Kinetic Aspects of Chrysotile Asbestos Thermal Decomposition Process
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
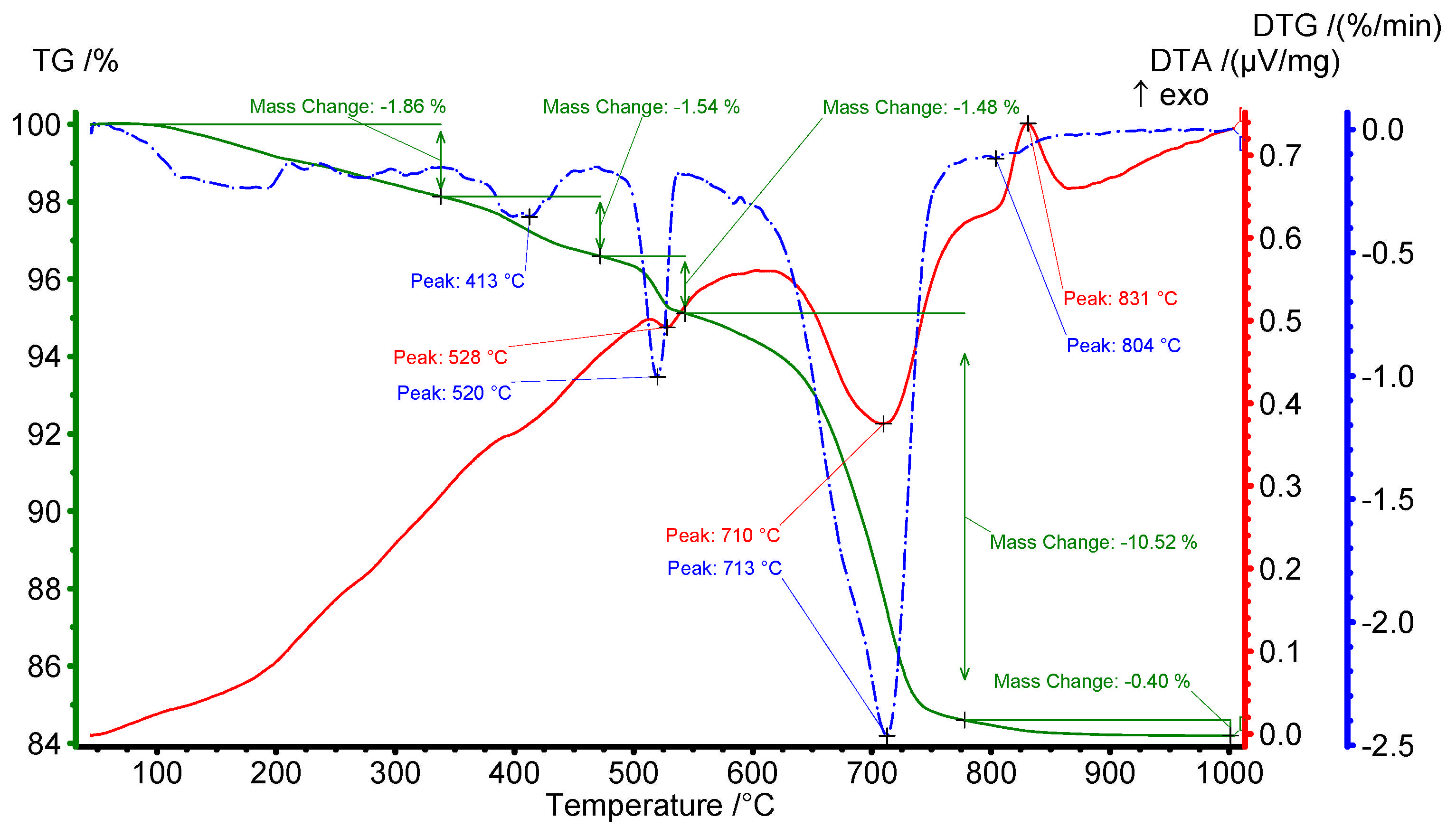
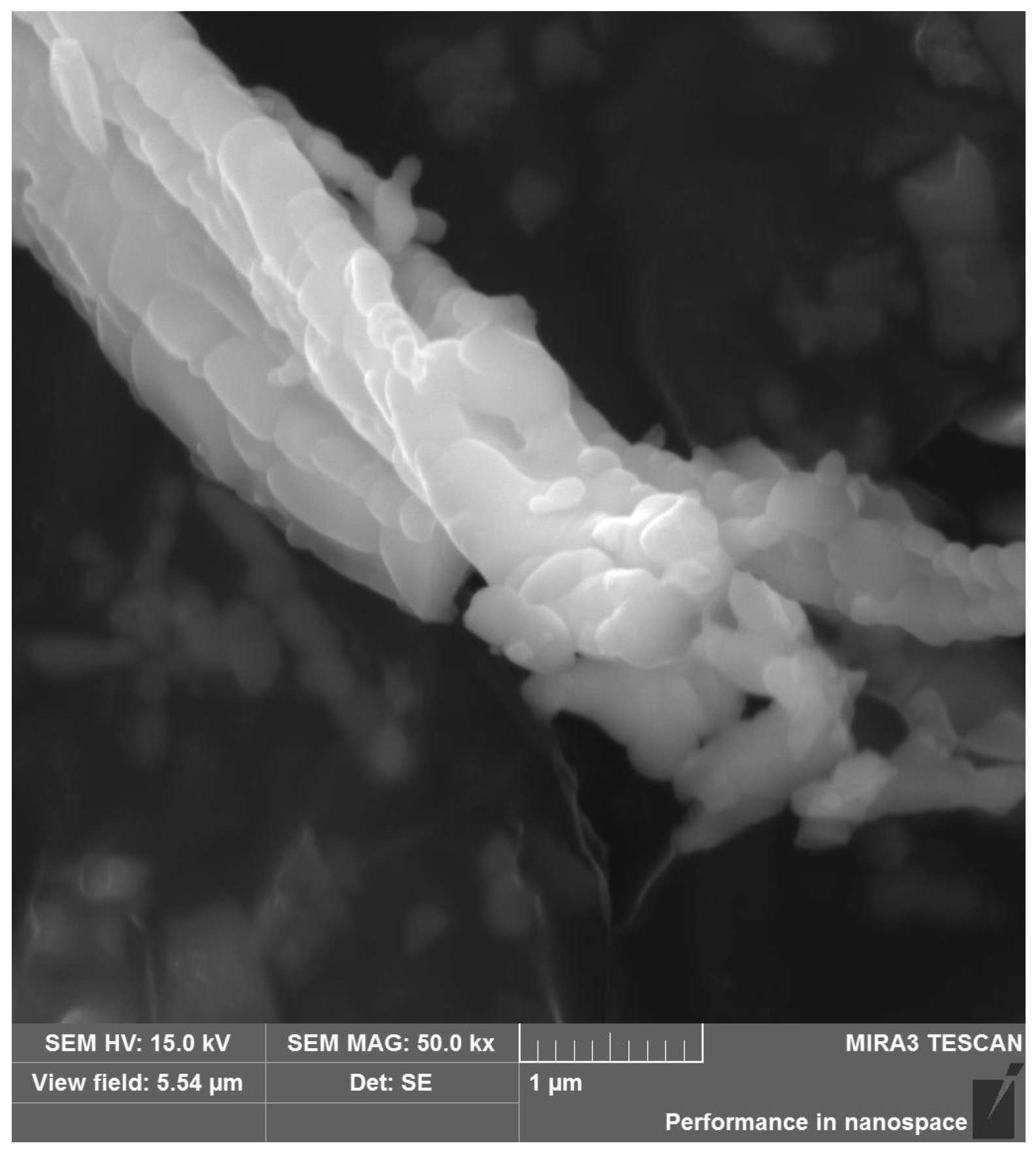
3.1. Characteristics of Chrysotile Asbestos
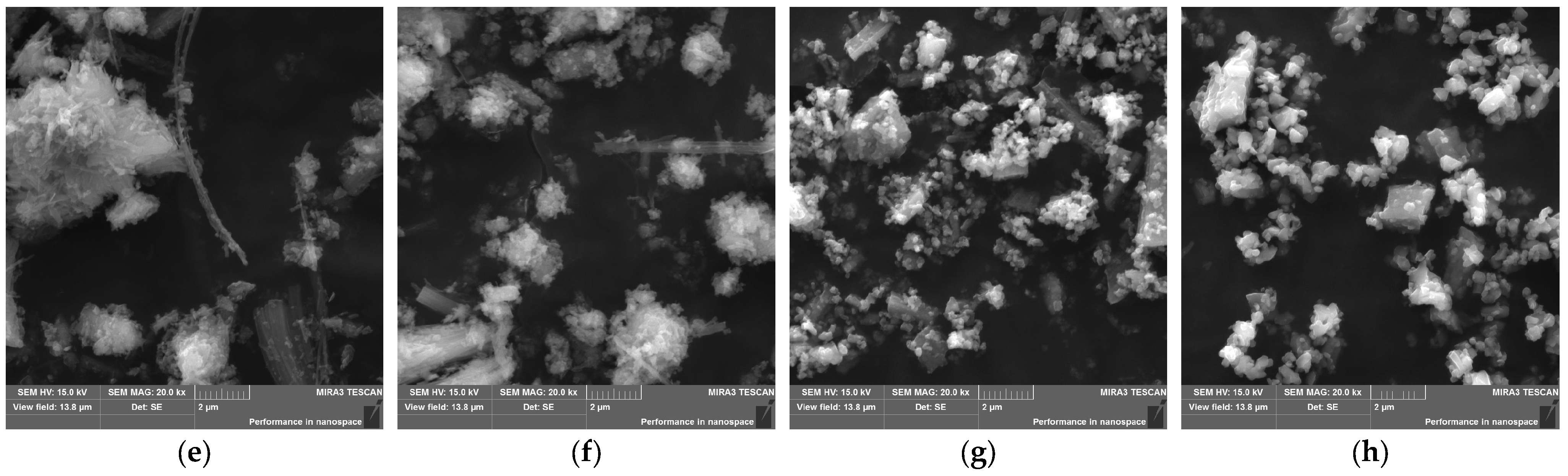
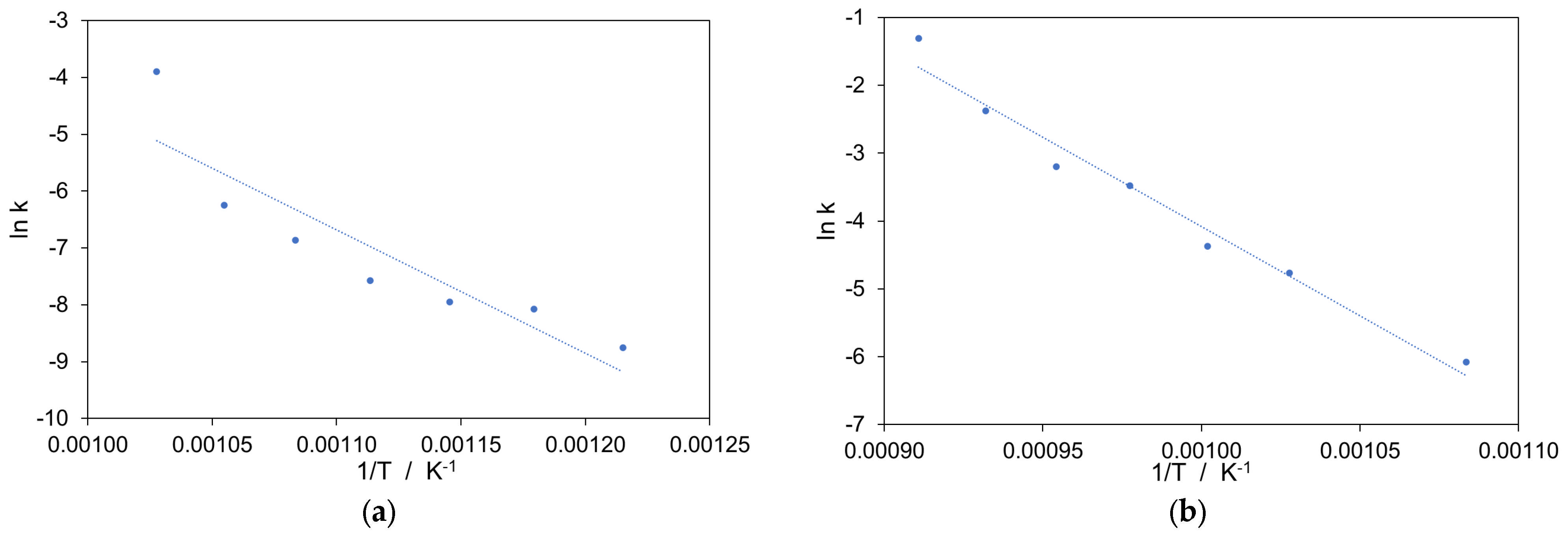
3.2. Kinetic Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virta, R.L. Mineral Commodity Profiles—Asbestos; Circular 1255-KK; U.S. Geological Survey: Reston, VA, USA, 2005.
- Sporn, T.A. Mineralogy of asbestos. In Malignant Mesothelioma; Tannapfel, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–11. [Google Scholar]
- Bartrip, P.W. History of asbestos related disease. Postgrad. Med. J. 2004, 80, 72–76. [Google Scholar] [CrossRef]
- Musk, A.W.; de Klerk, N.; Reid, A.; Hui, J.; Franklin, P.; Brims, F. Asbestos-related diseases. Tuber. Lung Dis. 2020, 24, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Stayner, L.; Welch, L.S.; Lemen, R. The worldwide pandemic asbestos-related diseases. Annu. Rev. Public Health 2013, 34, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Kwak, K.; Kang, D.; Paek, D. Environmental exposure to asbestos and the risk of lung cancer: A systematic review and meta-analysis. J. Occup. Environ. Med. 2022, 79, 207–214. [Google Scholar] [CrossRef] [PubMed]
- The International Ban Asbestos Secretariat IBAS. Available online: www.ibasecretariat.org/index.htm (accessed on 10 January 2025).
- Lin, R.T.; Chien, L.C.; Jimba, M.; Furuya, S.; Takahashi, K. Implementation of national policies for a total asbestos ban: A global comparison. Lancet Planet. Health 2019, 3, 341–348. [Google Scholar] [CrossRef]
- Paglietti, F.; Malinconico, S.; Conestabile della Staffa, B.; Bellagamba, S.; De Simone, P. Classification and management of asbestos-containing waste: European legislation and the Italian experience. Waste Manag. 2016, 50, 130–150. [Google Scholar] [CrossRef]
- Promentilla, M.A.B.; Peralta, G.L. An Evaluation of landfill disposal of asbestos-containing waste and geothermal residues within a risk-assessment framework. J. Mater. Cycles Waste Manag. 2003, 5, 13–21. [Google Scholar] [CrossRef]
- Macher, G.Z.; Torma, A.; Beke, D. Examining the environmental ramifications of asbestos fiber movement through the water–soil continuum: A review. Int. J. Environ. Res. Public Health 2025, 22, 505. [Google Scholar] [CrossRef]
- Paolini, V.; Tomassetti, L.; Segreto, M.; Borin, D.; Liotta, F.; Torre, M.; Petracchini, F. Asbestos treatment technologies. J. Mater. Cycles Waste Manag. 2019, 21, 205–226. [Google Scholar] [CrossRef]
- Spasiano, D.; Pirozzi, F. Treatments of asbestos containing wastes. J. Environ. Manag. 2017, 204, 82–91. [Google Scholar] [CrossRef]
- Zaremba, T.; Krząkała, A.; Piotrowski, J.; Garczorz, D. Utilization of chrysotile asbestos for sintering ceramics production. Ceram. Mater. 2010, 62, 149–155. (In Polish) [Google Scholar]
- Gualtieri, A.F.; Tartaglia, A. Thermal decomposition of asbestos and recycling in traditional ceramics. J. Eur. Ceram. Soc. 2000, 20, 1409–1418. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Cavenati, C.; Zanatto, I.; Meloni, M.; Elmi, G.; Gualtieri, M.L. The transformation sequence of cement–asbestos slates up to 1200 °C and safe recycling of the reaction product in stoneware tile mixtures. J. Hazard. Mater. 2008, 152, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Kusiorowski, R.; Zaremba, T.; Piotrowski, J.; Podwórny, J. Utilisation of cement-asbestos wastes by thermal treatment and the potential possibility use of obtained product for the clinker bricks manufacture. J. Mater. Sci. 2015, 50, 6757–6767. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Boccaletti, M. Recycling of the product of thermal inertization of cement–asbestos for the production of concrete. Constr. Build. Mater. 2011, 25, 3561–3569. [Google Scholar] [CrossRef]
- Kusiorowski, R.; Zaremba, T.; Piotrowski, J. The potential use of cement-asbestos waste in the ceramic masses destined for sintered wall clay brick manufacture. Ceram. Int. 2014, 40, 11995–12002. [Google Scholar] [CrossRef]
- Obmiński, A. Asbestos waste recycling using the microwave technique—Benefits and risks. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100577. [Google Scholar] [CrossRef]
- Carneiro, G.O.; Santos, T.A.; Simonelli, G.; Ribeiro, D.V.; Cilla, M.S.; Dias, C.M.R. Thermal treatment optimization of asbestos cement waste (ACW) potentializing its use as alternative binder. J. Clean. Prod. 2021, 320, 128801. [Google Scholar] [CrossRef]
- Capitani, G.; Dalpiaz, M.; Vergani, F.; Campanale, F.; Conconi, R.; Odorizzi, S. Recycling thermally deactivated asbestos cement in mortar: A possible route towards a rapid conclusion of the “asbestos problem”. J. Environ. Manag. 2024, 355, 120507. [Google Scholar] [CrossRef]
- Singh, R.; Rao, B.; Asolekar, S.R. Geopolymer-based solidification and stabilization for environmentally sound disposal of asbestos-containing waste. J. Mater. Cycles Waste Manag. 2025, 27, 75–90. [Google Scholar] [CrossRef]
- Gualtieri, A.F. Mineral Fibers: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction, and Toxicity; European Mineralogical Union: London, UK, 2017. [Google Scholar]
- Bloise, A.; Catalano, M.; Barrese, E.; Gualtieri, A.F.; Gandolfi, N.B.; Capella, S.; Belluso, E. TG/DSC study of the thermal behavior of hazardous mineral fibres. J. Therm. Anal. Calorim. 2016, 123, 2225–2239. [Google Scholar] [CrossRef]
- Kusiorowski, R.; Zaremba, T.; Piotrowski, J.; Adamek, J. Thermal decomposition of different types of asbestos. J. Therm. Anal. Calorim. 2012, 109, 693–704. [Google Scholar] [CrossRef]
- Bloise, A. Thermal behaviour of actinolite asbestos. J. Mater. Sci. 2019, 54, 11784–11795. [Google Scholar] [CrossRef]
- Bloise, A. On the thermal breakdown of tremolite: A new method for distinguishing between asbestos and non-asbestos tremolite samples. J. Mater. Sci. 2023, 58, 8779–8795. [Google Scholar] [CrossRef]
- Carmignano, O.R.; Vieira, S.S.; Brandão, P.R.; Bertoli, A.C.; Lago, R.M. Serpentinites: Mineral structure, properties and technological applications. J. Braz. Chem. Soc. 2020, 31, 2–14. [Google Scholar] [CrossRef]
- Flanagan, D.M. Mineral Commodity Summaries 2025, Ver. 1.2; U.S. Geological Survey: Reston, VA, USA, 2025; p. 38.
- Bloise, A.; Critelli, T.; Catalano, M.; Apollaro, C.; Miriello, D.; Croce, A.; Barrese, E.; Liberi, F.; Piluso, E.; Rinaudo, C.; et al. Asbestos and other fibrous minerals contained in the serpentinites of the Gimigliano-Mount Reventio Unit (Calabria, Italy). Environ. Earth Sci. 2014, 71, 3773–3786. [Google Scholar] [CrossRef]
- Teixeira, A.P.C.; Santos, E.M.; Vieira, A.F.P.; Lago, R.M. Use of chrysotile to produce highly dispersed K-doped MgO catalyst for biodiesel synthesis. Chem. Eng. J. 2013, 232, 104–110. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Niedojadło, J.; Buszewski, B. Structural features of natural and acids modified chrysotile nanotubes. J. Phys. Chem. Solids 2011, 72, 1015–1026. [Google Scholar] [CrossRef]
- Jeyaratnam, M.; West, N.G. A study of heat-degraded chrysotile, amosite and crocidolite by X-ray diffraction. Ann. Occup. Hyg. 1994, 38, 137–148. [Google Scholar] [CrossRef]
- Cattaneo, A.; Gualtieri, A.F.; Artioli, G. Kinetic study of the dehydroxylation of chrysotile asbestos with temperature by in situ XRPD. Phys. Chem. Miner. 2003, 30, 177–183. [Google Scholar] [CrossRef]
- Hashimoto, S.; Yamaguchi, A. Detoxification technique of asbestos using low-temperature heating and grinding. J. Ceram. Soc. Jpn. 2006, 41, 856–858. [Google Scholar]
- Dellisanti, F.; Minguzzi, V.; Morandi, N. Experimental results from thermal treatment of asbestos containing materials. GeoActa 2001–2002, 1, 61–70. [Google Scholar]
- Brindley, G.W.; Hayami, R. Mechanism of formation of forsterite and enstatite from serpentine. Mineral. Mag. 1965, 35, 189–195. [Google Scholar] [CrossRef]
- Langer, A.M. Reduction of the biological potential of chrysotile asbestos arising from conditions of service on brake pads. Regul. Toxicol. Pharmacol. 2003, 38, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Takata, A.; Yamauchi, H.; Yamashita, K.; Aminaka, M.; Hitomi, T.; Toya, T.; Kohyama, N. Mesothelioma carcinogenesis of chrysotile and forsterite compared and validated by intraperitoneal injection in rat. Ind. Health 2025, 63, 14–28. [Google Scholar] [CrossRef]
- Zaremba, T.; Krząkała, A.; Piotrowski, J.; Garczorz, D. Study on the thermal decomposition of chrysotile asbestos. J. Therm. Anal. Calorim. 2010, 101, 479–485. [Google Scholar] [CrossRef]
- PN-EN ISO 12677:2011; Chemical Analysis of Refractory Products by X-Ray Fluorescence (XRF)—Fused Cast-Bead Method. ISO: Geneva, Switzerland, 2011.
- Bloise, A.; Parisi, F.; La Russa, M.F.; Apollaro, C.; Godbert, N.; Aiello, I.; Giorno, E.; Croce, A.; Cagna, L.; López, A.J.; et al. Evaluation of asbestos dispersion during laser ablation of rocks containing Naturally Occurring Asbestos (NOA). Heliyon 2024, 10, e39624. [Google Scholar] [CrossRef]
- Łącki, J.W. Wydobycie i Zastosowanie Azbestu (Mining and Use of Asbestos), 1st ed.; Wydawnictwo Śląsk: Katowice, Poland, 1974. (In Polish) [Google Scholar]
- Shayakhetova, R.A.; Mukhametzhanova, A.A.; Akbayeva, D.N.; Terlikbaeva, A.Z.; Osipov, P.A.; Alimzhanova, A.M.; Zharmenov, A.A. Magnesium and silicon recovery from chrysotile asbestos waste of the deposit Zhitikara, Kazakhstan. Sci. Rep. 2024, 14, 31866. [Google Scholar]
- Földvári, M. Handbook of Thermogravimetric System of Minerals and Its Use in Geological Practice; Geological Institute of Hungary: Budapest, Hungary, 2011. [Google Scholar]
- Strzałkowska, E. Thermal Analysis of Minerals, Rocks and Mineral Waste Materials; Silesian University of Technology: Gliwice, Poland, 2019. (In Polish) [Google Scholar]
- Sanders, J.P.; Brosnan, D. Modelling the Thermal Decomposition of Chrysotile. 2005. Available online: https://www.jurispro.com/files/documents/doc-1066205184-article-1610.pdf (accessed on 14 April 2025).
- Khorami, J.; Choquette, D.; Kimmerle, F.M.; Gallagher, P.K. Interpretation of EGA and DTG analyses of chrysotile asbestos. Thermochim. Acta 1984, 76, 87–96. [Google Scholar] [CrossRef]
- Bloise, A.; Kusiorowski, R.; Lassinantti Gualtieri, M.; Gualtieri, A.F. Thermal behaviour of mineral fibres. In Mineral Fibers: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Gualtieri, A.F., Ed.; European Mineralogical Union: London, UK; Mineralogical Society of Great Britain & Ireland Minerals: Twickenham, UK, 2017. [Google Scholar]
- Gualtieri, A.F.; Giacobbe, C.; Viti, C. The dehydroxylation of serpentine group minerals. Am. Mineral. 2012, 97, 666–680. [Google Scholar] [CrossRef]
- Belardi, G.; Piga, L. Influence of calcium carbonate on the decomposition of asbestos contained in end-of-life products. Thermochim. Acta 2013, 573, 220–228. [Google Scholar] [CrossRef]
- Malkov, A.A.; Korytkova, E.N.; Maslennikova, T.P.; Shtykhova, A.M.; Gusarov, V.V. Effect of heat treatment on structural-chemical transformations in magnesium hydrosilicate Mg3Si2O5(OH)4 nanotubes. Russ. J. Appl. Chem. 2009, 82, 2079–2086. [Google Scholar] [CrossRef]
- Leonelli, C.; Veronesi, P.; Boccaccini, D.N.; Rivasi, M.R.; Barbieri, L.; Andreola, F.; Lancellotti, I.; Rabitti, D.; Pellacani, G.C. Microwave thermal inertisation of asbestos containing waste and its recycling in traditional ceramics. J. Hazard. Mater. 2006, 135, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Vast, P.; Andries, V.; Martines, M.A.U.; Auffredic, J.P.; Poulain, M.; Messaddeq, Y. Treatment and destruction of inorganic fibers wastes like asbestos by sodium polyphosphate. Phosphorus Res. Bull. 2004, 15, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Dlugogorski, B.Z.; Balucan, R.D. Dehydroxylation of serpentine minerals: Implications for mineral carbonation. Renew. Sustain. Energy Rev. 2014, 31, 353–367. [Google Scholar] [CrossRef]
- Viti, C. Serpentine minerals discrimination by thermal analysis. Am. Mineral. 2010, 95, 631–638. [Google Scholar] [CrossRef]
- Martinez, E. The effect of particle size on the thermal properties of serpentine minerals. Am. Mineral. 1961, 46, 901–912. [Google Scholar]
- Coats, C.J.A. Serpentine minerals from Manitoba. Can. Mineral. 1968, 9, 322–347. [Google Scholar]
- Rao, V.A.N.; Murty, M.S. Thermal studies on soft and brittle asbestos. Curr. Sci. 1979, 48, 760–761. [Google Scholar]
- Perez-Rodriguez, J.L.; Franco, F.; Ramirez-Valle, V.; Perez-Maqueda, L.A. Modification of the thermal dehydroxylation of antigorite by ultrasound treatment. J. Therm. Anal. Calorim. 2005, 82, 769–774. [Google Scholar] [CrossRef]
- Viti, C.; Giacobbe, C.; Gualtieri, A.F. Quantitative determination of chrysotile in massive serpentinites using DTA: Implications for asbestos determinations. Am. Mineral. 2011, 96, 1003–1011. [Google Scholar] [CrossRef]
- Jeong, H.; Moon, W.; Roh, Y. Characterization of mineralogical changes of chrysotile and its thermal decomposition by heat treatment. Econ. Environ. Geol. 2016, 49, 77–88. [Google Scholar] [CrossRef]
- Long, J.; Liu, W.; Zhang, N.; Zhang, H.; Xiao, Q.; Huang, S. New insight into the phase transition and kinetics of the dehydroxylation of bulk-to-nano chrysotile. Phys. Chem. Miner. 2024, 51, 28. [Google Scholar] [CrossRef]
- Kim, C.; Kim, Y.; Roh, Y. Thermal decomposition and phase transformation of chrysotile in asbestos-containing waste. Minerals 2025, 15, 344. [Google Scholar] [CrossRef]
- Viani, A.; Gualtieri, A.F.; Mácová, P.; Pollastri, S. Transformations through pseudomorphosis of asbestos minerals in thermally processed asbestos-containing materials investigated through SEM/EDS and micro-Raman spectroscopy: Implications for recycling of hazardous wastes. In Proceedings of the 18th International Microscopy Congress, Prague, Czech Republic, 7–12 September 2014. [Google Scholar]
- Gualtieri, A.F. Recycling asbestos-containing material (ACM) from construction and demolition waste (CDW). In Handbook of Recycled Concrete and Demolition Waste; Woodhead Publishing: Sawston, UK, 2013; pp. 500–525. [Google Scholar]
- Giacobbe, C.; Gualtieri, A.F.; Quartieri, S.; Rinaudo, C.; Allegrina, M.; Andreozzi, G.B. Spectroscopic study of the product of thermal transformation of chrysotile-asbestos containing materials (ACM). Eur. J. Mineral. 2010, 22, 535–546. [Google Scholar] [CrossRef]
- Joraid, A.A.; Alamri, S.N.; Abu-Sehly, A.A.; Al-Raqa, S.Y.; Shipman, P.O.; Shipley, P.R.; Abd-El-Aziz, A.S. Isothermal kinetics and thermal degradation of an aryl azo dye-containing polynorbornene. Thermochim. Acta 2011, 515, 38–42. [Google Scholar] [CrossRef]
- Chen, G.; Shi, Z.; Yu, J.; Wang, Z.; Xu, J.; Gao, B.; Hu, X. Kinetic analysis of the non-isothermal decomposition of carbon monofluoride. Thermochim. Acta 2014, 589, 63–69. [Google Scholar] [CrossRef]
- Wang, S.; Shen, F.; Zheng, H.; Nie, X.; Jiang, X.; Gao, Q. Isothermal reduction and Avrami-Erofeev kinetic model for reducing iron ore pellets in a 70% N2-30%H2/CO atmosphere. J. Sustain. Metall. 2024, 10, 2337–2351. [Google Scholar] [CrossRef]
- Kusiorowski, R.; Gerle, A.; Kujawa, M. Kinetic analysis of cement-asbestos materials’ thermal decomposition process by an ex situ technique. Fibers 2025, 13, 43. [Google Scholar] [CrossRef]
- Gasparini, E.; Tarantino, S.C.; Ghigna, P.; Pia Riccardi, M.; Cedillo-González, E.I.; Siligardi, C.; Zema, M. Thermal dehydroxylation of kaolinite under isothermal conditions. Appl. Clay Sci. 2013, 80–81, 417–425. [Google Scholar] [CrossRef]
- Gurgul, S.J.; Seng, G.; Williams, G.R. A kinetic and mechanistic study into the transformation of calcium sulfate hemihydrate to dihydrate. J. Synchrotron Radiat. 2019, 26, 774–784. [Google Scholar] [CrossRef]
- Giacobbe, C. High temperature reactions of serpentine group minerals. Plinius 2012, 38, 93. [Google Scholar]
- Trittschack, R.; Grobéty, B.; Brodard, P. Kinetics of the chrysotile and brucite dehydroxylation reaction: A combined non-isothermal/isothermal thermogravimetric analysis and high-temperature X-ray powder diffraction study. Phys. Chem. Miner. 2014, 41, 197–214. [Google Scholar] [CrossRef]

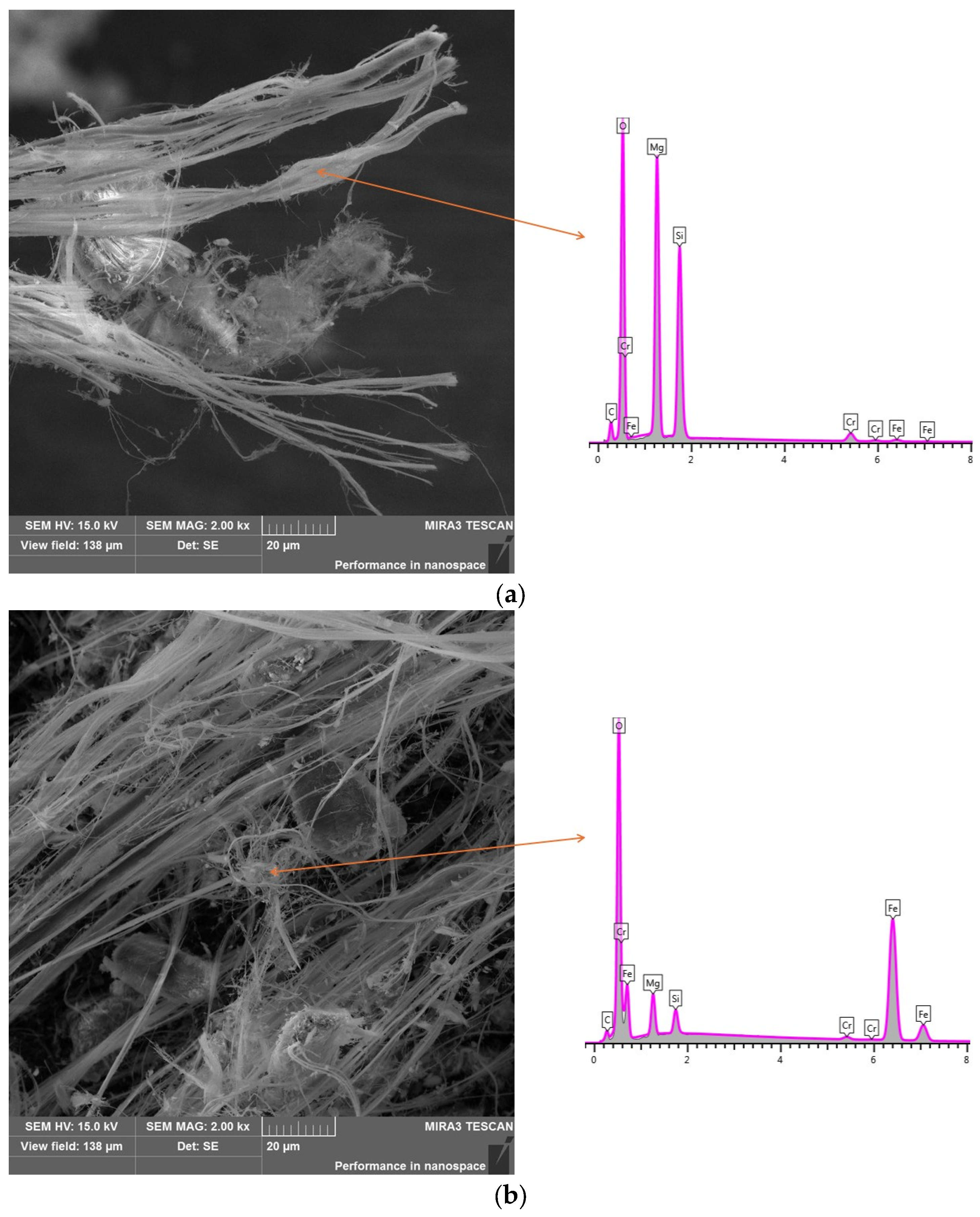





| SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | Cr2O3 | L.O.I. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 38.08 | 0.01 | 0.21 | 4.21 | 0.11 | 41.94 | 0.12 | 0.04 | bdl | bdl | 0.06 | 16.14 |
| Ex Situ Method | |||||||
| Temperature, °C | 550 | 575 | 600 | 625 | 650 | 675 | 700 |
| Value of reaction order, n | 0.69 | 0.70 | 0.82 | 0.80 | 0.85 | 0.89 | 0.87 |
| Rate coefficient 10−4, k | 1.57 | 3.12 | 3.53 | 5.15 | 10.4 | 19.3 | 20.3 |
| Correlation coefficient | 0.991 | 0.995 | 0.994 | 0.988 | 0.988 | 0.987 | 0.977 |
| In Situ Method | |||||||
| Temperature, °C | 650 | 700 | 725 | 750 | 775 | 800 | 825 |
| Value of reaction order, n | 0.48 | 0.57 | 0.55 | 0.55 | 0.45 | 0.55 | 0.41 |
| Rate coefficient 10−3, k | 2.2 | 8.5 | 12.6 | 30.8 | 40.9 | 92.6 | 271.1 |
| Correlation coefficient | 0.988 | 0.988 | 0.982 | 0.991 | 0.977 | 0.966 | 0.970 |
| Method | Ex Situ | In Situ |
|---|---|---|
| Activation energy, Ea, kJ mol−1 | 180.6 ± 14.5 | 218.8 ± 15.3 |
| Correlation coefficient | 0.821 | 0.974 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusiorowski, R.; Gerle, A.; Kujawa, M.; Bloise, A. Kinetic Aspects of Chrysotile Asbestos Thermal Decomposition Process. Minerals 2025, 15, 609. https://doi.org/10.3390/min15060609
Kusiorowski R, Gerle A, Kujawa M, Bloise A. Kinetic Aspects of Chrysotile Asbestos Thermal Decomposition Process. Minerals. 2025; 15(6):609. https://doi.org/10.3390/min15060609
Chicago/Turabian StyleKusiorowski, Robert, Anna Gerle, Magdalena Kujawa, and Andrea Bloise. 2025. "Kinetic Aspects of Chrysotile Asbestos Thermal Decomposition Process" Minerals 15, no. 6: 609. https://doi.org/10.3390/min15060609
APA StyleKusiorowski, R., Gerle, A., Kujawa, M., & Bloise, A. (2025). Kinetic Aspects of Chrysotile Asbestos Thermal Decomposition Process. Minerals, 15(6), 609. https://doi.org/10.3390/min15060609











