Microstructural Characterization of Expansive Soil Stabilized with Coconut Husk Ash: A Multi-Technique Investigation into Mineralogy, Pore Architecture, and Surface Interactions
Abstract
1. Introduction
2. Materials
2.1. Black Cotton Soil
2.2. Coconut Husk Ash
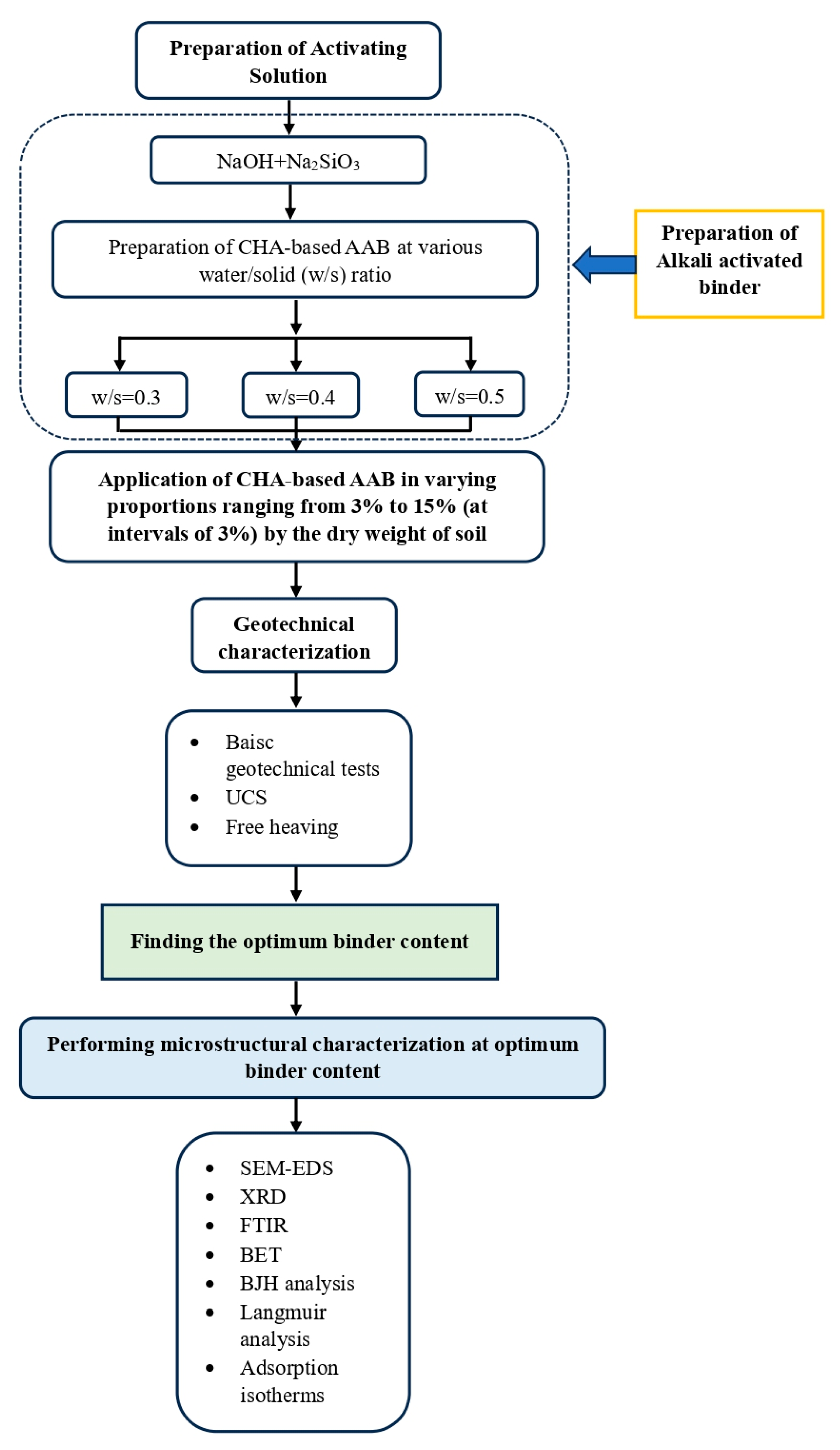
3. Methodology
4. Results
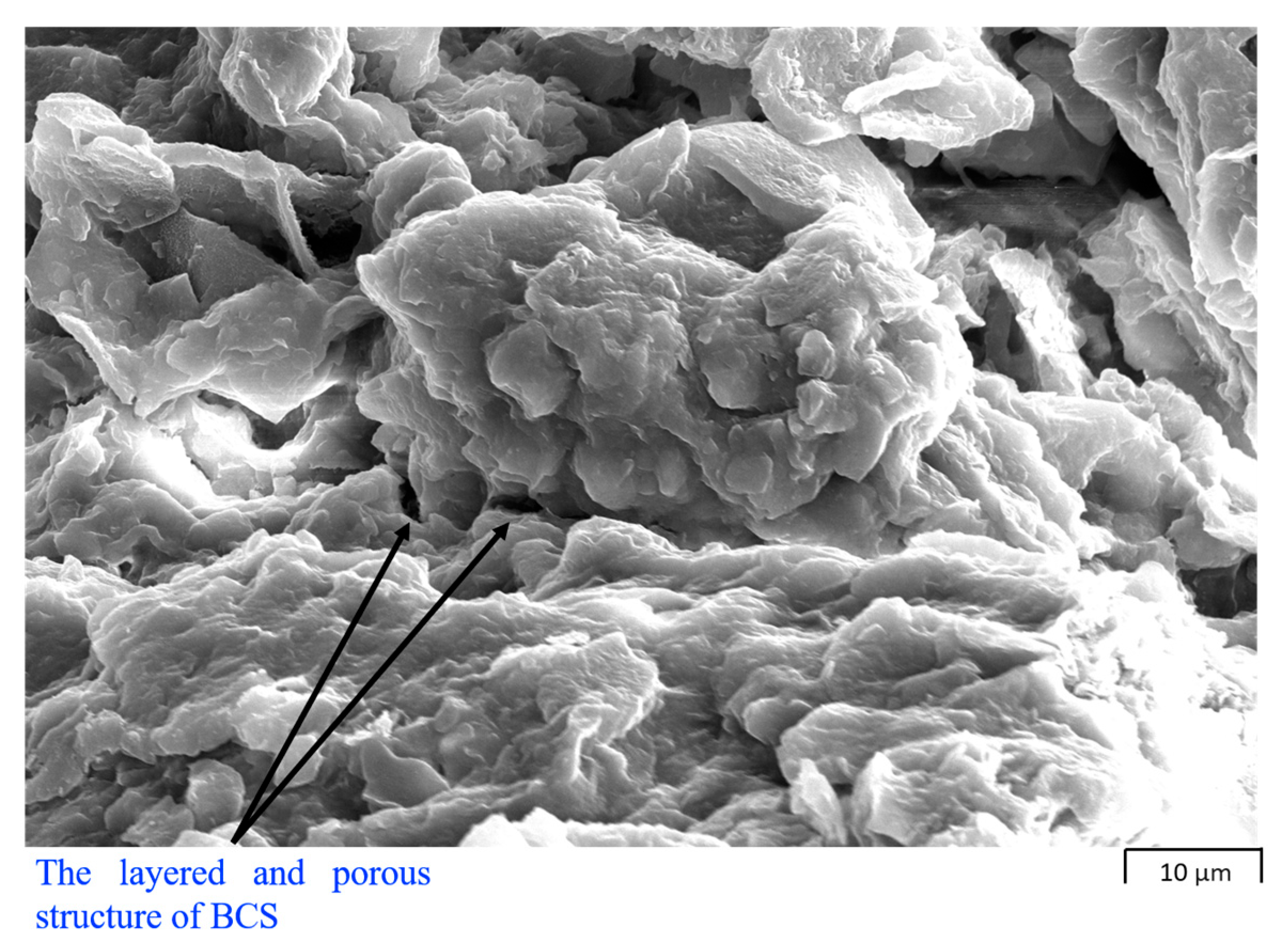
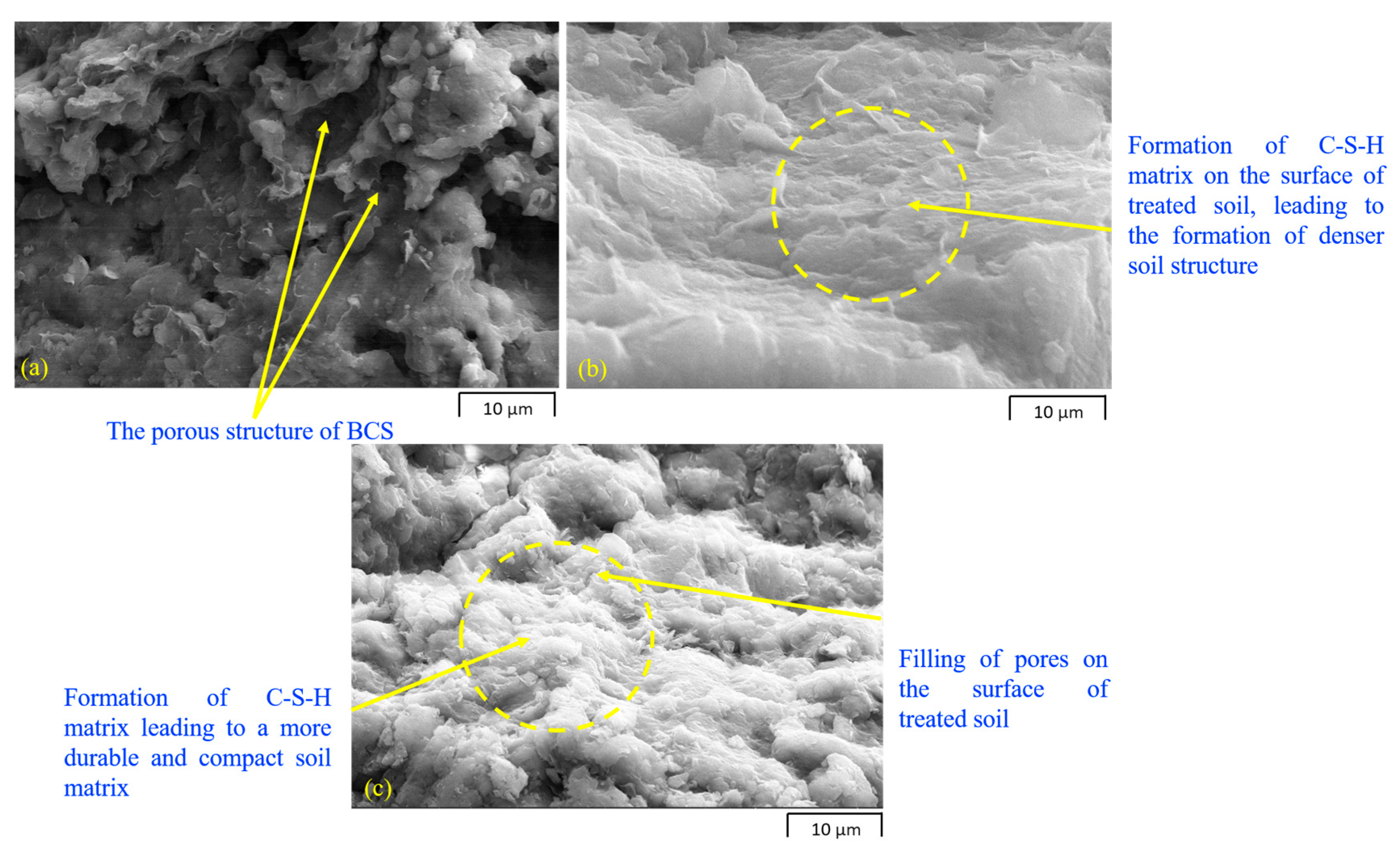
4.1. Scanning Electron Microscopy
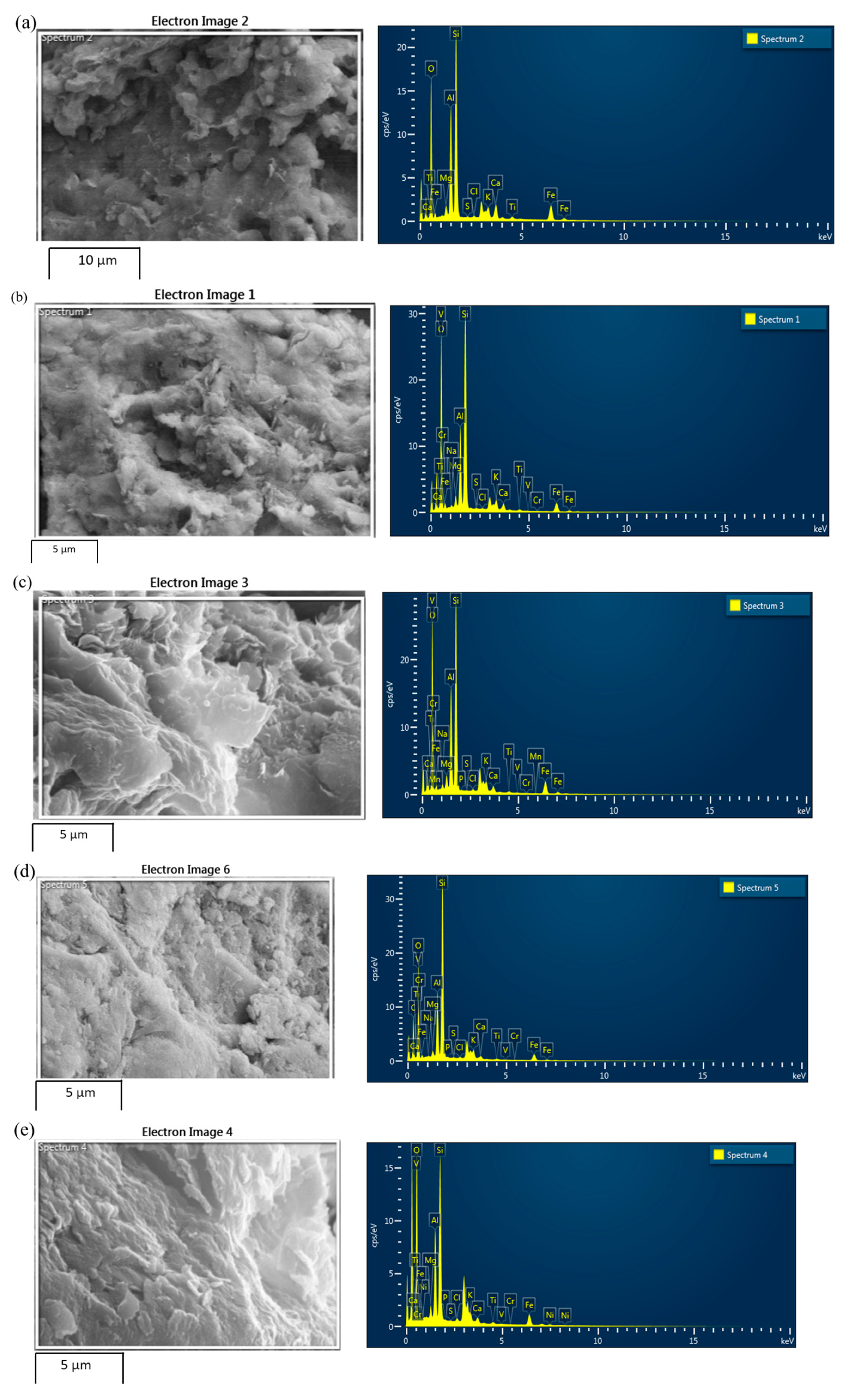
4.2. Energy Dispersive Spectroscopy
4.3. Monolayer Adsorption Capacity
4.4. Adsorption Isotherm, Pore Volume, Pore Diameter and SSA
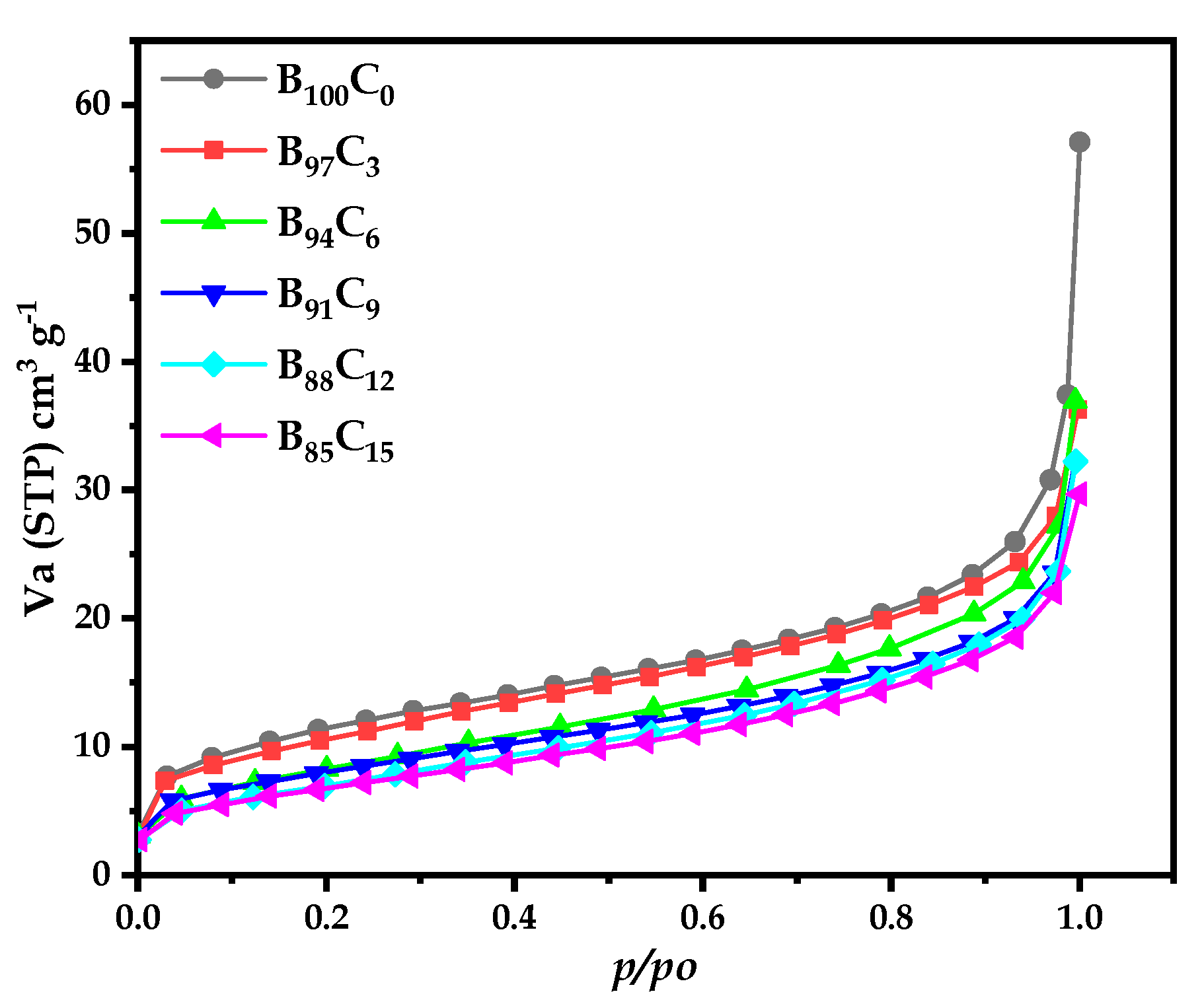
4.4.1. Adsorption Isotherm
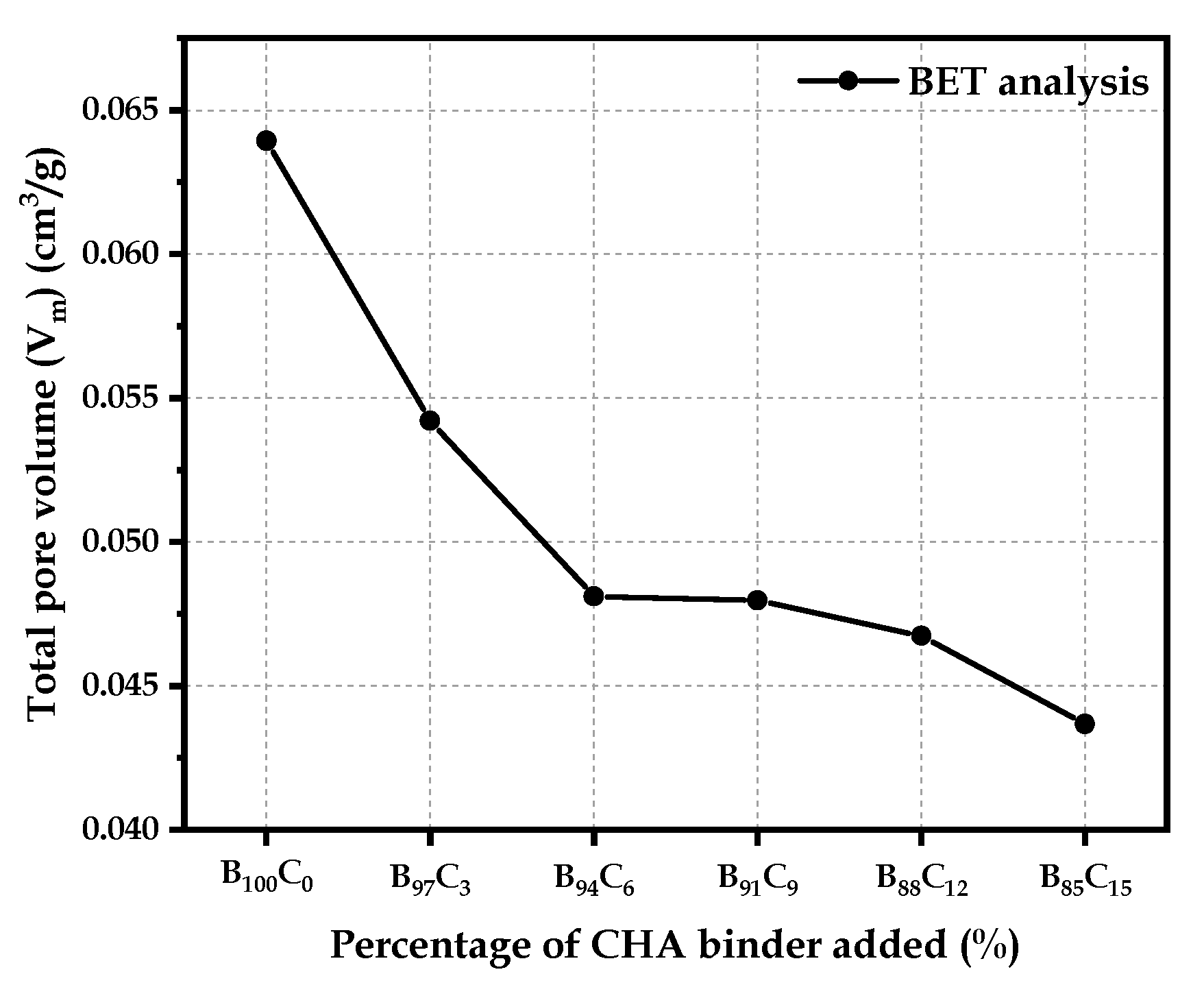
4.4.2. Total Pore Volume
4.4.3. Mean Pore Diameter
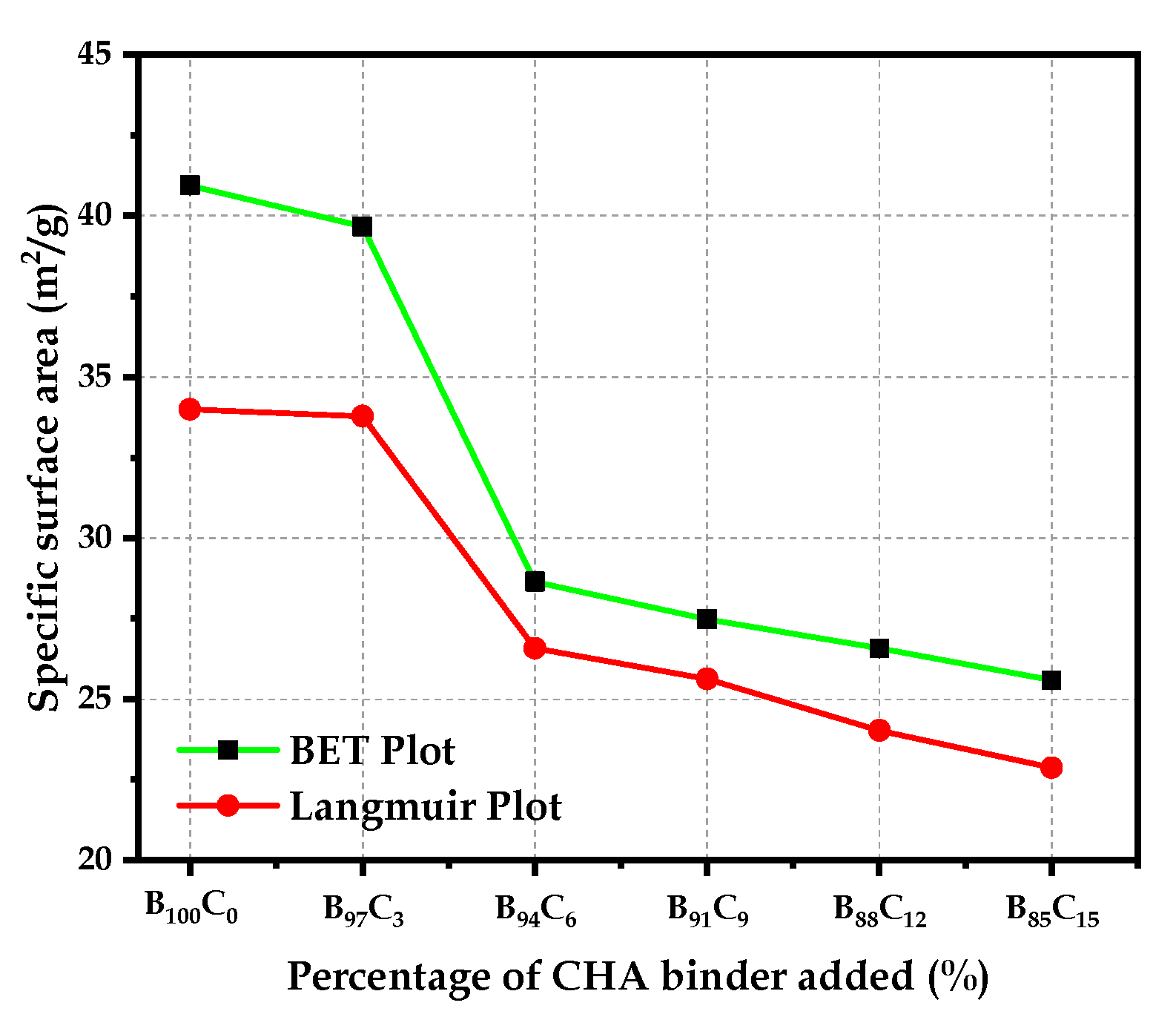
4.4.4. Specific Surface Area
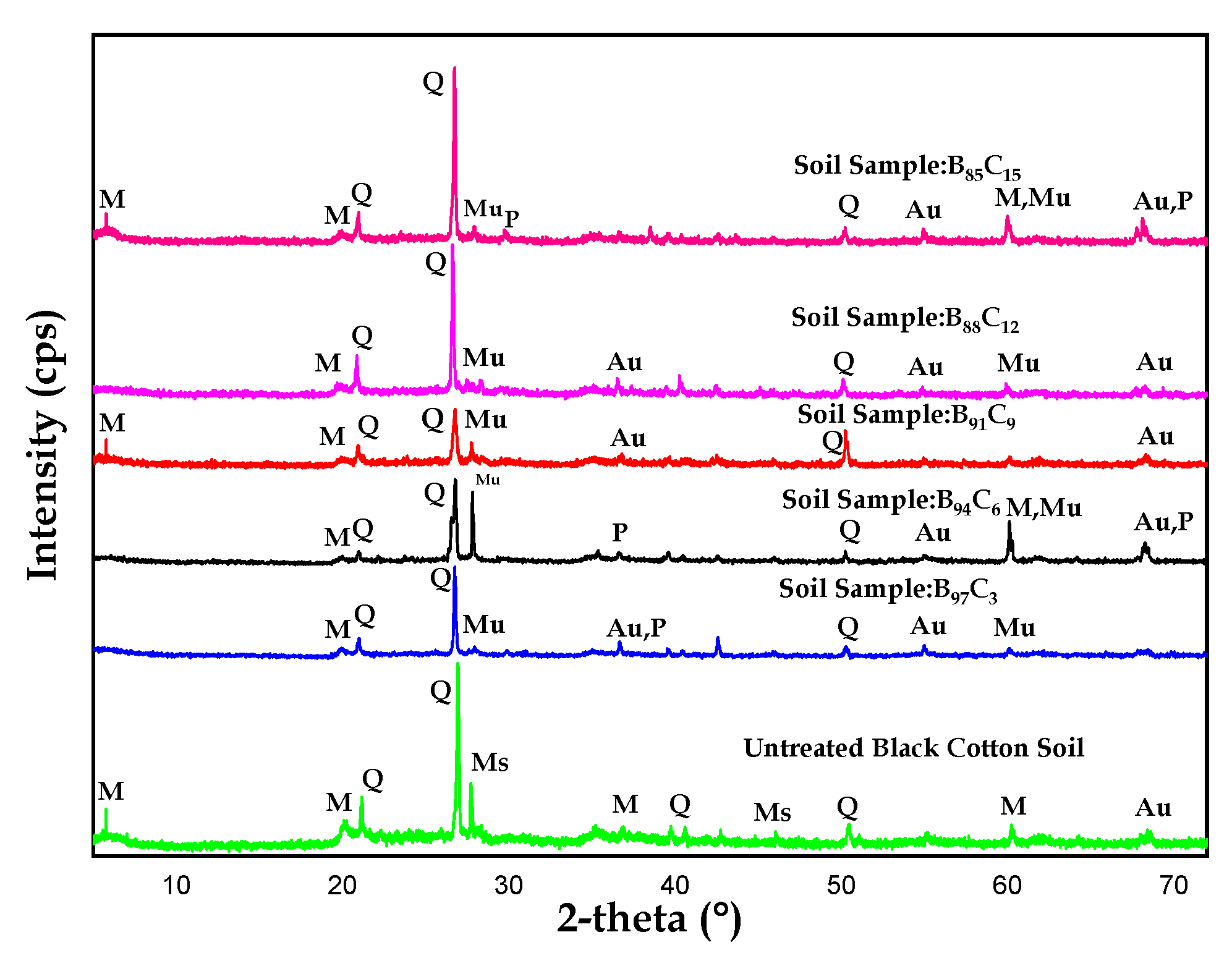
4.5. XRD
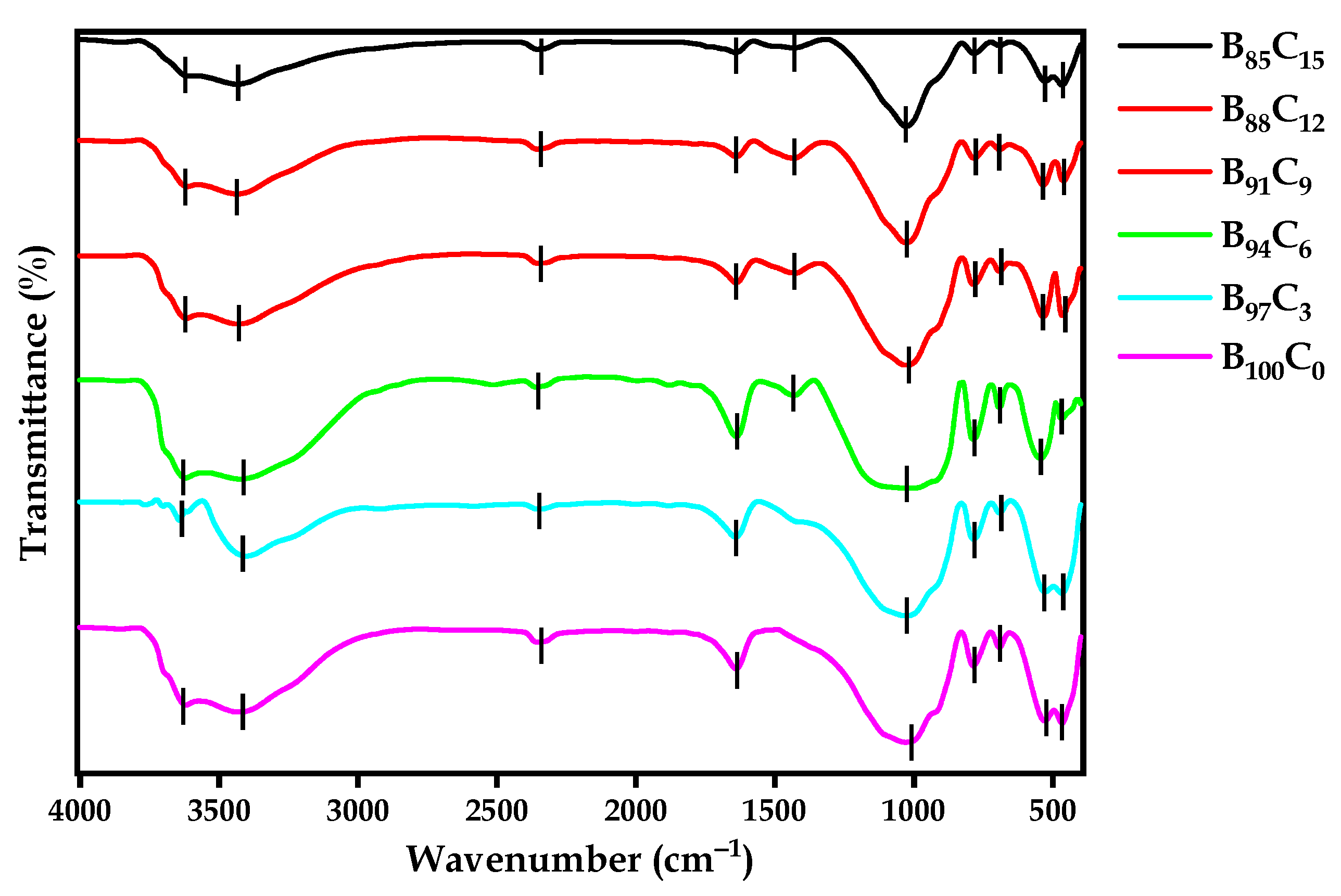
4.6. FTIR
5. Geotechnical Characterization
5.1. Basic Geotechnical Tests
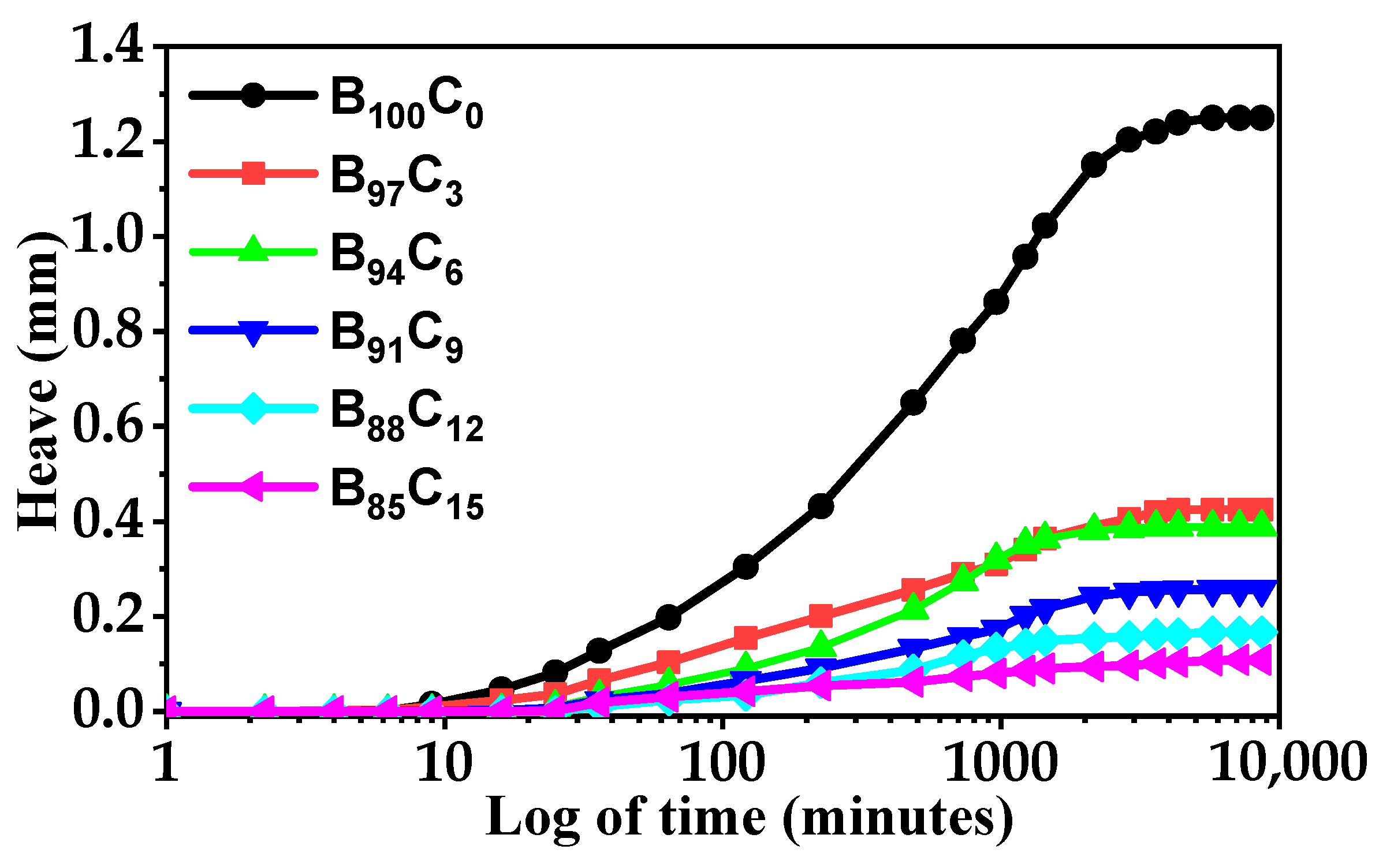
5.2. Free Heaving
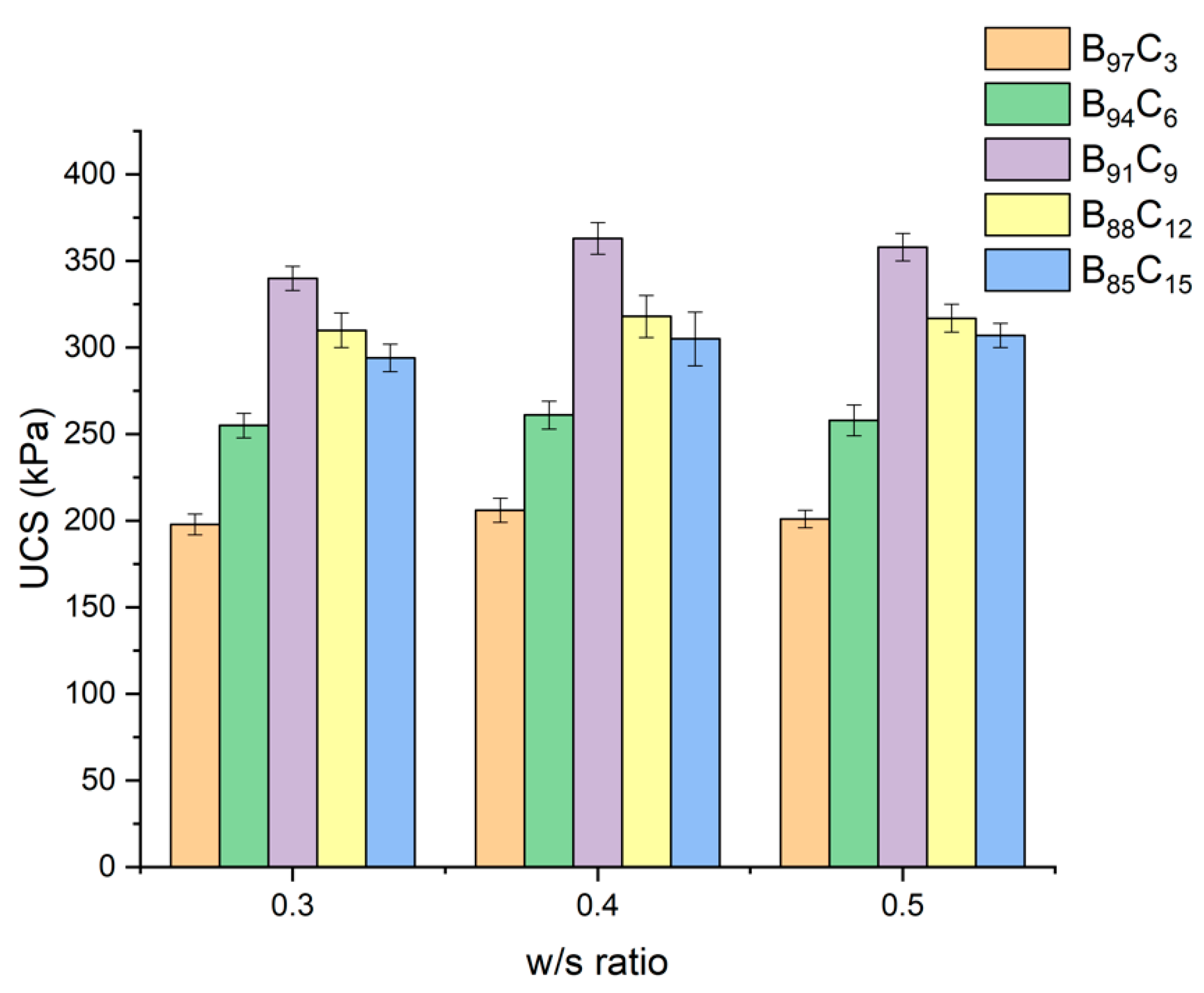
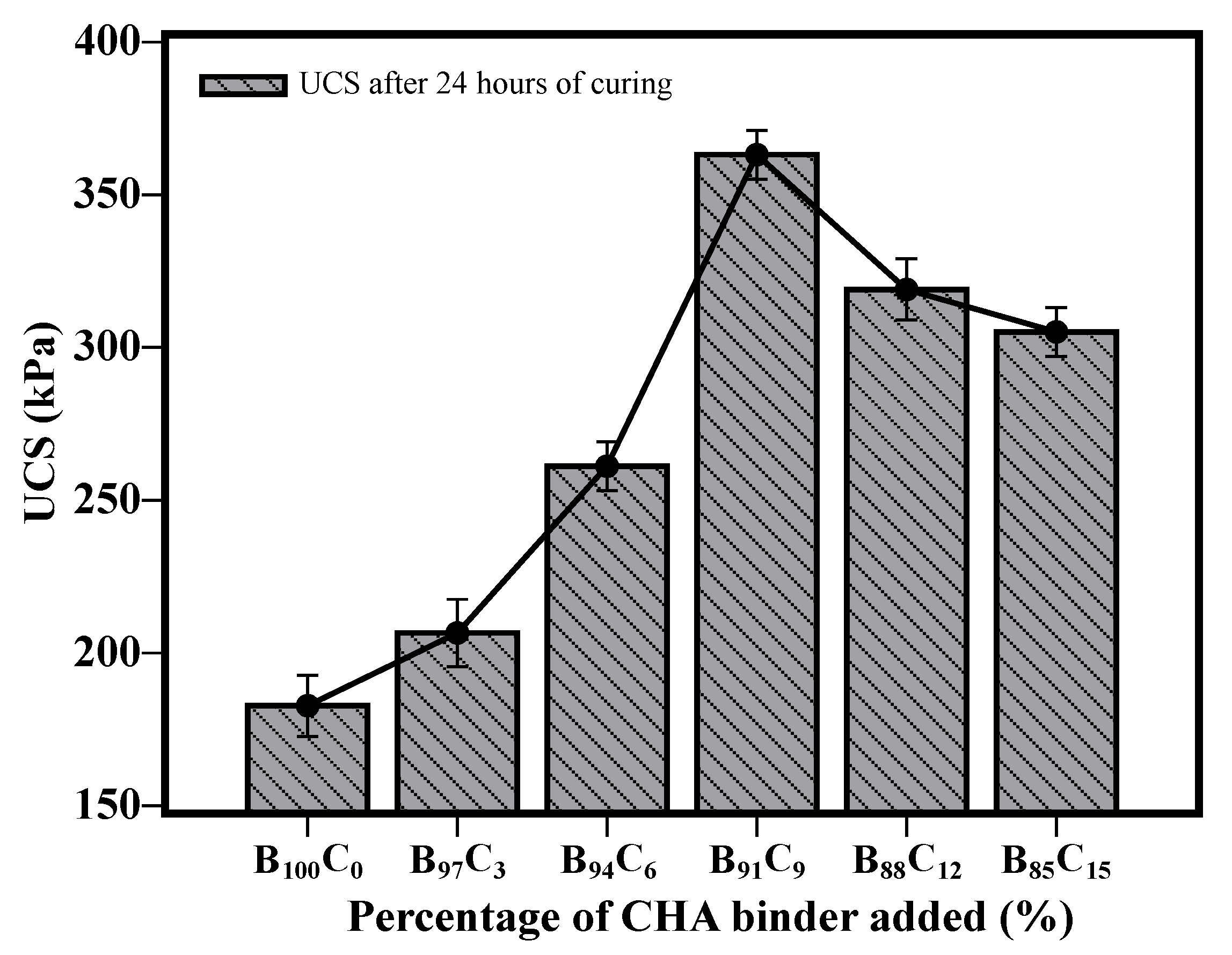
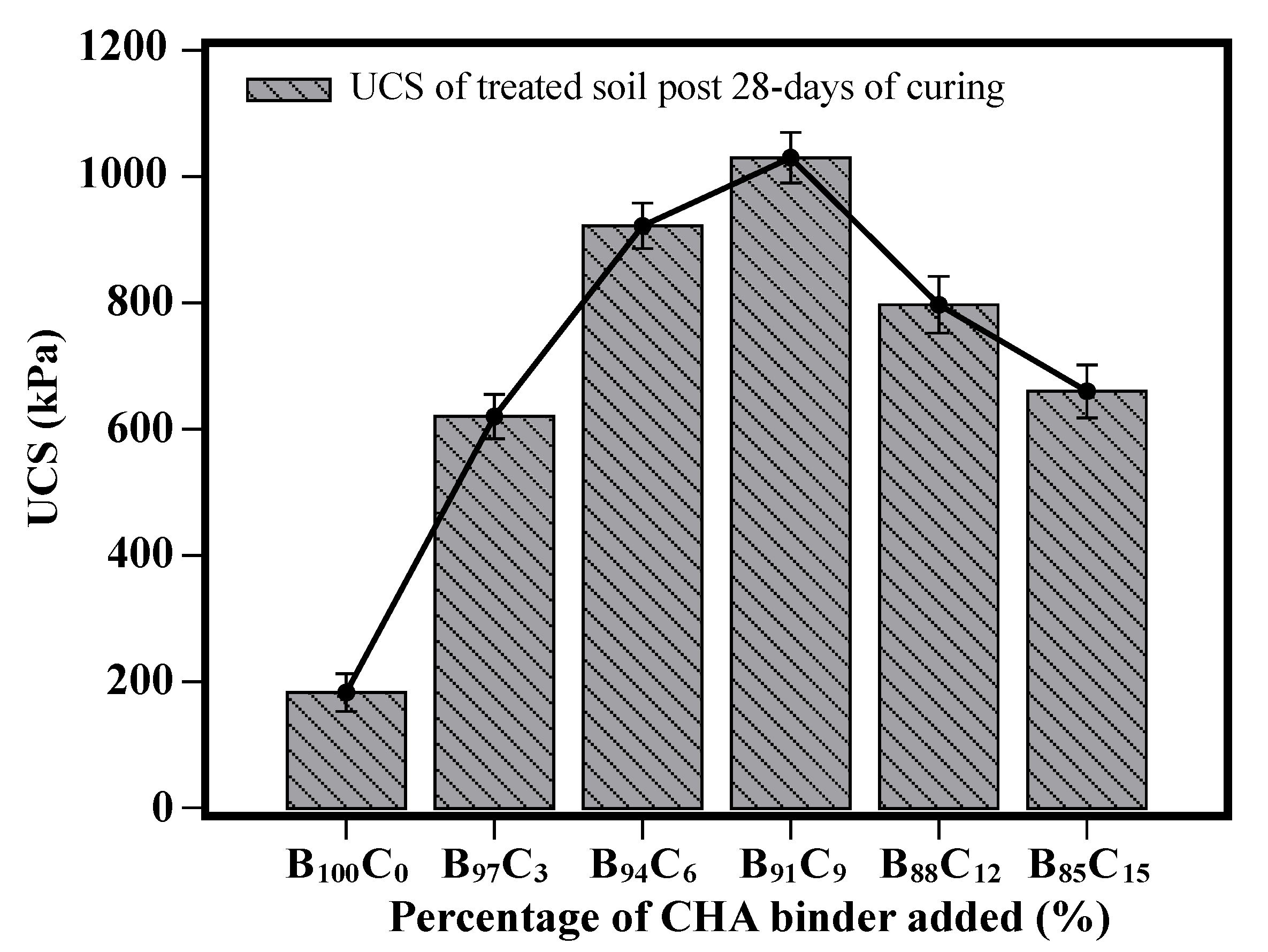
5.3. UCS
6. Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raji, A.K.; Karthika, R.; Amruthalekshmi, G.R.; Peter, A.K.; Sajeer, M.M. Study of Rut Behaviour of Coir Reinforced Black Cotton Soil Using Wheel Tracking Apparatus. In Proceedings of the Indian Geotechnical Conference, Kochi, India, 15–17 December 2011; p. J-258. [Google Scholar]
- Mehta, B.; Sachan, A. Effect of Mineralogical Properties of Expansive Soil on Its Mechanical Behavior. Geotech. Geol. Eng. 2017, 35, 2923–2934. [Google Scholar] [CrossRef]
- Malkawi, D.A.; Rabab’ah, S.R.; AlSyouf, M.M.; Aldeeky, H. Utilizing Expansive Soil Treated with Phosphogypsum and Lime in Pavement Construction. Results Eng. 2023, 19, 101256. [Google Scholar] [CrossRef]
- Mansour, E.; Kinuthia, J.; Oti, J.; Al-Waked, Q. Sulfate Soil Stabilisation with Binary Blends of Lime–Silica Fume and Lime–Ground Granulated Blast Furnace Slag. Transp. Geotech. 2022, 37, 100888. [Google Scholar] [CrossRef]
- Niraula, U.; Dahal, B.K.; Acharya, S.; Phuyal, P. High-Plasticity Silt Stabilization: Role of Waste Stone Dust, Cement, and Curing Time. Results Eng. 2025, 26, 104877. [Google Scholar] [CrossRef]
- Wei, Z.; Deng, Z. Research Hotspots and Trends of Comprehensive Utilization of Phosphogypsum: Bibliometric Analysis. J. Environ. Radioact. 2022, 242, 106778. [Google Scholar] [CrossRef]
- Mazlan, S.A.; Abang Hasbollah, D.Z.; Legiman, M.K.A.; Mohd Taib, A.; Ibrahim, A.; Ramli, A.B.; Jusoh, S.N.; Abdul Rahman, N.; Md Dan, M.F.; Zukri, A. Effectiveness of Coffee Husk Ash and Coconut Fiber in Improving Peat Properties. Phys. Chem. Earth 2023, 130, 103361. [Google Scholar] [CrossRef]
- Anuar, M.F.; Fen, Y.W.; Zaid, M.H.M.; Matori, K.A.; Khaidir, R.E.M. Synthesis and Structural Properties of Coconut Husk as Potential Silica Source. Results Phys. 2018, 11, 1–4. [Google Scholar] [CrossRef]
- Oza, J.B.; Gundaliya, P.J. Study of Black Cotton Soil Characteristics with Cement Waste Dust and Lime. Procedia Eng. 2013, 51, 110–118. [Google Scholar] [CrossRef]
- Puneeth, A.; Nagaraj, A.; Sagar, A.B. Stabilization of Black Cotton Soil Using Portland Pozzolana Cement and GGBS—A Case Study. Int. J. Sci. Res. Civ. Eng. 2021, 7, 7–13. [Google Scholar] [CrossRef]
- Zha, F.; Liu, S.; Du, Y.; Cui, K. Behavior of Expansive Soils Stabilized with Fly Ash. Nat. Hazards 2008, 47, 509–523. [Google Scholar] [CrossRef]
- Kim, T.; Lim, M.-N.; Kim, W.J.; Ho, T.T.; Lee, C.H.; Chae, K.J.; Bak, S.H.; Jin, G.Y.; Park, E.K.; Choi, S. Structural and Functional Alterations of Subjects with Cement Dust Exposure: A Longitudinal Quantitative Computed Tomography-Based Study. Sci. Total Environ. 2022, 837, 155812. [Google Scholar] [CrossRef] [PubMed]
- Dave, N.; Misra, A.K.; Srivastava, A.; Kaushik, S.K. Setting Time and Standard Consistency of Quaternary Binders: The Influence of Cementitious Material Addition and Mixing. Int. J. Sustain. Built Environ. 2017, 6, 30–36. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, Z.; Luo, X. An Improved Mechanistic-Empirical Creep Model for Unsaturated Soft and Stabilized Soils. Materials 2021, 14, 4146. [Google Scholar] [CrossRef]
- Khushbu, B.; Parmar, N.B. Use of Ground Nut Shell Ash and Coal Ash To Modify the Properties of Soil. Int. J. Adv. Eng. Res. Dev. 2017, 4, 991–994. [Google Scholar]
- Krishna, T.M.; Beebi, S.S. Soil Stabilization by Groundnut Shell Ash and Waste Fiber Material. Int. J. Innov. Eng. Technol. 2015, 5, 52–57. Available online: http://ijiet.com/wp-content/uploads/2015/06/811.pdf (accessed on 8 May 2025).
- Sharma, G.; Kaur, M.; Punj, S.; Singh, K. Biomass as a Sustainable Resource for Value-Added Modern Materials: A Review. Biofuels, Bioprod. Biorefin. 2020, 14, 673–695. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, M. Clayey Soil Stabilization Using Flyash and Jute Fibre. Mater. Today Proc. 2021, 48, 1205–1210. [Google Scholar] [CrossRef]
- Syed, M.; GuhaRay, A. Effect of Natural Fiber Reinforcement on Strength Response of Alkali Activated Binder Treated Expansive Soil: Experimental Investigation and Reliability Analysis. Constr. Build. Mater. 2021, 273, 121743. [Google Scholar] [CrossRef]
- Hills, T.P.; Sceats, M.; Rennie, D.; Fennell, P. LEILAC: Low Cost CO2 Capture for the Cement and Lime Industries. Energy Procedia 2017, 114, 6166–6170. [Google Scholar] [CrossRef]
- Lankaran, Z.E.; Nik Daud, N.N.; Rostami, V.; Yusoff, Z.M. Consolidated Drained Triaxial Test on Treated Coastal Soil and Finite Element Analysis Using PLAXIS 2D. Adv. Mater. Sci. Eng. 2022, 2022, 7263333. [Google Scholar] [CrossRef]
- Ahmed, I.; Sharma, A. Use of Coir Fiber And Wheat Husk Ash To Improve the Characteristics of Clayey Soil. Int. J. Eng. Trends Technol. 2019, 67, 85–89. [Google Scholar] [CrossRef]
- Onyelowe, K.C.; Onyia, M.E.; Van, D.B.; Firoozi, A.A.; Uche, O.A.; Kumari, S.; Oyagbola, I.; Amhadi, T.; Dao-Phuc, L. Shrinkage Parameters of Modified Compacted Clayey Soil for Sustainable Earthworks. J. Kejuruter. 2021, 33, 133–140. [Google Scholar] [CrossRef]
- Maruthi, I.; Peter, P. A Comprehensive Study on the Issue of Coconut Production in Karnataka; Agricultural Development and Rural Transformation Centre: Bengaluru, India, 2019; pp. 1–82. Available online: https://desagri.gov.in/aer/2018-19-a-comprehensive-study-on-the-issue-of-coconut-production-in-karnataka/ (accessed on 8 May 2025).
- Foo, K.Y.; Hameed, B.H. Coconut Husk Derived Activated Carbon via Microwave Induced Activation: Effects of Activation Agents, Preparation Parameters and Adsorption Performance. Chem. Eng. J. 2012, 184, 57–65. [Google Scholar] [CrossRef]
- Oluremi, J.R.; Adedokun, S.I.; Osuolale, O.M. Stabilization of Poor Lateritic Soils with Coconut Husk Ash. Int. J. Eng. Res. Technol. 2012, 1, 1–9. [Google Scholar]
- Yusuf, I.T. Investigating the Suitability of Coconut Husk Ash as a Road Soil Stabilizer. Int. J. Technol. 2020, 10, 27–35. [Google Scholar] [CrossRef]
- Bheel, N.; Mahro, S.K.; Adesina, A. Influence of Coconut Shell Ash on Workability, Mechanical Properties, and Embodied Carbon of Concrete. Environ. Sci. Pollut. Res. 2021, 28, 5682–5692. [Google Scholar] [CrossRef]
- Anuar, M.F.; Fen, Y.W.; Zaid, M.H.M.; Matori, K.A.; Khaidir, R.E.M. The Physical and Optical Studies of Crystalline Silica Derived from the Green Synthesis of Coconut Husk Ash. Appl. Sci. 2020, 10, 2128. [Google Scholar] [CrossRef]
- Jain, A.K.; Jha, A.K. Shivanshi Geotechnical Behaviour and Micro-Analyses of Expansive Soil Amended with Marble Dust. Soils Found. 2020, 60, 737–751. [Google Scholar] [CrossRef]
- Malgotra, H.; Deb, P. Characterization of Black Cotton Soil by Using Granulated Blast Furnace Slags. Mater. Today Proc. 2023, 12, 30. [Google Scholar] [CrossRef]
- Ashiru, A.M.; Sanni, J.E.; Mohammed, S. Slope Stability Analysis Using Computer Software for Black Cotton Soil of North-Eastern Nigeria. J. Sci. Multidiscip. Res. 2014, 6, 60–77. [Google Scholar]
- Gobinath, R.; Raja, G.; Prasath, E.; Shyamala, G.; Viloria, A.; Varela, N. Studies on Strength Characteristics of Black Cotton Soil by Using Novel SiO2 Combination as a Stabilizing Agent. Mater. Today Proc. 2020, 27, 657–663. [Google Scholar] [CrossRef]
- Nanda, B.; Mishra, J.; Patro, S.K. Synthesis of Rice Husk Ash-Based Alkaline Activators for Geopolymer Binder Systems: A Review. J. Build. Eng. 2024, 91, 109694. [Google Scholar] [CrossRef]
- Supriya; Chaudhury, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. Low-CO2 Emission Strategies to Achieve Net Zero Target in Cement Sector. J. Clean. Prod. 2023, 417, 137466. [Google Scholar] [CrossRef]
- Pu, B.-C.; Liu, B.; Li, L.; Jiang, L.; Zhou, J.; Ding, P. Using Rice Husk Ash in Alkali-Activated Ultra-High-Performance Concrete: Flowability, Early Age Strength and Elasticity Modulus. Constr. Build. Mater. 2024, 443, 137771. [Google Scholar] [CrossRef]
- Mavroulidou, M.; Gray, C.; Gunn, M.J.; Pantoja-Muñoz, L. A Study of Innovative Alkali-Activated Binders for Soil Stabilisation in the Context of Engineering Sustainability and Circular Economy. Circ. Econ. Sustain. 2022, 2, 1627–1651. [Google Scholar] [CrossRef]
- Lin, Y.; Alengaram, U.J.; Ibrahim, Z. Effect of Treated and Untreated Rice Husk Ash, Palm Oil Fuel Ash, and Sugarcane Bagasse Ash on the Mechanical, Durability, and Microstructure Characteristics of Blended Concrete—A Comprehensive Review. J. Build. Eng. 2023, 78, 107500. [Google Scholar] [CrossRef]
- Aziz, M.; Saleem, M.; Irfan, M. Engineering Behavior of Expansive Soils Treated with Rice Husk Ash. Geomech. Eng. 2015, 8, 173–186. [Google Scholar] [CrossRef]
- Salimzadehshooiili, M. Investigation of the Effect of Frequency on Shear Strength and Damping of Pure Sand and Sand Stabilised with Rice Husk Ash Using Cyclic Triaxial Tests. Adv. Civ. Archit. Eng. 2023, 14, 25–39. [Google Scholar] [CrossRef]
- Darsi, B.P.; Molugaram, K.; Madiraju, S.V.H. Subgrade Black Cotton Soil Stabilization Using Ground Granulated Blast-Furnace Slag (GGBS) and Lime, an Inorganic Mineral. Environ. Sci. Proc. 2021, 6, 15. [Google Scholar] [CrossRef]
- Gingine, V.; Mohammad, W.; Sudheer, Y.; Krishna, P.H. Electrokinetic Treatment on Black Cotton Soil of Warangal, India. In Proceedings of the Indian Geotechnical Conference, Roorkee, India, 22–24 December 2013. [Google Scholar] [CrossRef]
- IS 2720 (Part 4); Indian Standard, Methods of Test for Soils, Grain Size Analysis. Bureau of Indian Standards: New Delhi, India, 1985; pp. 1–38. Available online: https://ia600808.us.archive.org/31/items/gov.in.is.2720.4.1985/is.2720.4.1985.pdf (accessed on 8 May 2025).
- IS 2720 (Part 3); Indian Standard, Determination of Specific Gravity, Fine, Medium and Coarse Grained Soils. Bureau of Indian Standards: New Delhi, India, 1980; pp. 1–8. Available online: https://law.resource.org/pub/in/bis/S03/is.2720.3.2.1980.pdf (accessed on 8 May 2025).
- IS 2720 (Part XL); Indian Standard, Determination of Free Swell Index of Soils. Bureau of Indian Standards: New Delhi, India, 1977; pp. 1–6. Available online: https://ia600206.us.archive.org/17/items/gov.in.is.2720.40.1977/is.2720.40.1977.pdf (accessed on 8 May 2025).
- IS 2720 (Part 5); Indian Standard, Determination of Liquid Limit and Plastic Limit of Soil. Bureau of Indian Standards: New Delhi, India, 1985; pp. 1–16. Available online: https://ia600207.us.archive.org/21/items/gov.in.is.2720.5.1985/is.2720.5.1985.pdf (accessed on 8 May 2025).
- IS 2720 (Part 7); Indian Standard, Determination of Water Content-Dry Density Relation Using Light Compaction. Bureau of Indian Standards: New Delhi, India, 1980. Available online: https://archive.org/details/gov.in.is.2720.7.1980 (accessed on 8 May 2025).
- IS 2720 (Part 20); Indian Standard, Determination of Linear Shrinkage. Bureau of Indian Standards: New Delhi, India, 1997. Available online: https://dn790002.ca.archive.org/0/items/gov.in.is.2720.20.1992/is.2720.20.1992.pdf (accessed on 8 May 2025).
- IS 2720 (Part 10); Indian Standard, Methods of Test for Soils, Determination of Unconfined Compressive Strength. Bureau of Indian Standards: New Delhi, India, 1991; pp. 1–6. Available online: https://law.resource.org/pub/in/bis/S03/is.2720.10.1991.pdf (accessed on 8 May 2025).
- IS 2720 (Part 16); Indian Standard, Method of Test for Soils, Laboratory Determination of CBR. Bureau of Indian Standards: New Delhi, India, 2002; pp. 1–10. Available online: https://dn790008.ca.archive.org/0/items/gov.in.is.2720.16.1987/is.2720.16.1987.pdf (accessed on 8 May 2025).
- IS 27270-41; Indian Standard, Methods of Test for Soils, Measurement of Swelling Pressure of Soils. Bureau of Indian Standards: New Delhi, India, 1977. Available online: https://law.resource.org/pub/in/bis/S03/is.2720.41.1977.pdf (accessed on 8 May 2025).
- IS 2720 (Part 15); Indian Standard, Methods of Test for Soils, Determination of Consolidation Properties. Bureau of Indian Standards: New Delhi, India, 1965. Available online: https://law.resource.org/pub/in/bis/S03/is.2720.15.1965.pdf (accessed on 8 May 2025).
- Shimizu, S.; Matubayasi, N. Surface Area Estimation: Replacing the Brunauer-Emmett-Teller Model with the Statistical Thermodynamic Fluctuation Theory. Langmuir 2022, 38, 7989–8002. [Google Scholar] [CrossRef]
- De Mastro, F.; Cacace, C.; Traversa, A.; Pallara, M.; Cocozza, C.; Mottola, F.; Brunetti, G. Influence of Chemical and Mineralogical Soil Properties on the Adsorption of Sulfamethoxazole and Diclofenac in Mediterranean Soils. Chem. Biol. Technol. Agric. 2022, 9, 34. [Google Scholar] [CrossRef]
- Awad, A.M.; Shaikh, S.M.R.; Jalab, R.; Gulied, M.H.; Nasser, M.S.; Benamor, A.; Adham, S. Adsorption of Organic Pollutants by Natural and Modified Clays: A Comprehensive Review. Sep. Purif. Technol. 2019, 228, 115719. [Google Scholar] [CrossRef]
- Muliwa, A.M.; Oyewo, O.A.; Maity, A. Recent Progress on the Removal of Aqueous Mercury by Carbon-Based Adsorbents: A Review. Inorg. Chem. Commun. 2023, 156, 111207. [Google Scholar] [CrossRef]
- Xiao, W.; Jiang, X.; Liu, X.; Zhou, W.; Garba, Z.N.; Lawan, I.; Wang, L.; Yuan, Z. Adsorption of Organic Dyes from Wastewater by Metal-Doped Porous Carbon Materials. J. Clean. Prod. 2021, 284, 124773. [Google Scholar] [CrossRef]
- Lin, G.; Zeng, B.; Liu, X.; Li, J.; Zhang, B.; Zhang, L. Enhanced Performance of Functionalized MOF Adsorbents for Efficient Removal of Anthropogenic Hg(II) from Water. J. Clean. Prod. 2022, 381 Pt 1, 134766. [Google Scholar] [CrossRef]
- Dollimore, D.; Spooner, P.; Turner, A. The Bet Method of Analysis of Gas Adsorption Data and Its Relevance to the Calculation of Surface Areas. Surf. Technol. 1976, 4, 121–160. [Google Scholar] [CrossRef]
- Jaroniec, M.; Kruk, M.; Sayari, A. Recent Advances in Adsorption Characterization of Mesoporous Molecular Sieves. Stud. Surf. Sci. Catal. 2000, 129, 587–596. [Google Scholar] [CrossRef]
- Haghighatju, F.; Rafsanjani, H.H.; Esmaeilzadeh, F. Estimation of the Dimension of Micropores and Mesopores in Single Walled Carbon Nanotubes Using the Method Horvath-Kawazoe, Saito and Foley and BJH Equations. Micro Nano Lett. 2017, 12, 1–5. [Google Scholar] [CrossRef]
- Awad, M.A.; Khalaf, M.M.; Arbili, M.M. Experimental Measurement of Undrained Shear Strength of Fine Grained Soil-Crude Oil Mixtures Using Different Techniques. J. Eng. Sci. Technol. 2022, 17, 3128–3148. [Google Scholar]
- Sivapullaiah, P.V.; Prasad, B.G.; Allam, M.M. Effect of Sulfuric Acid on Swelling Behavior of an Expansive Soil. Soil Sediment Contam. 2009, 18, 121–135. [Google Scholar] [CrossRef]
- Lin, B.; Cerato, A.B.; Andrew, S.M.; Madden, M.E.E. Effect of Fly Ash on the Behavior of Expansive Soils: Microscopic Analysis. Environ. Eng. Geosci. 2013, 19, 85–94. [Google Scholar] [CrossRef]
- Suluguru, A.K.; Surana, S.R.; GuhaRay, A.; Kar, A.; Muktinutalapati, J. Experimental Investigations on Building Derived Materials in Chemically Aggressive Environment as a Partial Replacement of Soil in Geotechnical Applications. Geotech. Geol. Eng. 2019, 37, 947–963. [Google Scholar] [CrossRef]
- Syed, M.; GuhaRay, A.; Kar, A. Stabilization of Expansive Clayey Soil with Alkali Activated Binders. Geotech. Geol. Eng. 2020, 38, 6657–6677. [Google Scholar] [CrossRef]
- Ping, Y.; Kirkpatrick, R.J.; Brent, P.; McMillan, P.F.; Cong, X. Structure of Calcium Silicate Hydrate (C-S-H): Near-, Mid-, and Far-Infrared Spectroscopy. J. Am. Ceram. Soc. 1999, 82, 742–748. [Google Scholar] [CrossRef]
- Abou-Mesalam, M.M. Sorption Kinetics of Copper, Zinc, Cadmium and Nickel Ions on Synthesized Silico-Antimonate Ion Exchanger. Colloids Surfaces A Physicochem. Eng. Asp. 2003, 225, 85–94. [Google Scholar] [CrossRef]
- Kamseu, E.; Beleuk à Moungam, L.M.; Cannio, M.; Billong, N.; Chaysuwan, D.; Melo, U.C.; Leonelli, C. Substitution of Sodium Silicate with Rice Husk Ash-NaOH Solution in Metakaolin-Based Geopolymer Cement Concerning Reduction in Global Warming. J. Clean. Prod. 2017, 142, 3050–3060. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Satyanarayana, K.G.; Pramada, P.N.; Raghavan, P. Review Processing, Properties and Applications of Reactive Silica from Rice Husk—An Overview. Outlook Agric. 2019, 48, 117–125. [Google Scholar] [CrossRef]
- Dávila-Jiménez, M.M.; Elizalde-González, M.P.; Peláez-Cid, A.A. Adsorption Interaction between Natural Adsorbents and Textile Dyes in Aqueous Solution. Colloids Surfaces A Physicochem. Eng. Asp. 2005, 254, 107–114. [Google Scholar] [CrossRef]
- Nakbanpote, W.; Goodman, B.A.; Thiravetyan, P. Copper Adsorption on Rice Husk Derived Materials Studied by EPR and FTIR. Colloids Surfaces A Physicochem. Eng. Asp. 2007, 304, 7–13. [Google Scholar] [CrossRef]
- Nakbanpote, W.; Thiravetyan, P.; Kalambaheti, C. Preconcentration of Gold by Rice Husk Ash. Miner. Eng. 2000, 13, 391–400. [Google Scholar] [CrossRef]
- Joni, I.M.; Nulhakim, L.; Vanitha, M.; Panatarani, C. Characteristics of Crystalline Silica (SiO2) Particles Prepared by Simple Solution Method Using Sodium Silicate (Na2SiO3) Precursor. Available online: https://iopscience.iop.org/article/10.1088/1742-6596/1080/1/012006 (accessed on 8 May 2025).
- Chandrasekhar, S.; Pramada, P.N.; Praveen, L. Effect of Organic Acid Treatment on the Properties of Rice Husk Silica. J. Mater. Sci. 2005, 40, 6535–6544. [Google Scholar] [CrossRef]
- Wattez, T.; Patapy, C.; Frouin, L.; Waligora, J.; Cyr, M. Interactions between Alkali-Activated Ground Granulated Blast Furnace Slag and Organic Matter in Soil Stabilization/Solidification. Transp. Geotech. 2021, 26, 100412. [Google Scholar] [CrossRef]


| Soil Properties | Values | Standard | |
|---|---|---|---|
| Soil classification (ISSCS) | CH | IS 2720-4 (1985) [43] | |
| Specific gravity (G) | 2.6 | IS 2720-3-2 (1980) [44] | |
| Free swell index (%) | 80 | IS 2720-40 (1977) [45] | |
| Liquid limit (%) | 67 | IS 2720-5 (1985) [46] | |
| Plasticity index (%) | 42 | IS 2720-5 (1985) [46] | |
| Light compaction test | OMC (%) | 23.12 | IS 2720-7 (1980) [47] |
| MDD (g/cc) | 1.66 | ||
| Linear shrinkage (%) | 16.4 | IS 2720-20 (1997) [48] | |
| UCS (kN/m2) | 187 | IS 2720-10 (1991) [49] | |
| CBR (%) | Unsoaked | 1.93 | IS 2720-16 (1987) [50] |
| Soaked | 0.91 | ||
| Swell pressure (kN/m2) | 112 | IS 2720-41 (1977) [51] |
| Property | CHA |
|---|---|
| Color | Black |
| Specific Gravity | 1.83 |
| FSI (%) | 6 |
| Specific surface area (SSA) (m2/g) | 88.9 |
| Total pore volume (cm3/g) | 0.015 |
| Mean pore diameter (nm) | 56.8 |
| Loss on ignition at 100 °C (%) | 3.67 |
| Bulk density (loose) (g/cm3) | 0.71 |
| Material passing through the 75-micron mesh (%) | 53 |
| Oxide (%) | BCS | CHA |
|---|---|---|
| SiO2 | 54.24 | 11.98 |
| Al2O3 | 16.01 | 0.29 |
| Fe2O3 | 9.19 | 2.02 |
| MgO | 8.36 | 3.83 |
| CaO | 6.18 | 8.11 |
| K2O | 1.23 | 42.37 |
| Cl | 0.78 | 31.35 |
| P2O5 | 1.16 | 0.05 |
| Others (SO3 + MnO2 + TiO2) | 2.85 | - |
| Elements | Untreated BCS | 3% AAB | 6% AAB | 9% AAB | 12% AAB | 15% AAB |
|---|---|---|---|---|---|---|
| Si | 34.2 | 41.3 | 46.68 | 51.27 | 56.6 | 59.5 |
| Na | 0.22 | 1.51 | 1.78 | 1.89 | 1.87 | 1.84 |
| Al | 6.31 | 8.71 | 17.55 | 21.27 | 22.6 | 23.8 |
| Mg | 0.82 | 0.85 | 2.25 | 2.83 | 2.93 | 1.74 |
| Ca | 0.77 | 3.68 | 4.62 | 6.98 | 7.21 | 9.2 |
| K | 1.02 | 1.06 | 1.32 | 1.92 | 2.27 | 1.41 |
| Ti | 0.58 | 0.25 | 0.57 | 0.95 | 0.87 | 0.65 |
| Bond Formation | Wave Number (cm−1) | Reference |
|---|---|---|
| -OH (stretching) alcohol | 3619.73 | [68] |
| -OH (stretching) alcohol | 3424.96 | [68,75] |
| -S-H (stretching) thiol | 2513.76 | - |
| O=C=O (stretching) | 2357.55 | [75] |
| H-O-H (bending) | 1639.2 | [72] |
| C-H (bending) alkane | 1430.92 | [71] |
| -OH/Si-O-Si (silanol) | 1077.05 | [69,74] |
| -Si-H | 784.886 | [74] |
| -OH | 466.689 | [76] |
| Properties | BCS | B97C3 | B94C6 | B91C9 | B88C12 | B85C15 |
|---|---|---|---|---|---|---|
| G | 2.6 | 2.47 | 2.32 | 2.28 | 2.1 | 1.98 |
| MDD | 1.66 | 1.69 | 1.75 | 1.86 | 1.85 | 1.81 |
| OMC | 23.12 | 23.68 | 24.1 | 24.69 | 25.89 | 25.91 |
| FSI (%) | 80.0 | 50.0 | 37.5 | 22.22 | 15.79 | 10.0 |
| LL (%) | 66.54 | 60.7 | 56.79 | 55.87 | 53.53 | 52.09 |
| PI (%) | 41 | 34 | 32 | 30 | 26 | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abhishek, A.; GuhaRay, A.; Hata, T.; Abuel-Naga, H. Microstructural Characterization of Expansive Soil Stabilized with Coconut Husk Ash: A Multi-Technique Investigation into Mineralogy, Pore Architecture, and Surface Interactions. Minerals 2025, 15, 516. https://doi.org/10.3390/min15050516
Abhishek A, GuhaRay A, Hata T, Abuel-Naga H. Microstructural Characterization of Expansive Soil Stabilized with Coconut Husk Ash: A Multi-Technique Investigation into Mineralogy, Pore Architecture, and Surface Interactions. Minerals. 2025; 15(5):516. https://doi.org/10.3390/min15050516
Chicago/Turabian StyleAbhishek, Ankur, Anasua GuhaRay, Toshiro Hata, and Hossam Abuel-Naga. 2025. "Microstructural Characterization of Expansive Soil Stabilized with Coconut Husk Ash: A Multi-Technique Investigation into Mineralogy, Pore Architecture, and Surface Interactions" Minerals 15, no. 5: 516. https://doi.org/10.3390/min15050516
APA StyleAbhishek, A., GuhaRay, A., Hata, T., & Abuel-Naga, H. (2025). Microstructural Characterization of Expansive Soil Stabilized with Coconut Husk Ash: A Multi-Technique Investigation into Mineralogy, Pore Architecture, and Surface Interactions. Minerals, 15(5), 516. https://doi.org/10.3390/min15050516






