Effect of Calcium Chloride on the Reinforcement of Uranium Tailings with Sodium Hydroxide–Sodium Silicate–Metakaolin
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and Sample Descriptions
2.2. Batch Grouting Experiments
2.3. Test of Uranium Mill Tailings Solidified by Grouting
2.4. Triaxial Strength Test
2.5. Uranium Concentration Content Change Test
2.6. Radon Exhalation Rate Test
2.7. XRD, XPS Test Experiment
3. Results and Discussions
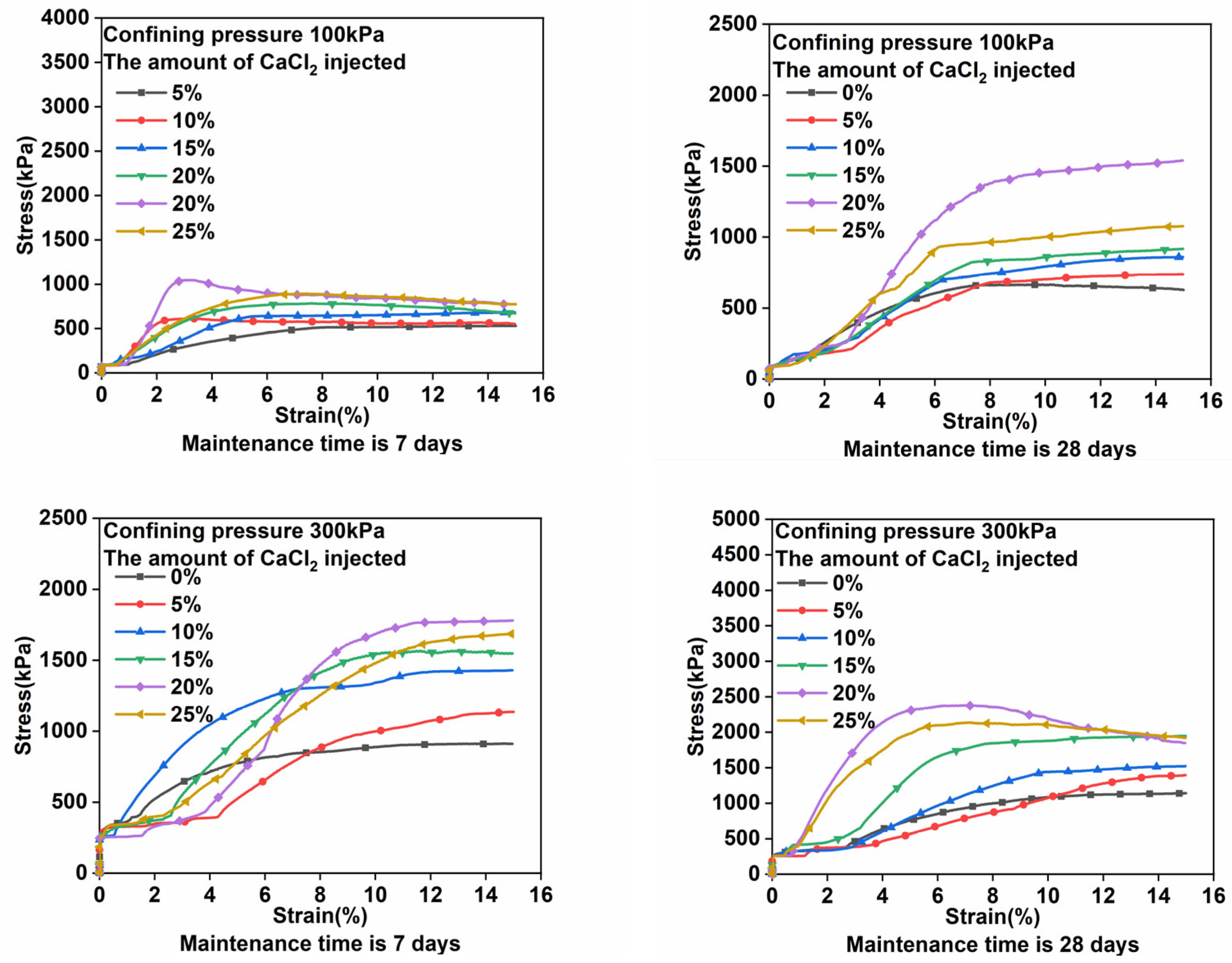
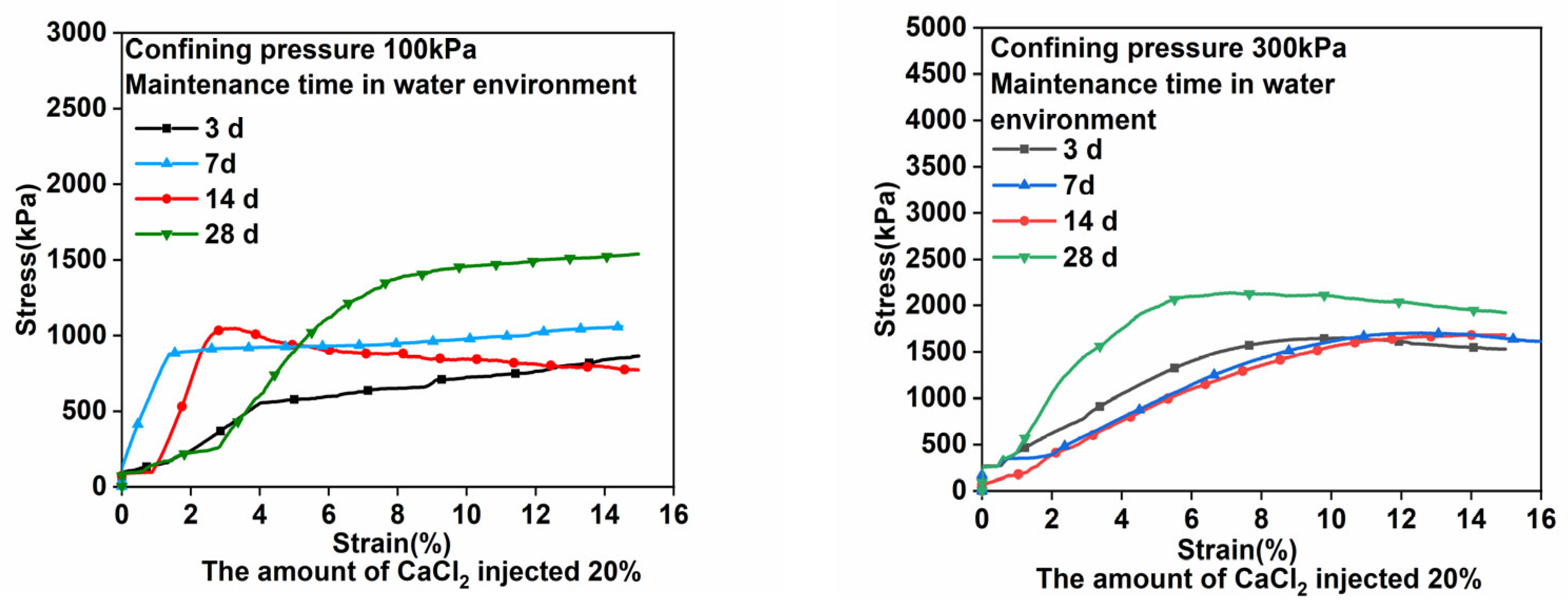
3.1. Triaxial Compression Test Results and Analyses
3.2. Hydraulicity Strength Test
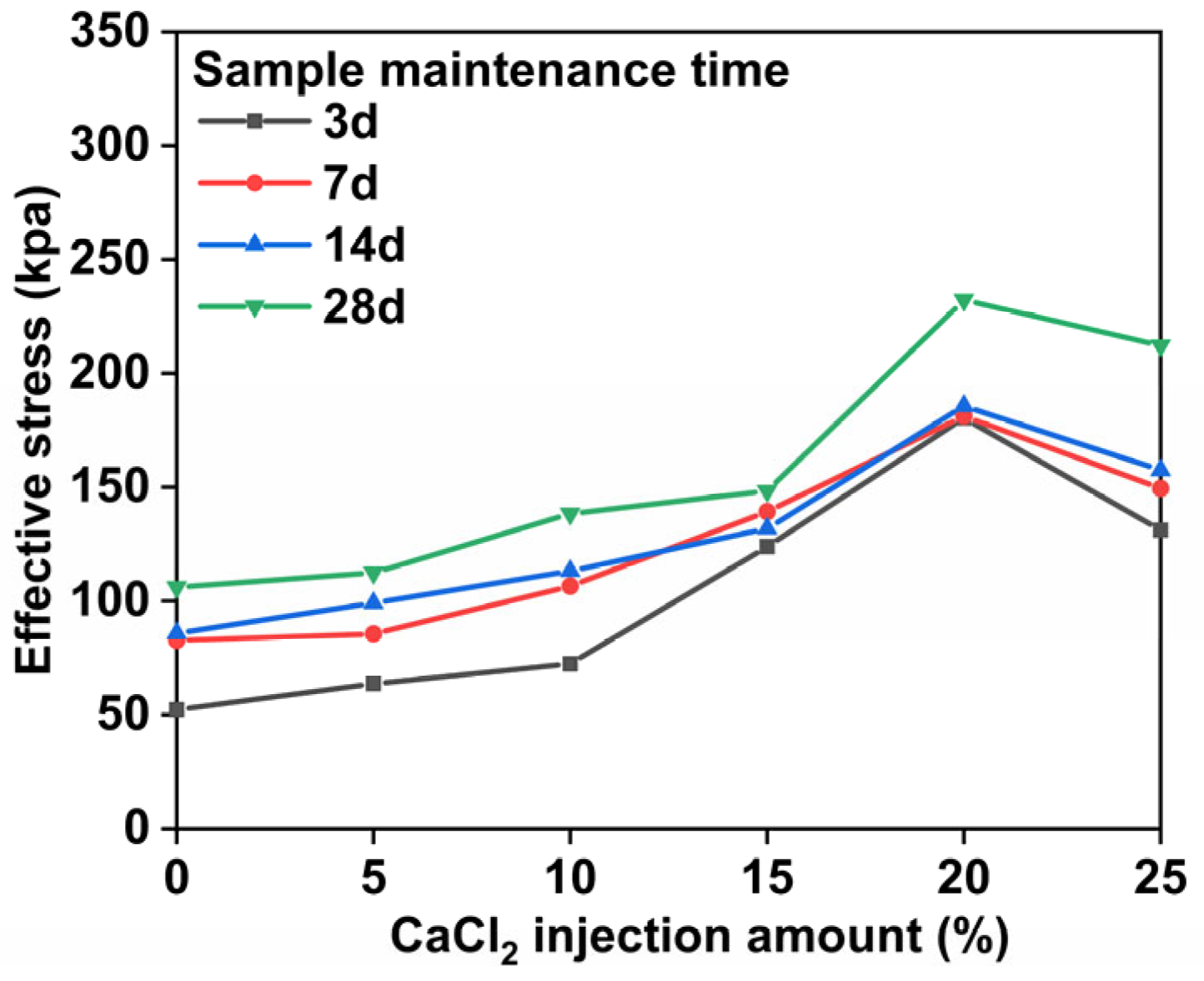
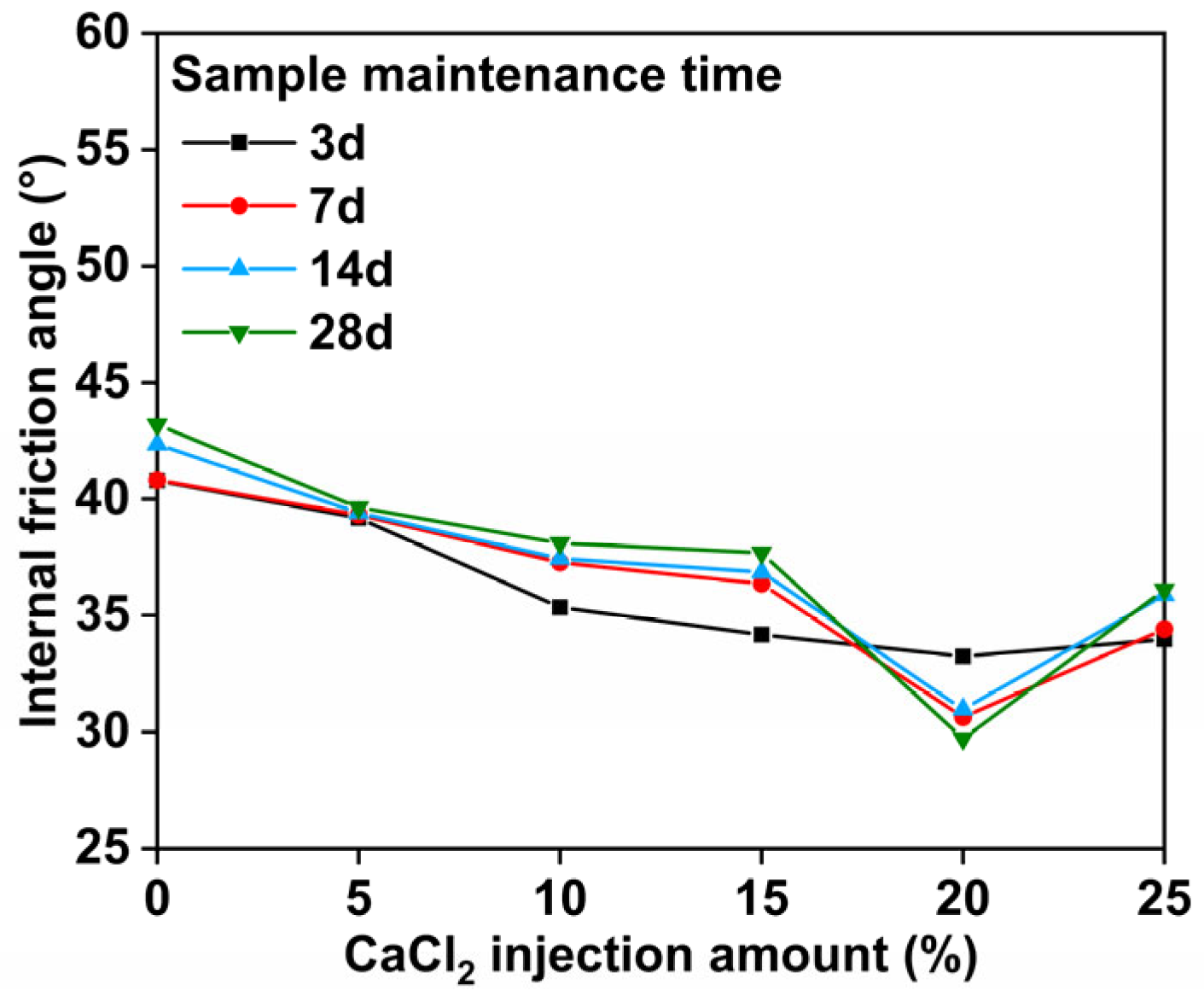
3.3. Analysis of Effective Stress and Internal Friction Angle Results
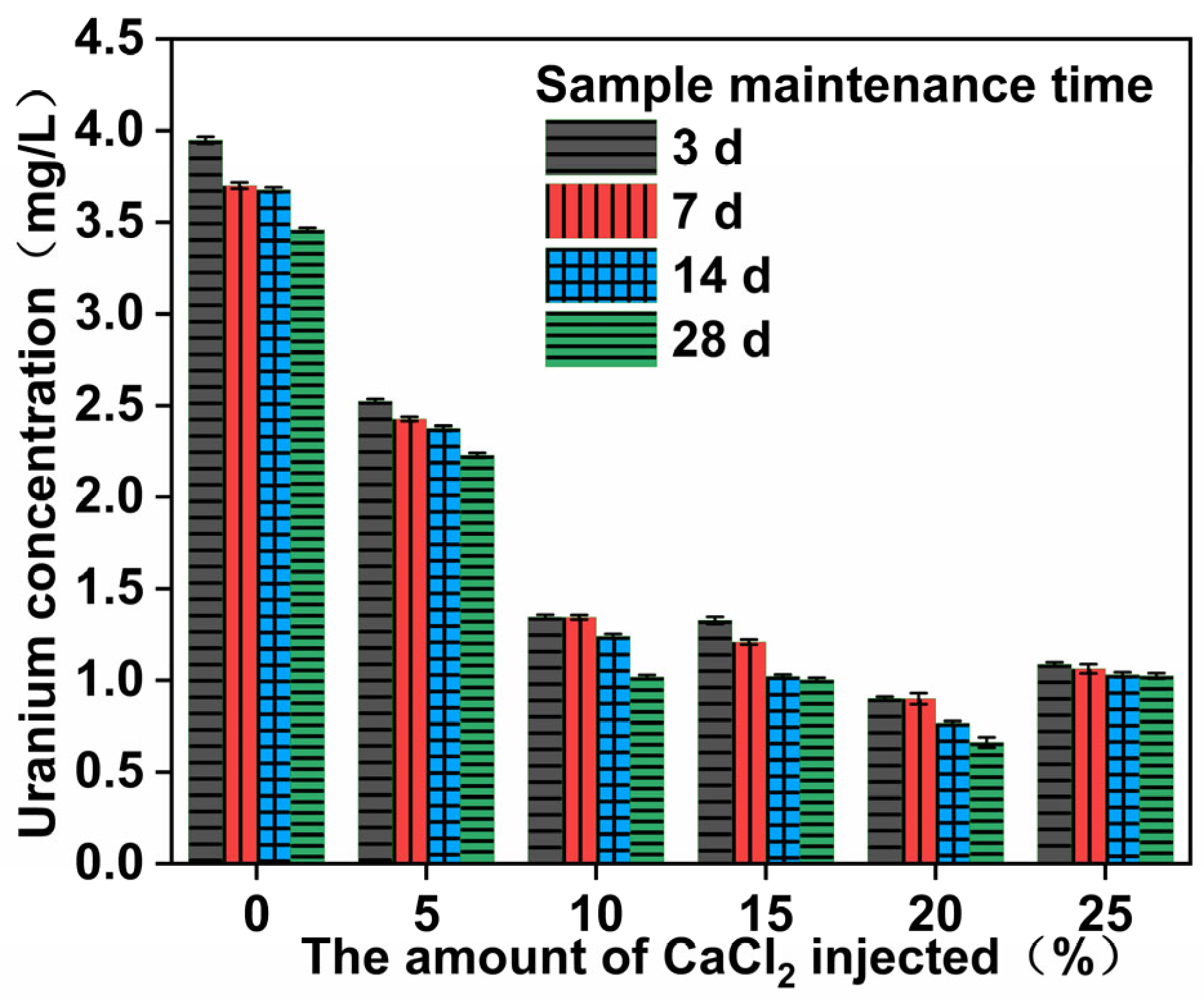
3.4. Dissolved U Concentration and Analysis
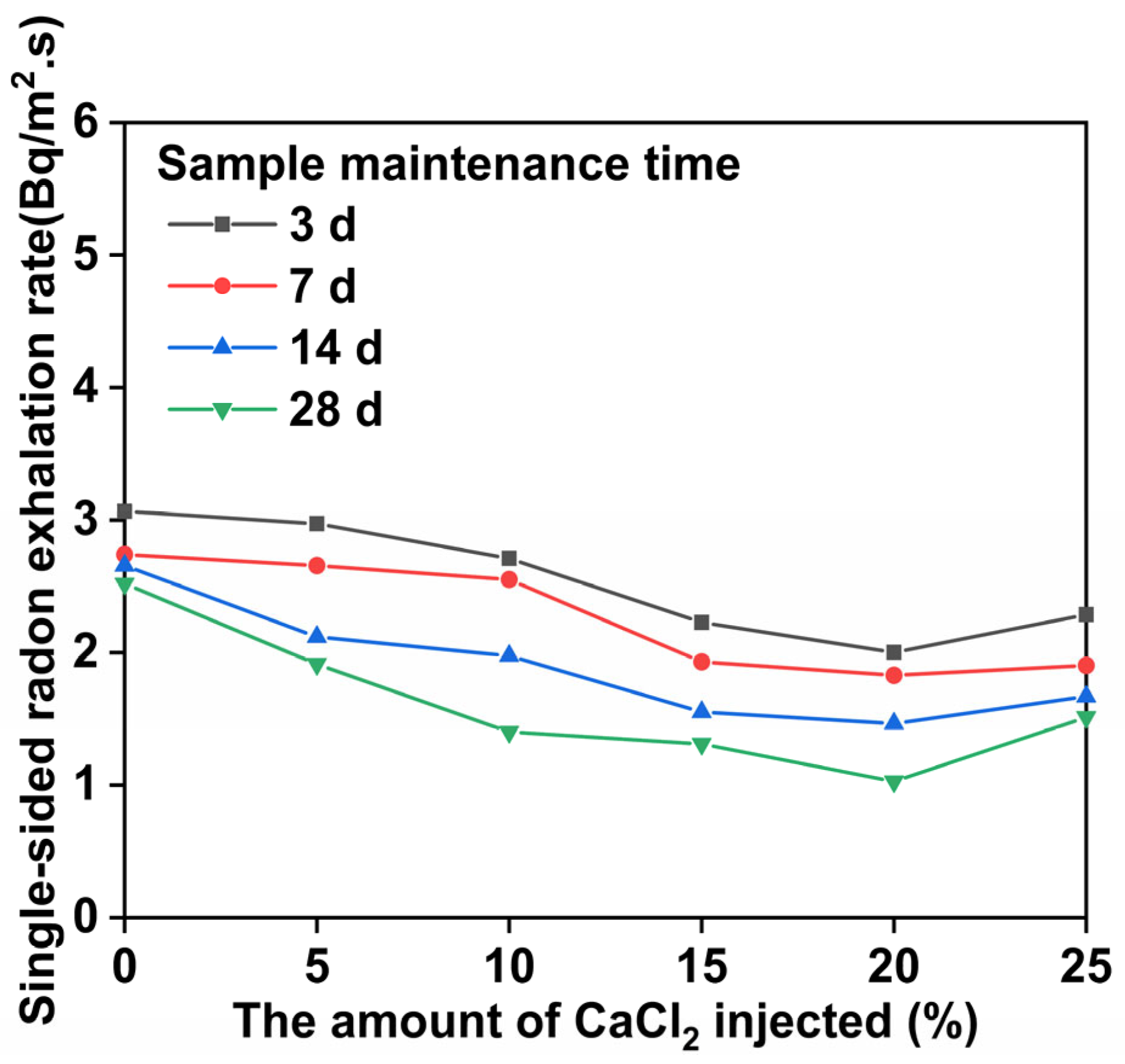
3.5. Results and Analysis of the Rn Exhalation Test
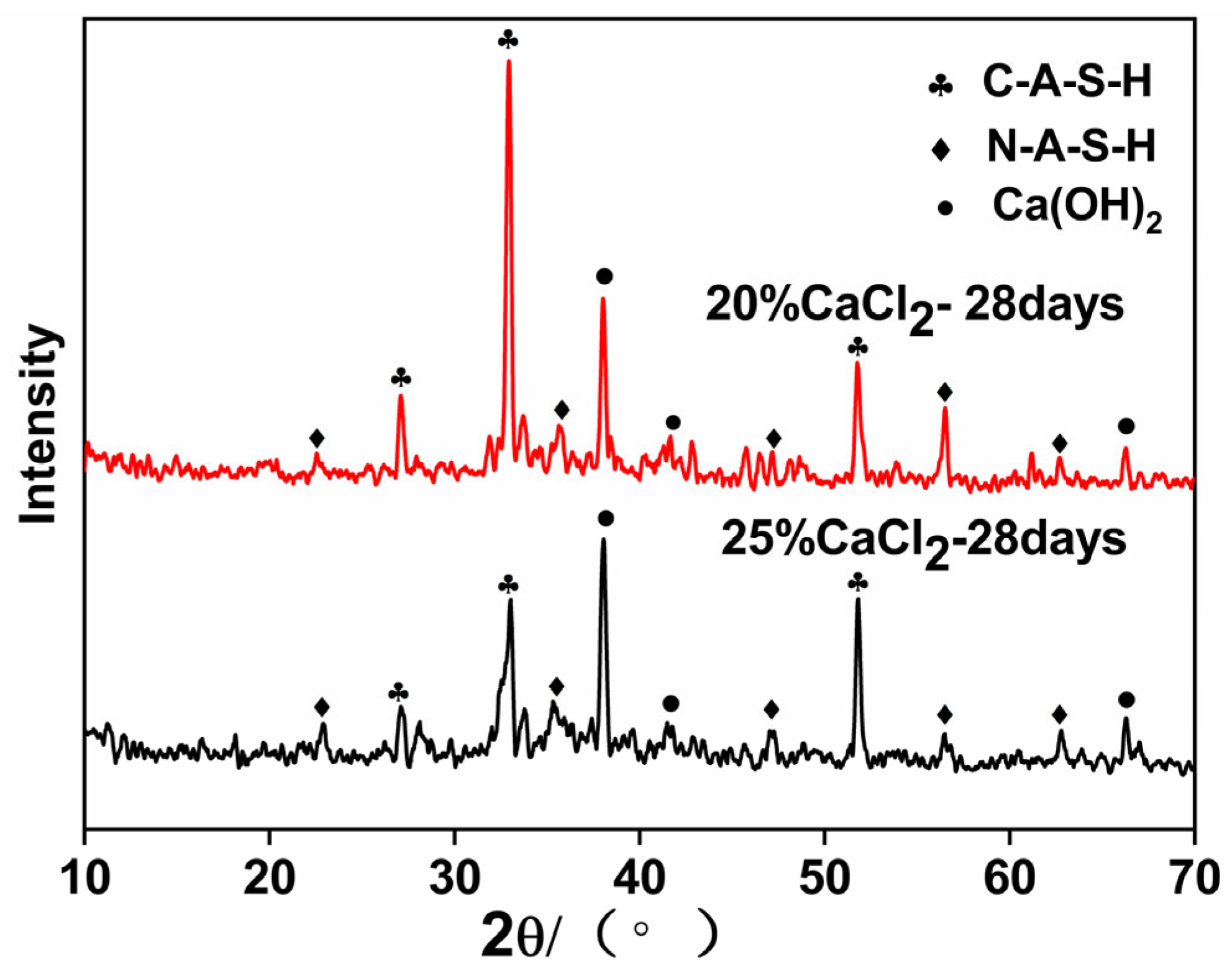
3.6. XRD Test Results Analysis
3.7. Microstructure Analysis
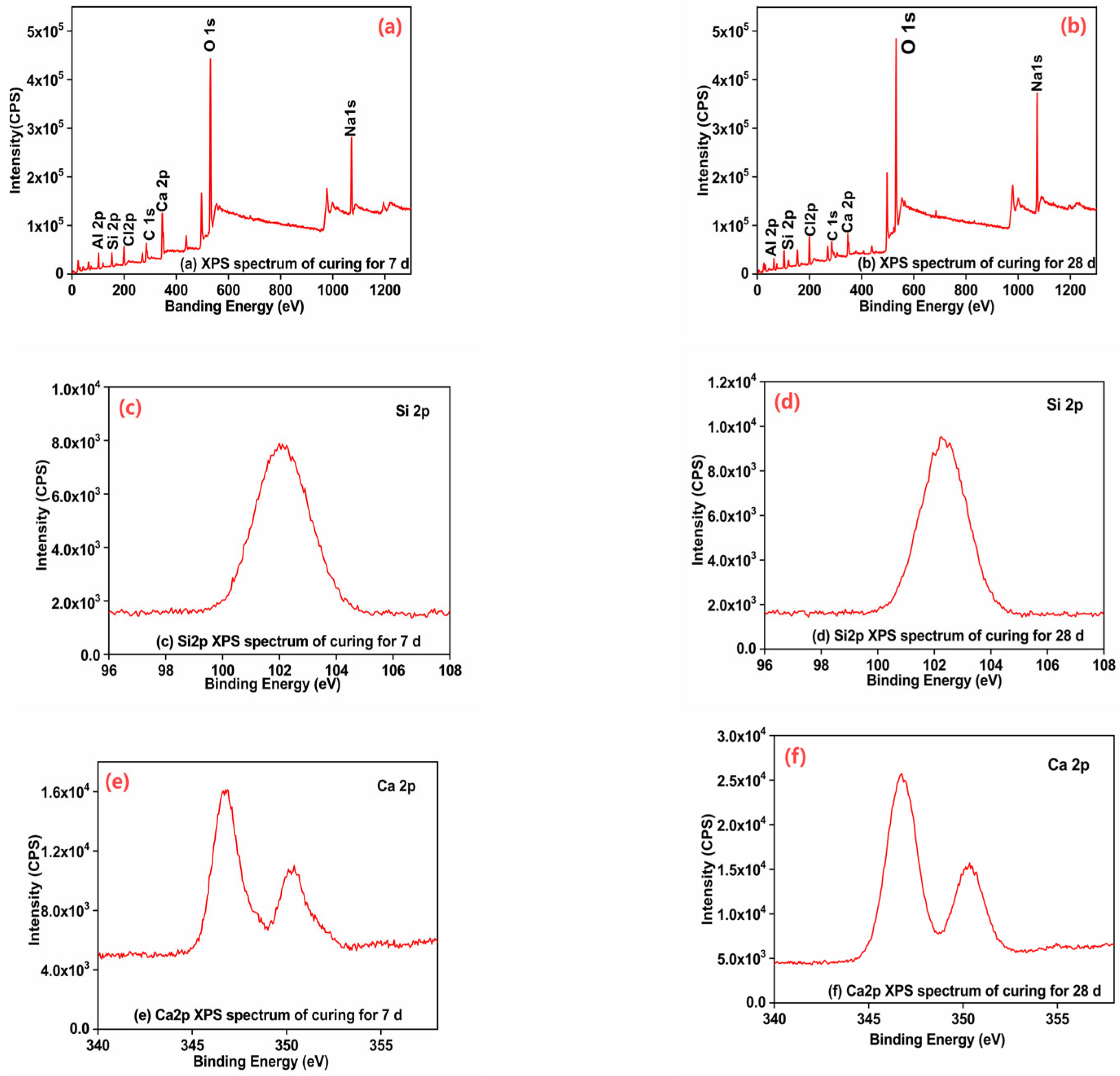
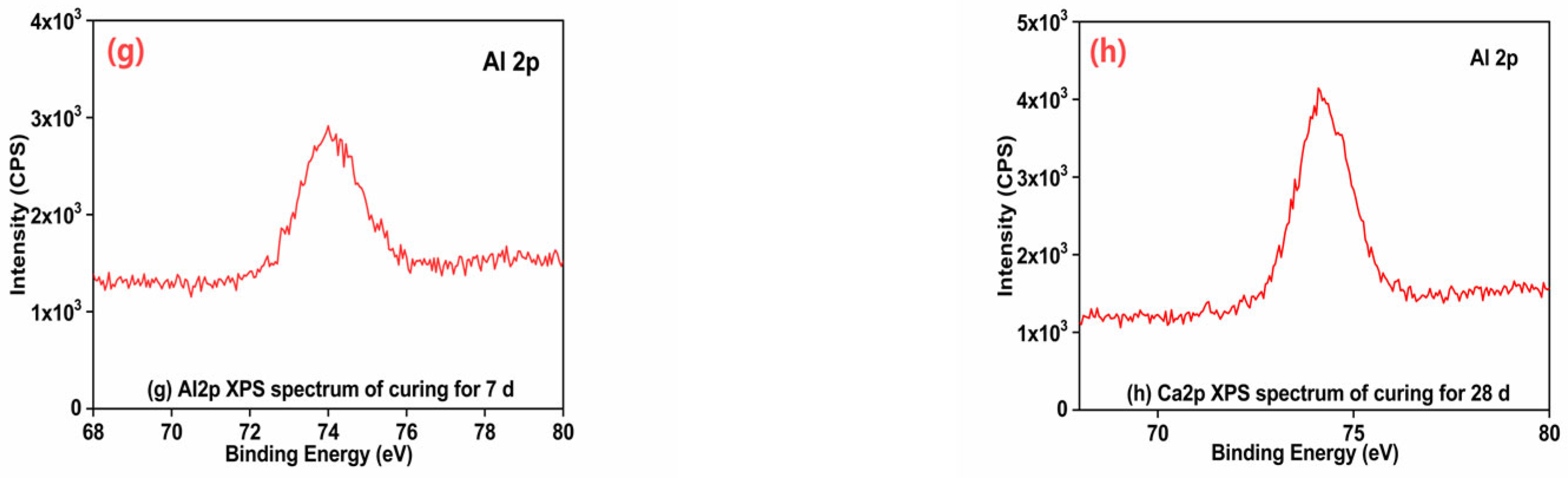
3.8. Analysis of the XPS Test Results
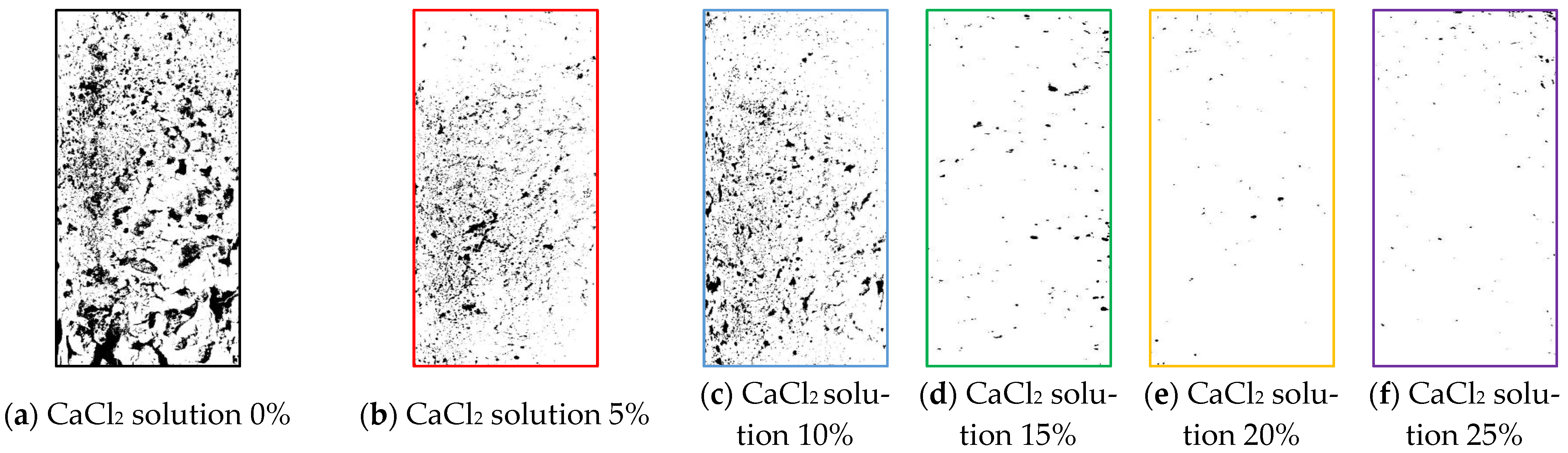
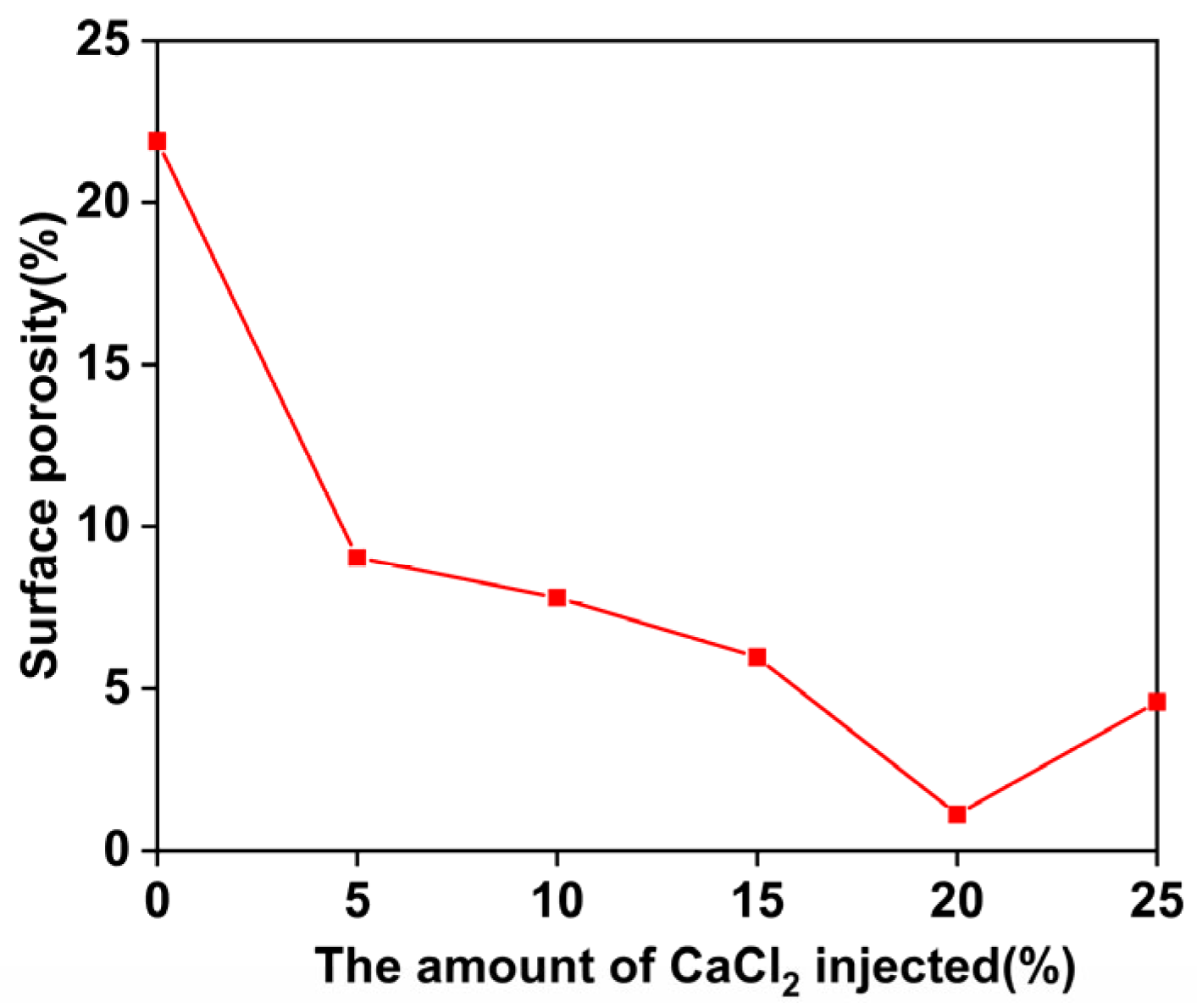
3.9. Microscopic Detection Image Analysis
4. Conclusions
- (1)
- The stronger the alkali-excited uranium tailing solidified body is, the higher the injection amount of CaCl2. If CaCl2 is injected at excessively high concentrations, the gel will erode and the mechanical properties of the solidified body will be affected. Therefore, the mass fraction of the injected amount of CaCl2 should be controlled at about 20%.
- (2)
- Under the assumption of the use of an appropriate amount of CaCl2, the longer the curing time, the higher the degree of polymerization, and the stronger the solidified body of alkali-activated uranium tailings. Compared with the strength before curing, the strength of the sample is doubled when the CaCl2 injection amount is 20% and the curing time is 28 days.
- (3)
- By adding excessive amounts of CaCl2, the cementation force and chemical bond force between the particles of the solidified body are both weakened, the structure of the solidified body is altered, and its mechanical properties are reduced. With a 25% injection of CaCl2, there is a 241 kPa difference between the 20% injection and the 25% injection.
- (4)
- The strengthening of uranium tailings using CaCl2–NaOH–sodium silicate–metakaolin can enhance the material’s mechanical properties, reduce the radon exhalation rate, and reduce the uranium leaching rate. Radon exhalation is reduced to 51.6% of the original sample, while uranium leaching is reduced by 88%.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horpibulsuk, S.; Rachan, R.; Chinkulkijniwat, A.; Raksachon, Y.; Suddeepong, A. Analysis of strength development in cement-stabilized silty clay from microstructural considerations. Constr. Build. Mater. 2010, 24, 2011–2021. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, X.; Hu, R.; Shi, Y.; Han, T.; Yu, J. Experimental study on caustic soda-excited steel slag powder in silty soil. Chin. J. Geotech. Eng. 2017, 39, 2187–2194. [Google Scholar]
- Wang, D.; Wang, H.; Zou, W.; Wang, X.; Li, L. Study on the Microscopic Mechanism of Alkali-Excited Fly Ash Solidification Sludge. Chin. J. Rock Mech. Eng. 2019, 38, 3197–3205. [Google Scholar] [CrossRef]
- Yang, A.; Yan, S.; Du, D.; Zhao, R.; Liu, J. Experimental study on the effect of alkaline environment on the strength of solidified marine soft soil in Tianjin. Geotech. Mech. 2010, 31, 2930–2934. [Google Scholar] [CrossRef]
- Cuccia, V.; Freire, C.B.; Ladeira, A.C.Q. Radwaste oil immobilization in geopolymer after non-destructive treatment. Prog. Nucl. Energy 2020, 122, 103246. [Google Scholar] [CrossRef]
- Galhardi, J.A.; García-Tenorio, R.; Bonotto, D.M.; Francés, I.D.; Motta, J.G. Natural radionuclides in plants, soils, and sediments affected by uranium-rich coal mining activities in Brazil. J. Environ. Radioact. 2017, 177, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ma, Q. Test and analysis of compressive performance of alkali-metakaolin-modified soil cement. J. Anhui Univ. Sci. Technol. (Nat. Sci. Ed.) 2020, 40, 35–40. [Google Scholar]
- Jiang, F.; Tan, B.; Wang, Z.; Liu, Y.; Hao, Y.; Zhang, C.; Wu, H.; Hong, C. Preparation and related properties of geopolymer-solidified uranium tailings bodies with various fibers and fiber content. Environ. Sci. Pollut. Res. 2022, 29, 20603–20616. [Google Scholar] [CrossRef]
- Zhang, K.; Yin, Z.; Cheng, G.; Zhou, J. Experimental study on the durability of concrete with polycarboxylate superplasticizer and different admixtures. Silic. Bull. 2018, 37, 2974–2978+2984. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Han, B. Analysis of Factors Affecting Bonding Properties of Water Glass-Calcium Chloride. Zhongzhou Coal 2012, 5–7. [Google Scholar]
- Zhang, F.; Yang, J.; Jin, W.; Zhang, L. Experimental study on the change law of permeability coefficient of water glass-calcium chloride solidified sandy soil. Sci. Technol. Bull. 2019, 35, 143–146+156. [Google Scholar] [CrossRef]
- Sawada, K.; Enokida, Y.; Tsukada, T. Oxidation and vitrification of aluminum with lead borate glass for low level radioactive waste treatment. J. Nucl. Sci. Technol. 2020, 57, 671–677. [Google Scholar] [CrossRef]
- Han, X.; Zhang, T.; He, B.; Xu, S. Analysis of Factors Affecting Strength of Fine Sand Body Reinforced by Sodium Glass-Calcium Chloride. Shaanxi Coal 2013, 32, 8–11. [Google Scholar]
- Southeast University; Zhejiang University. Soil Mechanics; China Construction Industry Press: Beijing, China, 2010; pp. 63–67. [Google Scholar]
- Thevanayagam, S.; Martin, G.R. Liquefaction in silty soils—Screening and remediation issues. Soil Dyn. Earthq. Eng. 2002, 22, 1035–1042. [Google Scholar] [CrossRef]
- Fredlund, D.G.; Morgenstern, N.R.; Widger, R.A. The shear strength of unsaturated soils. Can. Geotech. J. 1978, 15, 313–321. [Google Scholar] [CrossRef]
- Yang, B.; Liu, Y.; Wan, F.; Yang, T.; Feng, J.; Zhao, X.; Zheng, D. Experimental study on the influence of gradation characteristics on the permeability coefficient of sandy soil. J. Southwest Jiaotong Univ. 2016, 51, 855–861. [Google Scholar]
- Dang, F.; Liu, H.; Wang, X.; Xue, H.; Ma, Z. Research on empirical formula of permeability coefficient of cohesive soil based on effective void ratio. Chin. J. Rock Mech. Eng. 2015, 34, 1909–1917. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S. Geopolymerisation of multiple minerals. Miner. Eng. 2002, 15, 1131–1139. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Lee, W.K.W.; Van Deverter, J.S.J. The effect of inorganic salt contamination on the strength and durability of geopolymers. Colloids Surf. A 2002, 211, 115–126. [Google Scholar] [CrossRef]
- Panagiotopoulou, C.; Kontori, E.; Perraki, T.; Kakali, G. Dissolution of alumisilicate and by-products in alkaline media. J. Mater. Sci. 2007, 42, 2967–2973. [Google Scholar] [CrossRef]
- Joseph, D.; Linwood, S.J. Early High-Strength Concrete Composition. EP Patent EP0153097 A2, 13 December 1989. [Google Scholar]
- Ye, J.; Zhang, W.; Shi, D. The coagulation-enhancing effect of calcium on alkali-stimulated cementitious materials. J. Silic. 2017, 45, 1101–1112. [Google Scholar] [CrossRef]
- Jaarsveld, J.; Deventer, J.; Lukey, G.C. The characterization of source materials in fly ash-based geopolymers. Mater. Lett. 2003, 57, 1272–1280. [Google Scholar] [CrossRef]
- Rakhimova, N.R.; Rakhimov, R.Z. Hydrated portland cement as an admixture to alkali-activated slag cement. Adv. Cem. Res. 2015, 27, 107–117. [Google Scholar] [CrossRef]
- Yliniemi, J.; Pesonen, J.; Tiainen, M.; Illikainen, M. Alkali activation of recovered fuel-biofuel fly ashfrom fluidised-bed combustion: Stabilisation/solidification of heavy metals. Waste Manag. 2015, 43, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, Q.; Zhou, Y.; Yang, Q.; Guo, H. Modification of glass structure via Cao addition in granulated copper slag to enhance its pozzolanic activity. Constr. Build. Mater. 2020, 240, 117970. [Google Scholar] [CrossRef]
- Jiao, H.Z.; Wang, S.F.; Wu, A.X.; Shen, H.M.; Wang, J.D. Cementitious property of NaAlO2-activated Ge slag as cement supplement. Int. J. Miner. Metall. Mater. 2019, 26, 1594–1603. [Google Scholar] [CrossRef]
- Mobasher, N.; Bernal, S.A.; Provis, J.L. Structural evolution of an alkali sulfate activated slag cement. J. Nucl. Mater. 2016, 468, 97–104. [Google Scholar] [CrossRef]
- Tantawy, M.A.; Ahmed, S.A.; Abdalla, E.M.; Qassim, M.I. Immobilization of copper ions-laden kaolin waste: Influence of thermal treatment on its immobilization in cement paste. J. Mater. Cycles Waste Manag. 2016, 18, 263–272. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Criado, M. Microstructure development of alkali-activated fly ash cement: A descriptive model. Cem. Concr. Res. 2005, 35, 1204–1209. [Google Scholar] [CrossRef]
- Glukhovsky, V.D.D. Soil Silicates, Their Properties, Technology and Manufacturing, and Fields of Application; Civil Engineering Institute: Kiev, Ukraine, 1965. [Google Scholar]
- Duxson, P.; Mallicoat, S.W.; Lukey, G.C.; Kriven, W.M.; Deventer, J.S.J.V. The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Colloids Surf. A 2007, 292, 8–20. [Google Scholar] [CrossRef]
- Ilia, D. Study on the Workability of Alkali-Slag Cementitious Materials. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2015. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Shi, C. Strength, pore structure and permeability of alkali-activated slag mortars. Cem. Concr. Res. 1996, 26, 1789–1799. [Google Scholar] [CrossRef]
- Davidovist, J. Early High-Strength Mineral Polymer. US Patent No. 4509985, 9 April 1985. [Google Scholar]
- Davidovits, J. Synthetic Mineral Polymer Compound of the Silicoaluminates Family and Preparation Process. US Patent US4472199 A, 18 September 1984. [Google Scholar]
- Vandevenne, N.; Iacobescu, R.I.; Carleer, R.; Samyn, P.; D’Haen, J.; Pontikes, Y.; Schreurs, S.; Schroeyers, W. Alkali-activated materials for radionuclide immobilization and the effect of precursor composition on Cs/Sr retention. J. Nucl. Mater. 2018, 510, 575–584. [Google Scholar] [CrossRef]
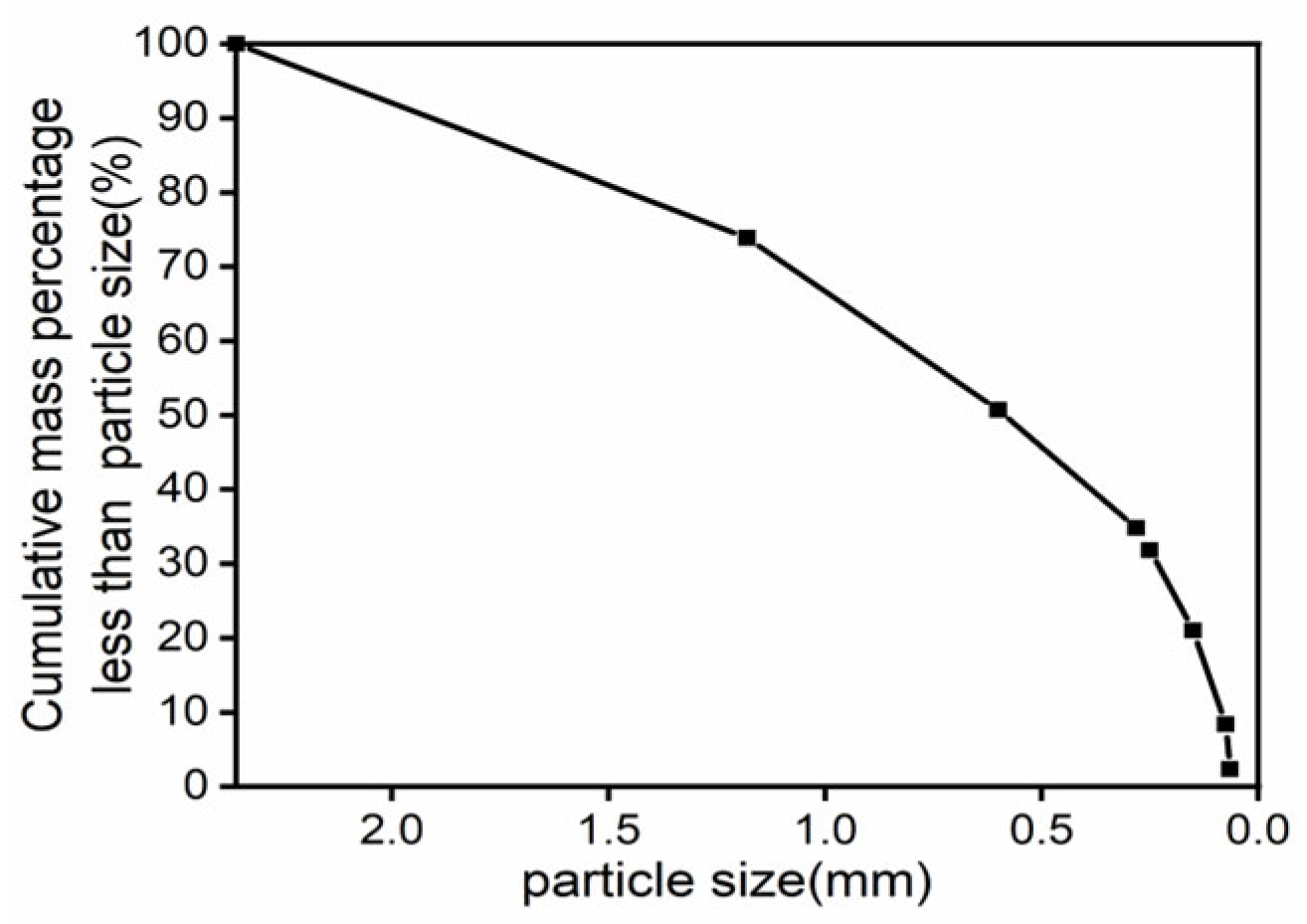
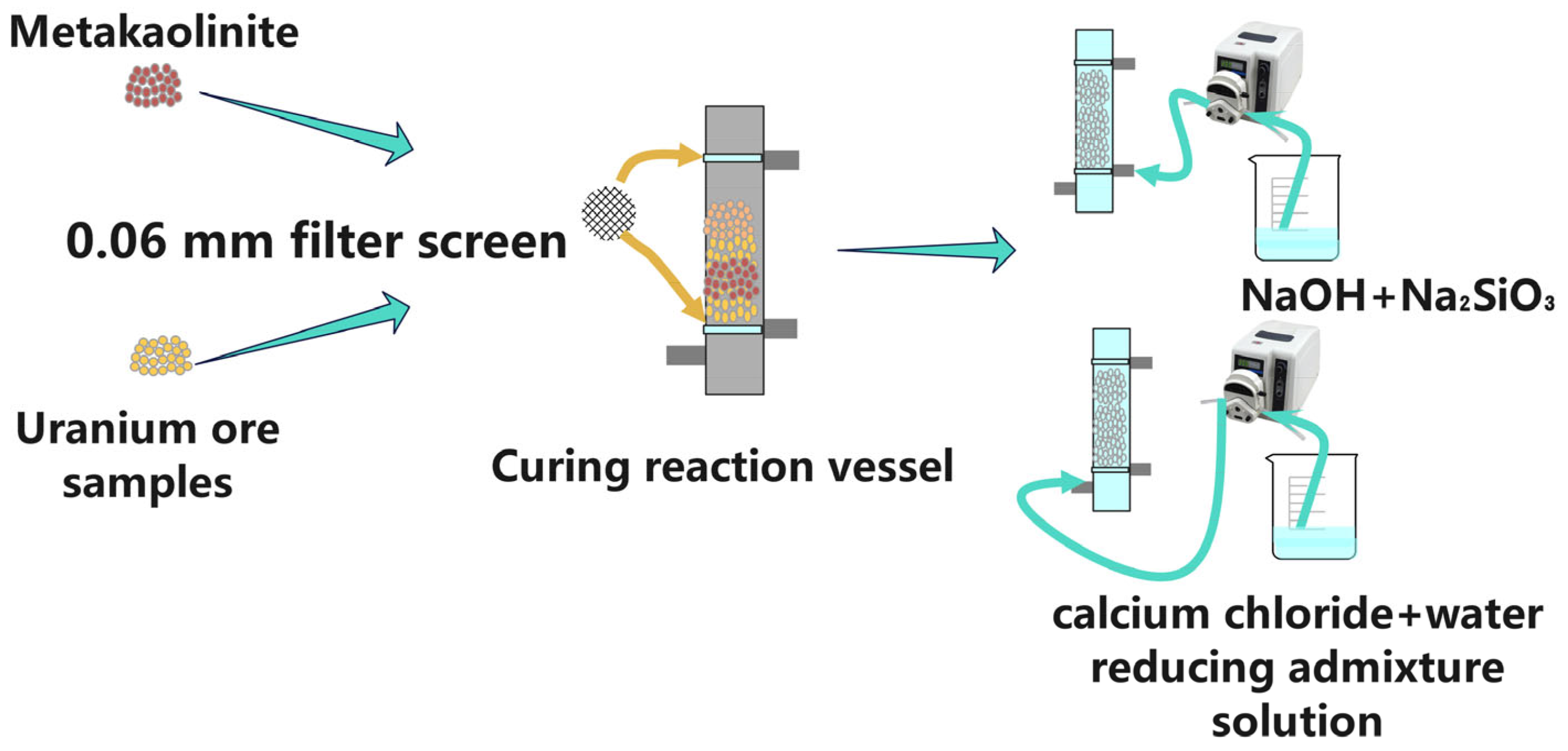








| Composition | SiO2 | Fe2O3 | Al2O3 | U | BeO | Others |
|---|---|---|---|---|---|---|
| Percentage/% | 74.35 | 8.53 | 7.18 | 0.022 | 0.072 | 8.93 |
| Composition | SiO2 | Fe2O3 | Al2O3 | NaO + K2O |
|---|---|---|---|---|
| Percentage/% | 54 | 0.8 | 43 | 0.3 |
| Categories | SiO2 | Na2O | Be/20 °C | Modulus |
|---|---|---|---|---|
| Parameters | 27.3% | 8.54% | 38.5Be | 3.3 |
| Categories | Uranium Tailings | Metakaolin | NaOH | Water Reducing Agent | Sodium Silicate |
|---|---|---|---|---|---|
| Parameters | 200 g | 54 g | 35 g | 2 g | 32 g |
| Weight Percentage (%) | 5 | 10 | 15 | 20 | 25 |
|---|---|---|---|---|---|
| Parameters (g) | 10 | 20 | 30 | 40 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Q.; Feng, X. Effect of Calcium Chloride on the Reinforcement of Uranium Tailings with Sodium Hydroxide–Sodium Silicate–Metakaolin. Minerals 2025, 15, 526. https://doi.org/10.3390/min15050526
Niu Q, Feng X. Effect of Calcium Chloride on the Reinforcement of Uranium Tailings with Sodium Hydroxide–Sodium Silicate–Metakaolin. Minerals. 2025; 15(5):526. https://doi.org/10.3390/min15050526
Chicago/Turabian StyleNiu, Qianjin, and Xiujuan Feng. 2025. "Effect of Calcium Chloride on the Reinforcement of Uranium Tailings with Sodium Hydroxide–Sodium Silicate–Metakaolin" Minerals 15, no. 5: 526. https://doi.org/10.3390/min15050526
APA StyleNiu, Q., & Feng, X. (2025). Effect of Calcium Chloride on the Reinforcement of Uranium Tailings with Sodium Hydroxide–Sodium Silicate–Metakaolin. Minerals, 15(5), 526. https://doi.org/10.3390/min15050526





