Nioboixiolite-(□),(Nb0.8□0.2)4+O2, a New Mineral Species from the Bayan Obo World-Class REE-Fe-Nb Deposit, Inner Mongolia, China
Abstract
1. Introduction
2. Analytical Methods
2.1. Chemical Composition Analysis
2.2. Crystal Structural Analysis
2.3. Infrared Absorption Spectroscopy Analysis
2.4. Raman Spectroscopy Analysis
2.5. Reflectance Test Analysis
2.6. X-Ray Photoelectron Spectroscopy (XPS) Analysis
3. Results
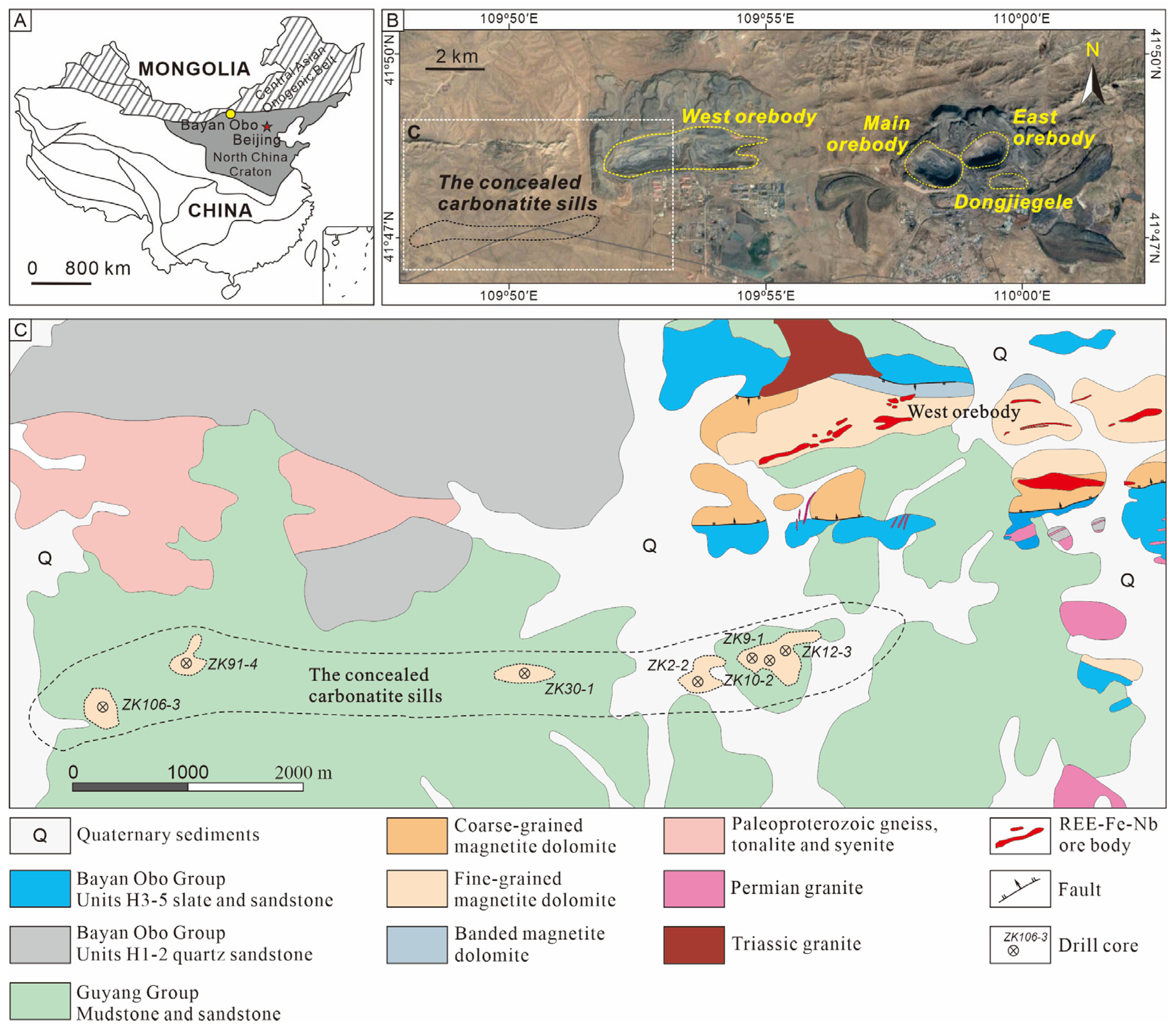
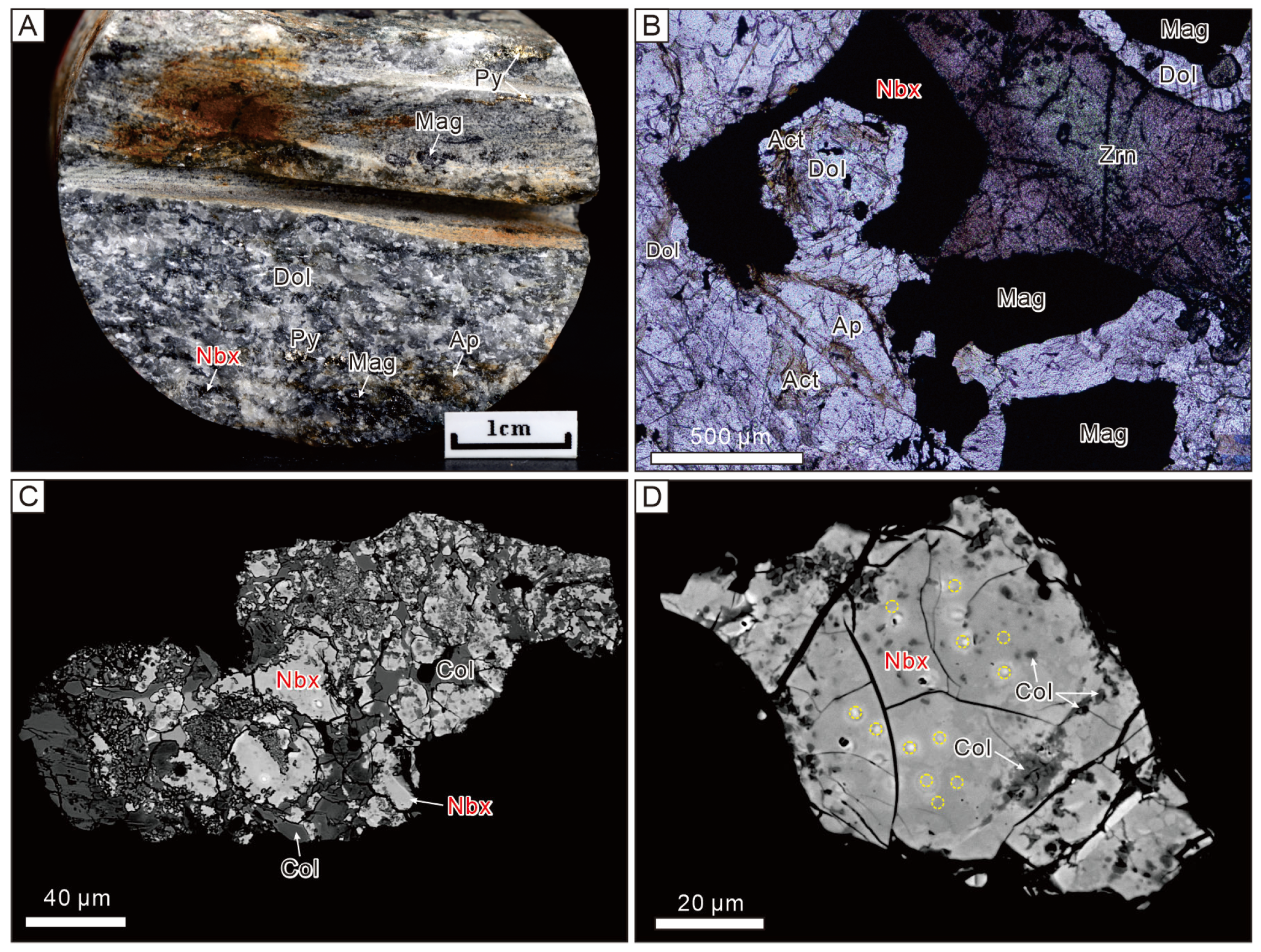
3.1. Occurrence and Associated Minerals
3.2. Optical, Morphological and Physical Properties of Nioboixiolite-(□)
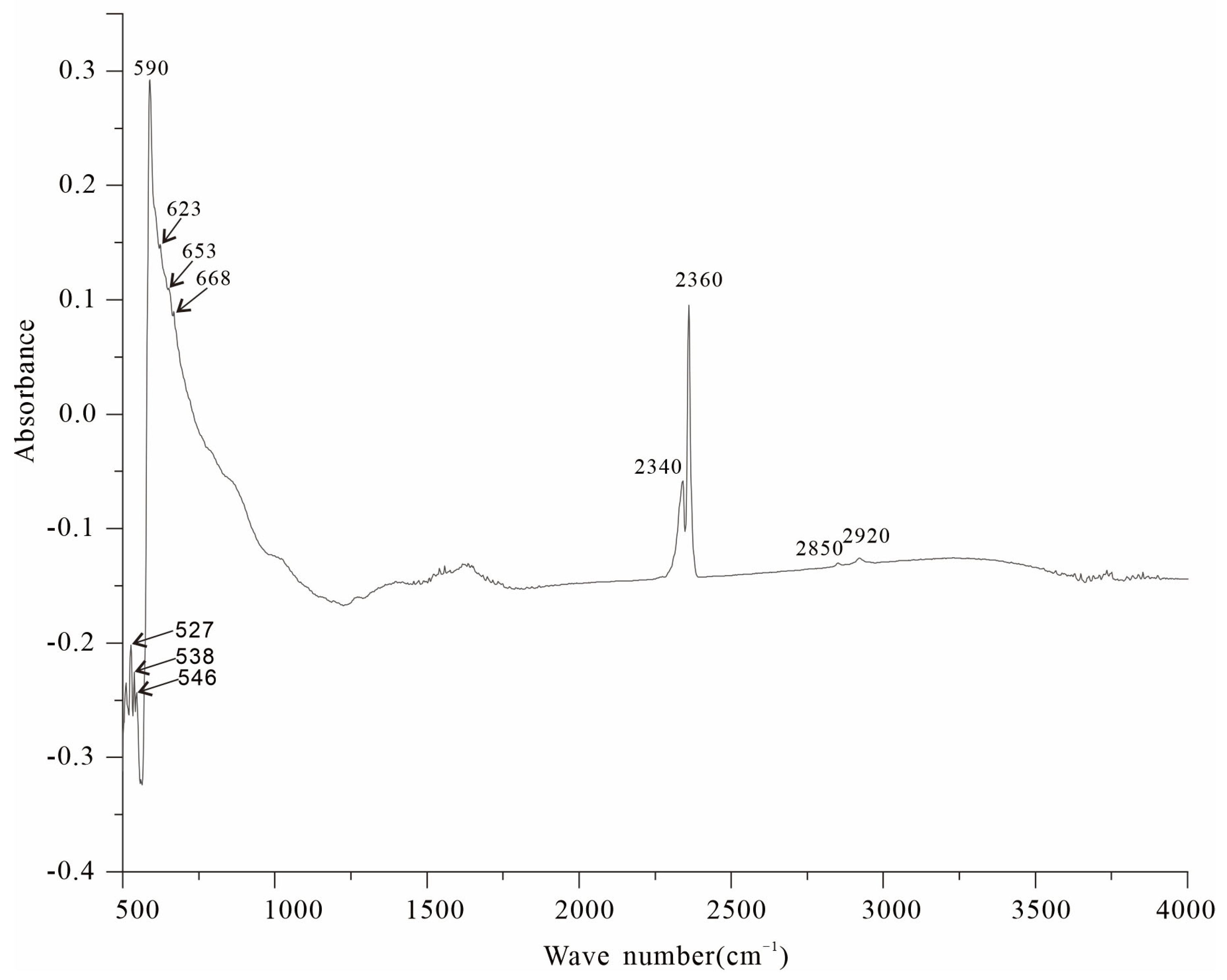
3.3. Infrared Absorption Spectroscopy
3.4. Raman Spectroscopy
3.5. Chemical Composition
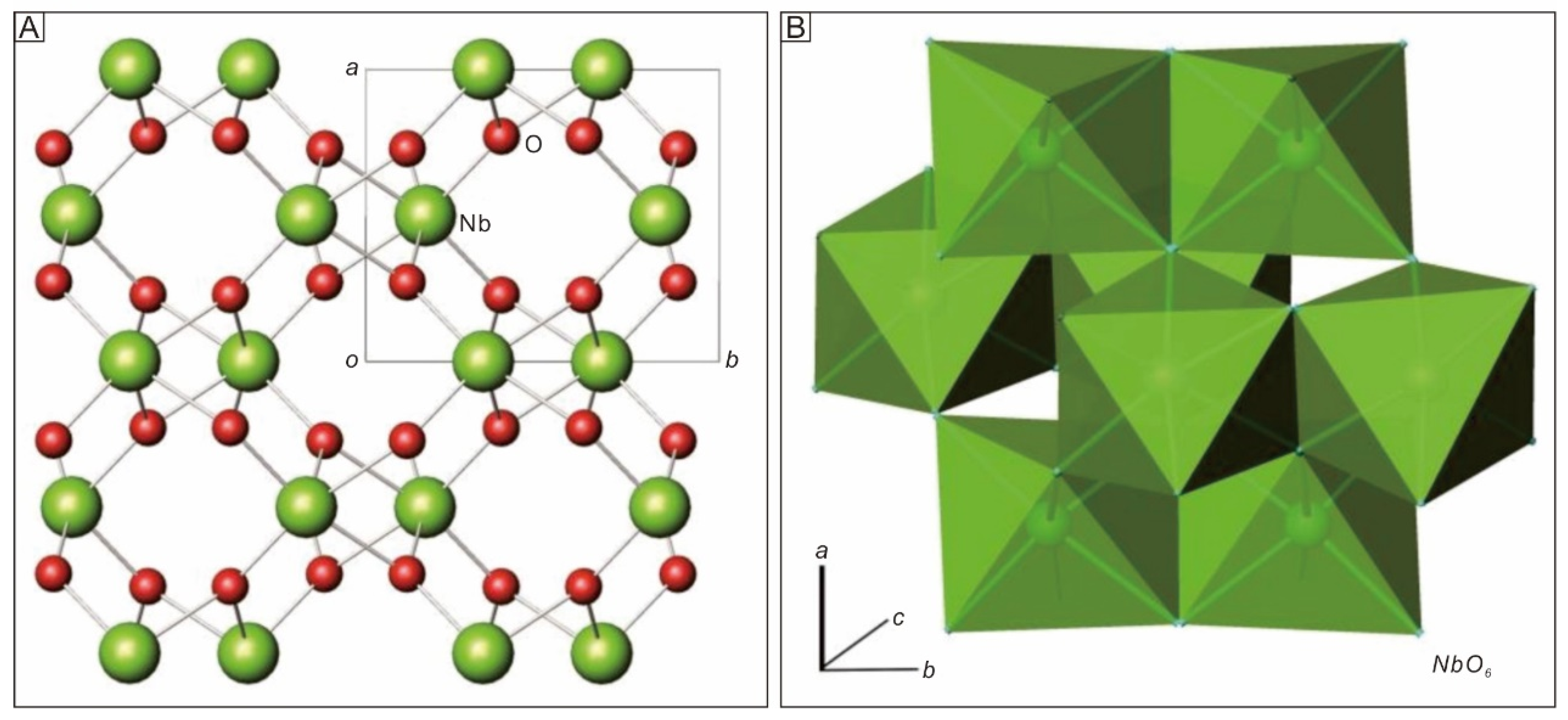
3.6. Crystal Structure
4. Discussion
| Contents | Nioboixiolite-(□) | Nioboixiolite-(Mn2+) | Ixiolite-(Fe2+) | Columbite-(Fe) | Scandian Ixiolite | Rossovskyite | Wodginite |
|---|---|---|---|---|---|---|---|
| Nb2O5 | 47.04 | 42.80 | 10.50 | 73.18 | 63.28 | 26.59 | 1.35 |
| Ta2O5 | 13.95 | 26.77 | 61.47 | 6.12 | 5.82 | 37.51 | 70.05 |
| UO3 | 21.56 | 1.44 | - | 0.02 | - | ||
| TiO2 | 3.68 | 7.66 | 0.38 | 0.26 | 6.54 | 7.69 | 2.39 |
| Fe2O3 | 3.57 | 0.2 | 8.08 | 15.03 b | 8.16 f | 20.58 a | 1.87 |
| CaO | 2.76 | - | 0.11 | 0.89 | - | ||
| SiO2 | 1.69 | - | 0.12 | 0.15 | 0.6 | ||
| REE2O3 | 1.58 | 1.34 d | - | 0.07 | 2.12 | - | |
| MnO | 0.12 | 14.94 | 5.40 | 1.63 | 9.65 | 1.68 | 9.04 |
| PbO | 0.91 | - | 0.26 | ||||
| ThO2 | 0.11 | 0.26 | - | 0.06 | - | ||
| MgO | 0.15 | - | 1.90 | - | |||
| ZrOb2 | - | 1.74 | 0.60 | - | - | ||
| F | 0.01 | - | 0.21 | ||||
| SnO2 | - | 1.01 | 12.27 | - | 0.2 | 13.2 | |
| Al2O3 | 0.01 | 0.16 | - | - | |||
| Sc2O3 | 1.80 | 2.1 | |||||
| WO3 | 0.30 | - | 5.61 | - | |||
| BaO | 0.62 | ||||||
| SrO | 1.49 | ||||||
| H2O+ | 0.16 | - | |||||
| H2O(−) | 0.08 | - | |||||
| Total | 99.25 | 99.96 | 99.63 | 99.78 | 99.37 | 99.66 | 98.50 |
| Reference | This study | [2] | [21] | This study | [3] | [22] | [23] |
| Mineral | Nioboixiolite-(□) Bayan Obo, China | Nioboixiolite-(Mn2+), Sosedka, Russia | Ixiolite-(Fe2+) Skogsböle, Finland | Columbite-(Fe) Bayan Obo, China |
|---|---|---|---|---|
| a (Å) | 5.7097 | 4.7559 | 5.731 | 5.709 |
| b (Å) | 4.7071 | 5.7318 | 4.742 | 14.150 |
| c (Å) | 5.1111 | 5.1344 | 5.152 | 5.094 |
| β (°) | 90 | 90 | 90 | 90 |
| V (Å3) | 137.37 | 139.97 | 140 | 414 |
| Symmetry | Orthorhombic | Orthorhombic | Orthorhombic | Orthorhombic |
| Space Group | Pbcn | Pbcn | Pbcn | Pbcn |
| Simplified Formula | (Nb0.8□0.2)4+O2 | (Nb2/3Mn2+1/3)O2 | (Ta2/3Fe2+1/3)O2 | Fe2+Nb2O6 |
| Reference | This study | [2] | [23] | This study |
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chukanov, N.V.; Pasero, M.; Aksenov, S.M.; Britvin, S.N.; Zubkova, N.V.; Li, Y.K.; Witzke, T. Columbite supergroup of minerals: Nomenclature and classification. Mineral. Mag. 2023a, 87, 18–33. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Pekov, I.V.; Zubkova, N.V.; Yapaskurt, V.O.; Shelukhina, Y.; Britvin, S.N.; Pushcharovsky, D.Y. Nioboixiolite-(Mn2+), (Nb2/3Mn2+1/3) O2, a new ixiolite-group mineral from the Malkhan pegmatite field, Transbaikal region, Russia. Proc. Russ. Mineral. Soc. 2023b, CLⅡ, 8–17. [Google Scholar]
- von Knorring, O.V.; Sahama, T.G. Scandian ixiolite from Mozambique and Madagascar. Bull. Geol. Soc. Finl. 1969, 41, 75–77. [Google Scholar] [CrossRef]
- Wise, M.A.; Černý, P.; Falster, A.U. Scandium substitution in columbite-group minerals and ixiolite. Can. Minerogist 1998, 36, 673–680. [Google Scholar]
- Zubkova, N.V.; Chukanov, N.V.; Pekov, I.V.; Ternes, B.; Schüller, W.; Pushcharovsky, D.Y. Tantalum-free Nb-dominant analogue of ixiolite from the Eifel paleovolcanic region, Germany, and its crystal structure: On the problem of “ashanite”. Zap. Ross. Mineral. Obs. 2020, 149, 125–134. (In Russian) [Google Scholar]
- Grice, J.D.; Ferguson, R.B.; Hawthorne, F.C. The crystal structures of tantalite, ixiolite and wodginite from Bernic Lake, Manitoba. I. Tantaliteand ixiolite. Can. Mineral. 1976, 14, 540–549. [Google Scholar]
- Nordenskiöld, A.E. Beitrag zu Finnlands Mineralogie. Ann. Phys. Chem. 1857, 11, 625–642. [Google Scholar] [CrossRef]
- Taggart, J.E., Jr.; Foord, E.E.; Rosenzweig, A.; Hanson, T. Scrutinyite, natural occurrences of PbO2 from Bingham, New Mexico, U.S.A., and Mapimi, Mexico. Can. Mineral. 1988, 26, 905–910. [Google Scholar]
- Willgallis, A.; Siegmann, E.; Hettiaratchi, T. Srilankite, a new Zr-Ti-oxide mineral. Neues Jahrb. Mineral. Monatshefte 1983, 1983, 151–157. [Google Scholar]
- Dera, P.; Prewitt, C.T.; Boctor, N.Z.; Hemley, R.J. Characterization of a high-pressure phase of silica from the Martian meteorite Shergotty. Am. Mineral. 2002, 87, 1018–1023. [Google Scholar] [CrossRef]
- Hu, L.; Li, Y.; Sun, S.; Li, R.; Ke, C.; Wang, A. Identification of new igneous carbonatites in the Bayan Obo area, Inner Mongolia. Geol. China 2023, 50, 1788–1803, (In Chinese with English Abstract). [Google Scholar]
- Strehle, M. X-Ray Photoelectron Spectroscopy (XPS) Study of Single Crystal UO2 and U3O8 on R-Plane Sapphire and Yttrium Stabilized Zirconium (YSZ) Substrates (Mater Dissertation); University of Illinois Urbana-Champaign: Champaign, IL, USA, 2011; pp. 1–64. [Google Scholar]
- Palacio, C.; Mathieu, H.J.; Stambouli, V.; Landolt, D. XPS study of in-situ oxidation of an Fe-Cr alloy by low pressure oxygen in the presence of water vapor. Surf. Ence Lett. 1993, 295, 251–262. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELX. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Hatert, F.; Burke, E.A.J. The IMA-CNMNC dominant-constituent rule revisited and extended. Can. Mineral. 2008, 46, 717–728. [Google Scholar] [CrossRef]
- Harrison, W.T.A.; Cheetham, A.K. Structural and magnetic properties of FeNbO4-II. Mater. Res. Bull. 1989, 24, 523–527. [Google Scholar] [CrossRef]
- Pourroy, G.; Lutanie, E.; Poix, P. Transition orthorhombic ↔ rutile in xMFeO4-(1 − x) ZnF2 phases (M = Ta, Nb and x > 0.7). J. Solid State Chem. 1990, 86, 41–49. [Google Scholar] [CrossRef]
- Baumgarte, A.; Blachnik, R. Phase relations in the system titaniumdioxide-diniobium-zinc-hexoxide. Mater. Res. Bull. 1992, 27, 1287–1294. [Google Scholar] [CrossRef]
- Hadamek, T.; Posadas, A.B.; Dhamdhere, A.; Smith, D.J.; Demkov, A.A. Spectral identification scheme for epitaxially grown single-phase niobium dioxide. J. Appl. Phys. 2016, 119, 095308. [Google Scholar] [CrossRef]
- Nickel, E.H.; Rowland, J.F.; McAdam, R.C. Ixiolite—A columbite substructure. Am. Mineral. 1963, 48, 961–979. [Google Scholar]
- Konovalenko, S.I.; Ananyev, S.A.; Chukanov, N.V.; Rastsvetaeva, R.K.; Aksenov, S.M.; Baeva, A.A.; Gainov, R.R.; Vagizov, F.G.; Lopatin, O.N.; Nebera, T.S. A new mineral species rossovskyite, (Fe3+,Ta)(Nb,Ti)O4: Crystal chemistry and physical properties. Phys. Chem. Miner. 2015, 42, 825–833. [Google Scholar] [CrossRef]
- Nickel, E.H.; Rowland, J.F.; McAdam, R.C. Wodginite—A new tin-manganese tantalate from Wodgina, Australia and Bernic lake, Manitoba. Can. Mineral. 1963, 7, 390–402. [Google Scholar]
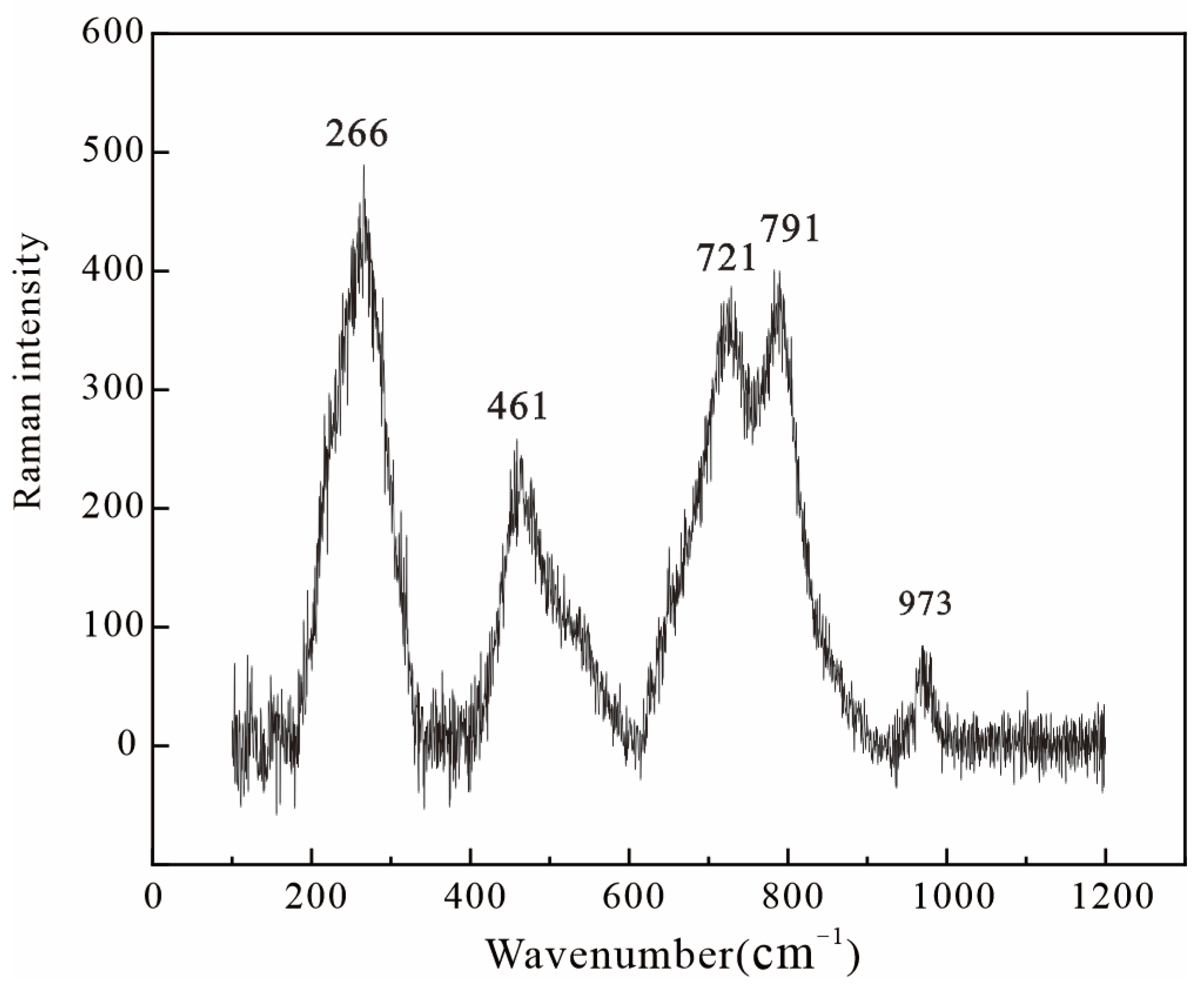


| Peak | Type | Amplitude | Center | FWHM | Asym50 | FW Base | Asym10 |
|---|---|---|---|---|---|---|---|
| 1 | Gauss + Lor Amp | 418.03 | 266.179 | 69.19 | 1 | 138.50 | 1 |
| 2 | Gauss + Lor Amp | 193.65 | 461.561 | 55.69 | 1 | 112.75 | 1 |
| 3 | Gauss + Lor Amp | 293.17 | 720.66 | 68.69 | 1 | 137.50 | 1 |
| 4 | Gauss + Lor Amp | 344.33 | 790.68 | 60.57 | 1 | 188.18 | 1 |
| Constituent | Wt.% * | Range | Stand. Dev. | Probe Standard |
|---|---|---|---|---|
| Nb2O5 | 47.04 | 43.86–51.80 | 2.09 | KNbO3 |
| Ta2O5 | 13.95 | 10.26–15.22 | 1.25 | LiTaO3 |
| UO3 * | 21.56 | 19.12–21.56 | 1.32 | thorianite |
| TiO2 | 3.68 | 3.05–4.91 | 0.53 | rutile |
| Fe2O3 * | 3.57 | 2.41–4.77 | 0.81 | hematite |
| CaO | 2.76 | 2.22–3.44 | 0.37 | wollastonite |
| MgO | 0.15 | 0.0–0.43 | 0.13 | forsterite |
| SiO2 | 1.69 | 0.80–3.86 | 0.96 | Jade |
| SrO | 1.49 | 1.11–2.16 | 0.31 | SrSO4 |
| BaO | 0.62 | 0.07–1.34 | 0.46 | BaSO4 |
| La2O3 | 0.08 | 0.00–0.14 | 0.04 | LaP5O14 |
| Ce2O3 | 0.68 | 0.29–1.13 | 0.23 | CeP5O14 |
| Pr2O3 | 0.10 | 0.00–0.22 | 0.07 | PrP5O14 |
| Nd2O3 | 0.25 | 0.00–0.37 | 0.12 | NdP5O14 |
| Sm2O3 | 0.04 | 0.00–0.09 | 0.04 | SmP5O14 |
| Eu2O3 | 0.02 | 0.00–0.11 | 0.04 | EuP5O14 |
| Gd2O3 | 0.06 | 0.00–0.15 | 0.05 | GdP5O14 |
| Tb2O3 | 0.13 | 0.00–0.36 | 0.14 | Tb3Ga5O12 |
| Dy2O3 | 0.05 | 0.00–0.17 | 0.06 | DyP5O14 |
| Ho2O3 | 0.04 | 0.00–0.18 | 0.06 | HoP5O14 |
| Er2O3 | 0.02 | 0.00–0.11 | 0.03 | ErP5O14 |
| Tm2O3 | 0.02 | 0.00–0.14 | 0.04 | Tm P5O14 |
| Yb2O3 | 0.04 | 0.00–0.29 | 0.08 | Yb P5O14 |
| Lu2O3 | 0.03 | 0.00–0.15 | 0.05 | LuSiO5 |
| Y2O3 | 0.02 | 0.00–0.13 | 0.04 | Y P5O14 |
| MnO | 0.12 | 0.00–0.29 | 0.08 | MnTiO3 |
| PbO | 0.91 | 0.76–1.06 | 0.08 | PbCr2O4 |
| ThO2 | 0.11 | 0.07–0.16 | 0.03 | thorianite |
| Al2O3 | 0.01 | 0.00–0.03 | 0.01 | jadeite |
| F | 0.01 | 0–0.09 | 0.02 | phlogopite |
| Total | 99.25 | 98.72–99.93 | 0.38 |
| h | k | l | d(obs) | d(calc) | I/I0 |
|---|---|---|---|---|---|
| 1 | 1 | 0 | 3.6621 | 3.6417 | 20 |
| 1 | 1 | 1 | 2.9746 | 2.9675 | 100 |
| 0 | 2 | 0 | 2.8603 | 2.8613 | 4 |
| 0 | 0 | 2 | 2.566 | 2.5596 | 10 |
| 0 | 2 | 1 | 2.5008 | 2.4976 | 20 |
| 2 | 0 | 0 | 2.3637 | 2.3606 | 2 |
| 1 | 0 | 2 | 2.2605 | 2.2502 | 2 |
| 1 | 2 | 1 | 2.2104 | 2.2077 | 6 |
| 1 | 1 | 2 | 2.0958 | 2.0941 | 10 |
| 0 | 2 | 2 | 1.9075 | 1.9077 | 6 |
| 2 | 2 | 0 | 1.8234 | 1.8209 | 2 |
| 1 | 2 | 2 | 1.7702 | 1.7687 | 20 |
| 2 | 2 | 1 | 1.7177 | 1.7156 | 15 |
| 1 | 1 | 3 | 1.5453 | 1.5452 | 10 |
| 0 | 2 | 3 | 1.4581 | 1.4656 | 20 |
| 0 | 4 | 1 | 1.3793 | 1.3778 | 10 |
| 3 | 1 | 2 | 1.3058 | 1.3053 | 1 |
| 0 | 4 | 2 | 1.2465 | 1.2488 | 2 |
| 3 | 3 | 0 | 1.2108 | 1.2139 | 2 |
| 2 | 4 | 1 | 1.19 | 1.19 | 2 |
| 4 | 1 | 1 | 1.1267 | 1.1276 | 2 |
| 0 | 4 | 3 | 1.0973 | 1.0963 | 8 |
| 1 | 3 | 4 | 1.0365 | 1.0368 | 2 |
| 2 | 4 | 3 | 0.9952 | 0.9943 | 2 |
| Structural formula | Nb0.88O2 | θ range for data collection/ ° | 5.615–29.335o |
| Formula weight | 124.91 | Index ranges | −6 ≤ h ≤ 6, −7 ≤ k ≤ 7, −6 ≤ l ≤ 6 |
| Crystal system | orthorhombic | Reflections collected | 2422 |
| Space group | Pbcn | Independent reflections | 2422 [R (int) = 0.0226] |
| a/Å | 4.7071 (5) | Completeness to θ = 29.33o/% | 97 |
| b/Å | 5.7097 (7) | Absorption correction | Semi-empirical from equivalents |
| c/Å | 5.1111 (6) | Refinement method | Full-matrix least-squares on F2 |
| Volume/nm3 | 137.37 (3) | ||
| Z | 4 | Goodness-of-fit on F2 | 0.94 |
| Dc/(g·cm−3) | 2.571 | Final R indices [I > 2σ (I)] | R1 = 0.031, wR2 = 0.110 |
| Absorption coefficient/mm−1 | 8.139 | Largest diff. peak and hole/(e·nm−3) | 1.32 and −1.25 |
| F (000) | 228 |
| Atom coordinates and displacement parameters (Å2) | ||||||
| Atom | Wyck. | Occupancy | x | y | z | Uiso |
| Nb1 | 4c | 0.877(19) | 1/2 | 0.83206(10) | 1/4 | 0.0199(4) |
| O1 | 8d | 1 | 0.2288(5) | 0.6169(4) | 0.4165(8) | 0.0190(11) |
| Anisotropic displacement parameters (in Å2) | ||||||
| Atom | U11 | U22 | U33 | U23 | U13 | U12 |
| Nb1 | 0.0238(6) | 0.0169(6) | 0.0190(6) | 0.000 | 0.00020(18) | 0.000 |
| O1 | 0.0225(16) | 0.0145(14) | 0.0200(17) | 0.0021(13) | −0.0035(13) | 0.0000(9) |
| Bond distances (Å) | ||||||
| Nb1–O1 | 1.965(3) × 2 | |||||
| –O1 | 2.037(4) × 2 | |||||
| –O1 | 2.128(3) × 2 | |||||
| Mean | 2.043 | |||||
| Bond Distances (Å) | Nioboixiolite-(□) | Nioboixiolite-(Mn2+) | Ixiolite-(Fe2+) |
|---|---|---|---|
| M–O1 | 1.965(3) × 2 | 1.984(7) × 2 | 2.04(4) × 2 |
| –O1 | 2.037(4) × 2 | 2.052(8) × 2 | 1.99(4) × 2 |
| –O1 | 2.128(3) × 2 | 2.137(7) × 2 | 2.16(4) × 2 |
| Mean | 2.043 | 2.058 | 2.06 |
| Reference | This study | [2] | [6] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ke, C.; Wang, D.; Peng, Z.; Zhao, Y.; Li, R.; Chen, Z.; Li, G.; Yu, H.; Zhang, L.; et al. Nioboixiolite-(□),(Nb0.8□0.2)4+O2, a New Mineral Species from the Bayan Obo World-Class REE-Fe-Nb Deposit, Inner Mongolia, China. Minerals 2025, 15, 88. https://doi.org/10.3390/min15010088
Li Y, Ke C, Wang D, Peng Z, Zhao Y, Li R, Chen Z, Li G, Yu H, Zhang L, et al. Nioboixiolite-(□),(Nb0.8□0.2)4+O2, a New Mineral Species from the Bayan Obo World-Class REE-Fe-Nb Deposit, Inner Mongolia, China. Minerals. 2025; 15(1):88. https://doi.org/10.3390/min15010088
Chicago/Turabian StyleLi, Yike, Changhui Ke, Denghong Wang, Zidong Peng, Yonggang Zhao, Ruiping Li, Zhenyu Chen, Guowu Li, Hong Yu, Li Zhang, and et al. 2025. "Nioboixiolite-(□),(Nb0.8□0.2)4+O2, a New Mineral Species from the Bayan Obo World-Class REE-Fe-Nb Deposit, Inner Mongolia, China" Minerals 15, no. 1: 88. https://doi.org/10.3390/min15010088
APA StyleLi, Y., Ke, C., Wang, D., Peng, Z., Zhao, Y., Li, R., Chen, Z., Li, G., Yu, H., Zhang, L., Guo, B., & Gao, Y. (2025). Nioboixiolite-(□),(Nb0.8□0.2)4+O2, a New Mineral Species from the Bayan Obo World-Class REE-Fe-Nb Deposit, Inner Mongolia, China. Minerals, 15(1), 88. https://doi.org/10.3390/min15010088







