slime. The Ti content in this fine fraction accounts for an approximate loss of 15.71%. Consequently, this study focuses on the beneficiation of fine size ore containing Ti-bearing particles to mitigate such losses and improve overall efficiency.
The chemical analysis results of the rutile ore sample (−63 µm) illustrate the presence of a high Si
2O content, which is associated with TiO
2 (total and in rutile), Fe
2O
3, Al
2O
3, CaO, MgO, metal oxides, and some other minerals (
Table 1).
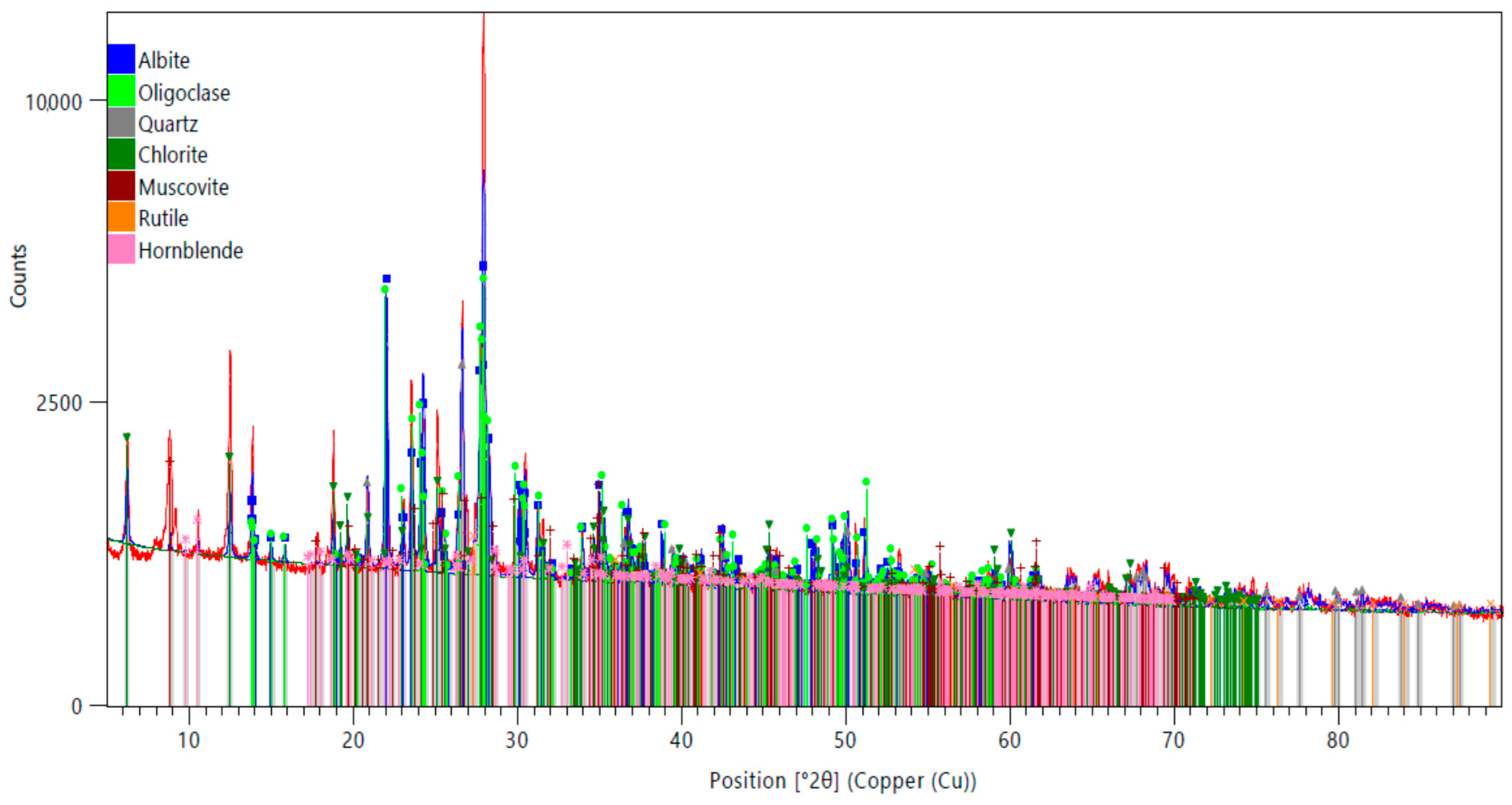
The X-ray diffraction (XRD) pattern of the ore sample, illustrated in
Figure 1, reveals distinct and well-defined peaks corresponding to several mineral phases, including Albite, Oligoclase (a feldspar group mineral), Quartz, Muscovite, Hornblende, and Rutile. The sharpness and relatively high intensity of these diffraction peaks indicate that the ore possesses a predominantly crystalline structure, reflecting well-ordered atomic arrangements within its mineral constituents. The dominance of silicate minerals such as feldspars, Quartz, and Muscovite suggests that the sample has a silicate-rich composition, which is in strong agreement with the findings from the chemical analysis. Additionally, notable diffraction peaks related to the rutile (TiO
2) were observed at 2θ positions around 27.1°, 35.5°, and 54.3°, consistent with the standard data provided by JCPDS card no. 71–116. These peaks confirm that rutile is the primary Ti-bearing phase within the ore, making it a significant component in terms of both mineralogical composition and potential economic value.
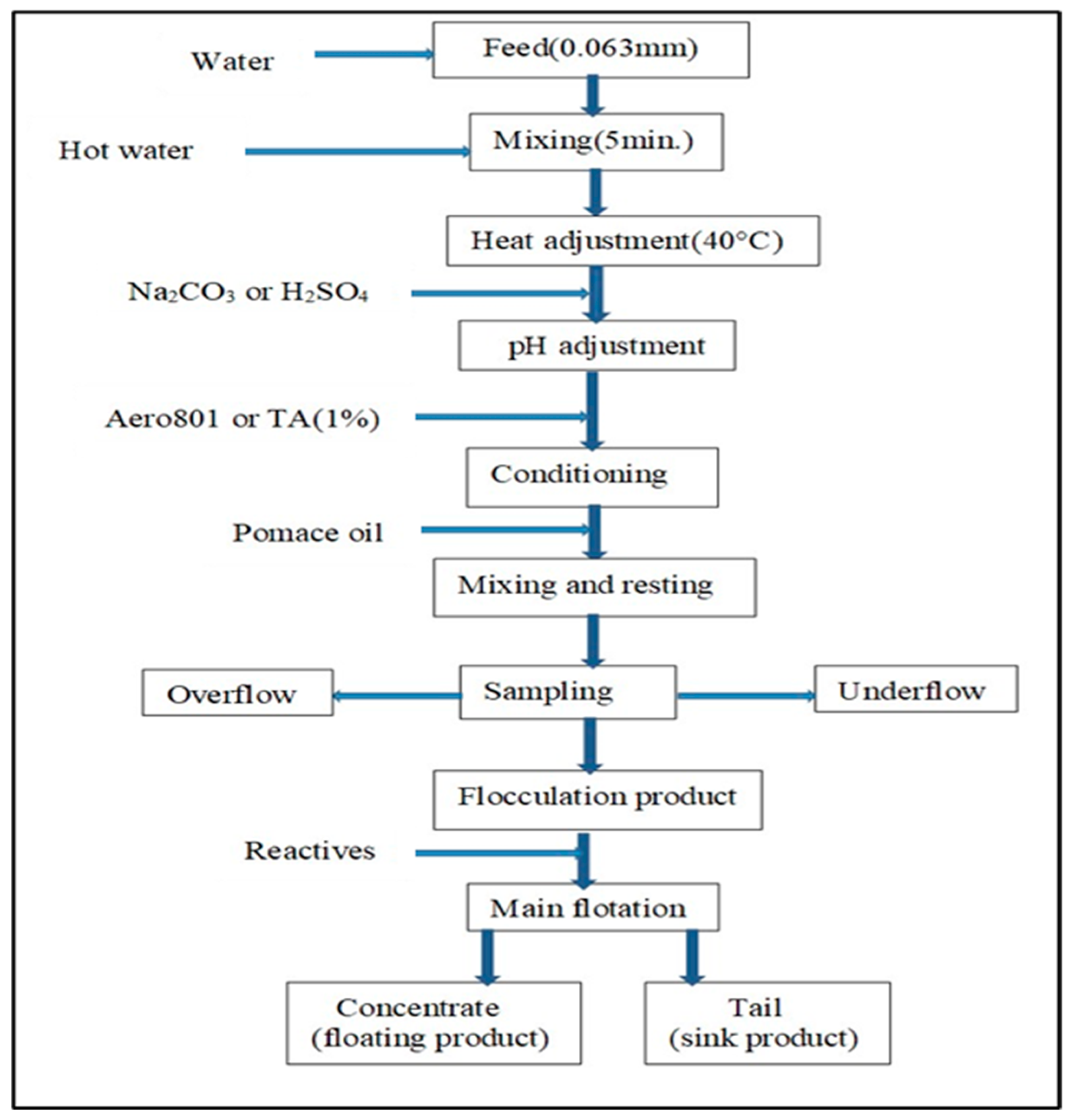
Figure 2 presents the enrichment flow diagram of the rutile sample. In this experiment, we prepared 500 g feed sample with a particle size below 63 μm with the pulp density adjusted to 50%. Then, this pulp was heated to 40 °C by mixing with hot water for 5 min at 1500 rpm. Then, either Na
2CO
3 (Merck (İstanbul, Turkey), 99%) or H
2SO
4 (Merck, 95%) was added to adjust the pH levels determined in the experimental conditions. Afterwards, Aero801 (Cytec (Drogenbos, Belgium); 600 g/t) or 1% tannic acid (TA (İstanbul, Turkey); 600 g/t) was added, followed by, pomace oil (domestic supplier; 30 kg/t) after conditioning, and this was mixed and left to rest for 3–5 min. In order to collect more representative ones, samples were taken from the top and bottom of the obtained flocculation product. Afterwards, the flocculated product was subjected to a basic flotation enrichment process by adding 1% Aero801 (Cytec, Sodium isopropyl xanthate (SIPX, Belgium), 400 g/t), 1% Aero825 (Cytec, Dithiophosphate, 400 g/t), 0.1% Aerofroth 88 (Cytec, Methyl isobutyl carbinol (MIBC), 100 g/t), 1% tannic acid (TA; 400 g/t), and pomace oil (50 kg/t) reagents, which were selected based on preliminary tests and modified due to experimental conditions. In the flotation method, the conditioning interval was performed for 5 min, followed by the duration of the foam removal process lasting 15 to 20 min.
The selected pH range (2.5–12) was designed to encompass both acidic and alkaline conditions that critically influence the surface chemistry of rutile and its associated gangue minerals during flotation. The isoelectric point (IEP) of rutile typically falls within the range of pH 4.5 to 6.5, at which the mineral surface carries minimal net charge, making surface interactions with reagents particularly sensitive to pH variations [
27,
28]. Exploring a wide pH spectrum allows for the evaluation of mineral surface charge behavior, reagent adsorption efficiency, and potential electrostatic interactions. Moreover, the reagents selected for this study, such as Aero801, TA, and pomace oil, are widely used in flotation practice and have demonstrated effective performance across a varied pH range, particularly in the beneficiation of oxide and silicate minerals [
29,
30]. Zeta potential plays an important role in determining which types of reagents can be used and at pH values the minerals which can be floated. Evaluating their combined effects under diverse chemical environments helps establish a broader understanding of reagent selectivity and process efficiency, especially for fine-grained titanium ores where surface reactivity is critical.
3. Results
In this study, the enrichment of rutile sample raw material was experimentally investigated using combined flocculation and flotation methods under different pH and reagent conditions. In the preliminary examinations, it was observed that the ore was mostly liberated in a particle size under 63 μm, thus all the experiments were carried out using this particle size. Then, the rutile sample was subjected to flocculation and flotation experiments, respectively, and promising results have been obtained using this enrichment method.
3.1. Flocculation Test
Rutile samples were subjected to the flocculation method using different pH values and reagents. The test temperature, mixing speed, mixing time, and waiting times were kept constant. Meanwhile, the pH values applied during these tests were 2.5-7-8-10-11-12. While experimental temperatures and stirring speeds were set at 40 °C and 1500 rpm, respectively, conditioning and mixing times were set as 5 min, and settling time was set to 3–5 min. In Test 1, 15% H
2SO
4, 1% Aero 801 (600 g/t), pomace oil (30 kg/t), and hot water were used as reagents. In Tests 2–4, the reagents added were Na
2CO
3, 1% Aero 801 (600 g/t), pomace oil (30 kg/t) and hot water. Finally, Tests 5–7, used 1%TA (600 g/t), pomace oil (30 kg/t) and hot water as added reagents.
Table 2 shows the detailed flocculation test conditions applied to rutile samples with a particle size of −63 μm.
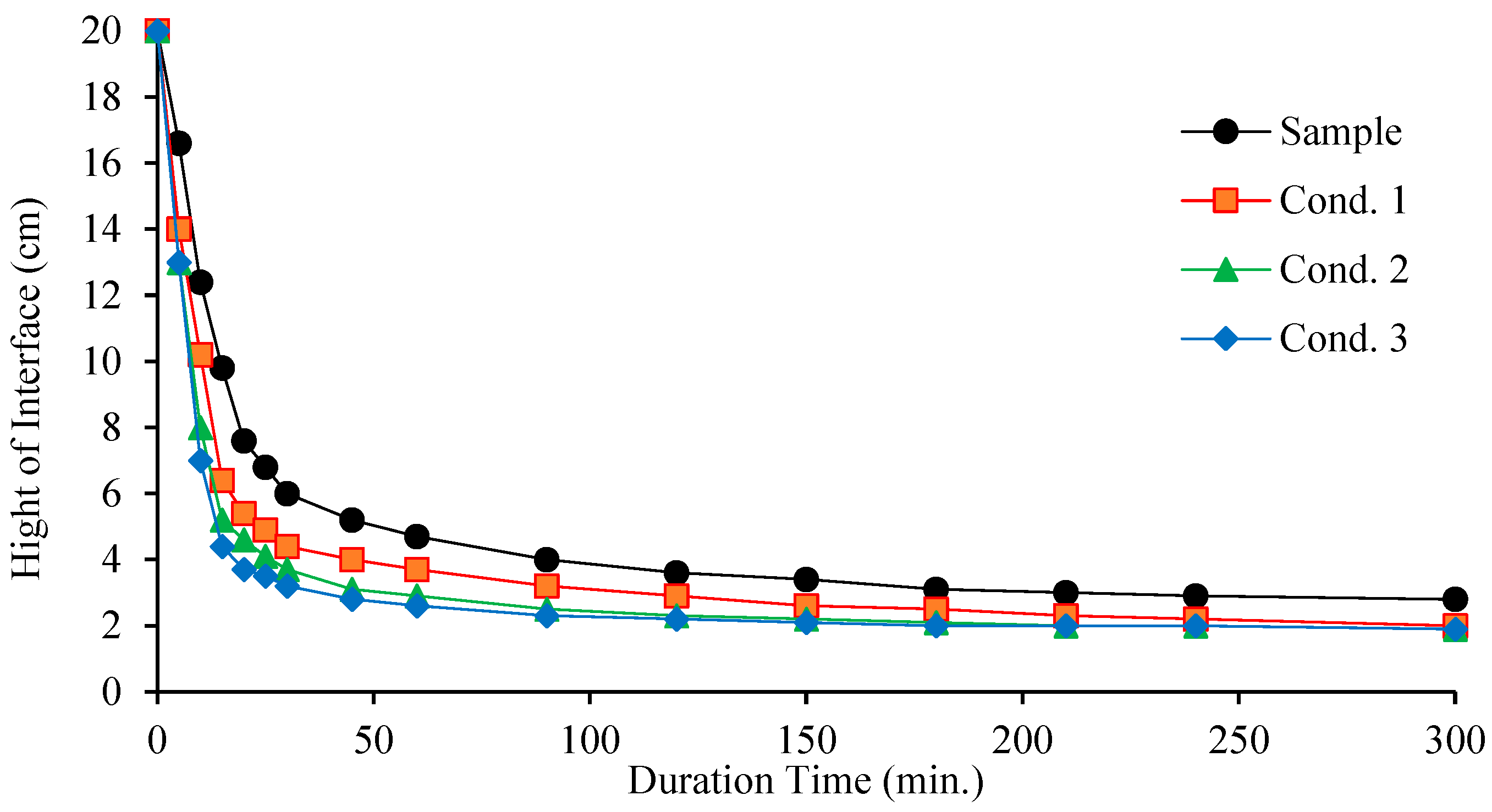
The effect of various flocculants on the settling rate of the sample was evaluated through settling tests. As shown in
Figure 3, all three flocculation conditions—(1) H
2SO
4, Aero 801, and pomace oil; (2) Na
2CO
3, Aero 801, and pomace oil; and (3) tannic acid, and pomace oil—enhance the settling ratio of the sample. The results also indicate that the use of flocculants significantly promotes the settling of ultra-fine particles in the ore sample, resulting in noticeably clearer supernatant water.
3.2. Flotation Test
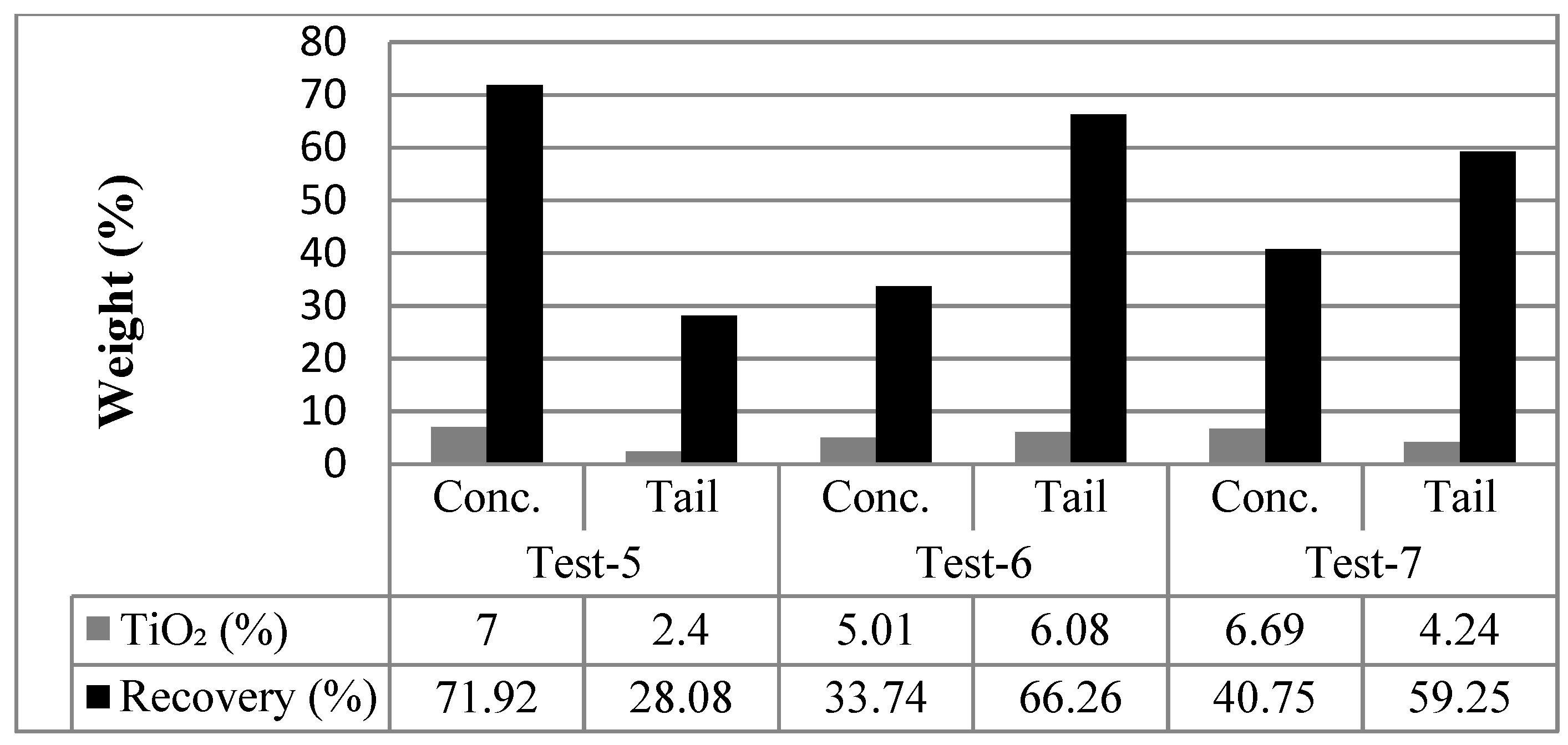
Following the flocculation process, the rutile samples were individually subjected to flotation enrichment. Flotation was also carried out on samples using different pH levels (2.5, 8, 11, 12, 7, 10, and 2.5, respectively) and different reagent combinations. Accordingly, 1% Aero801 (400 g/t), 1% Aero825 (400 g/t), and 0.1% Aerofroth88 (100 g/t) were used for Tests 1–4. For Tests 5–7, the reagents used were 1% TA (400 g/t), pomace oil (50 kg/t), and 0.1% Aerofroth88 (100 g/t).
Table 3 illustrates the conditions of the flotation tests applied to the rutile ore sample (−63 μm).
3.3. Enrichment Test Products of Rutile Sample
The weight percentage (%), content (g), TiO
2 grade (%), and recovery (%) values of both the concentrate and tail products obtained from flocculation and flotation enrichment (Tests 1–7) were calculated.
Table 4 shows information such as the metal content of the products obtained from the enrichment of the rutile sample.
It should be noted that the TiO2 concentrate grade achieved in this study (maximum 8.99%) remained below industrial-grade specifications. This result reflects the initial stage of beneficiation from low-grade rutile ore with a complex mineral matrix. While the enrichment step shows promising selectivity and recovery, further upgrading involving roasting, acid leaching, or multi-stage flotation will be necessary to achieve industrially viable concentrate. These follow-up processes are recommended for future work.
3.4. Microscopic Examination of Enrichment Test Products
Microscopic analysis of the flotation test products was conducted to evaluate the mineralogical composition and distribution of rutile and gangue minerals in both the concentration and tailings. This investigation shed light on the efficiency of flocculation and flotation processes, as well as the behavior of minerals during separation. The observations made in each test condition are presented below.
3.4.1. Test 1
Flotation concentrate: When the basic flotation concentrate (floating product) was examined under the microscope, it is revealed that flocculated and fine-grained gangue minerals were carried in the froth. Also, small amounts of rutile grains and coarse-grained green gangue minerals were present in the froth, indicating partial entrainment during flotation.
Tail: The examination of the basic flotation residue (sinking fraction) showed that while partially flocculated fine particles were present, the tailings predominantly consisted of coarse-grained gangue minerals (green and rutile + ilmenite). These results indicated limited selectivity under basic flotation conditions, likely due to incomplete flocculation and insufficient liberation.
3.4.2. Test 2
Flotation concentrate: The flotation concentrate predominantly consisted of rutile liberated from the flocculated phase, with minimal inclusion of coarse green gangue minerals.
Tail: Flocculant formation was not observed in the basic flotation residue (sinking). In general, coarse-grained, green and white gangue minerals and coarse-grained biotite, and some rutile, were collected in this product without floating. This was because most of the rutile remained between the micro-agglomerates and passed into the floating product. According to the results of this experiment, using selective suppressants during agglomerate formation is recommended to enhance the efficiency of the enrichment process by minimizing the unintended inclusion of gangue minerals in the concrete.
3.4.3. Test 3
Flotation concentrate: Following flocculation, gangue minerals floated with the foam and moved to the floating section. However, rutile particles also escaped into the product due to the effect of Aero801 and Aero825. Meanwhile, fine-grained white flocculated gangue minerals were generally dominant.
Tail: In the sinking section, we observed the flocculation-induced formation of gangue mineral chain bonds. The presence of a concentration consisting of rutile, garnet, and ilmenite was also observed.
3.4.4. Test 4
Flotation concentrate: In the flotation stage, the flocculated product contained fine gangue minerals and partially coarse gangue minerals.
Tail: Heavy minerals, including coarse gangue minerals and rutile, remained in the residue.
Both fine and partially coarse gangue minerals were present in the concentrate, indicating incomplete separation. The presence of heavy minerals like rutile in the tailings suggests potential recovery losses and insufficient collector performance.
3.4.5. Test 5
Flotation concentrate: During the flotation phase of this experiment, the hydrocarbon radical root of the collector combined with the hydrocarbon root of the pomace, and a paste-like product was obtained by floating in the foam phase, taking in small amounts of flocculated particles. This phenomenon is entirely due to the use of excessive reagent (TA) and pomace instead of flocculated grains.
Tail: Although there were large grains in the sinking product due to excessive use of reagents, very rapid agglomeration occurred (including large grains). This phenomenon disrupted selectivity. In the flocculation stage, as written in the test process, TA 600 g/t was applied instead of 200 g/t and pomace oil 20 g/t, while in the flotation stage, TA 100 g/t and pomace oil 10 g/t (pH: 7) was recommended as a new test condition.
3.4.6. Test 6
Flotation concentrate: Microscope examination of the flotation froth revealed that there was selective clustering in the flotation froth (floating product) predominantly consisting of black heavy minerals. This indicated that a selective separation of valuable minerals from their gangues was successfully achieved.
Tail: The tailing contained non-flocculated, coarse-grained, white and green gangue minerals associated with some rutile particles.
3.4.7. Test 7
Flotation concentrate: The concentrate contained finely flocculated gangue minerals, traces of pyrite, and small amounts of rutile grains, which were recovered via froth flotation. Additionally, some coarse-grained rutile and pyrite were observed in this phase.
Tail: The materials that remained during the flotation stage consisted mainly of coarse-grained, white and green gangue minerals, as well as rutile grains. The rutile present in the tailing was considered a pre-concentrate, which could be removed with gangue minerals, under these conditions, and particularly when present in fine grains. These results suggest that mechanical entrainment and particle size distribution also played an important role in recovery efficiency.
Overall, these results underscore the critical role of pH control, reagent selection, and reagents dosage optimization in achieving efficient rutile recovery through flotation and flocculation. Tests 2 and 6 demonstrated the best performance, pointing toward the effectiveness of reagent under moderate pH conditions. Conversely, Tests 1, 3, and 5 revealed common issues such as gangue entrainment, poor selectivity, or over-flocculation. Future work should focus on refining reagent interactions, particularly through the use of selective depressants and controlled flocculation strategies, to further improve concentrate quality and rutile recovery.
4. Discussion
According to the experimental results, Aero801 (SIPX) and Aero825 (Dithiophosphate) were used as collectors and Aerofroth88 (MIBC) as a frother in the flotation stage wherein Aero801 and pomace oil were used as flocculants: in Tests 1 (pH: 2.5), 2 (pH: 8), 3 (pH: 11), and 4 (pH: 12). Among these tests, the highest concentrated TiO
2 grade obtained was 8.99% TiO
2, with a recovery of 89.51% at pH: 8 (Test 2).
Figure 4 shows the enrichment comparison results for Tests 1–4.
In Tests 5 (pH: 7), 6 (pH: 10), and 7 (pH: 2.5), TA and olive pomace oil were used as flocculants during the flocculation stage, and again in the experimental series where Aerofroth88 served as a frother in combination with cationic and oil-based collectors. Under neutral pH conditions (pH: 7), the process yielded a concentrate with a 47% weight recovery, 7% TiO
2 grade, and 72% TiO
2 recovery. A comparative summary of the enrichment results for Tests 5–7 is presented in
Figure 5.
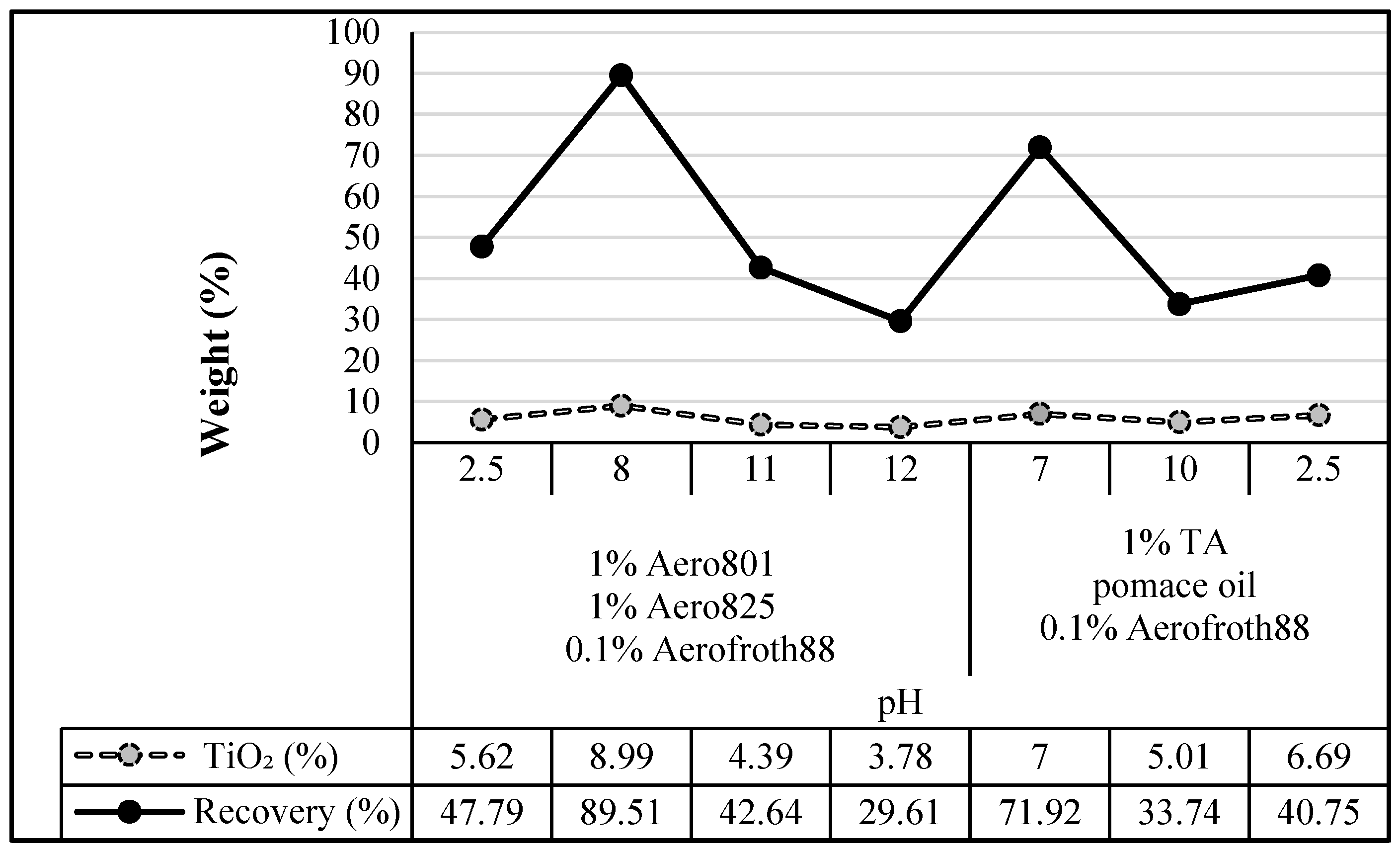
Figure 6 illustrates the variation in TiO
2 recovery across different pH levels and reagent combinations during the flotation stage. The graph clearly shows that the highest TiO
2 recovery, at approximately 89.5%, was achieved under pH: 8, corresponding to the conditions of Test 2, where Aero801 and pomace oil were used in the flocculation stage, followed by Aero801, Aero825, and Aerofroth88 in the flotation stage. A secondary peak in recovery was observed around neutral pH (Test 5), reaching about 72%, under conditions utilizing TA and pomace oil. The overall trend demonstrates that pH significantly influences recovery efficiency, with both strongly acidic and highly alkaline conditions resulting in lower TiO
2 recovery values. This highlights the importance of optimizing the chemical environment and reagent selection to maximize flotation performance, particularly for fine-grained rutile ores.
Across the wide pH spectrum experiment in which Aerofroth88+pomace oil was used as a flocculant, Aero801 and Aero825 were used as collectors, and Aerofroth88 was used as a frother, it was determined that the most optimum concentrate results were obtained with TiO
2 grades of 7.3% and 8.99%, and approximately 79% and 90% TiO
2 recovery at moderate pH levels (pH: 7 and pH: 8).
Figure 6 shows the recovery graph of concentrated TiO
2, which was the enrichment flotation product. In experiments where TA and pomace oil were used as alternative flocculants depending on pH in the flocculation stage, Aero801 and Aero825 were used as collectors, and Aerofroth88 was used as a frother in the flotation stage, the optimum values were obtained at pH: 7 with a 43%–47% weight ratio, 7.00%–7.76% TiO
2 grade, and 71.92%–77.97% TiO
2 recovery concentrates.
While the current results are based on single experimental runs for each condition, the comparative framework provides valuable insight into the process response under a range of parameters. Future studies will include repeat experiments, error analysis, and statistical evaluation to validate and refine the observed trends. This study contributes to a relatively underexplored area in rutile beneficiation, particularly for fine-grained ores, and lays the groundwork for more detailed mechanistic and process optimization research. Future studies should investigate several processes, such as multi-stage cleaning flotation, acid leaching [
31], roasting, or a combination of these to achieve industrial grade concentrate purity (>90%).
5. Conclusions
In this study, it was proven in the flotation enrichment stage that the best flocculant type was provided using Aero801+pomace oil at pH: 8 with the same collector and frother conditions and a concentrate gain of approximately 56% weight ratio, 8.99% TiO2 grade, and 89.5% recovery. As a result, the most efficient and highest-grade rutile sample was increased from 5.10% to 8.99% TiO2 under Test 2 experimental conditions. In the flocculation stage, it was determined that Aero801+pomace oil was used consecutively with Aero801 (SIPX), Aero825, and Aerofroth88 in the flotation stage, and the highest TiO2 grade and TiO2 recovery was again provided at pH: 8 (55.8% weight ratio, 8.99% grade, 89.5% recovery). In this study, successful separation was achieved according to the enrichment results applied depending on the pH and the type of reagent used in both flocculation and flotation stages. Further research should focus on optimizing these downstream processing steps and evaluating their impact on overall recovery, efficiency, and final product quality.