Achyrophanite, (K,Na)3(Fe3+,Ti,Al,Mg)5O2(AsO4)5, a New Mineral with the Novel Structure Type from Fumarolic Exhalations of the Tolbachik Volcano, Kamchatka, Russia
Abstract
1. Introduction
2. Occurrence and Mineral Association
3. Methods
4. Results
4.1. General Appearance and Physical Properties
4.2. Optical Data
4.3. Raman Spectroscopy
4.4. Chemical Data
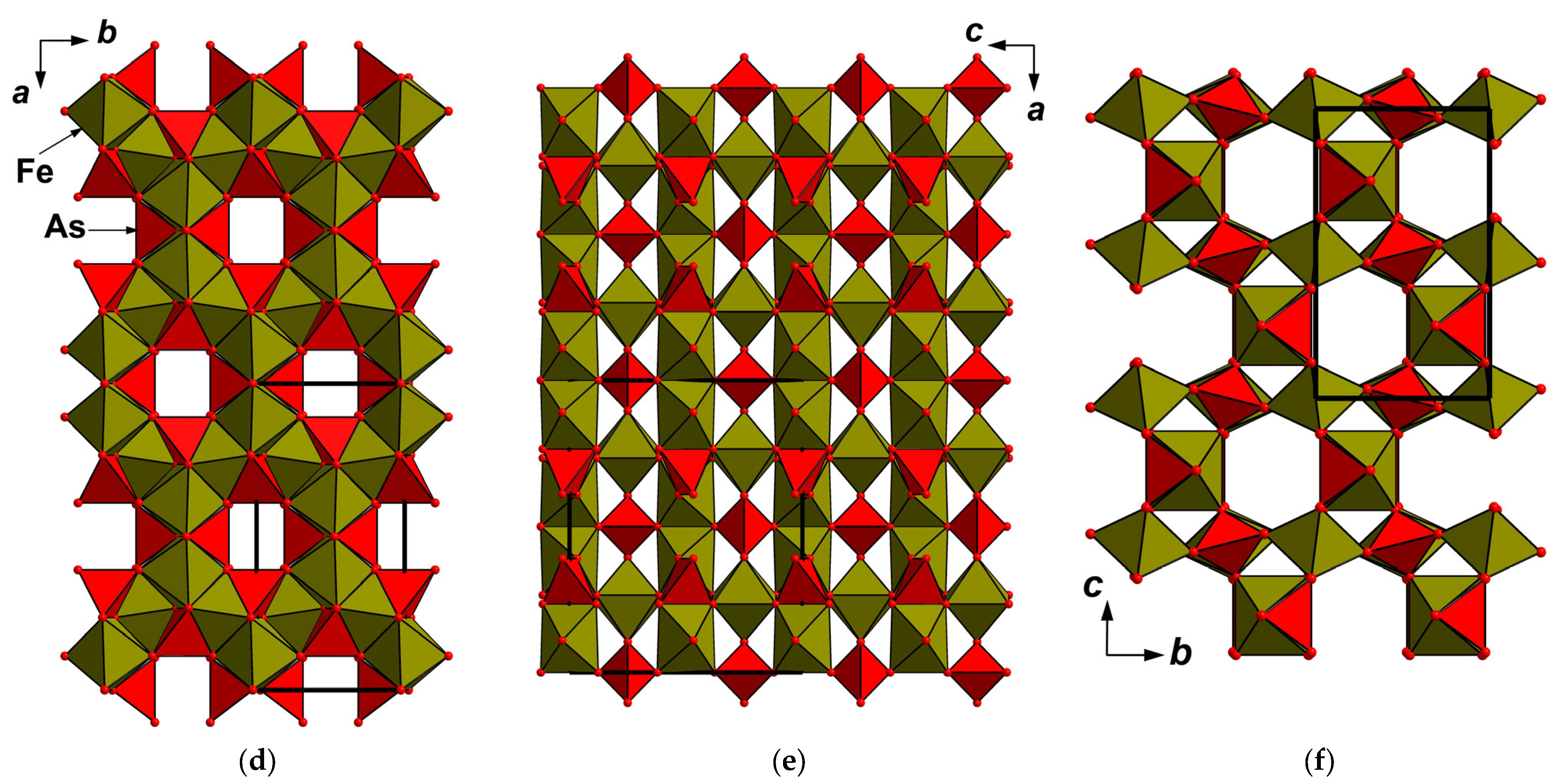
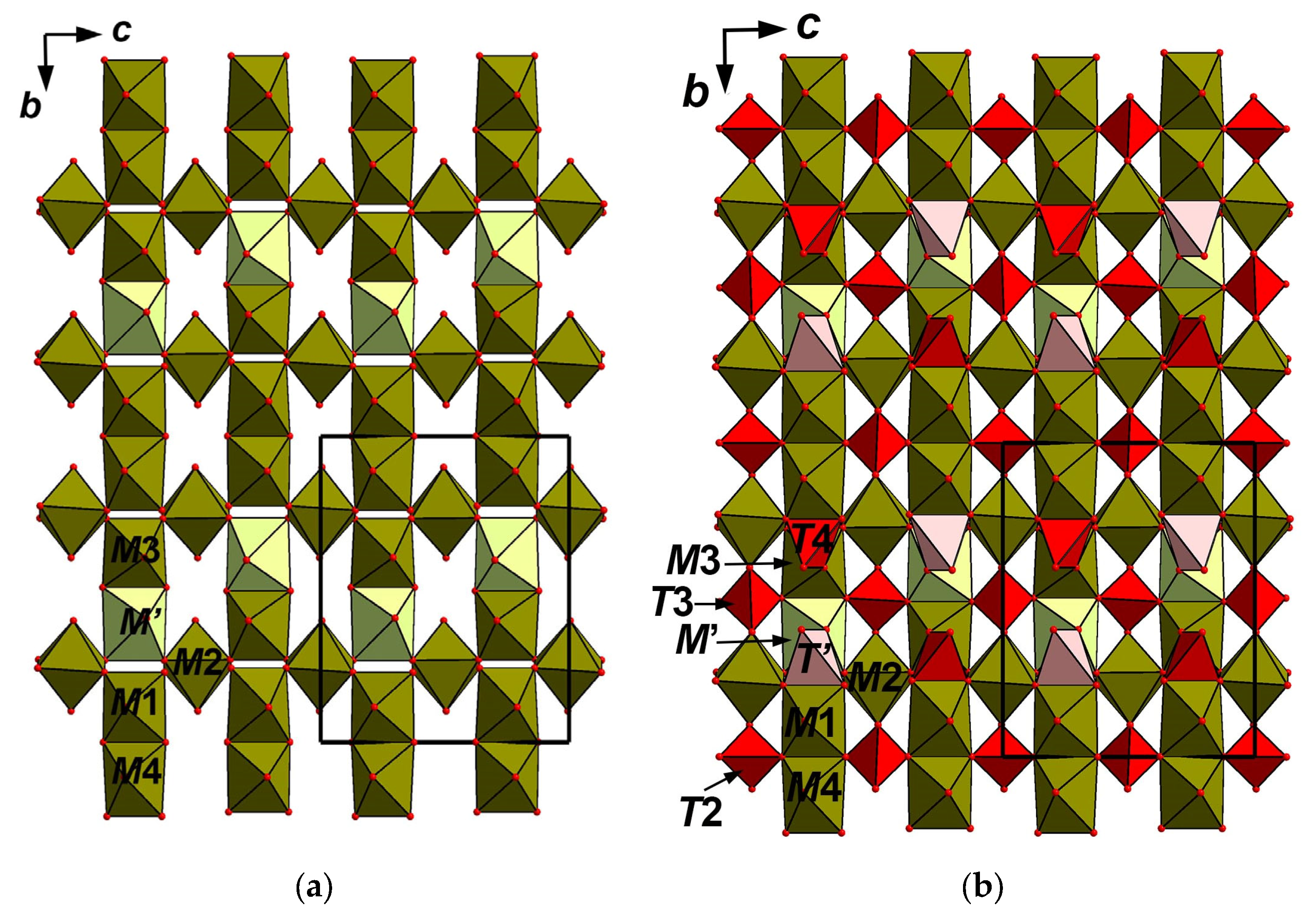
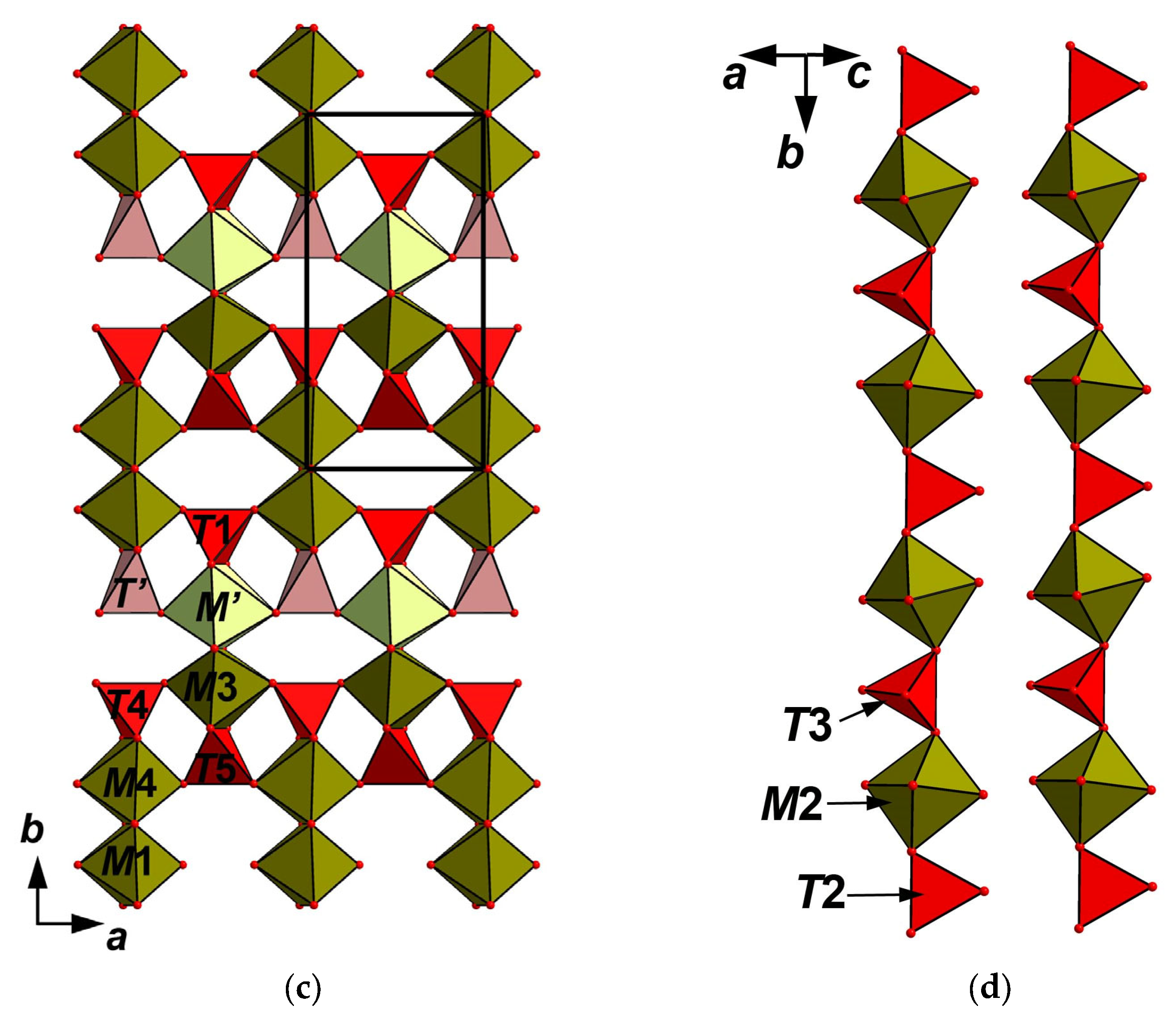
4.5. X-Ray Crystallography and Crystal Structure
5. Discussion
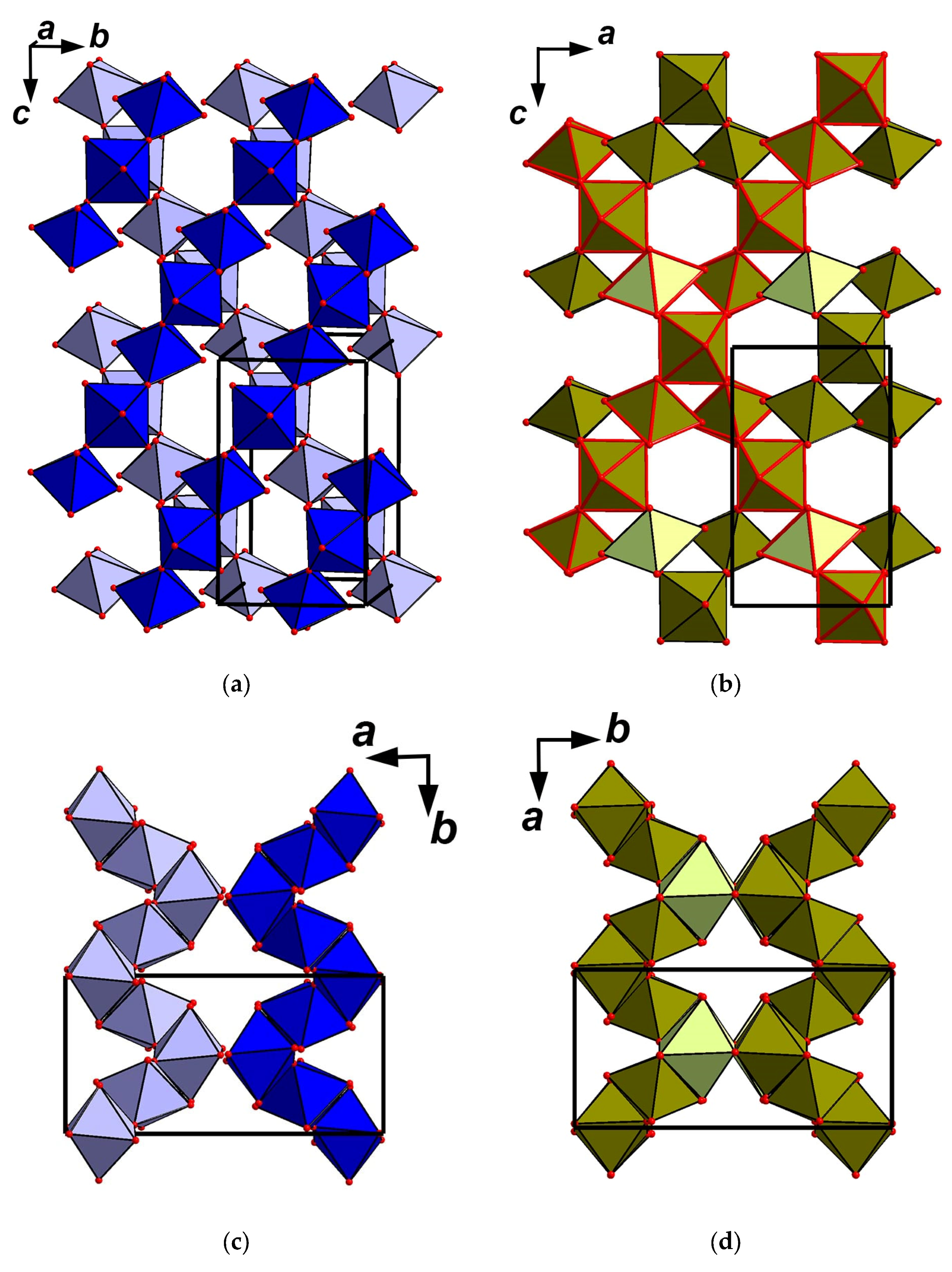
5.1. Structure Description
5.2. Comparative Crystal Chemistry
5.3. Genetic Notes
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pekov, I.V.; Koshlyakova, N.N.; Zubkova, N.V.; Lykova, I.S.; Britvin, S.N.; Yapaskurt, V.O.; Agakhanov, A.A.; Shchipalkina, N.V.; Turchkova, A.G.; Sidorov, E.G. Fumarolic Arsenates—A Special Type of Arsenic Mineralization. Eur. J. Mineral. 2018, 30, 305–322. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Agakhanov, A.A.; Vigasina, M.F.; Yapaskurt, V.O.; Britvin, S.N.; Turchkova, A.G.; Sidorov, E.G.; Zhitova, E.S.; Pushcharovsky, D.Y. New arsenate minerals from the Arsenatnaya fumarole, Tolbachik volcano, Kamchatka, Russia. XX. Evseevite, Na2Mg(AsO4)F, the first natural arsenate with antiperovskite structure. Mineral. Mag. 2023, 87, 839–848. [Google Scholar] [CrossRef]
- The Great Tolbachik Fissure Eruption; Fedotov, S.A., Markhinin, Y.K., Eds.; Cambridge University Press: New York, NY, USA, 1983. [Google Scholar]
- Vergasova, L.P.; Filatov, S.K. A study of volcanogenic exhalation mineralization. J. Volcanol. Seismol. 2016, 10, 71–85. [Google Scholar] [CrossRef]
- Pekov, I.V.; Agakhanov, A.A.; Zubkova, N.V.; Koshlyakova, N.V.; Shchipalkina, N.V.; Sandalov, F.D.; Yapaskurt, V.O.; Turchkova, A.G.; Sidorov, E.G. Oxidizing-type fumaroles of the Tolbachik Volcano, a mineralogical and geochemical unique. Russ. Geol. Geophys. 2020, 61, 675–688. [Google Scholar] [CrossRef]
- Shchipalkina, N.V.; Pekov, I.V.; Koshlyakova, N.N.; Britvin, S.N.; Zubkova, N.V.; Varlamov, D.A.; Sidorov, E.G. Unusual Silicate Mineralization in Fumarolic Sublimates of the Tolbachik Volcano, Kamchatka, Russia—Part 1: Neso-, Cyclo-, Ino- And Phyllosilicates. Eur. J. Mineral. 2020, 32, 101–119. [Google Scholar] [CrossRef]
- Britvin, S.N.; Dolivo-Dobrovolsky, D.V.; Krzhizhanovskaya, M.G. Software for processing the X-ray powder diffraction data obtained from the curved image plate detector of Rigaku RAXIS Rapid II diffractometer. Zap. Ross. Mineral. Obs. 2017, 146, 104–107. (In Russian) [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Mandarino, J.A. The Gladstone-Dale relationship, Part IV. The compatibility concept and its application. Can. Mineral. 1981, 19, 441–450. [Google Scholar]
- Agilent Technologies. CrysAlisPro Software System; Version 1.171.37.34; Agilent Technologies UK Ltd.: Oxford, UK, 2014. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Crystallogr. 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Bazán, B.; Mesa, J.L.; Pizarro, J.L.; Aguayo, A.T.; Arriortua, M.I.; Rojo, T. Fe(AsO4): A new iron(III) arsenate synthesized from thermal treatment of (NH4)[Fe(AsO4)F]. Chem. Commun. 2003, 5, 622–623. [Google Scholar] [CrossRef]
- Bazán, B.; Mesa, J.L.; Pizarro, J.L.; Rodríguez-Fernández, J.; Sánchez-Marcos, J.; Roig, A.; Molins, E.; Arriortua, M.I.; Rojo, T. Thermal transformation of (NH4)[Fe(AsO4)F] into the new textural porous orthorhombic Fe(AsO4) phase. Crystal structures, thermal behavior, spectroscopic and magnetic properties. Chem. Mater. 2004, 16, 5249–5259. [Google Scholar] [CrossRef]
- Glaum, R.; Gruehn, R.; Möller, M. Darstellung und Struktur von α-CrPO4. Beiträge zum thermischen Verhalten von wasserfreien Phosphaten. I. Z. Anorg. Allg. Chem. 1986, 543, 111–116. [Google Scholar] [CrossRef]
- Attfield, J.P.; Cheetham, A.K.; Cox, D.E.; Sleight, A.W. Synchrotron X-ray and neutron powder diffraction studies of the structure of α-CrPO4. J. Appl. Crystallogr. 1988, 21, 452–457. [Google Scholar] [CrossRef]
- Attfield, J.P.; Battle, P.D.; Cheetham, A.K.; Johnson, D.C. Magnetic structures and properties of α-CrPO4 and α-CrAsO4. Inorg. Chem. 1989, 28, 1207–1213. [Google Scholar] [CrossRef]
- Rittner, P.; Glaum, R. Kristallzüchtung und Einkristallstrukturverfeinerungen der Rhodium(III)-phosphate RhPO4 und RhP3O9. Z. Krist. 1994, 209, 162–169. [Google Scholar] [CrossRef]
- Nespolo, M.; Ferraris, G. Twinning by syngonic and metric merohedry. Analysis, classification and effects on the diffraction pattern. Z. Krist. 2000, 215, 77–81. [Google Scholar] [CrossRef]
- Pekov, I.V.; Yapaskurt, V.O.; Britvin, S.N.; Zubkova, N.V.; Vigasina, M.F.; Sidorov, E.G. New arsenate minerals from the Arsenatnaya fumarole, Tolbachik volcano, Kamchatka, Russia. V. Katiarsite, KTiO(AsO4). Mineral. Mag. 2016, 80, 639–646. [Google Scholar] [CrossRef]
- Mayo, S.C.; Thomas, P.A.; Teat, S.J.; Loiacono, G.M.; Loiacono, D.N. Structure and nonlinear optical properties of KTiOAsO4. Acta Crystallogr. 1994, 50, 655–662. [Google Scholar] [CrossRef]
- Northrup, P.A.; Parise, J.B.; Cheng, L.K.; Cheng, L.T.; McCarron, E.M. High-temperature single-crystal X-ray diffraction studies of potassium and (cesium, potassium) titanyl arsenates. Chem. Mater. 1994, 6, 434–440. [Google Scholar] [CrossRef]
- Novikova, N.E.; Verin, I.A.; Sorokina, N.I.; Alekseeva, O.A.; Tseitlin, M.; Roth, M. Structure of KTiOAsO4 single crystals at 293 and 30 K. Crystallogr. Rep. 2010, 55, 412–423. [Google Scholar] [CrossRef]
- Symonds, R.B.; Reed, M.H. Calculation of multicomponent chemical equilibria in gas-solid-liquid systems: Calculation methods, thermochemical data, and applications to studies of high-temperature volcanic gases with examples from Mount St. Helens. Am. J. Sci. 1993, 293, 758–864. [Google Scholar] [CrossRef]
- Churakov, S.V.; Tkachenko, S.I.; Korzhinskii, M.A.; Bocharnikov, R.E.; Shmulovich, K.I. Evolution of composition of high-temperature fumarolic gases from Kudryavy volcano, Iturup, Kuril Islands: The thermodynamic modeling. Geochem. Int. 2000, 38, 436–451. [Google Scholar]
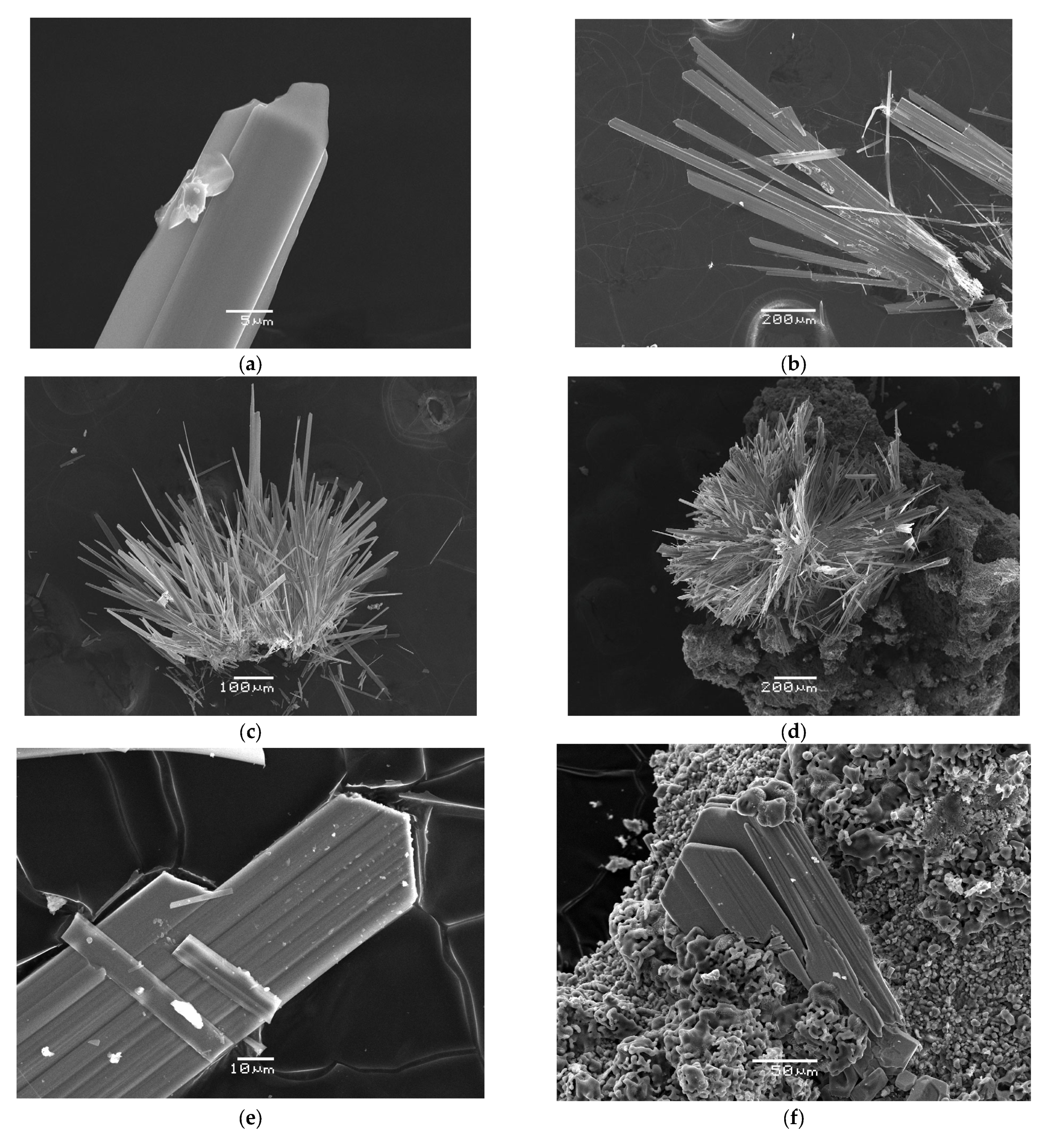





| Constituent | Mean (for 8 Spot Analyses) | Range | Standard Deviation |
|---|---|---|---|
| Na2O | 3.68 | 3.41–3.92 | 0.20 |
| K2O | 9.32 | 8.98–9.66 | 0.23 |
| CaO | 0.38 | 0.30–0.44 | 0.05 |
| MgO | 1.27 | 1.04–1.60 | 0.22 |
| MnO | 0.08 | 0.06–0.11 | 0.02 |
| CuO | 0.82 | 0.71–0.95 | 0.13 |
| ZnO | 0.48 | 0.37–0.58 | 0.08 |
| Al2O3 | 2.09 | 1.53–2.81 | 0.47 |
| Fe2O3 * | 20.42 | 18.64–22.71 | 1.59 |
| TiO2 | 7.35 | 6.60–7.96 | 0.55 |
| SiO2 | 0.12 | 0.04–0.19 | 0.05 |
| P2O5 | 0.14 | 0.04–0.20 | 0.05 |
| V2O5 | 0.33 | 0.14–0.50 | 0.12 |
| As2O5 | 51.88 | 50.61–54.25 | 1.17 |
| SO3 | 1.04 | 0.21–1.91 | 0.70 |
| Total | 99.40 |
| Iobs | dobs | Icalc * | dcalc ** | h k l |
|---|---|---|---|---|
| 5 | 13.21 | 13 | 13.249 | 010 |
| 2 | 8.36 | 8 | 8.353 | 011 |
| 9 | 6.62 | 8, 1 | 6.624, 6.582 | 020, 100 |
| 11 | 5.900 | 11 | 5.894 | 110 |
| 59 | 5.615 | 6, 88 | 5.641, 5.615 | 021, 101 |
| 9 | 5.376 | 10 | 5.381 | 002 |
| 4 | 4.980 | 2 | 4.985 | 012 |
| 3 | 4.683 | 1 | 4.669 | 120 |
| 3 | 4.417 | 1 | 4.416 | 030 |
| 1 | 4.278 | 1 | 4.283 | 121 |
| 42 | 4.174 | 41 | 4.177 | 022 |
| 2 | 4.077 | 1 | 4.086 | 031 |
| 3 | 3.972 | 2 | 3.974 | 112 |
| 31 | 3.669 | 39 | 3.667 | 130 |
| 6 | 3.527 | 5 | 3.527 | 122 |
| 4 | 3.469 | 3 | 3.471 | 131 |
| 14 | 3.413 | 13 | 3.414 | 032 |
| 6 | 3.315 | 1, 6 | 3.312, 3.291 | 040, 200 |
| 33 | 3.148 | 5, 37 | 3.154, 3.150 | 023, 103 |
| 8 | 3.064 | 1, 17 | 3.064, 3.062 | 113, 211 |
| 5 | 3.032 | 6 | 3.030 | 132 |
| 11 | 2.951 | 2, 17 | 2.959, 2.947 | 140, 220 |
| 43 | 2.852 | 45 | 2.853 | 141 |
| 100 | 2.814 | 61, 100 | 2.821, 2.808 | 042, 202 |
| 29 | 2.689 | 43 | 2.690 | 004 |
| 5 | 2.642 | 2, 4, 2 | 2.650, 2.639, 2.637 | 050, 230, 014 |
| 7 | 2.587 | 9 | 2.585 | 222 |
| 5 | 2.565 | 1, 9 | 2.564, 2.563 | 133, 231 |
| 1 | 2.490 | 1 | 2.490 | 104 |
| 2 | 2.443 | 1 | 2.447 | 114 |
| 1 | 2.398 | 1 | 2.396 | 151 |
| 1 | 2.371 | 1 | 2.369 | 232 |
| 2 | 2.296 | 2 | 2.298 | 034 |
| 2 | 2.284 | 3, 1 | 2.282, 2.282 | 143, 241 |
| 28 | 2.237 | 37 | 2.236 | 152 |
| 2 | 2.210 | 1 | 2.208 | 060 |
| 9 | 2.169 | 11, 1 | 2.169, 2.165 | 134, 310 |
| 2 | 2.144 | 1 | 2.142 | 242 |
| 6 | 2.127 | 8 | 2.126 | 233 |
| 4 | 2.087 | 3, 1, 1 | 2.088, 2.083, 2.083 | 044, 204, 320 |
| 4 | 2.041 | 3 | 2.046 | 105 |
| 5 | 2.029 | 9 | 2.027 | 251 |
| 5 | 2.009 | 4 | 2.008 | 312 |
| 1 | 1.929 | 2 | 1.927 | 252 |
| 2 | 1.884 | 2 | 1.884 | 234 |
| 3 | 1.873 | 6 | 1.872 | 303 |
| 5 | 1.847 | 12 | 1.846 | 332 |
| 2 | 1.834 | 3 | 1.834 | 260 |
| 10 | 1.815 | 1, 17 | 1.819, 1.815 | 170, 154 |
| 4 | 1.789 | 6, 3 | 1.789, 1.785 | 253, 215 |
| 17 | 1.764 | 31 | 1.763 | 244 |
| 6 | 1.739 | 8, 5 | 1.740, 1.736 | 145, 262 |
| 2 | 1.707 | 3 | 1.707 | 064 |
| 5 | 1.692 | 16, 2 | 1.690, 1.687 | 350, 314 |
| 2 | 1.667 | 2 | 1.668 | 235 |
| 4 | 1.658 | 1, 5 | 1.662, 1.656 | 036, 080 |
| 2 | 1.647 | 1, 10 | 1.647, 1.646 | 324, 400 |
| 2 | 1.631 | 4 | 1.630 | 343 |
| 2 | 1.614 | 3, 3, 1 | 1.615, 1.612, 1.611 | 411, 352, 136 |
| 7 | 1.585 | 6, 7 | 1.588, 1.583 | 181, 082 |
| 10 | 1.576 | 10, 17, 6 | 1.577, 1.575, 1.574 | 046, 206, 402 |
| 2 | 1.539 | 2, 1 | 1.539, 1.536 | 182, 305 |
| 1 | 1.530 | 3 | 1.526 | 431 |
| 2 | 1.515 | 3 | 1.515 | 264 |
| 2 | 1.509 | 2 | 1.507 | 174 |
| 3 | 1.495 | 6 | 1.497 | 107 |
| 3 | 1.481 | 1, 1, 4 | 1.483, 1.482, 1.479 | 236, 432, 280 |
| 4 | 1.467 | 7 | 1.466 | 183 |
| 3 | 1.449 | 12 | 1.449 | 156 |
| 4 | 1.438 | 6 | 1.437 | 190 |
| 8 | 1.432 | 27, 1 | 1.431, 1.428 | 354, 363 |
| 2 | 1.419 | 5, 4 | 1.421, 1.417 | 442, 433 |
| 2 | 1.405 | 5 | 1.404 | 404 |
| 2 | 1.393 | 4 | 1.394 | 345 |
| 3 | 1.388 | 3, 1, 3 | 1.388, 1.386, 1.385 | 192, 451, 372 |
| 2 | 1.374 | 5 | 1.373 | 424 |
| Structural Formula | (K0.47Na0.47?0.06)2(K0.50?0.50)4(?0.75K0.25)4 (?0.94Ca0.03Na0.03)4(Fe0.48Ti0.30Al0.22)2 (Fe0.70Ti0.30)4(Fe0.36Mg0.28Al0.20Ti0.16)2(Fe0.74Mg0.05Na0.17Al0.04)2(?0.75Cu0.11Na0.08Zn0.06)2 (AsO4)8[(As0.68S0.32)O4]2[(?0.75As0.25)O0.5 (?0.75O0.25)2]2O3 |
| Formula weight | 2171.74 |
| Temperature, K | 293(2) |
| Radiation and wavelength, Å | MoKα; 0.71073 |
| Crystal system, space group, Z | Orthorhombic, P2221, 1 |
| Unit-cell dimensions, Å/° | a = 6.5824(2) b = 13.2488(4) c = 10.7613(3) |
| V, Å3 | 938.48(5) |
| Absorption coefficient μ, mm−1 | 12.140 |
| F000 | 1022 |
| Crystal size, mm | 0.02 × 0.17 × 0.77 |
| Diffractometer | Xcalibur S CCD |
| θ range for data collection, °/Collection mode | 3.09–28.27/full sphere |
| Index ranges | −8 ≤ h ≤ 8, −17 ≤ k ≤ 17, −14 ≤ l ≤ 14 |
| Reflections collected | 15,862 |
| Independent reflections | 2335 (Rint = 0.0626) |
| Independent reflections with I > 2σ(I) | 2088 |
| Data reduction | CrysAlisPro, Agilent Technologies (Oxfordshire, UK), v. 1.171.37.34 [10] |
| Absorption correction | gaussian |
| Structure solution | direct methods |
| Refinement method | full-matrix least-squares on F2 |
| Number of refined parameters | 190 |
| Final R indices [I > 2σ(I)] | R1 = 0.0447, wR2 = 0.1065 |
| R indices (all data) | R1 = 0.0507, wR2 = 0.1102 |
| GoF | 1.089 |
| Largest diff. peak and hole, e/Å3 | 1.76 and −1.59 |
| Site | x | y | z | Ueq | s.o.f. | Q |
|---|---|---|---|---|---|---|
| A(1) | 0.6728(7) | 0.0 | 0.5 | 0.0545(15) | K0.47Na0.47 | 2 |
| A(2) | 0.198(2) | 0.3889(8) | 0.828(2) | 0.035(4) | K0.25 | 4 |
| A(3) | 0.2239(7) | 0.3724(5) | 0.9404(7) | 0.0472(17) | K0.50 | 4 |
| A’ | 0.730(7) | 0.184(4) | 0.500(6) | 0.033(10) * | Ca0.03Na0.03 | 4 |
| M(1) | 0.0 | 0.13054(17) | 0.75 | 0.0113(4) | Fe3+0.48Ti0.30Al0.22 | 2 |
| M(2) | 0.24503(16) | 0.25063(9) | 0.50445(16) | 0.0082(2) | Fe3+0.70Ti0.30 | 4 |
| M(3) | 0.5 | 0.39561(17) | 0.25 | 0.0077(5) | Fe3+0.36Mg0.28Al0.20Ti0.16 | 2 |
| M(4) | 0.0 | 0.11671(15) | 0.25 | 0.0087(4) | Fe3+0.74Na0.17Mg0.05Al0.04 | 2 |
| M’ | 0.5 | 0.6055(6) | 0.25 | 0.025(2) | Cu0.11Na0.08Zn0.06 | 2 |
| T(1) | 0.5 | 0.18623(9) | 0.75 | 0.0084(2) | As1.00 | 2 |
| T(2) | 0.18208(15) | 0.0 | 0.50 | 0.0070(2) | As1.00 | 2 |
| T(3) | 0.31817(15) | 0.5 | 0.50 | 0.0088(2) | As1.00 | 2 |
| T(4) | 0.0 | 0.32747(10) | 0.25 | 0.0087(3) | As0.68S0.32 ** | 2 |
| T(5) | 0.5 | 0.18211(9) | 0.25 | 0.0084(2) | As1.00 | 2 |
| T’ | 0.0 | 0.3259(4) | 0.75 | 0.0174(10) | As0.25 | 2 |
| O(1) | 0.2967(10) | 0.1146(5) | 0.7845(6) | 0.0125(14) | O1.00 | 4 |
| O(2) | 0.4763(10) | 0.4944(6) | 0.3774(5) | 0.0223(14) | O1.00 | 4 |
| O(3) | 0.6994(11) | 0.1136(5) | 0.2905(5) | 0.0121(14) | O1.00 | 4 |
| O(4) | 0.1940(8) | 0.3968(5) | 0.2086(6) | 0.0205(16) | O1.00 | 4 |
| O(5) | 0.0245(8) | 0.0004(5) | 0.3750(4) | 0.0123(10) | O1.00 | 4 |
| O(6) | 0.3291(8) | 0.1020(4) | 0.4949(7) | 0.0142(11) | O1.00 | 4 |
| O(7) | 0.4531(10) | 0.2698(4) | 0.3642(6) | 0.0115(15) | O1.00 | 4 |
| O(8) | 0.1699(8) | 0.3972(4) | 0.5045(8) | 0.0188(12) | O1.00 | 4 |
| O(9) | 0.4580(10) | 0.2679(4) | 0.6306(5) | 0.0109(15) | O1.00 | 4 |
| O(10) | 0.0404(9) | 0.2279(4) | 0.6294(5) | 0.0116(14) | O1.00 | 4 |
| O(11) | 0.0396(13) | 0.2421(4) | 0.3606(5) | 0.029(2) | O1.00 | 4 |
| O(12) | 0.175(8) | 0.405(3) | 0.799(8) | 0.035(4) | O0.25 | 4 |
| T(1)—O(1) | 1.682(6) × 2 | T(4)—O(4) | 1.635(3) × 2 |
| —O(9) | 1.702(6) × 2 | —O(11) | 1.663(3) × 2 |
| T(2)—O(6) | 1.663(5) × 2 | T(5)—O(3) | 1.655(7) × 2 |
| —O(5) | 1.698(5) × 2 | —O(7) | 1.719(6) × 2 |
| T(3)—O(8) | 1.677(5) × 2 | M’—O(12) | 1.649(11) × 2 |
| —O(2) | 1.682(6) × 2 | —O(10) | 1.855(4) × 2 |
| M(1)—O(10) | 1.849(6) × 2 | M(3)—O(2) | 1.901(7) × 2 |
| —O(1) | 1.999(7) × 2 | —O(4) | 2.063(6) × 2 |
| —O(5) | 2.201(6) × 2 | —O(7) | 2.093(6) × 2 |
| M(2)—O(10) | 1.927(6) | M(4)—O(3) | 2.026(7) × 2 |
| —O(9) | 1.965(6) | —O(5) | 2.052(6) × 2 |
| —O(8) | 2.004(6) | —O(11) | 2.060(7) × 2 |
| —O(6) | 2.048(6) | ||
| —O(7) | 2.054(6) | A(1)—O(6) | 2.636(7) × 2 |
| —O(11) | 2.058(7) | —O(5) | 2.677(7) × 2 |
| —O(3) | 2.716(6) × 2 | ||
| M’—O(2) | 2.018(9) × 2 | —O(1) | 2.780(6) × 2 |
| —O(9) | 2.131(9) × 2 | ||
| —O(12) | 2.21(7) × 2 | A(2)—O(10) | 2.689(13) |
| —O(2) | 2.695(12) | ||
| A’—O(3) | 2.45(6) | —O(9) | 2.810(15) |
| —O(1) | 2.50(6) | —O(12) | 2.82(3) |
| —O(9) | 2.53(5) | —O(4) | 2.867(11) |
| —O(10) | 2.54(5) | —O(8) | 3.02(3) |
| —O(7) | 2.60(5) | ||
| —O(11) | 2.64(5) | A(3)—O(9) | 2.624(8) |
| —O(6) | 2.86(5) | —O(8) | 2.680(7) |
| —O(10) | 2.695(8) | ||
| —O(2) | 2.733(9) | ||
| —O(4) | 2.911(10) |
| T(1) | T(2) | T(3) | T(4) | T(5) | T’ 0.25 | M(1) | M(2) | M(3) | M(4) | M’ | A(1) | A(2) | A(3) | Σ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O(1) | 1.26 x2↓ | 0.52 ×2↓ | 0.11 ×2↓ | 1.89 | |||||||||||
| O(2) | 1.26 ×2↓ | 0.64 ×2↓ | 0.11 ×2↓ | 0.05 | 0.10 | 2.16 | |||||||||
| O(3) | 1.35 ×2↓ | 0.48 ×2↓ | 0.14 ×2↓ | 1.97 | |||||||||||
| O(4) | 1.28 ×2↓ | 0.41 ×2↓ | 0.03 | 0.06 | 1.78 | ||||||||||
| O(5) | 1.20 ×2↓ | 0.31 ×2↓ | 0.46 ×2↓ | 0.15 ×2↓ | 2.12 | ||||||||||
| O(6) | 1.32 ×2↓ | 0.48 | 0.17 ×2↓ | 1.97 | |||||||||||
| O(7) | 1.14 ×2↓ | 0.47 | 0.43 ×2↓ | 2.04 | |||||||||||
| O(8) | 1.28 ×2↓ | 0.54 | 0.02 | 0.11 | 1.95 | ||||||||||
| O(9) | 1.19 ×2↓ | 0.60 | 0.08 ×2↓ | 0.04 | 0.13 | 2.04 | |||||||||
| O(10) | 0.20 ×2↓ | 0.78 ×2↓ | 0.66 | 0.06 | 0.11 | 1.81 | |||||||||
| O(11) | 1.19 ×2↓ | 0.47 | 0.44 ×2↓ | 2.10 | |||||||||||
| O(12) | 0.34 ×2↓ | 0.08 ×2↓ | 0.04 | 0.46 | |||||||||||
| Σ | 4.90 | 5.04 | 5.08 | 4.94 * | 4.98 | 1.08 | 3.22 | 3.22 | 2.76 | 2.76 | 0.60 | 1.14 | 0.24 | 0.51 |
| Mineral/ Compound | Achyrophanite | Synthetic Fe3+AsO4 | Katiarsite KTiO(AsO4) | Synthetic KTiO(AsO4) (KTA) | Synthetic (NH4)Fe3+(AsO4)F |
|---|---|---|---|---|---|
| Sp. gr. Unit cell a (Å) b (Å) c (Å) V (Å3) | P2221 6.5824(2) 13.2488(4) 10.7613(3) 938.48(5) | Imam 13.468(2) 6.525(1) 10.768(2) 946.3(3) | Pna21 13.174(4) 6.5635(10) 10.805(2) 934.3(3) | Pna21 13.1185(5) 6.5656(2) 10.7804(3) 928.5 | Pna21 13.270(2) 6.629(1) 10.866(1) 955.8(2) |
| Reference | This work | [13,14] | [20] | [22] | [13,14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pekov, I.V.; Zubkova, N.V.; Koshlyakova, N.N.; Belakovskiy, D.I.; Vigasina, M.F.; Agakhanov, A.A.; Britvin, S.N.; Turchkova, A.G.; Sidorov, E.G.; Zhegunov, P.S.; et al. Achyrophanite, (K,Na)3(Fe3+,Ti,Al,Mg)5O2(AsO4)5, a New Mineral with the Novel Structure Type from Fumarolic Exhalations of the Tolbachik Volcano, Kamchatka, Russia. Minerals 2025, 15, 706. https://doi.org/10.3390/min15070706
Pekov IV, Zubkova NV, Koshlyakova NN, Belakovskiy DI, Vigasina MF, Agakhanov AA, Britvin SN, Turchkova AG, Sidorov EG, Zhegunov PS, et al. Achyrophanite, (K,Na)3(Fe3+,Ti,Al,Mg)5O2(AsO4)5, a New Mineral with the Novel Structure Type from Fumarolic Exhalations of the Tolbachik Volcano, Kamchatka, Russia. Minerals. 2025; 15(7):706. https://doi.org/10.3390/min15070706
Chicago/Turabian StylePekov, Igor V., Natalia V. Zubkova, Natalia N. Koshlyakova, Dmitry I. Belakovskiy, Marina F. Vigasina, Atali A. Agakhanov, Sergey N. Britvin, Anna G. Turchkova, Evgeny G. Sidorov, Pavel S. Zhegunov, and et al. 2025. "Achyrophanite, (K,Na)3(Fe3+,Ti,Al,Mg)5O2(AsO4)5, a New Mineral with the Novel Structure Type from Fumarolic Exhalations of the Tolbachik Volcano, Kamchatka, Russia" Minerals 15, no. 7: 706. https://doi.org/10.3390/min15070706
APA StylePekov, I. V., Zubkova, N. V., Koshlyakova, N. N., Belakovskiy, D. I., Vigasina, M. F., Agakhanov, A. A., Britvin, S. N., Turchkova, A. G., Sidorov, E. G., Zhegunov, P. S., & Pushcharovsky, D. Y. (2025). Achyrophanite, (K,Na)3(Fe3+,Ti,Al,Mg)5O2(AsO4)5, a New Mineral with the Novel Structure Type from Fumarolic Exhalations of the Tolbachik Volcano, Kamchatka, Russia. Minerals, 15(7), 706. https://doi.org/10.3390/min15070706








