1. Introduction
The six platinum group metals (PGMs), ruthenium (Ru), rhodium (Rh), palladium (Pd), osmium (Os), iridium (Ir), and platinum (Pt), together with gold (Au) and silver (Ag), are considered “precious metals”. The stable nature and attractive metallic luster of these elements make them well known as raw materials for jewelry [
1]. In addition, they are valuable for their resistance to corrosion and oxidation, high melting point, electrical conductivity, and catalytic activity, and these elements also have a wide range of industrial applications [
2]. These applications include industrial consumables [
3], electronics [
4,
5], and biomedical components [
6]. Most notably, PGMs are widely used as catalysts in a variety catalytic chemical processes [
7], especially in catalytic converters, which are used to reduce hazardous and polluting emissions from vehicle exhaust systems [
8].
According to the 2024 Johnson Matthey PGM market reports [
9], the total supply of PGMs as of 2024 is 569.6 t, which includes 399.3 t of primary supply. Hence, the production of PGMs is dominated by the ore industry. The primary PGM-rich deposits include the Bushveld Complex in South Africa, the Great Dyke in Zimbabwe, the Stillwater deposit in the USA, and the Lac des Isles deposit in Canada [
10]. The platinum-group elements have complex mineralogies; they do not occur as independent natural ores but are mostly found in association with tellurides, arsenides, antimonides, sulfides, and various complicated mineral phases [
11]. Generally, PGM ores are grouped into three primary classes: PGM-dominant ores, Ni-Cu-dominant ores, and miscellaneous ores [
12].
PGM concentrates (~100–400 g/t) are generally recovered by the traditional matte-smelting refining technique to remove gangue material and produce an iron-nickel-copper-PGM matte. Slag formation may be facilitated by the addition of flux(es). The flotation concentrates with various fluxes are added to the electrical furnace. Typically, furnaces are operated at around 1350 °C. Special cases, such as smelting UG2 concentrates, which are extracted from the UG2 chromitite layer in South Africa, may require temperatures up to 1600 °C due to their high Cr
2O
3 content [
13]. The fluxes include those of limestone, silicates, carbonaceous reductants, and sulfides to ensure that fluid slag is produced at the desired temperature. If ores with low sulfide contents are processed, additional base metal concentrates need to be added to provide sufficient matte to allow for effective coalescence of the droplets and collection of valuable metals. Lead collection is the most well-known traditional smelting process [
14]. Lead oxide is utilized in conjunction with carbon as a reducing agent to produce the collector metal. However, health and safety issues restrict this process. Iron oxide serves as the most commonly employed collector. Under smelting conditions, a majority of the iron is transferred to the slag as FeO, while some iron oxide is reduced to metal, leading to the formation of a densely dispersed iron-nickel-copper-cobalt matte phase that interacts with PGMs and gold [
15].
Mintek has introduced an innovative method for processing nickel-copper and platinum group metal (PGM) sulfide concentrates, referred to as the ConRoast process [
16]. This technique involves roasting the sulfide concentrates to eliminate sulfur, followed by smelting the resultant dead-roasted concentrate in a DC arc furnace, using an iron-based alloy as a collector. This process notably provides significant flexibility in ore type selection, imposes no minimum quantity restrictions on base metals or sulfur, and can handle very high levels of chromite in the concentrate [
17]. The environmental advantages regarding sulfur emissions are substantial, as nearly all sulfur is extracted from enclosed roasting systems in a continuous stream of SO
2, which is suitable for feeding into a sulfuric acid plant [
18].
Another method does not limit the amount of base metal and sulfur in the concentrate. In their study, low-grade concentrates from Lonmin’s Rowland and Eastern operations were smelted with SiC as a reductant [
19]. The addition of SiC causes FeO to be reduced to iron, increasing the degree of metal fall and efficiently collecting PGMs. The Cr
2O
3 is reduced to CrO, which is highly miscible in the slag phase. To decrease the viscosity of the slag, Peirce–Smith converter slag from Lonmin was used as an additive to increase the FeO/SiO
2 ratio. The shortcomings of this process are the extra emission of CO
2 and high smelting temperature (>1600 °C).
In recent years, research on the pyrometallurgical recovery of PGMs’ (platinum group metals’) ore has been relatively rare. A major focus of research has been on optimizing the slag composition, particularly factors such as the melting point, viscosity, surface tension, and other slag physicochemical properties. Additionally, studies have explored how these properties impact smelting temperatures, the settling behavior of matte and alloy droplets, and the distribution of precious metals [
15,
20]. In conclusion, the slag primarily consists of CaO, FeO, SiO
2, and MgO. Changes in its composition can significantly alter various physical properties of the molten slag. For example:
An increase in MgO leads to higher electrical conductivity, melting temperature, and viscosity.
An increase in basicity (CaO/SiO2 ratio) reduces viscosity and electrical conductivity but raises the melting temperature.
An increase in FeO lowers the melting temperature but increases viscosity.
The melting temperature and electrical conductivity directly influence the operating temperature of the smelting process, which further affects the viscosity and surface tension of slag. These properties, in turn, impact the coalescence and settling efficiency of the matte prills. Due to these complex interactions, optimizing the slag composition requires balancing multiple factors. The ideal slag should have a low melting temperature, low viscosity, low surface tension and high electrical conductivity (to optimize heat generation in electric arc furnaces). Achieving this balance ensures high platinum group metal (PGM) recovery rates and low energy consumption during the smelting process.
After smelting, the furnace matte is further processed by converting. The air is blown into the molten matte to oxidize the iron and sulfur. Fluxing agents (mainly silica sand) are added to form an iron-rich slag that is skimmed off and returned to the smelting process. The converse matte undergoes hydrometallurgical treatment whereby the base metals dissolve, leaving an enriched PGM residue for refining the precious metals [
13,
21].
In PGM processes, hydrometallurgy is generally considered ineffective for the direct extraction of PGMs from concentrates due to the significant volume of solvent required in relation to the typically low quantities of PGMs involved [
22]. However, for some PGM ores with high chromite contents (>2.8%), high talc contents, and low sulfur contents, which form highly refractory chromium-bearing spinel layers during the smelting process, the smelting difficulties and high processing costs per unit of PGM are difficulties [
23]. A low sulfur content also reduces the number of droplets available for the capture of PGMs, which require more trapping agents. The decline in the quality of PGMs has resulted in the search for alternative hydrometallurgical treatments.
Hydrometallurgical treatments are generally divided into precious metal-rich solution processes and precious metal-rich residue processes. The different PGMs and/or gold are leached into the solution by single-step leaching or remain concentrated in the insoluble leach residue [
24]. The precious metal-rich solution industrial processes include chloride-based atmospheric leaching processes (Intec copper process) [
25], sulfate-based pressure leaching processes (Platsol
@ process) [
26], thiourea leaching processes, and halide system (bromide, chloride, and iodide) leaching processes. These methods require extreme reaction conditions to ensure that the base metals and precious metals are codissolved in a single step. For example, the Platsol
@ process is a high-temperature (>200 °C) pressure oxidation (~700 kPa O
2, 3200 kPa total pressure) sulfate-based process with the addition of 5–20 g/L NaCl to promote PGM mineral dissolution [
22]. The precious metal-rich residue processes include cyanide-based atmospheric/pressure leaching [
27,
28], chloride-based processes (Kell process [
29], Outokumpu HydroCopper
® process) [
30], sulfate-based leaching processes (ActivOx
® processes [
31], Albion process [
32]), and the Galvanox™ process (galvanically assisted atmospheric leaching in a ferric/ferrous sulfate medium) [
33].
However, the leaching of PGM from ore also faces many challenges. These include the passivation of sulfide minerals (chalcopyrite, covellite and millerite) in oxidative leaching processes [
34] and the presence of refractory PGM-sulfide/oxide species [
23]. These mineralogical compositions cause poor direct leachability. The pyro-hydrometallurgy procedure has been applied to promote the dissolution of sulfide minerals and PGMs. For example, the Stillwater flotation concentrate was roasted at 1050 °C to convert base metal sulphides into oxides and PGM sulphides into metals [
35].
The Jinbaoshan platinum-palladium deposit is the largest independent PGMs deposit found in China. There are eleven kinds of useful chemical components in the ore, including eight types of precious metals (Pt, Pd, Rh, Ru, Ir, Os, Au, and Ag) and valuable metals (Cu, Ni, and Co) [
36]. The content of Pt and Pd in the Jinbaoshan Pt-Pd deposit is 42 t, and the average grade is 1.77 g/t [
37]. The main gangue components of the ore are SiO
2, MgO, Fe
2O
3, FeO, CaO, and S [
38]. The nature of the ore results in many problems with mineral separation and postprocessing: The ore contains a variety of minerals with a complicated symbiotic relationship, the copper and nickel minerals are lean, and useful minerals, especially platinum and palladium minerals, in the microgranular material are disseminated to other minerals [
39]. Thus, the Jinbaoshan Pt-Pd deposit has not been utilized until now. Although pyrometallurgical smelting is the most mature process for handling platinum-palladium-containing ores, the high MgO content in the Jinbaoshan platinum-palladium deposit necessitates either high smelting temperatures or stringent flotation requirements [
40]. As a result, current research on the utilization of Jinbaoshan Pt-Pd deposits has focused primarily on hydrometallurgical leaching. After flotation, the concentrate was subjected to methods such as acidic pressure leaching [
36] and alkaline leaching [
40]. However, these methods often require extreme leaching conditions, consume large amounts of reagents, and produce significant quantities of wastewater.
In this study, a smelting process is proposed to treat high-MgO-content and low-precious-metal-containing concentrates obtained from flotation of the Jinbaoshan Pt-Pd deposit. The slag composition was designed to ensure a lower smelting temperature and sufficient collector, which allows for efficient separation of precious metals from gangue components in the concentrate. Moreover, this approach also achieves high extraction efficiency and high enrichment ratios for precious metals.
3. Results and Discussion
3.1. Slag Design and Phase Transformation
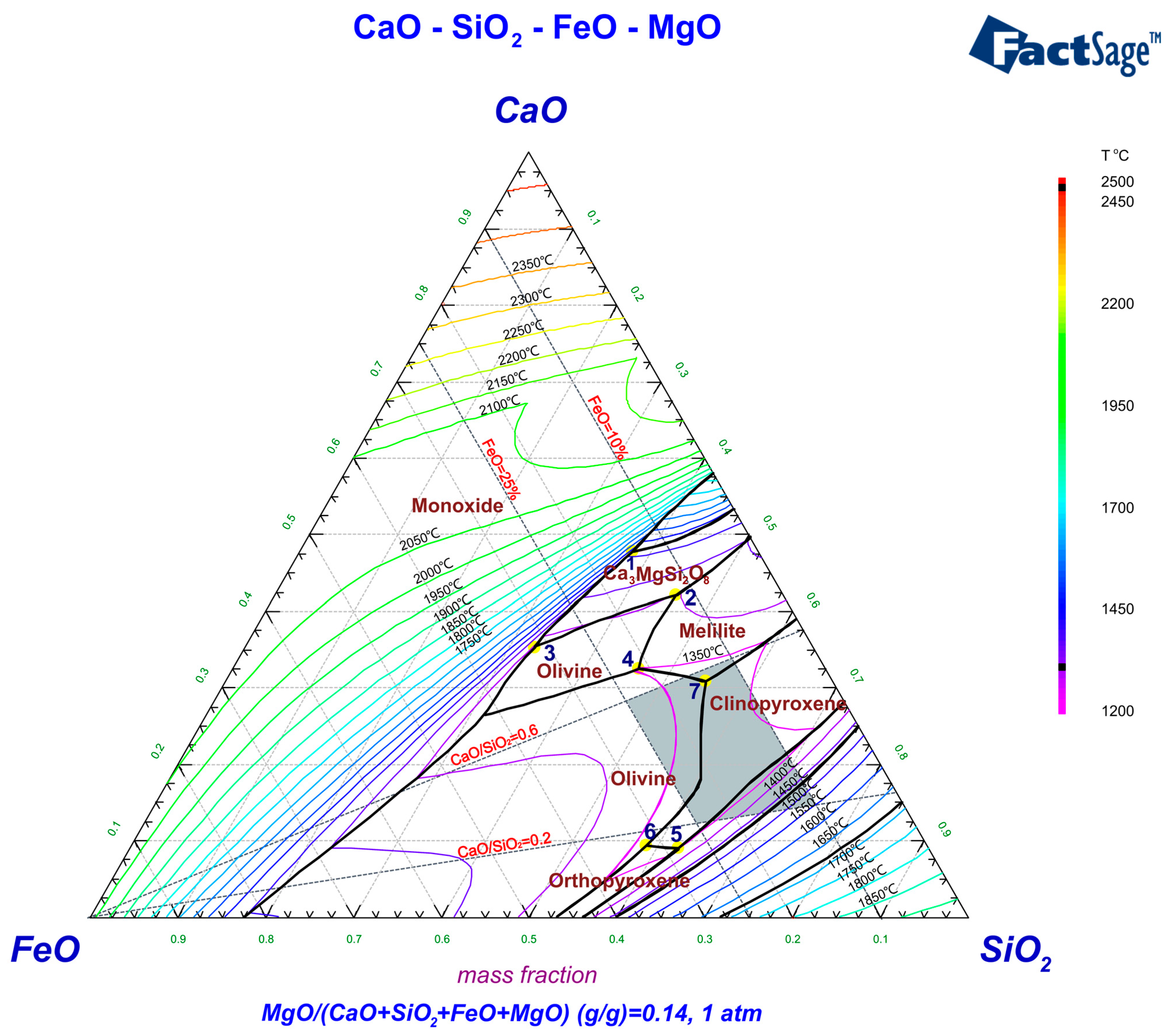
The melting point of slag typically denotes the temperature at which it transitions from a solid phase to a homogeneous liquid state, which can be approximately assessed by the liquidus temperature on the phase diagram. Based on composition analysis, the PGM concentrate is primarily comprised of SiO
2, MgO, and FeS
2. An increased concentration of MgO in the concentrate indicates a higher melting point, necessitating the use of fluxing agents to reduce this melting point. To avoid the introduction of new elements, SiO
2 and CaO found in the concentrate are selected as fluxing agents, along with the addition of FeO. FeO functions not only as a flux to modify the melting point of the slag but is also partially transformed to metallic iron or iron sulfide, which acts as a collector for precious metals. Therefore, the slag composition is established as CaO-SiO
2-FeO-MgO. Given that the MgO content in the slag can range from 12% to 16% depending on the experimental conditions, a MgO content of 14% is designated as representative of the discussion of the low melting point range of the slag, as illustrated in
Figure 2. The minimum melting point for this system is 1311 °C at eutectic point 7. To ensure a lower melting temperature and conserve energy, a smelting temperature of 1350 °C was selected. At this temperature, the slag composition within the 1350 °C liquidus range exhibited better melting performance. Therefore, the range of smelting experimental conditions can be determined as follows: CaO/SiO
2 from 0.2 to 0.6 and FeO content from 15% to 25%. This slag composition range is represented in the gray area on the phase diagram. The slag composition basically fits the liquidus at 1350 °C, and the slag is composed of clinopyroxene (MgCaSi
2O
6, FeCaSi
2O
6), melilite (Ca
2Mg (Si
2O
7)), and olivine (Fe
2SiO
4).
A preliminary smelting experiment was conducted by mixing 100 g of concentrate with 18 g of CaO, 22 g of SiO
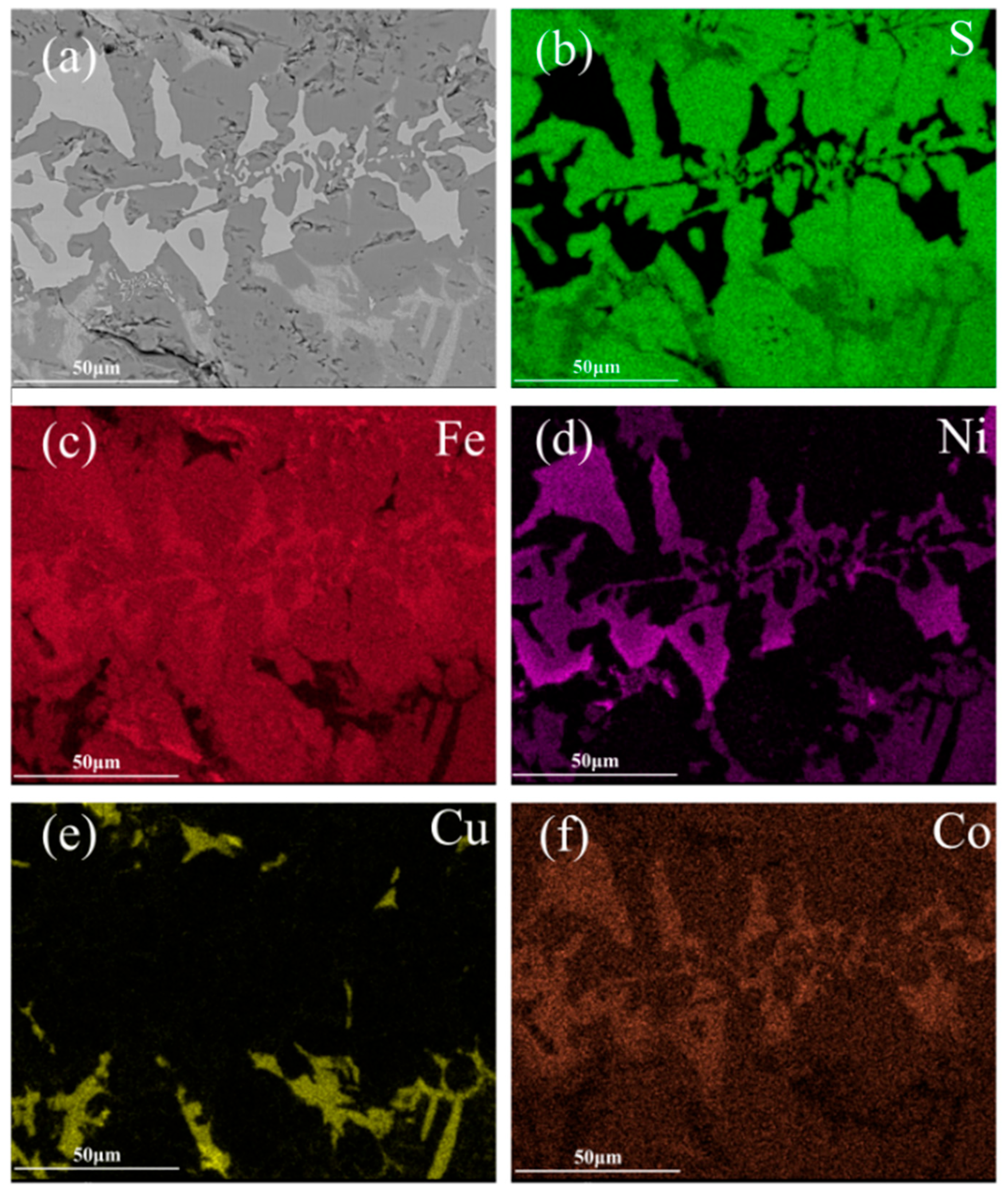
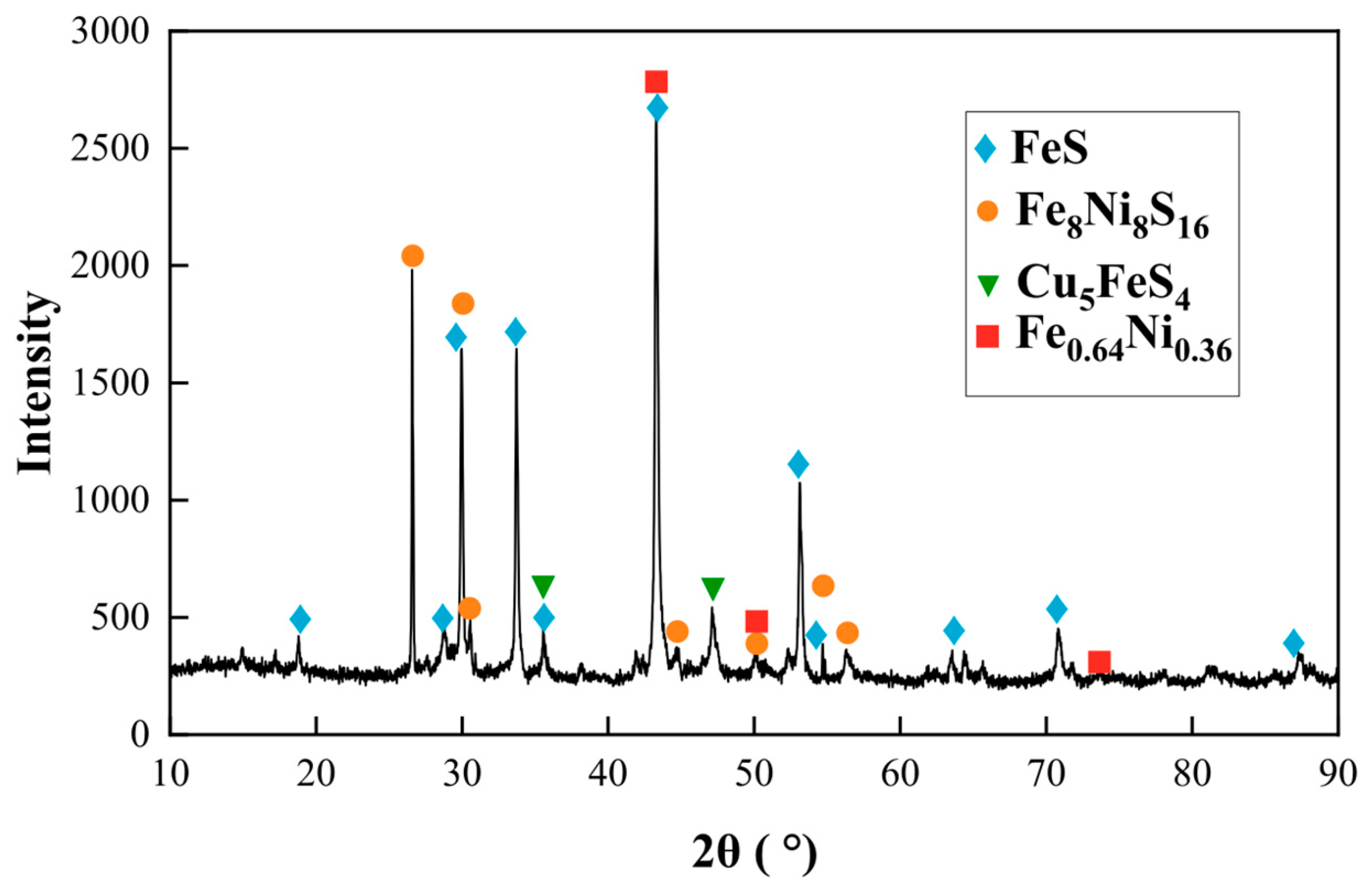
2, and 30 g of iron ore. The mixture was smelted at 1350 °C for 2 h. Microstructural morphology and phase identification of the matte sample were performed utilizing SEM-EDS and XRD techniques, respectively. The results are shown in
Figure 3 and
Figure 4, respectively.
On the basis of the XRD patterns, the phases identified in the iron matte include FeS, Fe
8Ni
8S
16, Cu
5FeS
4, and FeNi alloys. These results combined with the mapping micrographs suggest that the CuS and FeNiCo alloys were also present in the iron matte. The Fe, Cu, Ni, and Co contents in slag were 13.45%, 0.05%, 0.01%, and <0.01%, respectively. The distribution analysis revealed that Fe partitioned between the smelting slag and matte phases, while Cu, Ni, and Co predominantly reported to the matte phase. Further, FeS
2 from the concentrate and Fe
3O
4 from the iron ore react to form FeS and Fe, which enter the low-grade matte, whereas FeO enters the slag phase. From this, the main reactions are determined to be as follows:
During the smelting process, FeS
2 is first oxidized to FeS and SO
2 [
43]. Then, FeS reacts with Fe
3O
4 to produce FeO and Fe [
43]. Some of the FeO reacts with SiO
2 and enters the slag phase [
15], whereas the remaining FeO reacts with FeS to generate Fe and SO
2 [
44]. The Fe, FeS, CuS, and NiS droplets collect PGMs and increase in size by coalescing with other prills. They then settle out of the slag under the influence of gravity at a rate dependent on the viscosity of the slag [
15]. Ultimately, an iron matte enriched with PGMs is formed at the bottom of the crucible.
3.2. Effects of the MgO Content on PGM Recovery
According to previous studies, the MgO content significantly increases the liquidus temperature [
15,
20]. To clarify the effect of the MgO concentrate, a range of 12%–16% was selected by controlling the additional amount of CaO and SiO
2. In each experiment, 100 g of the Pt-Pd concentrate and 20 g of iron mine were mixed with CaO and SiO
2, the total masses of which were 30 g, 35 g, 40 g, 45 g, and 50 g, respectively, and the CaO/SiO
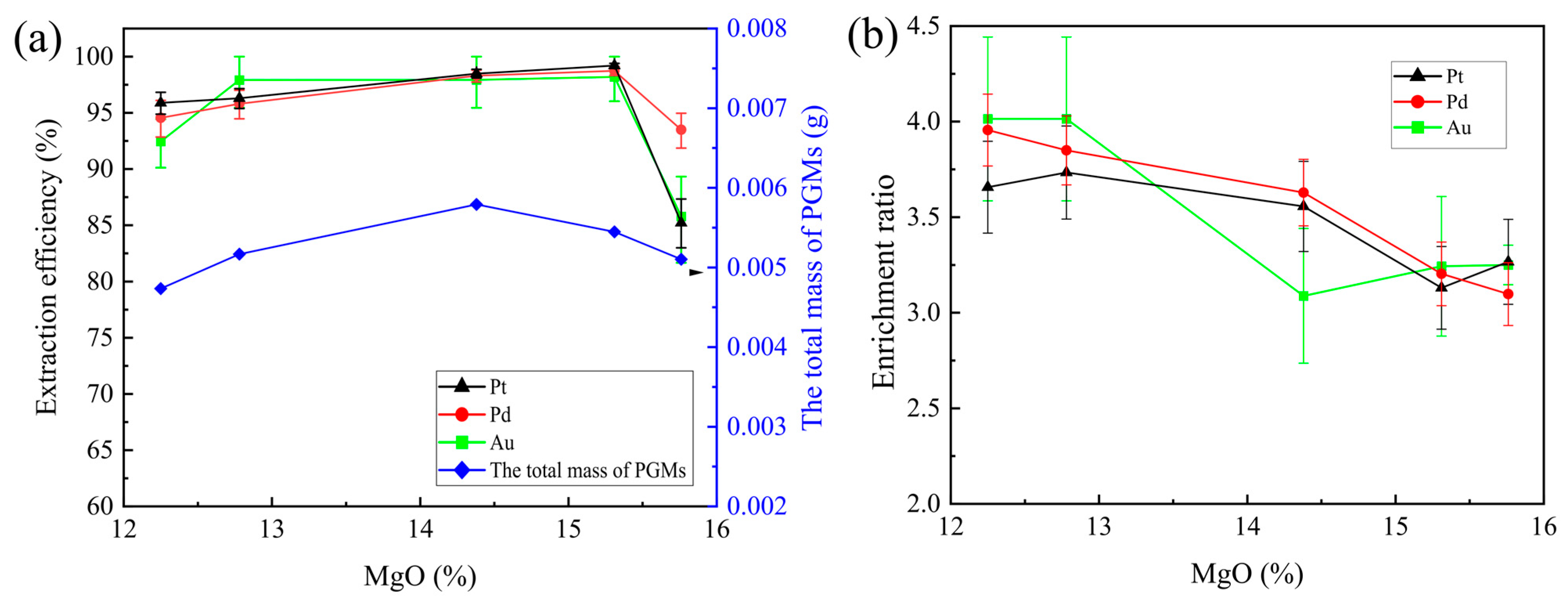
2 ratio was fixed at 0.2. The smelting experiments were conducted at 1350 °C for 2 h. The effects of MgO on the extraction efficiency of Au, Pt, and Pd and the total mass of PGMs in the matte are shown in
Figure 5a. The enrichment ratios of Au, Pt, and Pd are shown in
Figure 5b.
The extraction efficiency of Pt, Pd, and Au and the total mass of the PGMs increased as the MgO% in the slag increased. When the MgO% reached 14%, the extraction of Pt, Pd, and Au continued to increase, but the total mass of the PGMs decreased. When the MgO% reached 15%, the extraction of Pt, Pd, and Au and the total mass of the PGMs decreased. The enrichment ratio of Pt, Pd, and Au decreased as the MgO% increased.
The quantity of slag and matte, the composition of the slag, and the PGM content in the slag are shown in
Table 3.
The ternary slag compositions were basically unchanged when the MgO% changed. However, the quality of the slag and matte decreased and increased, respectively, when the MgO% increased. The decrease in slag mass contributed to the change in the flux (CaO + SiO2) dose. As the amount of silica decreased, the amount of FeO entering the slag phase decreased according to Reaction 6, whereas the amount of Fe increased, resulting in a greater mass of the matte. This increase in collector mass allowed more iron matte droplets to capture precious metals during the smelting process.
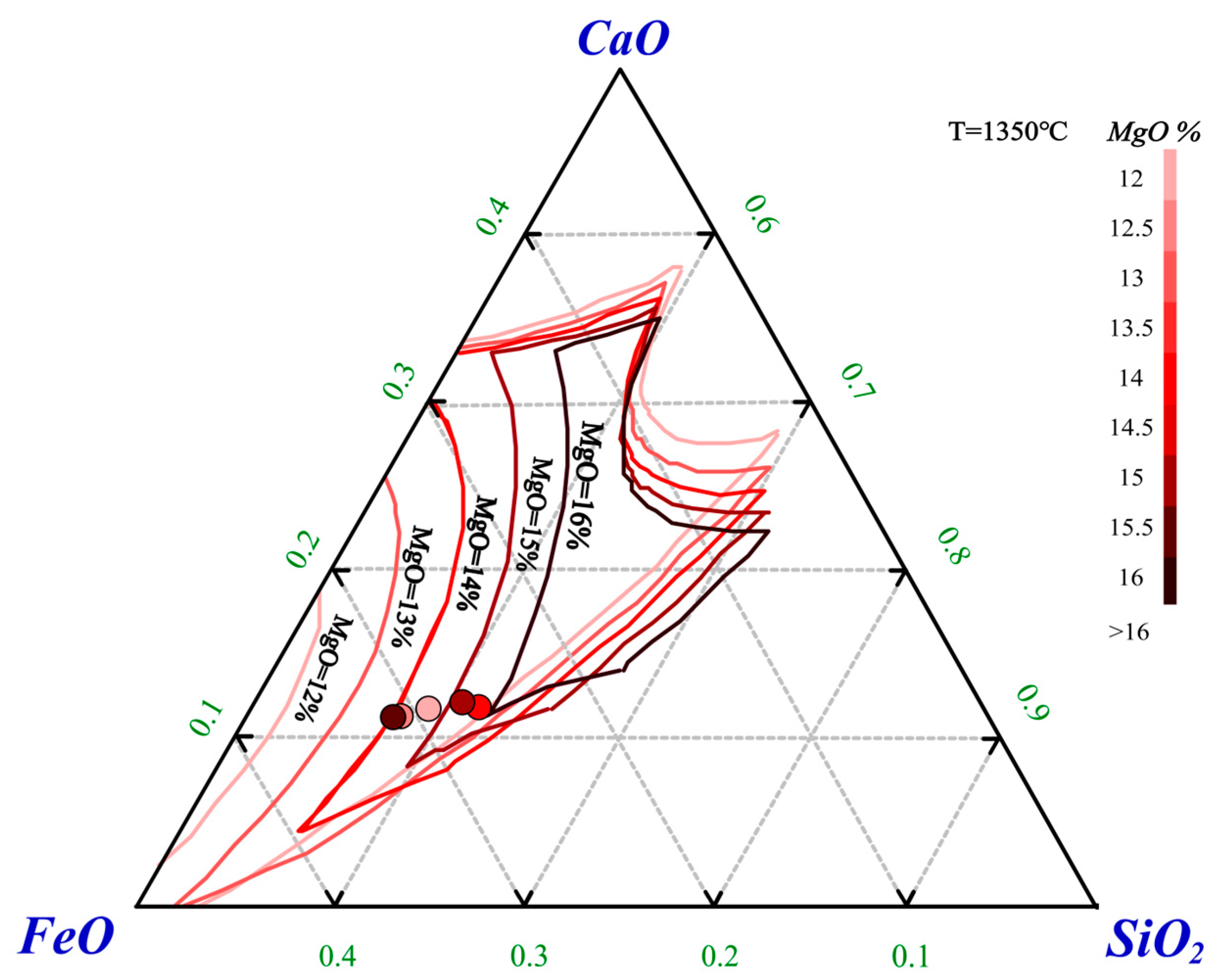
Ternary phase diagrams for CaO-FeO-SiO
2 with MgO contents of 12%, 13%, 14%, 15%, and 16% were plotted using FactSage. The liquidus at 1350 °C for different MgO contents was overlaid on a single-phase diagram, as shown in
Figure 6. The experimental data were plotted according to the ternary composition, with the MgO content was distinguished by color.
As the MgO content varies, the range of the 1350 °C liquidus becomes narrower, indicating that an increase in the MgO content significantly increases the melting point of the slag. When the MgO content is 15.31%, the ternary composition aligns precisely with the 1350 °C liquidus for 15% MgO, suggesting that the slag’s melting point is about 1350 °C. Conversely, when the MgO content reaches 15.76%, the ternary composition exceeds the 1350 °C liquidus for 16% MgO, suggesting that the melting point of the slag is above 1350 °C, which inhibits the complete melting of the experimental sample. Consequently, with an increase in MgO content, the volume of slag is reduced while the amount of matte increases, resulting in a higher number of matte droplets capable of capturing PGMs, thereby enhancing the collection rate of these metals. When the MgO content increased to 15.76%, the slag melting point surpassed the reaction temperature of 1350 °C, resulting in incomplete melting and a reduced collection rate for precious metals. However, high-matte collection dilutes the PGM grade of the alloy, which affects the PGM recoveries in the subsequent refining processes. Considering both the extraction efficiency and the enrichment factor, the optimal MgO content was determined to be 14.38%, with a flux addition of 40 g.
3.3. Effects of Iron Ore Consumption on PGM Recovery
To clarify the effect of the iron or iron additive amount, in each experiment, 100 g of the Pt-Pd concentrate, 32.5 g of SiO
2 and 7.5 g of CaO were mixed with iron ore, the quantities of which were 20 g, 25 g, 30 g, 35 g, 40 g, and 45 g, respectively. The smelting experiments were conducted at 1350 °C for 2 h. The effects of the amount of iron or additives on the extraction efficiency of Au, Pt, and Pd and the total mass of PGMs in the matte are shown in
Figure 7a. The enrichment ratios of Au, Pt, and Pd are shown in
Figure 7b.
With increasing amounts of iron ore, the enrichment efficiency of Pt, Pd, and Rh, as well as the total mass of the precious metals, tended to increase, peaked when the iron oxide amount was 30 g, and then slightly declined. The enrichment factors of Pt and Pd showed an overall decreasing trend with increasing FeO content, while Au exhibited significant fluctuation.
Table 4 presents data on various parameters associated with different amounts of iron ore added, including the masses of slag and matte, the slag ternary composition, the MgO content, and the PGM content in the slag. The table indicates that while the ternary composition of the smelting slag remained relatively stable with increasing iron ore content, both the masses of the smelting slag and matte exhibited an increase. However, when the iron ore quantity increased from 20 g to 25 g, the mass of low-basicity slag did not show a significant rise, whereas the mass of smelting slag increased substantially. This suggests that the iron ore predominantly enters the smelting slag phase. Under these circumstances, the enrichment rates of Pt, Pd, and Au were also lower, indicating that Reaction 2 occurs preferentially during the smelting process, resulting in the formation of slag from most of the FeO without generating sufficient matte droplets to capture the precious metals.
When the iron ore amount reached 30 g, a noticeable increase in the mass of matte was observed, indicating that the added iron ore primarily entered the matte phase in accordance with Reaction 5. Furthermore, the enrichment rates of precious metals improved significantly. An additional increase in iron ore led to a smaller rise in alloy mass and a larger increase in smelting slag mass, indicating that the majority of the iron oxide was incorporated into the slag phase. The rise in slag volume resulted in a reduction in MgO content and a lower melting point, but also increased the slag output, leading to greater energy consumption required to maintain the slag temperature during smelting. Considering these factors, 30 g was identified as the optimal amount of iron ore to be added.
3.4. Effect of the CaO/SiO2 Ratio on PGM Recovery
Basicity is one of the most influential and important factors because of its significant effects on the physical and chemical properties of slag. To clarify the effect of the basicity, a range of 0.2–0.6 was selected by controlling the added amount of CaO and SiO
2. In each experiment, 100 g of the Pt-Pd concentrate and 20 g of iron ore were mixed with CaO and SiO
2, and the total mass was held constant at 40 g. The smelting experiments were conducted at 1350 °C for 2 h. The effects of basicity on the extraction efficiency of Au, Pt, and Pd and the total mass of the PGMs in the matte are shown in
Figure 8a. The enrichment ratios of Au, Pt, and Pd are shown in
Figure 8b.
The extraction efficiency of Au, Pt, and Pd decreased as the basicity increased from 0.2 to 0.5 and reached a minimum at a basicity of 0.5. When the basicity increased from 0.5 to 0.6, the extraction efficiency increased. The total mass of the precious metals and enrichment factors of Au, Pt, and Pd exhibited the same trend.
Table 5 shows the mass of the slag and matte, the composition of the slag, and the PGM content in the slag. The mass of the smelting slag and matte exhibited opposite trends: The slag mass decreased while the matte mass increased. According to Reaction 6, the amount of FeO in the smelting slag decreased, whereas the remaining FeO, as described in Reaction 7, was reduced to metallic iron and entered the matte phase. This results in a reduction in the mass of the smelting slag and an increase in the mass of the matte.
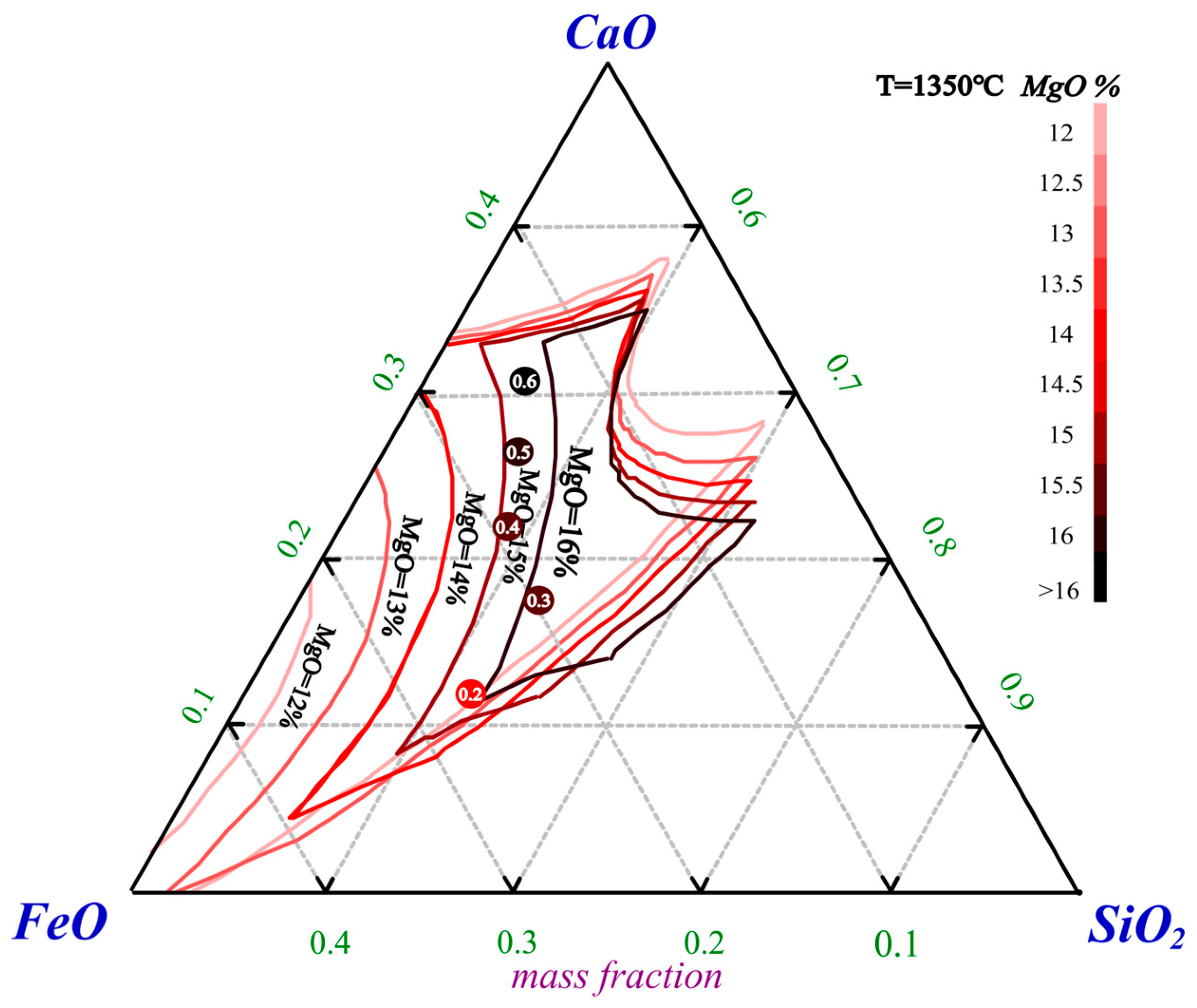
The ternary compositions with different basicities were plotted with the liquidus at 1350 °C for different MgO contents on a CaO-SiO
2-FeO phase diagram, which is shown in
Figure 9 [
41]. Each experimental point is labeled with its corresponding basicity, and the MgO content is distinguished by color.
Figure 9 shows that changes in basicity caused significant alterations in the slag phase composition and MgO content. As the basicity increased, the MgO content in the slag increased, leading to a reduction in the liquidus region, which represented an increase in the slag melting point and was detrimental to precious metal capture. However, the increase in basicity also resulted in a greater matte mass, which facilitated the formation of more droplets for capturing precious metals. Additionally, increased basicity disrupts the structure of the silicon-oxygen tetrahedron, reduces the viscosity of molten slag, and enhances its fluidity, thus improving the extraction efficiency of PGMs [
13].
In summary, when the basicity increased from 0.2 to 0.5, the meltability variation was dominant. The melting point of the slag increased, resulting in poorer meltability and a decrease in precious metal capture efficiency. However, when the basicity further increased to 0.6, the viscosity of the slag decreased significantly, leading to improved PGM extraction efficiency. The basicity is clearly a critical parameter in the smelting process, as it not only affects the viscosity of the slag but also leads to changes in the slag composition and variations in both the slag mass and matte mass. Considering the extraction effect, enrichment ratio, and residual amount of PGMs in the slag, a basicity of 0.2 was chosen as the optimal value for smelting.
3.5. Effects of Temperature on PGM Recovery
To investigate the effect of smelting temperature on PGM extraction efficiency, in each experiment, 100 g of the Pt-Pd concentrates, 32.5 g of SiO
2 and 7.5 g of CaO were mixed with 30 g of iron ore. The smelting experiments were conducted at 1300 °C, 1350 °C, 1400 °C, and 1450 °C for 2 h. The effects of temperature on the extraction efficiency of Au, Pt, and Pd and the total mass of PGMs in the matte are shown in
Figure 10a. The enrichment ratios of Au, Pt, and Pd are shown in
Figure 10b.
As the smelting temperature increased, the extraction efficiency of Pt, Pd, and Rh, as well as the total mass of PGMs in the matte, initially increased significantly. However, once the temperature reached 1350 °C, the changes became relatively stable. The enrichment factors of Au, Pt, and Pd exhibited an overall decreasing trend with increasing temperature.
Table 6 shows the masses of the smelting slag and iron matte at different temperatures, as well as the contents of the precious metals in the slag. At 1300 °C, the mass of matte recorded was only 8.05 g, indicating that the slag did not achieve sufficient melting at this temperature. The viscosity of the slag is influenced by both the temperature of the melt and its composition [
20]. The elevated viscosity resulted in the matte droplets remaining dispersed within the smelting slag instead of settling at the bottom of the crucible, which contributed to a higher PGM content in the slag. As the temperature rose, a slight increase in the mass of matte was observed, suggesting that elevated temperatures facilitate the reduction of FeO into slag. Taking into account the extraction efficiency and enrichment ratios of Pt, Pd, and Au, along with the precious metal content in the slag, 1350 °C yielded the most favorable outcomes. Given that higher smelting temperatures also result in increased energy consumption, 1350 °C was identified as the optimal smelting temperature.
3.6. Effect of Time on PGM Recovery
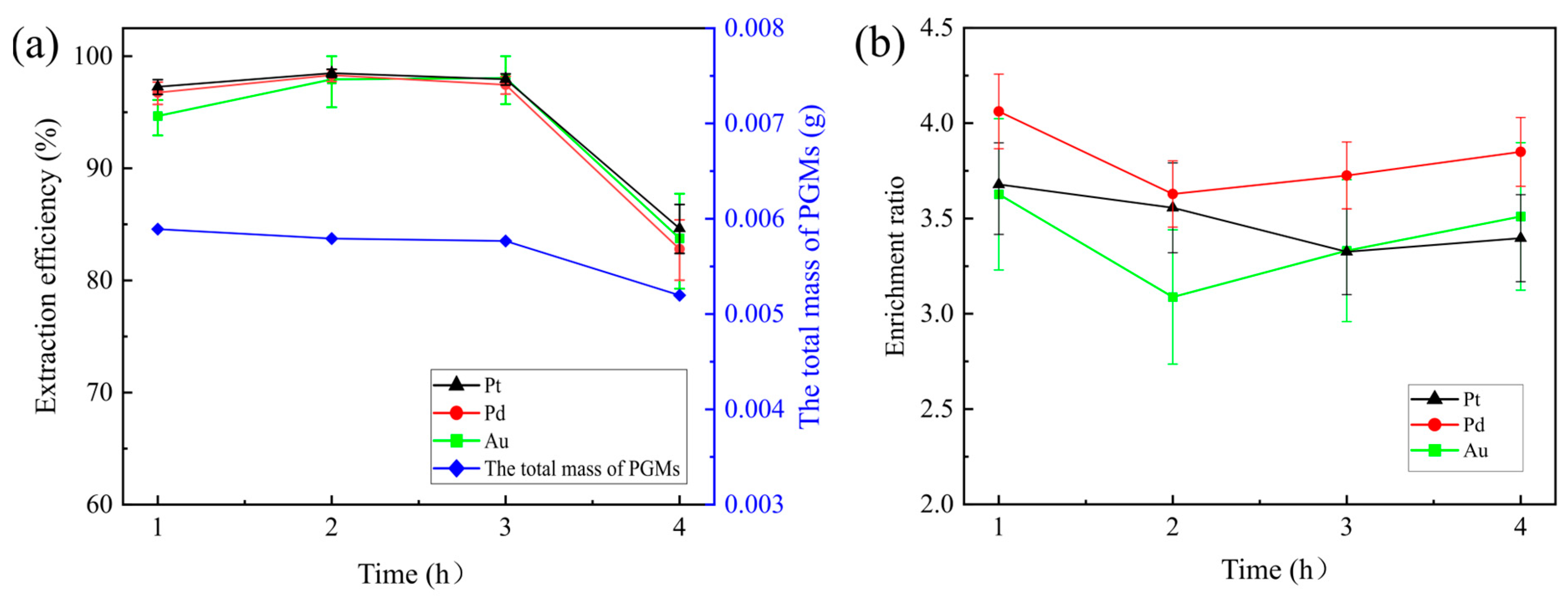
To investigate the effect of smelting time on PGM extraction efficiency, in each experiment, 100 g of the Pt-Pd concentrate, 32.5 g of SiO
2, and 7.5 g of CaO were mixed with 30 g of iron ore. The smelting experiments were conducted at 1350 °C for 1 h, 2 h, 3 h, or 4 h. The effects of temperature on the extraction efficiency of Au, Pt, and Pd and the total mass of the PGMs in the matte are shown in
Figure 11a, and the enrichment ratios of Pt, Pd, and Au are shown in
Figure 11b.
The figure indicates that as time increased, the enrichment rates of Pt, Pd, and Rh remained relatively constant. However, when the smelting time reached 4 h, there was a noticeable decrease in both the extraction efficiency of Pt, Pd, and Rh and the mass of PGMs in the matte. The enrichment factors of Au, Pt, and Pd exhibited an initial decrease followed by an increase with increasing time.
On the basis of the changes in the masses of the smelting slag and matte at different smelting times shown in
Table 7, the mass of the matte decreased after 4 h of smelting. Similar phenomena were reported in the literature, where extended smelting times led to the oxidation of FeS in the matte into the slag phase, resulting in increased PGM content in the slag [
45]. Considering the extraction efficiency and enrichment factors for Pt, Pd, and Rh, as well as the content of PGMs in the slag, a 1 h smelting time was chosen as the optimal time.
3.7. Comparative Analysis and Future Perspectives
The authors summarized the typical smelting parameters of PGM concentrates from South African companies in
Table 8 and compared them with the Jinbaoshan Pt-Pd concentrate (used in this study) in the last row. It can be observed that most concentrates require smelting temperatures above 1500 °C.
The Merensky concentrate processed by Impala shares similar MgO content and smelting temperature with the Jinbaoshan concentrate in this study. However, its SiO
2 (41%) and FeO (23%) levels are significantly higher than those of Jinbaoshan (SiO
2 25.97%, Fe 15.32%). Since both SiO
2 and FeO act as fluxing agents, this suggests that the Merensky concentrate inherently exhibits better meltability and would require lower flux additions compared to Jinbaoshan Pt-Pd concentrates. The furnaces in South Africa usually run with a slag-to-matte production ratio of between 3.5 and 9.0. In this paper, the mass ratio of slag to matte is 4.15. This indicates that the slag quality is appropriate, while the flux addition amount falls within a reasonable range. More flux costs money, calls for more energy to heat and melt, and higher slag temperatures cost more in terms of energy and refractory wear. However, in the smelting process, temperature is the dominant energy consumer. In the copper industry, the energy consumption required for heating to high temperatures (1300 °C) and maintaining thermal losses accounts for 60%–70% of the total energy consumption, while expanding production scale helps reduce energy consumption [
47]. As a result, the energy consumption of Jinbaoshan Pt-Pd concentrates is slightly higher than that of Merensky concentrate smelting but substantially lower than that of conventional smelting processes operating at 1500 °C.
From a solely pyrometallurgical perspective, this process is feasible for commercial use. However, due to the low grade of precious metals in the concentrates (75.53 g/t), the resulting matte also has a relatively low grade, posing challenges for subsequent matte processing. This remains an area for further research in the future.
This paper proposes a pyrometallurgical smelting approach for low-grade refractory precious metal concentrates. This method is applicable to difficult-to-process concentrates with high SiO
2 contents as well as elevated levels of Fe, Cu, and S—such as flotation concentrates from the Ghanaian gold province [
48,
49].