Zoharite, (Ba,K)6 (Fe,Cu,Ni)25S27, and Gmalimite, K6□Fe2+24S27—New Djerfisherite Group Minerals from Gehlenite-Wollastonite Paralava, Hatrurim Complex, Israel
Abstract
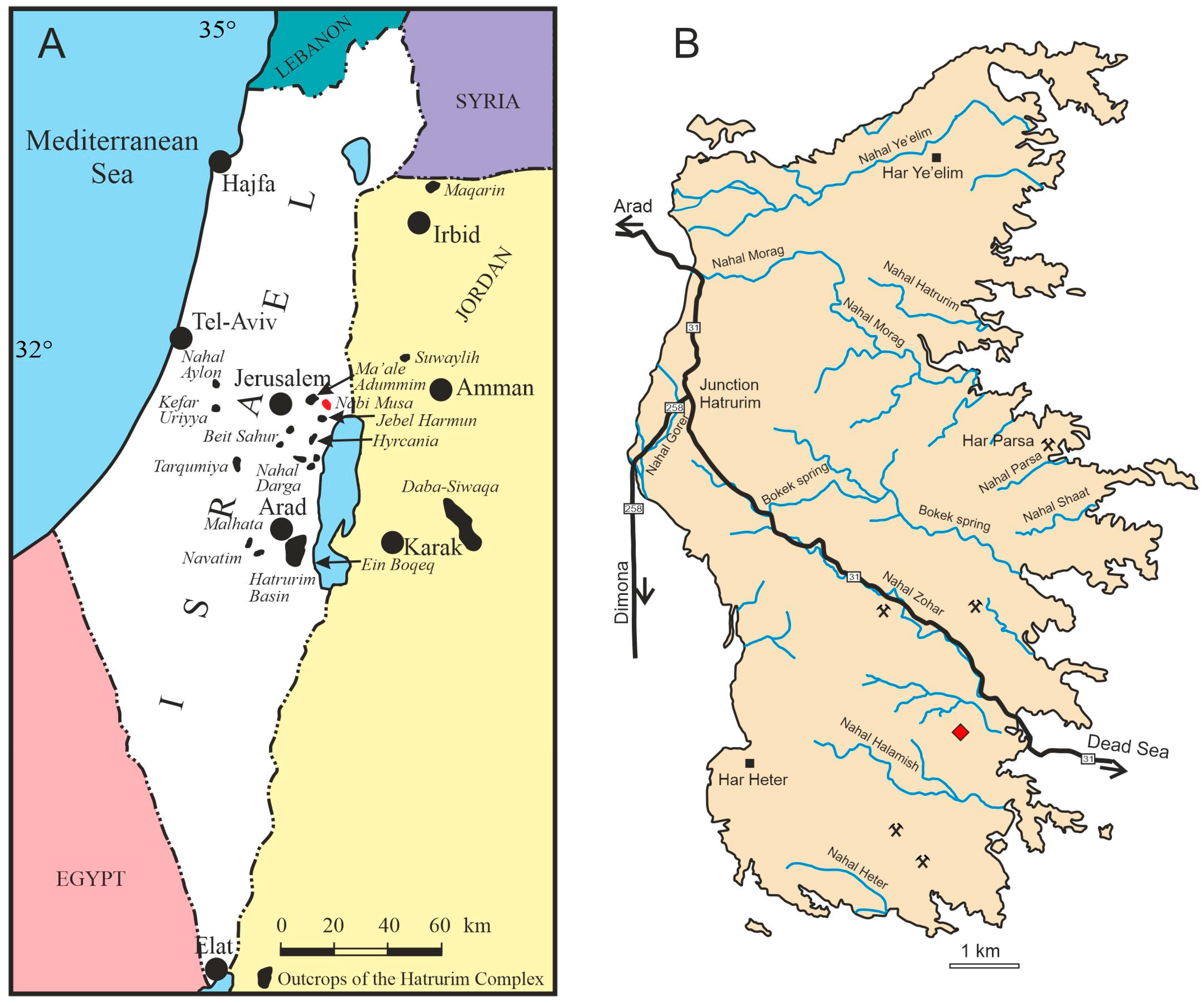
1. Introduction
2. Materials and Methods
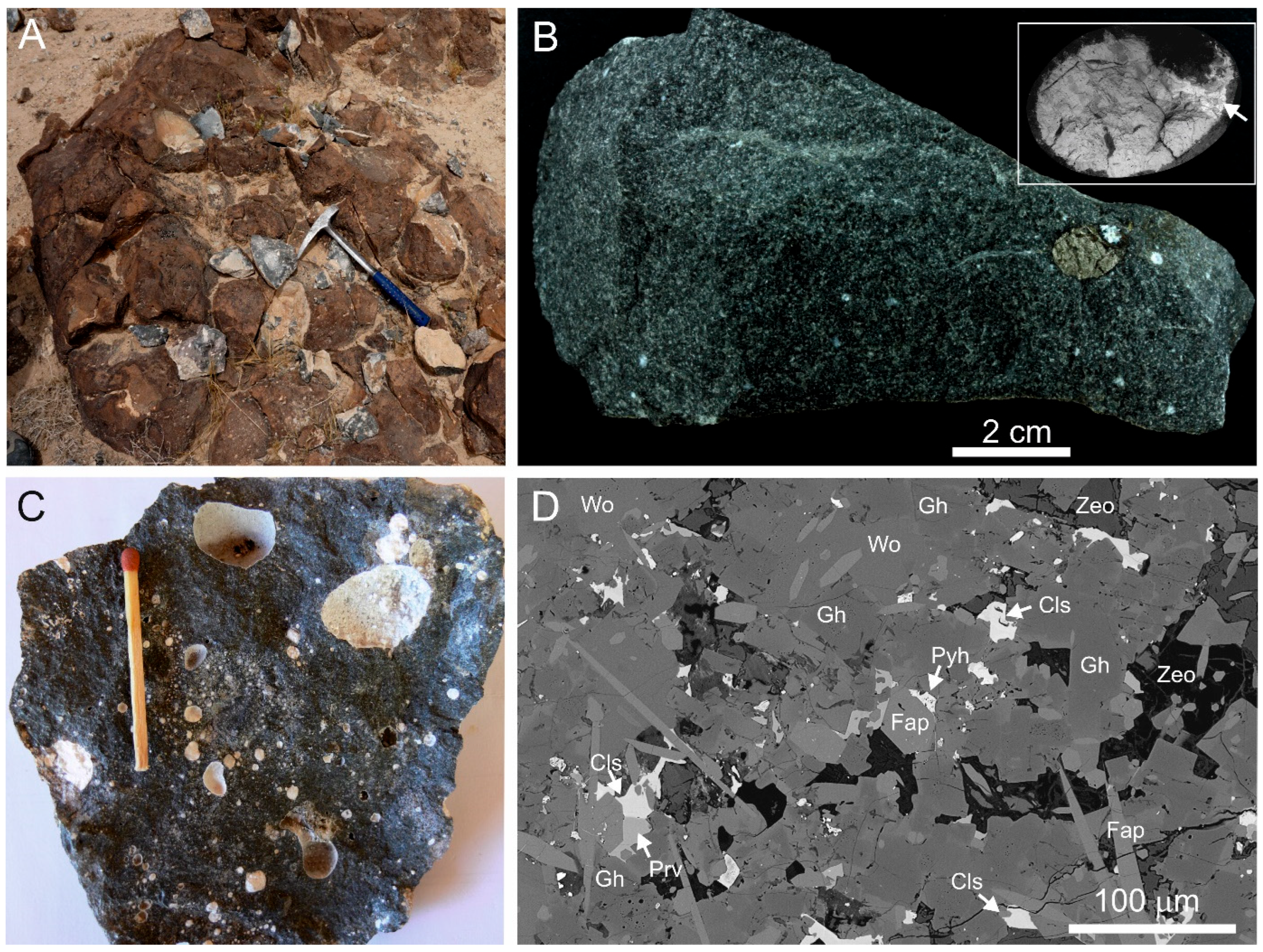
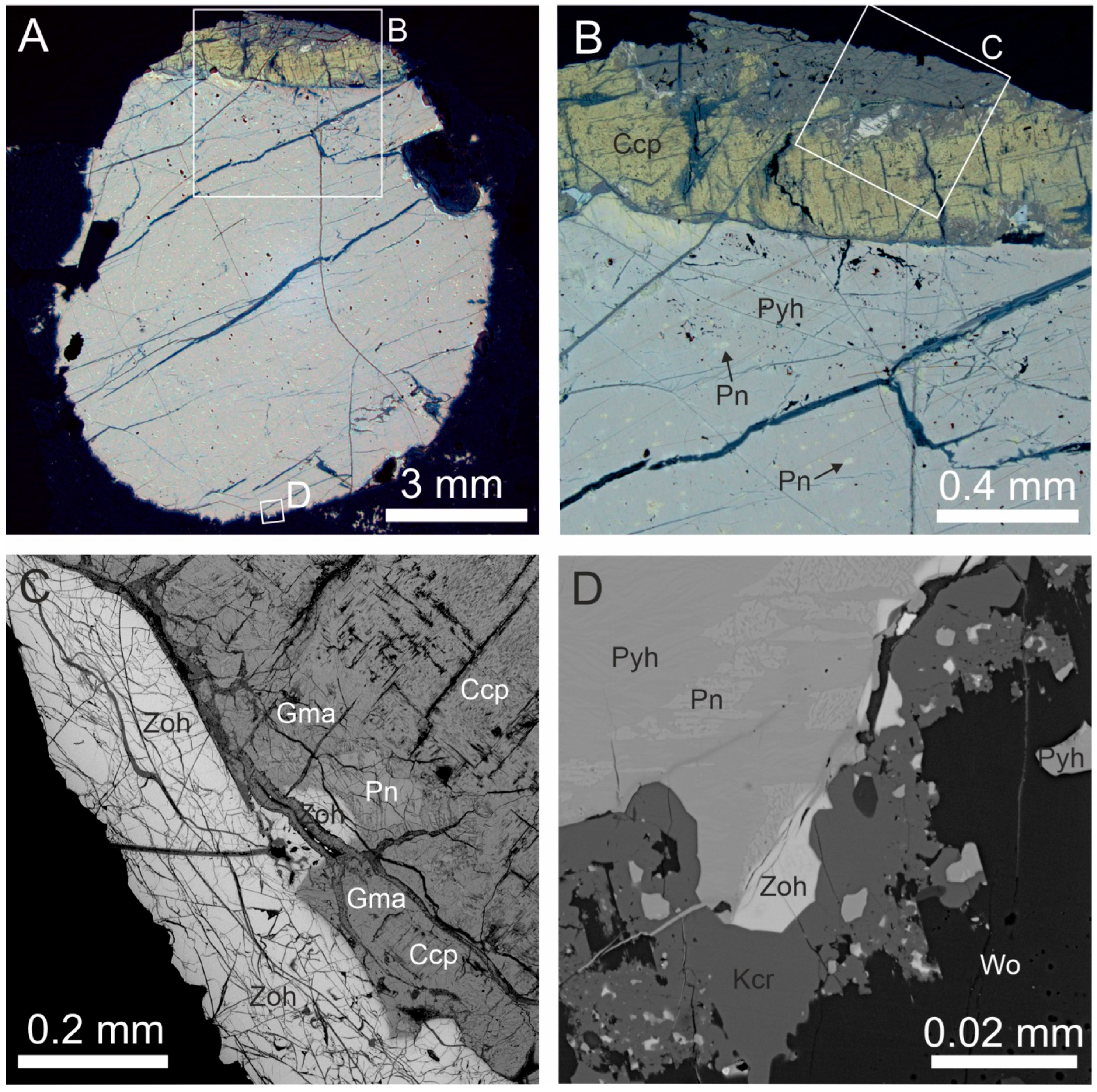
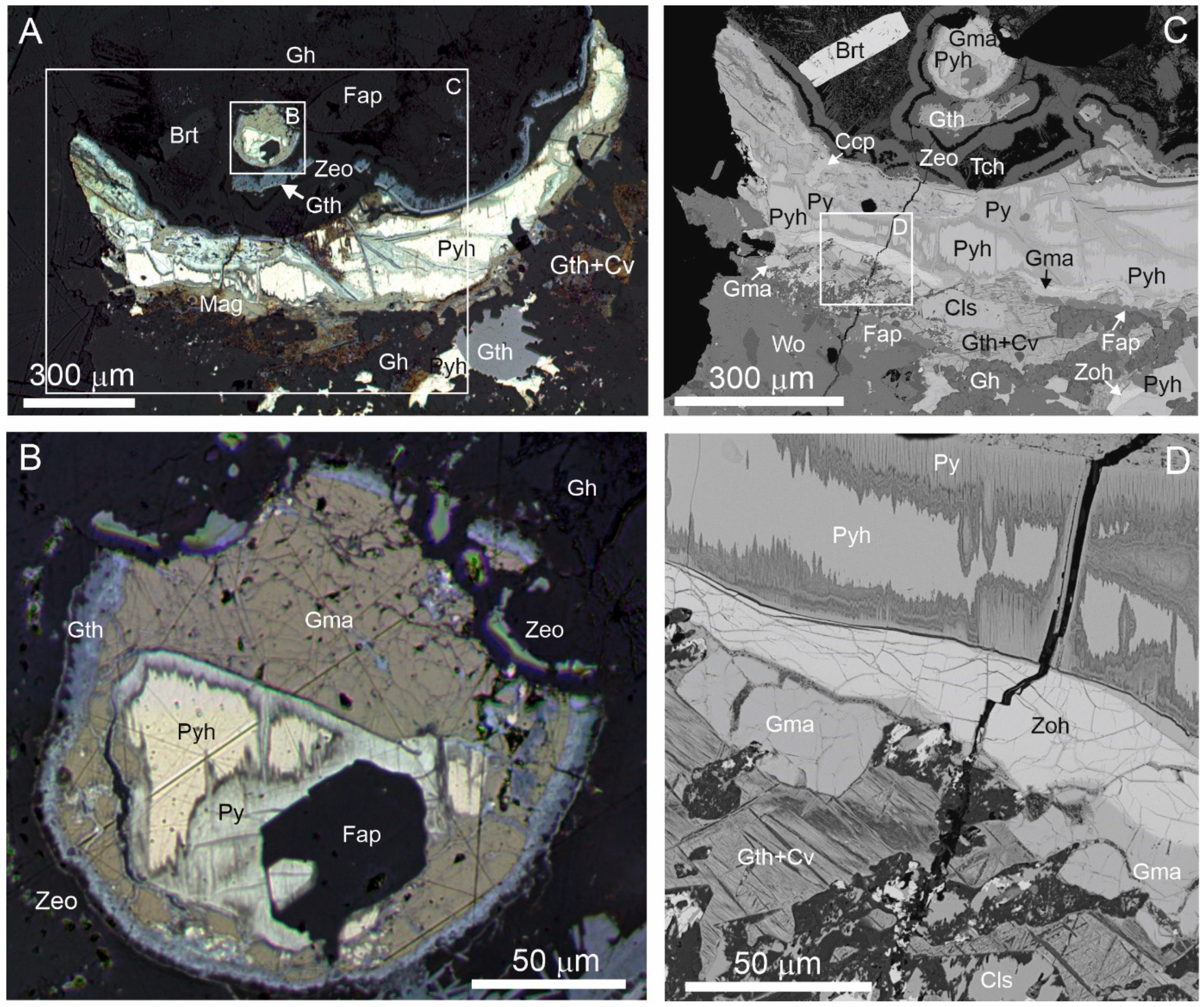
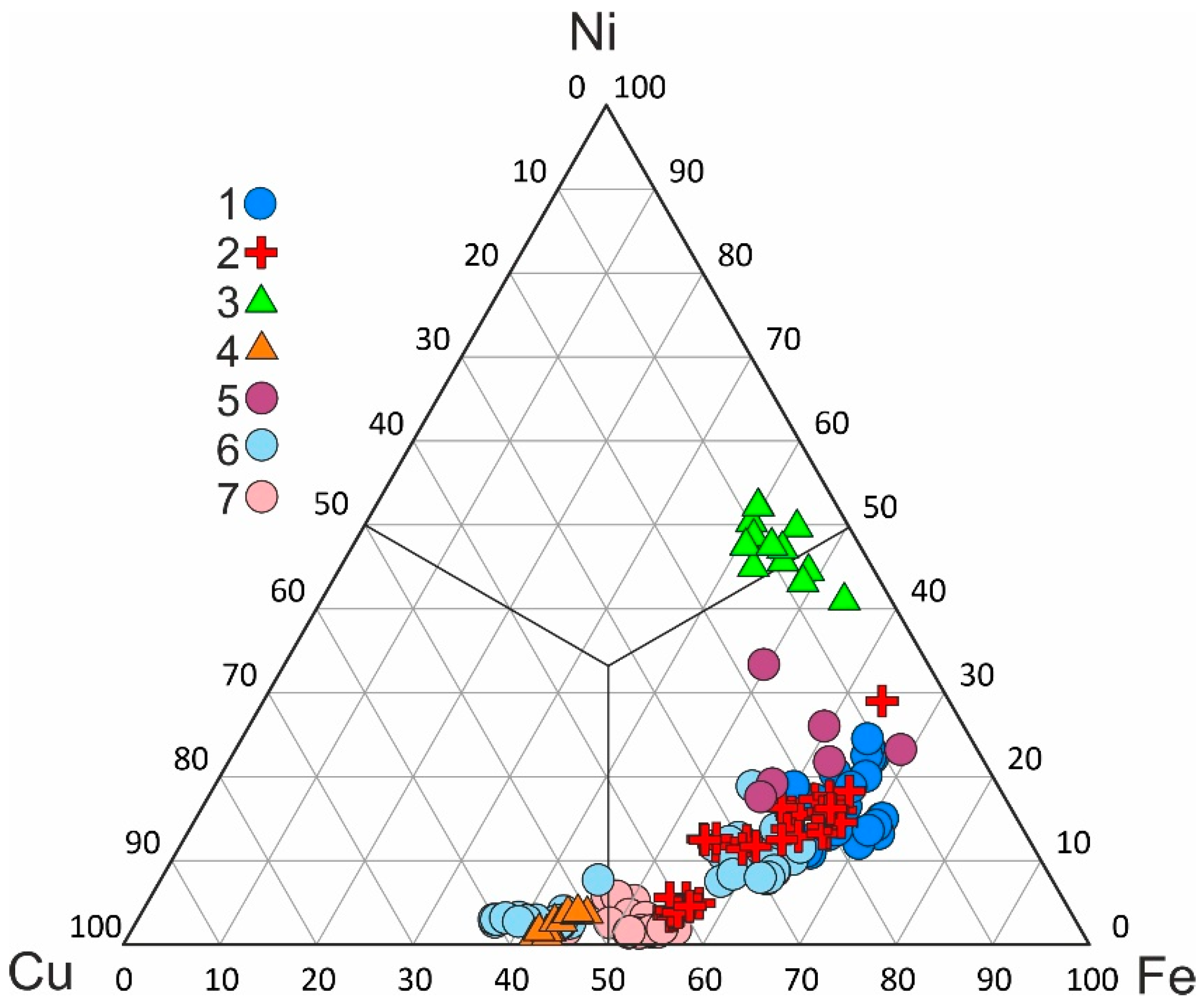
3. Occurrence
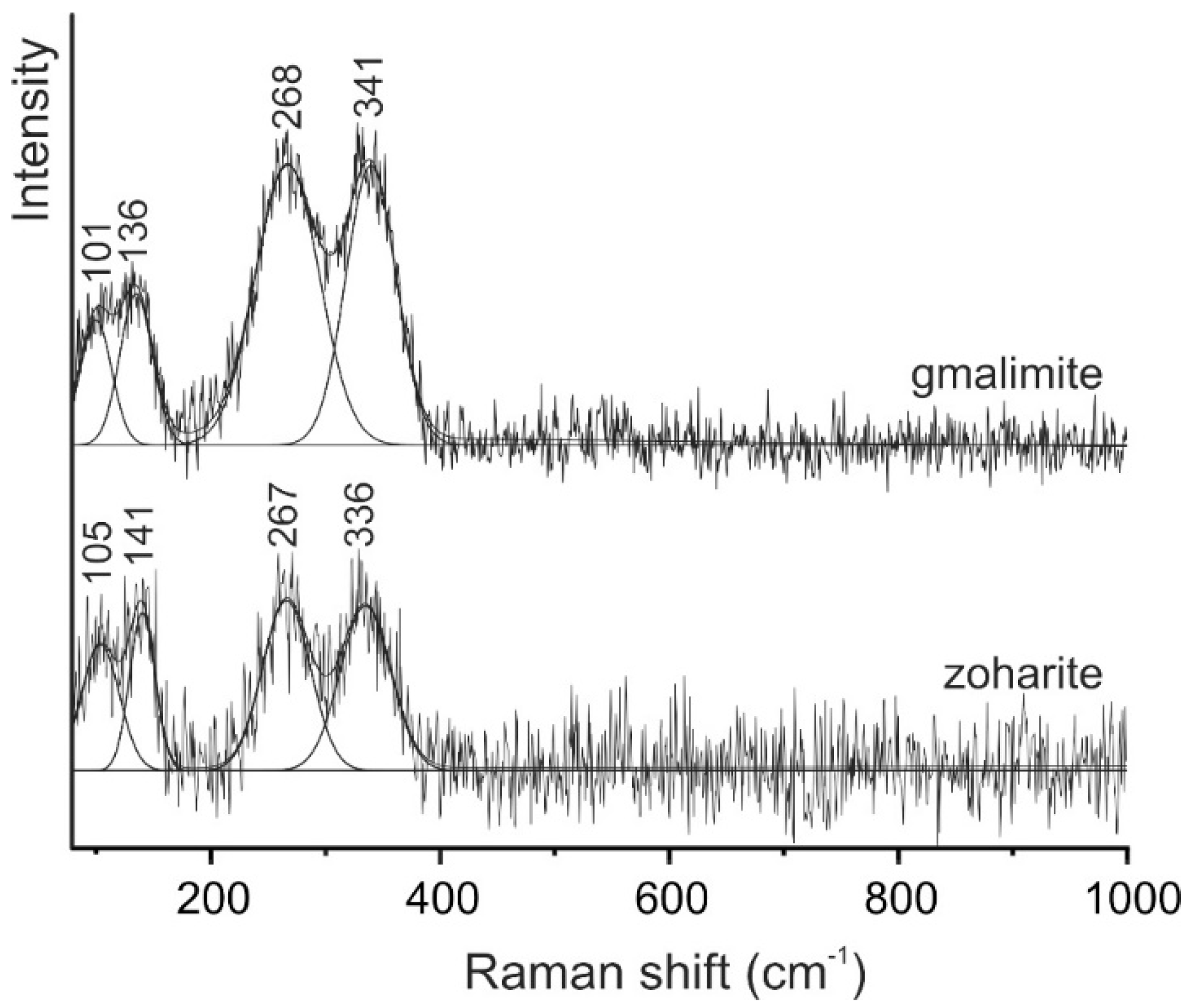
4. Raman Spectroscopy Study of Zoharite and Gmalimite
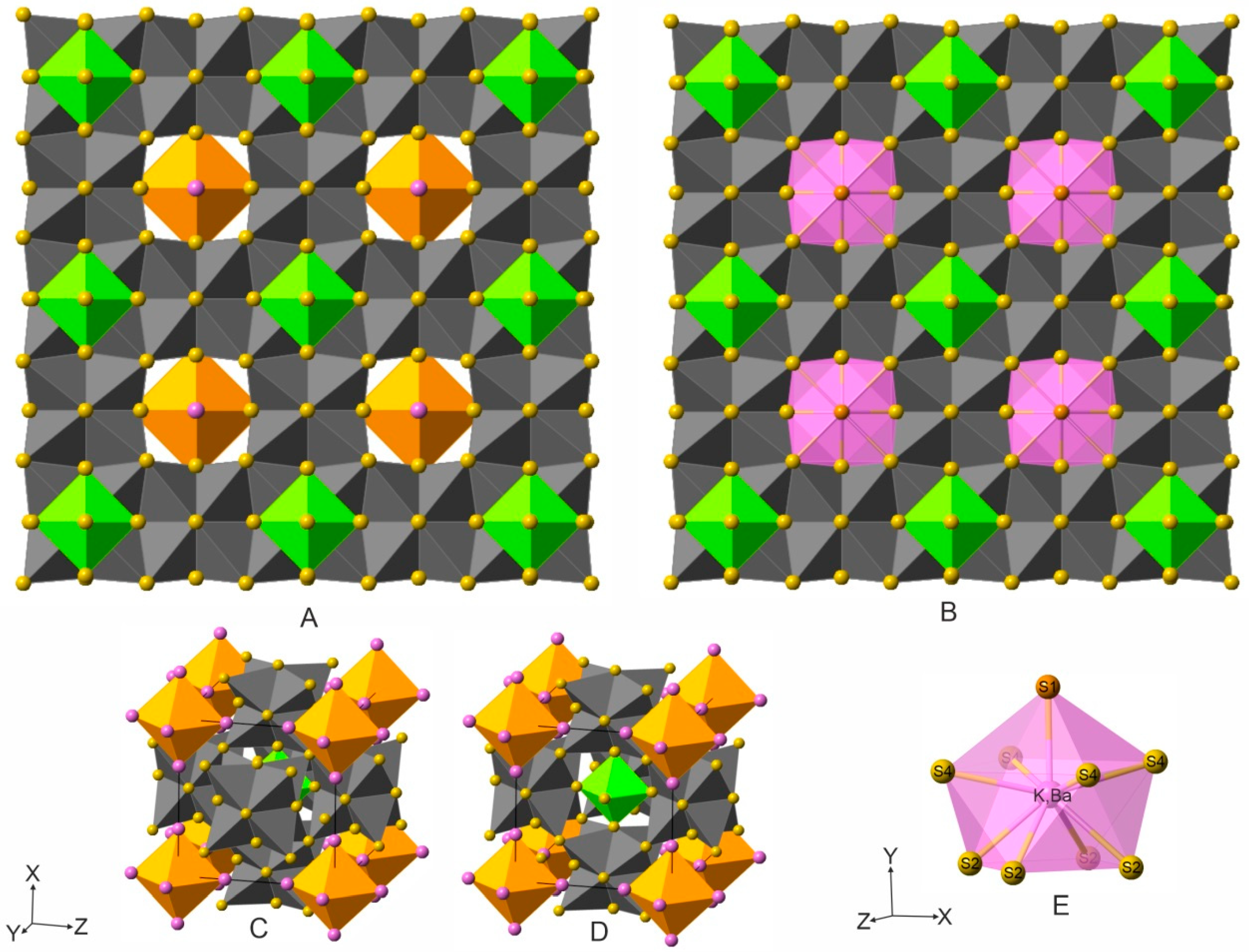
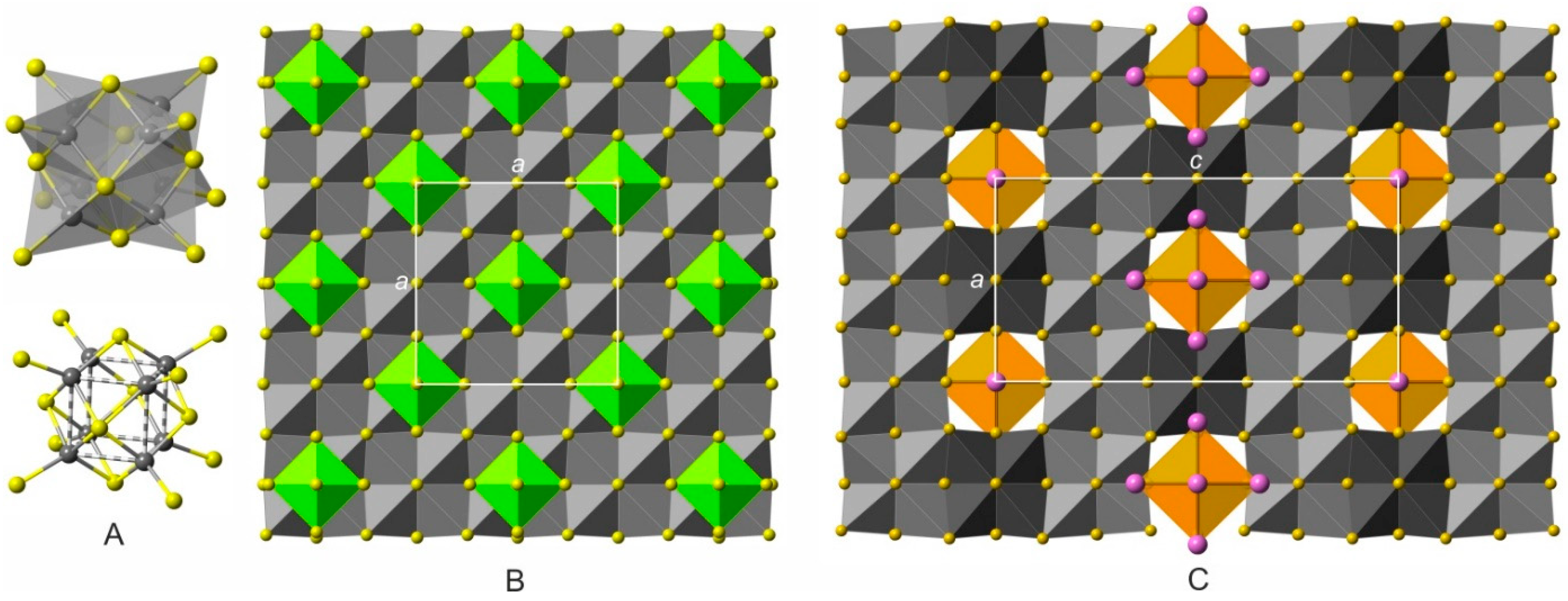
5. Crystal Structure of Zoharite and Gmalimite
6. Discussion
6.1. Comments on Crystal Chemistry of Minerals of the Djerfisherite Group
6.2. Comments on Genesis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuchs, L.H. Djerfisherite, alkali copper–iron sulfide: A new mineral from enstatite chondrites. Science 1966, 153, 166–167. [Google Scholar] [CrossRef]
- Rudashevskii, N.S.; Karpenov, A.M.; Shipova, G.S.; Shishkin, N.N.; Ryabkin, V.A. Thalfenisite, the thallium analog of djerfisherite. ZVMO 1979, 108, 696–701. (In Russian) [Google Scholar] [CrossRef]
- Laflamme, J.H.G.; Roberts, A.C.; Criddle, A.J.; Cabri, L.J. Owensite, (Ba,Pb)6(Cu, Fe, Ni)25S27, a new mineral species from the Wellgreen Cu-Ni-Pt-Pd deposit, Yukon. Can. Mineral. 1995, 33, 665–670. [Google Scholar]
- Szymański, J.T. The crystal structure of owensite, (Ba,Pb)6(Cu, Fe, Ni)25S27, a new member of the djerfisherite group. Can. Mineral. 1995, 33, 671–677. [Google Scholar]
- Genkin, A.D.; Troneva, N.V.; Zhuravlev, N.N. The first finding in ore a K-Fe-Cu sulfide—Djerfisherite. Geol. Ore Deposits 1969, 5, 57–64. (In Russian) [Google Scholar]
- Sharygin, V.V.; Golovin, A.V.; Pokhilenko, N.P.; Sobolev, N.V. Djerfisherite in unaltered kimberlites of the Udachnaya-East pipe, Yakutia. Dokl. Earth Sci. 2003, 390, 554–557. [Google Scholar]
- Sharygin, V.V.; Golovin, A.V.; Pokhilenko, N.P.; Kamenetsky, V.S. Djerfisherite in the Udachnaya-East pipe kimberlites (Sakha-Yakutia,Russia): Paragenesis, composition and origin. Eur. J. Mineral. 2007, 19, 51–63. [Google Scholar] [CrossRef]
- Sharygin, I.S.; Golovin, A.V.; Pokhilenko, N.P. Djerfisherite in kimberlites of the Kuoyksky field as an indicator of chlorine enrichment of kimberlite melts. Dokl. Akad. Nauk. Geochem. 2011, 436, 820–826. [Google Scholar]
- Abersteiner, A.; Kamenetsky, V.S.; Goemann, K.; Golovin, A.V.; Sharygin, I.S.; Giuliani, A.; Rodemann, T.; Spetsius, Z.V.; Kamenetsky, M. Djerfisherite in kimberlites and their xenoliths: Implications for kimberlite melt evolution. Contrib. Mineral. Petr. 2019, 174, 8. [Google Scholar] [CrossRef]
- Xu, J.; Melgarejo, J.C.; Li, Q.; Torró i Abat, L.; Castillo-Oliver, M. Magma mingling in kimberlites: Evidence from the groundmass cocrystallization of two spinel-group minerals. Minerals 2020, 10, 829. [Google Scholar] [CrossRef]
- Zaccarini, F.; Thalhammer, O.A.R.; Princivalle, F.; Lenaz, D.; Stanley, C.J.; Garuti, D. Djerfisherite in the Guli dunite complex, Polar Siberia: A primary or metasomatic phase? Can. Mineral. 2007, 45, 1201–1211. [Google Scholar] [CrossRef]
- Sharygin, V. Mineralogy of metacarbonate xenolith from alkali basalt, E. Eifel, Germany. Conference: Geochemistry of magmatic rocks-2012. In Proceedings of the 29th International Conference, School “Geochemistry of Alkaline Rocks”, Moscow, Russia, September 2012; pp. 95–97. [Google Scholar] [CrossRef]
- Sokol, E.V.; Deviatiiarova, A.S.; Kokh, S.N.; Reutsky, V.N.; Abersteiner, A.; Philippova, K.A.; Artemyev, D.A. Sulfide minerals as potential tracers of isochemical processes in contact metamorphism: Case study of the Kochumdek Aureole, East Siberia. Minerals 2021, 11, 17. [Google Scholar] [CrossRef]
- Kogarko, L.N.; Plant, D.A.; Henderson, C.M.B.; Kjarsgaard, B.A. Na-rich carbonate inclusions in perovskite and calzirtite from the Guli intrusive Ca-carbonatite, Polar Siberia. Contrib. Mineral. Petr. 1991, 109, 124–129. [Google Scholar] [CrossRef]
- Takechi, Y.; Kusachi, I.; Nakamuta, Y.; Kase, K. Nickel-bearing djerfisherite in gehlenite-spurrite skarn at Kushiro, Hiroshima prefecture, Japan. Resour. Geol. 2000, 50, 179–184. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Laajoki, K.V.O.; Gehor, S.A.; Yakovlev, Y.N.; Taikina-Aho, O. Chlorine-poor an analogues of djerfisherite-thalfenisite from Noril’sk, Siberia and Salmagorsky, Kola Peninsula, Russia. Can. Mineral. 1997, 35, 1421–1430. [Google Scholar]
- Barkov, A.Y.; Martin, R.F.; Cabri, L.J. Rare sulfides enriched in K, Tl and Pb from the Noril’sk and Salmagorsky complexes, Russia: New data and implications. Mineral. Mag. 2015, 79, 799–808. [Google Scholar] [CrossRef]
- Sharygin, V.V. K-Pb- and K-Ba-Phases of the Djerfisherite Group in High-Calcium Rocks. In Proceedings of the XXXIII International Conference “Alkaline Magmatism of the Earth and Related Strategic Metal Deposits”, School “Alkaline Magmatism of the Earth”, Moscow, Russia, 27 May 2016; pp. 150–152. [Google Scholar]
- Savina, E.A.; Peretyazhko, I.S.; Khromova, E.A.; Glushkova, V.E. Melted Rocks (Clinkers and Paralavas) from the Khamaryn-Khural-Khiid Combustion Metamorphic Complex in Eastern Mongolia: Mineralogy, Geochemistry and Genesis. Petroleum 2020, 28, 431–457. [Google Scholar] [CrossRef]
- Czamanske, G.K.; Erd, R.C.; Sokolova, M.N.; Dobovol’skaya, M.G.; Dmitrieva, M.T. New data on rasvumite and djerfisherite. Am. Mineral. 1979, 64, 776–778. [Google Scholar]
- Czamanske, G.K.; Erd, R.C.; Leonard, B.F.; Clark, J.R. Bartonite, a new potassium iron sulfide mineral. Am. Mineral. 1981, 66, 369–375. [Google Scholar]
- Dobrovolskaya, M.G.; Tsepin, A.I.; Evstigneeva, T.L. Muruskite K2Cu3FeS4—A new patasium, cuprum and iron sulfide. ZVMO 1979, 110, 468–473. (In Russian) [Google Scholar]
- Yakovenchuk, V.N.; Pakhomovsky, Y.P.; Men’shikov, Y.P.; Ivanyuk, G.Y.; Krivovichev, S.V.; Burns, P.C. Chlorbartonite, K6Fe24S26(Cl,S), a new mineral species from a hydrothermal vein in the Khibina massif, Kola Peninsula, Russia: Description and crystal structure. Can. Mineral. 2003, 41, 503–511. [Google Scholar] [CrossRef]
- Ostrooumov, M.; Arellano-Jimenez, M.; Ponce, A.; Taran, Y.; Reyes-Gasga, J. La colimaíta, K3VS4, un nuevo mineral del volcán Colima (México). Bol. Mineral. 2008, 18, 7–8. [Google Scholar]
- Burg, A.; Starinsky, A.; Bartov, Y.; Kolodny, Y. Geology of the Hatrurim Formation (“Mottled Zone”) in the Hatrurim basin. Israel J. Earth Sci. 1992, 40, 107–124. [Google Scholar]
- Bentor, Y.K.; Vroman, A.; Zak, I. Geological Map of Israel. Scale 1:250,000. Southern Sheet. Geol. Surv. Israel 1965, Jerusalem, sheets 1–2. [Google Scholar]
- Wojdyla, J.A.; Kaminski, J.W.; Panepucci, E.; Ebner, S.; Wang, X.; Gabadinho, J.; Wang, M. DA+ data acquisition and analysis softwareat the Swiss Light Source macromolecularcrystallography beamlines. J. Synchrotron. Radiat. 2018, 25, 293–303. [Google Scholar] [CrossRef]
- Rigaku. CrysAlisPro, 171.42.70a; Rigaku Oxford Diffraction Ltd.: Oxfordshire, UK, 2016. [Google Scholar]
- Kabsch, W. XDS. Acta Crystallogr. D 2010, D66, 125–132. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Gross, S. The mineralogy of the Hatrurim Formation, Israel. Geol. Surv. Israel B 1977, 70, 1–80. [Google Scholar]
- Vapnik, Y.; Sharygin, V.V.; Sokol, E.V.; Shagam, R. Paralavas in a combustion metamorphic complex: Hatrurim Basin, Israel. In GSA Reviews in Engineering Geology; Geological Society of America: Boulder, CO, USA, 2007; Volume 18, pp. 1–22. [Google Scholar]
- Geller, Y.I.; Burg, A.; Halicz, L.; Kolodny, Y. System closure during the combustion metamorphic “Mottled Zone” event, Israel. Chem. Geol. 2012, 334, 25–36. [Google Scholar] [CrossRef]
- Sharygin, V.V.; Vapnik, Y.; Sokol, E.V.; Kamenetsky, V.S.; Shagam, R. Melt Inclusions in Minerals of Schorlomite-Rich Veins of the Hatrurim Basin, Israel: Composition and Homogenization Temperatures. In Proceedings of the ACROFI-1, Nanjing, China, 26–28 May 2006; pp. 189–192. [Google Scholar]
- Krzątała, A.; Krüger, B.; Galuskina, I.; Vapnik, Y.; Galuskin, E. Walstromite, BaCa2(Si3O9), from rankinite paralava within gehlenite hornfels of the Hatrurim Basin, Negev Desert, Israel. Minerals 2020, 10, 407. [Google Scholar] [CrossRef]
- Sharygin, V.V. A hibonite-spinel-corundum-hematite assemblage in plagioclase-clinopyroxene pyrometamorphic rocks, Hatrurim Basin, Israel: Mineral chemistry, genesis and formation temperatures. Mineral. Mag. 2018, 83, 123–135. [Google Scholar] [CrossRef]
- Galuskina, I.O.; Galuskin, E.V.; Vapnik, Y.; Prusik, K.; Stasiak, M.; Dzierżanowski, P.; Murashko, M. Gurimite, Ba3(VO4)2, and hexacelsian, BaAl2Si2O8—Two new minerals from schorlomite-rich paralava of the Hatrurim Complex, Negev Desert, Israel. Mineral. Mag. 2016, 81, 1009–1019. [Google Scholar] [CrossRef]
- Golovin, A.V.; Goryainov, S.V.; Kokh, S.N.; Sharygin, I.S.; Rashchenko, S.V.; Kokh, K.A.; Sokol, E.V.; Devyatiyarova, A.S. The application of Raman spectroscopy to djerfisherite identification. J. Raman Spectrosc. 2017, 48, 1574–1582. [Google Scholar] [CrossRef]
- Ilinca, G. Distribution and Bond Valence Sum analysis of sulfosalts—The ECoN21 Computer Program. Minerals 2022, 12, 924. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Für Krist. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Stacey, T.E.; Borg, C.K.H.; Zavalijb, P.J.; Rodriguez, E.E. Magnetically stabilized Fe8(μ4-S)6S8 clusters in Ba6Fe25S27. Dalton Trans. 2014, 43, 14612. [Google Scholar] [CrossRef]
- Gelabert, M.C.; Ho, M.H.; Malik, A.-S.; DiSalvo, F.J.; Deniard, P.; Brec, R. Structure and properties of Ba6Ni25S27. Chem. Eur. J. 1997, 3, 1884–1889. [Google Scholar] [CrossRef]
- Snyder, G.J.; Badding, M.E.; Di Salvo, F.J. Synthesis, structure, and properties of Ba6Co25S27: A Perovskite-like superstructure of Co8S6 and Ba6S clusters. Inorg. Chem. 1992, 31, 2107–2110. [Google Scholar] [CrossRef]
- Burdett, J.K.; Miller, G.J. Polyhedral clusters insolids. The electronic structure of pentlandite. J. Am. Chem. Soc. 1987, 109, 4081–4091. [Google Scholar] [CrossRef]
- Patten, C.; Barnes, S.-J.; Mathez, E.A.; Jenner, F.E. Partition coefficients of chalcophile elements between sulfide and silicate melts and the early crystallization history of sulfide liquid: LA-ICP-MS analysis of MORB sulfide droplets. Chem. Geol. 2013, 358, 170–188. [Google Scholar] [CrossRef]
| Mineral | Gmalimite | Zoharite |
|---|---|---|
| Refined formula | (K5.05 Ba0.04□0.91) Σ6(Fe0.39□0.61) Σ1 (Fe15.31Cu5.76 Ni2.93)Σ24S27 | (Ba3.91K2.09)(Fe0.97□0.03)Σ1 (Fe15.1Cu6.5□2.4)Σ24 S27 |
| Crystal system | cubic | |
| Unit cell dimensions (Å) | a = b = c = 10.34863(8) α = β = γ = 90° | a = b = c = 10.3137(1) α = β = γ = 90° |
| Space group | m (no. 221) | |
| Volume (Å3) | 1108.28(3) | 1097.09(3) |
| Z | 1 | |
| Density (calculated) g/cm3 | 3.720 | 4.227 |
| Crystal size (μm) | 50 × 40 × 20 | 50 × 40 × 40 |
| Data collection | ||
| Diffractometer | beamline X06DA, Swiss Light Source | |
| SLS one-axis goniometer Aerotech | multi-axis goniometer PRIGo | |
| λ = 0.70849 Å | ||
| Detector/det. distance | PILATUS 2M-F/80 mm | |
| Exposure time (s)/step size (°) | 0.1/0.1 | |
| Number of frames | 1800 | |
| Max. θ range for data collection (°) | 33.28 | 31.92 |
| Index ranges | −15 ≤ h ≤ 15 | −10 ≤ h ≤ 11 |
| −14 ≤ k ≤ 14 | −14 ≤ k ≤ 8 | |
| −15 ≤ l ≤ 16 | −15 ≤ l ≤ 15 | |
| No. of measured reflections,I > 2\σ(I) | 4636 | 6508 |
| No. of unique reflections,I > 2\σ(I) | 428 | 436 |
| Refinement of the structure | ||
| no. of parameters | 24 | 24 |
| Rint | 0.0397 | 0.0112 |
| R1(obs)/R1(all) | 0.0364/0.0408 | 0.0122/0.0123 |
| wR2(obs)/wR2(all) | 0.0974/0.1020 | 0.0286/0.0286 |
| GOF | 1.22 | 1.193 |
| Δρ min. (e Å−3) | −1.74 (1.75 Å from S4) | −0.50 from S3 |
| Δρ max. (e Å−3) | 1.85 (0.38 Å from S1) | 0.30 from S2 |
| λ (nm) | Zoharite R (%) | Gmalimite R (%) | λ (nm) | Zoharite R (%) | Gmalimite R (%) |
|---|---|---|---|---|---|
| 400 | 18.5 | 17.9 | 560 | 25.4 | 25.1 |
| 410 | 18.8 | 17.9 | 570 | 25.7 | 25.5 |
| 420 | 19.1 | 18.1 | 580 | 26.0 | 25.6 |
| 430 | 19.5 | 18.7 | 589 (COM) | 26.3 | 25.9 |
| 440 | 19.9 | 18.9 | 590 | 26.3 | 26.0 |
| 450 | 21.0 | 19.9 | 600 | 26.7 | 26.0 |
| 460 | 21.3 | 20.9 | 610 | 26.8 | 26.0 |
| 470 (COM) | 22.2 | 21.5 | 620 | 27.1 | 26.1 |
| 480 | 22.5 | 22.0 | 630 | 27.1 | 26.1 |
| 490 | 22.8 | 22.4 | 640 | 27.6 | 26.1 |
| 500 | 23.3 | 22.9 | 650 (COM) | 27.7 | 26.3 |
| 510 | 23.6 | 23.4 | 660 | 27.7 | 26.4 |
| 520 | 24.2 | 23.7 | 670 | 27.6 | 26.3 |
| 530 | 24.4 | 24.1 | 680 | 27.7 | 26.2 |
| 540 | 24.8 | 24.4 | 690 | 27.8 | 26.1 |
| 546 (COM) | 25.1 | 24.6 | 700 | 27.9 | 26.2 |
| 550 | 25.2 | 24.8 |
| 1 | 2 | 3 | |||||
|---|---|---|---|---|---|---|---|
| wt.% | n = 3 | n = 13 | s.d. | range | n = 14 | s.d. | range |
| S | 33.77 | 33.58 | 0.36 | 32.74–34.06 | 30.87 | 0.63 | 29.92–32.07 |
| Se | n.d. | n.d. | 0.17 | 0.05 | 0.08–0.27 | ||
| K | 7.58 | 7.61 | 0.24 | 7.39–8.36 | 2.51 | 0.08 | 2.33–2.63 |
| Ba | 0.12 | 0.14 | 0.08 | 0–0.30 | 18.68 | 0.60 | 17.00–19.52 |
| Na | n.d. | n.d. | 0.18 | 0.05 | 0.08–0.33 | ||
| Fe | 36.19 | 36.84 | 1.27 | 34.48–38.85 | 28.35 | 1.43 | 24.85–30.98 |
| Ni | 6.71 | 7.56 | 1.22 | 6.25–10.28 | 6.85 | 0.97 | 4.01–8.95 |
| Cu | 14.33 | 12.58 | 2.50 | 7.51–15.43 | 12.38 | 2.62 | 8.05–17.78 |
| Total | 98.70 | 98.32 | 99.99 | ||||
| Calculated on cations and normalized on 27(S + Se) | |||||||
| K | 4.97 | 5.02 | 1.80 | ||||
| Ba | 0.02 | 0.03 | 3.81 | ||||
| Na | 0.22 | ||||||
| □ | 1.01 | 0.95 | 0.17 | ||||
| A | 6.00 | 6.00 | 6.00 | ||||
| Fe2+ | 16.61 | 17.01 | 14.21 | ||||
| Ni | 2.93 | 3.32 | 3.27 | ||||
| Cu | 5.78 | 5.11 | 5.45 | ||||
| □ | - | 2.07 | |||||
| M + M’ | 25.32 | 25.44 | 25.00 | ||||
| S | 27.00 | 27.00 | 26.94 | ||||
| Se | 0.06 | ||||||
| X + Y | 27.00 | 27.00 | 27.00 | ||||
| Cation charge (Cu2+) | 55.65 | 55.96 | 55.50 | ||||
| Cation charge (Cu+) | 49.87 | 50.85 | 50.05 | ||||
| (a) | |||||
| Site | x | y | z | Uiso | Occupancy |
| K1 | 0 | 0 | 0.2954(2) | 0.0406(6) | (a) 0.842(3)K + 0.006(3)Ba + 0.281□ |
| Fe1 | 0.5 | 0.5 | 0.5 | 0.0340(18) | (b) 0.387(11)Fe + 0.613□ |
| Fe2 | 0.36849(3) | 0.36849(3) | 0.13333(4) | 0.0261(2) | (c) 0.638 Fe + 0.24 Cu + 0.122 Ni |
| S1 | 0 | 0 | 0 | 0.0449(10) | 1S |
| S2 | 0.24863(10) | 0.50 | 0 | 0.0244(3) | 1S |
| S3 | 0.5 | 0.5 | 0.25487(14) | 0.0241(3) | 1S |
| S4 | 0.23025(8) | 0.23025(8) | 0.23025(8) | 0.0275(3) | 1S |
| (a) occupancy parameters of K and Ba are refined to matching a total scattering of 16 electrons. (b) occupancy parameter of Fe1 is refined to matching a total scattering of 10 electrons. (c) occupancy parameters of Fe, Cu, and Ni are set to matching chemical analysis and total scattering of the site. | |||||
| (b) | |||||
| Site | x | y | z | Uiso | Occupancy |
| Ba1/K1 | 0 | 0 | 0.30022(2) | 0.01716(8) | 0.652(2) Ba + 0.348(2) K |
| Fe1 | 0.5 | 0.5 | 0.5 | 0.0153(2) | (a) 0.97 Fe + 0.03 □ |
| Fe2/Cu2 | 0.36685(2) | 0.36685(2) | 0.13604(2) | 0.0181(2) | (b) 0.628(2) Fe + 0.27 Cu + 0.102□ |
| S1 | 0 | 0 | 0 | 0.0184(3) | 1S |
| S2 | 0.24794(5) | 0.50 | 0 | 0.01621(12) | 1S |
| S3 | 0.5 | 0.5 | 0.25988(7) | 0.01587(15) | 1S |
| S4 | 0.22326(4) | 0.22326(4) | 0.22326(4) | 0.01912(14) | 1S |
| (a) total scattering on this site equals 25 electrons. (b) total scattering on this site equals 24 electrons. | |||||
| (a) | ||||||
| Site | U11 | U22 | U33 | U12 | U13 | U23 |
| K1 | 0.0368(7) | 0.0368(7) | 0.0483(11) | 0 | 0 | 0 |
| Fe1 | 0.0340(18) | 0.0340(18) | 0.0340(18) | 0 | 0 | 0 |
| Fe2 | 0.0258(2) | 0.0258(2) | 0.0267(3) | −0.00051(9) | −0.00051(9) | 0.00026(12) |
| S1 | 0.0449(10) | 0.0449(10) | 0.0449(10) | 0 | 0 | 0 |
| S2 | 0.0229(4) | 0.0261(4) | 0.0241(4) | 0 | 0 | 0 |
| S3 | 0.0250(4) | 0.0250(4) | 0.0221(5) | 0 | 0 | 0 |
| S4 | 0.0275(3) | 0.0275(3) | 0.0275(3) | 0.0027(3) | 0.0027(3) | 0.0027(3) |
| (b) | ||||||
| Atom | U11 | U22 | U33 | U23 | U13 | U12 |
| Ba1 | 0.01774(9) | 0.01774(9) | 0.01600(12) | 0 | 0 | 0 |
| Fe1 | 0.0153(2) | 0.0153(2) | 0.0153(2) | 0 | 0 | 0 |
| Fe2 | 0.0172(4) | 0.0172(4) | 0.0200(3) | −0.00057(18) | −0.00057(18) | 0.00000(15) |
| S1 | 0.0184(3) | 0.0184(3) | 0.0184(3) | 0 | 0 | 0 |
| S2 | 0.0157(2) | 0.0160(2) | 0.0169(2) | 0 | 0 | 0 |
| S3 | 0.0166(2) | 0.0166(2) | 0.0144(3) | 0 | 0 | 0 |
| S4 | 0.01912(14) | 0.01912(14) | 0.01912(14) | 0.00231(14) | 0.00231(14) | 0.00231(14) |
| Zoharite | Gmalimite | ||||
|---|---|---|---|---|---|
| Site 1 | Site 2 | Bond Lengths (Å) | Site 1 | Site 2 | Bond Lengths (å) |
| Ba1/K1 (Ba0.652(2)K0.348(2)) | -S1 | 3.0964(2) | K1 (K0.842(3)Ba0.006(3)□0.281) | -S1 | 3.057(2) |
| -S2 | 3.2840(4) ×4 | -S2 | 3.333(2) × 4 | ||
| -S4 | 3.3517(5) × 4 | -S4 | 3.436(1) × 4 | ||
| mean | 3.293(1) | mean | 3.348(1) | ||
| BVS | 1.94 | BVS | 1.05 | ||
| Fe1(Fe0.97□0.03) | -S3 | 2.4765(7) × 6 | Fe1 (Fe0.387(11) □0.613) | -S3 | 2.537(1) × 6 |
| BVS | 2.25 | BVS | 0.76 | ||
| Fe2/Cu2 (Fe0.629(2)Cu0.27□0.102) | -S4 | 2.2794(4) | Fe2 (Fe0.638Cu0.240Ni0.122) | -S4 | 2.258(1) |
| -S2 | 2.3149(3) × 2 | -S2 | 2.301(1) × 2 | ||
| -S3 | 2.3244(5) | -S3 | 2.299(1) | ||
| mean | 2.308 | mean | 2.290(1) | ||
| BVS | 2.03 | BVS | 2.29 | ||
| S1 | Ba1/K1 | 3.0964(2) × 6 | S1 | K1 | 3.057(2) × 6 |
| BVS | 2.15 | BVS | 1.45 | ||
| S2 | Ba1/K1 | 3.2840(4) × 2 | S2 | K1 | 3.333(2) × 2 |
| Fe2/Cu2 | 2.3149(3) × 4 | Fe2 | 2.301(1) × 4 | ||
| mean | 2.638 | mean | 2.645 | ||
| BVS | 2.43 | BVS | 2.45 | ||
| S3 | Fe1 | 2.4765(7) × 4 | S3 | Fe1 | 2.537(1) × 4 |
| Fe2/Cu2 | 2.3244(5) | Fe2 | 2.299(1) | ||
| mean | 2.446 | mean | 2.4894 | ||
| BVS | 2.32 | BVS | 2.36 | ||
| S4 | Ba1/K1 | 3.3517(5) × 3 | S4 | K1 | 3.436(1) × 3 |
| Fe2/Cu2 | 2.2794(4) × 3 | Fe2 | 2.258(1) × 3 | ||
| mean | 2.816 | mean | 2.847 | ||
| BVS | 2.19 | BVS | 2.13 | ||
| Zoharite | Gmalimite | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| dhkl [Å] | Iref [%] | h | k | l | dhkl [Å] | Iref [%] | h | k | l |
| 10.3218 | 10 | 0 | 0 | 1 | 10.3567 | 91 | 0 | 0 | 1 |
| 7.2986 | 6 | 1 | 0 | 1 | 7.3233 | 33 | 1 | 0 | 1 |
| 5.9593 | 12 | 1 | 1 | 1 | 5.9795 | 59 | 1 | 1 | 1 |
| 3.4406 | 15 | 2 | 1 | 2 | 3.4522 | 9 | 0 | 0 | 3 |
| 3.4406 | 25 | 0 | 0 | 3 | 3.2751 | 28 | 1 | 0 | 3 |
| 3.264 | 65 | 1 | 0 | 3 | 3.1227 | 56 | 1 | 1 | 3 |
| 3.1121 | 45 | 1 | 1 | 3 | 2.9897 | 58 | 2 | 2 | 2 |
| 2.9796 | 80 | 2 | 2 | 2 | 2.5892 | 6 | 0 | 0 | 4 |
| 2.8628 | 9 | 2 | 0 | 3 | 2.5119 | 11 | 1 | 0 | 4 |
| 2.5034 | 14 | 1 | 0 | 4 | 2.376 | 51 | 3 | 1 | 3 |
| 2.368 | 66 | 3 | 1 | 3 | 2.0713 | 11 | 3 | 0 | 4 |
| 2.308 | 9 | 2 | 0 | 4 | 2.0311 | 6 | 3 | 1 | 4 |
| 2.2524 | 10 | 2 | 1 | 4 | 1.9932 | 11 | 1 | 1 | 5 |
| 1.9864 | 21 | 1 | 1 | 5 | 1.9932 | 6 | 3 | 3 | 3 |
| 1.9167 | 7 | 2 | 0 | 5 | 1.8308 | 100 | 4 | 0 | 4 |
| 1.8845 | 6 | 2 | 1 | 5 | 1.8029 | 5 | 4 | 1 | 4 |
| 1.8247 | 100 | 4 | 0 | 4 | 1.7762 | 6 | 3 | 3 | 4 |
| 1.7702 | 17 | 3 | 3 | 4 | 1.7506 | 9 | 3 | 1 | 5 |
| 1.7203 | 8 | 0 | 0 | 6 | 1.5613 | 12 | 2 | 2 | 6 |
| 1.5561 | 13 | 2 | 2 | 6 | 1.4502 | 5 | 1 | 1 | 7 |
| 1.5387 | 7 | 4 | 2 | 5 | 1.3483 | 7 | 3 | 1 | 7 |
| 1.5387 | 7 | 3 | 0 | 6 | 1.2946 | 10 | 0 | 0 | 8 |
| 1.5219 | 5 | 3 | 1 | 6 | 1.2653 | 7 | 3 | 3 | 7 |
| 1.3553 | 10 | 3 | 0 | 7 | 1.1368 | 4 | 5 | 3 | 7 |
| 1.2902 | 10 | 0 | 0 | 8 | 1.057 | 14 | 4 | 4 | 8 |
| 1.261 | 13 | 3 | 3 | 7 | 0.9154 | 4 | 8 | 0 | 8 |
| 1.2517 | 5 | 4 | 4 | 6 | 0.8188 | 8 | 4 | 0 | 12 |
| 1.0535 | 12 | 4 | 4 | 8 | 0.7741 | 14 | 7 | 3 | 11 |
| 0.9584 | 7 | 4 | 0 | 10 | 0.7719 | 14 | 8 | 4 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galuskina, I.O.; Krüger, B.; Galuskin, E.V.; Krüger, H.; Vapnik, Y.; Murashko, M.; Banasik, K.; Agakhanov, A.A. Zoharite, (Ba,K)6 (Fe,Cu,Ni)25S27, and Gmalimite, K6□Fe2+24S27—New Djerfisherite Group Minerals from Gehlenite-Wollastonite Paralava, Hatrurim Complex, Israel. Minerals 2025, 15, 564. https://doi.org/10.3390/min15060564
Galuskina IO, Krüger B, Galuskin EV, Krüger H, Vapnik Y, Murashko M, Banasik K, Agakhanov AA. Zoharite, (Ba,K)6 (Fe,Cu,Ni)25S27, and Gmalimite, K6□Fe2+24S27—New Djerfisherite Group Minerals from Gehlenite-Wollastonite Paralava, Hatrurim Complex, Israel. Minerals. 2025; 15(6):564. https://doi.org/10.3390/min15060564
Chicago/Turabian StyleGaluskina, Irina O., Biljana Krüger, Evgeny V. Galuskin, Hannes Krüger, Yevgeny Vapnik, Mikhail Murashko, Kamila Banasik, and Atali A. Agakhanov. 2025. "Zoharite, (Ba,K)6 (Fe,Cu,Ni)25S27, and Gmalimite, K6□Fe2+24S27—New Djerfisherite Group Minerals from Gehlenite-Wollastonite Paralava, Hatrurim Complex, Israel" Minerals 15, no. 6: 564. https://doi.org/10.3390/min15060564
APA StyleGaluskina, I. O., Krüger, B., Galuskin, E. V., Krüger, H., Vapnik, Y., Murashko, M., Banasik, K., & Agakhanov, A. A. (2025). Zoharite, (Ba,K)6 (Fe,Cu,Ni)25S27, and Gmalimite, K6□Fe2+24S27—New Djerfisherite Group Minerals from Gehlenite-Wollastonite Paralava, Hatrurim Complex, Israel. Minerals, 15(6), 564. https://doi.org/10.3390/min15060564









