Mechanisms and Genesis of Acidic Goaf Water in Abandoned Coal Mines: Insights from Mine Water–Surrounding Rock Interaction
Abstract
1. Introduction
2. Geologic Setting
3. Materials and Methods
3.1. Sample Collection and Characterization
3.2. Sequential Extraction
3.3. Batch Test
3.4. Column Tests
4. Results
4.1. Hydrochemistry of Goaf Water
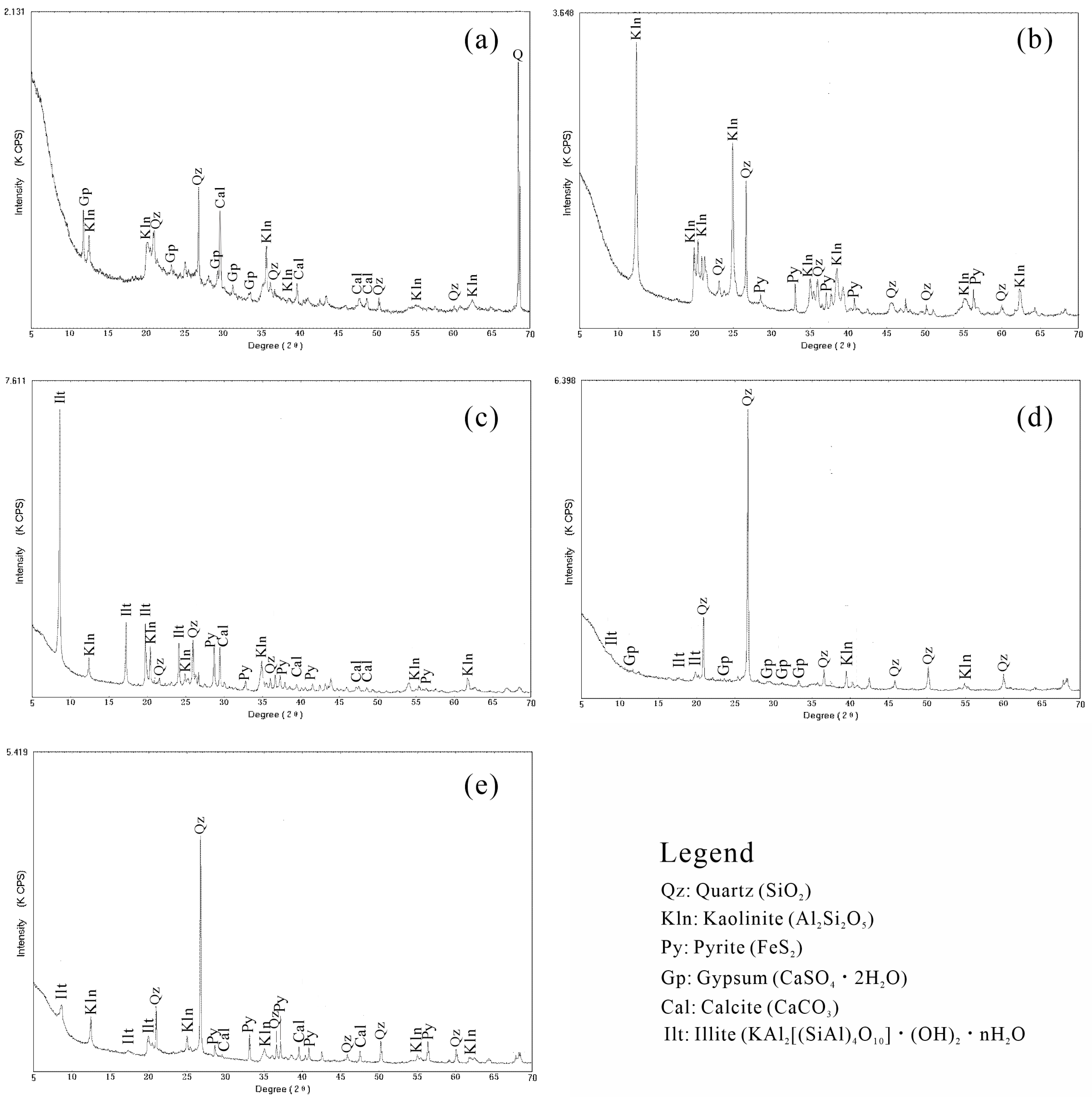
4.2. Mineralogy of CMSR
4.3. Heavy Metals and Trace Elements in CMSR
4.4. Occurrence of Fluorine in CMSR
4.4.1. Total Fluorine
4.4.2. Speciation of Fluorine
5. Discussion
5.1. Water–Surrounding Rock Interaction
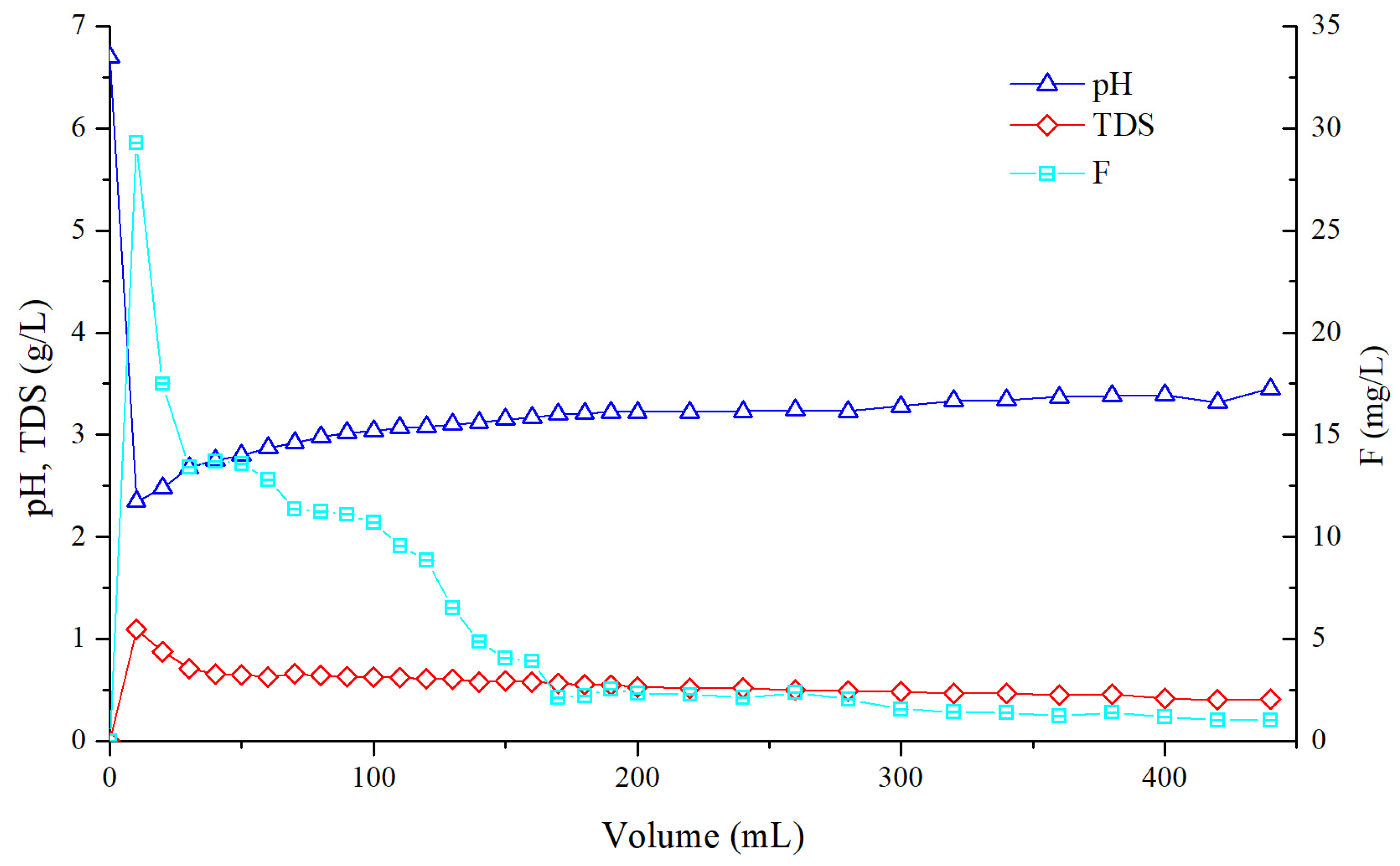
5.2. Variation of pH, TDS and Fluorine in Leachate Solution
5.3. Major Cations in Leaching Solution
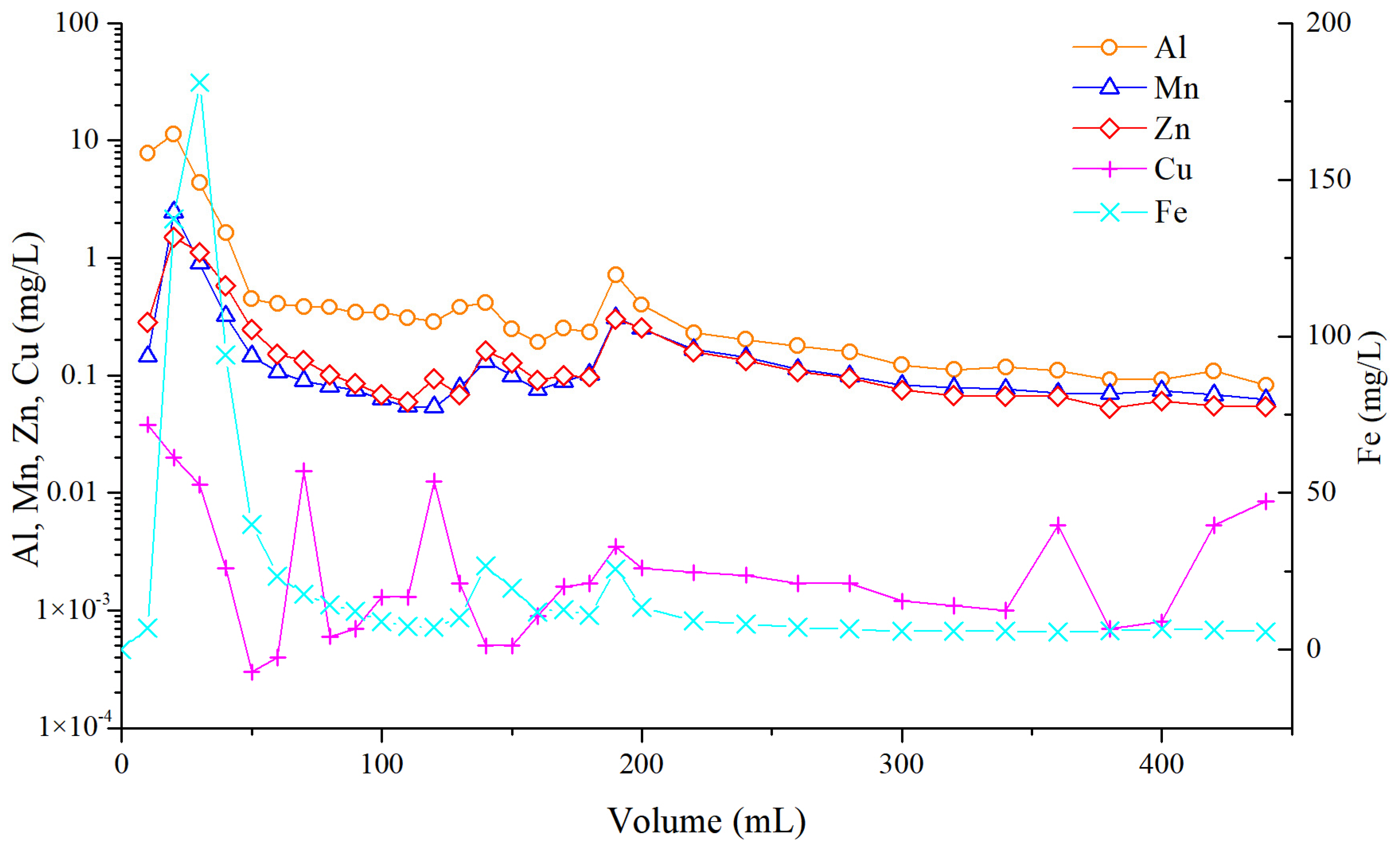
5.4. Dynamic Leaching of Major Heavy Metals and Fluorine
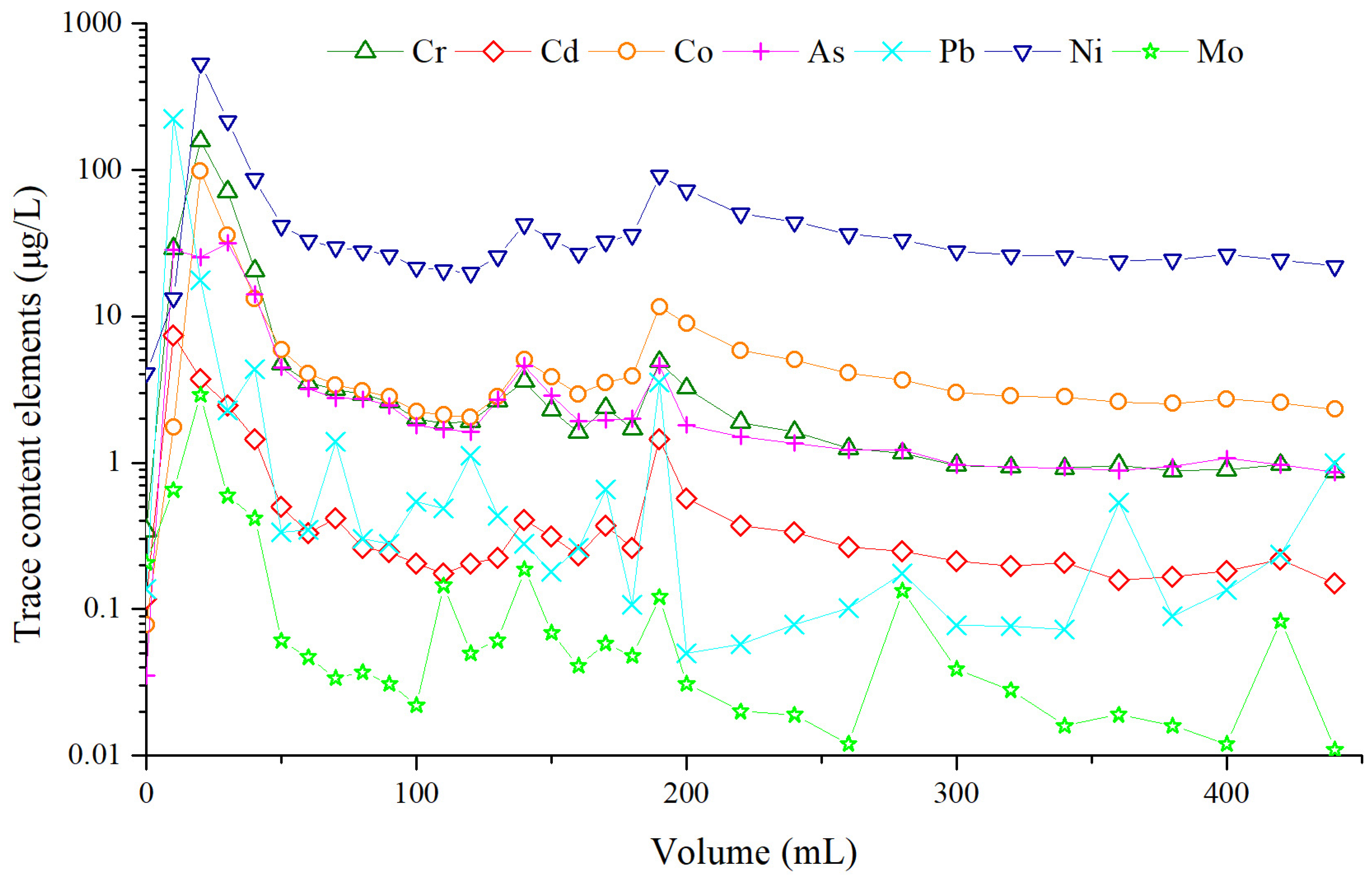
5.5. Trace Content Elements During Leaching Process
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- BP. Statistical Review of World Energy; BP PLC: London, UK, 2023. [Google Scholar]
- IEA (International Energy Agency). Coal 2023: Analysis and Forecast to 2026; IEA: Paris, France, 2023. [Google Scholar]
- Liu, H.B.; Liu, Z.L. Recycling utilization patterns of coal mining waste in China. Resour. Conserv. Recycl. 2010, 54, 1331–1340. [Google Scholar] [CrossRef]
- Zhao, H.R.; Li, B.K.; Wang, X.J.; Lu, H.; Li, H.Z. Evaluating the performance of China’s coal-fired power plants considering the coal depletion cost: A system dynamic analysis. J. Clean. Prod. 2020, 275, 122809. [Google Scholar] [CrossRef]
- Tai, X.; Xiao, W.; Tang, Y. A quantitative assessment of vulnerability using social-economic-natural compound ecosystem framework in coal mining cities. J. Clean. Prod. 2020, 258, 120969. [Google Scholar] [CrossRef]
- Tiwary, R.K. Environmental impact of coal mining on water regime and its management. Water Air Soil Pollut. 2001, 132, 185–199. [Google Scholar] [CrossRef]
- Xie, J.; Xin, L.; Hu, X.M.; Cheng, W.M.; Liu, W.T.; Wang, Z.G. Technical application of safety and cleaner production technology by underground coal gasification in China. J. Clean. Prod. 2020, 250, 119487. [Google Scholar] [CrossRef]
- Equeenuddin, S.M. Leaching of trace elements from Indian coal. J. Geol. Soc. India 2015, 86, 102–106. [Google Scholar] [CrossRef]
- Hua, C.Y.; Zhou, G.Z.; Yin, X.; Wang, C.Z.; Chi, B.R.; Cao, Y.Y.; Wang, Y.; Zheng, Y.; Cheng, Z.R.; Li, R.Y. Assessment of heavy metal in coal gangue: Distribution, leaching characteristic and potential ecological risk. Environ. Sci. Pollut. Res. 2018, 25, 32321–32331. [Google Scholar] [CrossRef]
- Huang, Y.L.; Li, J.M.; Song, T.Q.; Sun, Q.; Kong, G.Q.; Wang, F.W. Microstructure of coal gangue and precipitation of heavy metal elements. J. Spectrosc. 2017, 2017, 3128549. [Google Scholar] [CrossRef]
- Rashid, A.; Ayub, M.; Gao, X.B.; Xu, Y.Y.; Ullah, Z.; Zhu, Y.G.; Ali, L.; Li, C.C.; Ahmad, A.; Rinklebe, J.; et al. Unraveling the impact of high arsenic, fluoride and microbial population in community tubewell water around coal mines in a semiarid region: Insight from health hazards, and geographic information systems. J. Hazard. Mater. 2024, 480, 136064. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid mine drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.J.; Wu, Q.; Liu, H.L.; Jiao, J. Prediction and assessment of the disturbances of the coal mining in Kailuan to karst groundwater system. Phys. Chem. Earth A/B/C/ 2015, 89–90, 136–144. [Google Scholar] [CrossRef]
- Ahmad, N.; Niamatullah; Hussain, J.; Ahmad, I.; Asif, M. Estimation of health risk to humans from heavy metals in soil of coal mines in Harnai, Balochistan. Int. J. Environ. Anal. Chem. 2022, 102, 3894–3905. [Google Scholar] [CrossRef]
- Ahn, Y.; Han, M.; Choi, J. Monitoring the mobility of heavy metals and risk assessment in mine-affected soils after stabilization. J. Hazard. Mater. 2020, 400, 123231. [Google Scholar] [CrossRef]
- Favas, P.J.C.; Sarkar, S.K.; Rakshit, D.; Venkatachalam, P.; Prasad, M.N.V. Acid mine drainages from abandoned mines: Hydrochemistry, environmental impact, resource recovery, and prevention of pollution. In Environmental Materials and Waste: Resource Recovery and Pollution Prevention; Academic Press: New York, NY, USA, 2016; pp. 413–462. [Google Scholar] [CrossRef]
- Zhang, X.B.; Li, X.; Gao, X.B. Hydrochemistry and coal mining activity induced karst water quality degradation in the Niangziguan karst water system, China. Environ. Sci. Pollut. Res. 2016, 23, 6286–6299. [Google Scholar] [CrossRef]
- Li, C.C.; Gao, X.B.; Wang, W.Z.; Zhang, X.; Zhang, X.B.; Jiang, C.F.; Wang, Y.X. Hydro-biogeochemical processes of surface water leakage into groundwater in large scale karst water system: A case study at Jinci, northern China. J. Hydrol. 2021, 596, 125691. [Google Scholar] [CrossRef]
- Tao, L.C.; Zhang, Y.H.; Yuan, X.C.; Chen, Q.S.; Yu, J.H.; Ma, Y.Q.; Liu, H.H.; Tu, C.L. Hydrological processes in multi-layered aquifers of a karst watershed with coal mining activity: Insights from hydrochemistry and isotopes. J. Hydrol. Reg. Stud. 2024, 56, 102016. [Google Scholar] [CrossRef]
- Fang, L.; Zhou, A.G.; Li, X.Q.; Zhou, J.W.; Pan, G.F.; He, N.J. Response of antimony and arsenic in karst aquifers and groundwater geochemistry to the influence of mine activities at the world’s largest antimony mine, central China. J. Hydrol. 2021, 603, 127131. [Google Scholar] [CrossRef]
- Ding, L.J.; Wang, F.G.; Yuan, J.F.; Liu, H.Z.; Cheng, Z.L.; Cao, Y.Q. Spatial variability of hydrochemistry in coal-bearing karst areas considering sulfur pollution and underground engineering effects. Environ. Pollut. 2025, 371, 125957. [Google Scholar] [CrossRef]
- Peng, G.Z.; Gao, X.B.; Naseem, A.; Zhang, Y.F.; Wang, X.J.; Fu, W.X.; Yu, F.Z.; Ma, S.Y.; Shi, W.P.; Yi, L.; et al. Karst water quality, source of pollution, and health risk assessment in China. Sci. Total Environ. 2025, 973, 179120. [Google Scholar] [CrossRef]
- Huang, X.J.; Wang, G.C.; Liang, X.Y.; Cui, L.F.; Ma, L.; Xu, Q.Y. Hydrochemical and Stable Isotope (δD and δ18O) Characteristics of Groundwater and Hydrogeochemical Processes in the Ningtiaota Coalfield, Northwest China. Mine Water Environ. 2018, 37, 119–136. [Google Scholar] [CrossRef]
- Lazareva, E.V.; Myagkaya, I.N.; Kirichenko, I.S.; Gustaytis, M.A.; Zhmodik, S.M. Interaction of natural organic matter with acid mine drainage: In-situ accumulation of elements. Sci. Total Environ. 2019, 660, 468–483. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gao, X.B.; Tan, T.; Li, C.C.; Yan, R.Y.; Chi, Z.Y.; Feng, Y.; Gong, P.L.; Fang, J.C.; Zhang, X.Z.; et al. Sources and pollution path identification of PAHs in karst aquifers: An example from Liulin karst water system, northern China. J. Contam. Hydrol. 2021, 241, 103810. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Zhang, X.; Gao, X.B.; Qi, S.H.; Wang, Y.X. The Potential Environmental Impact of PAHs on Soil and Water Resources in Air Deposited Coal Refuse Sites in Niangziguan Karst Catchment, Northern China. Int. J. Environ. Res. Public Health 2019, 16, 1368. [Google Scholar] [CrossRef]
- Qiao, X.J.; Li, G.M.; Li, M.; Zhou, J.L.; Du, J.; Du, C.Y.; Sun, Z.H. Influence of coal mining on regional karst groundwater system: A case study in West Mountain area of Taiyuan City, northern China. Environ. Earth Sci. 2011, 64, 1525–1535. [Google Scholar] [CrossRef]
- Wu, Q.; Xing, L.T.; Ye, C.H.; Liu, Y.Z. The influences of coal mining on the large karst springs in North China. Environ. Earth Sci. 2011, 64, 1513–1523. [Google Scholar] [CrossRef]
- Xiao, W.; Zhang, W.K.; Ye, Y.M.; Lv, X.J.; Yang, W.F. Is underground coal mining causing land degradation and significantly damaging ecosystems in semi-arid areas? A study from an Ecological Capital perspective. Land Degrad. Dev. 2020, 31, 1969–1989. [Google Scholar] [CrossRef]
- Shen, S.H. Coal-mining technology in China. Energy 1986, 11, 1141–1145. [Google Scholar] [CrossRef]
- He, Q.; An, Y.F.; Sun, F.J.; Lai, C. Genesis of Pyrite Concretions: Constraints from Mineral and Geochemical Features of Longtan Formation in Anhui Province, Eastern China. Minerals 2019, 9, 467. [Google Scholar] [CrossRef]
- Zhu, G.; Wu, X.; Ge, J.P.; Liu, F.; Zhao, W.G.; Wu, C. Influence of mining activities on groundwater hydrochemistry and heavy metal migration using a self-organizing map (SOM). J. Clean. Prod. 2020, 257, 120664. [Google Scholar] [CrossRef]
- Mokgehle, T.M.; Gitari, W.M.; Tavengwa, N.T. Synthesis of di-carboxylic acid functionalized zeolites from coal fly ash for Cd (II) removal from acid mine drainage using column studies approach. J. Environ. Chem. Eng. 2019, 7, 103473. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Mamba, B.B. Acid mine drainage: Prevention, treatment options, and resource recovery: A review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Chen, G.; Ye, Y.C.; Yao, N.; Hu, N.Y.; Zhang, J.; Huang, Y. A critical review of prevention, treatment, reuse, and resource recovery from acid mine drainage. J. Clean. Prod. 2021, 329, 129666. [Google Scholar] [CrossRef]
- Christensen, B.; Laake, M.; Lien, T. Treatment of acid mine water by sulfatereducing bacteria; results from a bench scale experiment. Water Res. 1996, 30, 1617–1624. [Google Scholar] [CrossRef]
- Sebogodi, K.R.; Johakimu, J.K.; Sithole, B.B. Beneficiation of pulp mill waste green liquor dregs: Applications in treatment of acid mine drainage as new disposal solution in South Africa. J. Clean. Prod. 2020, 246, 118979. [Google Scholar] [CrossRef]
- Bradl, H.B. Chapter 1 Sources and origins of heavy metals. In Interface Science and Technology; Bradl, H.B., Ed.; Elsevier: Birkenfeld, Germany, 2005; Volume 6, pp. 1–27. ISBN 1573-4285. [Google Scholar] [CrossRef]
- Li, Q.G.; Wu, P.; Zha, X.F.; Li, X.X.; Wu, L.N.; Gu, S.Y. Effects of mining activities on evolution of water chemistry in coal-bearing aquifers in karst region of Midwestern Guizhou, China: Evidences from δ13C of dissolved inorganic carbon and δ34S of sulfate. Environ. Sci. Pollut. Res. 2018, 25, 18038–18048. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.L.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, S.; Igarashi, T.; Tabelin, C.B.; Tangviroon, P.; Li, H. Acid mine drainage sources and hydrogeochemistry at the Yatani mine, Yamagata, Japan: A geochemical and isotopic study. J. Contam. Hydrol. 2019, 225, 103502. [Google Scholar] [CrossRef]
- Sun, J.; Kobayashi, T.; Strosnider, W.H.J.; Wu, P. Stable sulfur and oxygen isotopes as geochemical tracers of sulfate in karst waters. J. Hydrol. 2017, 551, 245–252. [Google Scholar] [CrossRef]
- Candeias, C.; Melo, R.; Avila, P.F.; Da Silva, E.F.; Salgueiro, A.R.; Teixeira, J.P. Heavy metal pollution in mine-soil-plant system in S. Francisco de Assis- panasqueira mine (Portugal). Appl. Geochem. 2014, 44, 12–26. [Google Scholar] [CrossRef]
- Halim, M.A.; Majumder, R.K.; Zaman, M.N. Paddy soil heavy metal contamination and uptake in rice plants from the adjacent area of Barapukuria coal mine, northwest Bangladesh. Arab. J. Geosci. 2015, 8, 3391–3401. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, W.X.; Zhao, H.Q.; Yang, Q.C.; Yang, Z.P. Potential ecological risk assessment and prediction of soil heavy-metal pollution around coal gangue dump. Nat. Hazards Earth Syst. Sci. 2014, 14, 1599–1610. [Google Scholar] [CrossRef]
- Nordstrom, D.K. Hydrogeochemical processes governing the origin, transport and fate of major and trace elements from mine wastes and mineralized rock to surface waters. Appl. Geochem. 2011, 26, 1777–1791. [Google Scholar] [CrossRef]
- Hao, H.Y.; Hao, X.X.; Wu, P.; Li, X.X.; Han, Z.W.; Cao, X.X.; Yang, B.; Zhang, R.X.; Zhang, K.F.; Chen, M.Z.; et al. Different hydrogeological characteristics of springs associated with abandoned coal mines in well-developed karst area. J. Hydrol. 2025, 653, 132683. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Li, J.H.; Hao, S.J.; Wen, L.; Ma, Q.; Liu, C.J.; Shen, W. Temporal and Spatial Analysis of Water Resources under the Influence of Coal Mining: A Case Study of Yangquan Basin, China. Water 2023, 15, 3058. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, X.B.; Jiang, C.F.; Li, C.C.; Zhang, X.; Wang, W.Z.; Duan, Y.; Luo, W.T.; Mao, Z.F.; Wang, Y.X. Multiple contamination sources, pathways and conceptual model of complex buried karst water system: Constrained by hydrogeochemistry and δ2H, δ18O, δ34S, δ13C and 87Sr/86Sr isotopes. J. Hydrol. 2024, 639, 131614. [Google Scholar] [CrossRef]
- Gao, X.B.; Wang, Y.X.; Ma, T.; Hu, Q.H.; Xing, X.L.; Yu, Q. Anthropogenic impact assessment of Niangziguan karst water. Proc. Inst. Civ. Eng. Water Manag. 2011, 164, 495–510. [Google Scholar] [CrossRef]
- Ma, L.Y.; Lin, L.F.; Wang, X.J.; Zheng, Z.K.; Zhang, X.; Srivastava, P.; Gao, X.B. Sulfate and pH drive microbial assembly and coexistence in hyporheic zone contaminated by acid coal mine drainage. J. Hydrol. 2025, 652, 132703. [Google Scholar] [CrossRef]
- Feng, Q.Y.; Li, T.; Qian, B.; Zhou, L.; Gao, B.; Yuan, T. Chemical Characteristics and Utilization of Coal Mine Drainage in China. Mine Water Environ. 2014, 33, 276–286. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, H.X.; Xie, Z.H.; Huang, H.Y.; Zang, S.Y.; Lian, B. Heavy metal pollution characteristics in the Kaili coal mining region, Guizhou Province, China. J. Residuals Sci. Technol. 2015, 12, 123–131. [Google Scholar] [CrossRef]
- Wang, M.C.; Gui, H.R.; Hu, R.J.; Zhao, H.H.; Li, J.; Yu, H.; Fang, H.X. Hydrogeochemical Characteristics and Water Quality Evaluation of Carboniferous Taiyuan Formation Limestone Water in Sulin Mining Area in Northern Anhui, China. Int. J. Environ. Res. Public Health 2019, 16, 2512. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.X.; Zhou, P.J. Water damage analysis and governing in liuhuanggou coalmine, Xinjiang. Coal Geol. China 2015, 27, 39–42+62. [Google Scholar]
- Fang, X.Q.; Fu, Y.J. Impact of coal mining on karst water system in north China. Procedia Earth Planet. Sci. 2011, 3, 293–302. [Google Scholar] [CrossRef]
- Jiao, X.Y.; Wang, Y. Depositional environments of the coal-bearing strate and their controls on coal seams in the Yangquan mining district, Shanxi. Facies Palaeogeogr. 1999, 19, 30–39. [Google Scholar]
- Bryant, R.B.; Curi, N.; Roth, C.B.; Franzmeier, D.P. Use of an Internal Standard with Differential X-ray Diffraction Analysis for Iron Oxides. Soil Sci. Soc. Am. J. 1983, 47, 168–173. [Google Scholar] [CrossRef]
- Gao, X.B.; Xu, M.; Hu, Q.H.; Wang, Y.X. Leaching behavior of trace elements in coal spoils from Yangquan coal mine, Northern China. J. Earth Sci. 2016, 27, 891–900. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Zhang, J.Y.; Chou, C.L.; Li, Y.; Wang, Z.H.; Ge, Y.T.; Zheng, C.G. Trace element emissions from spontaneous combustion of gob piles in coal mines, Shanxi, China. Int. J. Coal Geol. 2008, 73, 52–62. [Google Scholar] [CrossRef]
- Dai, S.F.; Ren, D.Y. Fluorine concentration of coals in China—An estimation considering coal reserves. Fuel 2006, 85, 929–935. [Google Scholar] [CrossRef]
- Luo, K.L.; Ren, D.Y.; Xu, L.R.; Dai, S.F.; Cao, D.Y.; Feng, F.J.; Tan, J.A. Fluorine content and distribution pattern in Chinese coals. Int. J. Coal Geol. 2004, 57, 143–149. [Google Scholar] [CrossRef]
- Wiggering, H. Sulfide oxidation—An environmental problem within colliery spoil dumps. Environ. Geol. 1993, 22, 99–105. [Google Scholar] [CrossRef]
- Dong, J.H.; Li, J.B.; Huang, Y.; Zhong, J.Y.; Dun, K.; Wu, M.; Zhang, L.J.; Chen, Q.; Pan, B. Understanding the release, migration, and risk of heavy metals in coal gangue: An approach by combining experimental and computational investigations. J. Hazard. Mater. 2024, 461, 132707. [Google Scholar] [CrossRef]
- Lin, H.; Li, G.Y.; Dong, Y.B.; Li, J. Effect of pH on the release of heavy metals from stone coal waste rocks. Int. J. Miner. Process. 2017, 165, 1–7. [Google Scholar] [CrossRef]
- Shan, Y. Multivariate analysis of trace elements leaching from coal and host rock. Groundw. Sustain. Dev. 2019, 8, 402–412. [Google Scholar] [CrossRef]
- Yue, M.; Zhao, F.H. Leaching experiments to study the release of trace elements from mineral separates from Chinese coals. Int. J. Coal Geol. 2008, 73, 43–51. [Google Scholar] [CrossRef]
- Tang, Q.; Li, L.Y.; Zhang, S.; Zheng, L.G.; Miao, C.H. Characterization of heavy metals in coal gangue-reclaimed soils from a coal mining area. J. Geochem. Explor. 2018, 186, 1–11. [Google Scholar] [CrossRef]
- Bonnet, M.; Robin, V.; Parrotin, F.; Grozeva, N.; Seigneur, N.; Batbaatar, M.E.; Descostes, M. Influence of clay minerals on pH and major cation concentrations in acid-leached sands: Column experiments and reactive-transport modeling. J. Contam. Hydrol. 2024, 264, 104363. [Google Scholar] [CrossRef]
- Florence, A.; Ransom, M.; Mengel, D. Potassium Fixation by Oxidized and Reduced Forms of Phyllosilicates. Soil Sci. Soc. Am. J. 2017, 81, 1247–1255. [Google Scholar] [CrossRef]
- Min, Y.J.; Kim, D.; Jun, Y.S. Effects of Na+ and K+ Exchange in Interlayers on Biotite Dissolution under High-Temperature and High-CO2-Pressure Conditions. Environ. Sci. Technol. 2018, 52, 13638–13646. [Google Scholar] [CrossRef]
- Yan, S.H.; Zhang, T.B.; Zhang, B.B.; Zhang, T.G.; Cheng, Y.; Wang, C.; Luo, M.; Feng, H.; Siddique, K.H.M. The higher relative concentration of K+ to Na+ in saline water improves soil hydraulic conductivity, salt-leaching efficiency and structural stability. Soil 2023, 9, 339–349. [Google Scholar] [CrossRef]
- Lukman, S.; Essa, M.; Mu’azu, N.; Bukhari, A.; Basheer, C. Adsorption and Desorption of Heavy Metals onto Natural Clay Material: Influence of Initial pH. J. Environ. Sci. Technol. 2013, 6, 1–15. [Google Scholar] [CrossRef]
- Mao, L.C.; Ye, H. Influence of Redox Potential on Heavy Metal Behavior in Soils: A Review. Res. Environ. Sci. 2018, 31, 1669–1676. [Google Scholar] [CrossRef]
- Bakircioglu, D.; Kurtulus, Y.B.; Ibar, H. Investigation of trace elements in agricultural soils by BCR sequential extraction method and its transfer to wheat plants. Environ. Monit. Assess. 2011, 175, 303–314. [Google Scholar] [CrossRef]
- Nemati, K.; Bakar, N.K.A.; Abas, M.R.; Sobhanzadeh, E. Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. J. Hazard. Mater. 2011, 192, 402–410. [Google Scholar] [CrossRef]
- Fathollahzadeh, H.; Kaczala, F.; Bhatnagar, A.; Hogland, W. Speciation of metals in contaminated sediments from Oskarshamn Harbor, Oskarshamn, Sweden. Environ. Sci. Pollut. Res. 2014, 21, 2455–2464. [Google Scholar] [CrossRef] [PubMed]
- Tokalioglu, Ş.; Kartal, Ş.; Elci, L. Determination of heavy metals and their speciation in lake sediments by flame atomic absorption spectrometry after a four-stage sequential extraction procedure. Anal. Chim. Acta 2000, 413, 33–40. [Google Scholar] [CrossRef]
- Cubillas, P.; Hu, X.M.; Higgins, S.R. Strontium incorporation during calcite growth: Implications for chemical mapping using friction force microscopy. Chem. Geol. 2015, 411, 274–282. [Google Scholar] [CrossRef]
- Li, G.H.; Feng, Q.Y.; Deng, X.L.; Cui, Y.H.; Li, W.B.; Wang, H.; Gao, B.; Zhou, L.; Wang, X. Geochemical characteristics of fluorine in coal within Xiangning mining area, China, and associated mitigation countermeasures. Energy Explor. Exploit. 2019, 37, 1737–1751. [Google Scholar] [CrossRef]
- Jia, Z.Q.; Hao, S.; Lu, X.Y. Exfoliated Mg–Al–Fe layered double hydroxides/polyether sulfone mixed matrix membranes for adsorption of phosphate and fluoride from aqueous solutions. J. Environ. Sci. 2018, 70, 63–73. [Google Scholar] [CrossRef]
- Sujana, M.G.; Soma, G.; Vasumathi, N.; Anand, S. Studies on fluoride adsorption capacities of amorphous Fe/Al mixed hydroxides from aqueous solutions. J. Fluor. Chem. 2009, 130, 749–754. [Google Scholar] [CrossRef]
- Baruah, B.P.; Khare, P. Mobility of trace and potentially harmful elements in the environment from high sulfur Indian coal mines. Appl. Geochem. 2010, 25, 1621–1631. [Google Scholar] [CrossRef]

| Heavy Metals | Shale | Sandstone | Limestone | Mudstone | MG | FMT | LG |
|---|---|---|---|---|---|---|---|
| pH | 2.80–6.04 | 2.95–5.37 | 4.98–6.42 | 5.50–6.13 | 3.26 | 6.82 | 4.71 |
| TDS (mg/L) | 1115 | 2309 | 571 | 515 | 2528 | 3026 | 3672 |
| Al (mg/L) | 8.6 | 2.8 | 2.0 | 3.3 | 191.4 | 0.2 | 141.0 |
| Fe (mg/L) | 3.5 | 4.4 | 3.2 | 69.3 | 272.0 | 0.4 | 412.0 |
| Mn (mg/L) | 2.5 | 4.6 | 1.2 | 3.2 | 60.2 | 10.7 | 30.7 |
| Zn (mg/L) | 0.8 | 0.2 | <0.1 | 1.9 | 10.3 | 0.9 | 2.5 |
| F (mg/L) | 2.58 | 8.76 | 0.75 | 2.51 | 0.14 | 0.86 | 0.89 |
| Cu | 3.30 | 1.11 | 0.43 | 2.30 | 152.0 | 2.78 | 10.60 |
| Sr | 31.6 | 9.6 | 123.0 | 34.8 | 4450 | 7160 | 3950 |
| As | 0.02 | 1.25 | 0.07 | 10.65 | 2.87 | 3.12 | 3.54 |
| Pb | 1.07 | 0.52 | 0.12 | 0.03 | 2.70 | 2.13 | 1.45 |
| Cr | 0.29 | 0.42 | 0.01 | 0.29 | 3.21 | 2.58 | 0.98 |
| Cd | 1.36 | 2.60 | 0.30 | 3.03 | 13.70 | 1.80 | 1.97 |
| Co | 6.70 | 0.47 | 0.33 | 7.0 | 1.95 | 0.21 | 0.38 |
| Ni | 5.24 | 2.17 | 1.07 | 7.85 | 29.90 | 9.44 | 12.0 |
| Mo | 0.05 | 1.33 | 0.56 | 0.66 | 4.52 | 3.28 | 4.29 |
| V | 0.57 | 1.79 | 0.10 | 0.56 | 3.20 | 1.82 | 3.10 |
| Sample ID | Lithology | Quartz | Kaolinite | Illite | Calcite | Pyrite | Gypsum | Others |
|---|---|---|---|---|---|---|---|---|
| YKA | Mudstone | 22.63 | 59.39 | 14.80 | BDL | 0.20 | 0 | 3.00 |
| YKC | Shale | 0.31 | 1.67 | 95.63 | 0.98 | 0.35 | 0 | 1.05 |
| YKD | Sandstone | 48.48 | 21.80 | 17.82 | 0 | 11.90 | 0 | 0 |
| YKE | Limestone | 20.15 | 5.47 | BDL | 35.26 | 0.24 | 39.12 | 0 |
| YKF | Shale | 42.32 | 21.77 | 31.92 | 0.49 | 3.51 | 0 | 0 |
| YKG | Shale | 26.21 | 31.06 | 34.43 | 0 | 4.36 | 0 | 3.93 |
| YKH | Sandstone | 59.87 | 20.78 | 11.85 | 0 | 7.12 | 0 | 0.38 |
| YKI | Sandstone | 46.27 | 24.15 | 16.84 | 0.48 | 12.26 | 0 | 0 |
| YKJ | Mudstone | 7.30 | 89.17 | BDL | 0 | 3.54 | 0 | 0 |
| EKA | Sandstone | 50.00 | 6.70 | 37.00 | 4.00 | 0.10 | 0 | 2.20 |
| EKB | Mudstone | 29.22 | 61.42 | 8.82 | 0.53 | 0.14 | 0 | 0 |
| EKC | Shale | 1.17 | 19.13 | 77.78 | 0 | 0.16 | 0 | 1.92 |
| EKE | Sandstone | 86.96 | BDL | 3.30 | 1.04 | 8.70 | 0 | 0 |
| EKF | Sandstone | 96.07 | 1.79 | 1.09 | 0 | 0.08 | 1.04 | 0 |
| EKG | Mudstone | 23.73 | 70.91 | 2.70 | 0 | 2.56 | 0 | 0 |
| EKH | Mudstone | 14.08 | 69.73 | 11.81 | 0.49 | 2.07 | 0 | 1.82 |
| SDA | Limestone | 27.49 | 3.70 | 1.49 | 44.38 | 0.17 | 12.10 | 9.17 |
| XGA | Limestone | 1.35 | 0.65 | 4.94 | 48.67 | 0.22 | 43.24 | 1.58 |
| Heavy Metals | Sandstone | Limestone | Mudstone | Shale | Abundance in Continental Crust |
|---|---|---|---|---|---|
| Al (g/kg) | 116.1 | 20.2 | 108.9 | 137.4 | |
| Fe (g/kg) | 63.9 | 30.4 | 76.9 | 101.7 | 51 |
| Mn | 149.9 | 40.8 | 204 | 214.1 | 100 |
| Cu | 37.9 | 19.8 | 45.3 | 46.7 | 54 |
| Zn | 198.6 | 73.7 | 159.8 | 120.9 | 85 |
| As | 18.1 | 11.0 | 14.5 | 11.6 | 2.2 |
| Pb | 78.3 | 113.5 | 69.5 | 89.4 | 13 |
| Cr | 39.2 | 12.3 | 33.4 | 25.4 | 90 |
| Cd | 1.7 | 0.6 | 1.9 | 2.6 | 0.14 |
| Sr | 104.9 | 85.0 | 121.3 | 147.1 | 480 |
| Ba | 26.3 | 31.4 | 48.9 | 24.0 | |
| Co | 7.1 | 4.2 | 8.0 | 9.1 | 20 |
| Ni | 36.0 | 6.8 | 44.5 | 11.8 | 71 |
| Mo | 11.7 | 1.9 | 1.5 | 1.5 | 1.2 |
| V | 149.8 | 151.9 | 134.6 | 138.3 |
| Sample ID | Total Fluorine (mg/kg) | Water Soluble Fluorine (F1) | Exchangeable Fluorine Fraction (F2) | Amorphous Fe/Al Oxide Bound Fluorine (F3) | Crystalline Fe/Al Oxide Bound Fluorine (F4) | Fluorine Fraction Bound to Organics (F5) | Fluorine Fraction in Recalcitrant Phases (F6) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (mg/kg) | % | Content (mg/kg) | % | Content (mg/kg) | % | Content (mg/kg) | % | Content (mg/kg) | % | Content (mg/kg) | % | ||
| YKA | 416.7 | 16.5 | 3.96 | 7.9 | 1.90 | 14.3 | 3.42 | 6.0 | 1.43 | 6.4 | 1.52 | 365.7 | 87.77 |
| YKC | 273.5 | 7.5 | 2.74 | 9.1 | 3.31 | 16.1 | 5.87 | 5.0 | 1.81 | 6.8 | 2.51 | 229.1 | 83.76 |
| SDA | 106.1 | 2.3 | 2.12 | 8.1 | 7.59 | 10.2 | 9.60 | 3.8 | 3.61 | 3.8 | 3.61 | 77.9 | 73.47 |
| YKD | 539.2 | 28.8 | 5.34 | 12.2 | 2.27 | 22.4 | 4.15 | 11.2 | 2.08 | 11.6 | 2.15 | 453.1 | 84.01 |
| YKE | 190.0 | 3.5 | 1.82 | 6.7 | 3.51 | 20.0 | 10.53 | 9.0 | 4.74 | 3.8 | 1.98 | 147.2 | 77.42 |
| YKF | 453.8 | 16.7 | 3.68 | 7.5 | 1.66 | 5.0 | 1.09 | 9.2 | 2.03 | 8.7 | 1.92 | 406.7 | 89.62 |
| YKG | 616.0 | 22.4 | 3.64 | 17.7 | 2.87 | 5.7 | 0.92 | 4.6 | 0.75 | 8.6 | 1.40 | 557.1 | 90.42 |
| YKH | 494.9 | 18.1 | 3.67 | 7.5 | 1.52 | 6.1 | 1.22 | 11.2 | 2.27 | 7.3 | 1.46 | 444.7 | 89.86 |
| YKI | 539.7 | 6.2 | 1.14 | 9.8 | 1.81 | 18.5 | 3.43 | 4.2 | 0.78 | 10.3 | 1.90 | 490.8 | 90.94 |
| YKJ | 155.9 | 3.7 | 2.38 | 23.2 | 14.89 | 16.8 | 10.74 | 7.4 | 4.76 | 15.0 | 9.60 | 89.8 | 57.63 |
| XGA | 208.1 | 5.5 | 2.65 | 6.1 | 2.95 | 10.7 | 5.16 | 4.3 | 2.04 | 6.6 | 3.15 | 174.9 | 84.05 |
| EKA | 175.3 | 7.1 | 4.04 | 9.9 | 5.66 | 6.3 | 3.56 | 13.0 | 7.39 | 11.7 | 6.65 | 127.5 | 72.70 |
| EKB | 296.9 | 16.7 | 5.61 | 8.4 | 2.83 | 14.2 | 4.78 | 3.2 | 1.09 | 12.8 | 4.31 | 241.6 | 81.38 |
| EKC | 309.8 | 5.0 | 1.61 | 14.1 | 4.55 | 7.3 | 2.37 | 6.8 | 2.20 | 14.5 | 4.69 | 262.0 | 84.58 |
| EKE | 1885 | 200.0 | 10.60 | 9.8 | 0.52 | 18.8 | 1.00 | 86.6 | 4.60 | 39.5 | 2.10 | 1530 | 81.18 |
| EKF | 232.4 | 2.8 | 1.21 | 6.5 | 2.78 | 29.6 | 12.71 | 33.9 | 14.60 | 23.1 | 9.96 | 136.5 | 58.74 |
| EKG | 273.5 | 11.6 | 4.23 | 9.3 | 3.39 | 6.7 | 2.46 | 7.1 | 2.61 | 27.8 | 10.16 | 211.0 | 77.15 |
| EKH | 454.0 | 14.4 | 3.18 | 8.5 | 1.87 | 16.2 | 3.56 | 5.9 | 1.31 | 13.3 | 2.93 | 395.7 | 87.15 |
| Mean value | 423.4 | 21.6 | 3.53 | 10.1 | 3.66 | 13.6 | 4.81 | 12.9 | 3.34 | 12.9 | 4.00 | 352.3 | 80.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Gao, X.; Li, C.; Huang, H.; Bai, X.; Zheng, L.; Shi, W.; Han, J.; Tan, T.; Chen, S.; et al. Mechanisms and Genesis of Acidic Goaf Water in Abandoned Coal Mines: Insights from Mine Water–Surrounding Rock Interaction. Minerals 2025, 15, 753. https://doi.org/10.3390/min15070753
Wu Z, Gao X, Li C, Huang H, Bai X, Zheng L, Shi W, Han J, Tan T, Chen S, et al. Mechanisms and Genesis of Acidic Goaf Water in Abandoned Coal Mines: Insights from Mine Water–Surrounding Rock Interaction. Minerals. 2025; 15(7):753. https://doi.org/10.3390/min15070753
Chicago/Turabian StyleWu, Zhanhui, Xubo Gao, Chengcheng Li, Hucheng Huang, Xuefeng Bai, Lihong Zheng, Wanpeng Shi, Jiaxin Han, Ting Tan, Siyuan Chen, and et al. 2025. "Mechanisms and Genesis of Acidic Goaf Water in Abandoned Coal Mines: Insights from Mine Water–Surrounding Rock Interaction" Minerals 15, no. 7: 753. https://doi.org/10.3390/min15070753
APA StyleWu, Z., Gao, X., Li, C., Huang, H., Bai, X., Zheng, L., Shi, W., Han, J., Tan, T., Chen, S., Ma, S., Li, S., Zhu, M., & Li, J. (2025). Mechanisms and Genesis of Acidic Goaf Water in Abandoned Coal Mines: Insights from Mine Water–Surrounding Rock Interaction. Minerals, 15(7), 753. https://doi.org/10.3390/min15070753







