Exploring the Potential of Granite Sawing Sludge from Cuasso Al Monte (Italy) for the Development of Aluminosilicate Gel for a Sustainable Industry
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Mineralogical and Molecular Characterization
3.2. Morphological and Mechanical Characterization
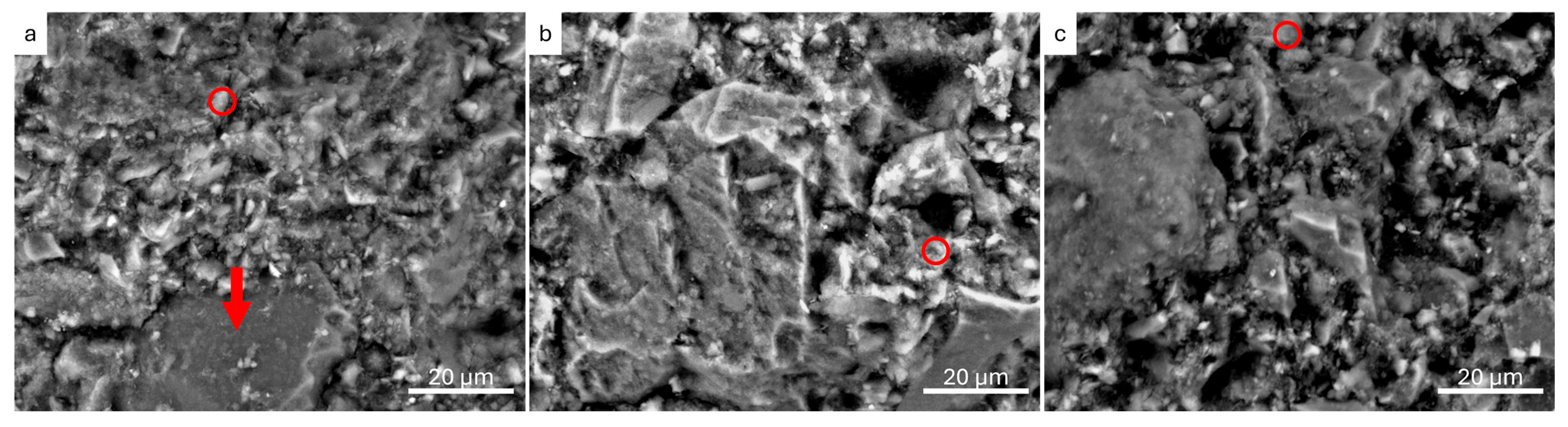
3.2.1. Scanning Electron Microscopy and Energy-Dispersion Spectroscopy (SEM-EDS)
3.2.2. Mechanical Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramón-Álvarez, I.; Batuecas, E.; Sánchez-Delgado, S.; Torres-Carrasco, M. Mechanical performance after high-temperature exposure and Life Cycle Assessment (LCA) according to unit of stored energy of alternative mortars to Portland cement. Constr. Build. Mater. 2023, 365, 130082. [Google Scholar] [CrossRef]
- Arora, N.K.; Mishra, I. United Nations Sustainable Development Goals 2030 and environmental sustainability: Race against time. Environ. Sustain. 2019, 2, 339–342. [Google Scholar] [CrossRef]
- Brilha, J.; Gray, M.; Pereira, D.; Pereira, P. Geodiversity: An integrative review as a contribution to the sustainable management of the whole of nature. Environ. Sci. Policy 2018, 86, 19–28. [Google Scholar] [CrossRef]
- Coppola, B.; Palmero, P.; Montanaro, L.; Tulliani, J.M. Alkali-activation of marble sludge: Influence of curing conditions and waste glass addition. J. Eur. Ceram. Soc. 2020, 40, 3776–3787. [Google Scholar] [CrossRef]
- Montani, C. Congiuntura internazionale Produzione Scambi Consumi Tecnologie Beni strumentali Schede dei Paesi Leader. In XXXII Rapporto Marmo e Pietre nel Mondo 2021 Marble and Stones in the World XXXII Report; International Situation Production Interchange Consumption Technology Tools Profiles of Leading Countries; Aldus: Venice, Italy, 2021. [Google Scholar]
- Jalalian, M.H.; Bagherpour, R.; Khoshouei, M. Wastes production in dimension stones industry: Resources, factors, and solutions to reduce them. Environ. Earth Sci. 2021, 80, 560. [Google Scholar] [CrossRef]
- Mosaferi, M.; Dianat, I.; Khatibi, M.S.; Mansour, S.N.; Fahiminia, M.; Hashemi, A.A. Review of Environmental Aspects and Waste Management of Stone Cutting and Fabrication Industries. J. Mater. Cycles Waste Manag. 2014, 16, 721–730. [Google Scholar] [CrossRef]
- Kramer, M.R.; Blanc, P.D.; Fireman, E.; Amital, A.; Guber, A.; Rhahman, N.A.; Shitrit, D. Artificial Stone Silicosis: Disease Resurgence Among Artificial Stone Workers. Chest 2012, 142, 419–424. [Google Scholar] [CrossRef]
- Zari, M.; Smith, R.; Ferrari, R. Evaluation of Dust Emission Rate from Landfill Mining Activities. Detritus 2023, 25, 78–89. [Google Scholar] [CrossRef]
- Sivacoumar, R.; Jayabalou, R.; Swarnalatha, S.; Balakrishnan, K. Particulate Matter from Stone Crushing Industry: Size Distribution and Health Effects. J. Environ. Eng. 2006, 132, 405–414. [Google Scholar] [CrossRef]
- Piccini, L.; Di Lorenzo, T.; Costagliola, P.; Galassi, D.M.P. Marble Slurry’s Impact on Groundwater: The Case Study of the Apuan Alps Karst Aquifers. Water 2019, 11, 2462. [Google Scholar] [CrossRef]
- Montero, M.J.; Araque, R.A.; Rey, J.M. Occupational health and safety in the framework of corporate social responsibility. Saf. Sci. 2009, 47, 1440–1445. [Google Scholar] [CrossRef]
- Construction & Demolition Debris Management for Sustainable Reconstruction After Disasters. Italian Case Studies. Available online: https://www.researchgate.net/publication/236648331_Construction_Demolition_debris_management_for_sustainable_reconstruction_after_disasters_Italian_case_studies (accessed on 6 April 2024).
- Chen, J.; Wang, Y.; Shi, Q.; Peng, X.; Zheng, J. An international comparison analysis of CO2 emissions in the construction industry. Sustain. Dev. 2021, 29, 754–767. [Google Scholar] [CrossRef]
- Surra, E.; Sousa, J.; Correia, M.; Carvalheiras, J.; Labrincha, J.A.; Marques, J.C.; Lapa, N.; Delerue-Matos, C. Technical, Environmental, and Cost Assessment of Granite Sludge Valorisation. Appl. Sci. 2023, 13, 4513. [Google Scholar] [CrossRef]
- Uysal, M.; Aygörmez, Y.; Canpolat, O.; Cosgun, T.; Kuranlı, Ö. Investigation of using waste marble powder, brick powder, ceramic powder, glass powder, and rice husk ash as eco-friendly aggregate in sustainable red mud-metakaolin based geopolymer composites. Constr. Build. Mater. 2022, 361, 129718. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Stroscio, A.; Barone, G.; Fernàndez-Jimenez, A.; Lancellotti, I.; Leonelli, C.; Mazzoleni, P. Sicilian clay sediments as precursor for alkali activated materials. Appl. Clay Sci. 2024, 253, 107350. [Google Scholar] [CrossRef]
- Lancellotti, I.; Piccolo, F.; Traven, K.; Češnovar, M.; Ducman, V.; Leonelli, C. Alkali Activation of Metallurgical Slags: Reactivity, Chemical Behavior, and Environmental Assessment. Materials 2021, 14, 639. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L. Durability of alkali-activated materials: Progress and perspectives. J. Am. Ceram. Soc. 2014, 97, 997–1008. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and related alkali-activated materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Portale, S.; Finocchiaro, C.; Occhipinti, R.; Mazzoleni, P.; Barone, G. Feasibility study about the use of basalt sawing sludge in building and restoration. Mater. Lett. 2023, 333, 133624. [Google Scholar] [CrossRef]
- Salihoglu, N.K.; Salihoglu, G. Marble Sludge Recycling by Using Geopolymerization Technology. J. Hazard. Toxic Radioact. Waste 2018, 22, 04018019. [Google Scholar] [CrossRef]
- Aruntaş, H.Y.; Gürü, M.; Dayı, M.; Tekin, I. Utilization of waste marble dust as an additive in cement production. Mater. Des. 2010, 31, 4039–4042. [Google Scholar] [CrossRef]
- Corinaldesi, V.; Moriconi, G.; Naik, T.R. Characterization of marble powder for its use in mortar and concrete. Constr. Build. Mater. 2010, 24, 113–117. [Google Scholar] [CrossRef]
- Alyamaç, K.E.; Ince, R. A preliminary concrete mix design for SCC with marble powders. Constr. Build. Mater. 2009, 23, 1201–1210. [Google Scholar] [CrossRef]
- Lozano-Lunar, A.; Dubchenko, I.; Bashynskyi, S.; Rodero, A.; Fernández, J.M.; Jiménez, J.R. Performance of Self-Compacting Mortars with Granite Sludge as Aggregate. Constr. Build. Mater. 2020, 251, 118998. [Google Scholar] [CrossRef]
- Zichella, L.; Dino, G.A.; Bellopede, R.; Marini, P.; Padoan, E.; Passarella, I. Environmental Impacts, Management and Potential Recovery of Residual Sludge from the Stone Industry: The Piedmont Case. Resour. Policy 2020, 65, 101562. [Google Scholar] [CrossRef]
- Portale, S.; Mazzoleni, P.; Barone, G. Alkali activated binders based on rock sawing sludges: Synthesis, characterization and future perspectives. Constr. Build. Mater. 2024, 450, 138669. [Google Scholar] [CrossRef]
- Gramaccioli, C.M.; Diella, V.; Demartin, F.; Orlandi, P.; Campostrini, I. Cesian Bazzite and Thortveitite from Cuasso Al Monte, Varese, Italy: A Comparison with the Material from Baveno, and Inferred Origin. Can. Mineral. 2000, 38, 1409–1418. [Google Scholar]
- Capitani, G.; Mugnaioli, E.; Gentile, P. Submicrometer yttrian zircon coating and arborescent aeschynite microcrystals on truncated bipyramidal anatase: An electron microscopy study of miarolitic cavities in the Cuasso al Monte granophyre (Varese, Italy). Am. Mineral. 2023, 103, 480–488. [Google Scholar] [CrossRef]
- Pezzotta, F.; Diella, V.; Guastoni, A. Scandium silicates from the Baveno and Cuasso al Monte NYF-granites, Southern Alps (Italy): Mineralogy and genetic inferences. Am. Mineral. 2005, 90, 1442–1452. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Labrincha, J.; Leonelli, C.; Palomo, A.; Chindaprasirt, P. Handbook of Alkali-Activated Cements, Mortars and Concretes, 1st ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 1–830. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Investigations of tungsten mine waste geopolymeric binder: Strength and microstructure. Constr. Build. Mater. 2008, 22, 2212–2219. [Google Scholar] [CrossRef]
- Finocchiaro, C.; Barone, G.; Mazzoleni, P.; Leonelli, C.; Gharzouni, A.; Rossignol, S. FT-IR study of early stages of alkali activated materials based on pyroclastic deposits (Mt. Etna, Sicily, Italy) using two different alkaline solutions. Constr. Build. Mater. 2020, 262, 120095. [Google Scholar] [CrossRef]
- Lancellotti, I.; Catauro, M.; Ponzoni, C.; Bollino, F.; Leonelli, C. Inorganic polymers from alkali activation of metakaolin: Effect of setting and curing on structure. J. Solid State Chem. 2013, 200, 341–348. [Google Scholar] [CrossRef]
- BGMN Home. Available online: http://www.bgmn.de/index.html (accessed on 18 December 2024).
- Longhi, M.A.; Rodríguez, E.D.; Walkley, B.; Eckhard, D.; Zhang, Z.; Provis, J.L.; Kirchheim, A.P. Metakaolin-based geopolymers: Efflorescence and its effect on microstructure and mechanical properties. Ceram. Int. 2022, 48, 2212–2229. [Google Scholar] [CrossRef]
- Provis, J.L. Geopolymers and other alkali activated materials: Why, how, and what? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Yang, J.; Xu, L.; Wu, H.; Jin, J.; Liu, L. Microstructure and mechanical properties of metakaolin-based geopolymer composites containing high volume of spodumene tailings. Appl. Clay Sci. 2022, 218, 106412. [Google Scholar] [CrossRef]
- Palmero, P.; Formia, A.; Tulliani, J.M.; Antonaci, P. Valorisation of alumino-silicate stone muds: From wastes to source materials for innovative alkali-activated materials. Cem. Concr. Compos. 2017, 83, 251–262. [Google Scholar] [CrossRef]
- Ghorbani, S.; Stefanini, L.; Sun, Y.; Walkley, B.; Provis, J.L.; De Schutter, G.; Matthys, S. Characterisation of alkali-activated stainless steel slag and blast-furnace slag cements. Cem. Concr. Compos. 2023, 143, 105230. [Google Scholar] [CrossRef]
- Onutai, S.; Osugi, T.; Sone, T. Alumino-Silicate Structural Formation during Alkali-Activation of Metakaolin: In-Situ and Ex-Situ ATR-FTIR Studies. Materials 2023, 16, 985. [Google Scholar] [CrossRef]
- Zhang, D.W.; Zhao, K.F.; Wang, D.M.; Li, H. Relationship of amorphous gel-microstructure-elastoviscosity properties of alkali-activated materials fresh pastes with different Ms waterglass. Constr. Build. Mater. 2021, 287, 123023. [Google Scholar] [CrossRef]
- Zhang, D.W.; Zhao, K.F.; Xie, F.Z.; Li, H.; Wang, D.M. Effect of water-binding ability of amorphous gel on the rheology of geopolymer fresh pastes with the different NaOH content at the early age. Constr. Build. Mater. 2020, 261, 120529. [Google Scholar] [CrossRef]
- Pascual, A.B.; Tognonvi, T.M.; Tagnit-Hamou, A. Optimization study of waste glass powder-based alkali activated materials incorporating metakaolin: Activation and curing conditions. J. Clean. Prod. 2021, 308, 127435. [Google Scholar] [CrossRef]
- Bignozzi, M.C.; Manzi, S.; Lancellotti, I.; Kamseu, E.; Barbieri, L.; Leonelli, C. Mix-design and characterization of alkali activated materials based on metakaolin and ladle slag. Appl. Clay Sci. 2013, 73, 78–85. [Google Scholar] [CrossRef]
- Kuenzel, C.; Neville, T.; Donatello, S.; Vandeperre, L.; Boccaccini, A.; Cheeseman, C. Influence of metakaolin characteristics on the mechanical properties of geopolymers. Appl. Clay Sci. 2013, 83–84, 308–314. [Google Scholar] [CrossRef]
- Vogt, O.; Ukrainczyk, N.; Ballschmiede, C.; Koenders, E. Reactivity and Microstructure of Metakaolin Based Geopolymers: Effect of Fly Ash and Liquid/Solid Contents. Materials 2019, 12, 3485. [Google Scholar] [CrossRef]
- Li, L.; Xie, J.; Zhang, B.; Feng, Y.; Yang, J. A state-of-the-art review on the setting behaviours of ground granulated blast furnace slag- and metakaolin-based alkali-activated materials. Constr. Build. Mater. 2023, 368, 130389. [Google Scholar] [CrossRef]
- Pundienė, I.; Pranckevičienė, J.; Zhu, C.; Kligys, M. The role of temperature and activator solution molarity on the viscosity and hard structure formation of geopolymer pastes. Constr. Build. Mater. 2021, 272, 121661. [Google Scholar] [CrossRef]
- Bersani, D.; Aliatis, I.; Tribaudino, M.; Mantovani, L.; Benisek, A.; Carpenter, M.A.; Gatta, G.D.; Lottici, P.P. Plagioclase composition by Raman spectroscopy. J. Raman Spectrosc. 2018, 49, 684–698. [Google Scholar] [CrossRef]
- Freeman, J.J.; Wang, A.; Kuebler, K.E.; Jolliff, B.L.; Haskin, L.A. Characterization of Natural Feldspars by Raman Spectroscopy for Future Planetary Exploration. Can. Mineral. 2008, 46, 1477–1500. [Google Scholar] [CrossRef]
- Jovanovski, G.; Šijakova-Ivanova, T.; Boev, I.; Boev, B.; Makreski, P. Intriguing minerals: Quartz and its polymorphic modifications. ChemTexts 2022, 8, 14. [Google Scholar] [CrossRef]
- Krishnamurti, D. The Raman Spectrum of Quartz and Its Interpretation. Proc. Indian Acad. Sci. 1985, 47, 276–291. [Google Scholar]
- Sato, R.K.; McMillan, P.F. An infrared and Raman study of the isotopic species of α-quartz. J. Phys. Chem. 1987, 91, 3494–3498. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Bersani, D.; Lottici, P.P.; Montenero, A. Micro-Raman investigation of iron oxide films and powders produced by sol-gel syntheses. J. Raman Spectrosc. 1999, 30, 355–360. [Google Scholar] [CrossRef]
- Jentzsch, P.V.; Kampe, B.; Ciobotă, V.; Rösch, P.; Popp, J. Inorganic salts in atmospheric particulate matter: Raman spectroscopy as an analytical tool. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 697–708. [Google Scholar] [CrossRef]
- Frost, R.L.; Dickfos, M. Hydrated double carbonates–A Raman and infrared spectroscopic study. Polyhedron 2007, 26, 4503–4508. [Google Scholar] [CrossRef]
- Gómez-Laserna, O.; Arrizabalaga, I.; Prieto-Taboada, N.; Olazabal, M.Á.; Arana, G.; Madariaga, J.M. In situ DRIFT, Raman, and XRF implementation in a multianalytical methodology to diagnose the impact suffered by built heritage in urban atmospheres. Anal. Bioanal. Chem. 2015, 407, 5635–5647. [Google Scholar] [CrossRef]
- Caggiani, M.C.; Coccato, A.; Barone, G.; Finocchiaro, C.; Fugazzotto, M.; Lanzafame, G.; Occhipinti, R.; Stroscio, A.; Mazzoleni, P. Raman spectroscopy potentiality in the study of geopolymers reaction degree. J. Raman Spectrosc. 2022, 53, 617–629. [Google Scholar] [CrossRef]
- Kosor, T.; Nakić-Alfirević, B.; Gajović, A. Geopolymerization index of fly ash geopolymers. Vib. Spectrosc. 2016, 85, 104–111. [Google Scholar] [CrossRef]
- Mirmoghtadaei, R.; Shen, L.; Li, Y.; Li, M.; Wang, B. Enhancing alkali-activated materials with low quality fly ash: A novel mixing approach for robust construction materials. J. Clean. Prod. 2023, 421, 138261. [Google Scholar] [CrossRef]
- Ramteke, D.D.; Hujova, M.; Kraxner, J.; Galusek, D.; Romero, A.R.; Falcone, R.; Bernardo, E. Up-cycling of ‘unrecyclable’ glasses in glass-based foams by weak alkali-activation, gel casting and low-temperature sintering. J. Clean. Prod. 2021, 278, 123985. [Google Scholar] [CrossRef]
- Ben Messaoud, I.; Hamdi, N.; Srasra, E.; Lavorgna, M. Physicochemical Characterization of Geopolymer Binders and Foams Made from Tunisian Clay. Adv. Mater. Sci. Eng. 2018, 2018, 9392743. [Google Scholar] [CrossRef]
- Yusan, S.; Bampaiti, A.; Aytas, S.; Erenturk, S.; Aslani, M.A.A. Synthesis and structural properties of ZnO and diatomite-supported ZnO nanostructures. Ceram. Int. 2016, 42, 2158–2163. [Google Scholar] [CrossRef]
- Azhar, N.S.D.M.; Zainal, F.F.; Abdullah, M.M.A.B. Bonding and Phases Analysis of Geopolymer Materials. In Proceedings of the IOP Conference Series: Materials Science and Engineering, International Conference on Sustainable Materials (ICoSM 2020), Pahang, Malaysia, 30 March 2020; Volume 957, p. 012052. [Google Scholar] [CrossRef]
- Khan, M.I.; Azizli, K.; Sufian, S.; Siyal, A.A.; Man, Z. Sodium Silicate Free Geopolymer as Coating Material: Adhesion to Steel. In Proceedings of the 1st International Electronic Conference on Materials, Online, 26 May–10 June 2014. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. In situ ATR-FTIR study of the early stages of fly ash geopolymer gel formation. Langmuir 2007, 23, 9076–9082. [Google Scholar] [CrossRef] [PubMed]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. Attenuated total reflectance fourier transform infrared analysis of fly ash geopolymer gel aging. Langmuir 2007, 23, 8170–8179. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Provis, J.L.; van Deventer, J.S.J. Time-resolved and spatially-resolved infrared spectroscopic observation of seeded nucleation controlling geopolymer gel formation. J. Colloid Interface Sci. 2011, 357, 384–392. [Google Scholar] [CrossRef]
- Christophliemk, M.; Pikkarainen, A.; Heponiemi, A.; Tuomikoski, S.; Runtti, H.; Hu, T.; Kantola, A.; Lassi, U. Preparation and characterization of porous and stable sodium- and potassium-based alkali activated material (AAM). Appl. Clay Sci. 2022, 230, 106697. [Google Scholar] [CrossRef]
- Shariati, M.; Shariati, A.; Trung, N.T.; Shoaei, P.; Ameri, F.; Bahrami, N.; Zamanabadi, S.N. Alkali-activated slag (AAS) paste: Correlation between durability and microstructural characteristics. Constr. Build. Mater. 2021, 267, 120886. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S.J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Ceramic-like inorganic polymers. J. Ceram. Sci. Technol. 2017, 8, 335–350. [Google Scholar] [CrossRef]
- Occhipinti, R.; Caggiani, M.C.; de Ferri, L.; Xu, Z.; Steindal, C.C.; Razavi, N.; Andriulo, F.; Mazzoleni, P.; Barone, G. Structural properties of volcanic precursors-based geopolymers before and after natural weathering. Ceram. Int. 2023, 49, 21892–21902. [Google Scholar] [CrossRef]
- Finocchiaro, C.; Barone, G.; Mazzoleni, P.; Cultrone, G. Insight on physical–mechanical properties of one-part alkali-activated materials based on volcanic deposits of Mt. Etna (Italy) and their durability against ageing tests. Mater. Struct. 2024, 57, 198. [Google Scholar] [CrossRef]

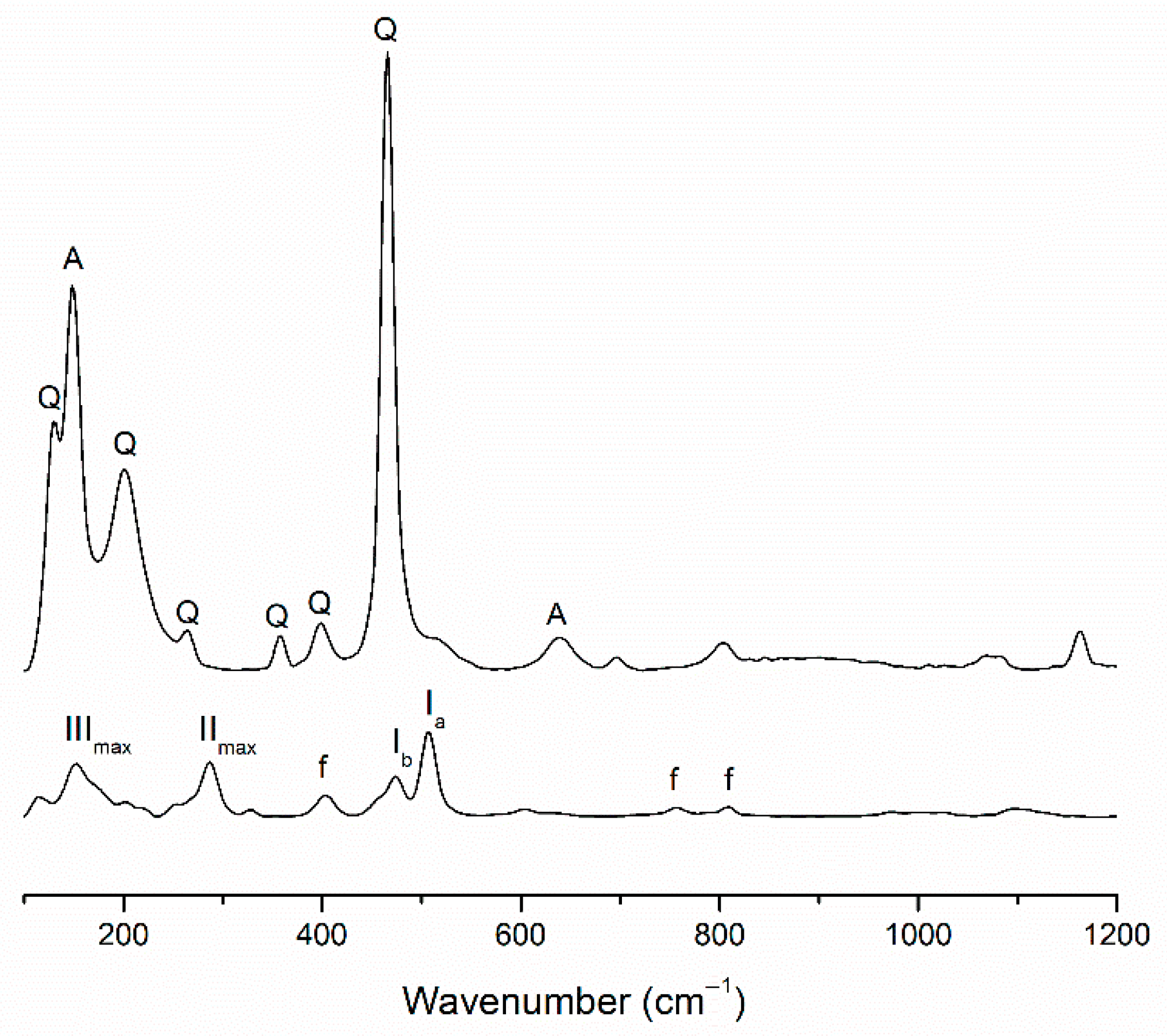

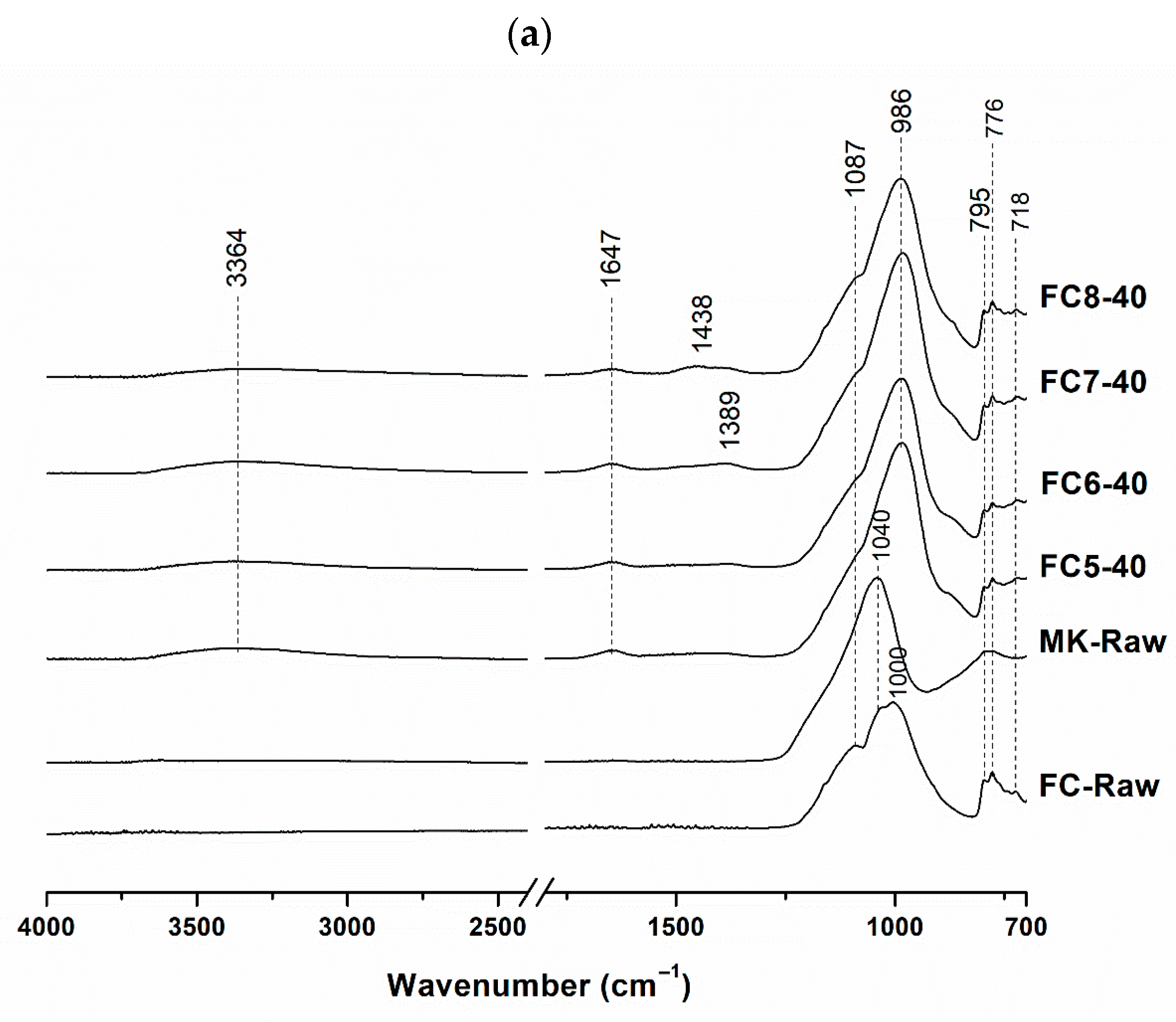
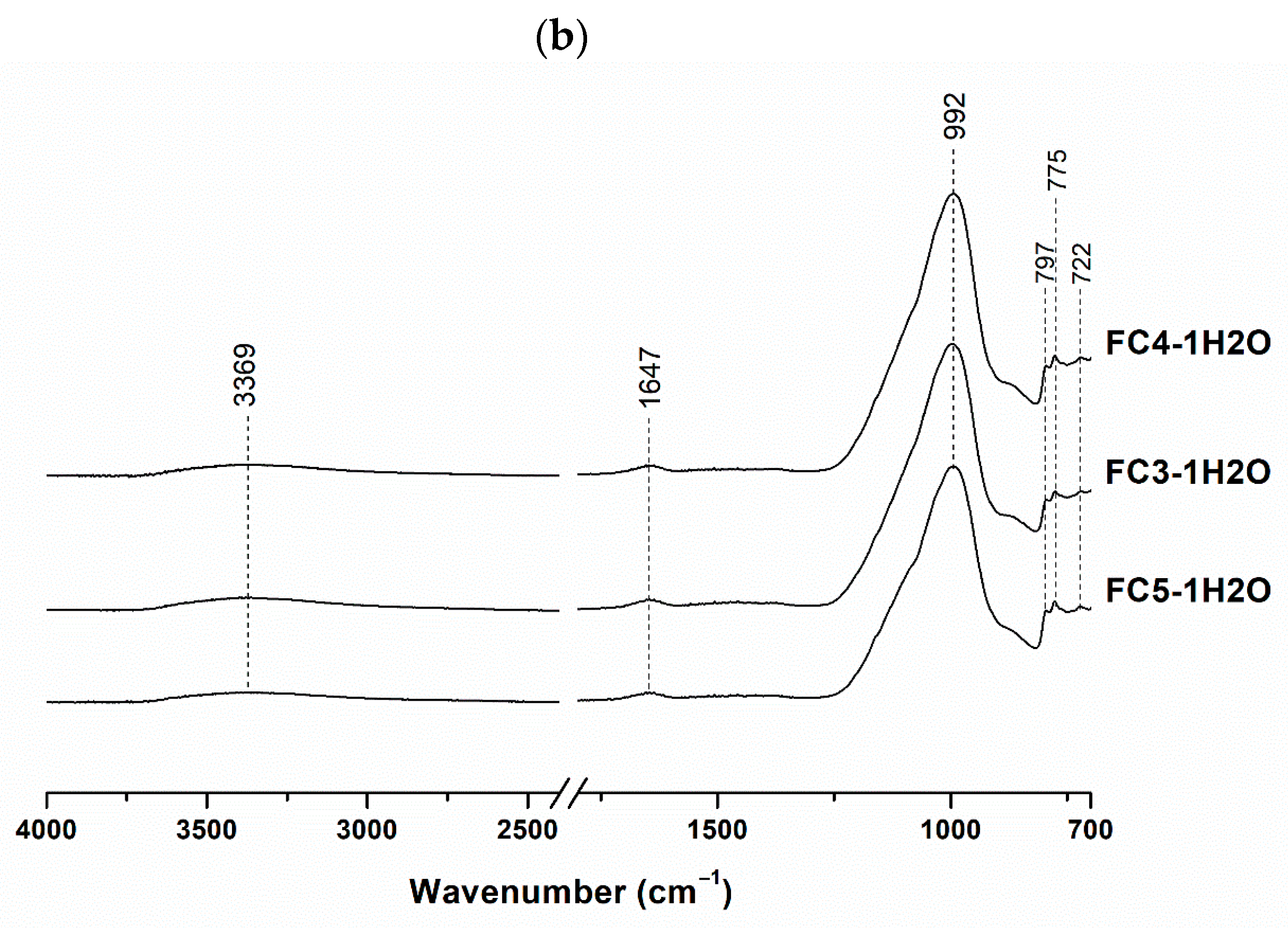
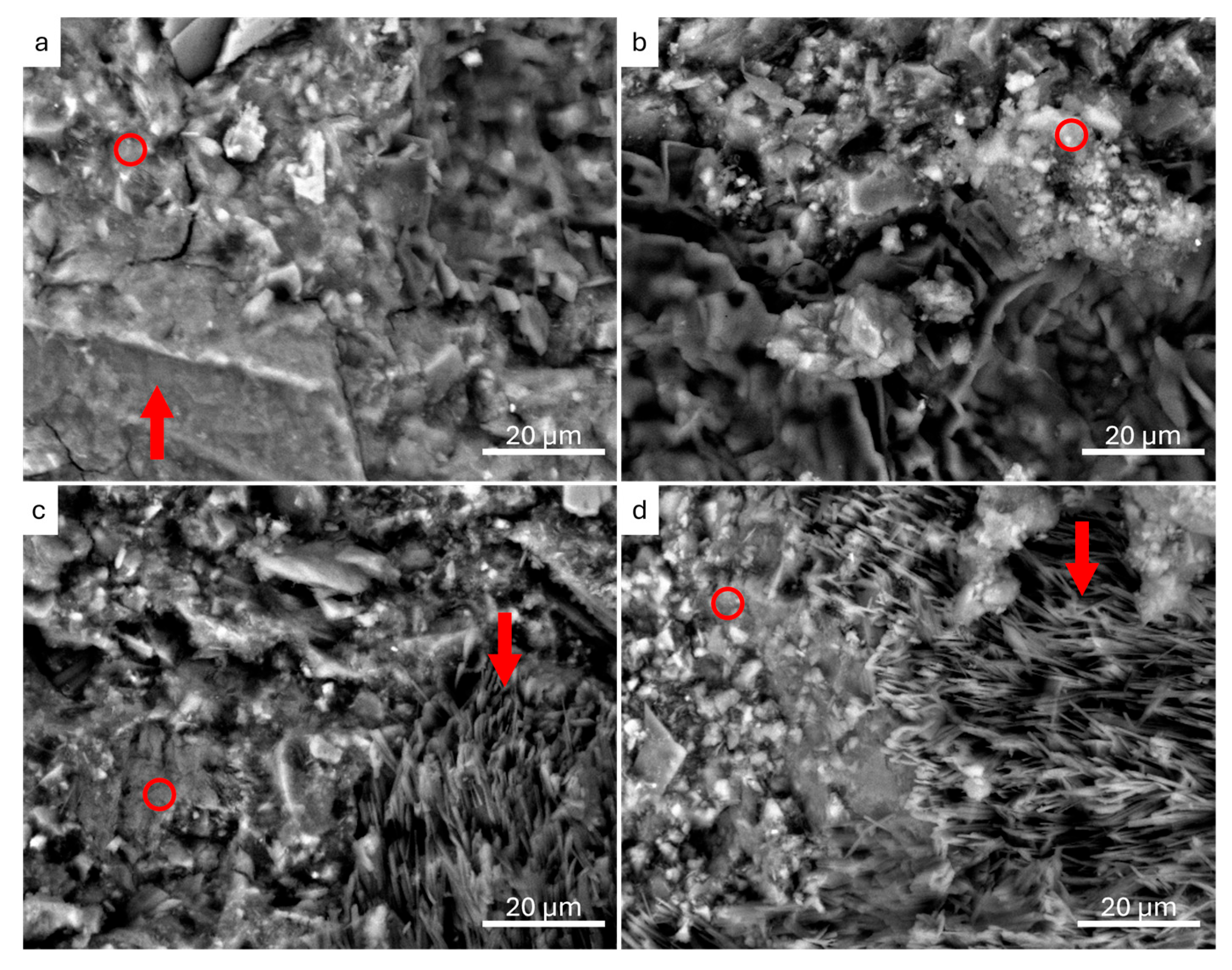
| Sample | FC (wt.%) | MK (wt.%) | NaOH 8 M (wt.%) | Na2SiO3 (wt.%) | H2O (wt.%) | Integrity Test |
|---|---|---|---|---|---|---|
| FC10-4.6 | 100 | - | 100 | - | - | Not passed |
| FC10-20 | 100 | - | 20 | 80 | - | Not passed |
| FC10-30 | 100 | - | 30 | 70 | - | Not passed |
| FC10-40 | 100 | - | 40 | 60 | - | Not passed |
| FC10-50 | 100 | - | 50 | 50 | - | Not passed |
| FC10-60 | 100 | - | 60 | 40 | - | Not passed |
| FC10-70 | 100 | - | 70 | 30 | - | Not passed |
| FC10-80 | 100 | - | 80 | 20 | - | Not passed |
| FC5-40 | 50 | 50 | 40 | 60 | - | Passed |
| FC6-40 | 60 | 40 | 40 | 60 | - | Passed |
| FC7-40 | 70 | 30 | 40 | 60 | - | Passed |
| FC8-40 | 80 | 20 | 40 | 60 | - | Passed |
| FC9-40 | 90 | 10 | 40 | 60 | - | Not passed |
| FC5-50 | 50 | 50 | 50 | 50 | - | Not passed |
| FC6-50 | 60 | 40 | 50 | 50 | - | Not passed |
| FC7-50 | 70 | 30 | 50 | 50 | - | Not passed |
| FC8-50 | 80 | 20 | 50 | 50 | - | Not passed |
| FC9-50 | 90 | 10 | 50 | 50 | - | Not passed |
| FC5-60 | 50 | 50 | 60 | 40 | - | Not passed |
| FC6-60 | 60 | 40 | 60 | 40 | - | Not passed |
| FC7-60 | 70 | 30 | 60 | 40 | - | Not passed |
| FC8-60 | 80 | 20 | 60 | 40 | - | Not passed |
| FC9-60 | 90 | 10 | 60 | 40 | - | Not passed |
| NaOH 9.9 M (wt.%) | ||||||
| FC5-1H2O | 50 | 50 | 25 | 50 | 25 | Passed |
| FC3-1H2O | 30 | 70 | 25 | 50 | 25 | Passed |
| FC4-1H2O | 40 | 60 | 25 | 50 | 25 | Passed |
| FC4-1.5H2O | 40 | 60 | 22.2 | 44.4 | 33.3 | Not passed |
| Sample | Al2O3 | SiO2 | CaO + MgO | Na2O + K2O | Fe2O3 | TiO2 |
|---|---|---|---|---|---|---|
| FC | 12.9 ± 0.8 | 76.36 ± 0.4 | 0.74 ± 0.05 | 8.7 ± 0.4 | 1.3 ± 0.1 | - |
| MK | 35 ± 3.0 | 61 ± 1.7 | 0.26 ± 0.06 | 0.56 ± 0.04 | 1.3 ± 0.1 | 1.7 ± 0.2 |
| Phase [wt.%] | FC-Raw | MK-Raw | FC5-40 | FC6-40 | FC7-40 | FC8-40 | FC5-1H2O | FC3-1H2O | FC4-1H2O |
|---|---|---|---|---|---|---|---|---|---|
| Amorphous | 0.00 | 76.80 | 34.13 | 31.33 | 24.97 | 25.88 | 42.24 | 61.94 | 53.20 |
| Plagioclase | 17.96 | 1.49 | 18.16 | 15.14 | 14.16 | 14.85 | 8.22 | 12.25 | 11.13 |
| Microcline | 35.69 | 0.44 | 13.28 | 17.98 | 21.80 | 22.08 | 14.92 | 4.9 | 8.62 |
| Kaolinite | - | 2.88 | 2.81 | 3.05 | 2.51 | 2.26 | 2.95 | 1.9 | 1.98 |
| Muscovite | 1.24 | 1.67 | 1.69 | 0.94 | 1.03 | 1.70 | 0.64 | 1.13 | 1.15 |
| Quartz | 42.71 | 14.46 | 28.80 | 30.39 | 33.86 | 32.06 | 29.88 | 16.95 | 23.22 |
| Biotite | 1.49 | 1.49 | 0.59 | 0.27 | 0.9 | 0.35 | 0.41 | 0.30 | 0.36 |
| Anatase | - | 0.77 | 0.34 | 0.63 | 0.64 | 0.61 | 0.70 | 0.45 | 0.21 |
| Hematite | 0.91 | - | 0.18 | 0.26 | 0.13 | 0.19 | 0.04 | 0.18 | 0.13 |
| Sample | wt.% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | Si | Al | Si/Al | Mg | P | K | Ca | Ti | Mn | Fe | |
| FC5-40 | 6.1 | 62.8 | 25.9 | 2.42 | 0.2 | 0.3 | 2.1 | 0.5 | 0.6 | 0.2 | 1.3 |
| FC6-40 | 4.4 | 61.7 | 19.5 | 3.16 | 0.1 | 0.05 | 13.3 | 0.3 | 0.2 | 0.07 | 0.4 |
| FC7-40 | 4.5 | 64 | 19.3 | 3.31 | 0.1 | 0.4 | 11.5 | 0 | 0 | 0 | 0.2 |
| FC8-40 | 3.3 | 58.2 | 13.7 | 4.24 | 0.2 | 0.1 | 21.4 | 0.6 | 0.7 | 0.3 | 1.5 |
| FC5-1H2O | 8.1 | 74 | 15.5 | 4.77 | 0.2 | 0 | 1.1 | 0.1 | 0.2 | 0.08 | 0.7 |
| FC3-1H2O | 3.1 | 80.1 | 14.7 | 5.44 | 0.2 | 0.05 | 0.6 | 0.1 | 0.4 | 0.09 | 0.7 |
| FC4-1H2O | 4.6 | 56 | 34.8 | 1.60 | 0.3 | 0.2 | 1.1 | 0.3 | 1.1 | 0 | 1.6 |
| ID | Compressive Strength [MPa] | Standard Deviation |
|---|---|---|
| FC5-40 | 27.63 | 2.95 |
| FC6-40 | 29.37 | 2.77 |
| FC7-40 | 23.07 | 1.73 |
| FC8-40 | 22.23 | 3.52 |
| FC5-1H2O | 18.63 | 2.2 |
| FC3-1H2O | 24.87 | 0.95 |
| FC4-1H2O | 22.64 | 2.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafarana, S.E.; Achilli, A.; Barone, G.; Bersani, D.; Finocchiaro, C.; Fornasini, L.; Portale, S.; Mazzoleni, P. Exploring the Potential of Granite Sawing Sludge from Cuasso Al Monte (Italy) for the Development of Aluminosilicate Gel for a Sustainable Industry. Minerals 2025, 15, 718. https://doi.org/10.3390/min15070718
Zafarana SE, Achilli A, Barone G, Bersani D, Finocchiaro C, Fornasini L, Portale S, Mazzoleni P. Exploring the Potential of Granite Sawing Sludge from Cuasso Al Monte (Italy) for the Development of Aluminosilicate Gel for a Sustainable Industry. Minerals. 2025; 15(7):718. https://doi.org/10.3390/min15070718
Chicago/Turabian StyleZafarana, Sabrina Elettra, Alessandro Achilli, Germana Barone, Danilo Bersani, Claudio Finocchiaro, Laura Fornasini, Silvia Portale, and Paolo Mazzoleni. 2025. "Exploring the Potential of Granite Sawing Sludge from Cuasso Al Monte (Italy) for the Development of Aluminosilicate Gel for a Sustainable Industry" Minerals 15, no. 7: 718. https://doi.org/10.3390/min15070718
APA StyleZafarana, S. E., Achilli, A., Barone, G., Bersani, D., Finocchiaro, C., Fornasini, L., Portale, S., & Mazzoleni, P. (2025). Exploring the Potential of Granite Sawing Sludge from Cuasso Al Monte (Italy) for the Development of Aluminosilicate Gel for a Sustainable Industry. Minerals, 15(7), 718. https://doi.org/10.3390/min15070718









