Element Mobility in a Metasomatic System with IOCG Mineralization Metamorphosed at Granulite Facies: The Bondy Gneiss Complex, Grenville Province, Canada
Abstract
1. Introduction
2. Geological Setting
2.1. The Grenville Province
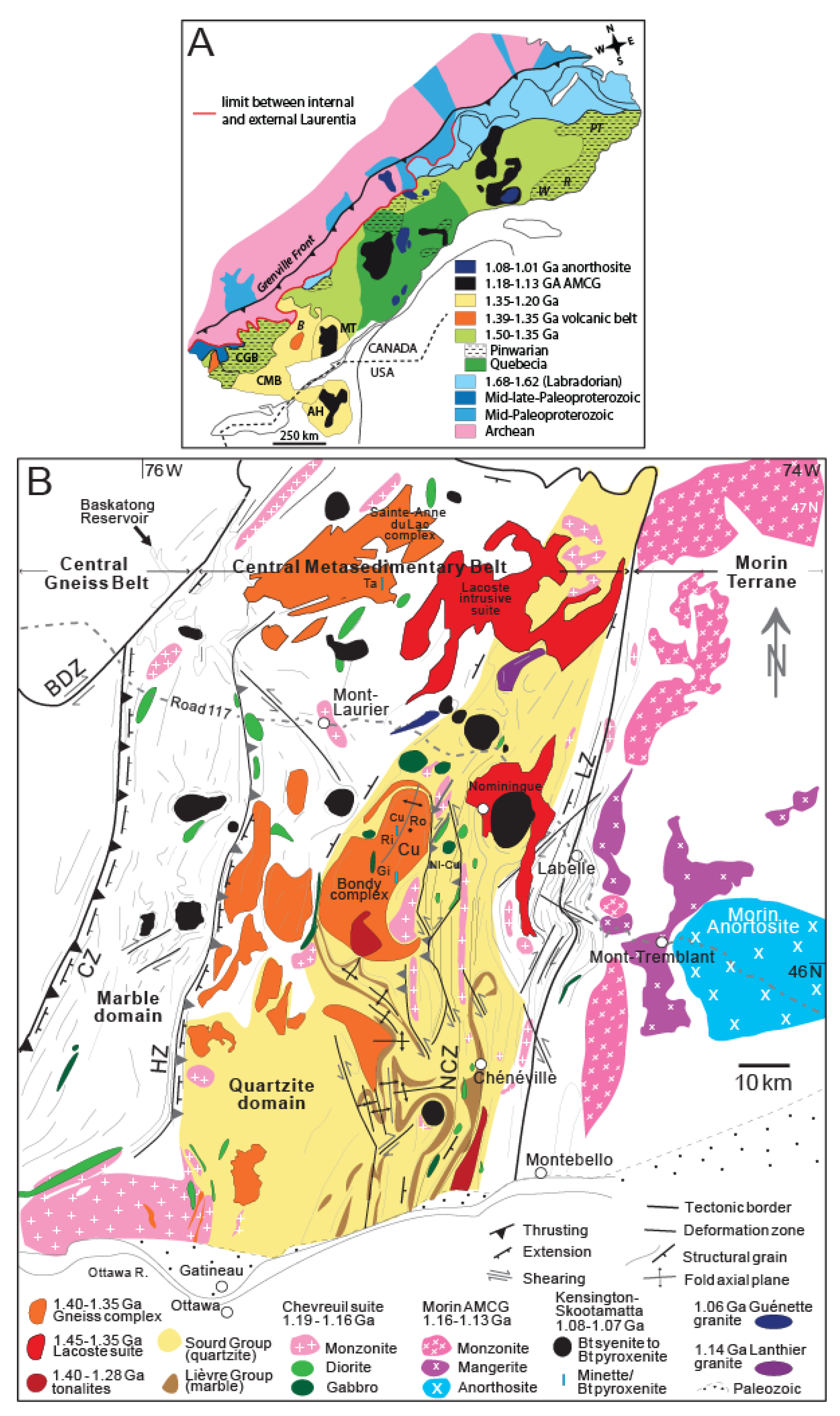
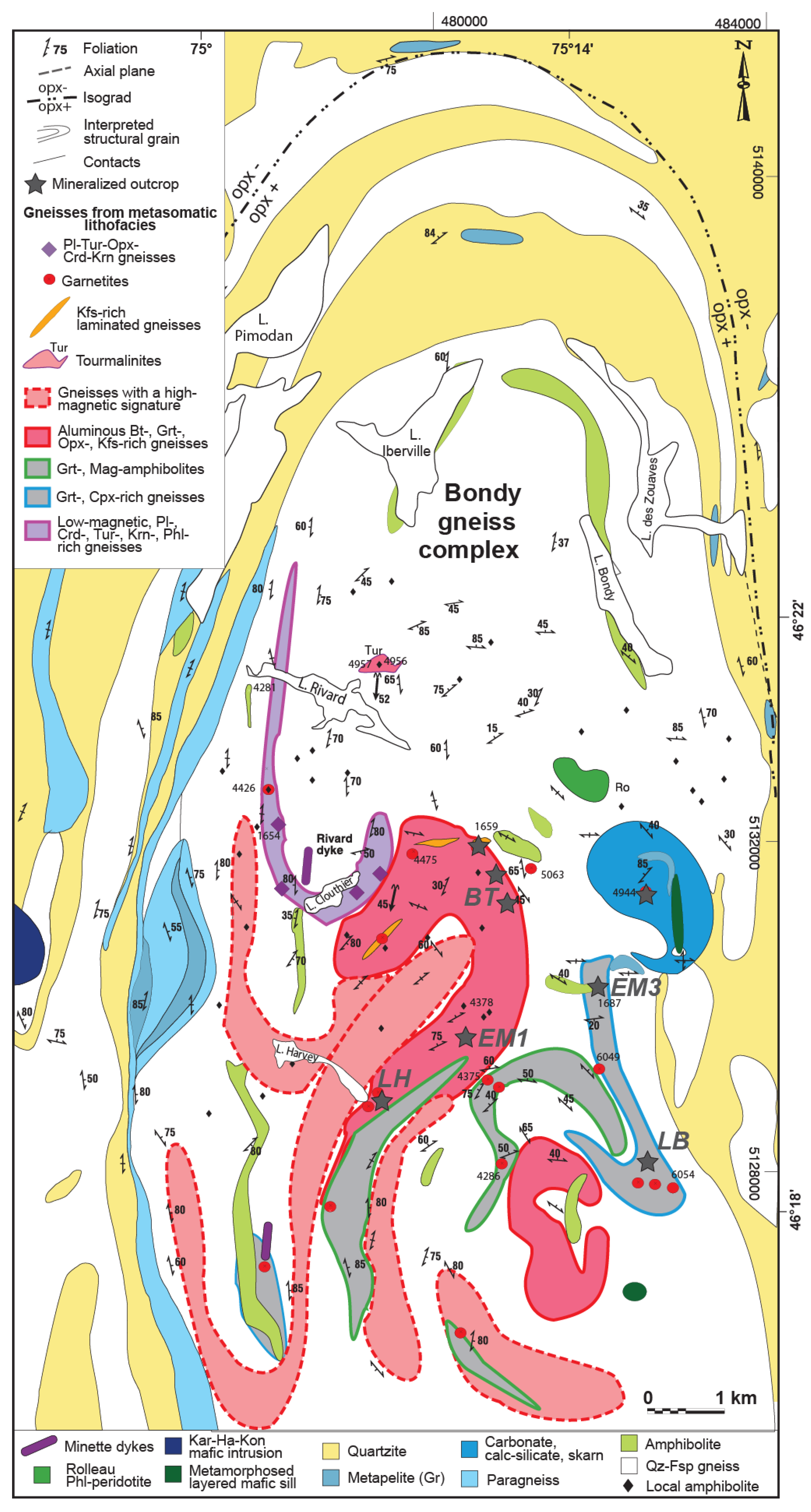
2.2. The Bondy Gneiss Complex
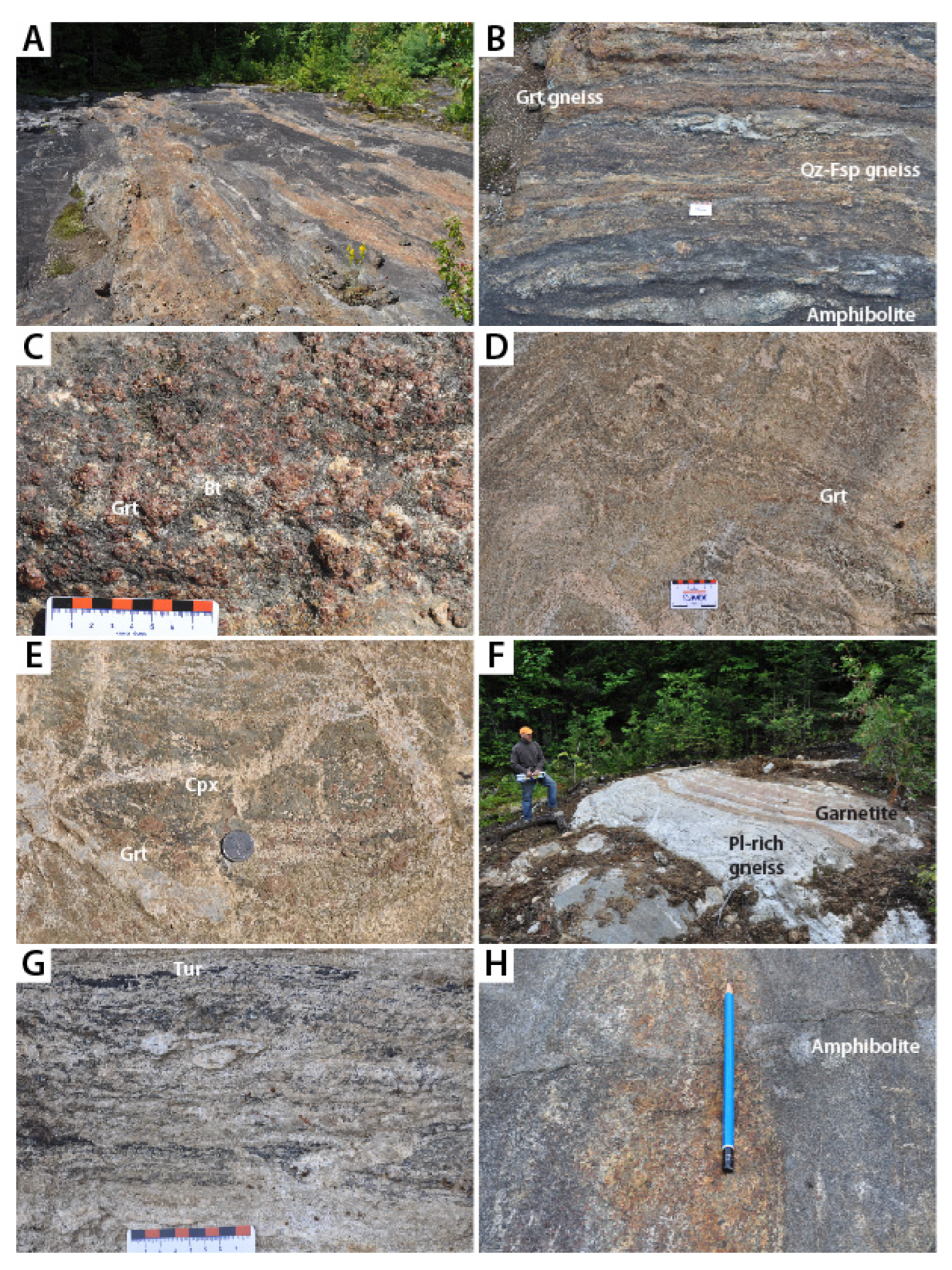
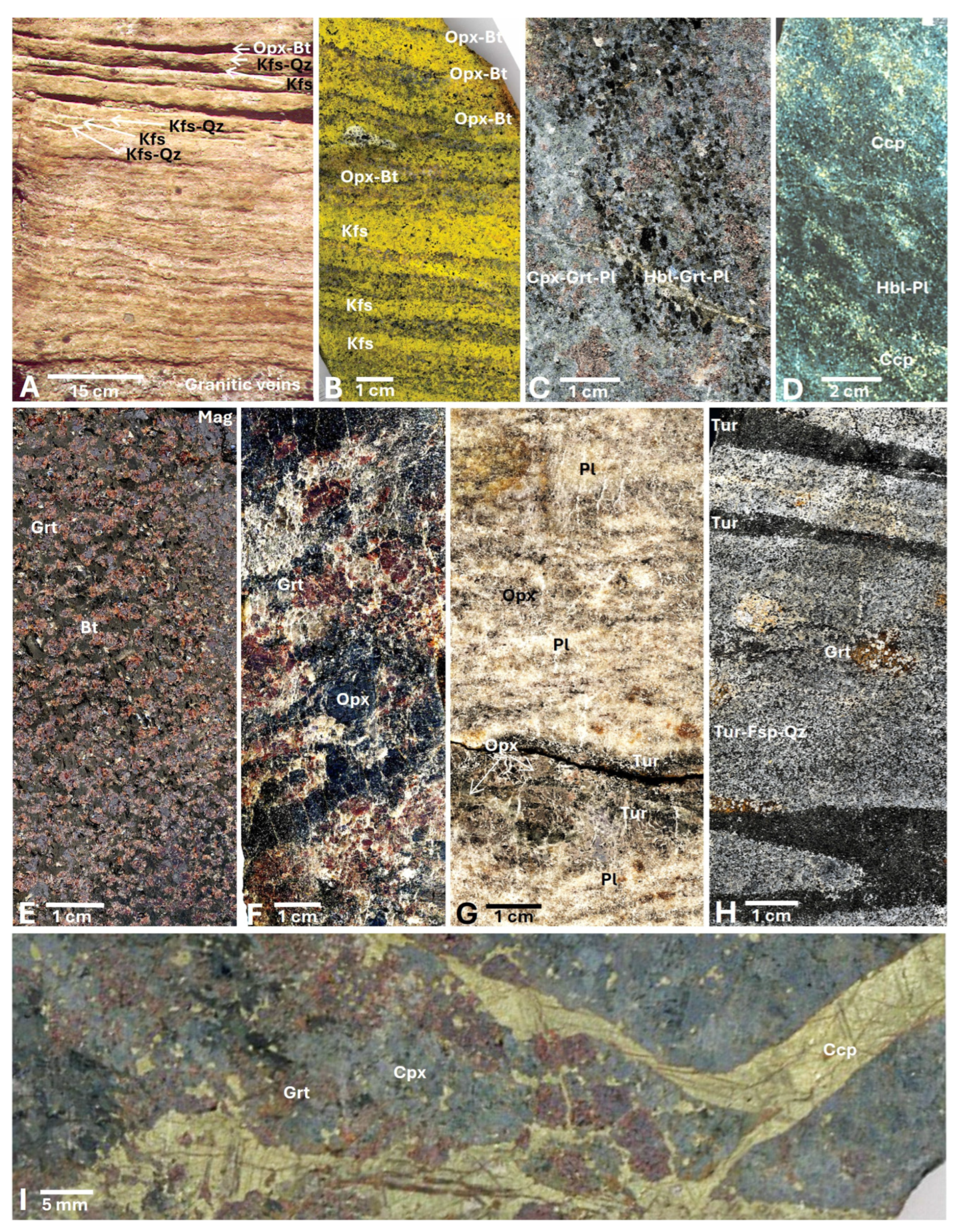
3. Description of Lithotypes
4. Lithogeochemistry
4.1. Analytical Methods
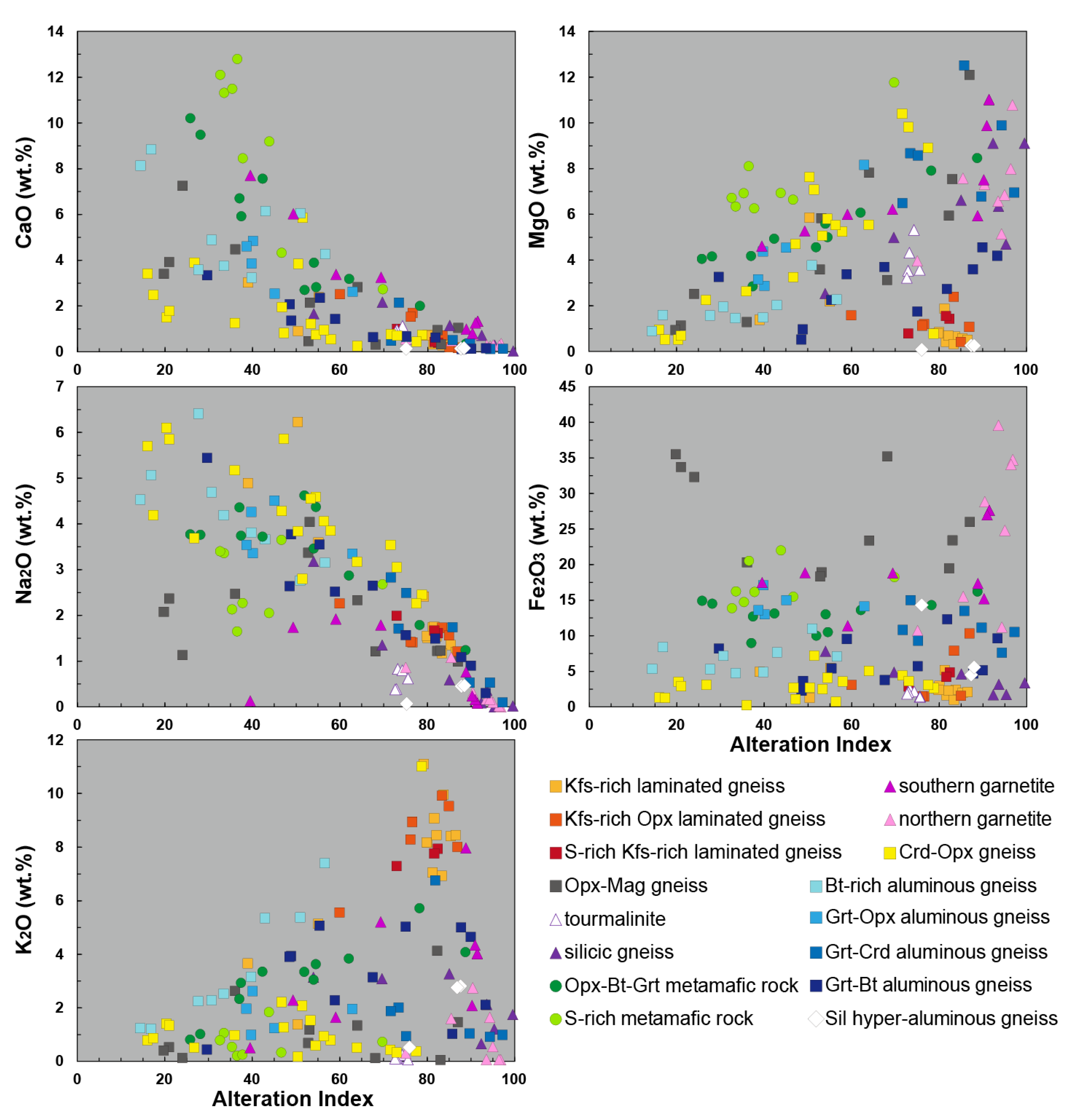
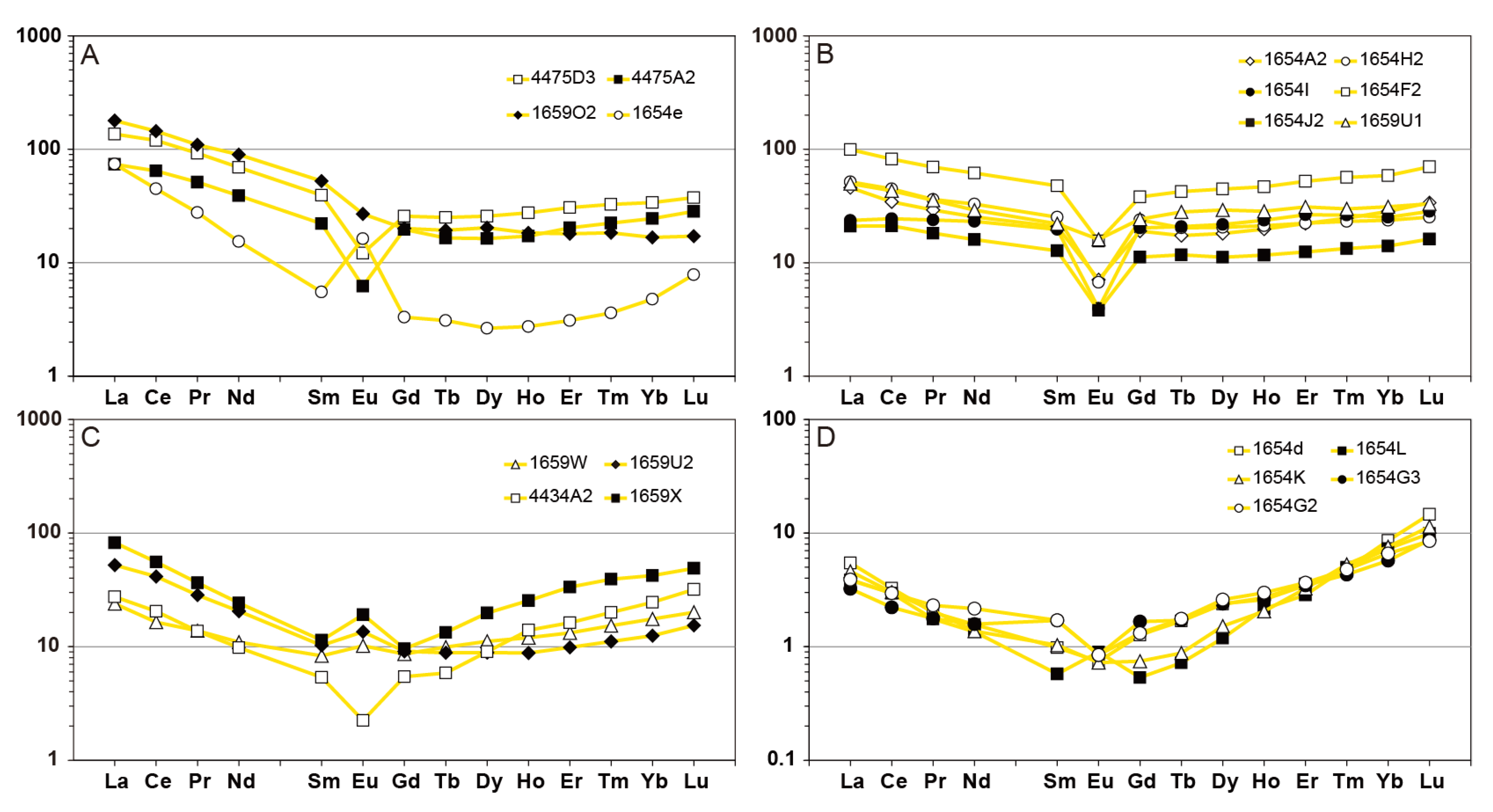
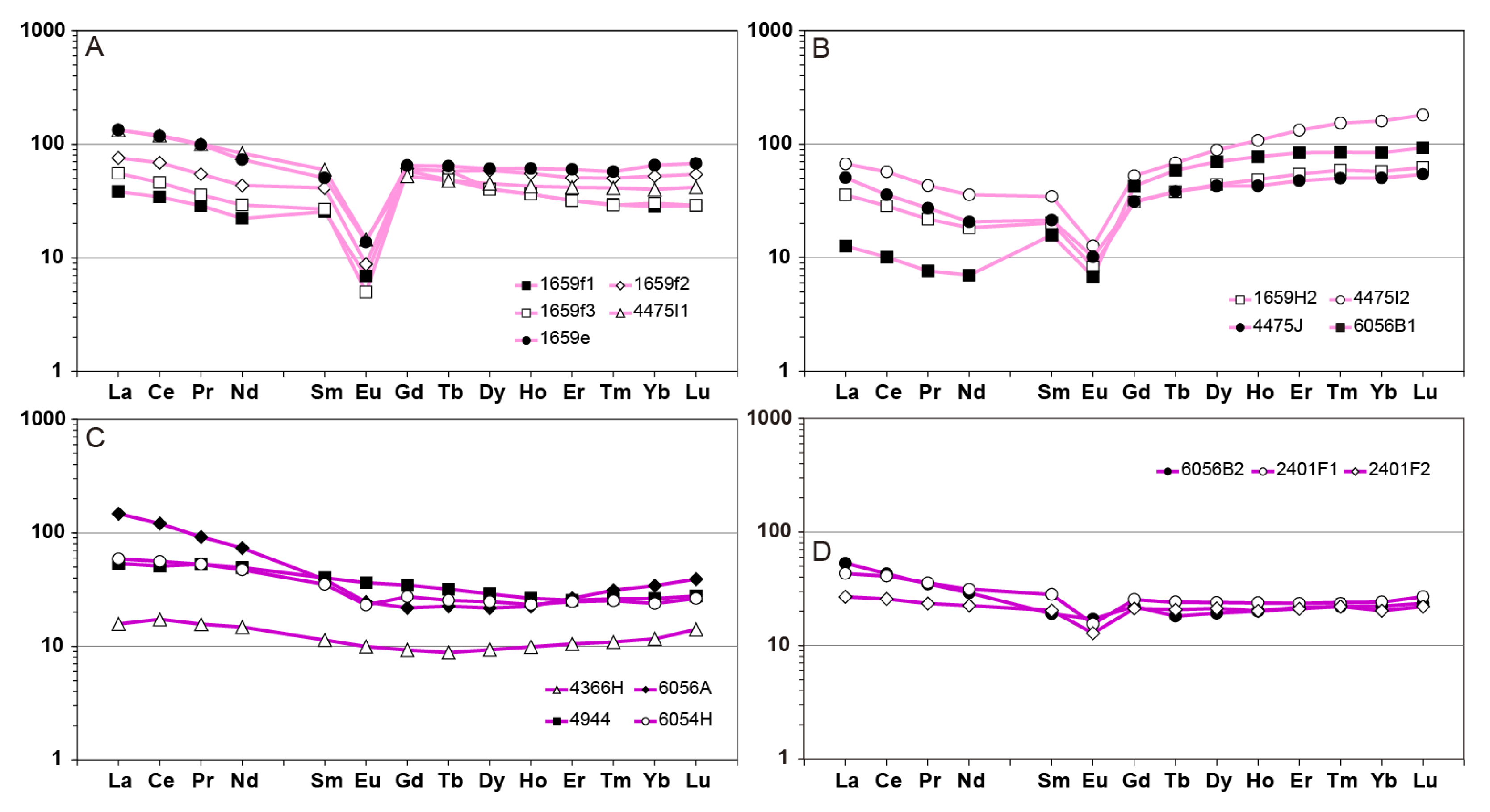
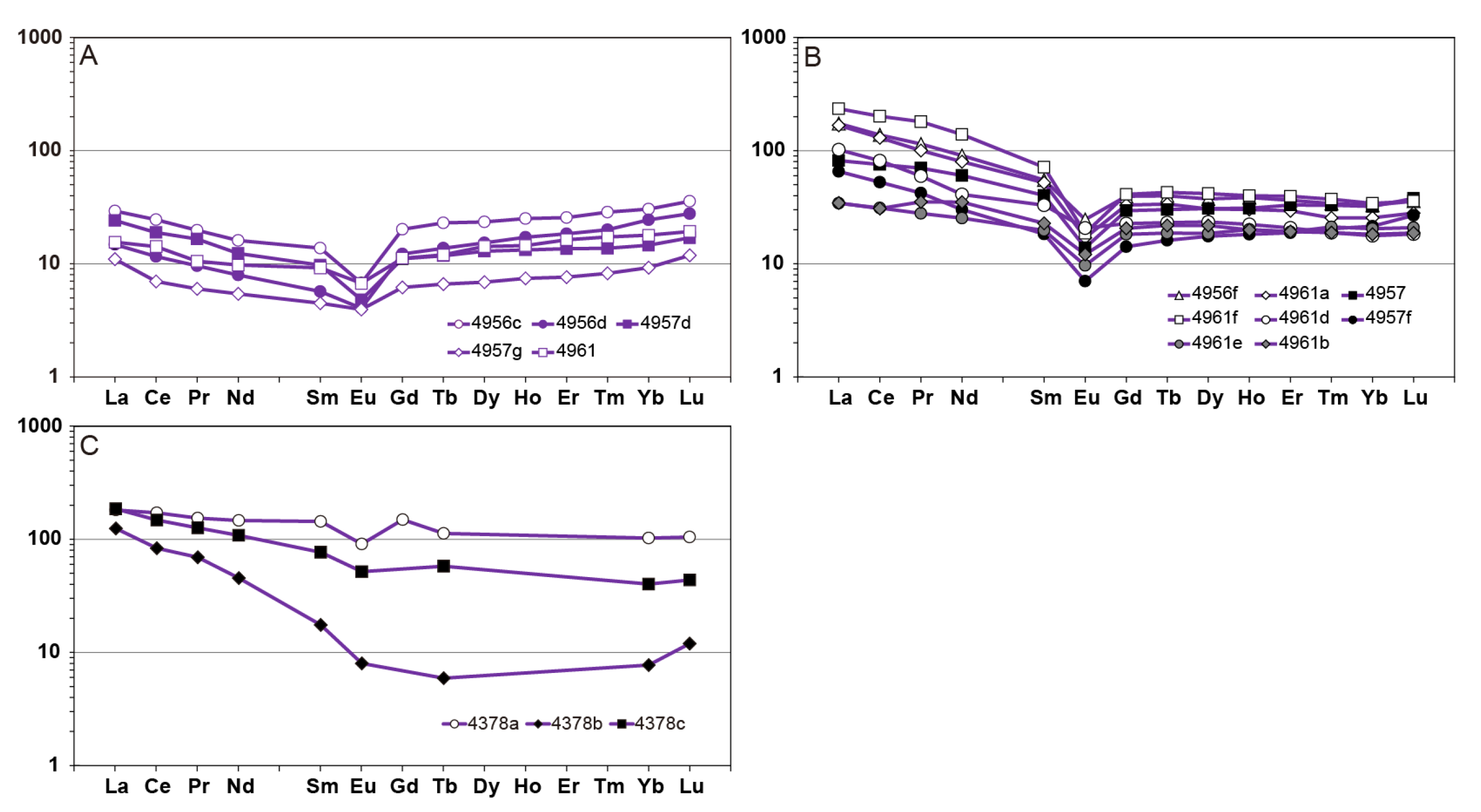
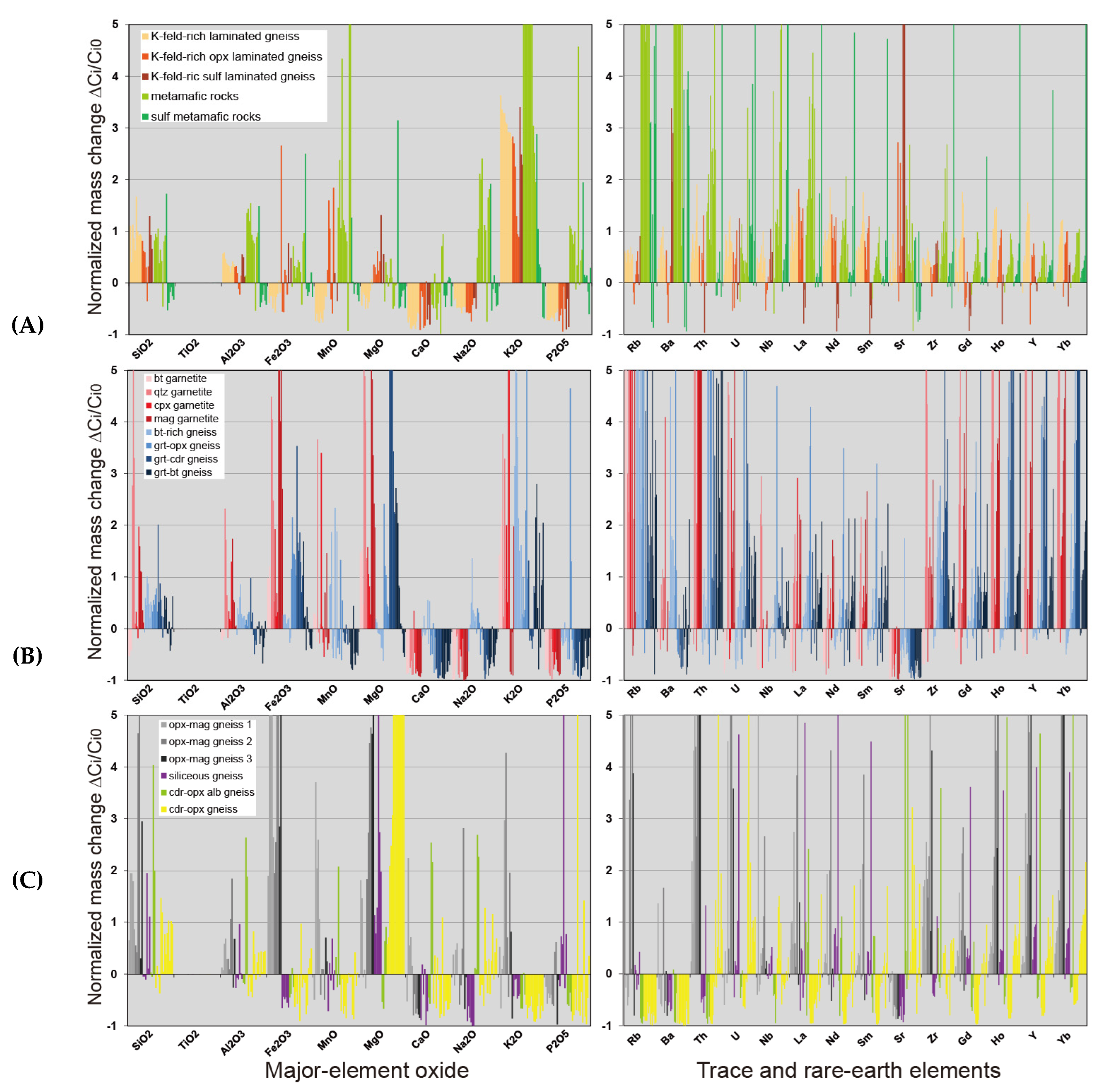
4.2. Mobility of Major, Trace and Rare Earth Elements Through Hydrothermal Alteration
4.3. Geochemistry of Hydrothermal Lithofacies
4.3.1. Metamafic Rocks
4.3.2. K-Feldspar-Rich Laminated Gneisses
4.3.3. Aluminous Gneisses
4.3.4. Orthopyroxene-Magnetite-Rich Gneisses
4.3.5. Plagioclase-Rich Cordierite-Orthopyroxene Gneisses
4.3.6. Garnet-Rich Rocks
4.3.7. Tourmalinites and Siliceous Gneisses
4.3.8. Hyperaluminous Gneisses
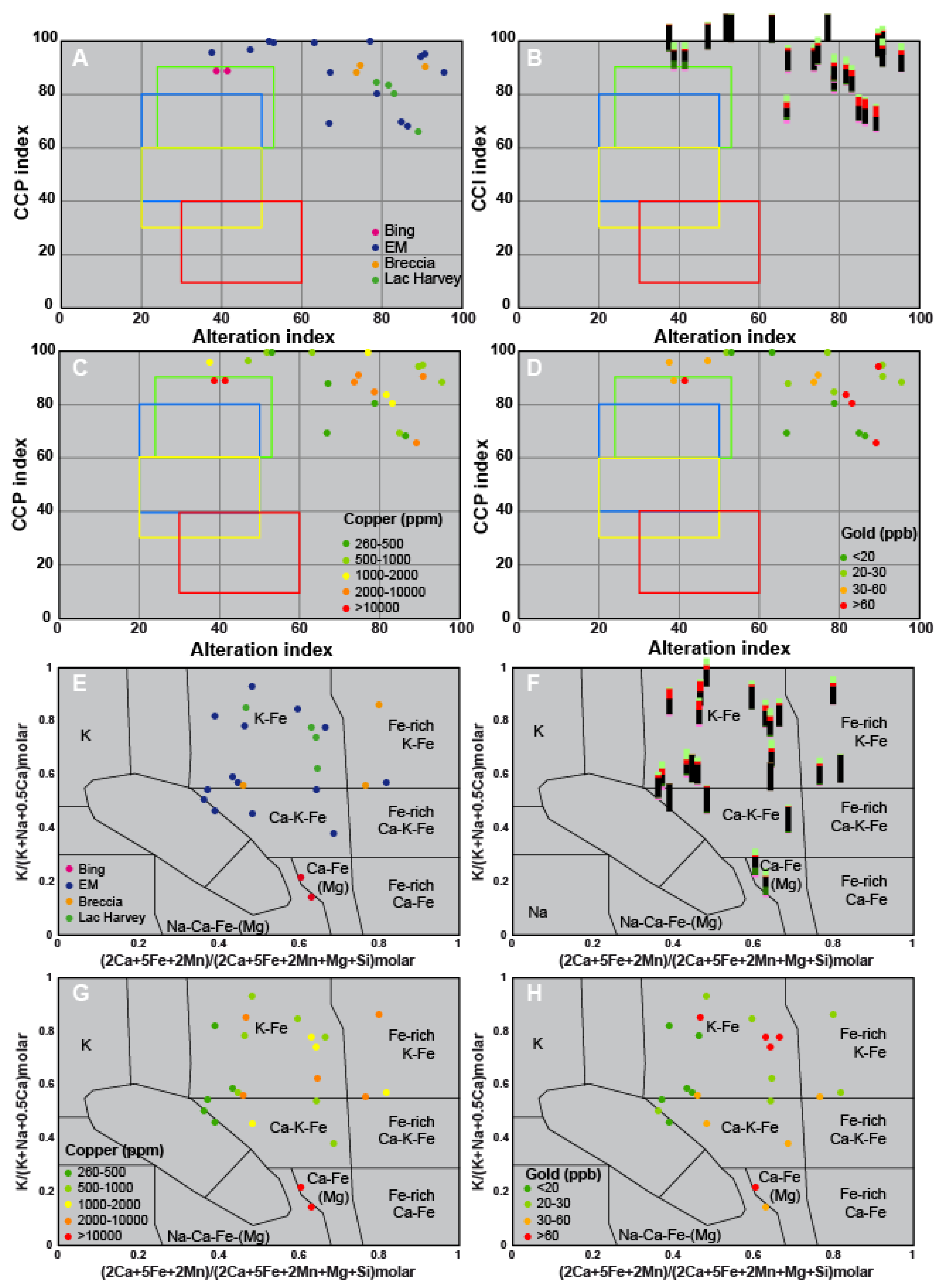
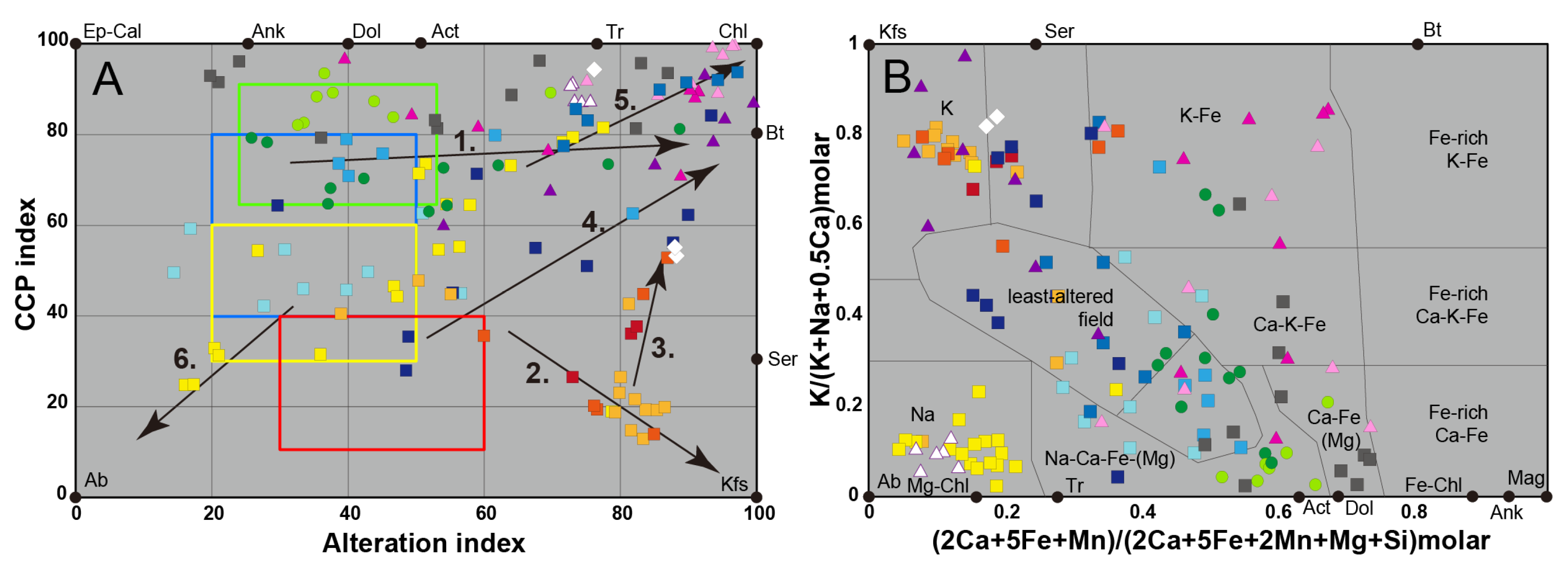
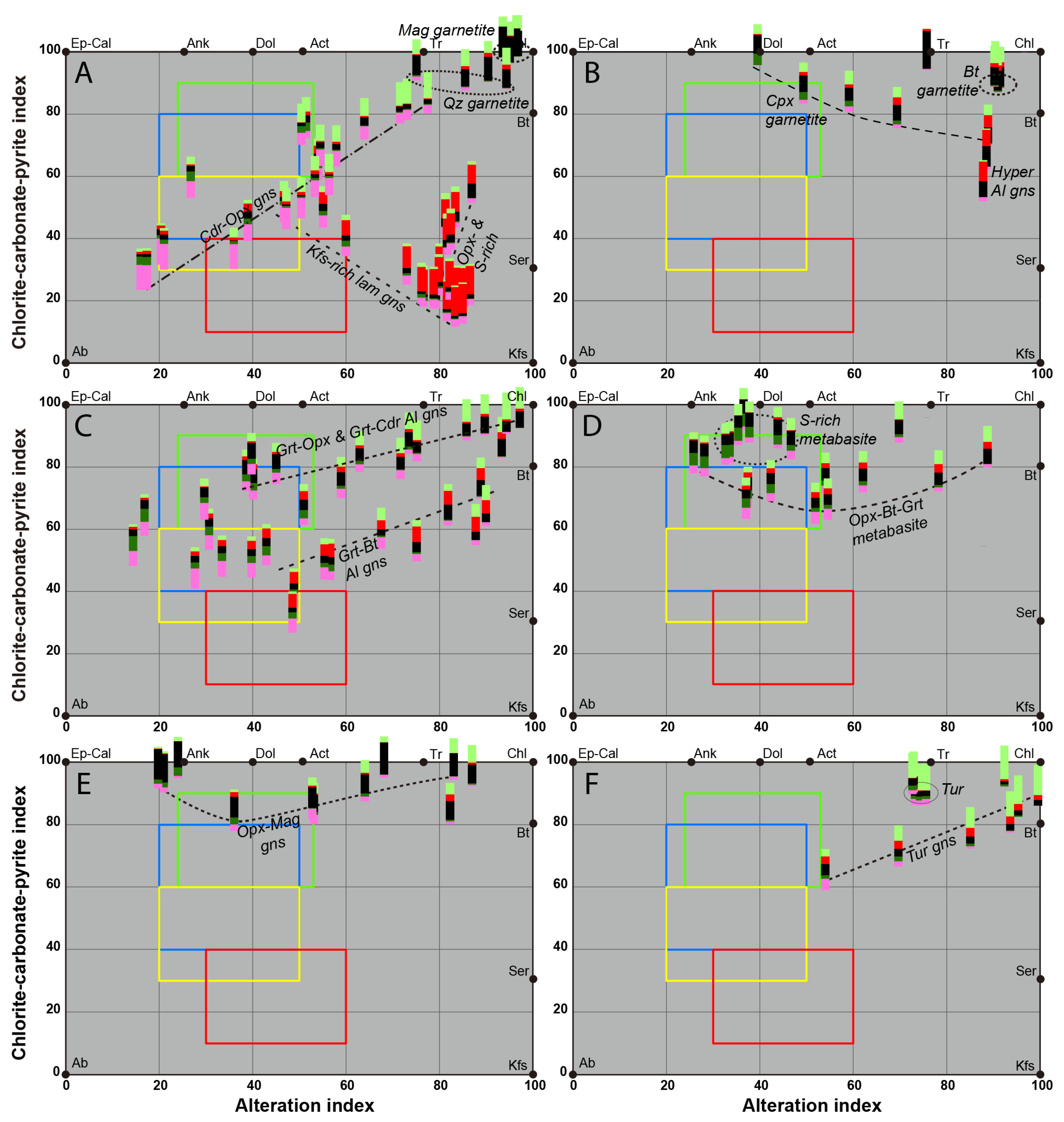
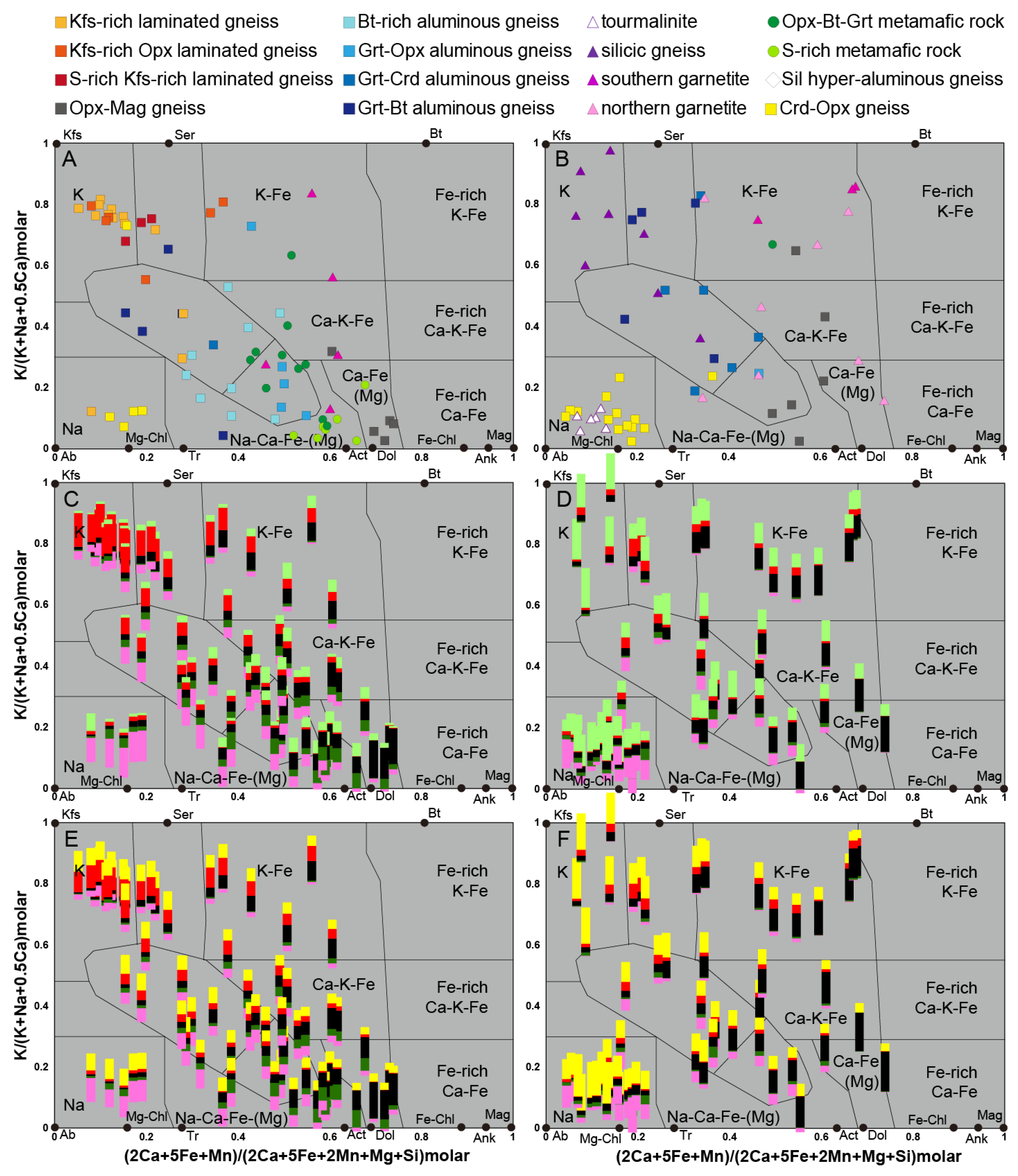
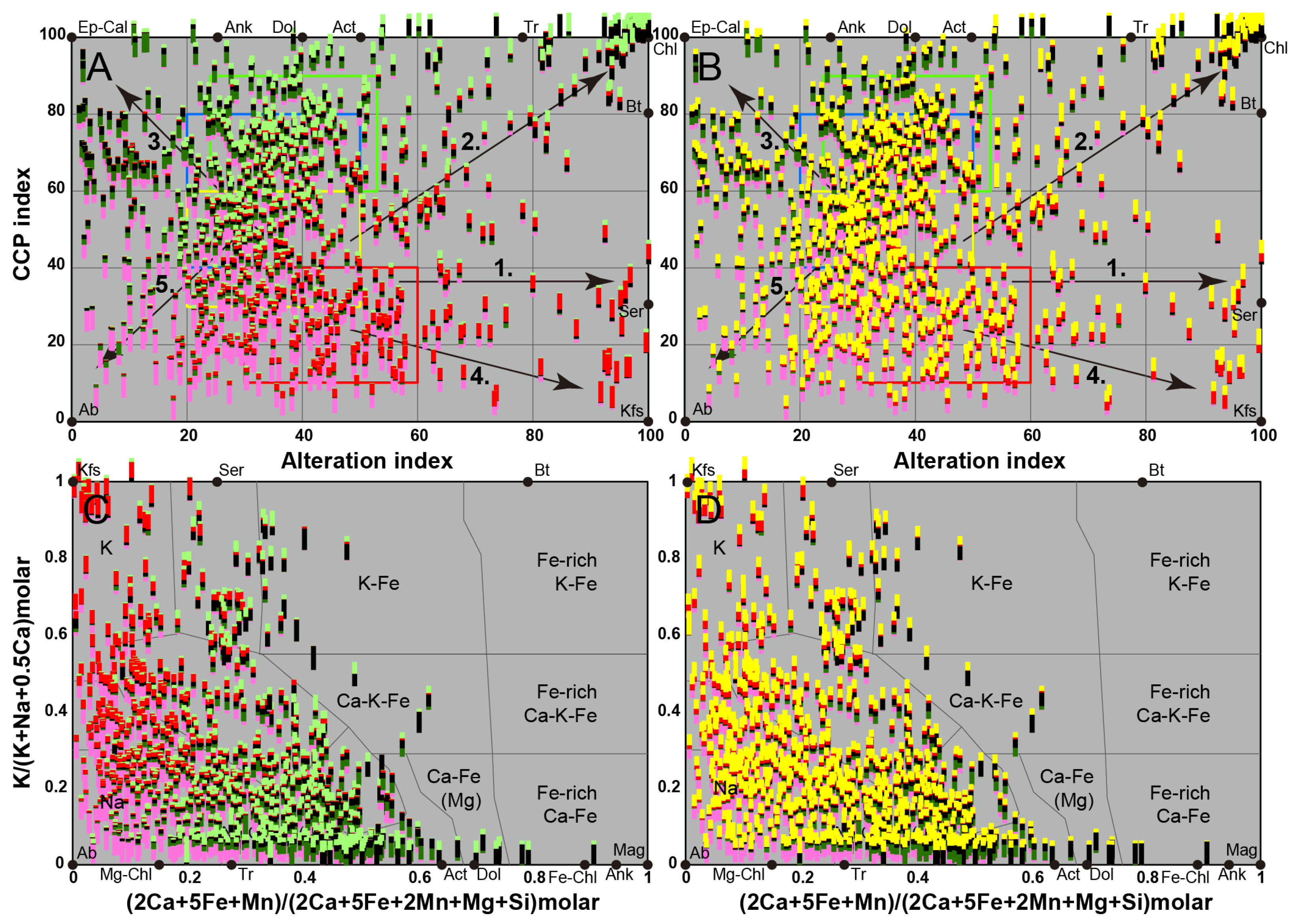
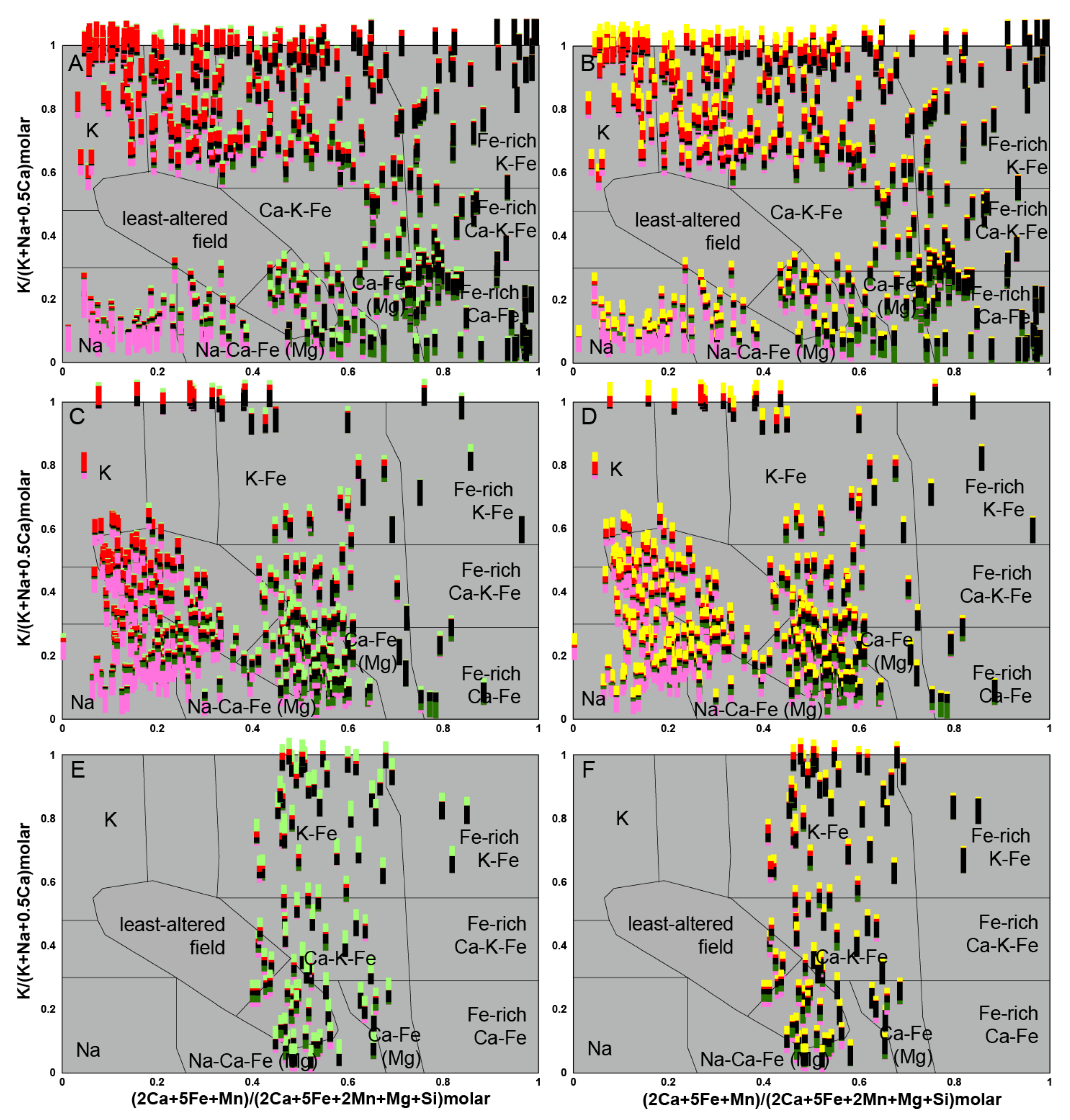
5. Identification of the Main Alteration Types
5.1. Petrological Interpretation of Alteration Facies
5.2. Lithogeochemical Footprint of the BGC Lithotypes Induced by Major Elements Mobility During Metasomatism
5.2.1. Rationale
5.2.2. Lithogeochemical Footprints, Element Mobility and Alteration Trends
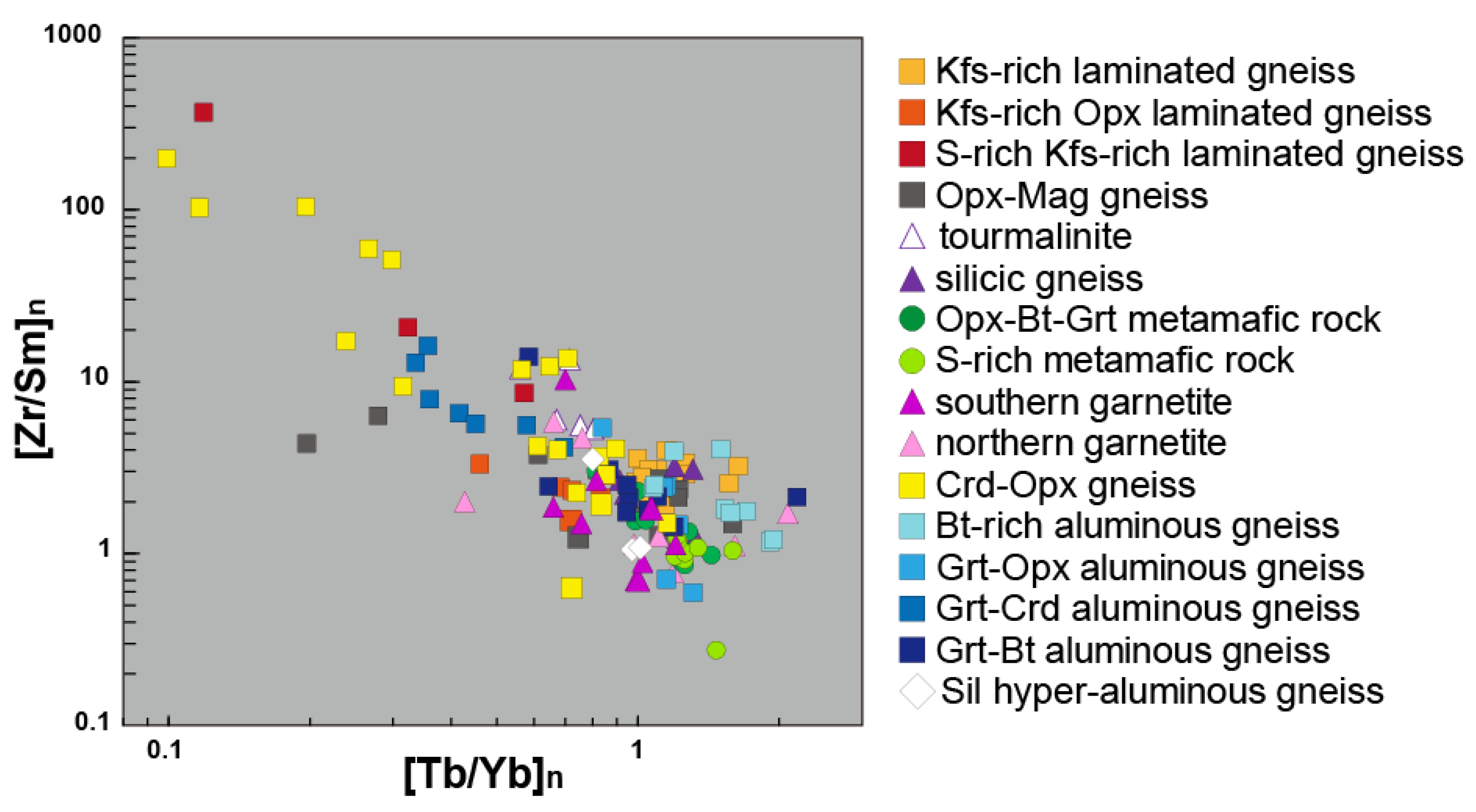
6. Trace and Rare Earth Elements Mobility
6.1. Mass-Balance Calculations
6.2. Rare Earth Elements Mobility During Metasomatism
7. Metallogenic Model and Implications for Exploration
7.1. A MIAC Lithogeochemical Signature
7.2. Implications for Exploration of High-Grade Metamorphic Terranes
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
References
- McFarlane, C.; Mavrogenes, J.A.; Tomkins, A. Recognizing hydrothermal alteration through a granulite facies metamorphic overprint at the Challenger Au deposit, South Australia. Chem. Geol. 2007, 243, 64–89. [Google Scholar] [CrossRef]
- Corriveau, L.; Spry, P.G. Metamorphosed hydrothermal ore deposits. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 13, pp. 175–194. [Google Scholar] [CrossRef]
- Corriveau, L.; Blein, O.; Laflèche, M. Progress Report on the Bondy Gneiss Complex and Its Cupriferous Hydrothermal System, Mont-Laurier Area; GM 54808; Ministère des Ressources Naturelles: Québec, QC, Canada, 1997. [Google Scholar]
- Corriveau, L.; Blein, O.; Gervais, F.; Trapy, P.H.; De Souza, S.; Fafard, D. Iron-Oxide and Alkali-Calcic Alteration, Skarn and Epithermal Mineralizing Systems of the Grenville Province: The Bondy Gneiss Complex in the Central Metasedimentary Belt of Quebec as a Case Example—A Field Trip to the 14th Society for Geology Applied to Mineral Deposits (SGA) Biennial Meeting; Open File 8349; Geological Survey of Canada: Ottawa, ON, Canada, 2018. [Google Scholar] [CrossRef]
- Allard, G.O.; Carpenter, R.H. Mineralogical anomalies in metamorphosed terranes, a neglected but promising exploration tool. In Proceedings of the International Conference of Geochemical Evolution: Continental Crust, Pocos de Caldas, Brazil, 11–16 July 1988; pp. 229–236. [Google Scholar]
- Tomkins, A.G.; Mavrogenes, J.A. Mobilization of gold as a polymetallic melt during pelite anatexis at the Challenger gold deposit, South Australia: A metamorphosed Archean gold deposit. Econ. Geol. 2002, 97, 1249–1271. [Google Scholar] [CrossRef]
- Drejing-Carroll, D.; Hitzman, M.W.; Coller, D. Geology of the Nautanen North Cu-Au-Ag-(Mo) deposit, Norrbotten, Sweden. Econ. Geol. 2023, 118, 1765–1794. [Google Scholar] [CrossRef]
- Harris, L.B. Research Applied to Exploration for IOCG-Style Mineralization in the Grenville Province, SW Quebec - Progress Report and Research Plan (Spring 2007 - Winter 2008); GM 65498; Ministère des Ressources Naturelles: Québec, QC, Canada, 2007. [Google Scholar]
- Blein, O.; Corriveau, L.; Laflèche, M.R. Cordierite-orthopyroxenes white gneiss: A key to unveiling pre-metamorphic hydrothermal activity in the Bondy gneiss complex, Grenville Province, Quebec. In Proterozoic Tectonic Evolution of the Grenville Orogen in North America; Tollo, R.P., Corriveau, L., McLelland, J., Bartholomew, M.J., Eds.; The Geological Society of America: Boulder, CO, USA, 2004; Memoir 197; pp. 19–33. [Google Scholar]
- Bonnet, A.-L.; Corriveau, L. Alteration vectors to metamorphosed hydrothermal systems in gneiss terranes. In Mineral Deposits of Canada: A Synthesis of Major Deposit-Types, District Metallogeny, the Evolution of Geological Provinces, and Exploration Methods; Goodfellow, W.D., Ed.; Geological Association of Canada, Mineral Deposits Division: St. John’s, NL, Canada, 2007; Special Publication 5. [Google Scholar]
- Spry, P.G.; McFadden, S.; Teale, G.S.; Alers, B.; Shallow, J.M.; Glenn, J.M. Nodular sillimanite rocks as field indicators to metamorphosed massive sulfide deposits. Ore Geol. Rev. 2022, 141, 104632. [Google Scholar] [CrossRef]
- Stevens, B.P.J.; Barron, L.M. Volcanic Textures in the Palaeoproterozoic Hores Gneiss, Broken Hill, Australia; Quarterly Notes 113; Geological Survey of New South Wales: Maitland, NSW, Australia, 2002; pp. 1–22. [Google Scholar]
- Gifkins, C.; Herman, W.; Large, R. Altered Volcanic Rocks: A Guide to Description and Interpretation; Centre for Ore Deposit Research (CODES), University of Tasmania: Hobart, TAS, Australia, 2005; pp. 241–246. [Google Scholar]
- Corriveau, L. Architecture de la Ceinture Métasédimentaire Centrale au Québec, Province de Grenville: Un Exemple de l’Analyse de Terrains de Métamorphisme Élevé; Bulletin 586; Geological Survey of Canada: Ottawa, ON, Canada, 2013. [Google Scholar]
- Corriveau, L.; Montreuil, J.-F. Metasomatic mineral systems with IOA, IOCG and affiliated critical and precious metal deposits: A review from a field geology perspective. Minerals 2025, 15, 365. [Google Scholar] [CrossRef]
- Hamilton, M.; Montreuil, J.-F.; Adlakha, E.; Corriveau, L.; Bain, W. Base, Critical, and Precious Metals Mineralization in the Metasomatic Iron and Alkali-calcic Systems of the Southern Province in the Sudbury Area: A Geological Guidebook; Open File Report 6391 Ontario Geological Survey: Sudbury, ON, Canada, 2023. [Google Scholar]
- Blein, O.; Harlaux, M.; Corriveau, L.; Niiranen, T.; Lynch, E.P.; Lisitsin, V.; Ehrig, K.; Montreuil, J.F.; Gourcerol, B. Geochemical footprints of IOA and IOCG deposits in Northern Norrbotten, Sweden, and Cloncurry District, Australia. J. Geochem. Explor. 2025, 277, 107820. [Google Scholar] [CrossRef]
- Corriveau, L.; Montreuil, J.-F.; Potter, E.G. Alteration facies linkages among IOCG, IOA and affiliated deposits in the Great Bear magmatic zone, Canada. Econ. Geol. 2016, 111, 2045–2072. [Google Scholar] [CrossRef]
- Corriveau, L.; Montreuil, J.-F.; Blein, O.; Ehrig, K.; Potter, E.G. Mineral systems with IOCG and affiliated deposits: Part 2—Geochemical Footprints. In Mineral Systems with Iron Oxide Copper-Gold (IOCG) and Affiliated Deposits; Corriveau, L., Potter, E.G., Mumin, A.H., Eds.; Special Paper 52; Geological Association of Canada: St. John’s, NL, Canada, 2022; pp. 159–204. [Google Scholar]
- Montreuil, J.-F.; Corriveau, L.; Grunsky, E.C. Compositional data analysis of IOCG systems, Great Bear magmatic zone, Canada: To each alteration types its own geochemical signature. Geochem. Explor. Envir. Anal. 2013, 13, 229–247. [Google Scholar] [CrossRef]
- Acosta-Góngora, P.; Potter, E.G.; Lawley, C.J.M.; Corriveau, L.; Sparkes, G. Geochemical characterization of the Central Mineral Belt U±Cu±Mo±V mineralization, Labrador, Canada: Evaluation of IOCG potential and application of unsupervised machine-learning. J. Geochem. Expl. 2022, 237, 106995. [Google Scholar] [CrossRef]
- Blein, O.; Corriveau, L.; Montreuil, J.-F.; Ehrig, K.; Fabris, A.; Reid, A.; Pal, D. Geochemical signatures of metasomatic ore systems hosting IOCG, IOA, albite-hosted uranium and affiliated deposits: A tool for process studies and mineral exploration. In Mineral Systems with Iron Oxide Copper-Gold (IOCG) and Affiliated Deposits; Corriveau, L., Potter, E.G., Mumin, A.H., Eds.; Special Paper 52; Geological Association of Canada: St. John’s, NL, Canada, 2022; pp. 263–298. [Google Scholar]
- Boggs, K.; Corriveau, L. Granulite-facies P-T-t paths and the influence of retrograde cation diffusion during polyphase orogenesis, western Grenville Province, Québec. In Proterozoic Tectonic Evolution of the Grenville Orogen in North America; Tollo, R.P., Corriveau, L., McLelland, J., Bartholomew, M.J., Eds.; The Geological Society of America: Boulder, CO, USA, 2004; Memoir 197; pp. 35–64. [Google Scholar]
- Corriveau, L.; Morin, D. Modelling 3D architecture of western Grenville from surface geology, xenoliths, styles of magma emplacement, and Lithoprobe reflectors. Can. J. Earth Sci. 2000, 37, 235–251. [Google Scholar] [CrossRef]
- Warren, R.G.; Shaw, R.D. Volcanogenic Cu-Pb-Zn bodies in granulites of the central Arunta Block, central Australia. J. Metamorph. Geol. 1985, 3, 481–499. [Google Scholar] [CrossRef]
- Hussey, K.J.; Huston, D.L.; Claoue-Long, J.C. Geology and Origin of Some Cu-Pb-Zn (Au-Ag) Deposits in the Strangways Metamorphic Complex, Arunta Region, Northern Territory; Report 17; Northern Territory Geological Survey: Darwin, NT, Australia, 2006. [Google Scholar]
- McGloin, M.V.; Creaser, R.A. Summary of Results. Re–Os Molybdenite Dating of the Mount Hardy Deposit, Aileron Province; Record 2017–014; Northern Territory Geological Survey: Darwin, NT, Australia, 2017. [Google Scholar]
- McGloin, M.V.; Weisheit, A.; Trumbull, R.B.; Maas, R. Using Tourmaline to Identify Base Metal and Tungsten Mineralising Processes in the Jervois Mineral Field and Bonya Hills, Aileron Province; Rec 2019-001; Northern Territory Geological Survey: Darwin, NT, Australia, 2019. [Google Scholar]
- Large, R.R.; Gemmell, J.B.; Paulick, H.; Huston, D.L. The alteration box plot: A simple approach to understanding the relationship between alteration mineralogy and lithogeochemistry associated with volcanic-hosted massive sulfide deposits. Econ. Geol. 2001, 96, 957–971. [Google Scholar] [CrossRef]
- Gower, C.F.; Krogh, T.E. A U–Pb geochronological review of the Proterozoic history of the eastern Grenville Province. Can. J. Earth Sci. 2002, 39, 795–829. [Google Scholar] [CrossRef]
- Slagstad, T.; Easton, R.M.; Huyskens, M.; Culshaw, N. Complementarity of Lu–Hf isotopes from detrital and igneous zircon: An example from the Central Gneiss Belt, Grenville Province, Ontario. Can. J. Earth Sci. 2024, 62, 192–210. [Google Scholar] [CrossRef]
- Moukhsil, A.; El Bourki, M. Synthèse Géologique et Métallogénique de la Région de Clova Jusqu’au Nord du Lac-Saint-Jean, Parties Ouest et Centrale de la Province de Grenville, Québec, Canada; BG 2024-08; Ministère des Ressources Naturelles et des Forêts: Québec, QC, Canada, 2024. Available online: https://gq.mines.gouv.qc.ca/bulletins-geologiques/bg-2024-08-synthese-grenville/ (accessed on 26 February 2025).
- Dickin, A.P.; McNutt, R.H.; Martin, C.; Guo, A. The extent of juvenile crust in the Grenville Province: Nd isotope evidence. Geol. Soc. Am. Bull. 2010, 122, 870–883. [Google Scholar] [CrossRef]
- Dickin, A.P.; Hynes, E.; Landry, E.; Vautour, S. The significance of Nd isotope mapping in Precambrian orogenic belts: A case study from the Saguenay region of the Quebec Grenville Province. Precambrian Res. 2023, 394, 107–126. [Google Scholar] [CrossRef]
- Vautour, S.; Dickin, A. Nd isotope mapping of a Pinwarian-age composite arc belt in the Quebecia terrane of the central Grenville Province, Canada. Precambrian Res. 2019, 332, 105409. [Google Scholar] [CrossRef]
- Culshaw, N.; Van De Kerckhove, S.; Slagstad, T.; Marsh, J.; Easton, R.M. Crustal evolution of the Laurentian continental margin from the Paleo through Mesoproterozoic: A zircon U–Pb and Hf transect through the western Grenville Province, Ontario, Canada. Precambrian Res. 2023, 386, 106963. [Google Scholar] [CrossRef]
- Indares, A.; Moukhsil, A.; Groulier, P.A. Geon 14 to early geon 13 granitoid magmatism in. the Grenville Province of Canada, northeastern Laurentia: Distribution, geochemical patterns, and links with an active-margin setting. In Laurentia: Turning Points in the Evolution of a Continent; Whitmeyer, S.J., Williams, M.L., Kellett, D.A., Tikoff, B., Eds.; The Geological Society of America: Boulder, CO, USA, 2004; Volume 220. [Google Scholar] [CrossRef]
- Dickin, A.; Hynes, E.; Strong, J.; Wisborg, M. Testing a back-arc ‘aulacogen’ model for the Central Metasedimentary Belt of the Grenville Province. Geol. Mag. 2016, 153, 681–695. [Google Scholar] [CrossRef]
- Wodicka, N.; Corriveau, L.; Stern, R.A. SHRIMP U-Pb zircon geochronology of the Bondy gneiss complex: Evidence for circa 1.39 Ga arc magmatism and polyphase Grenvillian metamorphism in the Central Metasedimentary Belt, Grenville Province, Quebec. In Proterozoic Tectonic Evolution of the Grenville Orogen in North America; Tollo, R.P., Corriveau, L., McLelland, J., Bartholomew, M.J., Eds.; The Geological Society of America: Boulder, CO, USA, 2004; Memoir 197; pp. 243–266. [Google Scholar]
- Corriveau, L.; van Breemen, O. Docking of the Central Metasedimentary Belt to Laurentia: Evidence from the 1.17-1.16 Ga Chevreuil intrusive suite and host gneisses, Quebec. Can. J. Earth Sci. 2000, 37, 253–269. [Google Scholar] [CrossRef]
- Davis, D.W.; Nantel, S. Datations U-Pb dans la Partie Nord de la Ceinture Centrale des Métasédiments, Province de Grenville, Région de Mont-Laurier; MB 2016-04; Ministère des Ressources Naturelles et des Forêts: Québec, QC, Canada, 2016. [Google Scholar]
- Blein, O.; Laflèche, M.R.; Corriveau, L. Geochemistry of the granulitic Bondy gneiss complex: A 1.4 Ga arc in the central metasedimentary belt, Grenville Province, Canada. Precambrian Res. 2003, 102, 193–218. [Google Scholar] [CrossRef]
- Nantel, S. Géologie et Aperçu de la Géochronologie et des Indices Métalliques Découverts Entre 1996 et 2007 dans la Partie Nord de la Ceinture Centrale des Métasédiments, Province de Grenville, Région de Mont-Laurier; RG2008-04; Ministère des Ressources Naturelles: Québec, QC, Canada, 2008. [Google Scholar]
- Dickin, A.P.; McNutt, R.H. The Central Metasedimentary Belt (Grenville Province) as a failed back-arc rift zone: Nd isotope evidence. Earth Planet. Sci. Lett. 2007, 259, 97–106. [Google Scholar] [CrossRef]
- Richmond Minerals Inc. Report on the Geophysical Surveying and Ground Sampling at the Bondy Gneiss Complex; GM 65497; Ministère des Ressources Naturelles: Québec, QC, Canada, 2010. [Google Scholar]
- Dufréchou, G.; Harris, L.B.; Corriveau, L.; Antonoff, V. Regional and local controls on mineralization and pluton emplacement in the Bondy gneiss complex, Grenville Province, Canada interpreted from aeromagnetic and gravity data. J. Appl. Geophys. 2015, 116, 192–205. [Google Scholar] [CrossRef]
- Boggs, K. Retrograde Cation Exchange in Garnets During Slow Cooling of Mid Crustal Granulites and the P-T-t Trajectories from the Mont-Laurier Region, Grenville Province, Québec. Master’s Thesis, Université du Québec à Chicoutimi, Saguenay, QC, Canada, 1996. [Google Scholar]
- Carrington, D.P.; Harley, S.L. Partial melting and phase relations in high-grade metapelites: An experimental petrogenetic grid in the KFMASH system. Contrib. Mineral. Petrol. 1995, 120, 270–291. [Google Scholar] [CrossRef]
- Vallance, T.G. Mafic rock alteration and isochemical development of some cordierite-anthophyllite rocks. J. Petrol. 1967, 8, 84–96. [Google Scholar] [CrossRef]
- Franklin, J.M.; Lydon, J.W.; Sangster, D.F. Volcanic-associated massive sulfide deposits. Econ. Geol. 1981, 75, 486–627. [Google Scholar]
- Schandl, E.S.; Gorton, M.P.; Wasteneys, H.A. Rare-earth element geochemistry of the metamorphosed volcanogenic massive sulphide deposits of the Manitouwadge mining camp, Superior Province, Canada: A potential exploration tool? Econ. Geol. 1995, 90, 1217–1236. [Google Scholar] [CrossRef]
- Zaleski, E.; Peterson, V.L. Depositional setting and deformation of massive sulfide deposits, iron-formation, and associated alteration in the Manitouwadge Greenstone Belt, Superior Province, Ontario. Econ. Geol. 1995, 90, 2244–2261. [Google Scholar] [CrossRef]
- Hashiguchi, H.; Yamada, R.; Inoue, T. Practical application of low Na2O anomalies in footwall acid lava for delimiting promising areas around the Kosaka and Fukazawa Kuroko deposits, Akita Prefectural, Japan. In The Kuroko Massive Sulfide Deposits; Ohmoto, H., Skinner, B.J., Eds.; Monograph 5 Society of Economic Geologists: Littleton, CO, USA, 1983; pp. 387–394. [Google Scholar]
- Warr, L.M. IMA–CNMNC approved mineral symbols. Min. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Roberts, M.D.; Oliver, N.H.S.; Fairclough, M.C.; Hölttä, P.S.; Lahtinen, R. Geochemical and oxygen isotope signature of sea-floor alteration associated with a polydeformed and highly metamorphosed massive sulphide deposit, Ruostesuo, Central Finland. Econ. Geol. 2003, 98, 535–556. [Google Scholar] [CrossRef]
- Taylor, B.E. Epithermal gold deposits. In Mineral Deposits of Canada: A Synthesis of Major Deposit-Types, District Metallogeny, the Evolution of Geological Provinces, and Exploration Methods; Goodfellow, W.D., Ed.; Special Publication 5; Geological Association of Canada, Mineral Deposits Division: St. John’s, NL, Canada, 2007; pp. 113–139. [Google Scholar]
- Hollis, S.P.; Yeats, C.J.; Wyche, S.; Barnes, S.J.; Ivanic, T.J.; Belford, S.M.; Davidson, G.J.; Roache, A.J.; Wingate, M.T.D. A review of volcanic-hosted massive sulfide (VHMS) mineralization in the Archean Yilgarn craton, Western Australia: Tectonic, stratifraphic and geochemical associations. Precambrian Res. 2015, 260, 113–135. [Google Scholar] [CrossRef]
- Grant, J.A. The isocon diagram—A simple solution to Gresens’ equation for metasomatic alteration. Econ. Geol. 1986, 81, 1976–1982. [Google Scholar] [CrossRef]
- Grant, J.A. Isocon analysis: A brief review of the method and applications. Phys. Chem. Earth 2005, 30, 997–1004. [Google Scholar] [CrossRef]
- Gresens, R.L. Composition-volume relationships of metasomatism. Chem. Geol. 1967, 2, 47–65. [Google Scholar] [CrossRef]
- Salvi, S.; Fontan, F.; Monchoux, O.; Williams-Jones, A.E.; Moine, B. Hydrothermal mobilization of high field strength elements in alkaline igneous systems: Evidence from the Tamazeght Complex (Morocco). Econ. Geol. 2000, 95, 559–576. [Google Scholar] [CrossRef]
- Jiang, S.-Y.; Wang, R.-C.; Xu, X.-S.; Zhao, K.-D. Mobility of high field strength elements (HFSE) in magmatic-, metamorphic-, and submarine-hydrothermal systems. Phys. Chem. Earth 2005, 30, 1020–1029. [Google Scholar] [CrossRef]
- Cann, J.R. Rb, Sr, Y, Zr and Nb in some ocean-floor basaltic rocks. Earth Planet. Sci. Lett. 1970, 10, 7–11. [Google Scholar] [CrossRef]
- van Dongen, M.; Weinberg, R.F.; Tomkins, A.G. REE-Y, Ti, and P remobilization in magmatic rocks by hydrothermal alteration during Cu–Au deposit formation. Econ. Geol. 2010, 105, 763–776. [Google Scholar] [CrossRef]
- Montreuil, J.-F.; Potter, E.G.; Corriveau, L.; Davis, W.J. Element mobility patterns in magnetite-group IOCG systems: The Fab IOCG system, Northwest Territories, Canada. Ore Geol. Rev. 2016, 72, 562–584. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Wood, S.A. A preliminary petrogenetic grid for rare earth element fluorocarbonate and related minerals. Geochim. Cosmochim. Acta 1992, 56, 725–738. [Google Scholar] [CrossRef]
- Gammons, C.H.; Wood, S.A.; Williams-Jones, A.E. The aqueous geochemistry of the rare earth elements and yttrium: VI. Stability of neodymium chloride complexes from 25 to 300 °C. Geochim. Cosmochim. Acta 1996, 60, 4615–4630. [Google Scholar] [CrossRef]
- Gysi, A.P.; Williams-Jones, A.E. Hydrothermal mobilization of pegmatite-hosted REE and Zr at Strange Lake, Canada: A reaction path model. Geochim. Cosmochim. Acta 2013, 122, 324–352. [Google Scholar] [CrossRef]
- Gysi, A.P.; Williams-Jones, A.E.; Collins, P. Lithogeochemical vectors for hydrothermal processes in the Strange Lake peralkaline granitic REE-Zr-Nb deposit. Econ. Geol. 2016, 111, 1241–1276. [Google Scholar] [CrossRef]
- Elliott, H.A.; Wall, F.; Chakhmouradian, A.R.; Siegfried, P.R.; Dahlgren, S.; Weatherley, S.; Finch, A.A.; Marks, M.A.; Dowman, E.; Deady, E. Fenites associated with carbonatite complexes: A review. Ore Geol. Rev. 2018, 93, 38–59. [Google Scholar] [CrossRef]
- Walter, B.F.; Giebel, R.J.; Steele-MacInnis, M.; Marks, M.A.; Kolb, J.; Markl, G. Fluids associated with carbonatitic magmatism: A critical review and implications for carbonatite magma ascent. Earth Sci. Rev. 2021, 215, 103509. [Google Scholar] [CrossRef]
- Kolonin, G.; Shironosova, G. Thermodynamic model for REE complexation in the course of interaction between REE-fluorite and hydrothermal fluid. Geochem. Int. 2002, 40, 103–112. [Google Scholar]
- Migdisov, A.A.; Williams-Jones, A.E. Hydrothermal transport and deposition of the rare earth elements by fluorine-bearing aqueous liquids. Miner. Depos. 2014, 49, 987–997. [Google Scholar] [CrossRef]
- Migdisov, A.A.; Williams-Jones, A.E.; Brugger, J.; Caporuscio, F.A. Hydrothermal transport, deposition, and fractionation of the REE: Experimental data and thermodynamic calculations. Chem. Geol. 2016, 439, 13–42. [Google Scholar] [CrossRef]
- Perry, E.; Gysi, A.P. Rare earth elements in mineral deposits: Speciation in hydrothermal fluids and partitioning in calcite. Geofluids 2018, 2018, 5382480. [Google Scholar] [CrossRef]
- Smith, M.; Henderson, P.; Campbell, L. Fractionation of the REE during hydrothermal processes: Constraints from the Bayan Obo Fe-REE-Nb deposit, Inner Mongolia, China. Geochim. Cosmochim. Acta 2000, 64, 3141–3160. [Google Scholar] [CrossRef]
- Smith, M.P.; Campbell, L.; Kynicky, J. A review of the genesis of the world class Bayan Obo Fe–REE–Nb deposits, Inner Mongolia, China: Multistage processes and outstanding questions. Ore Geol. Rev. 2015, 64, 459–476. [Google Scholar] [CrossRef]
- Gagnon, R.; Buro, Y.A.; Ibrango, S.; Gagnon, D.; Stapinsky, M.; Del Carpio, S.; Larochelle, E. Projet de Terres Rares Kwyjibo; Rapport technique NI 43-101 révisé, 2018; Dra Met-Chem: Montréal, QC, Canada, 2018; Available online: https://www.sedarplus.ca/csa-party/records/document.html?id=6fe7e177d4d03d91a694437e1ea496e30055103b092233ae7ba5039ea38c7265 (accessed on 15 December 2023).
- Sappin, A.-A.; Perreault, S. Drill Core Pictures and Description of Samples Collected from the REE Kwyjibo Deposit (SOQUEM Warehouse, Val d’Or, QC–October 2015); Open File 8794; Geological Survey of Canada: Ottawa, ON, Canada, 2021. [Google Scholar]
- Williams-Jones, A.E.; Samson, I.; Olivo, G. The genesis of hydrothermal fluorite-REE deposits in the Gallinas Mountains, New Mexico. Econ. Geol. 2000, 95, 327–341. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; O’Reilly, D.; Wilson, R.; Das, K.; Wade, B. Mineral chemistry of rare earth element (REE) mineralization, Browns Range, Western Australia. Lithos 2013, 172–173, 192–213. [Google Scholar] [CrossRef]
- Trofanenko, J.; Williams-Jones, A.E.; Simandl, G.J.; Migdisov, A.A. The nature and origin of the REE mineralization in the Wicheeda carbonatite, British Columbia, Canada. Econ. Geol. 2016, 111, 199–223. [Google Scholar] [CrossRef]
- Day, W.C.; Slack, J.F.; Ayuso, R.A.; Seeger, C.M. Regional geologic and petrologic framework for iron oxide ± apatite ± rare earth element and iron oxide copper-gold deposits of the Mesoproterozoic St. Francois Mountains Terrane, Southeast Missouri, USA. Econ. Geol. 2016, 111, 1825–1858. [Google Scholar] [CrossRef]
- Baker, J.H.; Hellingwerf, R.H. Rare-earth elements in lithochemical prospecting for W-Mo-Au mineralized granites and related high- and low-temperature skarns [abs.]. In Proceeding of the South Symposium on Exploration Geochemistry, Athens, Greece, 10–11 November 1986; Program with Abstract; Institute of Geology and Mineral Exploration and Association of Exploration Geologists Internat: Athens, Greece, 1986. [Google Scholar]
- Wood, S.A. The aqueous geochemistry of the rare-earth elements and yttrium 2. Theoretical predictions of speciation in hydrothermal solutions to 350 °C at saturated water vapour pressure. Chem. Geol. 1991, 88, 99–125. [Google Scholar] [CrossRef]
- Keppler, H. Influence of fluorine on the enrichment of high field strength trace elements in granitic rocks. Contrib. Mineral. Petrol. 1993, 114, 479–488. [Google Scholar] [CrossRef]
- Corriveau, L.; Montreuil, J.-F.; Blein, O.; Potter, E.; Ansari, M.; Craven, J.; Enkin, R.; Fortin, R.; Harvey, B.; Hayward, N.; et al. Metasomatic Iron and Alkali Calcic (MIAC) System Frameworks: A TGI-6 Task Force to Help De-Risk Exploration for IOCG, IOA and Affiliated Primary Critical Metal Deposits; Geological Survey of Canada: Ottawa, ON, Canada, 2021; Scientific Presentation 127; 105 p. [Google Scholar]
- Corriveau, L.; Montreuil, J.-F.; Hayward, N.; Tschirhart, V.; Blein, O.; Boulanger, O.; Adlakha, E.; Pippy, R. Metasomatic iron and alkali-calcic (MIAC) mineral systems: A TGI6 geoscience foundation for critical mineral wealth and Indigenous mineral resources development. In Targeted Geoscience Initiative 6: Volcanic, Sedimentary, and Hydrothermal Ore Systems Genesis and Exploration Methods; Peter, J.M., Gadd, M.G., Eds.; Open File; Geological Survey of Canada: Ottawa, ON, Canada, 2025; in press. [Google Scholar]
- Corriveau, L.; Lauzière, K.; Montreuil, J.-F.; Potter, E.; Prémont, S.; Hanes, R. Dataset of New Lithogeochemical Analysis in the Great Bear Magmatic Zone, Northwest Territories, Canada; Open File 7643; Geological Survey of Canada: Ottawa, ON, Canada, 2015. [Google Scholar]
- Lacasse, C.M.; Ganade, C.E.; Mathieru, L.; Teixeira, N.A.; Lopes, L.B.L.; Monteiro, C.F. Restoring original composition of hydrothermally altered Archean metavolcanic rocks of the Carajás Mineral Province (Brazil): Geodynamic implications for the transition from lid to mobile tectonics. Lithos 2020, 373, 105647. [Google Scholar] [CrossRef]
- Torresi, I.; Xavier, R.P.; Bortholote, D.F.A.; Monteiro, L.V.S. Hydrothermal alteration, fluid inclusions and stable isotope systematics of the Alvo 118 iron-oxide-copper-gold deposit, Carajás Mineral Province (Brazil): Implications for ore genesis. Miner. Depos. 2012, 47, 299–323. [Google Scholar] [CrossRef]
- Holdsworth, R.E.; Pinheiro, R.V.L. The anatomy of shallow-crustal transpressional structures: Insights from the Archaean Carajás fault zone, Amazon, Brazil. J. Struct. Geol. 2000, 22, 1105–1123. [Google Scholar] [CrossRef]
- Almeida, J.A.C.; Dall’Agnol, R.; Oliveira, M.A.; Macambira, M.J.B.; Pimentel, M.M.; Rämö, O.T.; Guimaraes, F.V.; Leite, A.A.S. Zircon geochronology and geochemistry of the TTG suites of the Rio Maria granite-greenstone terrane: Implications for the growth of the Archean crust of Carajás Province, Amazonian craton, Brazil. Precambrian Res. 2013, 227, 157–185. [Google Scholar] [CrossRef]
- Trunfull, E.F.; Hagemann, S.G.; Xavier, R.P.; Moreto, C.P.N. Critical assessment of geochronological data from the Carajás Mineral Province, Brazil: Implications for metallogeny and tectonic evolution. Ore Geol. Rev. 2020, 121, 103556. [Google Scholar] [CrossRef]
- Oliveira, R.G. Insights on the framework of the Carajás province, Amazonian craton, Brazil, and on the three-dimensional shape of the Carajás Basin, based on gravity data. J. Geol. Surv. Brazil 2018, 1, 101–112. [Google Scholar] [CrossRef]
- Spry, P.G.; Peter, J.M.; Slack, J.F. Meta-exhalites as exploration guides to ore. In Metamorphic and Metamorphogenic Ore Deposits; Spry, P.G., Marshall, B., Vokes, F.M., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2000; Reviews in Economic Geology 11; pp. 163–201. [Google Scholar]
- Thompson, J.A. Relationships of coticule geochemistry to stratigraphy in the Perry Mountain and Megunticook Formations, New England Appalachians. Can. Mineral. 2001, 39, 1021–1037. [Google Scholar] [CrossRef]
- Willner, A.P.; Pawlig, S.; Massonne, H.-J.; Hervé, F. Metamorphic evolution of spessartine quartzites (coticules) in the high-pressure, low-temperature complex at Bahia Mansa, Coastal Cordillera of South-Central Chile. Can. Mineral. 2001, 39, 1547–1569. [Google Scholar] [CrossRef]
- Renard, A. Sur la Structure et la Composition Minéralogique du Coticule et sur ses Rapports avec le Phyllade Oligistifère; Mémoires Couronnés de l’Académie Royale de Belgique; Editions de l’Académie Royale de Belgique: Brussels, Belgium, 1978; Volume 41. [Google Scholar]
- Spry, P.G. Geochemistry and origin of coticules (spessartine-quartz rocks) associated with metamorphosed massive sulfide deposits. In Regional Metamorphism of Ore Deposits and Genetic Implications; Spry, P.G., Bryndzia, L.T., Eds.; VSP: Utrecht, The Netherlands, 1990; pp. 49–75. [Google Scholar]
- Krosse, S.; Schreyer, W. Comparative geochemistry of coticules (spessartine-quartzites) and their red schist country rocks in the Ordovician of the Ardennes Mountains, Belgium. Chem. Erde 1993, 53, 1–20. [Google Scholar]
- Slack, J.F.; Shaw, D.R.; Leitch, C.H.B. Tourmalinites and coticules from Sullivan-type Pb-Zn-Ag deposit and vicinity, British Columbia: Geology, geochemistry and genesis. In Geological Environment of the Sullivan Deposit, British Columbia; Lydon, J.W., Hoy, T., Slack, J.F., Knapp, M.E., Eds.; Geological Association of Canada, Mineral Deposits Division: St John’s, NL, Canada, 2000; Special Volume 1. [Google Scholar]
- Martins, T.F.; Seoane, J.C.S.; Tavares, F.M. Cu–Au exploration target generation in the eastern Carajás Mineral Province using random forest and multi-class index overlay mapping. J. S. Am. Earth Sci. 2022, 116, 103790. [Google Scholar] [CrossRef]
- Nykänen, V.; Groves, D.I.; Ojala, V.J.; Eilu, P.; Gardoll, S.J. Reconnaissance scale conceptual fuzzy-logic prospectivity modeling for iron oxide copper-gold deposits in the northern Fennoscandian Shield, Finland. Aust. J. Earth Sci. 2008, 55, 25–38. [Google Scholar] [CrossRef]
- Cloutier, J.; Ford, A.; Huston, D.; Haynes, M.; Schofield, A.; Doublier, M.; Sanchez, G.; Duan, J.; Goodwin, J.; Beyer, E.; et al. Iron oxide copper–gold potential of Australia using a hybrid data- and knowledge-driven approach and its implications for the formation of IOCG mineral systems. Ore Geol. Rev. 2025, 185, 106802. [Google Scholar] [CrossRef]
- Trapy, P.-H. Modélisation d’Équilibre de Phase Prédictive des Faciès d’Altération Associés aux Gîtes à Oxydes de Fer-Cuivre-Or dans les Terrains de Hauts Grades Métamorphiques. Unpublished Master’s Thesis, École Polytechnique de Montréal, Montréal, QC, Canada, 2018. [Google Scholar]
- Ministère des Ressources Naturelles et des Forêts. SIGEOM Database. Available online: https://sigeom.mines.gouv.qc.ca/signet/classes/I1108_afchCarteIntr?l=A (accessed on 1 May 2025).
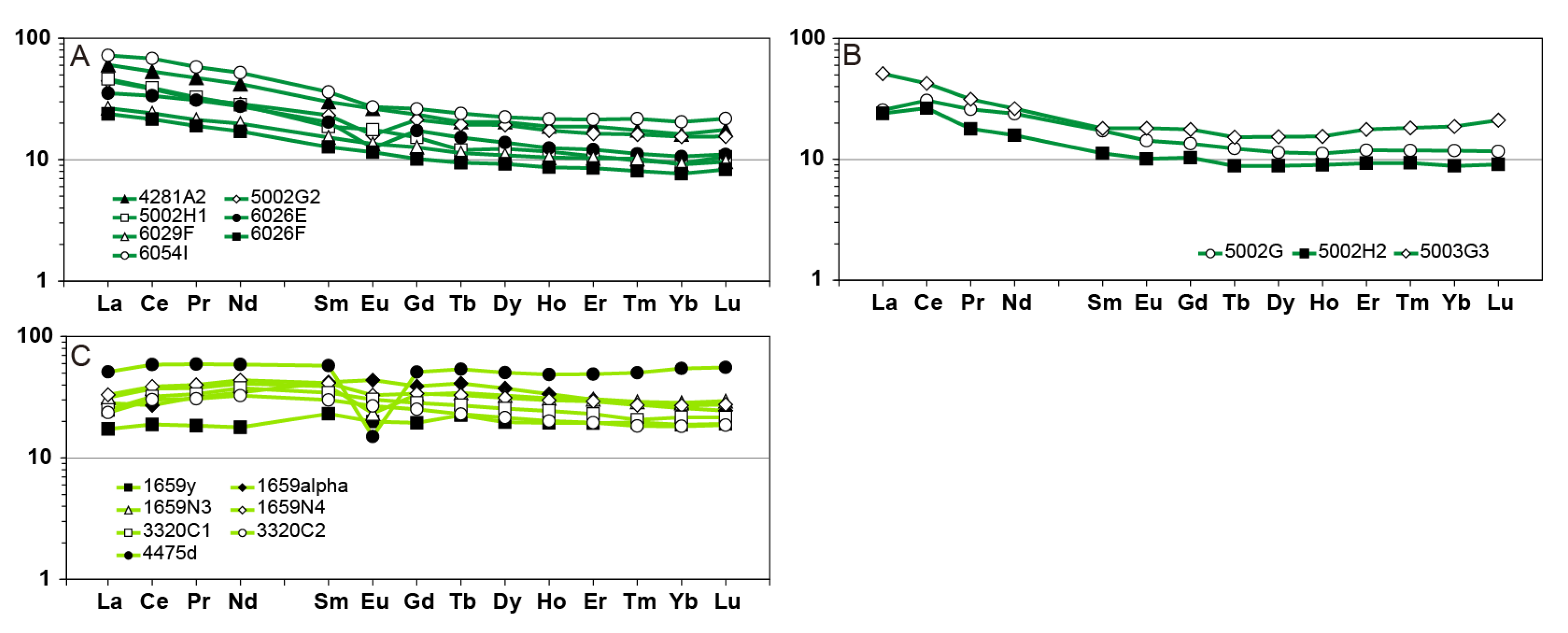
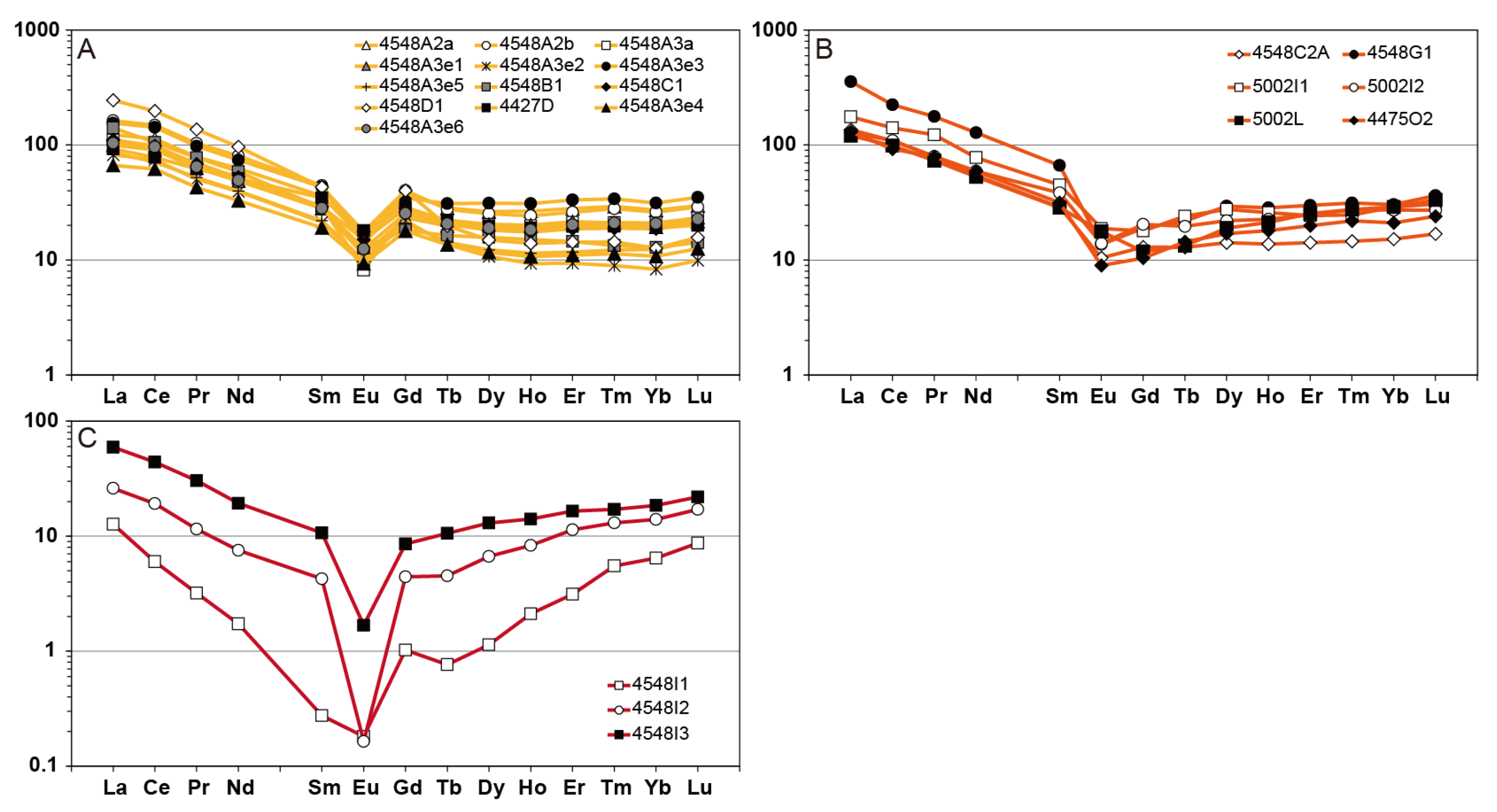
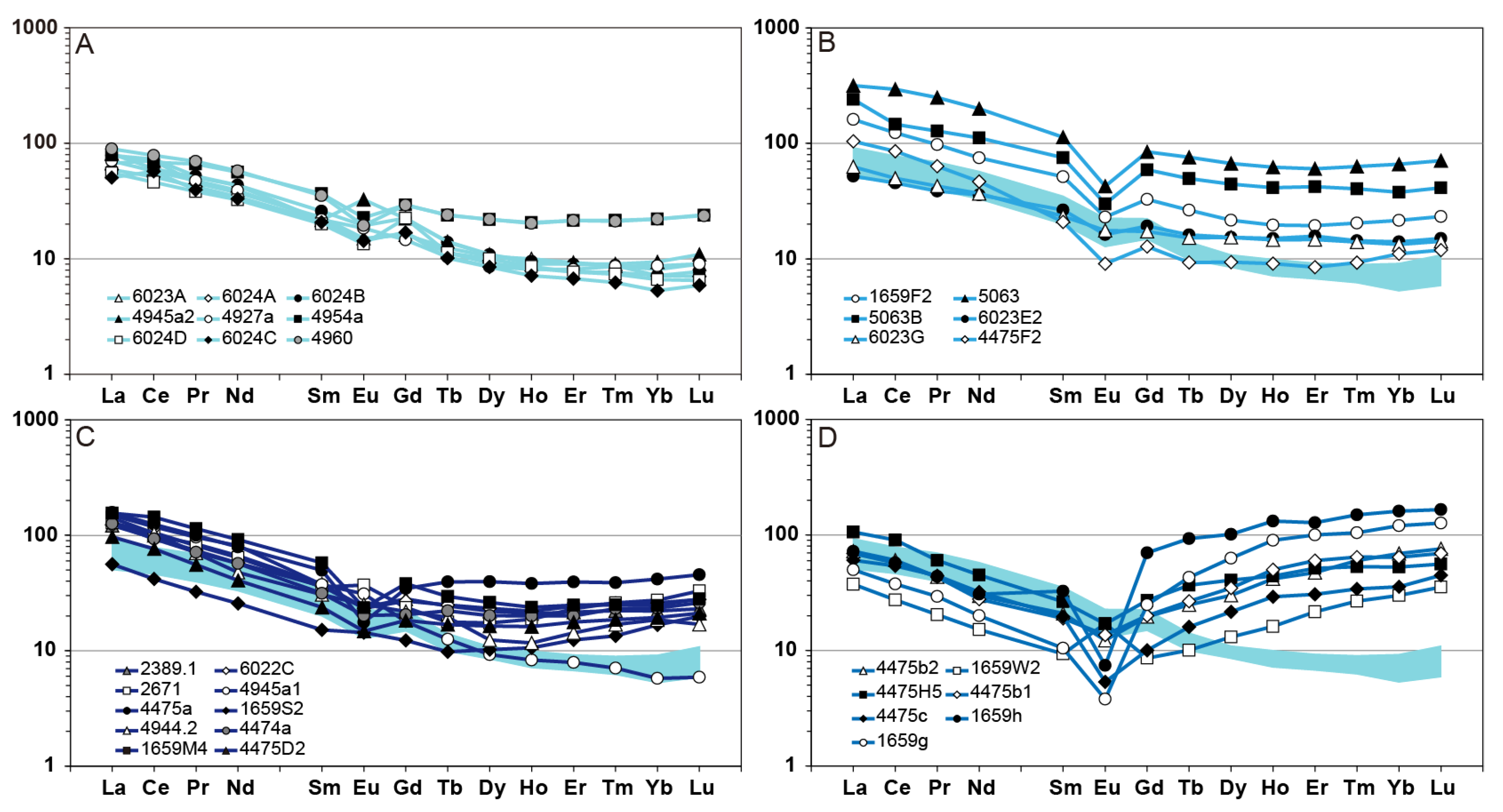
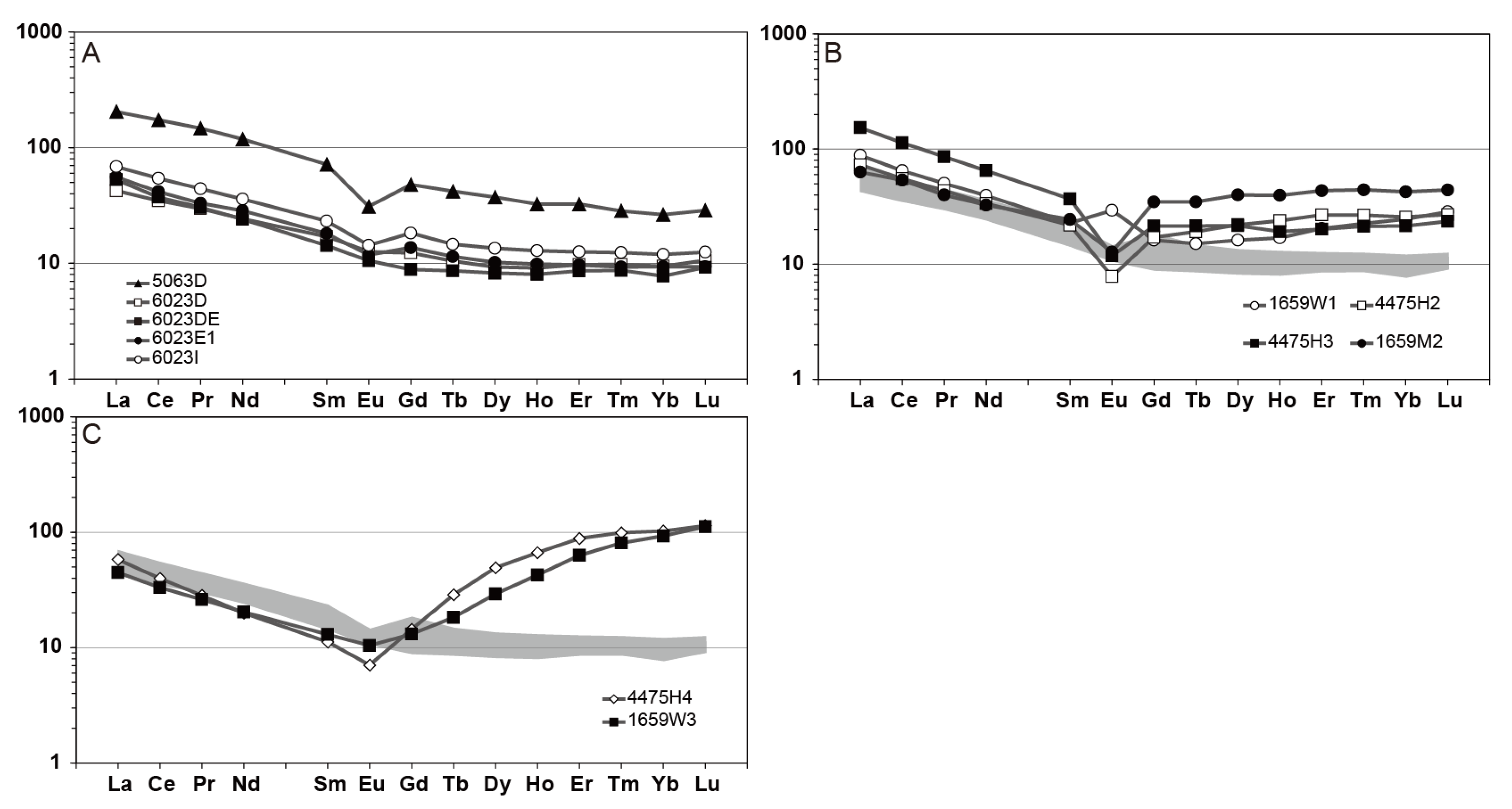
| Mineral System | Alteration Facies | Diagnostic Minerals |
|---|---|---|
| VMS | Advanced argillic | Kaolinite, alunite, opal, smectite |
| Argillic | Sericite, illite, smectite, pyrophyllite, opal | |
| Sericitic | Sericite, illite, opal | |
| Chloritic | Chlorite, opal, quartz, sericite | |
| Carbonate propylitic | Chlorite, epidote, chlorite, sericite, feldspar | |
| Silicic | Quartz | |
| Porphyry | Potassic | K-feldspar, biotite |
| Sericitic | Sericite | |
| Advanced argillic | Kaolinite, alunite, pyrophyllite | |
| Propylitic | Epidote, chlorite, actinolite, illite-sericite, smectite | |
| Sodic-calcic and sodic | Albite, actinolite, epidote, chlorite | |
| Greisen | Muscovite, quartz | |
| Epithermal | Propylitic | Quartz, K-feldspar, albite, illite, chlorite, calcite |
| Argillic | Smectite, illite, chlorite | |
| Advanced argillic | Quartz, alunite, kaolinite, pyrophyllite | |
| Potassic | K-feldspar, illite, quartz, carbonate | |
| Silicic | Quartz, chalcedony, opal | |
| MIAC | Na | Albite, scapolite |
| Na-Ca | Albite, oligoclase, scapolite, clinopyroxene, amphibole | |
| Na-Ca-Fe | Albite, amphibole, magnetite, scapolite | |
| High-temperature Ca-Fe | Fe-rich amphibole, apatite, magnetite, epidote | |
| High-temperature Ca-K-Fe | Fe-rich amphibole, biotite, magnetite, siderite, K-feldspar | |
| High-temperature K-Fe | Biotite, magnetite, K-feldspar | |
| Low-temperature K-Fe | Hematite, sericite | |
| Low-temperature Ca-Mg | Actinolite, carbonates, chlorite, epidote, fluorite |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Portions of this work are reproduced with permission under crown copyright, held by his Majesty the King in Right of Canada, and are made available under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blein, O.; Corriveau, L. Element Mobility in a Metasomatic System with IOCG Mineralization Metamorphosed at Granulite Facies: The Bondy Gneiss Complex, Grenville Province, Canada. Minerals 2025, 15, 803. https://doi.org/10.3390/min15080803
Blein O, Corriveau L. Element Mobility in a Metasomatic System with IOCG Mineralization Metamorphosed at Granulite Facies: The Bondy Gneiss Complex, Grenville Province, Canada. Minerals. 2025; 15(8):803. https://doi.org/10.3390/min15080803
Chicago/Turabian StyleBlein, Olivier, and Louise Corriveau. 2025. "Element Mobility in a Metasomatic System with IOCG Mineralization Metamorphosed at Granulite Facies: The Bondy Gneiss Complex, Grenville Province, Canada" Minerals 15, no. 8: 803. https://doi.org/10.3390/min15080803
APA StyleBlein, O., & Corriveau, L. (2025). Element Mobility in a Metasomatic System with IOCG Mineralization Metamorphosed at Granulite Facies: The Bondy Gneiss Complex, Grenville Province, Canada. Minerals, 15(8), 803. https://doi.org/10.3390/min15080803