Zircon out, Elpidite in: Deformation-Driven Zirconosilicate Evolution in Peralkaline Granites: A Case Study of the Papanduva Pluton (Brazil)
Abstract
1. Introduction
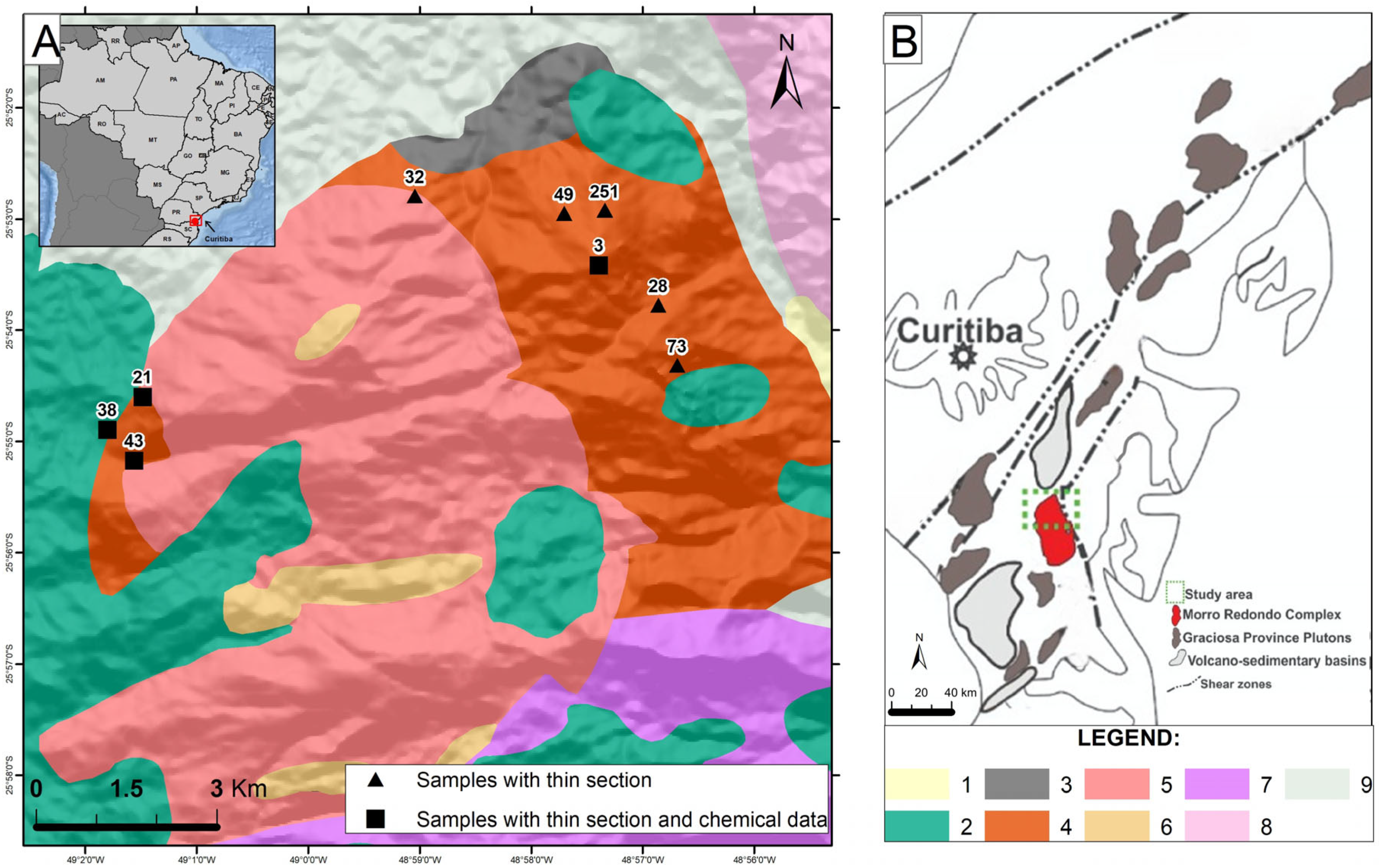
2. Geological Background
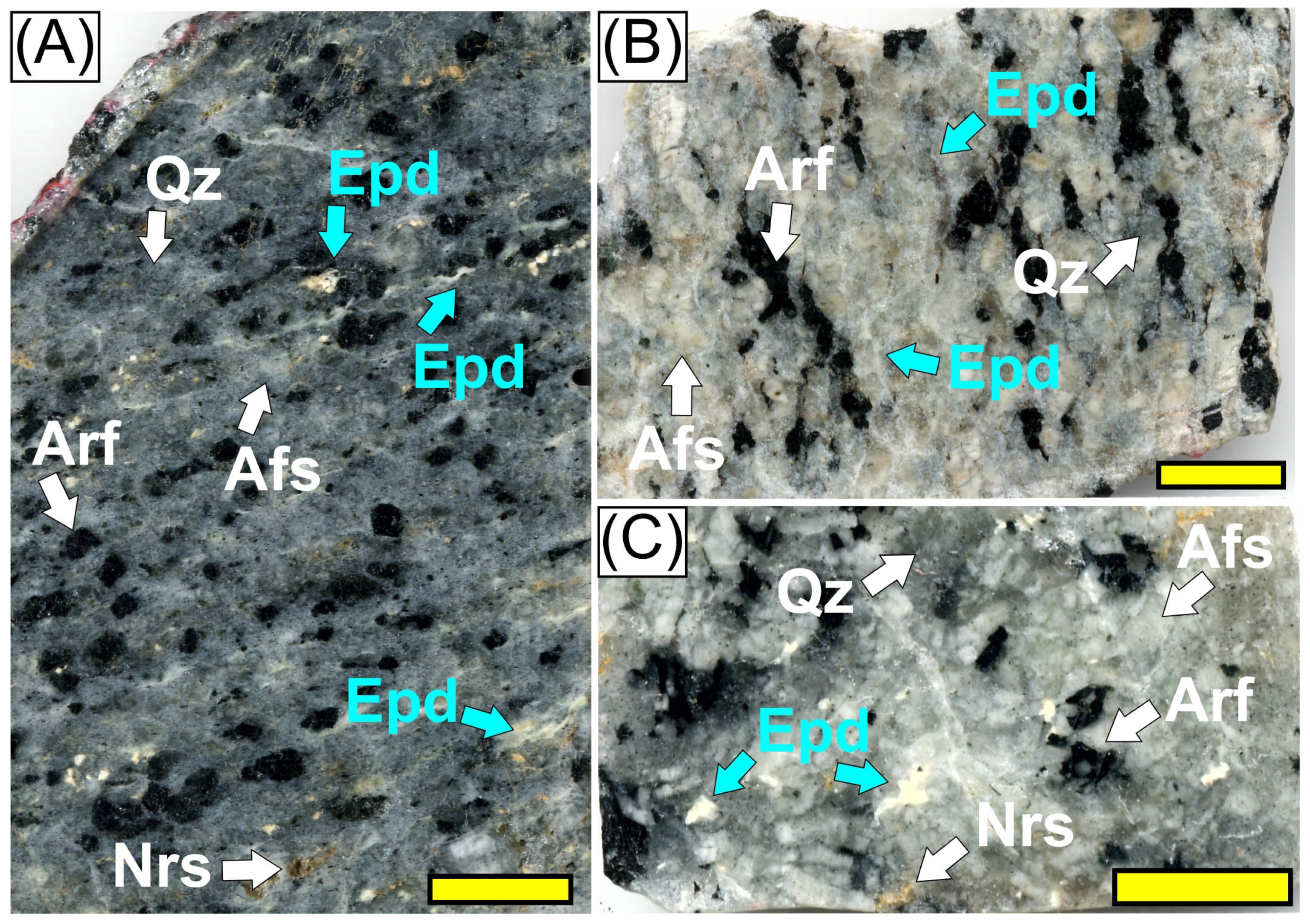
2.1. Petrographic and Mineralogical Characteristics
2.2. Geochemical Signature
3. Analytical Methods
3.1. Petrography
3.2. Electron Probe Microanalysis (EPMA)
3.3. LA-ICP-MS Analysis
4. Results
4.1. Textural and Compositional Classification of Zirconosilicates
4.1.1. Textural Characteristics
- A.
- Elpidite Textural Varieties:
- I.
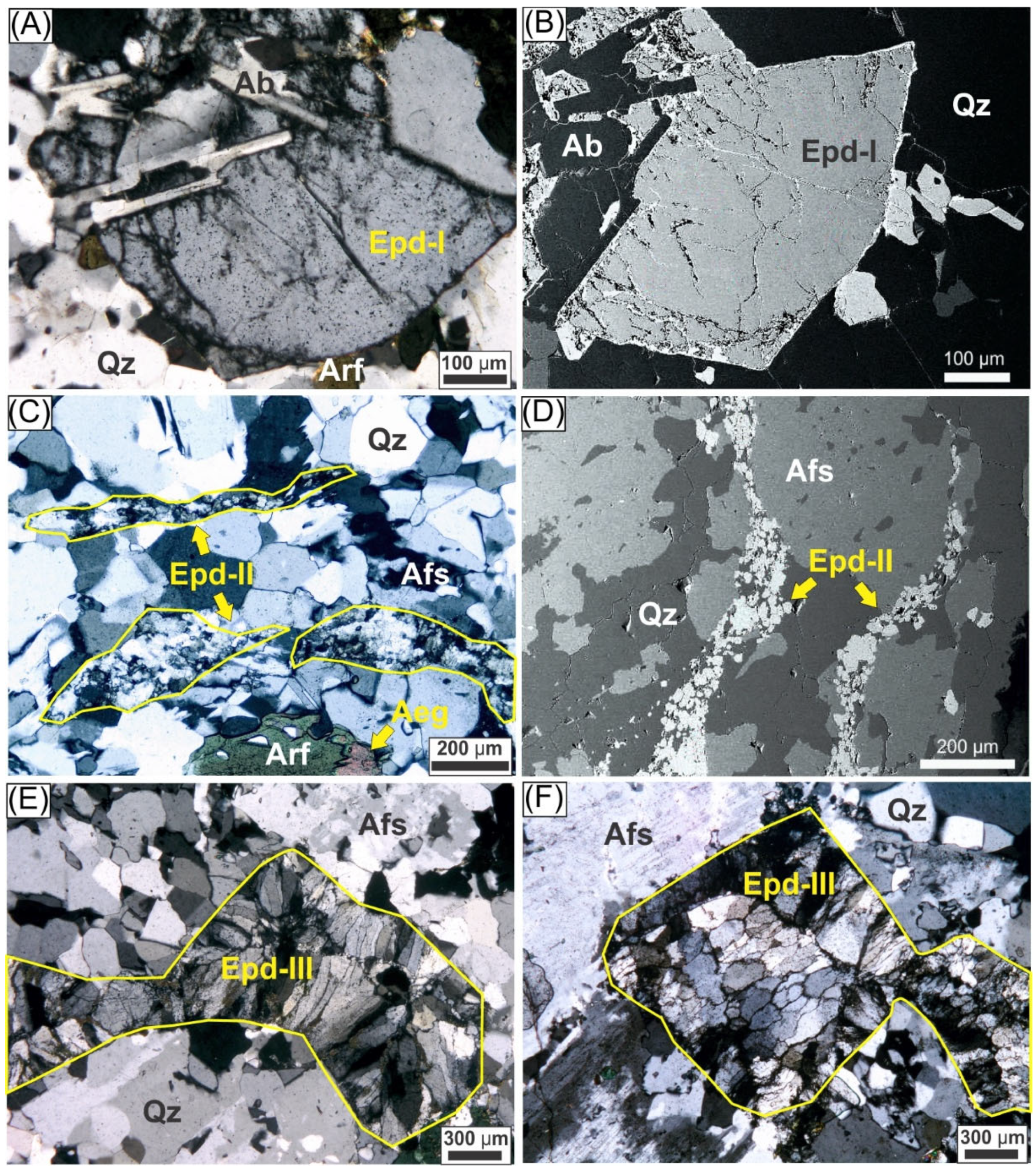
- Type-I (Euhedral Crystals): Subhedral to euhedral crystals ranging from 0.5–2.0 mm, with clearly defined orthorhombic shapes and uniform optical properties (Figure 3A). These crystals frequently display fractures filled with secondary phases appearing bright in BSE images (Figure 3B). Some crystals contain inclusions of albite laths (Figure 3A).
- II.
- Type-II (Vein-like Aggregates): Consist of elongated clusters of fine-grained crystals aligned parallel to protomylonitic foliation (Figure 3A,B). Compared to other types, they exhibit darker optical coloration and more extensive alterations.
- III.
- B.
- (Na,K)-Zirconosilicates textural varieties:
- I.
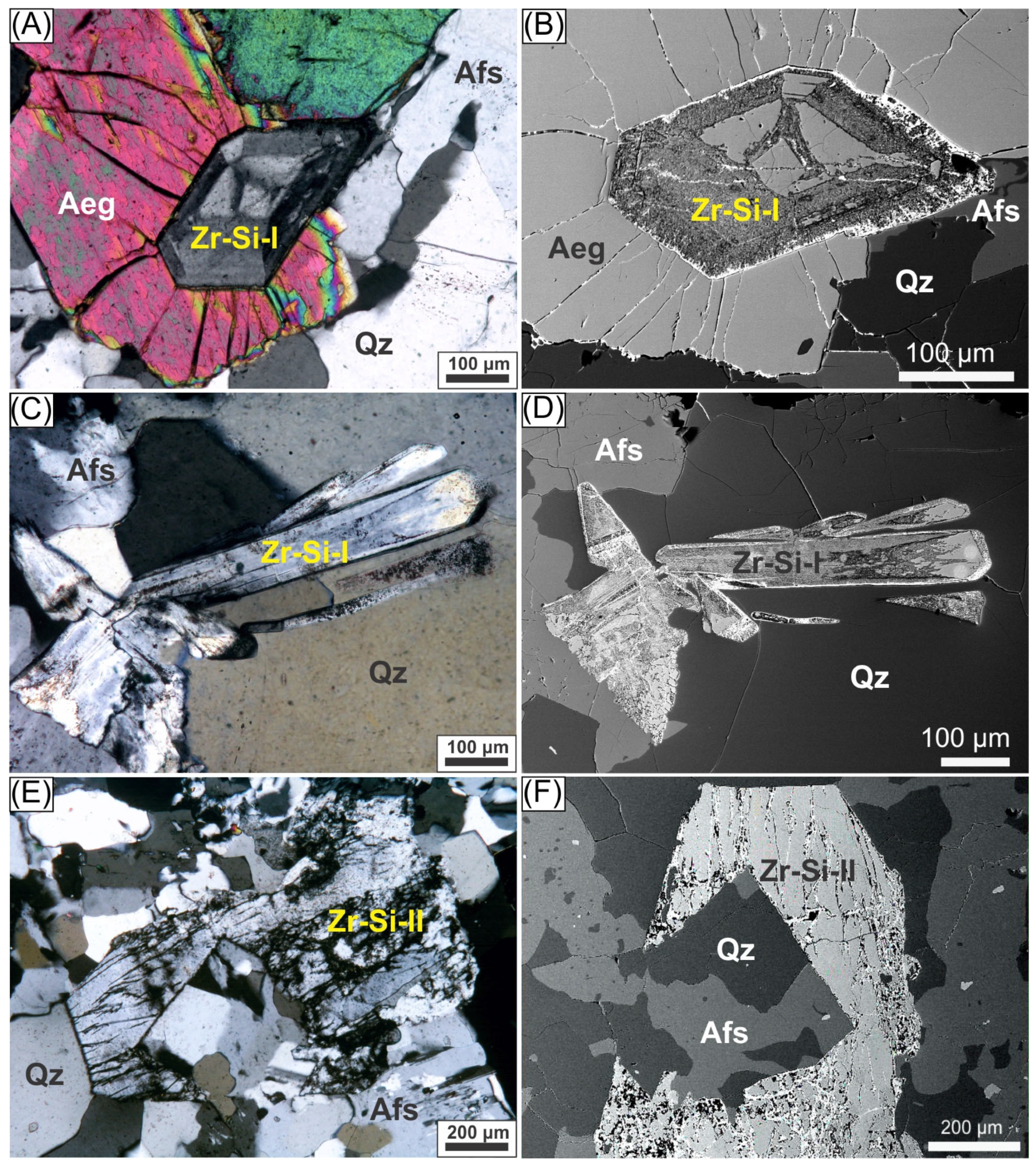
- (Na,K)Zr-Si-I (sample MR-38): Euhedral crystals exhibiting an apparent monoclinic habit with high relief, low birefringence, and a complex internal structure (Figure 4A,C). BSE imaging reveals concentric zoning patterns that preserved the original morphologies despite significant alterations (Figure 4B,D). Cathodoluminescence (CL) imaging shows Na-rich luminescent cores surrounded by darker, K-enriched rims, indicative of alteration-driven structural changes. CL elemental mapping (Supplementary Material S3) demonstrates pronounced chemical zoning, with Na concentrated in the crystal core, which corresponds to relics of the original crystal, and K enrichment along the intermediate and outer zones and fractures. The Zr concentrations increase towards the crystal edges, with Hf and Al showing similar patterns. Ca shows no rim enrichment and is randomly distributed along the internal fractures.
- II.
- (Na,K)Zr-Si-II (sample MR-26): Subhedral to euhedral elongated crystals with an apparent orthorhombic habit, B(-) optical sign, moderate relief, and first-order interference colors (Figure 4E). They appear homogeneous in BSE imaging but display extensive fracturing with secondary infill (Figure 4F).
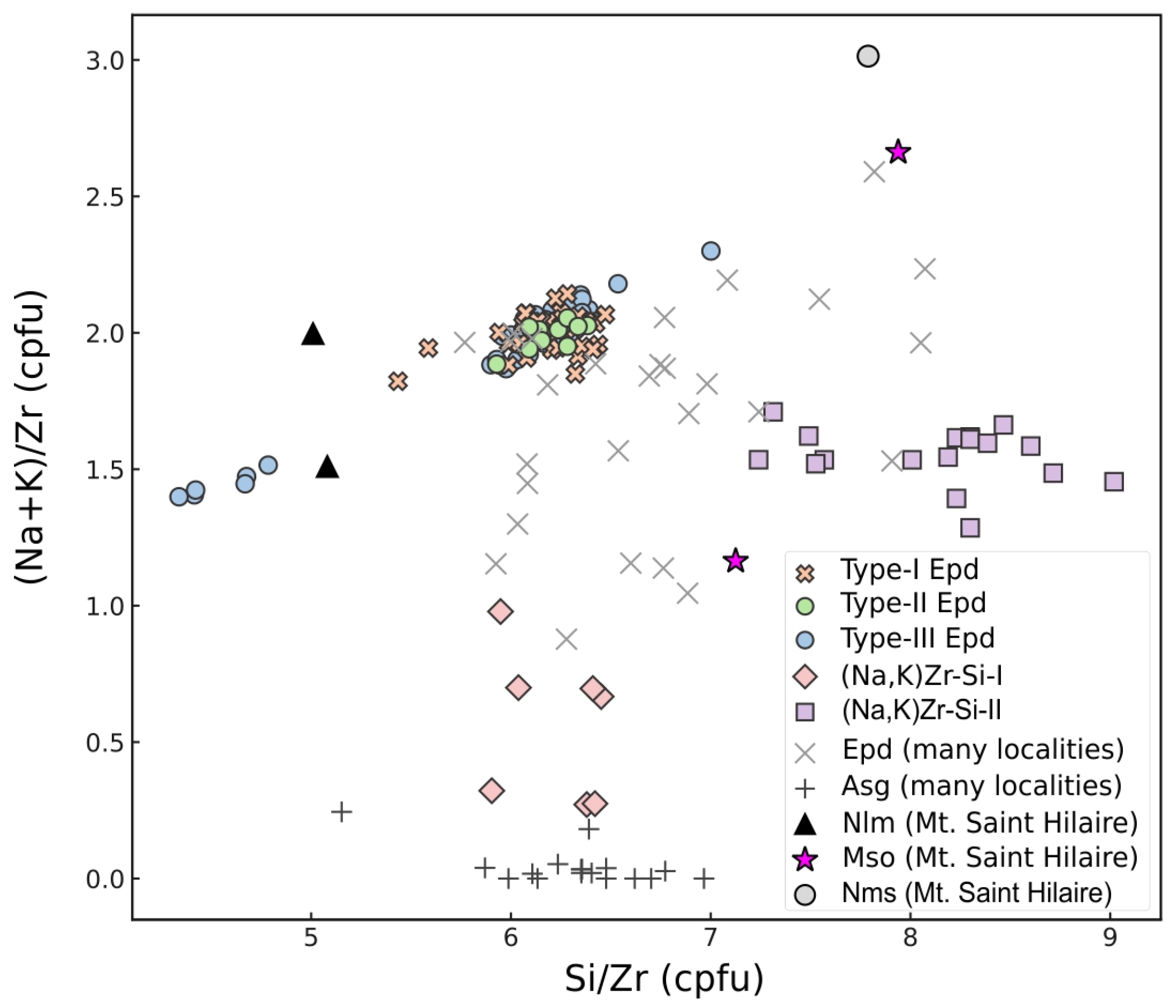
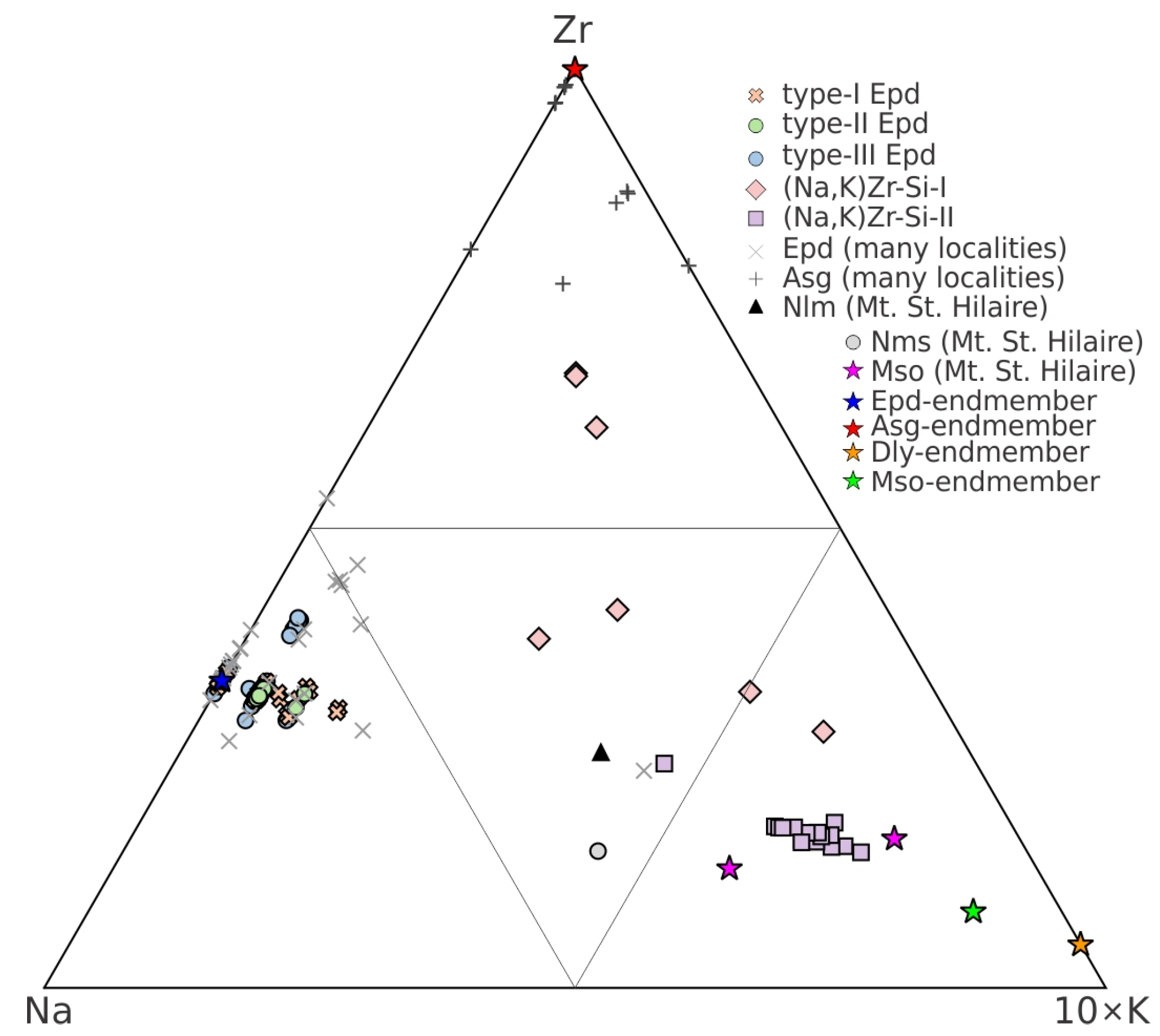
4.1.2. Quantitative Classification Framework
4.1.3. Chemical Composition
- A.
- Elpidite Chemical Characteristics:
- B.
- (Na,K)-Zirconosilicate Chemical Characteristics
4.2. Trace Element Systematics
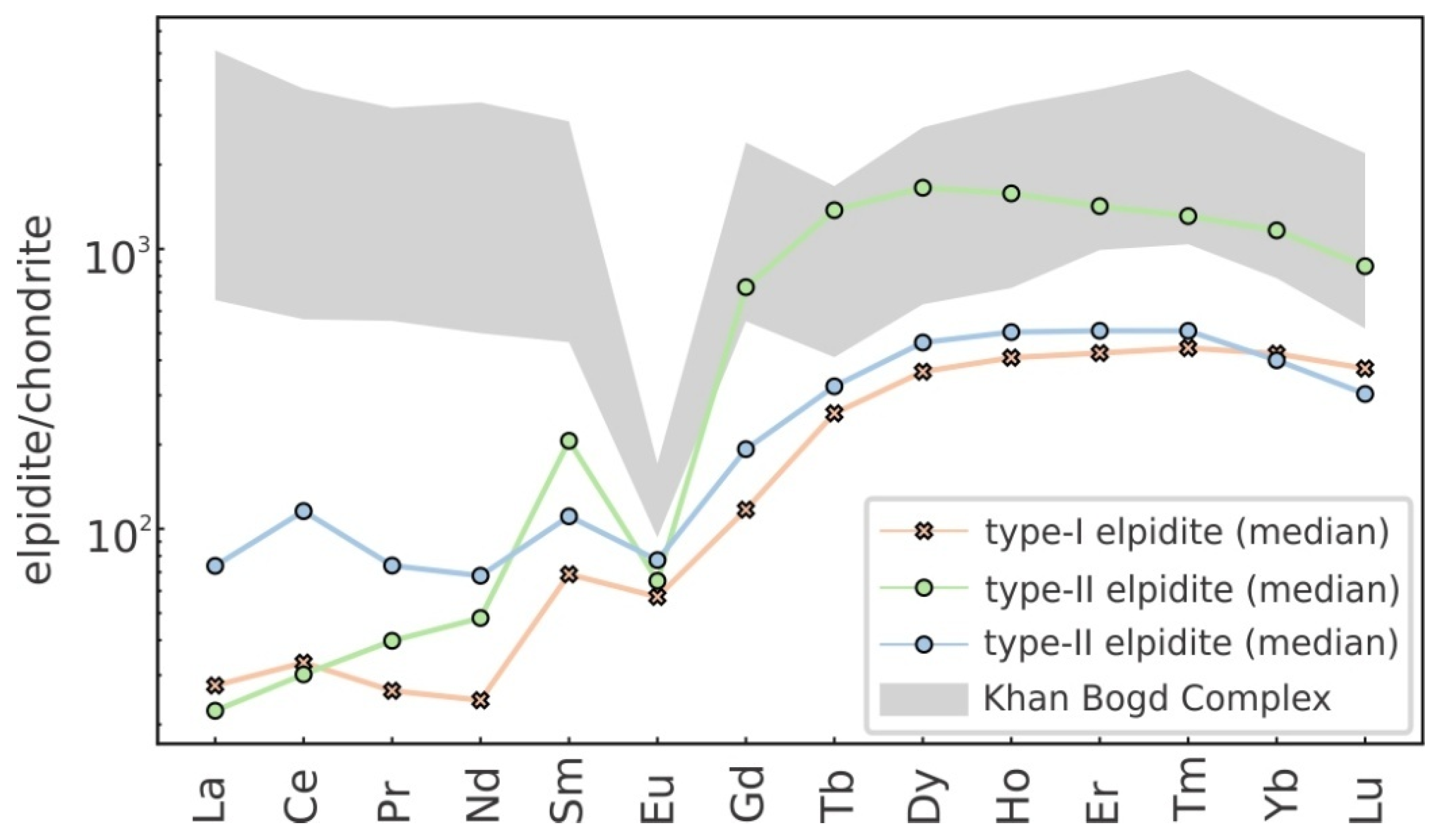
4.2.1. REE Content and Distribution Patterns
4.2.2. Trace Element Patterns and Element Correlations
5. Discussion
5.1. Facies Dichotomy: Causes and Significance
5.2. Compositional Architecture and Structural Controls
5.3. Deformation as a Primary Control
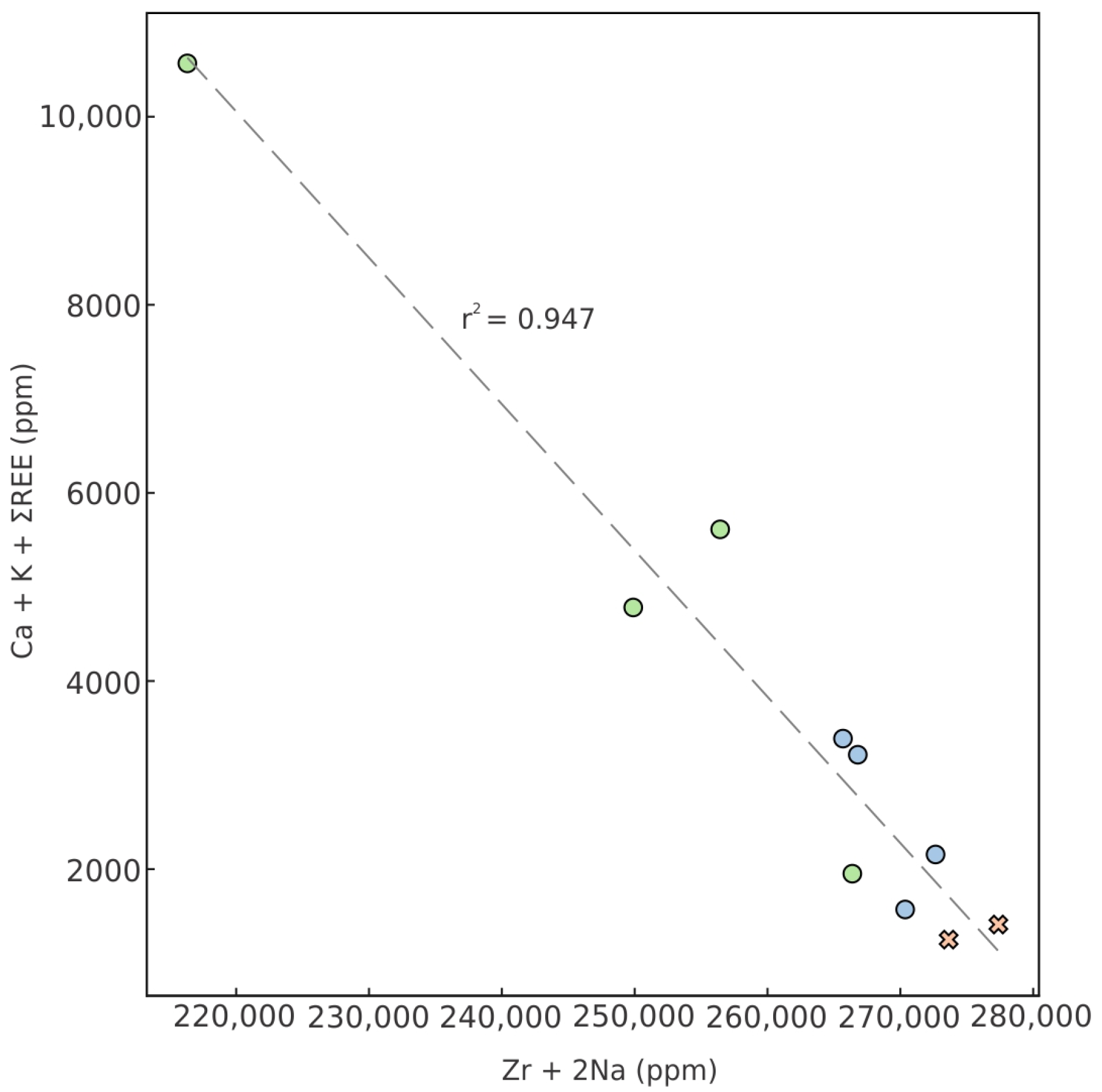
- Correlation strength gradients: The correlation between substitution components Zr4+ + 2Na+ and Ca2+ + K+ + REE3+ (Figure 9) varies directly with deformation intensity, being strongest in vein-like aggregates (r = −0.923; r2 = 0.947), intermediate in granular aggregates (r = −0.315), and weakest in euhedral crystals (r = −0.150).
- Variable Eu anomalies: The progressive weakening of positive Eu anomalies from euhedral crystals (Eu/Eu* = 0.646) to vein-like aggregates (Eu/Eu* = 0.228) indicates increasing fluid–rock interaction under progressively more reducing conditions along deformation-controlled pathways (Figure 8).
5.4. Integrated Evolutionary Model
- I.
- Stage 1—Primary magmatic crystallization: Located in the high µNa2O, low µK2O region of the diagram, this stage represents the initial magmatic crystallization under highly peralkaline conditions. Euhedral elpidite formed during the late-magmatic stage from highly peralkaline, zircon-undersaturated residual melt. The occasional inclusion of albite laths in euhedral elpidite indicates co-precipitation, confirming its primary late-magmatic origin. Positive Eu anomalies in euhedral crystals (Eu/Eu* = 0.646) reflect crystallization from magmatic melts with minimal fluid interaction.
- II.
- Stage 2— Deformation-enhanced fluid interaction: As the system evolved toward moderate K2O and still-high µNa2O, submagmatic deformation created microfractures that served as preferential fluid pathways. This stage produces two distinct alteration patterns:
- a.
- Foliation-controlled REE enrichment: Vein-like elpidite aggregates formed along deformation features, characterized by extreme HREE enrichment (median DyN = 1654.4) coupled with significant Na-leaching, as indicated by the strong negative correlation between the Na and REE contents in these samples.
- b.
- Pervasive alkali exchange: (Na,K)Zr-Si-I formed through preferential Na removal with minimal framework disruption, maintaining Si/Zr ≈ 6.2 while showing severe alkali depletion [(Na + K)/Zr ≈ 0.6].
- III.
- Stage 3—Late-stage recrystallization: The final stage occurred under high µK2O and low µNa2O conditions, representing the final phase of the system evolution. Two divergent pathways were developed:
- a.
- Framework-modifying recrystallization: (Na,K)Zr-Si-II formed through interactions with K-rich, Si-saturated fluids, resulting in both modified alkali content [(Na + K)/Zr ≈ 1.6] and elevated Si/Zr ratios (≈8.1).
- b.
- Hydrothermal elpidite precipitation: Granular elpidite aggregates crystallized under waning hydrothermal activity, showing intermediate REE levels (median = 1382 ppm) and higher (La/Yb)N ratios (0.191) than vein-like aggregates.

5.5. Reaction Mechanisms and Stability Fields
- Stage 1 Reactions (High µNa2O region):R0: Zircon + Na-rich fluid → Euhedral elpidite + ArfvedsoniteZrSiO4 + Na-rich fluid → Na2ZrSi6O15·3H2O + Na-amphiboleR1: Arfvedsonite + O2 → Aegirine + Na2O + H2ONa3Fe52+Fe23+Si8O22(OH)2 + 3O2 → 5NaFe3+Si2O6 + Na2O + 2H2OR2: Na2O + TiO2 + 4SiO2 → Narsarsukite2Na2O + TiO2 + 4SiO2 → Na2(Ti,Fe3+)Si4(O,F)11R3: Formation of euhedral elpiditeNa2O + ZrO2 + 6SiO2 + 3H2O → Na2ZrSi6O15·3H2O
- Stage 2 Reactions (Transition to intermediate µNa2O and µK2O):R4: Fluid-mediated crystallization along deformation features (vein-like elpidite)Na2O + ZrO2 + 6SiO2 + 3H2O + (REE,Ca,K) → (Na,K)2ZrSi6O15·3H2OR5: Euhedral elpidite + K-rich fluid → (Na,K)Zr-Si-I + Na-rich fluidNa2ZrSi6O15·3H2O + K,Ca-rich fluid → (Na,Ca,K)0.66ZrSi6O15·nH2O + Na+
- Stage 3 Reactions (High µK2O, low µNa2O region):R6: Euhedral elpidite + K-rich fluid + silica → (Na,K)Zr-Si-II + Na-rich fluidNa2ZrSi6O15·3H2O + K+ + 2SiO2 → (Na,K)1.5ZrSi8O19·nH2O + Na+R7: narsarsukite + Zr-rich fluid + K-rich fluid → (Na,K)Zr-Si-II + Al-bearing narsarsukite rimNa2(Ti,Fe3+)Si4(O,F)11 + Zr4+ + K+ + 2SiO2 → (Na,K)1.5ZrSi8O19·nH2O + Al-bearing narsarsukite rimR8: Formation of granular elpiditeNa2O + ZrO2 + 6SiO2 + K+ + Ca2+ + 3H2O → (Na,K)2ZrSi6O15·3H2O

5.6. Sources and Evolution of Potassium
- Primary magmatic enrichment: The foliated facies represents a more evolved magmatic composition than the massive facies, with higher initial K/Na ratios documented in whole-rock analyses [25]. As crystallization progressed, potassium became concentrated in the residual melts, whereas sodium-rich minerals preferentially crystallized [37].
- Autometasomatic processes: The crystallization sequence from Na-rich minerals (aegirine and arfvedsonite) to K-bearing zirconosilicates demonstrates systematic Na depletion from residual fluids, creating progressively higher K/Na ratios recorded in the mineral compositions.
- Structural control on fluid pathways: Submagmatic deformation created preferential fluid pathways along structural discontinuities, as evidenced by the occurrence of (Na,K)Zr-Si-I phases associated with deformation features.
- 4.
- 5.
- Ion-exchange processes: Experimental studies have demonstrated that elpidite exhibits high K+ exchangeability even at low temperatures (90–150 °C), with K+ preferentially occupying water molecule sites [60]. This mechanism explains the compositional evolution observed in our samples: the (Na,K)Zr-Si-I phase shows selective cation exchange with minimal framework disruption, whereas extensive exchange (>50% of Na+) causes severe framework distortion. This provides a mechanistic basis for the transition to (Na,K)Zr-Si-II, characterized by both modified alkali content and elevated Si/Zr ratios.
5.7. Broader Implications and Comparative Context
5.7.1. Mineralogical and Textural Comparisons
5.7.2. Trace Element Patterns and Mineral Competition
5.7.3. Structural Controls: A Distinctive Feature
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linthout, K. Alkali-Zirconosilicates in Peralkaline Rocks. Contrib. Mineral. Petrol. 1984, 86, 155–158. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Hettmann, K.; Schilling, J.; Frost, B.R.; Markl, G. The Mineralogical Diversity of Alkaline Igneous Rocks: Critical Factors for the Transition from Miaskitic to Agpaitic Phase Assemblages. J. Petrol. 2011, 52, 439–455. [Google Scholar] [CrossRef]
- Andersen, T.; Erambert, M.; Larsen, A.O.; Selbekk, R.S. Petrology of Nepheline Syenite Pegmatites in the Oslo Rift, Norway: Zirconium Silicate Mineral Assemblages as Indicators of Alkalinity and Volatile Fugacity in Mildly Agpaitic Magma. J. Petrol. 2010, 51, 2303–2325. [Google Scholar] [CrossRef]
- Gysi, A.P.; Williams-Jones, A.E.; Collins, P. Lithogeochemical Vectors for Hydrothermal Processes in the Strange Lake Peralkaline Granitic REE-Zr-Nb Deposit. Econ. Geol. 2016, 111, 1241–1276. [Google Scholar] [CrossRef]
- Salvi, S.; Williams-jones, A.E. Zirconosilicate Phase Relations in the Strange Lake (Lac Brisson) Pluton, Quebec-Labrador, Canada. Am. Mineral. 1995, 80, 1031–1040. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Markl, G. A Global Review on Agpaitic Rocks. Earth-Sci. Rev. 2017, 173, 229–258. [Google Scholar] [CrossRef]
- Aja, S.U.; Wood, S.A.; Williams-Jones, A.E. The Solubility of Some Alkali-Bearing Zr Minerals in Hydrothermal Solutions. MRS Proc. 1996, 432, 69. [Google Scholar] [CrossRef]
- Roelofsen, J.N.; Veblen, D.R. Relationships among Zirconosilicates: Examination by Cathodoluminescence and Transmission Electron Microscopy. Mineral. Petrol. 1999, 67, 71–84. [Google Scholar] [CrossRef]
- Cegiełka, M.; Bagiński, B.; Macdonald, R.; Belkin, H.E.; Kotowski, J.; Upton, B.G.J. Zirconium Silicates in a Peralkaline Granite: A Record of the Interplay of Magmatic and Hydrothermal Processes (Ilímaussaq complex, Greenland). Acta Geol. Pol. 2022, 72, 235–245. [Google Scholar] [CrossRef]
- Kynicky, J.; Chakhmouradian, A.R.; Xu, C.; Krmicek, L.; Galiova, M. Distribution and Evolution of Zirconium Mineralization in Peralkaline Granites and Associated Pegmatites of the Khan Bogd Complex, Southern Mongolia. Can. Mineral. 2011, 49, 947–965. [Google Scholar] [CrossRef]
- Paterson, S.R.; Vernon, R.H.; Tobisch, O.T. A Review of Criteria for the Identification of Magmatic and Tectonic Foliations in Granitoids. J. Struct. Geol. 1989, 1, 349–363. [Google Scholar] [CrossRef]
- Bouchez, J.L.; Delas, C.; Gleizes, G.; Nédélec, A.; Cuney, M. Submagmatic Microfractures in Granites. Geology 1992, 20, 35. [Google Scholar] [CrossRef]
- Blenkinsop, T.G. Deformation Microstructures and Mechanisms in Minerals and Rocks; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; ISBN 978-0-412-73480-9. [Google Scholar]
- Cannillo, E.; Rossi, G.; Ungaretti, L. The Crystal Structure of Elpidite. Am. Mineral. 1973, 58, 106–109. [Google Scholar]
- Day, M.C.; Hawthorne, F.C. A Structure Hierarchy for Silicate Minerals: Chain, Ribbon, and Tube Silicates. Mineral. Mag. 2020, 84, 165–244. [Google Scholar] [CrossRef]
- Zubkova, N.V.; Nikolova, R.P.; Chukanov, N.V.; Kostov-Kytin, V.V.; Pekov, I.V.; Varlamov, D.A.; Larikova, T.S.; Kazheva, O.N.; Chervonnaya, N.A.; Shilov, G.V.; et al. Crystal Chemistry and Properties of Elpidite and Its Ag-Exchanged Forms. Minerals 2019, 9, 420. [Google Scholar] [CrossRef]
- Bogdanov, A.; Kaneva, E.; Shendrik, R. New Insights into the Crystal Chemistry of Elpidite, Na2Zr[Si6O15]·3H2O and (Na1+YCax□1−X−Y)Σ=2Zr[Si6O15]·(3−X)H2O, and Ab Initio Modeling of IR Spectra. Materials 2021, 14, 2160. [Google Scholar] [CrossRef]
- Bernard, C. Concentration and Fractionation of Rare Earth Elements in Alkaline Complexes: The Role of Fluids. Ph.D. Thesis, Université Paul Sabatier—Toulouse III, Toulouse, France, 2020. [Google Scholar]
- Bonin, B. Peralkaline Granites in Corsica: Some Petrological and Geochemical Constraints. Rendiconti Della Soc. Ital. Mineral. E Petrol. 1988, 43, 281–306. [Google Scholar]
- Elliott, J.E. Peralkaline and Peraluminous Granites and Related Mineral Deposits of the Arabian Shield, Kingdom of Saudi Arabia; USGS: Reston, VA, USA, 1983; p. 37. [Google Scholar]
- Zubkova, N.V.; Pekov, I.V.; Chukanov, N.V.; Yapaskurt, V.O.; Turchkova, A.G.; Larikova, T.S.; Pushcharovsky, D.Y. A Highly Hydrated Variety of Elpidite from the Khibiny Alkaline Complex, Kola Peninsula, Russia. Mineral. Mag. 2021, 85, 627–633. [Google Scholar] [CrossRef]
- Mondal, S.; Upadhyay, D.; Banerjee, A. REE Mineralization in Siwana Peralkaline Granite, Western India-Role of Fractional Crystallization, Hydrothermal Remobilization, and Feldspar-Fluid Interaction. Lithos 2021, 396–397, 106240. [Google Scholar] [CrossRef]
- Buch, T. Caracterização mineralógica e geoquímica de ocorrências de elementos terras raras no maciço granítico Ouro Fino, Rondônia, Brasil. Master’s Thesis, Universidade de Brasília, Brasília, Brazil, 2018. [Google Scholar]
- Vilalva, F.C.J.; Vlach, S.R.F.; Simonetti, A. Nacareniobsite-(Ce) and britholite-(Ce) in Peralkaline Granites from the Morro Redondo Complex, Graciosa Province, Southern Brazil: Occurrence and Compositional data. Can. Mineral. 2013, 51, 313–332. [Google Scholar] [CrossRef]
- Vilalva, F.C.J.; Vlach, S.R.F. Geology, Petrography and Geochemistry of the A-Type Granites from the Morro Redondo Complex (PR-SC), Southern Brazil, Graciosa Province. An. Acad. Bras. Ciênc. 2014, 86, 85–116. [Google Scholar] [CrossRef]
- Vilalva, F.C.J. Petrografia e Mineralogia de Granitos Peralcalinos: O Plúton Papanduva, Complexo Morro Redondo (PR/SC). Master’s Thesis, Universidade de São Paulo, São Paulo, Brazil, 2007. [Google Scholar] [CrossRef]
- Oliveira, A.L.S.D. Elpidita, um Álcali-Zirconossilicato do Plúton Papanduva (PR-SC): Aspectos Texturais, Quimismo e Implicações Petrológicas. Bachelor’s Thesis, Universidade Federal do Rio Grande do Norte, Natal, Brazil, 2019. [Google Scholar]
- Grangeiro, L.P. Texturas e Química Mineral de Zirconosilicatos em Granitos Peralcalinos do Pluton Papanduva, PR-SC. Ph.D. Thesis, Universidade Federal do Rio Grande do Norte, Natal, Brazil, 2024. [Google Scholar]
- Vlach, S.R.F.; Vilalva, F.C.J. Narsarsukite in Peralkaline Granites from the Papanduva Pluton, Graciosa Province, South Brazil: Insights from Textural and Compositional Features. Mineral. Mag. 2023, 87, 896–907. [Google Scholar] [CrossRef]
- Gualda, G.A.R.; Vlach, S.R.F. The Serra da Graciosa A-Type Granites and Syenites, Southern Brazil. Part 1: Regional Setting and Geological Characterization. An. Acad. Bras. Ciênc. 2007, 79, 405–430. [Google Scholar] [CrossRef]
- Vlach, S.R.F.; Siga, O.; Harara, O.M.M.; Gualda, G.A.R.; Basei, M.A.S.; Vilalva, F.C.J. Crystallization Ages of the A-Type Magmatism of the Graciosa Province (Southern Brazil): Constraints from Zircon U-Pb (ID-TIMS) Dating of Coeval K-rich Gabbro-dioritic Rocks. J. S. Am. Earth Sci. 2011, 32, 407–415. [Google Scholar] [CrossRef]
- Hueck, M.; Oriolo, S.; Basei, M.A.S.; Oyhantçabal, P.; Heller, B.M.; Wemmer, K.; Siegesmund, S. Archean to Early Neoproterozoic Crustal Growth of the Southern South American Platform and its Wide-Reaching “African” Origins. Precambrian Res. 2022, 369, 106532. [Google Scholar] [CrossRef]
- Heller, B.M.; Hueck, M.; Passarelli, C.R.; Basei, M.A.S. Zircon U-Pb Geochronology and Hf Isotopes of the Luís Alves Terrane: Archean to Paleoproterozoic Evolution and Neoproterozoic Overprint. J. S. Am. Earth Sci. 2021, 106, 103008. [Google Scholar] [CrossRef]
- Basei, M.A.S.; Nutman, A.; Siga, O.; Passarelli, C.R.; Drukas, C.O. Chapter 7.2 The Evolution and Tectonic Setting of the Luis Alves Microplate of Southeastern Brazil: An Exotic Terrane during the Assembly of Western Gondwana. In Developments in Precambrian Geology; Elsevier: Amsterdam, The Netherlands, 2009; Volume 16, pp. 273–291, ISBN 978-0-444-53249-7. [Google Scholar]
- Basei, M.A.S.; Neves, B.B.B.; Siga, O.; Babinski, M.; Pimentel, M.M.; Gaeta Tassinari, C.C.; Hollanda, M.H.B.; Nutman, A.; Cordani, U.G. Contribution of SHRIMP U–Pb Zircon Geochronology to Unravelling the Evolution of Brazilian Neoproterozoic Fold Belts. Precambrian Res. 2010, 183, 112–144. [Google Scholar] [CrossRef]
- Vilalva, F.C.J.; Simonetti, A.; Vlach, S.R.F. Insights on the origin of the Graciosa A-Type Granites and Syenites (Southern Brazil) from Zircon U-Pb Geochronology, Chemistry, and Hf and O Isotope Compositions. Lithos 2019, 340–341, 20–33. [Google Scholar] [CrossRef]
- Vilalva, F.C.J.; Vlach, S.R.F.; Simonetti, A. Chemical and O-Isotope Compositions of Amphiboles and Clinopyroxenes from A-Type Granites of the Papanduva Pluton, South Brazil: Insights into Late- to Post-Magmatic Evolution of Peralkaline Systems. Chem. Geol. 2016, 420, 186–199. [Google Scholar] [CrossRef]
- Siachoque, A.; Santos, C.A.; Vlach, S.R.F. Amphiboles and Phyllosilicates in the A-Type Mandira Granite Massif, Graciosa Province, SE Brazil: Textures, Composition and Crystallisation Conditions. Mineral. Mag. 2021, 85, 784–807. [Google Scholar] [CrossRef]
- Vilalva, F.C.J.; Vlach, S.R.F. Major- and Trace-Element Composition of REE -Rich Turkestanite from Peralkaline Granites of the Morro Redondo Complex, Graciosa Province, South Brazil. Mineral. Mag. 2010, 74, 645–658. [Google Scholar] [CrossRef]
- Vilalva, F.C.J.; Vlach, S.R.F.; Peternell, M. Estimativa de temperatura de deformação em granitos peralcalinos do complexo Morro Redondo, província Graciosa, PR-SC por meio de análise quantitativa digital de texturas. In Anais do 46° Congresso Brasileiro de Geologia; SBG: Santos, Brazil, 2012. [Google Scholar]
- Vernon, R.H. Review of Microstructural Evidence of Magmatic and Solid-State Flow. Vis. Geosci. 2000, 5, 1–23. [Google Scholar] [CrossRef]
- Fazio, E.; Fiannacca, P.; Russo, D.; Cirrincione, R. Submagmatic to Solid-State Deformation Microstructures Recorded in Cooling Granitoids during Exhumation of Late-Variscan Crust in North-Eastern Sicily. Geosciences 2020, 10, 311. [Google Scholar] [CrossRef]
- Zubkova, N.V.; Ksenofontov, D.A.; Chukanov, N.V.; Pekov, I.V.; Artamonova, A.A.; Koshlyakova, N.N.; Bychkov, A.Y.; Pushcharovsky, D.Y. Crystal Chemistry of the Microporous Zirconosilicate Na6Zr3[Si9O27], a Product of High-Temperature Transformation of Catapleiite, and Its Ag-Exchanged Form. Minerals 2020, 10, 243. [Google Scholar] [CrossRef]
- Gore, T.E.; McDonald, A.M. Melansonite, (Na,□)□2KZrSi8O19·5H2O, a New Member of the Rhodesite Group, from Mont Saint-Hilaire, Québec, Canada: Characterization, Crystal-Structure Determination, and Origin. Can. J. Mineral. Petrol. 2023, 61, 387–400. [Google Scholar] [CrossRef]
- Andrade, S. Análises por LA-ICPMS em Zircões de Rochas Graníticas da Faixa Ribeira no Estado de São Paulo—SE do Brasil: Implicações Genéticas e Geocronológicas. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2016. [Google Scholar] [CrossRef]
- Griffin, W.; Powell, W.; Pearson, N.J.; O’Reilly, S. GLITTER: Data reduction software for laser ablation ICP-MS. In Laser Ablation ICP-MS in the Earth Sciences: Current Practices and Outstanding Issue; Sylvester, P.J., Ed.; Mineralogical Association of Canada Short Cours; Mineralogical Association of Canada: Vancouver, BC, Canada, 2008; pp. 308–311. [Google Scholar]
- Birkett, T.C.; Miller, R.R.; Roberts, A.C.; Mariano, A.N. Zirconium-Bearing Minerals of the Strange Lake Intrusive Complex, Quebec-Labrador. Can. Mineral. 1992, 30, 191–205. [Google Scholar]
- Cametti, G.; Armbruster, T.; Nagashima, M. Dehydration and Thermal Stability of Elpidite: An in-Situ Single Crystal X-ray Diffraction Study. Microporous Mesoporous Mater. 2016, 227, 81–87. [Google Scholar] [CrossRef]
- Mesto, E.; Kaneva, E.; Schingaro, E.; Vladykin, N.; Lacalamita, M.; Scordari, F. Armstrongite from Khan Bogdo (Mongolia): Crystal Structure Determination and Implications for Zeolite-Like Cation Exchange Properties. Am. Mineral. 2014, 99, 2424–2432. [Google Scholar] [CrossRef]
- Vladykin, N.V.; Kovalenko, V.I.; Kashaev, A.A.; Sapozhnikov, A.N.; Pisarskaya, V.A. A New Silicate of Calcium and Zirconium—Armstrongite. Dokl. Akad. Nauk SSSR 1973, 209, 1185–1188. [Google Scholar]
- Vladykin, N.; Kovalenko, V.I. Zirconium Silicates. Armstrongite. In Minerals of Mongolia; Novgorodova, M.I., Ed.; Fersman Mineralogical Museum of the Russian Academy of Sciences: Moscow, Russia, 2006; pp. 250–256. [Google Scholar]
- Jambor, J.L.; Roberts, A.C.; Grice, J.D. Armstrongite from the Strange Lake Alkalic Complex, on the Quebec—Labrador Boundary, Canada. Powder Diffr. 1987, 2, 2–4. [Google Scholar] [CrossRef]
- Lykova, I.; Rowe, R.; Poirier, G.; Friis, H.; Barnes, S. Natromelansonite, Na3 Zr[Si7 AlO19 ]⋅4–5H2 O, a New Member of the Rhodesite Mero-Plesiotype Series from Mont Saint-Hilaire, Quebec, Canada. Mineral. Mag. 2024, 88, 195–202. [Google Scholar] [CrossRef]
- McDonald, A.M.; Chao, G.Y. Natrolemoynite, a New Hydrated Sodium Zirconosilicate from MONT Saint-Hilaire, Quebec: Description and Structure Determination. Can. Mineral. 2001, 39, 1295–1306. [Google Scholar] [CrossRef][Green Version]
- McDonough, W.F.; Sun, S.-s. The Composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Marr, R.A.; Baker, D.R.; Williams-Jones, A.E. Alkali Zirconosilicate Speciation in Halogen-Rich, Felsic, Peralkaline Magmas. Mineral. Mag. 1994, 58A, 559–560. [Google Scholar] [CrossRef]
- Ferreira, P.; Ferreira, A.; Rocha, J.; Soares, M.R. Synthesis and Structural Characterization of Zirconium Silicates. Chem. Mater. 2001, 13, 355–363. [Google Scholar] [CrossRef]
- Jale, S.R.; Ojo, A.; Fitch, F.R. Synthesis of Microporous Zirconosilicates Containing ZrO6 Octahedra and SiO4 Tetrahedra. Chem. Commun. 1999, 411–412. [Google Scholar] [CrossRef]
- Delbove, F. Étude expérimentale de la distribution des alcalins K, Rb, Cs et des alcalino-terreux Ca, Sr, Ba, entre albite cristallisée et fondue et solution saline hydrothermale. Bull. Minéralogie 1978, 101, 317–333. [Google Scholar] [CrossRef]
- Grigor’eva, A.A.; Zubkova, N.V.; Pekov, I.V.; Kolitsch, U.; Pushcharovsky, D.Y.; Vigasina, M.F.; Giester, G.; Dordević, T.; Tillmanns, E.; Chukanov, N.V. Crystal Chemistry of Elpidite from Khan Bogdo (Mongolia) and Its K- and Rb-Exchanged Forms. Crystallogr. Rep. 2011, 56, 832–841. [Google Scholar] [CrossRef]
- Charoy, B.; Raimbault, L. Zr-, Th-, and REE-Rich Biotite Differentiates in the A-type Granite Pluton of Suzhou (Eastern China): The Key Role of Fluorine. J. Petrol. 1994, 35, 919–962. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.-F.M.; El-Kibbi, M.M. Anorogenic Magmatism: Chemical Evolution of the Mount El-Sibai A-Type Complex (Egypt), and Implications for the Origin of Within-Plate Felsic Magmas. Geol. Mag. 2001, 138, 67–85. [Google Scholar] [CrossRef]
- Vlach, S.R.F.; Gualda, G.A.R. Allanite and Chevkinite in A-Type Granites and Syenites of the Graciosa Province, Southern Brazil. Lithos 2007, 97, 98–121. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grangeiro, L.P.; Vilalva, F.C.J.; Vlach, S.R.F.; Oliveira, A.L.S.d. Zircon out, Elpidite in: Deformation-Driven Zirconosilicate Evolution in Peralkaline Granites: A Case Study of the Papanduva Pluton (Brazil). Minerals 2025, 15, 667. https://doi.org/10.3390/min15070667
Grangeiro LP, Vilalva FCJ, Vlach SRF, Oliveira ALSd. Zircon out, Elpidite in: Deformation-Driven Zirconosilicate Evolution in Peralkaline Granites: A Case Study of the Papanduva Pluton (Brazil). Minerals. 2025; 15(7):667. https://doi.org/10.3390/min15070667
Chicago/Turabian StyleGrangeiro, Larissa P., Frederico C. J. Vilalva, Silvio R. F. Vlach, and Armando L. S. de Oliveira. 2025. "Zircon out, Elpidite in: Deformation-Driven Zirconosilicate Evolution in Peralkaline Granites: A Case Study of the Papanduva Pluton (Brazil)" Minerals 15, no. 7: 667. https://doi.org/10.3390/min15070667
APA StyleGrangeiro, L. P., Vilalva, F. C. J., Vlach, S. R. F., & Oliveira, A. L. S. d. (2025). Zircon out, Elpidite in: Deformation-Driven Zirconosilicate Evolution in Peralkaline Granites: A Case Study of the Papanduva Pluton (Brazil). Minerals, 15(7), 667. https://doi.org/10.3390/min15070667






