Abstract
The use of widespread and inexpensive clay minerals as adsorptive agents, as well as materials obtained by their chemical modification, can contribute to the solution of the problem of environmental pollution with antibiotics. This review considers the structural features of various natural clay minerals and the effect of these features on their sorption capacity. Based on the analysis of available papers (over the last 15 years, also including some fundamental basics over the last 20–30 years), it has been established that the main property of an antibiotic molecule affecting the ability to be adsorbed by a clay mineral is the hydrophilicity of the organic substance molecule. The leading properties that determine the ability of clays to adsorb antibiotics are the charge and area of their surfaces. The ability of antibiotic molecules to protonate and a partial change in the edge charge of mineral layers is determined by the acidity of the sorption solution. In addition, empirical evidence is provided that the most important factors affecting adsorption are the ionic strength of the sorption solution, the concentration of the adsorbent and adsorbate, and the interaction temperature. The diversity of the composition, structure, and properties of clay minerals allows them to be effective sorbents for a wide range of antibiotics.
1. Introduction
Antibiotics are among the most significant discoveries of humanity, having saved countless lives and improved the quality of life for many people by significantly changing the way we treat a wide range of infectious diseases. However, the widespread use of antibiotics also leads to environmental pollution, including surface and groundwater, bottom sediments, and soils, where they act as toxins on native microorganisms [1].
Antibiotics used for medical purposes are not always completely absorbed by a human organism, and their active ingredients, as well as their metabolites, end up in wastewater and, after passing through treatment plants, can enter aquatic ecosystems [2]. Medical institutions and pharmaceutical companies are major sources of antibiotic pollution in wastewater [3]. Using wastewater for irrigation and sewage sludge as fertilizer can lead to the contamination of agricultural soil with antibiotics. Soil pollution is also caused by the extensive use of antibiotics in livestock farming and the use of animal waste (urine and manure) as fertilizers [4,5]. It has been estimated that the amount of antibiotics accumulated in agricultural soils can reach one kilogram per hectare or more, comparable to the concentrations of pesticides [6]. The migration of these antibiotics through food chains can lead to their accumulation in the bodies of both farm animals and humans.
The main classification schemes for antibiotics are based on their molecular structure, character of action, and activity spectrum. Antibiotics within a structural group generally exhibit similar patterns of action, toxicity, and potential side effects. The following main groups of antibiotics are distinguished based on their chemical or molecular structure: beta-lactam antibiotics, tetracyclines, macrolides, quinolones, aminoglycosides, sulfonamides, glycopeptides, and oxazolidinones [7].
Within the framework of the concept of sustainable development, an important issue is the development of materials and technologies that can minimize the risk of water and soil contamination with antibiotics and reclaim already contaminated sites [8]. Industrial techniques for cleaning water based on physical and chemical processes, such as oxidation and UV irradiation, show promising results in removing organic pollutants. However, these methods can be expensive and have side effects that are difficult to predict. Additionally, techniques such as sedimentation, coagulation–flocculation, ion exchange, solvent extraction, membrane filtration, reverse osmosis, and ozonation can be used to purify water from organic pollutants [9,10,11,12].
Adsorption methods based on porous materials are a simple and efficient way to remove organic pollutants from water [13]. Common sorbents such as zeolites, activated carbon, graphene oxide, hydrogels, and metal–organic frameworks can be used to adsorb antibiotics from water [14,15,16,17,18]. However, these sorbents have some disadvantages, including not always being highly efficient and specific in their adsorption, having high costs, and being difficult to regenerate or dispose of.
Sorption materials based on clay minerals, on the other hand, are economical, abundant, have a large specific surface area (SSA), and a high cation exchange capacity (CEC) [19,20,21,22]. They are also physically and chemically stable, non-toxic, and have the ability to desorb pollutants after use, making them effective sorbents for purifying water from antibiotics [23].
Although clay minerals are important in many environmental processes, especially in controlling the migration of molecules and ions in soil, they are also used as materials to create various substances that solve other environmental problems [24,25]. Modification of the properties of these minerals to increase their adsorption capacity for organic pollutants is often achieved by chemical methods [20].
Currently, active research is being conducted into the composition, properties, and adsorption capacity of natural clay minerals as potential sorbents of various pollutants. This research focuses on their ability to bind antibiotics, as demonstrated by a large number of publications on Sciencedirect, using the keywords “Antibiotics adsorption by clay minerals” (Figure 1).
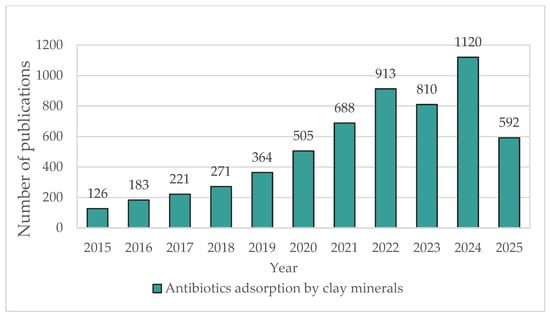
Figure 1.
Increase in publications over the last 10 years on the topic “Antibiotics adsorption by clay minerals” in the ScienceDirect database.
This review attempts to summarize the main patterns of antibiotic adsorption by various natural clay minerals based on the structures and characteristics of both adsorbents and adsorbates. The main properties of the adsorption solution that affect the interaction between antibiotics and natural clay minerals are described.
2. Natural Clay Minerals, Their Structure and Properties
Clay minerals are secondary hydrous silicates, aluminosilicates, and ferrosilicates with a standard structure consisting of layers comprising a tetrahedral silicate sheet bonded to an octahedral aluminum hydroxide sheet. In each unit of the tetrahedral sheet, one Si atom is connected to four O atoms or OH groups in a tetrahedral configuration.
The arrangement of tetrahedral units in the sheet results in the formation of a hexagonal network characterized by the chemical composition Si2O6(OH)4. The octahedral sheet is composed of Al or Mg and Fe atoms surrounded by a closely packed six OH groups or O atoms in an octahedral configuration (Figure 2). In the presence of Al atoms, the sheet is characterized by the chemical composition Al2(OH)6. When an Al3+ ion with a charge of 3+ is present in an octahedral sheet, only 2/3 of the possible positions are filled to balance the charges, and the mineral is called dioctahedral. In the presence of a Mg2+ ion with a charge of 2+, all three positions are filled to balance the structure, and the mineral is called trioctahedral.
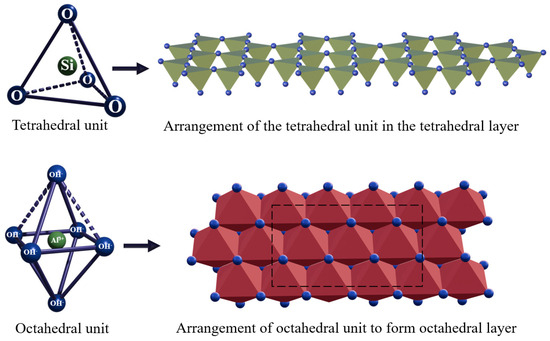
Figure 2.
Structure of the tetrahedral and octahedral unit.
A Si tetrahedral sheet and an Al octahedral sheet are connected together by sharing an apical O or OH to form a layer of a clay mineral. There are two layer types. Layers consisting of one tetrahedral sheet and one octahedral sheet form a 1:1 (or tetrahedrooctahedral, TO) layer type. The second type of layer includes two tetrahedral sheets that are on either side of an octahedral sheet—this is the 2:1 (or TOT) layer type. Each clay mineral layer has two basal surfaces. In 2:1 layer-type clay minerals, these surfaces are siloxane surfaces (i.e., planes of basal O atoms) (Figure 2). In TO-type clay minerals, one basal surface is a siloxane surface, and the other is a plane of protonated octahedral O atoms. The ratio of tetrahedral and octahedral sheets in the structural layer of the mineral is the basis of the most common classification of phyllosilicates (Table 1).

Table 1.
Classification of phyllosilicates based on their layer types [26].
By isomorphic substitution, the structural cations in the tetrahedral and octahedral sheets with low-valence metal ions, the surfaces of clay minerals, especially the 2:1 layer type, become negatively charged. In the minerals with dioctahedral structure, the most frequently encountered substitutions are of Si4+ by Al3+ in the tetrahedral sheets and of Al3+ by Mg2+, Fe2+, or Fe3+ in the octahedral sheets. In addition, the presence of vacant octahedral sites in trioctahedral minerals, the presence of trioctahedral units in dioctahedral minerals, and partial dehydroxylation of the octahedral sheets as a result of the oxidation/reduction of structural octahedral iron can affect the charge of the mineral layer [27]. The layer charge is not necessarily distributed spatially uniformly in the layer, since the localization of isomorphic substitutions can be ordered, clustered, or randomly distributed [28]. In addition, the pH of the solutions in which clay suspensions are formed can alter the charges at the edges of clay particles [29]. It is believed that the basal oxygen atoms on the siloxane surface cannot be protonated in the range of water pH, although experiments by Gupta and Miller (2010) may suggest otherwise [30]. Conversely, the Al2-OH functional groups on the octahedral basal surface of kaolinite have a well-established ability to gain or lose protons when exposed to liquid water that contributes to the observed pH-dependent surface charge of kaolinite [31,32]. The total negative charge of the layer is balanced by the presence of exchangeable, mainly alkaline (Na+, K+) and alkaline earth metals (Ca2+, Mg2+) on the basal surface of the minerals. Clay minerals of the 1:1 layer type (TO), including kaolinite, have a layer charge close to zero.
The stacking of up to tens of clay mineral layers forms a clay mineral particle. From crystallographic data, it can be determined that the distance between the planes of oxygen atoms on opposite surfaces of the layers is 6.54 Å for montmorillonite and 4.5 Å for kaolinite. Clay minerals generally have high aspect ratios with variable morphologies: kaolinite and well-crystallized illite tend to have hexagonal and elongated hexagonal morphologies, respectively, while montmorillonite and less well-crystallized illite have mostly irregular platy or lath-shaped morphologies [33]. When packed together, clay mineral particles form aggregates and their external surfaces delineate interparticle spaces. Assemblies of aggregates delineate interaggregate spaces [34].
Due to their unique structure, clay minerals have the highest specific surface area among known minerals in nature [33]. The total specific surface area of smectite minerals can be 450–850 m2 g−1, while the outer specific surface area of smectite group minerals reaches 40–70 m2 g−1 [35,36]. Figure 3 shows the structures of various clay minerals, which reflect the different arrangements of atoms and ions within the crystal due to chemical changes or thermal fluctuations that occur during weathering.
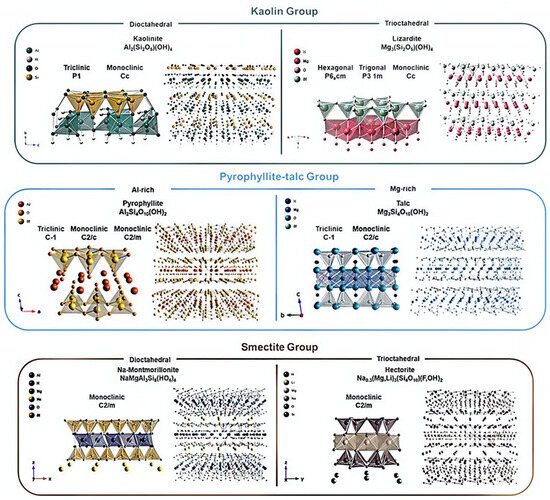
Figure 3.
Crystallographic structures of various clay minerals [37].
The surface properties of the minerals consist of the surface properties of the basal surfaces and the surface properties of the edges. As already noted, the negative charge of the layer, which arises mainly as a result of isomorphic substitutions, is compensated primarily by cations located in the interlayer space or on the outer basal surfaces. Unless these cations are in the closed interlayer spaces of non-swelling clay minerals, they exchange fairly easily with other cations in solution. In swelling clay minerals, cation exchange occurs simultaneously on both the internal and external basal surfaces. The size of the clay particle and the value of the interlayer distance can influence the ion exchange process [38].
In non-swelling clay minerals, the properties of the layers that form the outer basal surfaces of the clay particle may differ from those of the layers in the bulk of the particle [33]. However, this heterogeneity has little or no effect on the specific surface area but may strongly influence the surface charge and hence the surface reactivity of the mineral particles.
The negative charge of the basal surfaces of clay minerals is compensated by the adsorption of exchangeable cations and the exclusion of anions near the mineral surface in the electrical double layer (EDL). Measurements of anion exclusion and electrophoretic mobility in aqueous suspensions of clay particles indicate that the EDL is in the order of a few nanometers in thickness and is strongly dependent on the ionic strength [39]. The EDL can be theoretically divided into a Stern layer containing inner- and outer-sphere surface complexes and a diffuse layer containing ions interacting with the surface via long-range electrostatic forces [40,41]. The composition and structure of the diffuse layer have been intensively studied since they influence many macroscopically observable phenomena, including swelling, osmosis, and particle aggregation [42].
The edge structure of clay minerals has not been fully established and is apparently quite diverse. Traditionally, the edges have been assumed to have a TO or TOT structure similar to the bulk structure, with the surface elements (Si, Al, and their substitution atoms) having the same coordination as in the internal structure and with the outer oxygen atoms being undercoordinated [43]. However, a number of experimental and modeling results suggest that the octahedral and tetrahedral elements at the edges of clay layers may adopt a different stoichiometry and coordination than in the bulk mineral structure, at least under some conditions [44]. The edges of clay minerals carry a pH-dependent surface charge that arises from the acid–base properties of the functional groups present at the edges of the clay layers. The edge surfaces of clay minerals can bind inorganic or organic cations, anions, and molecules through short-range interactions [45,46]. Non-specific interactions that compensate for the charge of the edge surface can also be observed [39,47].
3. General Patterns of Adsorption of Different Antibiotics by Clay Materials
The overall negative surface charge on clay minerals, despite the presence of a number of pH-dependent positive charges at edges and cleavages, leads to preferential adsorption of hydrophilic, positively charged particles on clay minerals. The anionic adsorbing capacity of clay minerals is low: less than 5 cmol kg−1 for smectites [48] and not more than 2 cmol kg−1 for kaolinites [49]. Thus, a key factor in the adsorption of antibiotics by clay is the hydrophilicity of organic molecules, which can react with the negatively charged surface of the mineral.
The distribution coefficient in the octanol–water system (Kow or P) is an indicator of the hydrophilicity and hydrophobicity of a substance. It is defined as the ratio of the concentration of the substance in n-octanol, a lipophilic solvent, to its concentration in water, a hydrophilic solvent, under equilibrium conditions. Higher Kow values indicate a more hydrophobic substance, meaning it is more soluble in lipophilic media and less soluble in water.
For example, for tetracyclines, the log Kow values are −2.2 to −1.3 [50], and the solid/water distribution coefficients (Kd) are 300–2000 L kg−1 [51]. The low Kow values of tetracyclines suggested that the antibiotic molecule is hydrophilic with a high water solubility. A high affinity of tetracyclines for clay minerals was shown in many studies [52,53,54]. Kulshrestha et al. [53] reported a high adsorption capacity of oxytetracycline on montmorillonite of 800 mg g−1 at pH 5. Parolo et al. [54] obtained a tetracycline adsorption capacity of 0.9 mmol g−1, corresponding to 400 mg g−1, at pH 3 on a bentonite. Our own experiments show that the hydrophilic basal surfaces of clay layers of montmorillonite and kaolinite interact weakly with hydrophobic molecules of the antibiotic amoxicillin (log Kow = 0.87) and more effectively adsorb hydrophilic oxytetracycline molecules (log Kow = −0.90). Therefore, in aqueous solutions, molecules with negative Kow will have strong interaction with clay minerals, resulting in their adsorption on the surface and intercalation in the interlayers of the swelling minerals.
In the presence of a negative charge on the mineral surface, two important factors that affect the adsorption of antibiotics are the specific surface area and the cation-exchange capacity of the clay mineral. These values may depend on the deposit of an individual mineral, the characteristics of sample preparation (grinding), and the method of determining the parameter (using various swelling-inducing agents or adsorbents that do not cause swelling).
The specific surface area of certain minerals determined by the N2 gas adsorption method (BET) is shown in Table 2.

Table 2.
Specific surface area of some clay minerals from different deposits [55].
SSA data obtained using other methods may have different absolute values, but they are generally related to each other. In this study, the specific surface areas of illite and montmorillonite minerals were determined using atomic force microscopy (AFM) and compared to SSA values obtained by N2 gas adsorption (BET) and liquid adsorption using ethylene glycol monomethyl ether (EGME).
For illite, the SSA was estimated to be 41 ± 3 m2·g−1 by BET and 83 ± 5 m2 g−1 by AFM. For montmorillonite, BET estimated a SSA of 61 ± 2 m2 g−1, while the analysis of AFM images yielded a much higher mean SSA of 346 ± 37 m2 g−1. The authors suggest that the sample preparation for AFM imaging may have caused delamination of clay mineral particles. The specific surface area estimated by EGME for illite was 112 m2 g−1 and 475 m2 g−1 for montmorillonite, which is about 30%–40% greater than the AFM values [56].
There is a significant difference in the cation exchange capacity (CEC) of various clay minerals. For example, the CEC of montmorillonite typically ranges from 70 to 130 meq 100 g−1 of soil, while that of illite ranges from about 20 to 40 meq 100 g−1, and that of kaolinite ranges from 3 to 15 meq 100 g−1. The CEC values for halloysite, hectorite, palygorskite, sepiolite, and vermiculite are 5–10 meq, 80–130 meq, 30–40 meq, and 30–40 meq 100 g−1, respectively [57]. Chemical composition of the minerals, specific surface area, and acidic and alkaline environments are the main factors affecting CEC of clay minerals. Three-layer swelling minerals have the highest specific surface area and cation-exchange capacity. Below is a table of adsorption models for some antibiotics on clay minerals, both modified and native (Table 3).

Table 3.
Adsorption models of antibiotics with various Kow on clay minerals.
Based on various models describing the process of antibiotic adsorption by clays, the absorption mechanisms can be quite diverse (Figure 4).
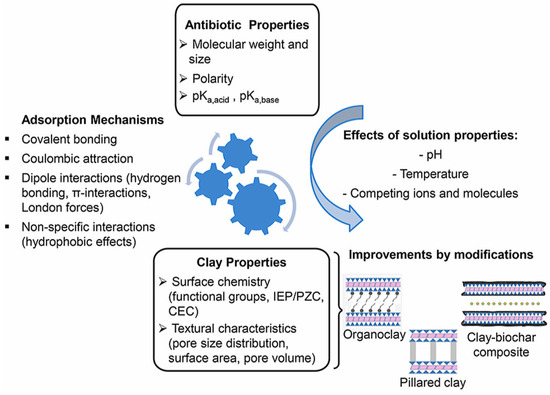
Figure 4.
Adsorption mechanisms of antibiotics by natural and modified clay minerals [20].
Ion exchange may be a dominant binding mechanism since clays carry a sufficient amount of metal ions and a number of anions silicates and carbonates). Clay minerals possess a low affinity for non-polar or aromatic antibiotics and are expected to sorb sufficient quantities of polar pharmaceuticals [66]. Some studies have suggested that the important mechanism of interaction between clay minerals and antibiotics is complexation [52]. However, the cation exchange mechanism for antibiotic adsorption on clay minerals has been supported by more researchers. Li et al. found that there is simultaneous H+ uptake during the process of tetracycline adsorption onto smectites, and cation exchange occurs even under neutral pH conditions [67]. Zhao et al. suggested that the adsorption of tetracycline on the kaolinite surface occurs through an outer-sphere cation exchange mechanism [68]. However, it is likely that several processes are occurring simultaneously, and the predominance of these processes depends on the properties of the mineral and the antibiotic.
4. Adsorption of Antibiotics by Various Natural Clay Minerals
4.1. Adsorption of Antibiotics by Minerals with a 1:1 Structure
A typical representative of phyllosilicates with a 1:1 structure is carolinite. This mineral is formed as a result of the weathering of feldspar and other aluminosilicates in acidic conditions. It is composed of a silica tetrahedral layer and an alumina octahedral layer, which are alternately arranged. The high stability of kaolinite contributes to its low plasticity, cohesion, and low swelling and shrinking properties [69].
Due to its properties, kaolinite is characterized by some features of antibiotic adsorption. Thus, the study of adsorption of various antibiotics, including tetracycline hydrochloride, neomycin sulfate, lincomycin hydrochloride, chloramphenicol, and ampicillin trihydrate, by kaolinite at different pH values (1.2, 3, and 5) revealed that chloramphenicol and ampicillin trihydrate were not adsorbed by this mineral. The adsorption of tetracycline hydrochloride at pH 1.2 could be better described by the Freundlich model, while at other pH values it was more accurately represented by the Langmuir model. This antibiotic’s adsorption reaction was found to be reversible. Neomycin sulfate and lincomycin hydrochloride’s adsorption isotherms were best described by the Langmuir equation, except for lincomycin hydrochloride at pH 1.2. Neomycin sulfate adsorption by kaolinite appeared to be an irreversible process, whereas lincomycin hydrochloride adsorption was partially reversible.
Ciprofloxacin (Cip) is a second-generation fluoroquinolone antibiotic with high solubility in water at both high and low pH levels. In sorption experiments, it showed a high affinity for the negatively charged surfaces of kaolinite, and quantitatively, a correlation between the desorbed exchangeable cations and the adsorbed Cip confirmed that cation exchange was the dominant mechanism for Cip adsorption on kaolinite.
It is suggested that the adsorption of Cip is charge-limited rather than surface-area-limited. The constant d-spacing after the adsorption of different amounts of Cip indicates that Cip adsorbs onto the outer surfaces of kaolinite. When the pH of the solution increases above 8, the amount of Cip adsorbing decreases significantly and reaches almost zero at pH 11 [70].
In the structure of halloysite, another mineral with a 1:1 structure, layers of tetrahedral and octahedral sheets are folded into tubular structures such that the outer surface is made of siloxane (Si-O-Si) and the inner surface is made of aluminol (Al-OH) [71]. Each tubular structure consists of 10–15 aluminosilicate bilayers and has a length of 1–5 μm, an outer diameter of 40–60 nm, and an inner diameter of 10–15 nm [72]. Due to its developed surface, which carries a positive electrical charge on the inner surface and a negative charge on the outer surface, halloysite has the ability to bind different ions.
Pure halloysite has been used as an adsorbent to remove antibiotic ciprofloxacin, and it has exhibited removal capacities of up to 21.7 mg g−1. The experimental results show that, compared to cyclosilicate tourmaline and sheet silicate biotite, halloysite with a nanotube structure has the highest adsorption capacity [73]. The authors [74] also used unmodified halloysite nanotubes as an effective adsorbent for the uptake of ciprofloxacin at low concentrations. Kinetic analysis showed that the adsorption of this antibiotic onto halloysite occurs as a monolayer process and follows a pseudo-second order rate equation. Zeta potential studies suggest that electrostatic interactions between the antibiotic and the mineral are likely responsible for the high adsorption efficiency. Furthermore, halloysite retains its adsorption capacity even after multiple adsorption/desorption cycles, indicating its potential for use in wastewater treatment applications.
The adsorption study of oxytetracycline on halloysite at an initial concentration of 25 mg L−1 and pH 3.0–9.0 revealed that the adsorption is strongly dependent on pH, and the optimal pH range is between 3.5 and 5.5. At a pH of 3.5, the maximum adsorption capacity of oxytetracycline was 21 mg g−1, which corresponds to 68% of the added concentration. This can be explained by the positively charged inner lumen and negatively charged outer lumen of the halloysite’s tubular structure, which leads to the formation of inner-sphere complexes with the anionic and cationic forms of oxytetracycline [75]. Halloysite nanoclay was tested as an adsorbent to remove the antibiotic enrofloxacin from aqueous solutions. The adsorption of enrofloxacin onto the clay was best described by the Langmuir model, with a maximum adsorption capacity of 34.80 mg g−1. The experimental data were satisfactorily fitted by a pseudo-second order model, suggesting that chemisorption (through electrostatic interactions) is the dominant adsorption mechanism [76].
4.2. Adsorption of Antibiotics by Minerals with a 2:1 Structure
The ideal unit structure of talc is Mg3Si4O10(OH)2. This structure consists of stacked sheets that are arranged with varying degrees of disorder, allowing them to slide easily, which gives talc its characteristic softness.
Talc sheets have two types of surfaces: “face” and “edge”. Face surfaces contain basal planes due to the sliding of sheets, and they have siloxane groups (-Si-O-Si-). These groups are electrically neutral, energetically weak, and hydrophobic.
Edge surfaces are formed during the milling process and contain silanol (SiOH) and magnesiol (MgOH) groups. These groups make the edges polar and hydrophilic, but they are more energetic than face surfaces. As a result, these surfaces are susceptible to hydrolysis and can form strong hydrogen bonds with water molecules and other polar substances [77].
The in vitro adsorption of ciprofloxacin onto talc was studied at various pH levels. The adsorption depended on the amount of adsorbent used and was completed within one hour. The adsorption followed the Langmuir adsorption isotherm, and maximal adsorption occurred in the pH range of 1.2–3. Talc adsorbs the antibiotic through an ion exchange mechanism and can also form complexes with ciprofloxacin, thus removing it from the solution [78]. Dube et al. (2018) [79] conducted a study on the removal of ciprofloxacin and isoniazid from aqueous solutions using talc as an adsorbent. The study found that the Langmuir model provided the best fit for both antibiotics’ sorption. The heat of sorption calculated from Temkin plots ranged from 0.018 to 10.460 J mol−1, suggesting a physical adsorption process. The kinetic data best conformed to the pseudo-second order equation, with R2 values ranging from 0.998 to 0.999. The removal of these antibiotics by the adsorbent was influenced by pH, indicating that electrostatic interactions played a significant role in the adsorption process.
Montmorillonite is a main clay mineral found in bentonite rocks, which are formed from volcanic ash. These rocks are less common in sedimentary rocks older than the Mesozoic era and represent an intermediate stage in the weathering process. Montmorillonite can be found in silty soils in temperate regions, where it is one of the most common clay fractions [80]. Bentonites can be divided into two main types: calcium bentonite, which is non-swelling, and sodium bentonite, also known as swelling bentonite.
Ciprofloxacin was removed from an aqueous solution by bentonite at a 99% efficiency with a contact time of 30 min at pH 4.5 [81]. When comparing the sorption efficiency of bentonite, activated carbon, zeolite, and pumice with respect to ciprofloxacin, it was noted that bentonite shows the best results [82]. Gulen and Demircivi (2020) [83] established, on the basis of thermodynamic data, that the adsorption of ciprofloxacin on montmorillonite clay is endothermic and spontaneous. The system reached equilibrium in 60 min. The maximum adsorption value at pH 7 reached 128 mg g−1.
The interactions of oxytetracycline with native montmorillonite and Na-montmorillonite were studied at various pH levels. The adsorption of oxytetracycline to the clay could be described by the Freundlich-type adsorption isotherms. It was observed that the adsorption of oxytetracycline in the native and sodium forms of montmorillonite decreases with increasing pH in the order pH 1.5 > 5.0 > 8.7 > 11.0. This trend is consistent with cationic exchange interactions that are dominant at lower pH values when oxytetracycline has a net positive charge. On the other hand, at pH 5.0, when oxytetracycline is zwitterionic, hydrophobic interactions are predominant over other mechanisms [53].
The adsorption of tetracycline onto smectite involves the active participation of surfaces located in the interlayer position, as confirmed by the expansion of the d001 spacing. The adsorption capacity of smectite for tetracycline is equivalent to 0.74–1.11 times its cation exchange capacity, depending on the specific smectite mineral studied.
Accompanying the adsorption of tetracycline, there is also simultaneous adsorption of protons, resulting in protonation of the tetracycline dimethylamine group. At higher concentrations of tetracycline, additional adsorption of protons occurs, leading to a ratio of adsorbed protons to adsorbed tetracycline greater than one. This suggests that the adsorbed protons may serve as counterions, partially offsetting the negative charges on tricarbonyl and phenolic diketone functional groups. The positive correlations between the desorption of cations and the adsorption of tetracycline, as well as between tetracycline adsorption and H+ adsorption, provided the first evidence to confirm the cation exchange as the main mechanism for tetracycline uptake, even at neutral pH conditions [67].
An increase in the interlayer spacing of montmorillonite from 12.6 Å to 19.5 Å was observed using X-ray diffraction in the presence of tetracycline [54]. The experiments and modeling suggest that not only cationic species, but also neutral and monoanionic forms of tetracycline, adsorb on the negatively charged montmorillonite surface. Although there is a significant electrostatic interaction between tetracycline and montmorillonite, there is also a rather strong non-electrostatic interaction. When bentonite was used to treat real wastewater contaminated with amoxicillin, the removal efficiency of bentonite (88%) was lower than that of activated carbon (95%), although none of the tested materials could completely remove amoxicillin due to competition from other pollutants in the wastewater for adsorption sites [84].
Ciprofloxacin was removed from an aqueous solution by bentonite with a 99% efficiency after 30 min of contact time at pH 4.5 [81]. When comparing the sorption efficiency of bentonite, activated carbon, zeolite, and pumice in terms of their ability to remove ciprofloxacin, bentonite was found to be the most effective [82]. Gulen and Demircivi [83], based on thermodynamic data, found that the adsorption of ciprofloxacin onto montmorillonite clay is an endothermic and spontaneous process. The system reaches equilibrium in 60 min. The maximum adsorption value at pH 7 is 128 mg g−1.
The interactions between oxytetracycline and native montmorillonite and Na-montmorillonite were studied at different pH levels. The adsorption of oxytetracycline on the clay can be described by the Freundlich adsorption isotherms. It was found that the adsorption of oxytetracycline in both native and sodium montmorillonite decreased with increasing pH, in the following order: pH 1.5 > 5.0 > 8.7 > 11.0. This trend can be explained by cationic exchange interactions, which are dominant at low pH values, when oxytetracycline has a positive net charge. At pH 5.0, however, zwitterionic hydrophobic interactions become predominant over other mechanisms [53].
The adsorption of tetracycline onto smectite occurs through the active participation of surfaces located in the interlayer position. This is confirmed by an increase in the d001 spacing. The adsorption capacity of smectite for tetracycline is equivalent to between 0.74 and 1.11 times its cation exchange capacity, depending on the type of smectite mineral studied. During the process of tetracycline adsorption, H+ ions are also adsorbed simultaneously, leading to the protonation of the dimethylamine group of tetracycline. At higher concentrations of tetracycline, additional H+ adsorption occurs, resulting in a ratio of adsorbed H+/adsorbed tetracycline greater than one. This suggests that the additional H+ ions may serve as counterions, partially offsetting the negative charges of the tricarbonyl and phenolic diketone groups of tetracycline. The positive correlations between the desorption of cations and the adsorption of tetracycline, as well as the adsorption of H+ and tetracycline, provide the first evidence to support cation exchange as the primary mechanism of tetracycline adsorption, even under neutral pH conditions [67].
An increase in the interlayer spacing of montmorillonite from 12.6 Å to 19.5 Å has also been shown in X-ray diffraction studies in the presence of tetracycline [54]. Experiments and simulations suggest that not only cationic species, but also neutral and monoanionic forms of tetracycline, adsorb onto the negatively charged surface of montmorillonite. Although there is a significant electrostatic interaction between tetracycline and montmorillonite, there is also a rather strong non-electrostatic interaction.
When bentonite was used to remove amoxicillin from real wastewater, its efficiency (88%) was lower than that of activated carbon (95%). Nevertheless, none of these adsorbents were able to completely remove amoxicillin from the water due to the competition for adsorption with other pollutants present in the wastewater [84].
The adsorption of trimethoprim on montmorillonite is better described by the Langmuir model, as shown by the determination coefficients R2 of 0.98 and 0.99. The adsorption increases with decreasing temperature, as indicated by the adsorption isotherms and kinetics. The maximum adsorption values vary from 0.1400 mmol·g−1 at 318 K to 0.4461 mmol·g−1 at 303 K. The sorption energies, obtained from the Dubinin–Radushkevich isotherm, range from 8 to 16 kJ·mol−1, indicating that the adsorption of trimethoprim occurs through an ion exchange mechanism.
The removal of the drug from aqueous solution depends on pH and ionic strength, with maximum adsorption occurring at pH 5.04. Low pH promotes high adsorption due to the attraction between the negative clay surface and the protonated form of the drug [85].
Vermiculite has a 2:1 layer structure similar to montmorillonite, but with a layer charge ranging from 0.6–0.9 per formula unit instead of 0.2–0.6. This lower charge allows the interlayer space to open, enabling exchangeable cations to enter and leave the material. As a result, vermiculite exhibits a high cation exchange capacity and a strong affinity for weakly hydrated ions. Vermiculite swells less extensively than smectite [86].
The adsorption of β-lactam antibiotics, including ampicillin, amoxicillin, and benzylpenicillin, on the surface of vermiculite was studied using density functional theory calculations. The C09 van der Waals density functional was used to account for van der Waals interactions in order to determine the most stable configurations that govern the interactions between the antibiotic molecules and the vermiculite surface [87].
Each molecule prefers to orient horizontally on the surface, forming both Mg∙∙S and Mg∙π contacts or two Mg∙O interactions between the S atom in the -CS group, the π electrons of the benzene ring, or the O atoms of the -COOH and -OH groups of the molecules and the Mg2+ sites on the surface. Interestingly, a significant role for the Mg∙π interaction in complex stabilization is observed for the first time. These processes are strong chemisorption events with adsorption energies ranging from −72 to −78 kcal mol−1. AIM calculations show a significant contribution of Mg-O/S/π interactions and O-H bonds to the complex stabilization. The formation and role of these interactions on the stability of the complexes were thoroughly examined using molecular orbital (MO) and total electron density transfer (EDT) analysis based on the natural bond orbital (NBO) approach [88].
The adhesion of benzylpenicillin to the vermiculite surface occurs through favorable >C=O…Mg interactions and O-H…O hydrogen bonds between the -COOH group of the molecule and Mg2+ ions on the surface, as well as O2- ions in the -SiO3 and -AlO3 groups. The benzylpenicillin molecule binds more favorably to the Mg2+ sites on the vermiculite surface than to Al3+ sites. Calculations suggest that both >C=O…Mg and O-H…O interactions contribute to the stability of the benzylpenicillin complex on vermiculite. Interestingly, the electron density transfer from the surface to the molecule is slightly stronger than that from the molecule to the surface. These results suggest that adsorption of benzylpenicillin onto vermiculite can be described as a strong chemisorption process [89].
Tylosin, a broad-spectrum macrolide veterinary antibiotic, is retained on vermiculite and montmorillonite through exchange and non-exchange mechanisms. Isotherms from exchange experiments show that tylosin preferentially binds to Na over a wide range of exchangeable cation compositions on montmorillonite. It also prefers Ca over this mineral, although only when Ca is the dominant cation. X-ray diffraction suggests that tylosin intercalates into montmorillonite but not vermiculite, and its overall adsorption on vermiculite is negligible [90].
Illite has a 2:1 layered structure with a general formula of ((K1–1.5A14[Si7–6.5A11–1.5O20](OH4)). Al3+ ions occupy a quarter of the tetrahedral positions, creating a negative charge within the layer structure that is balanced by monovalent cations, typically K+, occupying the interlayer positions. Poorly hydrated potassium ions are responsible for the lack of swelling in the mineral. The morphology of illite can be irregular, with granules or elongated spikes [91]. The cation-exchange capacity of illite is smaller than that of montmorillonite and is typically 20–30 meq 100 g−1.
The removal of tetracycline from an aqueous environment using non-swelling illite was investigated in a batch system under different pH and ionic strength conditions. The adsorption capacity of illite for tetracycline was found to be 32 mg·g−1 at a pH of 5–6. The adsorption equilibrium data followed the Freundlich isotherm, and the kinetics of tetracycline adsorption were moderately fast, reaching equilibrium in approximately 8 h. The results followed the pseudo-second order kinetic model and the Elovich model. X-ray diffraction patterns before and after adsorption showed no changes in the basal spacing or intensity, indicating that the absorbed antibiotic molecules were located on the external surface of the mineral and not within the interlayers of the clay mineral, such as in montmorillonite or rectorite. Despite its low tetracycline adsorption capacity, illite has been shown to be a promising candidate for removing tetracycline from wastewater with high concentrations of the antibiotic. The results suggest that illite exhibits lower adsorption of tylosin compared to montmorillonite, making it a potential alternative for tetracycline removal [92,93].
4.3. The Adsorption of Antibiotics by Mixed-Layer Minerals
Rectorite is a mineral that consists of alternating layers of mica and smectite, forming a pattern of ABABAB… with a 50/50 ratio of each layer. This ordered arrangement makes it a rare mineral in nature [94]. Tetracycline uptake into rectorite occurs through intercalation, where the antibiotic molecule inserts itself between the layers of the clay mineral. This process reduces the crystallinity of rectorite by 2–3 fundamental layers along the c-axis.
When tetracycline is adsorbed on rectorite, the maximum expansion of the interlayer occurs at higher pH values. At these pH levels, the antibiotic molecules adopt an expanded conformation. However, the amount of intercalated tetracycline at higher pH is lower compared to lower pH conditions. At low to neutral pH, the insertion of the twisted conformation of tetracycline into the interlayer space results in a gallery height of 9.2 Å. At high pH, however, the antibiotic adopts an extended conformation, together with counterions, and the gallery height increases to 10.3 Å [95]
4.4. The Adsorption of Antibiotics by Ribbon Minerals
Palygorskite and sepiolite are both phyllosilicate minerals, but they have distinct structural differences from typical 1:1 and 2:1 layered structures. Both contain continuous tetrahedral sheets, but the adjacent bands of tetrahedra within each tetrahedral sheet point in opposing directions rather than in the same direction. This results in a structure that resembles ribbons of 2:1 layers connected at their edges, with water molecules occupying the spaces between them. The 2:1 ribbons in sepiolite are wider than those in palygorskite, and both minerals have a fiber-like morphology, in contrast to the plate-like morphology of most other 1:1 and 2:1 phyllosilicates [86]. Palygorskite is a clay mineral with a microfiber morphology, low surface charge, a high magnesium content, and a large specific surface area due to its abundance of active sorption sites. However, its cation exchange capacity is relatively low compared to other minerals. The adsorption of cephalexin, a first-generation cephalosporin antibiotic, onto palygorskite has been better described using the Langmuir model. The adsorption capacity was found to be 112.36 mg·g−1, indicating a strong interaction between the antibiotic and the mineral surface. Thermodynamic analysis revealed that the process is endothermic, indicating an increase in energy during adsorption. This suggests that chemisorption is the main mechanism of adsorption. Additionally, the Weber–Morris model was used to describe the adsorption process, suggesting that pore diffusion may also play a role in the interaction between the two materials [96].
Also, like palygorskite, the tetrahedral sheets of sepiolite are divided into ribbons by inversion, although they are still linked in sheet form. The octahedral sheets, on the other hand, are continuous only in two dimensions.
Sepiolite has a structure made up of needle-like particles with talc-like layers. These layers consist of two layers of tetrahedral silica and a central octahedral magnesium layer.
The interaction between the electrokinetic surface of sepiolite and the functional groups in amoxicillin was studied. The pH of the environment and the adsorbent dosage, contact time, amoxicillin concentration, and temperature were all optimized to find the best conditions for amoxicillin adsorption. The optimized conditions were determined to be pH 3, 1 g·L−1 of adsorbent dosage, 30 min of contact time, 50 mg·L−1 amoxicillin concentration, and 35 °C temperature. In addition, the adsorption data indicated that amoxicillin adsorption was well described by the Freundlich isotherm model, and the pseudo-second order kinetic model was the best fit for amoxicillin on beige sepiolite (50% mineral). It was found that diffusion was the rate-limiting step in the adsorption process, but intraparticle liquid film diffusion played a more significant role. It was also concluded that the adsorption was physical and spontaneous [97].
5. Factors Affecting the Adsorption of Antibiotics on Clay Minerals
5.1. Acidity of a Sorption Solution
As mentioned previously, the hydrophilicity and charge of the antibiotic molecule, as well as the properties of non-basal clay mineral surfaces, are influenced by the acidity of the sorption solution. The maximum adsorption of different antibiotics varies depending on their composition and structure. For example, the maximum adsorption of trimethoprim on montmorillonite occurs at pH 5.04. At this pH, the molecule of trimethoprim is protonated and binds to the negatively charged surface of the mineral [85]. Tylosin has a methylamine functional group that is protonated in neutral to acidic solutions (pKa = 7.50 ± 0.13), and cation exchange of the antibiotic through this functional group has been identified as an important mechanism of drug immobilization in a soil [98]. The role of pH is crucial during antibiotics adsorption, since the majority of pharmaceuticals carry more than one ionic state, and protonation of the edge functional groups of minerals is possible.
For example, tetracycline has different charges at different sites, depending on the pH of the solution. At pH values below 3.3, the dimethylammonium group of the molecule is protonated, giving it a positive charge. Between pH values of 3.3 and 7.7, tetracycline exists as a zwitterion with both positive and negative charges, with the positive charge predominating at pH 5.0. This is due to the loss of a proton from the phenolic diketone group. When the pH is above 7.7, the molecule has either a monovalent negative charge or a divalent negative charge, depending on whether protons are lost from the tricarbonyl group or the phenolic diketone. The pKa values of tetracycline in aqueous solutions are approximately 3.3, 7.7, and 9.7 [53]. In addition, the structure of tetracycline also depends on the pH of the solution. In basic solutions, an extended structure is the predominant form, while in acidic to neutral solutions, a twisted structure prevails [99].
The study of the influence of pH on the adsorption of tetracyclines onto illite revealed a strong correlation between pH and the adsorption process. The maximum adsorption of tetracycline was found to occur at pH 3.5, while the maximum adsorption for oxytetracycline was observed at pH 3 and for chlortetracycline at pH 4. After these points, adsorption decreased with increasing pH values. As expected, the maximum adsorption occurred near the pKa1 values of the respective tetracyclines [100].
The mineral surfaces remain negatively charged globally along the whole pH range due to structural charges. However, the charge of the edge sites may change with pH. Rozalen et al. have shown that the proportion of AlOH2+ sites increases below pH 8 and reaches a plateau at pH 4 [101]. There are different points of view on the location of the isoelectric points (IEPs) of the edge charges [102]. Tombacz and Szekeres suggest that the edges are positively charged below pH 6.5, while Thomas et al. estimate that the IEP of the edges is around 3.6, and Lagaly proposes that it might be slightly above pH 5 [103]. Regardless of the exact value of the IEP, the relative number of positively charged edge sites increases at lower pH, which could facilitate ionic interactions with anionic groups [104].
There were substantial influences of pH on the adsorption capacities of kaolinite for oxytetracycline. With the increase in pH, the adsorption capacity increased and then decreased. A maximum adsorption capacity of kaolinite existed at a pH of approximately 5.5. The surface charges of kaolinite and oxytetracycline changed with different pH values, which influenced the adsorption properties. For amphoteric oxytetracycline, there are three pKa values (3.57, 7.49 and 9.88), and oxytetracycline was divided into four fractions as follows under different pH conditions: the cationic fraction (+00) fraction with pH < 3.57, the zwitterionic fraction (+−0) during pH of 3.57–7.49, the amination anionic fraction (+--) or bivalent anionic fraction (0--) with pH > 7.49. It is believed that the surface of kaolinite has a constant structural charge and edge charge depending on the solution pH [105]. The surface charge of kaolinite is normally considered to be a negative surface charge, and some positive charge exists on kaolinite under acidic conditions, while negative charges are present under alkaline conditions [68]. In this study, when the pH was greater than 7.49, the same charges existed on both oxytetracycline and kaolinite and resulted in electrostatic repulsion. Thus, the adsorption capacity of kaolinite for oxytetracycline in the pH range >7.49 was worse than that in the pH range <7.49. At pH < 3.57, the positive charge of oxytetracycline can be adsorbed by the negative charge of kaolinite; with the increase in pH value, the charge of oxytetracycline becomes neutral [106].
5.2. Ionic Strength
The ionic strength of the solutions has a significant impact on the adsorption of antibiotics, as it affects the competition between chemicals in the solution and drug molecules for sorption sites. In a binary system, the presence of methylene blue seems to act antagonistically towards the adsorption of enrofloxacin [76].
At all pH values, tetracycline species compete with sodium ions for surface sites. Therefore, there is an important effect of ionic strength on adsorption. The data indicate that the adsorption of tetracyclines decreases as the ionic strength of the solution increases. The adsorption of tetracycline, oxytetracycline, and chlortetracycline decreased from 32% to 29% on illites and from 33% to 31% on kaolinites at initial input, with an increase in ionic strength between 0.0 and 0.6 [54,100].
The presence of a high concentration of dissolved organic matter (DOM) has also been found to reduce the adsorption of oxytetracycline onto clay, suggesting that DOM may increase the mobility of the antibiotic in the natural environment [53].
5.3. Adsorbate Dosage
The amount of adsorbed substance and the rate of adsorption depend on the initial concentration of antibiotics. The initial concentration of the antibiotic reduces the resistance to mass transfer and provides the necessary driving force. An increase in the initial concentration of the antibiotic in the solution leads to a higher adsorption capacity at the beginning of absorption. As the adsorbent approaches saturation, the adsorption efficiency decreases, since the number of available sorption centers becomes smaller and competition between antibiotic molecules for these sites is observed.
At higher initial concentrations of the adsorbate, the absorption of antibiotics increases regardless of the nature of the adsorbent surface (microporous or mesoporous, negatively or positively charged). Thus, the influence of the initial concentration of tetracycline hydrochloride on the removal efficiency was studied using various concentrations ranging from 20 to 100 mg L−1. The results showed that at a concentration of 20 mg L−1, the adsorption efficiency was approximately 85%. As the antibiotic concentration increased, the uptake level decreased progressively. This indicates that a higher residual antibiotic concentration may be associated with higher initial antibiotic concentrations, due to a decrease in the number of free adsorption sites available to remove the antibiotic molecules [107]. For each specific adsorbent, there is a saturation limit, which can be calculated from the equilibrium adsorption concentration and using a number of adsorption models.
5.4. Adsorbent Dosage
Studies show that the amount of adsorbed pharmaceuticals can increase with increasing amount of adsorbent [108]. The increase in absorption can be explained by the increase in vacant sites at higher dosages of the adsorbent. However, it is believed that the adsorption of pharmaceuticals rarely reaches the saturation value [109]; therefore, a further increase in the adsorbent dosage may not have much measurable value. A better idea of the adsorption capacity of an adsorbent is given by the value of specific adsorption—the amount of antibiotic drugs adsorbed per unit mass of the adsorbent under different conditions. It is noted that the value of specific adsorption decreases with an increase in the amount of adsorbent. Ribeiro and Ribeiro [109] conducted an experiment on the adsorption of erythromycin on various polymeric sorbents. They found that the adsorbents reached their saturation values at different dosages in solution. They also found that with an increase in the dosage to 10 g L−1, the removal of erythromycin did not increase significantly. Excessive use of adsorbents can increase the overall cost of the adsorption process. Kim et al. [110] demonstrated that the adsorption of trimethoprim on activated carbon reached the saturation value at 1.0 g L−1 of adsorbent, and further increases in the amount of adsorbent did not affect the adsorption capacity. It is optimal to measure the best dosage of adsorbent for a given concentration of the pharmaceutical compound.
5.5. Temperature
Temperature is another important factor affecting the adsorption process of antibiotics on clays. Temperature can influence changes in activity at the solid–liquid interface, disruption of interactions between adsorbate molecules and functional groups of the adsorbent, and possibly changes in the nature of the adsorbent [93]. If the adsorption process is endothermic, an increase in temperature usually increases the molecular activity at the phase boundary, which can increase the diffusion rate of antibiotic molecules in solutions. Higher temperatures increase the kinetic energy of the antibiotic molecules, allowing them to move more quickly and interact more easily with the clay mineral surface [111]. The removal of enrofloxacin, a fluoroquinolone antibiotic, from aqueous solution by adsorption on bentonite was an endothermic process. Higher temperatures were favorable for the adsorption of the antibiotic [112].
If the adsorption process is exothermic, this can lead to a decrease in absorption due to the weakness of the interaction forces between the reactive groups on the adsorbent surface and the antibiotic molecules [85]. Bekci et al. [85] found that adsorption of trimethoprim by montmorillonite was exothermic and spontaneous. Authors reported that the adsorption decreased considerably by increasing the temperature of the solution (303–318 K). The authors point out the exothermic nature of adsorption and the decrease in absorption with increasing temperature during the adsorption of tetracycline on bentonite [107].
6. Conclusions
The widespread use of antibiotics has undoubtedly transformed medical treatment and significantly improved public health. However, this progress comes with significant environmental costs, including pollution of water bodies, soils, and agricultural ecosystems. Antibiotics in wastewater and their potential accumulation in the food chain pose a serious problem for animal and human health. In the context of the sustainable development of society, it is imperative to take immediate action to reduce antibiotic pollution and restore damaged environments. Technologies for the use of clay minerals and materials based on them for water purification and immobilization of antibiotics in soils can bring promising results.
Available literature data show that clay minerals of different structures and all groups can be effective sorbents of antibiotics, but the most successful results are shown for minerals of the 2:1 structure of the smectite group, which have an expanding structural cell (swelling), a high specific surface area, are widespread, and inexpensive.
The basic property of an antibiotic molecule that determines its ability to be absorbed by clay minerals is its hydrophilicity, which can be estimated by the value of the distribution coefficient in the octanol–water system (Kow). At the same time, the presence of aromatic fragments in the antibiotic molecule obviously complicates their absorption by three-layer swelling minerals. It is clear that the main mechanisms of antibiotic adsorption by clays are ion exchange and complex formation; the predominance of these processes depends on the properties of the mineral and the antibiotic.
The leading factors influencing the adsorption of antibiotics by natural clays are the pH and ionic strength of the sorption solution, the concentration of the adsorbate, the dosage of the adsorbent, and the interaction temperature. The role of pH is crucial during antibiotics adsorption, since the majority of their molecules carry more than one ionic state, and protonation of the edge functional groups of minerals is possible.
Author Contributions
Conceptualization and methodology, Y.A., S.M., L.P. and M.G.; data curation, I.K., S.M. and I.P.; investigation, T.D. and L.P.; writing—original draft, L.P., M.G. and V.S.; writing—review and editing, M.G., L.P., T.M., Y.A., I.K., V.S. and S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation grant № 25-17-20037, conducted jointly with the authorities of the subject of the Russian Federation (Tula region).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Godoy, M.; Sánchez, J. Antibiotics as emerging pollutants in water and its treatment. In Antibiotic Materials in Healthcare; Kokkarachedu, V., Kanikireddy, V., Sakidu, R., Eds.; Academic Press: London, UK, 2020; pp. 221–230. [Google Scholar] [CrossRef]
- Praveena, S.M.; Shaifuddin, S.N.M.; Sukiman, S.; Nasir, F.A.M.; Hanafi, Z.; Kamarudin, N.; Ismail, T.H.T.; Aris, A.Z. Pharmaceuticals residues in selected tropical surface water bodies from Selangor (Malaysia): Occurrence and potential risk assessments. Sci. Total Environ. 2018, 642, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Wei, Q.; Wei, D. A critical review on antibiotics and hormones in swine wastewater: Water pollution problems and control approaches. J. Hazard. Mater. 2020, 387, 121682. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Fernández-Calviño, D.; Arias-Estévez, M. Tetracycline and Sulfonamide Antibiotics in Soils: Presence, Fate and Environmental Risks. Processes 2020, 8, 1479. [Google Scholar] [CrossRef]
- Camacho-Arévalo, R.; García-Delgado, C.; Mayans, B.; Antón-Herrero, R.; Cuevas, J.; Segura, M.L.; Eymar, E. Sulfonamides in Tomato from Commercial Greenhouses Irrigated with Reclaimed Wastewater: Uptake, Translocation and Food Safety. Agronomy 2021, 11, 1016. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S. Pharmaceutical antibiotic compounds in soils–A review. J. Plant Nutr. Soil Sci. 2003, 166, 145–167. [Google Scholar] [CrossRef]
- Etebu, E.; Arikekpar, I. Antibiotics: Classification and mechanisms of action with emphasis on molecular perspectives. Int. J. Appl. Microbiol. Biotechnol. Res. 2016, 4, 90–101. [Google Scholar]
- López-Cabeza, R.; Cox, L.; Gámiz, B.; Galán-Pérez, J.A.; Celis, R. Adsorption of sulfamethoxazole and ethofumesate in biochar- and organoclay-amended soil: Changes with adsorbent aging in the laboratory and in the field. Sci. Total Environ. 2024, 939, 173501. [Google Scholar] [CrossRef]
- Somma, S.; Reverchon, E.; Baldino, L. Water Purification of Classical and Emerging Organic Pollutants: An Extensive Review. ChemEngineering 2021, 5, 47. [Google Scholar] [CrossRef]
- Kharkova, A.; Arlyapov, V.; Medvedeva, A.; Lepikash, R.; Melnikov, P.; Reshetilov, A. Mediator Microbial Biosensor Analyzers for Rapid Determination of Surface Water Toxicity. Sensors 2022, 22, 8522. [Google Scholar] [CrossRef]
- Arlyapov, V.A.; Khar’kova, A.S.; Abramova, T.N.; Kuznetsova, L.S.; Ilyukhina, A.S.; Zaitsev, M.G.; Machulin, A.V.; Reshetilov, A.N. A hybrid redox-active polymer based on bovine serum albumin, ferrocene, carboxylated carbon nanotubes, and glucose oxidase. J. Anal. Chem. 2020, 75, 1189–1200. [Google Scholar] [CrossRef]
- Perchikov, R.N.; Provotorova, D.V.; Kharkova, A.S.; Arlyapov, V.A.; Medvedeva, A.S.; Machulin, A.V.; Filonov, A.E.; Reshetilov, A.N. Bioanalytical System for Determining the Phenol Index Based on Pseudomonas putida BS394(pBS216) Bacteria Immobilized in a Redox-Active Biocompatible Composite Polymer “Bovine Serum Albumin–Ferrocene–Carbon Nanotubes”. Polymers 2022, 14, 5366. [Google Scholar] [CrossRef]
- Guégan, R.; De Oliveira, T.; Le Gleuher, J.; Sugahara, Y. Tuning down the environmental interests of organoclays for emerging pollutants: Pharmaceuticals in presence of electrolytes. Chemosphere 2020, 239, 124730. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Ye, J.; Yang, Q.; Hu, Y.; Zhang, T.; Jiang, L.; Munezero, S.; Lin, K.; Cui, C. Occurrence and removal of antibiotics, antibiotic resistance genes, and bacterial communities in hospital wastewater. Environ. Sci. Pollut. Res. 2021, 28, 57321–57333. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, L.; Chen, W.; Bao, Y.; Zheng, Y.; Huang, B.; Mu, Q.; Wen, D.; Feng, C. Antibiotics in coastal water and sediments of the East China Sea: Distribution, ecological risk assessment and indicators screening. Mar. Pollut. Bull. 2020, 151, 110810. [Google Scholar] [CrossRef] [PubMed]
- Premarathna, K.S.D.; Rajapaksha, A.U.; Adassoriya, N.; Sarkar, B.; Sirimuthu, N.M.S.; Cooray, A.; Ok, Y.S.; Vithanage, M. Clay-biochar composites for sorptive removal of tetracycline antibiotic in aqueous media. J. Environ. Manag. 2019, 238, 315–322. [Google Scholar] [CrossRef]
- de Sousa, D.N.R.; Insa, S.; Mozeto, A.A.; Petrovic, M.; Chaves, T.F.; Fadini, P.S. Equilibrium and kinetic studies of the adsorption of antibiotics from aqueous solutions onto powdered zeolites. Chemosphere 2018, 205, 137–146. [Google Scholar] [CrossRef]
- Imanipoor, J.; Mohammadi, D.; Dinari, M.; Ehsani, M.R. Adsorption and desorption of amoxicillin antibiotic from water matrices using an effective and recyclable MIL-53 (Al) metal–organic framework adsorbent. J. Chem. Eng. Data 2020, 66, 389–403. [Google Scholar] [CrossRef]
- Chauhan, V.; Pandey, A.; Bali, P.; Sharma, H.; Kanwar, S.S. Antibiotics contamination in the environment and its remediation. In Development in Wastewater Treatment Research and Processes; Shah, M.P., Shah, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 157–170. [Google Scholar] [CrossRef]
- Hacıosmanoğlu, G.G.; Mejias, C.; Martin, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Antibiotic adsorption by natural and modified clay minerals as designer adsorbents for wastewater treatment: A comprehensive review. J. Environ. Manag. 2022, 317, 115397. [Google Scholar] [CrossRef]
- Chang, P.H.; Li, Z.; Jiang, W.-T.; Sarkar, B. Clay minerals for pharmaceutical wastewater treatment. In Modified Clay and Zeolite Nanocomposite Materials; Mercurio, M., Sarkar, B., Langella, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 167–196. [Google Scholar] [CrossRef]
- Abd-ElFattah, H.; Kamel, R.; Maged, A.; Kharbish, S. Harnessing clays and clay composites for efficient removal of pharmaceutical contaminants from water: A review. Front. Sci. Res. Technol. 2025, 10, 85–100. [Google Scholar] [CrossRef]
- Yaulilahua-Huacho, R.; Sumarriva-Bustinza, L.A.; Huere-Peña, J.L.; Dueñas-Jurado, C.; Ccente-Chancha, E.J.; Ayuque-Rojas, J.C.; Castañeda-Campos, C.; Palacios-Mucha, M.L.; Garcia-Ticllacuri, R.; Rodas-Ccopa, H.; et al. Surface engineering of chak’o nano-clay with iron oxide and APTES for enhanced heavy metal adsorption in water treatment. F1000Research 2025, 14, 334. [Google Scholar] [CrossRef]
- Niramo, W.; Goodman, B.A. Clay mineral products for improving environmental quality. Appl. Clay Sci. 2023, 242, 106980. [Google Scholar] [CrossRef]
- Perelomov, L.; Mandzhieva, S.; Minkina, T.; Atroshchenko, Y.; Perelomova, I.; Bauer, T.; Pinsky, D.; Barakhov, A. The Synthesis of Organoclays Based on Clay Minerals with Different Structural Expansion Capacities. Minerals 2021, 11, 707. [Google Scholar] [CrossRef]
- Wang, A.; Wang, W. Introduction. In Nanomaterials from Clay Minerals A new Approach to Green Functional Materials, 1st ed.; Wang, A., Wang, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–20. [Google Scholar][Green Version]
- Yu, C.; Qian, A.; Lu, Y.; Liao, W.; Zhang, P.; Tong, M.; Dong, H.; Zeng, Q.; Yuan, S. Electron transfer processes associated with structural Fe in clay minerals. Crit. Rev. Environ. Sci. Technol. 2024, 54, 13–38. [Google Scholar] [CrossRef]
- Ngouana Wakou, B.F.; Kalinichev, A.G. Structural arrangements of isomorphic substitutions in Smectites: Mo-lecular simulation of the swelling properties, interlayer structure, and dynamics of hydrated Cs-Montmorillonite re-visited with new clay models. J. Phys. Chem. C 2014, 118, 12758–12773. [Google Scholar] [CrossRef]
- Awad, A.M.; Shaikh, S.M.R.; Jalab, R.; Gulied, M.H.; Nasser, M.S.; Benamor, A.; Adham, S. Adsorption of Organic Pollutants by Natural and Modified Clays: A Comprehensive Review. Sep. Purif. Technol. 2019, 228, 115719. [Google Scholar] [CrossRef]
- Gupta, V.; Miller, J.D. Surface force measurements at the basal planes of ordered kaolinite particles. J. Coll. Interface Sci. 2010, 344, 362–371. [Google Scholar] [CrossRef]
- Li, G.L.; Zhou, C.H.; Fiore, S.; Yu, W.H. Interactions between microorganisms and clay minerals: New insights and broader applications. Appl. Clay Sci. 2019, 177, 91–113. [Google Scholar] [CrossRef]
- Tombácz, E.; Szekeres, M. Surface charge heterogeneity of kaolinite in aqueous suspension in comparison with montmorillonite. Appl. Clay Sci. 2006, 34, 105–124. [Google Scholar] [CrossRef]
- Yang, C.; Gao, R.; Yang, H. Application of layered nanoclay in electrochemical energy: Current status and future. EnergyChem 2021, 3, 100062. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. General introduction: Clays, clay minerals, and clay science. In Handbook of Clay Science. Part A. Fundamentals, Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–19. [Google Scholar] [CrossRef]
- Fauchille, A.L.; Hedan, S.; Valle, V.; Prêt, D.; Cabrera, J.; Cosenza, P. Effect of microstructure on hydric strain in clay rock: A quantitative comparison. Appl. Clay Sci. 2019, 182, 105244. [Google Scholar] [CrossRef]
- Pokidko, B.V.; Bukanova, E.F.; Tutorsky, I.A.; Il‘ina, M.B. Influence of Ca2+ on the adsorption of different surfactants in the bentonite-water interface. Fine Chem. Technol. 2009, 4, 77–83. (In Russian) [Google Scholar]
- Ra, H.; Kim, S.; Kim, T.; Bae, J.; Shim, W. Phyllosilicate Clay Minerals: Principles and Applications. Mater. Matters 2022, 17. Available online: https://www.sigmaaldrich.com/NL/en/technical-documents/technical-article/materials-science-and-engineering/nanoparticle-and-microparticle-synthesis/phyllosilicate-clay-minerals-principles-applications (accessed on 9 July 2025).
- López-Rodríguez, D.; Micó-Vicent, B.; Jordán-Núñez, J.; Bonet-Aracil, M.; Bou-Belda, E. Uses of Nanoclays and Adsorbents for Dye Recovery: A Textile Industry Review. Appl. Sci. 2021, 11, 11422. [Google Scholar] [CrossRef]
- Kosmulski, M. Isoelectric points and points of zero charge of metal (hydr) oxides: 50 years after Parks’ review. Adv. Coll. Interface Sci. 2016, 238, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Henderson, D.; Boda, D. Insights from theory and simulation on the electrical double layer. Phys. Chem. Chem. Phys. 2009, 11, 3822–3830. [Google Scholar] [CrossRef]
- Wu, J. Understanding the electric double-layer structure, capacitance, and charging dynamics. Chem. Rev. 2022, 122, 10821–10859. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, L.; Li, R.; Zhang, Z.; Wang, Q.; Yang, J.; Guo, C.F.; Pan, T. First decade of interfacial iontronic sensing: From droplet sensors to artificial skins. Adv. Mat. 2021, 33, 2003464. [Google Scholar] [CrossRef]
- White, G.N.; Zelazny, L.W. Analysis and implications of the edge structure of dioctahedral phyllosilicates. Clays Clay Miner. 1988, 36, 141–146. [Google Scholar] [CrossRef]
- Yang, M.; Liang, X.; Ma, L.; Huang, J.; He, H.; Zhu, J. Adsorption of REEs on kaolinite and halloysite: A link to the REE distribution on clays in the weathering crust of granite. Chem. Geol. 2019, 525, 210–217. [Google Scholar] [CrossRef]
- Goldberg, S., Jr.; Criscenti, L.J. Modeling adsorption of metals and metalloids by soil components. In Biophysico-Chemical Processes of Heavy Metals and Metalloids in Soil Environments; Violante, A., Huang, P.M., Gadd, G.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 215–264. [Google Scholar] [CrossRef]
- Zeng, L.; Sarmadivaleh, M.; Saeedi, A.; Chen, Y.; Zhong, Z.; Xie, Q. Storage integrity during underground hydrogen storage in depleted gas reservoirs. Earth-Sci. Rev. 2023, 247, 104625. [Google Scholar] [CrossRef]
- Liu, X.; Tournassat, C.; Grangeon, S.; Kalinichev, A.G.; Takahashi, Y.; Fernandes, M.M. Molecular-level understanding of metal ion retention in clay-rich materials. Nat. Rev. Earth Environ. 2022, 3, 461–476. [Google Scholar] [CrossRef]
- Borchardt, G. Smectites. In Minerals in Soil Environments, 2nd ed.; Dixon, J.B., Weed, S.B., Eds.; Soil Science Society of America: Madison, WI, USA, 1989; pp. 675–727. [Google Scholar] [CrossRef]
- Dixon, J.B. Kaolin and Serpentine Group Minerals. In Minerals in Soil Environments, 2nd ed.; Dixon, J.B., Weed, S.B., Eds.; Soil Science Society of America: Madison, WI, USA, 1989; pp. 467–525. [Google Scholar] [CrossRef]
- Li, Y.; Fang, J.; Yuan, X.; Chen, Y.; Yang, H.; Fei, X. Distribution Characteristics and Ecological Risk Assessment of Tetracyclines Pollution in the Weihe River, China. Int. J. Environ. Res. Public Health 2018, 15, 1803. [Google Scholar] [CrossRef]
- Tolls, J. Sorption of veterinary pharmaceuticals in soils: A review. Environ. Sci. Technol. 2001, 35, 3397–3406. [Google Scholar] [CrossRef]
- Figueroa, R.A.; Leonard, A.; MacKay, A.A. Modeling tetracycline antibiotic sorption to clays. Environ. Sci. Technol. 2004, 38, 476–483. [Google Scholar] [CrossRef]
- Kulshrestha, P.; Giese, R.F., Jr.; Aga, D.S. Investigating the molecular interactions of oxytetracycline in clay and organic matter: Insights on factors affecting its mobility in soil. Environ. Sci. Technol. 2004, 38, 4097–4105. [Google Scholar] [CrossRef]
- Parolo, M.E.; Savini, M.C.; Vallés, J.M.; Baschini, M.T.; Avena, M.J. Tetracycline adsorption on montmorillonite: pH and ionic strength effects. Appl. Clay Sci. 2008, 40, 179–186. [Google Scholar] [CrossRef]
- Dogan, M.; Dogan, A.U.; Yesilyurt, F.I.; Alaygut, D.; Buckner, I.; Wurster, D.E. Baseline studies of The Clay Minerals Society special clays: Specific surface area by the Brunauer Emmett Teller (BET) method. Clays Clay Miner. 2006, 54, 534–541. [Google Scholar] [CrossRef]
- Macht, F.; Eusterhues, K.; Pronk, G.J.; Totsche, K.U. Specific surface area of clay minerals: Comparison between atomic force microscopy measurements and bulk-gas (N2) and -liquid (EGME) adsorption methods. Appl. Clay Sci. 2011, 53, 20–26. [Google Scholar] [CrossRef]
- Eslinger, E.; Pevear, D. Clay Minerals for Peteroleum Geologists and Engineers; SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 1985. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, S.; Jing, R.; Shao, Y.; Liu, X.; Lv, F.; Hu, X.; Zhang, Q.; Meng, Z.; Liu, A. Competitive adsorption of antibiotic tetracycline and ciprofloxacin on montmorillonite. Appl. Clay Sci. 2019, 180, 105175. [Google Scholar] [CrossRef]
- Jorge, N.L.; Garrafa, M.V.; Romero, J.M.; Jorge, M.J.; Jorge, L.C.; Delfino, M.R.; Meruvia-Rojas, Y.V.; Hernández-Laguna, A.; Sainz-Díaz, C.I. Adsorption of Ciprofloxacin on Clay Minerals in Argentinian Santa Rosa-Corrientes Soils. Molecules 2024, 29, 1760. [Google Scholar] [CrossRef]
- Antonelli, R.; Malpass, G.R.P.; da Silva, M.G.C.; Vieira, M.G.A. Fixed-bed adsorption of ciprofloxacin onto bentonite clay: Characterization, mathematical modeling, and DFT-based calculations. Ind. Eng. Chem. Res. 2021, 60, 4030–4040. [Google Scholar] [CrossRef]
- Jara-Cobos, L.; Peñafiel, M.E.; Montero, C.; Menendez, M.; Pinos-Vélez, V. Ciprofloxacin Removal Using Pillared Clays. Water 2023, 15, 2056. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, P.; Hrichi, A.; Garrido-Zoido, J.M.; Álvarez-Torrellas, S.; Larriba, M.; Gil, M.V.; Amor, H.B.; García, H. Natural clays as adsorbents for the efficient removal of antibiotic ciprofloxacin from wastewaters: Experimental and theoretical studies using DFT method. J. Ind. Eng. Chem. 2024, 134, 137–151. [Google Scholar] [CrossRef]
- Jin, X.; Zha, S.; Li, S.; Chen, Z. Simultaneous removal of mixed contaminants by organoclays—Amoxicillin and Cu(II) from aqueous solution. Appl. Clay Sci. 2014, 102, 196–201. [Google Scholar] [CrossRef]
- De Oliveira, T.; Fernandez, E.; Fougère, L.; Destandau, E.; Boussafir, M.; Sohmiya, M.; Sugahara, Y.; Guégan, R. Competitive association of antibiotics with a clay mineral and organoclay derivatives as a control of their lifetimes in the environment. ACS Omega 2018, 3, 15332–15342. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, R.; Martins, F.R.; Malpass, G.R.P.; da Silva, M.G.C.; Vieira, M.G.A. Ofloxacin Adsorption by Calcined Verde-Lodo Bentonite Clay: Batch and Fixed Bed System Evaluation. J. Mol. Liq. 2020, 315, 113718. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, Y.; Boyd, S.A.; Teppen, B.J.; Li, H. Sorption and desorption of carbamazepine from water by smectite clays. Chemosphere 2010, 81, 954–960. [Google Scholar] [CrossRef]
- Li, Z.; Chang, P.-H.; Jean, J.-S.; Jiang, W.-T.; Wang, C.-J. Interaction between tetracycline and smectite in aqueous solution. J. Colloid Interf. Sci. 2010, 341, 311–319. [Google Scholar] [CrossRef]
- Zhao, Y.; Geng, J.; Wang, X.; Gu, X.; Gao, S. Tetracycline adsorption on kaolinite: pH, metal cations and humic acid effects. Ecotoxicology 2011, 20, 1141–1147. [Google Scholar] [CrossRef]
- Nawaz, S.; Ahmad, M.; Asif, S.; Klemeš, J.J.; Mubashir, M.; Munir, M.; Zafar, M.; Bokhari, A.; Mukhtar, A.; Saqib, S.; et al. Phyllosilicate derived catalysts for efficient conversion of lignocellulosic derived biomass to biodiesel: A review. Bioresour. Technol. 2022, 343, 126068. [Google Scholar] [CrossRef]
- Li, Z.; Hong, H.; Liao, L.; Ackley, C.J.; Schulz, L.A.; MacDonald, R.A.; Mihelich, A.L.; Emard, S.M. A mechanistic study of ciprofloxacin removal by kaolinite. Colloids Surf. B Biointerfaces 2011, 88, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112–113, 75–93. [Google Scholar] [CrossRef]
- Ramadass, K.; Singh, G.; Lakhi, K.S.; Benzigar, M.R.; Yang, J.-H.; Kim, S.; Almajid, A.M.; Belperio, T.; Vinu, A. Halloysite nanotubes: Novel and eco-friendly adsorbents for high-pressure CO2 capture. Microporous Mesoporous Mater. 2019, 277, 229–236. [Google Scholar] [CrossRef]
- Duan, W.; Wang, N.; Xiao, W.; Zhao, Y.; Zheng, Y. Ciprofloxacin adsorption onto different micro-structured tourmaline, halloysite and biotite. J. Mol. Liq. 2018, 269, 874–881. [Google Scholar] [CrossRef]
- Cheng, R.; Li, H.; Liu, Z.; Du, C. Halloysite Nanotubes as an Effective and Recyclable Adsorbent for Removal of Low-Concentration Antibiotics Ciprofloxacin. Minerals 2018, 8, 387. [Google Scholar] [CrossRef]
- Ramanayaka, S.; Sarkar, B.; Cooray, A.T.; Ok, Y.S.; Vithanage, M. Halloysite nanoclay supported adsorptive removal of oxytetracycline antibiotic from aqueous media. J. Hazard. Mater. 2020, 384, 121301. [Google Scholar] [CrossRef]
- Giannoulia, S.; Triantaphyllidou, I.-E.; Tekerlekopoulou, A.G.; Aggelopoulos, C.A. Mechanisms of Individual and Simultaneous Adsorption of Antibiotics and Dyes onto Halloysite Nanoclay and Regeneration of Saturated Adsorbent via Cold Plasma Bubbling. Nanomaterials 2023, 13, 341. [Google Scholar] [CrossRef]
- Barbosa, S.E.; Castillo, L.A. Morphological and Physicochemical Properties of Macrocrystalline Talc from Argentine. Minerals 2023, 13, 683. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Thavorn-amornsri, T.; Pereira, M.F.R.; Serp, P.; Figueiredo, J.L. Comparison between activated carbon, carbon xerogel and carbon nanotubes for the adsorption of the antibiotic ciprofloxacin. Catal. Today 2012, 186, 29–34. [Google Scholar] [CrossRef]
- Dube, C.; Tandlich, R.; Wilhelmi, B. Adsorptive removal of ciprofloxacin and isoniazid from aqueous solution. Nova Biotechnol. Chim. 2018, 17, 16–28. [Google Scholar] [CrossRef]
- Raudkivi, A.J. Loose Boundary Hydraulics, 1st ed.; CRC Press: London, UK, 1998; 507p. [Google Scholar] [CrossRef]
- Genç, N.; Dogan, E.C.; Yurtsever, M. Bentonite for ciprofloxacin removal from aqueous solution. Water Sci. Technol. 2013, 68, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Genç, N.; Dogan, E.C. Adsorption kinetics of the antibiotic ciprofloxacin on bentonite, activated carbon, zeolite, and pumice. Desalin. Water Treat. 2015, 53, 785–793. [Google Scholar] [CrossRef]
- Gulen, B.; Demircivi, P. Adsorption properties of flouroquinolone type antibiotic ciprofloxacin into 2:1 dioctahedral clay structure: Box-Behnken experimental design. J. Mol. Struct. 2020, 1206, 127659. [Google Scholar] [CrossRef]
- Putra, E.K.; Pranowo, R.; Sunarso, J.; Indraswati, N.; Ismadji, S. Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: Mechanisms, isotherms and kinetics. Water Res. 2009, 43, 2419–2430. [Google Scholar] [CrossRef]
- Bekci, Z.; Seki, Y.; Yurdakoc, M. Equilibrium studies for trimethoprim adsorption on montmorillonite KSF. J. Hazard. Mater. 2006, 133, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Schulze, D.G. Mineral components of the soil clay fraction. In Encyclopedia of Soils in the Environment, 2nd ed.; Goss, M.J., Oliver, M., Eds.; Academic Press: London, UK, 2023; pp. 109–120. [Google Scholar] [CrossRef]
- Cooper, V.R. Van der Waals density functional: An appropriate exchange functional. Phys. Rev. B 2010, 81, 161104. [Google Scholar] [CrossRef]
- Tri, N.N.; Nguyen, M.T.; Trung, N.T. A molecular level insight into adsorption of β-lactam antibiotics on vermiculite surface. Surf. Sci. 2020, 695, 121588. [Google Scholar] [CrossRef]
- Tri, N.N.; Trung, N.T. Theoretical study on the adsorption of benzylpenicillin molecule onto vermiculite surface. Vietnam. J. Chem. 2019, 57, 514–519. [Google Scholar] [CrossRef]
- Call, J.J.; Rakshit, S.; Essington, M.E. The Adsorption of Tylosin by Montmorillonite and Vermiculite: Exchange Selectivity and Intercalation. Soil Sci. Soc. Am. J. 2019, 83, 584–596. [Google Scholar] [CrossRef]
- Hamza, A.; Hussein, I.A.; Mahmoud, M. Chapter 1—Introduction to reservoir fluids and rock properties. In Developments in Petroleum Science; Hussein, I.A., Mahmoud, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 78, pp. 1–19. [Google Scholar] [CrossRef]
- Chang, P.-H.; Li, Z.; Jean, J.-S.; Jiang, W.-T.; Wang, C.-J.; Lin, K.-H. Adsorption of tetracycline on 2:1 layered non-swelling clay mineral illite. Appl. Clay Sci. 2012, 67–68, 158–163. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S.; Shahzad, K. A review on removal of pharmaceuticals from water by adsorption. Desalination Water Treat. 2016, 57, 12842–12860. [Google Scholar] [CrossRef]
- Alekseeva, T.V.; Alekseev, A.O. Rectorite as a Syngenetic Component of a Soddy-Podzolic Soil. Eurasian Soil Sci. 2020, 53, 1502–1508. [Google Scholar] [CrossRef]
- Chang, P.-H.; Li, Z.; Jiang, W.-T.; Jean, J.-S. Adsorption and intercalation of tetracycline by swelling clay minerals. Appl. Clay Sci. 2009, 46, 27–36. [Google Scholar] [CrossRef]
- Giannoulia, S.; Tekerlekopoulou, A.G.; Aggelopoulos, C.A. Exploration of cephalexin adsorption mechanisms onto bauxite and palygorskite and regeneration of spent adsorbents with cold plasma bubbling. Appl. Water. Sci. 2024, 14, 51. [Google Scholar] [CrossRef]
- Bilgin, N.; Bulut, E.; Sabah, E. Mechanistic insight into amoxicillin removal by natural sepiolite. Int. J. Environ. Sci. Technol. 2023, 20, 8897–8912. [Google Scholar] [CrossRef]
- Qiang, Z.; Adams, C. Potentiometric determination of acid dissociation constants (pKa) for human and veterinary antibiotics. Water Res. 2004, 38, 2874–2890. [Google Scholar] [CrossRef] [PubMed]
- Othersen, O.G.; Beierlein, F.; Lanig, H.; Clark, T. Conformations and tautomers of tetracycline. J. Phys. Chem. B 2003, 107, 13743–13749. [Google Scholar] [CrossRef]
- Bansal, O.P. Sorption of Tetracycline, Oxytetracycline, and Chlortetracycline in Illite and Kaolinite Suspensions. ISRN Environ. Chem. 2013, 694681. [Google Scholar] [CrossRef]
- Rozalen, M.; Brady, P.V.; Huertas, F.J. Surface chemistry of K-montmorillonite: Ionic strength, temperature dependence and dissolution kinetics. J. Coll. Interface Sci. 2009, 333, 474–484. [Google Scholar] [CrossRef]
- Tombacz, E.; Szekeres, M. Colloidal behavior of aqueous montmorillonite suspensions: The specific role of pH in the presence of indifferent electrolytes. Appl. Clay Sci. 2004, 27, 75–94. [Google Scholar] [CrossRef]
- Thomas, F.; Michot, L.J.; Vantelon, D.; Montarges, E.; Prelot, B.; Cruchaudet, M.; Delon, J.F. Layer charge and electrophoretic mobility of smectites. Coll. Surf. A Physicochem. Eng. Asp. 1999, 159, 351–358. [Google Scholar] [CrossRef]
- Lagaly, G.; Dékány, I. Colloid clay science. In Handbook of Clay Science. Part A. Fundamentals, Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 243–345. [Google Scholar] [CrossRef]
- Ma, C.; Eggleton, R.A. Cation exchange capacity of kaolinite. Clay Clay Miner. 1999, 47, 174–180. [Google Scholar] [CrossRef]
- Song, Y.; Sackey, E.A.; Wang, H.; Wang, H. Adsorption of oxytetracycline on kaolinite. PLoS ONE 2019, 14, e0225335. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, S.M.; Al-Mahmoud, S.M. Adsorption of tetracycline antibiotic from aqueous solutions using natural Iraqi bentonite. Egypt. J. Chem. 2021, 64, 5511–5519. [Google Scholar] [CrossRef]
- Bui, T.X.; Choi, H. Adsorptive removal of selected pharmaceuticals by mesoporous silica SBA-15. J. Hazard. Mater. 2009, 168, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.L.; Ribeiro, I.C. Modelling the adsorption kinetics of erythromycin onto neutral and anionic resins. Bioprocess Biosyst. Eng. 2003, 26, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Shon, H.K.; Ngo, H.H. Adsorption characteristics of antibiotics trimethoprim on powdered and granular activated carbon. J. Ind. Eng. Chem. 2010, 16, 344–349. [Google Scholar] [CrossRef]
- Deng, H.; Song, Y.; Li, W.; Fatima, M.N.; Bibi, H.; Ye, S. Performance and economy of antibiotic adsorption by the composite of plant decomposed liquid, chemical modifier, and clay. Process Saf. Environ. Prot. 2024, 192, 1408–1419. [Google Scholar] [CrossRef]
- Zhang, J.X.; Zhou, Q.X.; Li, W. Adsorption of enrofloxacin from aqueous solution by bentonite. Clay Miner. 2013, 48, 627–637. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).