Assessment of Feldspars from Central Portugal Pegmatites for Sustainable Ceramic Applications
Abstract
1. Introduction
2. Materials and Methods
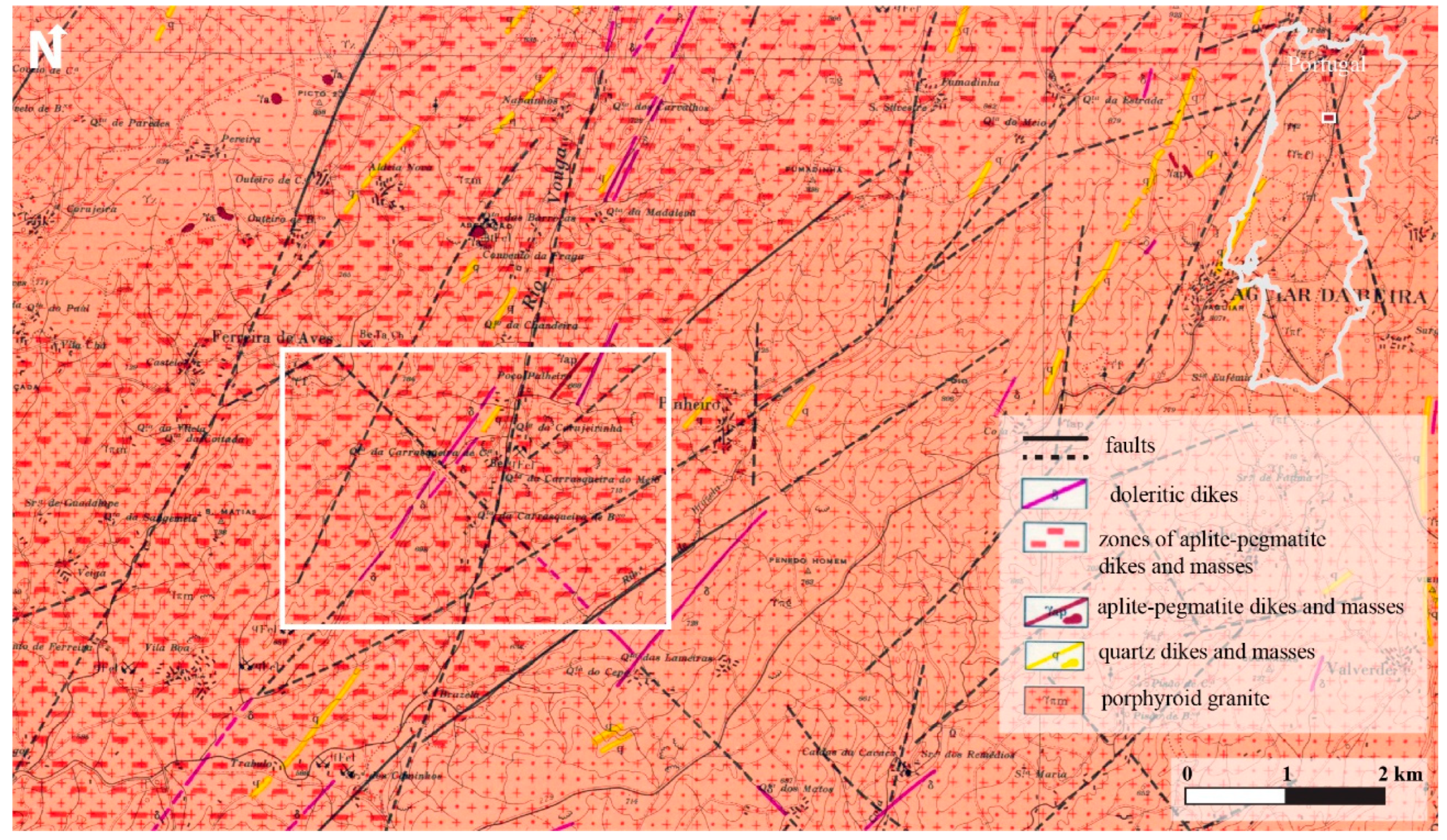
2.1. Study Area Context and Sampling
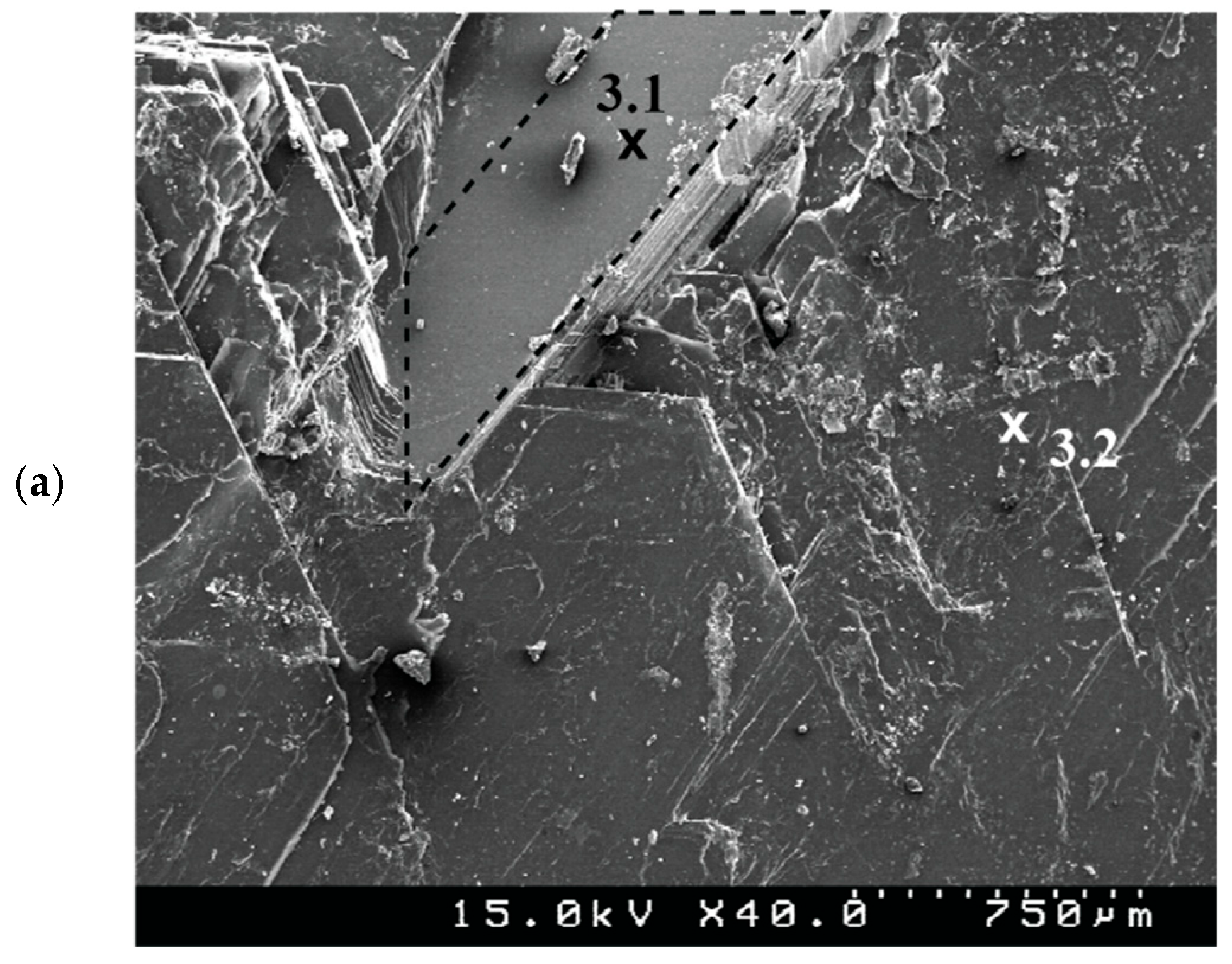
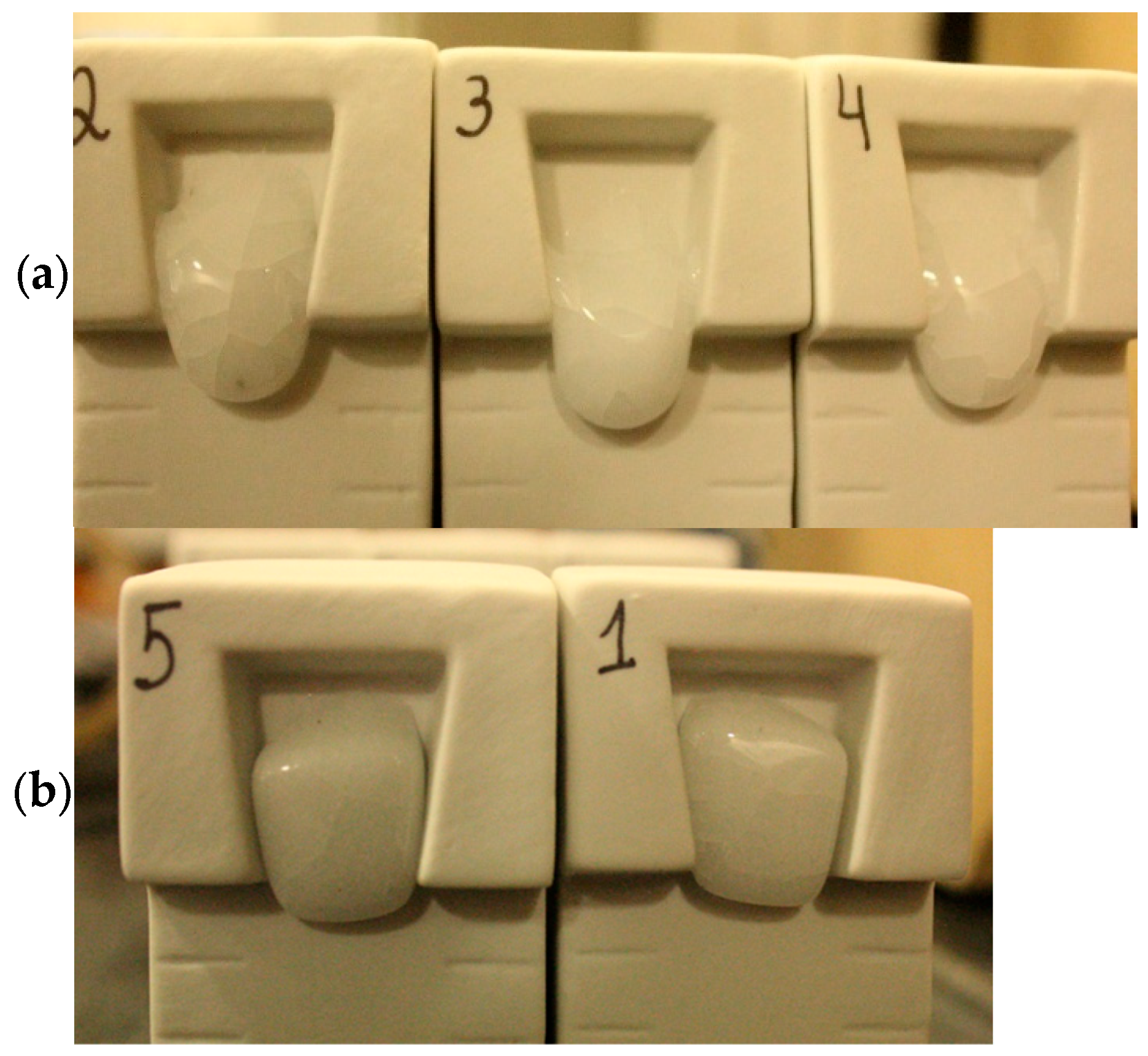
2.2. Samples Characterization
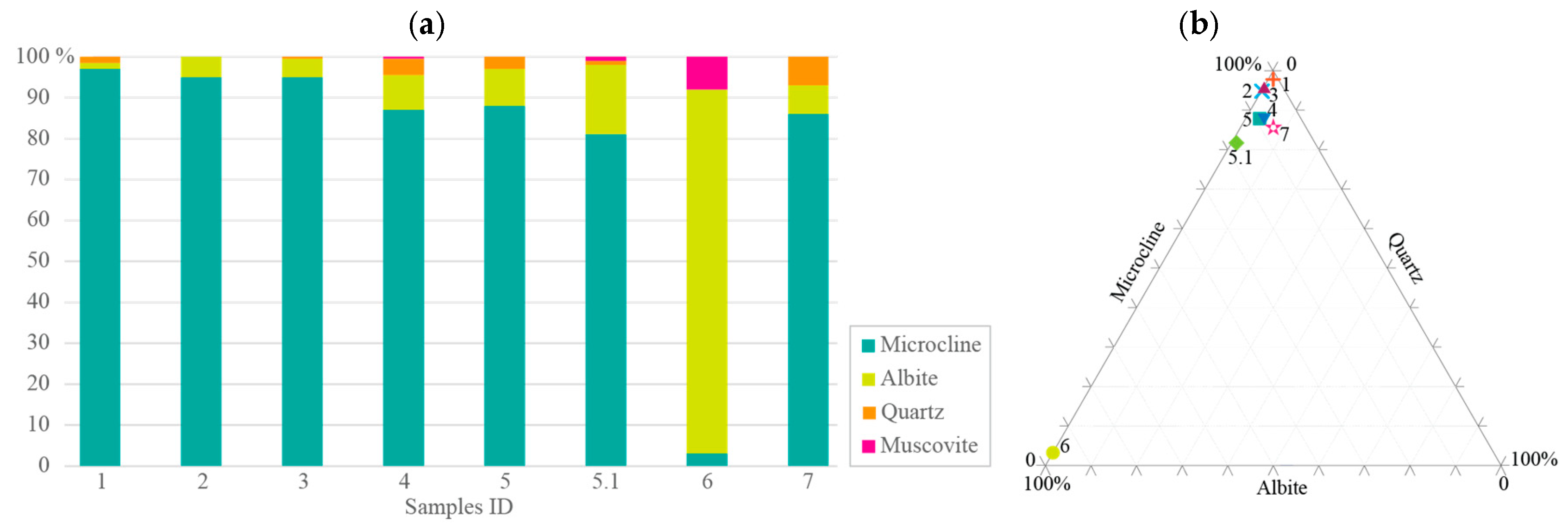
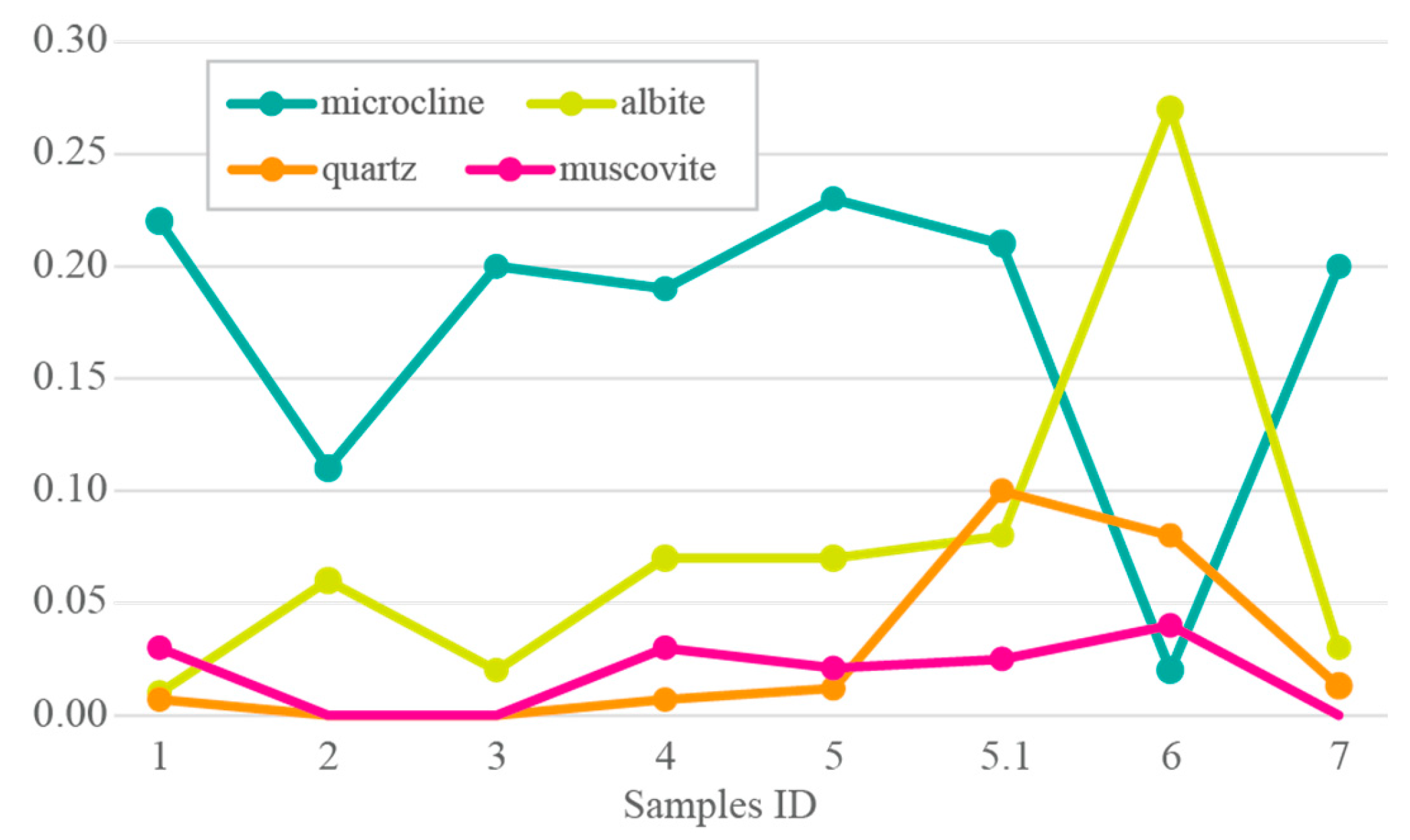
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carty, W.; Senapati, U. Porcelain-Raw materials, processing, phase evolution, and mechanical behavior. J. Am. Ceram. Soc. 1998, 81, 3–20. [Google Scholar] [CrossRef]
- Gaied, M.; Gallala, W. Beneficiation of feldspar ore for application in the ceramic industry: Influence of composition on the physical characteristics. Arab. J. Chem. 2015, 8, 186–190. [Google Scholar] [CrossRef]
- Das, S.K.; Dana, K. Differences in Densification Behaviour of K- and Na-Feldspar-Containing Porcelain Bodies. Thermochim. Acta 2003, 406, 199–206. [Google Scholar] [CrossRef]
- Lewicka, E.; Trenczek-Zajac, A. Investigations of feldspar-quartz raw materials after firing: Effect of various Na₂O/K₂O ratio and synthetic pigments addition. Minerals 2020, 10, 646. [Google Scholar] [CrossRef]
- Kayacı, K. The use of perlite as flux in the production of porcelain stoneware tiles. Bol. Soc. Esp. Cerám. V. 2021, 60, 283–290. [Google Scholar] [CrossRef]
- Lewicka, E. Rational use of selected mining by-products in the ceramic industry in Poland. Min. Res. Manag. 2020, 36, 59–76. [Google Scholar] [CrossRef]
- Li, Z.; Ma, G.; Zheng, D.; Zhang, X. Effect of Fe2O3 on the crystallization behavior, microstructure and properties of glass-ceramics prepared from the modified tailings after reduction of copper slag. Mater. Today Commun. 2022, 31, 103516. [Google Scholar] [CrossRef]
- Sawadogo, Y.; Sawadogo, M.; Sory, N.; Ouedraogo, M.; Dao, K.; Seynou, M.; Blanchart, P.; Zerbo, L. Porcelain: Raw materials, technological properties and applications-a review. J. Soc. Ouest Afr. Chim. 2024, 53, 29–44. [Google Scholar]
- Moore, D.; Reynolds, R. X-Ray Diffraction and the Identification and Analysis of Clay Minerals; Oxford University Press: Oxford, UK, 1989; ISBN 978-0-19-505170-4. [Google Scholar]
- Warr, L. A new collection of clay mineral ‘Crystallinity’ Index Standards and revised guidelines for the calibration of Kübler and Árkai indices. Clay Min. 2018, 53, 339–350. [Google Scholar] [CrossRef]
- Ozturk, Z.; Yildiz, B. The effect of different fluxes on thermal behavior of floor tile glazes. Acta Phys. Pol. A 2015, 127, 1183–1185. [Google Scholar] [CrossRef]
- Sokolář, R.; Keršnerová, L.; Šveda, M. The effect of different fluxing agents on the sintering of dry pressed porcelain bodies. J. Asian Ceram. Soc. 2017, 5, 241–247. [Google Scholar] [CrossRef]
- Liu, W.; Zuo, H. Mixed influence of Na2O and K2O on the viscous properties of aluminosilicate melt. Ceram. Int. 2025, 51, 17131–17137. [Google Scholar] [CrossRef]
- Dondi, M.; Conte, S.; Molinari, C.; Zanelli, C. Mineral Resources for the Ceramic Industry: Survey of Feldspathic Raw Materials in Italy. Minerals 2025, 15, 87. [Google Scholar] [CrossRef]
- Teixeira, C.; Medeiros, A.; Fernandes, A. Geological Map of Portugal, Scale of 1/50000: Explanatory News on the Sheet 14-D: Aguiar da Beira; Instituto Nacional de Engenharia, Tecnologia e Inovação: Lisbon, Portugal, 1972. [Google Scholar]
- Teixeira, J.; Chaminé, H.; Marques, J.; Carvalho, J.; Pereira, A.; Carvalho, M.; Fonseca, P.; Pérez-Alberti, A.; Rocha, F. A comprehensive analysis of groundwater resources using GIS and multicriteria tools (Caldas da Cavaca, Central Portugal): Environmental issues. Environ. Earth Sci. 2015, 73, 2699–2715. [Google Scholar] [CrossRef]
- Azevedo, M.R.; Aguado, B.V.; Nolan, J.; Martins, M.E.; Medina, J. Origin and emplacement of syn-orogenic Variscan granitoids in Iberia the Beiras massif. J. Virtual Explor. 2005, 19, 7. [Google Scholar] [CrossRef]
- Azevedo, M.; Aguado, B. Origem E Instalação de Granitóides Variscos na Zona Centro-Ibérica; Dias, R., Ed.; Geologia Pré-mesozóica de Portugal; Escolar Ed: Maputo, Mozambique, 2013; Volume 1, pp. 377–402. [Google Scholar]
- Pereira, R.; Alves, T.M. Tectono-stratigraphic signature of multiphased rifting on divergent margins (deep-offshore southwest Iberia, North Atlantic). Tectonics 2012, 31. [Google Scholar] [CrossRef]
- Portela, L.; Azevedo, M.R.; Medina, J.; Aguado, B.V. The Lusinde late-post-tectonic Variscan granite (Central Iberian Zone): Pluton emplacement at the termination of the Juzbado-Penalva do Castelo shear zone. J. Iber. Geol. 2024. [Google Scholar] [CrossRef]
- Santos, J.; Lopes, J.; Pereira, V. Geological Map of Portugal, Sheet 14-D, 1st ed.; Portuguese Geological Services: Lisbon, Portugal, 1972. (In Portuguese) [Google Scholar]
- Dinis, P. Prospeçao, Pesquisa e Caracterizaçao de Pegmatitos em Pinheiro-Aguiar da Beira. Master’s Thesis, University of Aveiro, Aveiro, Portugal, 1999. [Google Scholar]
- Trabulo, L.; Gomes, L.; Nunes, L. Enquadramento Geologico, Estrutura e Paragénese do Grupo Pegmatítico de Senhora de Assunçao-Aguiar da Beira-Centro de Portugal; Universidade do Porto: Porto, Portugal, 1995; Volume 4, pp. 837–841. (In Portuguese) [Google Scholar]
- Oliveira, A.; Rocha, F.; Rodrigues, A.; Jouanneau, J.; Dias, A.; Weber, O.; Gomes, C. Clay minerals from the sedimentary cover from the Northwest Iberian shelf. Prog. Oceanogr. 2002, 52, 233–247. [Google Scholar] [CrossRef]
- Singer, F.; Singer, S. Industrial Ceramics; Chapman & Hall: London, UK, 1979. [Google Scholar]
- Warr, L.; Rice, A. Interlaboratory standardization and calibration of clay mineral crystallinity and crystallite size data. J. Metamorph. Geol. 1994, 12, 141–152. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Sun, N.; Liu, R.; Wang, Z.; Wang, L.; Sun, W. Systematic review of feldspar beneficiation and its comprehensive application. Miner. Eng. 2018, 128, 141–152. [Google Scholar] [CrossRef]
- Ogbamikhumi, A.; Eguagie, A.J. Characterization of feldspars associated with pegmatite of Dagbala area for ceramics and glass production in southwestern Nigeria. J. Appl. Sci. Env. Manag. 2023, 27, 1009–1015. [Google Scholar] [CrossRef]
- Norton, F. Fine Ceramics: Technology and Applications; McGraw-Hill: New York, NY, USA, 1970. [Google Scholar]
- Abdel-Khalek, N.; Yehia, A.; Ibrahim, S. Beneficiation of Egyptian feldspar for application in the glass and ceramics industries. Miner. Eng. 1994, 7, 1193–1201. [Google Scholar] [CrossRef]
- Kyonka, J.; Cook, R. The Properties of Feldspars and Their Use in Whitewares; University of Illinois Bulletin: Champaign, IL, USA, 1954; Volume 51, p. 42. [Google Scholar]
- Silva, E.; Cunha, J.; Marinho, M. Pegmatitos da Região de Itambé, Bahia: Geologia de Potencialidade Económica; Série Arquivos Abertos 10; CBPM: Salvador, Brazil, 1996. (In Portuguese) [Google Scholar]
- Amaireh, M.; Aljaradin, M. Characterization of the Jordanian feldspar raw materials for application in the ceramic and glass industries. Int. J. Min. Eng. Min. Proc. 2014, 3, 28–31. [Google Scholar] [CrossRef]
- Kalyoncu, E.; Burat, F. Selective Separation of Coloring Impurities from Feldspar Ore by Innovative Single-stage Flotation. Miner. Process. Extr. Metall. Rev. 2021, 43, 910–915. [Google Scholar] [CrossRef]
- Liu, M.; Yang, X.; Zhao, L.; Guo, J.; Zhang, L.; Shao, Y. Effect of alkaline oxides (CaO and MgO) on the mechanical properties of SiC-based foam ceramics. Ceram. Int. 2024, 50, 10152–10159. [Google Scholar] [CrossRef]
- Baila, F.; Labbilta, T.; Darmane, Y. Feldspar Purification from Iron Impurities: A Review of Treatment Methods. Miner. Process. Extr. Metall. Rev. 2023, 45, 564–572. [Google Scholar] [CrossRef]
- Dong, W.; Bao, Q.; Zhou, J.-E.; Zhao, T.; Liu, K.; Hu, Z. Preparation of porcelain building tiles using “K2O–Na2O” feldspar flux as a modifier agent of low-temperature firing. J. Ceram. Soc. Jpn. 2017, 125, 690–694. [Google Scholar] [CrossRef]




| ID | Location | Coordinates |
|---|---|---|
| 1 | Quinta da Carrasqueira de Baixo | 40°47′52.6″ N 7°37′59.6″ W |
| 2 | Quinta da Carrasqueira | 40°48′09.0″ N 7°37′57.0″ W |
| 3 | Quinta da Carrasqueira de Cima I | 40°47′58.8″ N 7°37′39.9″ W |
| 4 | Quinta da Corujeirinha | 40°48′15.6″ N 7°37′36.9″ W |
| 5 | Poço Palheiros de Cima | 40°48′29.1″ N 7°37′19.6″ W |
| 5.1 | Poço Palheiros de Baixo | 40°48′26.2″ N 7°37′19.6″ W |
| 6 | Quinta da Carrasqueira de Cima II | 40°48′08.0″ N 7°37′19.8″ W |
| 7 | Pinheiro | 40°48′26.6″ N 7°36′50.8″ W |
| Variables | Samples ID | Ref. * | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 5.1 | 6 | 7 | ||
| SiO2 | 62.782 | 62.694 | 61.038 | 63.280 | 66.760 | 62.849 | 57.841 | 67.559 | 65–75 |
| Al2O3 | 18.334 | 19.211 | 21.378 | 18.893 | 16.955 | 18.841 | 21.997 | 17.060 | 17–24 |
| K2O | 16.887 | 13.764 | 12.788 | 13.773 | 12.610 | 13.637 | 2.165 | 11.208 | 3–15 |
| Na2O | 0.456 | 2.983 | 2.243 | 3.014 | 1.987 | 2.959 | 7.064 | 2.796 | 3–12 |
| Fe2O3 | 0.121 | 0.049 | 0.107 | 0.041 | 0.253 | 0.100 | 0.530 | 0.111 | <0.4 |
| P2O5 | 0.204 | 0.545 | 0.391 | 0.354 | 0.314 | 0.395 | 0.548 | 0.337 | <2 |
| SO3 | 0.021 | 0.009 | 0.011 | 0.009 | 0.010 | 0.007 | 0.013 | 0.006 | - |
| Cl- | nd | nd | 0.009 | nd | 0.006 | nd | nd | 0.010 | - |
| MgO | 0.229 | 0.137 | 0.487 | 0.099 | 0.117 | 0.098 | 0.246 | 0.167 | <0.5 |
| CaO | 0.140 | 0.066 | 0.041 | 0.062 | 0.230 | 0.071 | 0.405 | 0.064 | <2.5 |
| TiO2 | nd | nd | 0.009 | nd | nd | nd | 0.012 | nd | <0.2 |
| LOI | 0.620 | 0.370 | 1.320 | 0.360 | 0.600 | 0.890 | 9.060 | 0.570 | <2 |
| K2O/Na2O | 37.03 | 4.61 | 5.70 | 4.57 | 6.35 | 4.61 | 0.31 | 4.01 | 3.1 |
| Na2O + K2O | 17.34 | 16.75 | 15.03 | 16.79 | 14.60 | 16.60 | 9.23 | 14.00 | ≥11 |
| MgO + CaO | 0.369 | 0.203 | 0.528 | 0.161 | 0.347 | 0.169 | 0.651 | 0.231 | <2 |
| Variable | 1 | 2 | 3 | 4 | 5 | 5.1 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|
| Ba | 400.0 | 25.1 | 59.1 | 110.0 | 290.0 | 41.3 | 9.1 | 34.8 |
| Br | 2.7 | 2.4 | 4.2 | 1.5 | 3.8 | 4.9 | 4.2 | 1.8 |
| Cr | 5.3 | 4.3 | 8.8 | 4.3 | 8.1 | 12.9 | 6.1 | 16.7 |
| Cs | 28.7 | 34.2 | 44.1 | 12.7 | 19.3 | 14.8 | 35.5 | 25.4 |
| Cu | 7.5 | 3.2 | 3.8 | 4.6 | nd | 8.2 | 31.2 | nd |
| Ga | 11.9 | 18.6 | 19.3 | 17.3 | 17.3 | 12.7 | 33.9 | 15.2 |
| Mn | 41.3 | 19.5 | 26.6 | 15.5 | 29.3 | 53.6 | 260.0 | 25.2 |
| Pb | 19.9 | 29.9 | 38.2 | 46.6 | 49.5 | 9.8 | 11.2 | 28.6 |
| Sn | 7.1 | 17.7 | 17.1 | 12.5 | 14.2 | 9.9 | 34.9 | 12.1 |
| Sr | 32.7 | 8.5 | 8.1 | 31.7 | 44.4 | 14.4 | 21.3 | 11.9 |
| Tl | 8.9 | 8.5 | 7.6 | 4.2 | 5.9 | 7.0 | nd | 4.7 |
| U | 1.9 | 3.0 | 1.8 | 2.5 | 3.3 | 1.7 | 5.9 | 3.0 |
| Zn | 9.5 | 1.4 | nd | nd | 2.7 | 4.9 | 15.9 | 1.6 |
| Zr | nd | nd | nd | nd | nd | 8.0 | 25.5 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candeias, C.; Gomes, A.; Rocha, F. Assessment of Feldspars from Central Portugal Pegmatites for Sustainable Ceramic Applications. Minerals 2025, 15, 527. https://doi.org/10.3390/min15050527
Candeias C, Gomes A, Rocha F. Assessment of Feldspars from Central Portugal Pegmatites for Sustainable Ceramic Applications. Minerals. 2025; 15(5):527. https://doi.org/10.3390/min15050527
Chicago/Turabian StyleCandeias, Carla, Adga Gomes, and Fernando Rocha. 2025. "Assessment of Feldspars from Central Portugal Pegmatites for Sustainable Ceramic Applications" Minerals 15, no. 5: 527. https://doi.org/10.3390/min15050527
APA StyleCandeias, C., Gomes, A., & Rocha, F. (2025). Assessment of Feldspars from Central Portugal Pegmatites for Sustainable Ceramic Applications. Minerals, 15(5), 527. https://doi.org/10.3390/min15050527








